Summary
This review proposes definitions for key terms in the field of HIV-1 latency and eradication. In the context of eradication, a reservoir is a cell type that allows persistence of replication-competent HIV-1 on a time scale of years in patients on optimal antiretroviral therapy. Reservoirs act as a barrier to eradication in the patient population in whom cure attempts will likely be made. Halting viral replication is essential to eradication, and definitions and criteria for assessing whether this goal has been achieved are proposed. The cell types that may serve as reservoirs for HIV-1 are discussed. Currently, only latently infected resting CD4+ T cells fit the proposed definition of a reservoir, and more evidence is necessary to demonstrate that other cell types including hematopoietic stem cells and macrophages fit this definition. Further research is urgently required on potential reservoirs in the gut-associated lymphoid tissue and the central nervous system.
Introduction
In 1997, it became possible for the first time to reduce plasma HIV-1 levels to below the detection limit of clinical assays (50 copies of HIV-1 RNA/ml) with combinations of three antiretroviral drugs (Perelson et al., 1997). This approach, known as highly active antiretroviral therapy (HAART), dramatically decreased deaths from HIV-1 infection (Palella et al., 1998). With millions of life-years saved (Walensky et al., 2006), HAART is a major achievement of modern medicine, converting a uniformly fatal illness into one in which adherent patients starting treatment early now have near normal life expectancy (Mills et al., 2011).
The effective suppression of viremia initially inspired hopes that the virus could be eradicated with two to three years of HAART (Perelson et al., 1997). However, in 1995, a latent form of HIV-1 infection was demonstrated in vivo (Chun et al., 1995). A small fraction of resting memory CD4+ T cells were shown to carry integrated viral genomes. These cells do not release infectious virus in the resting state, but can do so following cellular activation (Chun et al., 1995; Chun et al., 1997). This latent reservoir in resting CD4+ T cells persists even in patients on HAART who have no clinically detectable viremia (Chun et al., 1997; Finzi et al., 1997; Wong et al., 1997). Longitudinal analysis demonstrated that in patients on HAART, the time to eradication of the population of latently infected cells would be over 60 years (Finzi et al., 1999; Siliciano et al., 2003; Strain et al., 2003). This latent reservoir is now recognized as the major barrier to HIV-1 eradication (Richman et al., 2009). Even patients who have been on HAART for several years experience viral rebound within weeks of treatment interruption (Davey et al., 1999), typically from an archival variant (Joos et al., 2008). Life-long HAART is therefore required.
The latent reservoir for HIV-1 in resting CD4+ T cells differs in important ways from classic forms of latency observed for viruses such as those of the Herpesviridae family. For herpes simplex viruses, latency allows immune evasion and viral persistence between episodes of active replication (Perng and Jones, 2010). In contrast, in untreated HIV-1 infection, there is continuous viral replication throughout the disease course (Figure 1) (Piatak et al., 1993). Rapid viral evolution allows escape from neutralizing antibodies (Richman et al., 2003) and cytolytic T lymphocyte (CTL) responses (Borrow et al., 1997) and provides the principal mechanism of immune evasion.
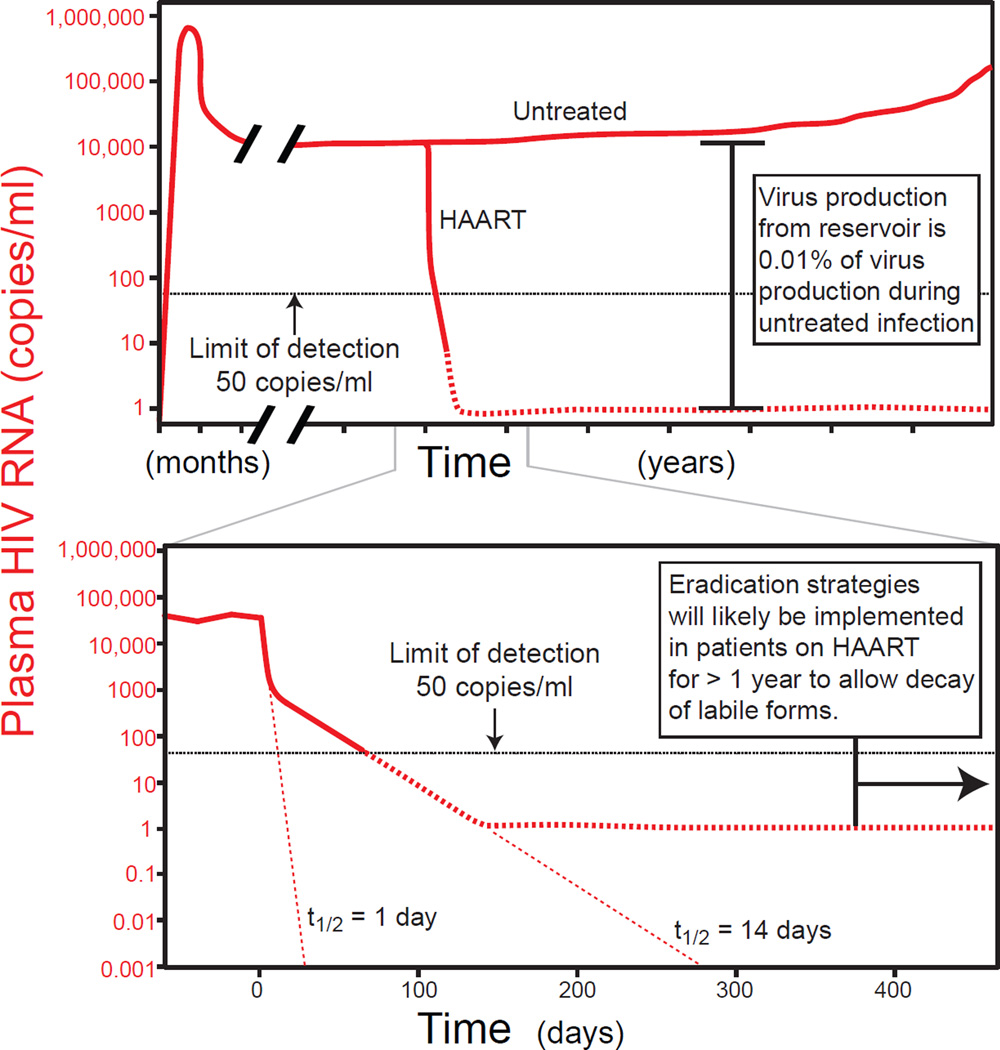
Figure 1.
