Abstract
The study of biological form and how it arises is the domain of the developmental biologists; but once the form is achieved, the organ poses a fascinating conundrum for all the life scientists: how are form and function maintained in adult organs throughout most of the life of the organism? That they do appears to contradict the inherently plastic nature of organogenesis during development. How do cells with the same genetic information arrive at, and maintain such different architectures and functions, and how do they keep remembering that they are different from each other? It is now clear that narratives based solely on genes and an irreversible regulatory dynamics cannot answer these questions satisfactorily, and the concept of microenvironmental signaling needs to be added to the equation. During development, cells rearrange and differentiate in response to diffusive morphogens, juxtacrine signals and the extracellular matrix (ECM). These components, which constitute the modular microenvironment, are sensitive to cues from other tissues and organs of the developing embryo as well as from the external macroenvironment. On the other hand, once the organ is formed, these modular constituents integrate and constrain the organ architecture, which ensures structural and functional homeostasis and therefore, organ specificity. We argue here that a corollary of the above is that once the organ architecture is compromised in adults by mutations or by changes in the microenvironment such as aging or inflammation, that organ becomes subjected to the developmental and embryonic circuits in search of a new identity. But since the microenvironment is no longer embryonic, the confusion leads to cancer: hence as we have argued, tumors become new evolutionary organs perhaps in search of an elusive homeostasis.
“It turns out that an eerie type of chaos can lurk just behind a facade of order---and yet, deep inside the chaos lurks an even eerier type of order.”
-Douglas Hofstadter1
INTRODUCTION
How a single fertilized egg develops into a multicellular animal, bearing tissues and organs with distinct morphologies and functions, is one of the most spectacular problems in organismal biology. The establishment of the animal body plan and organogenesis i.e. development of organs within it, is achieved within a time frame that is remarkably short relative to the life-time of the organism (developmental time tables for mouse and chicken are approximately 21 days, and for human is 9 months, in comparison to their average life spans, which are approximately 2-, 6- and 70–80 years). Whereas organ-specificity, the maintenance of organ structure once developed and its functional integrity is not visually as dramatic as the path of their development, how it is logistically maintained through such relatively long life spans, represents an equally fascinating puzzle.
Not surprisingly, most organs do not start functioning in their adult physiological state as they develop. Mammary epithelial cells do not start producing milk until long after puberty, when they proliferate and branch out within the mammary fat pad. Similarly, the pre-cartilage limb cells in vertebrate embryos undergo patterning and differentiation much before the appendages are called upon to function after birth. It is thus reasonable to suppose that the molecular mechanisms of organogenesis will differ from those involved in maintaining the structural and functional identity of the organ (organ specificity) throughout its post-natal period. In fact, organogenesis and organ specificity appear to have different end points: the first results in progressive emergence of divergent states from a single state and the second pertains to the persistence of a single state over time (i.e. homeostasis).
Developing tissues have to be exquisitely sensitive to cues from their external surroundings (macroenvironment) as well to their microenvironments: there has to be rapid and reciprocal cues and responses to allow the diverse changes in both tissue patterns and eventual differentiation. This context-dependent sensitivity in tissue- and organ- phenotype that occurs independent of genotypic changes such as mutation and is reversible with change in context, is known as phenotypic plasticity. The latter is an essential characteristic of development, and has been argued to be a crucial mechanism for the evolution of morphological diversity2, 3. On the other hand, once organogenesis stops, the organ has to become more constrained in architectural response to its external environmental conditions but still be responsive to organ-specific cues for functional performance. We argue that once this distinction gets blurred and organ architecture becomes compromised as well, the organ becomes subject to both developmental and evolutionary rules with the end result being a novel and rapidly evolving ‘tumor organ’2!
In this brief review we undertake a comparative analysis of the relation of organs (before and after they develop) to their micro/macro-environment, and propose how this contributes to making developmental systems malleable, organs constrained, and tumors subject to developmental chaos.
The first section of our review uncovers the dynamics of interaction between developing tissues and cues that come from outside and within the growing embryo. In the second section we discuss the adult organ microenvironment and how it contributes to organ specificity. We end by proposing that loss of organ architecture re-activates the embryonic response in a non-embryonic microenvironment, a root cause of organ confusion in developing cancer. Our arguments are predominantly based on experimental findings in mammary gland biology, especially in relation to specificity, but we believe the general principles would be applicable to any vertebrate organ.
1. DECODING THE DEVELOPMENTAL MICRO- AND MACRO-ENVIRONMENTS
In a theoretical paper in 1982 one of us argued that the unit of function in higher organisms is the cell plus the ECM since the latter sends signals to the nucleus/chromatin and receives signals back in a series of dynamic and reciprocal in interactions4. A later paper defined the unit of function as the organ itself5. The process of organogenesis also is a series of bi-directional and dynamic communications between a community of cells and their microenvironment, resulting in the modification of both: the cells become increasingly differentiated and the microenvironment becomes concurrently more organized to give the organ the final pattern and structure. This model of morphogenesis referred to as dynamic reciprocity4 provides a more realistic narrative than other deterministic models that view organogenesis as consisting of ‘blueprints’ and ‘programs’ of multigene interactions6. Using specific examples, the following subsection shows the nature of microenvironmental cues and how these may influence developmental outcome.
A. Developmental mechanisms and plasticity
In one of the most dramatic biological findings of the 20th century, Wilmut and coworkers did the ultimate experiment: they showed that the nucleus of an epithelial cell from a sheep mammary gland, when inserted into an enucleated embryonic cell in the gestational uterine environment could give rise to an entire mammal7. Precartilage cells of the avian limb in culture can be induced to differentiate into brown adipocyte-like cells despite the fact that the brown fat phenotype and the brown adipocyte differentiation pathway are absent in the avian clade,8 and there are literally ‘numerous’ told and untold other examples. Although nuclear reprogramming - as was performed in Wilmut’s experiments - and the absolute responsiveness of the intact cells within different microenvironmental contexts are viewed by many as distinct phenomena, we will argue here that both point to a fundamental biological principle, i.e. that development and the maintenance and/or re-acquisition of the differentiated state share the same biological plasticity. Evolution of form and function are highly context-dependent outcomes that are determined by the microenvironment in which the genome and the cell find themselves. What are the factors or processes that give rise to these contexts in the first place?
In addition to the microenvironment, the macroenvironment also plays surprising roles in the outcome of the offspring. The dissection of the mechanisms of macroenvironment-dependent variation in development is complicated by the fact that such cues often affect several morphological traits at the same time, and at the molecular level, simultaneously alter the dynamics of several genes9. That said, clear-cut examples do exist where a morphological trait shows variations specific to changes in its surroundings (e.g., temperature-dependent variation in centipede segment number10, the dependence on diet of the presence of horns in beetles11, production of winged offspring by pea aphids in response to predator attack on their colonies12, variation in digit number with alteration in the temperature of egg incubation in chickens13, and the size dependence of penises in intertidal barnacles on water wave dynamics14). How can the influence of the macroenvironment on tissue phenotype be explained at the molecular level? This is a current hot topic of research. One of the links proposed is the endocrine system: temperature regulates the temporal dynamics of ecdysone, an insect hormone that influences the size of the eye-spots in the wings of the butterfly B. anynana15. In an elegant example of predator-induced phenotypic change, juvenile Daphnia develop defensive neckteeth in response to chemical signals from predator insect larvae. This involves upregulation of genes transcribing sex hormones, developmental transcription factors as well as proteins constituting the extracellular milieu e.g., morphogens sonic hedgehog (SHH) and wingless, and epidermal growth factor receptor (EGFR))16. The structuralist school of natural philosophers represented first by Lamarck17 and St Hilaire and currently by Newman and Müller18 among others, ascribes a more direct developmental role of the macroenvironment, wherein biophysical and biochemical factors such as temperature, pH, ionic composition can influence the material (soft19 and excitable20) properties of the developing embryonic tissues thereby transforming cellular patterns.