Plasma virus levels in untreated and treated HIV-1 infection. Virus production continues throughout the course of untreated HIV-1 infection (top panel). Levels of viremia vary widely between patients but average between 10,000 and 100,000 copies/ml. Stable reservoirs make a very small contribution to overall virus production. Their contribution, apparent only when active replication is halted by HAART, is around 1 copy/ml (Palmer et al., 2003; Palmer et al., 2008). HAART induces a rapid biphasic drop in the level of viremia (bottom panel), reflecting the rapid turnover of the cells that produce most of the plasma virus (Ho et al., 1995; Perelson et al., 1996; Perelson et al., 1997; Wei et al., 1995). Eradication strategies will likely be implemented in patients who have had suppression of viremia to below the limit of detection for >1 year to allow decay of these labile infected cell populations.
Why then does latent HIV-1 infection occur? HIV-1 latency is best viewed as an accidental consequence of viral tropism for activated CD4+ T cells. Generally, infection leads to the rapid death of activated CD4+ T cells (Ho et al., 1995; Wei et al., 1995), but rarely, activated CD4+ T cells become infected as they are reverting back to a resting memory state. Resting CD4+ T cells are much less permissive for HIV-1 gene expression due to profound differences in the transcriptional environment. Thus, latency can be established. Latent HIV-1 is found in resting memory CD4+ T cells but not in naïve CD4+ T cells (Brenchley et al., 2004a; Chomont et al., 2009; Chun et al., 1997; Pierson et al., 2000), supporting the idea that the cells become infected during the transition from activated effectors to resting memory cells. The establishment of latent infection is a rare event; hence, the frequency of latently infected cells is extremely low, typically around 1/106 resting CD4+ T cells (Chun et al., 1997; Finzi et al., 1997). Encountering antigen or other activating stimuli can induce latently infected cells to produce virus, but the rate of virus production from this and other stable reservoirs is typically <0.01% of the rate of virus production due to ongoing replication in untreated patients (Figure 1). Thus, this small pool of latently infected cells likely plays only a minor role in the natural history of infection. Essentially, HIV-1 latency is an epiphenomenon, but one that is extremely important in the context of HAART because it represents a barrier to cure. This view of latency has significant implications for therapeutic efforts to purge the latent reservoir cells and for the search for other HIV-1 reservoirs.
In addition to the latent reservoir in resting CD4+ T cells, there is another important indication of viral persistence in patients on HAART. Although HAART suppresses viremia to <50 copies/ml, more sensitive assays with limits of detection as low as 1 copy/ml reveal trace levels of viremia in many patients (Dornadula et al., 1999; Palmer et al., 2003; Palmer et al., 2008). Because the half-life of plasma virus is on the order of minutes (Perelson et al., 1996; Ramratnam et al., 1999), this low-level viremia, referred to as “residual viremia”, indicates ongoing virus production in patients on HAART. At least some of these viruses appear to be replication-competent (Sahu et al., 2010). In principle, residual viremia could result from a low degree of ongoing viral replication, the release of virus from latently infected cells that have become activated, or the release of virus from other stable reservoirs.