Interestingly, phenotypic change can occur also due to inherent variation or noise that occurs in all living systems independent of changes in their environment and genotype. Considerable progress in understanding biological noise has been made through investigations on determination of cell fate decisions in prokaryotic systems (e.g. differentiation in Bacillus subtilis21) and simple eukaryotic systems (e.g. stress-response genes in Saccharomyces22). There are well-established examples from multicellular development as well, where stochasticity is employed in constructive ways to give rise to spatial heterogeneity in gene expression and cell fates23, although it is kept under stricter control by a host of regulatory mechanisms including, but not limited to, synchronous expression by stalled polymerases24 and inter-nuclear communication in order to minimize error in readout of signals from transcriptional factors25. In certain cases noisiness is actively recruited to give rise to patterns, for example the random patterning of color photopigments in the compound eye of fruitflies26 and cell fate determination of gonadal precursor cells in C. elegans27. There are excellent reviews for a more in-depth discussion of the role of stochasticity in cell state change23 and tissue patterning28. In summary, the state of being malleable (‘plastic’) to respond to external signals by changing shape, function or destiny through stochasticity and micro- and macro-environmental influences, is a key property of development. As Kirschner and colleagues state: “Interconvertible multi-statedness is a key aspect of multicellular self-organization”29, but we like to add that ‘interconvertibility’ would surely be subjected to microenvironmental influences.
The establishment of antero/posterior axis in drosophila embryo is in response to bicoid gradient30 and the transformation of mammalian foregut endodermal cells into liver progenitors is induced by signals from cardiac endoderm31. As these examples show, the developmental microenvironment includes both extracellular molecules, as well as cells that are adjacent to, but not necessarily part of the prospective organ parenchyma. In addition, as the variation in form and function mentioned above in B. anynana and Daphnia indicate, the developmental microenvironment is integrated with cues from outside of the embryo as well, in order to influence the phenotypic outcome of developing tissues. In the rest of this subsection we focus on the developmental microenvironment, specifically its three major and ubiquitous molecular components: morphogens, ECMs and cell membrane proteins that mediate juxtacrine interactions.
B. Morphogens
The discovery of developmental microenvironment can be traced to the classical transplantation experiments by Lewis, Spemann and Mangold that led to the discovery of embryonic induction, the ability of embryonic tissues to influence each other’s fate32, 33. Lewis’s remarkably prescient quote: “It will be natural to inquire into the nature of these influences to determine if they are mechanical, chemical, electrical or unknown influences still to be discovered” set the stage for the investigation of the makeup of the tissue microenvironment34. The first of these “influences”, revealed through rigorous biochemical studies of the 20th century, are morphogens, soluble molecules that are secreted from one tissue, diffuse within the extracellular space and bind to receptors in distal tissues altering their phenotype35.
Several vertebrate organs that show branching morphogenesis (e.g., mammary gland, lung, salivary gland, kidney) develop through interactions between the epithelial tissue and the surrounding stromal mesenchyme, involving an exchange of morphogenic signals between both tissues36. Mammary gland development follows this recipe, although it differs from other above-mentioned organs in that it develops postnatally. In preadolescence, a small rudimentary gland with a limited number of tiny branches is present within the fat pad and the principal morphogenesis is initiated by pubertal endocrine dynamics37. Studies on ovariectomized mice38 or mice with knockout of estrogen receptor ER-α39 reveals that the endocrine system stimulates the expression of the morphogen amphiregulin40 that interacts with epidermal growth factor receptor (EGFR) to induce elongation of mammary gland ducts. Experiments using 3D laminin-rich ECM (lrECM) culture assays with mammary gland organoids, established in the Bissell laboratory41 show that the morphogens FGF-2 and -7 induce branching in organoids of EGFR-null mice40, 41 suggesting that they lie downstream of the amphiregulin-EGFR pathway. TGF-β, another morphogen under the control of female sex hormones42 regulates both quiescence43 and branching pattern of the mammary epithelia in normal glands43–45 (see Figure 1 upper panel). Mammary branching morphogenesis is therefore a good example of how the endocrine cues influence the immediate components of the tissue microenvironment such as morphogens to influence spatial patterning of cells during development.
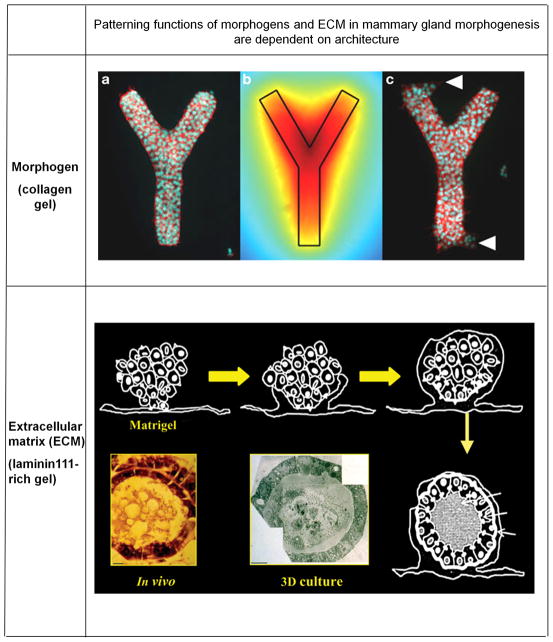
Figure 1. Patterning functions of morphogens and ECM in mammary gland morphogenesis.
Upper panel: Confocal image of an engineered Y-shaped mammary epithelial branching tubule with (a) cells stained for actin (red) and nuclei (green). The computed spatial map of inhibitory diffusible morphogen TGF-β, produced by mammary epithelial cells (and shown in the form of a heatmap in (b) predicts that the local concentration of the inhibitor is highest at the point of trifurcation (where initiation of branching does not take place) and lowest at the distal ends of the tubules, where branching is allowed (c). Branching is determined by the interplay of the local tissue geometry and the spatial geometry of morphogen diffusion. (Adapted from Nelson et al 200644; Hayes et al, 2006128)
Lower panel: Mouse mammary epithelial cells when cultured on top of laminin-111-rich ECM, develop into spheroid multicellular structures that are encased in basement membrane. These structures resemble in vivo mammary acini in size and shape. After four days, a lumen forms through epithelial cell death in the center. The lumen then fills up with milk, secreted by the peripheral epithelial cells. The lower left panel is a light microscope picture of an acinus from a section of a gland in vivo. The lower middle panel shows a low magnification transmission electron microscopic image of an acinus formed in culture. (Adapted from Barcellos-Hoff et al, 1989129)
C. Extra Cellular Matrices: Signaling scaffolds
ECM proteins, along with their membrane receptors such as integrins, dystroglycans46 and endopeptidases that cleave and remodel ECMs such as metalloproteinases (MMPs), constitute an important axis of the developing microenvironment. The role of the ECM as a mechanical link between the epithelial and mesenchymal tissues during their developmental interaction was first postulated by Clifford Grobstein in 195547. Since then, ECM has been shown to mediate tissue patterning and differentiation in almost all developmental systems; some of the earliest demonstrations included collagen-mediated differentiation of myoblasts in myotubes48, and the role of collagen in differentiation of corneal epithelia49. In an interesting switch, culture studies on how the differentiated state is maintained (which we will discuss in the subsequent section on organ-specificity), have provided additional clues to the contribution of ECM to organogenesis. The idea that ECM molecules in general, and the basement membrane (BM) in particular, would signal via their receptors to the nucleus and chromatin and thus would play a crucial role in organ-specificity and homeostasis was articulated in 1982, and then 1987, based on cell culture studies on liver hepatocytes and mammary epithelial cells4.
Briefly reviewing the developmental role of ECM in mammary morphogenesis, collagen I is one of the first ECM proteins that appears during ductal elongation in the pubertal mammary gland in vivo50. In fact the stromal collagen I achieves an orientation prior to branching morphogenesis that then determines the direction in which the invading epithelia branch and proliferate51. Collagen I is predominantly distributed around the large mammary ducts during postnatal development. During branching within the mammary gland of the virgin mice, the invading edges of the epithelia, also known as the terminal end buds, are surrounded by a BM that is predominantly composed of hyaluronic acid52. In contrast, the basement membrane surrounding the ducts consists of a distinct set of ECM molecules which includes laminin-111, collagen IV, laminin-322 and HSPGs53.
The most well-studied class of ECM receptors are integrins, a family of evolutionarily conserved proteins that function in almost every organogenetic process, and particularly in the development of branched organs such as salivary gland54, kidney and lung55. In vivo studies on mammary morphogenesis in mice show for instance, that the deletion of α2 integrin and the functional inhibition of β1 integrin attenuate mammary ductal branching56,57. We are still discovering the precise spatiotemporal expression of different integrins during mammary development58. Given that integrins have a large set of ligands they can bind to, it is possible that the same integrin subunit may participate in binding to different ligands in different locales of the gland.
The role of MMPs, the ECM remodeling enzymes, in mammary ductal morphogenesis is well documented. The membrane-bound MT1-MMP and diffusible MMP-2 are found at the invasive front of penetrating terminal end buds where MMP-2 facilitates the invasion of the TEBs into the stroma59. MT1-MMP has been shown also to facilitate the invasion of mammary epithelial cells by softening the collagenous microenvironment contiguous with the invading front of mammary cells in 3D culture60, but most importantly as mentioned above, it is responsible for the invasion of the epithelial cells through its transmembrane/cytoplasmic domain which interacts with β1 integrin for signaling58. MMP-2 represses lateral budding in mid-pubertal mammary glands. MMP-3 does not affect primary ductal invasion but promotes lateral branching both in vivo and in 3D gels in culture59.
ECM can bring about and transform tissue patterns by altering cellular motility (e.g., neural crest migration61) and by spatially modulating cellular quiescence and proliferation (presence of laminin-111 in the BMs of already-formed mammary epithelial ducts inhibits cellular proliferation whereas its absence at the TEBs allows for the growth and invasion62). In addition, ECM composition and its interaction with cells allows it to influence the rheological properties (such as viscosity and elasticity) of developing tissues influencing their phenotypic outcome (cells in tissues with ECM organized as basement membranes can exhibit buckling, wrinkling and folding effects63, unlike mesenchymal tissues where cells are embedded in a more rigid ECM36. In fact extensive exon/domain shuffling of ECM proteins coincided with64, and likely contributed to, the earliest events of metazoan evolution that witnessed an incredible plasticity in multicellular morphologies65.
D. Juxtacrine signaling
The third component of developmental microenvironment that we consider in this review consists of receptor-ligand pairs that reside on the cell surface and interact with each other to initiate signaling. This interaction occurs when one cell bearing a receptor and the other bearing its cognate ligand are next to each other, hence the signaling is referred to as juxtacrine. Two well-known examples of juxtacrine signaling are the interaction between the Notch family of receptors and its ligands, and the interaction between Eph receptor tyrosine kinases and their transmembrane ligands, Ephrins. Notch pathway contributes to the determination of cell fates through the process of lateral inhibition: it amplifies stochastic differences in expression of Notch and its receptors, which transforms a relatively uniform cell population into two distinct populations66. This is evidenced during Notch mediated conversion of proneural clusters into neuronal and glial cells during neurogenesis. Notch is also involved in tissue compartmentalization67 and during vertebrate somitogenesis67, processes where it is utilized to communicate cell-fate decisions across spatial scales. Ephrins and their counterreceptors Ephs have been traditionally described as essential cues for axon guidance68 but they influence cellular patterning in important developmental processes such as neurogenesis69, somitogenesis70 and angiogenesis71. Here we will briefly discuss the role of Eph-Ephrin in mammary branching morphogenesis.
Microarray analysis and in situ hybridization data72 suggest that Ephrin-B1 is enriched significantly in the stroma surrounding the TEBs relative to the cells surrounding the ducts. On the other hand, the transcript levels of ephrin receptor EphA2 is high in the TEB epithelia in comparison to ductal epithelial cells. Although there is no evidence that the epithelial EphA2 interacts with the stromal Ephrin-B1, it seems likely that an Eph-Ephrin juxtacrine interaction involving one or both of Ephrin-B1 and EphA2 is unique to the TEB microenvironment making it different from the ductal microenvironment. Mammary glands of mice with EphA2 deletion show decreased epithelial invasion into the mammary fat pad and impaired branching, consistent with the notion that juxtacrine signaling plays an important role in ductal patterning during mammary gland development73.
We have used the examples of morphogens, ECM and juxtacrine signaling to decode some of the characteristic interactions between cells and their microenvironment in a developmental context. These components interact with each other, recruit cues from other parts of the developing embryo as well as also respond to external macroenvironment. This makes the phenotype of developing tissues an emergent outcome that is shaped by the dynamic context surrounding the cells. In the case of postnatally developing mammary gland, cues from endocrine system and the surrounding stroma act on the mammary epithelial cells along with their microenvironmental components such as morphogens and ECM molecules in order to give rise to the mammary branching pattern.
Once the organ forms, and becomes functionally differentiated, its structure and pattern must be ‘locked in’ in vivo to maintain homeostasis. In the next section, we summarize work describing the microenvironment of the adult functioning organ and discuss how it may participate in this ‘locking in’ or ‘breaking out’.
2. DECODING THE ORGAN MICROENVIRONMENT
Organs in the post-developmental stage of an organism’s life history generally differ from their developmental stage in three aspects: 1. Architectural constraints: Organs achieve an anatomical architecture (through dynamic and reciprocal signaling between the chromatin and the extracellular components that make up the organ microenvironment) that makes them distinct from each other, and which are generally well insulated from external macroenvironmental influences relative to the embryonic tissues. 2. Integration: The organ microenvironment is integrated with other organs and tissues through the cardiovascular, nervous and endocrine systems. Some organs undergo changes in architecture in response to cues from these systems during the life of the organism. As a result, the term macroenvironment now refers to all the other tissues and organs of the adult organismal body that signal to the organ and influence its phenotype. 3. Delineation of labor: Cells and tissues within a given organ each perform a specialized function. For example, in the case of the mammary gland, luminal epithelial cells (LEPs) are involved in production and secretion of milk, and the myoepithelial cells (MEPs) contract to facilitate milk secretion. This is traditionally referred to as ‘terminal differentiation’, originally used by geologists to refer to specific chemical changes in rocks74 but appropriated by biologists at the turn of the 19th century to refer to what was perceived to be, ‘irreversible’ changes in fate of cells75. The rigidity of the term “terminal”, well suited for the rigid geology, is poorly suited for biology and has for too long prevented biologists from looking beyond the cell as a unit of function.
There is some old literature, unfortunately little read let alone cited, that demonstrates that differentiation is also entirely dependent on its context. Elegant experiments performed almost half a century ago by Deome and colleagues showed that even ‘terminally differentiated’ mammary epithelia, upon transplantation into mammary fat pads cleared of their epithelia, could recapitulate the formation of the entire mammary epithelial tree76. Recent work by Gilbert Smith’s group shows that ectodermal cells from semeniferous tubules of adult testes77 and in another instance neural stem cells78 (both from a murine strain expressing the Rosa26–LacZ reporter gene under Wap-Cre promoter during pregnancy), when mixed with mammary epithelial cells and placed in epithelia-free fat pads were redirected to exhibit mammary epithelial phenotype and function. Of course the ultimate example is Dolly: cells from a frozen mammary gland could recreate the entire animal7. Thus differentiation in an adult functioning organ is not intrinsically terminal; it is a contextual function of its organ microenvironment. The latter keeps the cell in its differentiated state. It also suggests that the microenvironment of an adult organ is essentially inhibitory: to maintain homeostasis, the microenvironment must prevent inappropriate growth79, apoptosis80 and trans-differentiation. The cell rarely, if ever, is autonomous within an organ. The next section presents evidence from mammary gland biology supporting this notion.
A. The mammary acinus - and not isolated mammary epithelial cells bereft of their ECM and tissue polarity - is the unit of lactating mammary function
Much of our knowledge regarding the molecular mechanisms responsible for the structure and function of the lactating mammary gland has come from a combination of engineered mice and 3D cultures of mammary epithelial cells in lrECM (see Figure 1 lower panel) or floating collagen gels81, 82. This is because when fully functioning tissues, including mammary epithelia are taken from the organ, dissociated and cultured on rigid and flat substrata, they lose form and function very rapidly83. The mammary cells lose their functional capacity to respond to lactogenic cues or to secrete milk proteins83. In contrast, appropriate microenvironmental contexts such as floating collagen gels allow expression of tissue-specific genes4, 81, 82, 84 or formation of a few of the milk proteins such as the caseins82. The latter was shown later to be a function of the formation of a basement membrane85, in particular the presence of laminin-111. Additionally we now know that the reason that the cells become functional in floating collagen gels and are able to form the BM is because in the detached gel, tension is released and the cells are able to become polarized86. That laminin-111 is sufficient for producing β-casein as long as the cells are within or on top of a gel was demonstrated by the fact that even single cells will express β-casein when laminin-111 was added to collagen gels87. Production of other milk proteins, however, requires an additional layer of complexity. Formation of acini with tight junctional complexes that form the lumen is required for expression of whey acidic protein, another milk protein in rodent milk88, 89.
In vivo, the acini form at the distal end of the ducts of the mammary arbor during mid-pregnancy and lactation. An acinus consists of luminal (LEPs) and myoepithelial cells (LEPs) and is surrounded by a BM consisting of laminin-111, laminin V, collagen IV and other globular proteins and glycosaminoglycans90. β-casein gene expression in the acini is dependent on the interaction between β1 integrin and laminin-11191. Using 3D cultures of LEPs embedded in collagen I, with and without MEPs, Gudjonnson and coworkers showed that MEPs are responsible for laminin-111 synthesis and therefore formation of laminin-111-containing BM92. The LEPs secrete milk in response to lactogenic cues from the endocrine system only when cells’ are provided correct cues by their microenvironment, in this case laminin-111. The latter includes MEPs in addition to the BM, in order to form the ‘double layered tube’ that exists in vivo.
There is another aspect to the acinar architecture: the cells have to be arranged in a way that makes the whole acinus polar. The LEPs always lie to the apical side of MEPs. Milk secretion is always directed centripetally from the apical surface of LEPs into the lumen and this polarity of the structure has profound implications for functional differentiation.
B. The central significance of acinar polarity
How is acinar polarity attained and maintained? Mammary epithelial cells cultured in 2D monolayers have ‘cellular polarity’, which is an ancient cellular behavior, predating multicellularity93, whereby the cells acquire distinct basal and apical surfaces. There is a difference, however, between the polarity of the tissues in an appropriate 3D context and cells in monolayers.
The comparative study of the formation of polar acini by culturing two well-established human mammary cell lines - S1 (the non-malignant component of the HMT3522 progression series94) and MCF-10A in lrECM demonstrates that basal and apical polarity are established via distinct but overlapping mechanisms95. Both S1- and MCF10A- derived acini show basal polarity when cultured in 3D lrECM, but only S1 acini express Zonula Occludens-1 (a marker for glandular apical polarity)92, 96. Using a high-throughput culture assay to estimate the relative contribution of different BM components to apical polarity, it was shown that the establishment of apical polarity requires also collagen IV95.
Moreover, acini formed by culturing S1 or MCF10A in 3D, using chicken BM do not achieve significant polarity even though CBM contains both laminin-111 and collagen IV. CBM unlike lrECM produced by a tumor from mice97 does not form a gel95. Therefore, in addition to chemical constitution, physical properties of the BM are important for tissue polarity86.
Which comes first: Cellular polarity or the acini? Tanner et al. showed recently that the ability to form acini from single adult epithelial cells involves a novel morphomechanical process98: Single mammary epithelial cells within an lrECM gel, undergoes multiple rotations, where actin is organized to one of the poles before dividing; as non-malignant cells divide, there is attachment and polarity between the mother/daughter cells as well as their progenies produced by the progressive cycles of cell division (Figure 2A). The motion is synchronous, hence referred to as coherent angular motion (CAMo); its perturbation by pharmacological inhibition of actomyosin network leads to non-polar amorphous cellular masses. Interestingly, the silencing of partitioning deficient 3 homolog (PAR3), a tight junction protein involved in cell polarity as well as inhibition of E-Cadherin resulted in termination of CAMo and formation of shapes other than acini, suggesting that acinar morphogenesis is dependent on cell-cell adhesion and polarity.
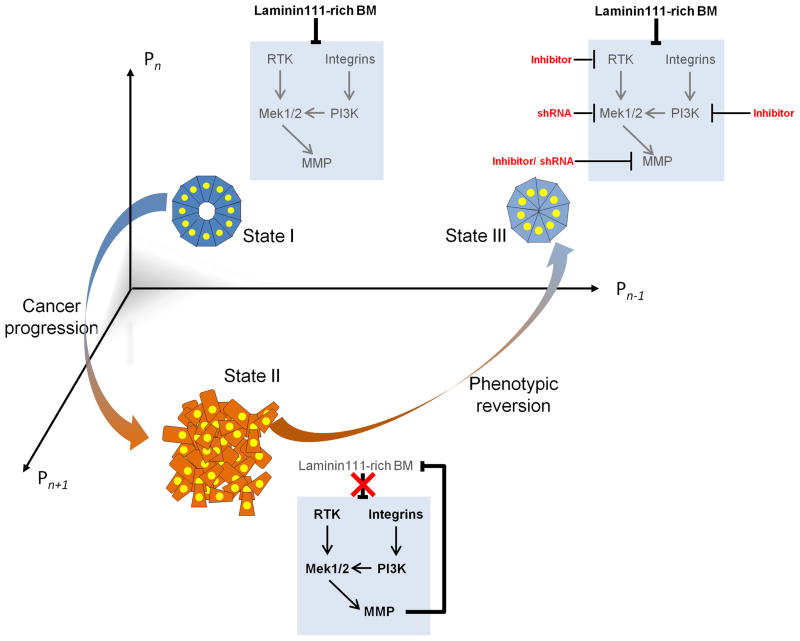
Figure 2. Organ specificity in 3D: an emergent property of a multicomponent interaction network.
Systems biology potentially reveals the relationship between homeostasis and cancer. Shown here is an n-dimensional interaction space with the axes representing protein expression levels.
State I depicts the phenotypic organization of normal/non-malignant mammary gland as an acinus that emerges if the mammary epithelial cells are placed in lrECM. The acinus is characterized by cellular quiescence, basal and apical polarity and lumen formation. An intact laminin-111-rich BM ensures repression of oncogenic signaling pathways and downregulation of a metalloproteinase MMP979, 101, 104.
State II depicts an amorphous phenotype with loss in acinar organization and polarity and cell proliferation, all of which occur when proteolytic MMPs are overexpressed in either non-malignant human breast cells (MMP9)104 or in mammary glands in vivo (MMP3)130. Overexpression of MMP results in proteolysis and destruction of the BM, leading to loss in tissue specificity and eventually tumors131.
State III depicts a reverted and re-organized mammary epithelial phenotype with growth arrest, a partial restoration of polarity (only basal polarity returns). The reversion occurs when one or more of the oncogenic signaling pathways or MMPs are inhibited99, 101.
C. What non-malignant and cancer cells grown in 3D teach us about organ specificity?
The HMT3522 human cancer progression model, consisting of the non-malignant S1 cells, malignant T4-2, and their isogenic intermediates is an ideal system with which to study the link between growth and polarity94.
Non-malignant S1 cells form organized polar acini in lrECM (Figure 3: State I); in contrast, T4-2 cancer cells in 3D show significantly higher expression of β1- and β4 integrins, higher ratio of cell-surface β1 integrin to β4 integrins and random non-polar distribution of β1-, β4-, α6- and α3 integrins in comparison to S1 cells99. Additionally a number of other oncogenic pathways such as EGFR, MAP kinase, PI3 Kinase are overexpressed (Figure 2: State II). Inhibiting overexpression of signaling in any of these pathways when cancer cells are placed in 3D, polarizes the cells, increases adhesion and leads to formation of growth-arrested and basally polarized T4-2 structures that resemble the non-malignant acini, although the apical polarity is not restored to the same degree as basal polarity100 (Figure 2: State III). The interesting phenomenon here is that all signaling pathways appear to integrate when any one of them is inhibited: For example, normalizing the hyperactive EGFR activity in T4-2, normalizes β1 integrin and vice versa101, revealing a tight reciprocal inter-modulation between tissue polarity and growth arrest, although the two are bifurcated downstream of PI3K signaling102.
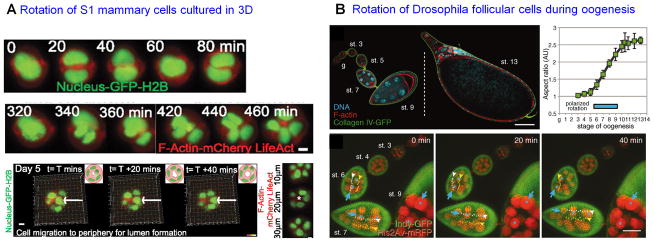
Figure 3. Coherent morphomechanics of cells in organ specificity and embryogenesis.
(A) Coherent angular motion (CAMo) of non-malignant S1 breast epithelial cells occurs when they are cultured in 3D gels leading to the formation of polar spheroidal acinar-like structures as revealed by live imaging of the cells with fluorescent signals from the nuclear histone (green) and actin cytoskeleton (red) (upper and middle panels). By day 5 the S1 acini growth-arrest (lowermost panel) with concurrent lumen formation. (Adapted from Tanner et al., 2012)
(B) Synchronized circumferential rotation of Drosophila follicular cells coincides with axis elongation of its eggs. The left and middle figures of upper panel show follicular elongation as Drosophila oogenesis proceeds. Upper right figure shows quantification of the aspect ratio of the elongating follicle. Lower panel shows the synchronized revolution of follicular cells (through yellow and white dots and arrows) and their germline nuclei (red, blue asterisks and arrow) against collagen IV (green) containing microenvironment as oogenesis takes place (reprinted from Haigo and Bilder, 2011, with permission).
It is possible that these same mechanisms apply to other acinar forming tissues such as prostate. Preliminary experiments using prostate cell lines under similar conditions also ‘reverted’ them toward a non-malignant phenotype103. Thus malignant cells with a grossly abnormal genome can be reverted to a normal architecture and in doing so, radically reduced tumorigenicity99, 101.
The reverse is also true: Loss of architecture leads to disorganized colonies, oncogenic pathway activation and increased growth. MMP9, a matrix metalloproteinase that is upregulated in several cancers, has the ability to cleave laminin-111 and remodel the mammary BM, is regulated by MEK/ERK pathway and in turn regulates that pathway104. Activation of MMP9 leads to disruption of polarity, activation of MEK/ERK, β1 integrin, EGFR and PI3Kinase pathways and forces the S1 cells into proliferation105. The BM is thus a prominent constituent of the adult organ microenvironment that contributes to organ specificity by integrating function, growth and structure. Among Eph-Ephrin family, EphA2 is upregulated in breast cancer specimens as well as aggressive cancer cell lines106. EphA2 overexpression in non-malignant MCF10A cells deregulates their signaling, resulting in aberrant cell adhesion, invasive behavior in culture and tumorigenicity in vivo106.
D. The evolutionary basis of organ homeostasis
In the previous section we described how the organ microenvironment, contributes to organ specificity by integrating aspects of function and architecture at several levels. What emerges out of a comparative study of organ development and specificity is that similar gene products are employed in both processes. Even some of the cellular and tissue level processes are similar although they may be directed towards opposite ends. For example, the cellular morphomechanics evidenced during the axis elongation of Drosophila eggs (Figure 3B) appears to be similar to CAMo (Figure 3A), but the result is topologically opposite – the follicles lose their sphericity and become ellipsoid107. Laminin-111 is involved in cellular quiescence of adult mammary epithelial cells62 but stimulates proliferation in mouse embryonic stem cells108. Epithelial-mesenchymal interactions during development consist of proliferative and morphogenetic signals that are reciprocally exchanged between the two cell types leading to organogenesis36. On the other hand the interactions between adult stromal mesenchyme and adult normal epithelia are often anti-proliferative: Mammary stromal fibroblasts and caveolin 1-expressing adipocytes both inhibit the mitogenic potential of mammary epithelia109,110.
How did the mechanism of organ specificity evolve? Were the molecular players and the interactions in the context of organogenesis ‘co-opted’ to give rise to, and maintain, the organ architecture? Clues to the resolution of these issues are not yet all here, and may have to come from systems analyses of analogously complex engineering and computational networks. In any given complex system that can assume a number of different states due to stochasticity or internal dynamics or extraneous impulses, any specific state or set of states, towards which the system gravitates from all adjacent neighboring states is defined as an attractor 111. In complex biological systems with multiple components and a greater number of spatially and temporally specific interactions, the probability of having more than one attractor is high. Not all these attractors are physiological. Those involving abnormal expression of components or anomalous interactions would be detrimental to the structure and functioning of the system and yet be stable. Otherwise seldom visited, these attractors represent potential traps wherein the system may be driven by pathological insults112–114. For example, the electrical dynamics of a normally pulsating heart, and two of its pathologies, myocardial infarction and atrial fibrillation, are very different but each represents distinct attractor states115. One of the ways in which a system may escape from one attractor and enter into another is if the system is inherently noisy or if an external perturbation manages to nudge it out from one into another. On the other hand, the attractor is made stable by constraining forces that attenuate the effects of external forces and/or decrease the noisiness of the system.
We suggest that the physiologically functioning adult organ keeps a stable architecture, representing an attractor, through the organ microenvironment. Developmental state of an organ primordium is another attractor. It is not as strong as the adult organ attractor since the developmental microenvironment is less constraining – phenotypic plasticity accorded to development as a result is the raw material for evolution of new organ types. Indeed the tumor microenvironment resembles the developmental microenvironment in a number of respects; both are less constraining with some morphological aspects that are more similar between the two states than that of the normal functioning organ. Cancer state of the organ represents a third attractor as has been pointed out by large-scale analysis of gene networks in pulmonary and hepatocellular cancers116. Gupta and coworkers postulated that individual tumors consist of more than one cell populations with distinct phenotypes and the relative proportions of these phenotypes are kept in equilibrium with each other through stochastic transitions between the phenotypes117. The work from the laboratories of Petersen, Bissell and coworkers provides a clear experimental basis for such a postulate118. Organ physiological and pathological states have been likened to attractors before119 but the association is based on the differentiated state of the normal and cancer cells and the gene networks responsible for it. As we discuss above, the differentiation state of the cell and its microenvironment are reciprocally and dynamically linked, and at any given time one or the other could be dominant over the other4. For example, Rous sarcoma virus, which is tumorigenic in chickens, is neither tumorigenic nor teratogenic in early chick embryos120. pp60v-src expression can become part of normal cells of a feather in a chick embryo when injected into embryonic stage 24 wing, is non-tumorigenic until very close to hatching, despite the fact that the virus replicates and also expresses src-specific kinase in the embryonic cells121. As we have shown, even cancer cells can be ‘reverted’ to an organized non-invasive phenotype by normalizing the dynamics of key transducers of microenvironment99, 100. The example of Wilmut’s Dolly discussed above shows that the embryonic environment helps ‘revert’ adult chromatin to an embryonic fate7. These examples strengthen our thesis that it is the microenvironment and not the differentiated state of given cells that defines and separates the developmental, physiological and cancer states of the organ.
The systems biology-based interpretation however allows us to hypothesize and explore other mechanisms by which organ specificity is established. Among the most interesting of these is the shrinking of ‘interaction space’ by global reduction of gene expression leading to a decreased probability that the organ state would enter abnormal and possibly pathological attractors. This is achieved by chromatin condensation associated with histone deacetylation in adult mammary epithelial nuclei and is triggered by ‘outside-in’ signaling from the laminin-111-rich BM via the actin cytoskeleton122. LrECM-induced repression of transcription is also mediated by higher DNA methylation brought about by the expression of methyl CpG binding protein (MeCP2)123. A second mechanism of maintaining specificity is the tight integration of organ microenvironmental constituents: bidirectional cross-modulation between the proliferation-promoting EGFR and the adhesion-inducing β1 integrin that is seen in acinar epithelial cells upon their being organized by their BM. Both EGFR and β1 integrin have abnormal expression in malignant cells and inhibiting either one of them down-regulates the other. This tight reciprocal cross-modulation of two tissue-level behaviors, proliferation and adhesion can potentially constrain the variability of the organ state and makes it specific and evolutionary stable124. Even widely conserved developmental mechanisms may lack such cross-modulation between key cellular and genetic components. An example is the disequilibrium between the proliferative rate of presomitic mesodermal cells and the periodicity of oscillation of the Notch pathway that has lead to remarkable inter-specific variability in the somite number (and consequently the number of vertebrae) within reptilian clade125.
We now know that the presence of regulatory feedback loops is a property that is ubiquitous among multicomponent systems and possibly necessary for normalizing homeostasis (e.g. see summary of interconnectedness of signaling pathways when cancer cells are reverted by using a single agent for a given pathway: all other known oncogenic pathways get corrected100). It was suggested to have been essential for organ specificity more than 30 years ago4 and shown by mathematical analyses to be essential for robustly functioning complex systems126. Given that laminin-111 plays a central and integrative role in the mammary organ microenvironment by linking phenotype-determining properties such as tissue polarity, BM formation, cell proliferation and gene expression, the study of potential regulatory feedbacks especially by laminin-111 on its deposition or gene expression, and on its interacting partners represents a future direction for research.
CONCLUSION
In recent times we have witnessed the emergence of multidisciplinary research in biology involving cell-, developmental-, cancer-, systems- biologists, bioengineers, biophysicists, as well as physicists and mathematicians in order to tackle questions that may be ripe for answers. Undertaking such an endeavor has required terms and concepts that in each of these disciplines may have different connotations. Thus the statement that cancer is a developmental disease or that tumors may be “developing organs”127 may be understood differently within each discipline. In this essay, we have tried to bring the concept of plasticity from developmental biology and specificity from cell and tumor biology within a single conceptual framework. Much more remains to be done and we look forward to a time that biology could gain a first principle. Given how little we still know about the mysteries of the organism and organ specificity though, we may have to contend with the possibility that the absence of a first principle may indeed turn out to be biology’s equivalence of its first principle!
Acknowledgments
The work from M.J.B.’s laboratory has been supported by grants from the U.S. Department of Energy, OBER Office of Biological and Environmental Research and Low Dose Scientific Focus Area, by multiple grants from the National Cancer Institute, by a grant from Breast Cancer Research Foundation and by two ‘Innovator awards’ from the U.S. Department of Defense. R.B. is supported by a postdoctoral fellowship from Susan G. Komen for the Cure. The authors would like to thank Stuart A. Newman, Irene Kuhn, Joni Mott, Kandice Tanner and the two anonymous reviewers of the first submission of this essay, for critical reading of this essay and providing helpful suggestions.
References
- 1.Hofstadter DR. Metamagical themas : questing for the essence of mind and pattern. New York, N.Y: Basic Books; 1985. [Google Scholar]
- 2.West-Eberhard MJ. Developmental plasticity and evolution. Oxford ; New York: Oxford University Press; 2003. [Google Scholar]
- 3.Newman SA, Bhat R, Mezentseva NV. Cell state switching factors and dynamical patterning modules: complementary mediators of plasticity in development and evolution. J Biosci. 2009;34:553–572. doi: 10.1007/s12038-009-0074-7. [DOI] [PubMed] [Google Scholar]
- 4.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 5.Bissell MJ, Hall HG. Form and function in the mammary gland: The role of extracellular matrix. In: Nevell MC, Neville CWD, editors. The Mammary Gland: Development, Regulation and Function. New York: Plenum Publishing Corp; 1987. pp. 97–146. [Google Scholar]
- 6.Davidson EH. The regulatory genome : gene regulatory networks in development and evolution. Oxford: Academic; 2005. New ed. [Google Scholar]
- 7.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 8.Mezentseva NV, Kumaratilake JS, Newman SA. The brown adipocyte differentiation pathway in birds: an evolutionary road not taken. BMC Biol. 2008;6:17. doi: 10.1186/1741-7007-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlichting CD, Smith H. Phenotypic plasticity: linking molecular mechanisms with evolutionary outcomes. Evolutionary Ecology. 2002;16:189–211. [Google Scholar]
- 10.Vedel V, Chipman AD, Akam M, Arthur W. Temperature-dependent plasticity of segment number in an arthropod species: the centipede Strigamia maritima. Evol Dev. 2008;10:487–492. doi: 10.1111/j.1525-142X.2008.00259.x. [DOI] [PubMed] [Google Scholar]
- 11.Moczek AP. Integrating micro- and macroevolution of development through the study of horned beetles. Heredity (Edinb) 2006;97:168–178. doi: 10.1038/sj.hdy.6800871. [DOI] [PubMed] [Google Scholar]
- 12.Weisser WW, Christian Braendle, Nicole Minoretti. Predator-induced morphological shift in the pea aphid. Proceedings of the Royal Society B-Biological Sciences. 1999;266:1175–1181. [Google Scholar]
- 13.Sturkie PD. Suppression of polydactyly in the domestic fowl by low temperature. Journal of Experimental Zoology. 1943;93:325–346. [Google Scholar]
- 14.Neufeld CJ, Palmer AR. Precisely proportioned: intertidal barnacles alter penis form to suit coastal wave action. Proceedings of the Royal Society B-Biological Sciences. 2008;275:1081–1087. doi: 10.1098/rspb.2007.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brakefield PM, Kesbeke F, Koch PB. The regulation of phenotypic plasticity of eyespots in the butterfly Bicyclus anynana. Am Nat. 1998;152:853–860. doi: 10.1086/286213. [DOI] [PubMed] [Google Scholar]
- 16.Miyakawa H, Imai M, Sugimoto N, Ishikawa Y, Ishikawa A, Ishigaki H, Okada Y, Miyazaki S, Koshikawa S, Cornette R, et al. Gene up-regulation in response to predator kairomones in the water flea, Daphnia pulex. BMC Dev Biol. 2010;10:45. doi: 10.1186/1471-213X-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamarck JBPAdMd. Philosophie zoologique, ou, Exposition des considérations relatives à l’histoire naturelle des animaux. Paris: 1809. [Google Scholar]
- 18.Newman SA, Forgacs G, Muller GB. Before programs: the physical origination of multicellular forms. Int J Dev Biol. 2006;50:289–299. doi: 10.1387/ijdb.052049sn. [DOI] [PubMed] [Google Scholar]
- 19.de Gennes PG. Soft matter. Science. 1992;256:495–497. doi: 10.1126/science.256.5056.495. [DOI] [PubMed] [Google Scholar]
- 20.Mikhailov AS. Foundations of synergetics. Berlin ; New York: Springer-Verlag; 1990. [Google Scholar]
- 21.Suel GM, Garcia-Ojalvo J, Liberman LM, Elowitz MB. An excitable gene regulatory circuit induces transient cellular differentiation. Nature. 2006;440:545–550. doi: 10.1038/nature04588. [DOI] [PubMed] [Google Scholar]
- 22.Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balazsi G, van Oudenaarden A, Collins JJ. Cellular decision making and biological noise: from microbes to mammals. Cell. 2011;144:910–925. doi: 10.1016/j.cell.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boettiger AN, Levine M. Synchronous and stochastic patterns of gene activation in the Drosophila embryo. Science. 2009;325:471–473. doi: 10.1126/science.1173976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregor T, Tank DW, Wieschaus EF, Bialek W. Probing the limits to positional information. Cell. 2007;130:153–164. doi: 10.1016/j.cell.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wernet MF, Mazzoni EO, Celik A, Duncan DM, Duncan I, Desplan C. Stochastic spineless expression creates the retinal mosaic for colour vision. Nature. 2006;440:174–180. doi: 10.1038/nature04615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karp X, Greenwald I. Post-transcriptional regulation of the E/Daughterless ortholog HLH-2, negative feedback, and birth order bias during the AC/VU decision in C. elegans. Genes Dev. 2003;17:3100–3111. doi: 10.1101/gad.1160803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston RJ, Jr, Desplan C. Stochastic mechanisms of cell fate specification that yield random or robust outcomes. Annu Rev Cell Dev Biol. 2010;26:689–719. doi: 10.1146/annurev-cellbio-100109-104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirschner M, Gerhart J, Mitchison T. Molecular “vitalism”. Cell. 2000;100:79–88. doi: 10.1016/s0092-8674(00)81685-2. [DOI] [PubMed] [Google Scholar]
- 30.Driever W, Nusslein-Volhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988;54:83–93. doi: 10.1016/0092-8674(88)90182-1. [DOI] [PubMed] [Google Scholar]
- 31.Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- 32.Lewis WH. Transplantation of the lips of the blastopore in rana palustris. American Journal of Anatomy. 1907;7:137–143. [Google Scholar]
- 33.Spemann H, Mangold H. The induction of embryonic predispositions by implantation of organizers foreign to the species. Archiv Fur Mikroskopische Anatomie Und Entwicklungsmechanik. 1924;100:599–638. [Google Scholar]
- 34.Lewis WH. Experimental studies on the development of the eye in Amphibia I On the origin of the lens Rana palustras. American Journal of Anatomy. 1904;3:505–536. [Google Scholar]
- 35.Slack JMW. From egg to embryo : determinative events in early development. Cambridge: Cambridge University Press; 1983. [Google Scholar]
- 36.Forgács G, Newman S. Biological physics of the developing embryo. Cambridge ; New York: Cambridge University Press; 2005. [Google Scholar]
- 37.Hens JR, Wysolmerski JJ. Key stages of mammary gland development: molecular mechanisms involved in the formation of the embryonic mammary gland. Breast Cancer Res. 2005;7:220–224. doi: 10.1186/bcr1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coleman S, Silberstein GB, Daniel CW. Ductal morphogenesis in the mouse mammary gland: evidence supporting a role for epidermal growth factor. Dev Biol. 1988;127:304–315. doi: 10.1016/0012-1606(88)90317-x. [DOI] [PubMed] [Google Scholar]
- 39.Kenney NJ, Bowman A, Korach KS, Barrett JC, Salomon DS. Effect of exogenous epidermal-like growth factors on mammary gland development and differentiation in the estrogen receptor-alpha knockout (ERKO) mouse. Breast Cancer Res Treat. 2003;79:161–173. doi: 10.1023/a:1023938510508. [DOI] [PubMed] [Google Scholar]
- 40.Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132:3923–3933. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fata JE, Mori H, Ewald AJ, Zhang H, Yao E, Werb Z, Bissell MJ. The MAPK(ERK-1,2) pathway integrates distinct and antagonistic signals from TGFalpha and FGF7 in morphogenesis of mouse mammary epithelium. Dev Biol. 2007;306:193–207. doi: 10.1016/j.ydbio.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ewan KB, Shyamala G, Ravani SA, Tang Y, Akhurst R, Wakefield L, Barcellos-Hoff MH. Latent transforming growth factor-beta activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation. Am J Pathol. 2002;160:2081–2093. doi: 10.1016/s0002-9440(10)61158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daniel CW, Robinson S, Silberstein GB. The role of TGF-beta in patterning and growth of the mammary ductal tree. J Mammary Gland Biol Neoplasia. 1996;1:331–341. doi: 10.1007/BF02017389. [DOI] [PubMed] [Google Scholar]
- 44.Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavlovich AL, Boghaert E, Nelson CM. Mammary branch initiation and extension are inhibited by separate pathways downstream of TGFbeta in culture. Exp Cell Res. 2011;317:1872–1884. doi: 10.1016/j.yexcr.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grobstein C. In: Aspects of synthesis and order in growth. Rudnik D, editor. Princeton: Princeton University Press; 1955. pp. 233–256. [Google Scholar]
- 48.Hauschka SD, Konigsberg IR. The influence of collagen on the development of muscle clones. Proc Natl Acad Sci U S A. 1966;55:119–126. doi: 10.1073/pnas.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meier S, Hay ED. Control of corneal differentiation by extracellular materials. Collagen as a promoter and stabilizer of epithelial stroma production. Dev Biol. 1974;38:249–270. doi: 10.1016/0012-1606(74)90005-0. [DOI] [PubMed] [Google Scholar]
- 50.Keely PJ, Wu JE, Santoro SA. The spatial and temporal expression of the alpha 2 beta 1 integrin and its ligands, collagen I, collagen IV, and laminin, suggest important roles in mouse mammary morphogenesis. Differentiation. 1995;59:1–13. doi: 10.1046/j.1432-0436.1995.5910001.x. [DOI] [PubMed] [Google Scholar]
- 51.Brownfield DG, Venugopalan G, Lo A, Mori H, Tanner K, Fletcher DA, Bissell MJ. Patterned Collagen Fibers Orient Branching Mammary Epithelium through Distinct Signaling Modules. Curr Biol. 2013 doi: 10.1016/j.cub.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silberstein GB, Daniel CW. Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev Biol. 1982;90:215–222. doi: 10.1016/0012-1606(82)90228-7. [DOI] [PubMed] [Google Scholar]
- 53.Lochter A, Bissell MJ. Involvement of extracellular matrix constituents in breast cancer. Semin Cancer Biol. 1995;6:165–173. doi: 10.1006/scbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- 54.Menko AS, Kreidberg JA, Ryan TT, Van Bockstaele E, Kukuruzinska MA. Loss of alpha 3 beta1 integrin function results in an altered differentiation program in the mouse submandibular gland. Developmental dynamics : an official publication of the American Association of Anatomists. 2001;220:337–349. doi: 10.1002/dvdy.1114. [DOI] [PubMed] [Google Scholar]
- 55.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 56.Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klinowska TC, Soriano JV, Edwards GM, Oliver JM, Valentijn AJ, Montesano R, Streuli CH. Laminin and beta1 integrins are crucial for normal mammary gland development in the mouse. Dev Biol. 1999;215:13–32. doi: 10.1006/dbio.1999.9435. [DOI] [PubMed] [Google Scholar]
- 58.Mori H, Lo AT, Inman JL, Alcaraz J, Ghajar CM, Mott JD, Nelson CM, Chen CS, Zhang H, Bascom JL, et al. Transmembrane/cytoplasmic, rather than catalytic, domains of Mmp14 signal to MAPK activation and mammary branching morphogenesis via binding to integrin beta1. Development. 2013;140:343–352. doi: 10.1242/dev.084236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alcaraz J, Mori H, Ghajar CM, Brownfield D, Galgoczy R, Bissell MJ. Collective epithelial cell invasion overcomes mechanical barriers of collagenous extracellular matrix by a narrow tube-like geometry and MMP14-dependent local softening. Integr Biol (Camb) 2011;3:1153–1166. doi: 10.1039/c1ib00073j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bronner-Fraser M. Mechanisms of neural crest cell migration. Bioessays. 1993;15:221–230. doi: 10.1002/bies.950150402. [DOI] [PubMed] [Google Scholar]
- 62.Spencer VA, Costes S, Inman JL, Xu R, Chen J, Hendzel MJ, Bissell MJ. Depletion of nuclear actin is a key mediator of quiescence in epithelial cells. J Cell Sci. 2011;124:123–132. doi: 10.1242/jcs.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gierer A. Biological features and physical concepts of pattern formation exemplified by hydra. Curr Top Dev Biol. 1977;11:17–59. doi: 10.1016/s0070-2153(08)60742-5. [DOI] [PubMed] [Google Scholar]
- 64.Hynes RO. The evolution of metazoan extracellular matrix. J Cell Biol. 2012;196:671–679. doi: 10.1083/jcb.201109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gould SJ. Wonderful life : the Burgess Shale and nature of history. 1. New York: W.W. Norton; 1989. [Google Scholar]
- 66.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nature reviews Neuroscience. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 67.Irvine KD. Fringe, Notch, and making developmental boundaries. Current opinion in genetics & development. 1999;9:434–441. doi: 10.1016/S0959-437X(99)80066-5. [DOI] [PubMed] [Google Scholar]
- 68.Pasquale EB, Deerinck TJ, Singer SJ, Ellisman MH. Cek5, a membrane receptor-type tyrosine kinase, is in neurons of the embryonic and postnatal avian brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:3956–3967. doi: 10.1523/JNEUROSCI.12-10-03956.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ashton RS, Conway A, Pangarkar C, Bergen J, Lim KI, Shah P, Bissell M, Schaffer DV. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat Neurosci. 2012;15:1399–1406. doi: 10.1038/nn.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Durbin L, Brennan C, Shiomi K, Cooke J, Barrios A, Shanmugalingam S, Guthrie B, Lindberg R, Holder N. Eph signaling is required for segmentation and differentiation of the somites. Genes & development. 1998;12:3096–3109. doi: 10.1101/gad.12.19.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Molecular cell. 1999;4:403–414. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- 72.Kouros-Mehr H, Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn. 2006;235:3404–3412. doi: 10.1002/dvdy.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vaught D, Chen J, Brantley-Sieders DM. Regulation of mammary gland branching morphogenesis by EphA2 receptor tyrosine kinase. Mol Biol Cell. 2009;20:2572–2581. doi: 10.1091/mbc.E08-04-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hubbard GD. Drumlinoids of the Catatonk Folio. Bulletin of the American Geographical Society of New York. 1906;38:355–365. [Google Scholar]
- 75.Stedman TL. Twentieth century practice; an international encyclopedia of modern medical science by leading authorities of Europe and America. New York: W. Wood and Company; 1895. [Google Scholar]
- 76.Daniel CW, Deome KB. Growth of Mouse Mammary Glands in Vivo after Monolayer Culture. Science. 1965;149:634–636. doi: 10.1126/science.149.3684.634. [DOI] [PubMed] [Google Scholar]
- 77.Boulanger CA, Mack DL, Booth BW, Smith GH. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci U S A. 2007;104:3871–3876. doi: 10.1073/pnas.0611637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Booth BW, Mack DL, Androutsellis-Theotokis A, McKay RD, Boulanger CA, Smith GH. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci U S A. 2008;105:14891–14896. doi: 10.1073/pnas.0803214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Michalopoulos G, Pitot HC. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975;94:70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- 82.Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- 83.Bissell MJ. The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. Int Rev Cytol. 1981;70:27–100. doi: 10.1016/s0074-7696(08)61130-4. [DOI] [PubMed] [Google Scholar]
- 84.Lee EY, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984;98:146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Streuli CH, Bissell MJ. Expression of extracellular matrix components is regulated by substratum. J Cell Biol. 1990;110:1405–1415. doi: 10.1083/jcb.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alcaraz J, Xu R, Mori H, Nelson CM, Mroue R, Spencer VA, Brownfield D, Radisky DC, Bustamante C, Bissell MJ. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 2008;27:2829–2838. doi: 10.1038/emboj.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen LH, Bissell MJ. A novel regulatory mechanism for whey acidic protein gene expression. Cell Regul. 1989;1:45–54. doi: 10.1091/mbc.1.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin CQ, Bissell MJ. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993;7:737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- 90.Fu HL, Moss J, Shore I, Slade MJ, Coombes RC. Ultrastructural localization of laminin and type IV collagen in normal human breast. Ultrastruct Pathol. 2002;26:77–80. doi: 10.1080/01913120252959245. [DOI] [PubMed] [Google Scholar]
- 91.Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, Bissell MJ. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dickinson DJ, Nelson WJ, Weis WI. A polarized epithelium organized by beta- and alpha-catenin predates cadherin and metazoan origins. Science. 2011;331:1336–1339. doi: 10.1126/science.1199633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rizki A, Weaver VM, Lee SY, Rozenberg GI, Chin K, Myers CA, Bascom JL, Mott JD, Semeiks JR, Grate LR, et al. A human breast cell model of preinvasive to invasive transition. Cancer Res. 2008;68:1378–1387. doi: 10.1158/0008-5472.CAN-07-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Plachot C, Chaboub LS, Adissu HA, Wang L, Urazaev A, Sturgis J, Asem EK, Lelievre SA. Factors necessary to produce basoapical polarity in human glandular epithelium formed in conventional and high-throughput three-dimensional culture: example of the breast epithelium. BMC Biol. 2009;7:77. doi: 10.1186/1741-7007-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Underwood JM, Imbalzano KM, Weaver VM, Fischer AH, Imbalzano AN, Nickerson JA. The ultrastructure of MCF-10A acini. J Cell Physiol. 2006;208:141–148. doi: 10.1002/jcp.20639. [DOI] [PubMed] [Google Scholar]
- 97.Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 98.Tanner K, Mori H, Mroue R, Bruni-Cardoso A, Bissell MJ. Coherent angular motion in the establishment of multicellular architecture of glandular tissues. Proc Natl Acad Sci U S A. 2012;109:1973–1978. doi: 10.1073/pnas.1119578109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bissell MJ, Kenny PA, Radisky DC. Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes. Cold Spring Harb Symp Quant Biol. 2005;70:343–356. doi: 10.1101/sqb.2005.70.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu H, Radisky DC, Wang F, Bissell MJ. Polarity and proliferation are controlled by distinct signaling pathways downstream of PI3-kinase in breast epithelial tumor cells. J Cell Biol. 2004;164:603–612. doi: 10.1083/jcb.200306090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang X, Fournier MV, Ware JL, Bissell MJ, Yacoub A, Zehner ZE. Inhibition of vimentin or beta1 integrin reverts morphology of prostate tumor cells grown in laminin-rich extracellular matrix gels and reduces tumor growth in vivo. Mol Cancer Ther. 2009;8:499–508. doi: 10.1158/1535-7163.MCT-08-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beliveau A, Mott JD, Lo A, Chen EI, Koller AA, Yaswen P, Muschler J, Bissell MJ. Raf-induced MMP9 disrupts tissue architecture of human breast cells in three-dimensional culture and is necessary for tumor growth in vivo. Genes Dev. 2010;24:2800–2811. doi: 10.1101/gad.1990410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pearson GW, Hunter T. PI-3 kinase activity is necessary for ERK1/2-induced disruption of mammary epithelial architecture. Breast Cancer Res. 2009;11:R29. doi: 10.1186/bcr2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. [PubMed] [Google Scholar]
- 107.Haigo SL, Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331:1071–1074. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suh HN, Kim MO, Han HJ. Laminin-111 Stimulates Proliferation of Mouse Embryonic Stem Cells Through a Reduction of Gap Junctional Intercellular Communication via RhoA-Mediated Cx43 Phosphorylation and Dissociation of Cx43/ZO-1/Drebrin Complex. Stem Cells Dev. 2012 doi: 10.1089/scd.2011.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res. 2001;61:1320–1326. [PubMed] [Google Scholar]
- 110.Williams TM, Sotgia F, Lee H, Hassan G, Di Vizio D, Bonuccelli G, Capozza F, Mercier I, Rui H, Pestell RG, et al. Stromal and epithelial caveolin-1 both confer a protective effect against mammary hyperplasia and tumorigenesis: Caveolin-1 antagonizes cyclin D1 function in mammary epithelial cells. The American journal of pathology. 2006;169:1784–1801. doi: 10.2353/ajpath.2006.060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strogatz SH. Nonlinear dynamics and chaos : with applications to physics, biology, chemistry, and engineering. 1. Cambridge, MA: Westview Press; 2000. [Google Scholar]
- 112.Haurie C, Dale DC, Mackey MC. Cyclical neutropenia and other periodic hematological disorders: a review of mechanisms and mathematical models. Blood. 1998;92:2629–2640. [PubMed] [Google Scholar]
- 113.Kaneko K. Characterization of stem cells and cancer cells on the basis of gene expression profile stability, plasticity, and robustness: dynamical systems theory of gene expressions under cell-cell interaction explains mutational robustness of differentiated cells and suggests how cancer cells emerge. Bioessays. 2011;33:403–413. doi: 10.1002/bies.201000153. [DOI] [PubMed] [Google Scholar]
- 114.Winfree AT. The geometry of biological time. 2. New York: Springer; 2001. [Google Scholar]
- 115.Kaneko K, Tsuda Io. Complex systems : chaos and beyond : a constructive approach with applications in life sciences. Berlin ; New York: Springer; 2001. [Google Scholar]
- 116.Shiraishi T, Matsuyama S, Kitano H. Large-scale analysis of network bistability for human cancers. PLoS Comput Biol. 2010;6:e1000851. doi: 10.1371/journal.pcbi.1000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 118.Kim J, Villadsen R, Sorlie T, Fogh L, Gronlund SZ, Fridriksdottir AJ, Kuhn I, Rank F, Wielenga VT, Solvang H, et al. Tumor initiating but differentiated luminal-like breast cancer cells are highly invasive in the absence of basal-like activity. Proc Natl Acad Sci U S A. 2012;109:6124–6129. doi: 10.1073/pnas.1203203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang S, Ernberg I, Kauffman S. Cancer attractors: a systems view of tumors from a gene network dynamics and developmental perspective. Semin Cell Dev Biol. 2009;20:869–876. doi: 10.1016/j.semcdb.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dolberg DS, Bissell MJ. Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature. 1984;309:552–556. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- 121.Stoker AW, Hatier C, Bissell MJ. The embryonic environment strongly attenuates v-src oncogenesis in mesenchymal and epithelial tissues, but not in endothelia. J Cell Biol. 1990;111:217–228. doi: 10.1083/jcb.111.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Le Beyec J, Xu R, Lee SY, Nelson CM, Rizki A, Alcaraz J, Bissell MJ. Cell shape regulates global histone acetylation in human mammary epithelial cells. Exp Cell Res. 2007;313:3066–3075. doi: 10.1016/j.yexcr.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Plachot C, Lelievre SA. DNA methylation control of tissue polarity and cellular differentiation in the mammary epithelium. Exp Cell Res. 2004;298:122–132. doi: 10.1016/j.yexcr.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 124.Torday JS, Rehan VK. Cell-cell signaling drives the evolution of complex traits: introduction-lung evo-devo. Integrative and Comparative Biology. 2009;49:142–154. doi: 10.1093/icb/icp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gomez C, Ozbudak EM, Wunderlich J, Baumann D, Lewis J, Pourquie O. Control of segment number in vertebrate embryos. Nature. 2008;454:335–339. doi: 10.1038/nature07020. [DOI] [PubMed] [Google Scholar]
- 126.Doyle JC, Csete M. Architecture, constraints, and behavior. Proc Natl Acad Sci U S A. 2011;108 (Suppl 3):15624–15630. doi: 10.1073/pnas.1103557108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Radisky D, Hagios C, Bissell MJ. Tumors are unique organs defined by abnormal signaling and context. Semin Cancer Biol. 2001;11:87–95. doi: 10.1006/scbi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 128.Hayes S. Shaped to split. Nat Cell Biol. 2006;8:1325. doi: 10.1038/ncb1206-1325. [DOI] [PubMed] [Google Scholar]
- 129.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]