Although the discovery of a stable latent reservoir for HIV-1 diminished hopes for eradication, the recent cure of a single patient using an ablative bone-marrow transplantation strategy has renewed interest in cure research (Hutter et al., 2009). The patient had stable suppression of viremia on HAART when he developed acute myelogenous leukemia (AML). As part of the treatment for AML, he received chemotherapy and radiation followed by a bone marrow transplant from an HLA-matched donor. The unique aspect of the case was the choice of a donor who was also homozygous for a 32-base pair deletion in the HIV-1 coreceptor CCR5. Thus, the patient’s hematopoietic compartment was reconstituted with HIV-1-resistant cells. At the time of initial transplantation, HAART was stopped, and there has been no rebound in viremia for over five years. This patient is thus considered to be the first and, to date, the only patient cured of HIV-1 infection. Although this approach will be difficult to generalize, the successful cure of a single patient has renewed interest in the possibility of eradicating HIV-1 infection. The purpose of this article is to precisely define the viral reservoirs that represent a barrier to eradication.
Definitions
Discussions of HIV-1 eradication have been hindered by a lack of precision and consensus regarding the meaning of key terms such as latency, viral replication, reservoir, sanctuary, compartment, and cure. Therefore, we propose here a set of definitions that can be used as a starting point for refining the terminology in cure research (Table 1).
Table 1.
Proposed definitions for key terms in eradication research.
| Term | Brief definition |
|---|---|
| Latency | Reversibly non-productive state of infection of individual cells. Latently infected cells retain the capacity to produce infectious virus particles. |
| Viral replication | New cycles of infection in which previously uninfected cells become infected and produce virus that goes on to infect additional cells. Evidence for viral replication includes the presence of labile products of reverse transcription, viral evolution during prolonged HAART with drugs at therapeutic concentrations, and a decrease in residual viremia levels with the addition of a fourth drug to a HAART regimen. |
| Reservoir (previous virologic definition) | Cell type or anatomical site in association with which a replication-competent form of the virus persists with more stable kinetics than the main pool of actively replicating virus |
| Reservoir (practical definition) | Infected cell population that allows persistence of replication-competent HIV-1 in patients on optimal HAART regimens on the order of years. |
| Compartment | An anatomical site for which there is limited exchange of viral genetic information with other sites; observable using phylogenetic tools. May contain compartment-specific viral sequences. |
| Sanctuary | An anatomical site with suboptimal free drug levels |
| Cure: | |
| Sterilizing | Complete eradication of all replication-competent forms of the virus. |
| Functional | Permanent viral suppression in the absence of therapy to levels that prevent immunodeficiency and transmission. |
Latency
The standard virologic definition of latency is a reversibly non-productive state of infection of individual cells. Latently infected cells do not produce infectious virus particles but retain the capacity to do so. Resting memory CD4+ T cells carrying transcriptionally silent HIV-1 genomes clearly represent a form of latency because a fraction of these cells produce infectious virus following cellular activation (Chun et al., 1995; Chun et al., 1997; Chun et al., 1997; Finzi et al., 1997; Wong et al., 1997). The term latency is sometimes used in clinical setting to refer to the prolonged asymptomatic phase between acute infection and the development of AIDS. This usage is incorrect because viral replication continues throughout the course of untreated HIV-1 infection (Figure 1) ((Piatak et al., 1993).
Viral replication
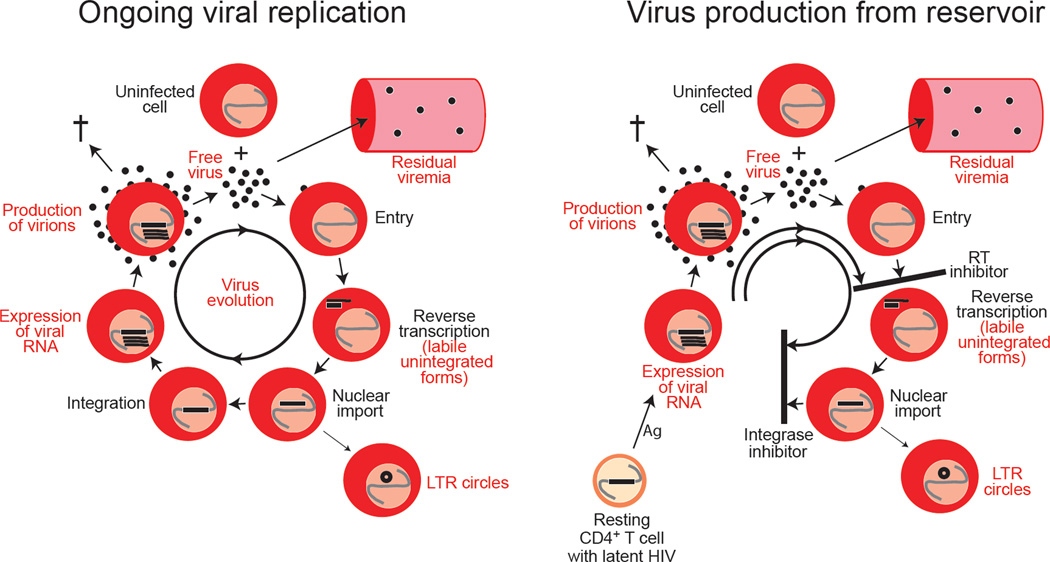
The first step in HIV-1 eradication is to stop the virus from replicating. Surprisingly, there is considerable confusion regarding the term “viral replication” and the measures that can be used to determine whether replication has been halted by HAART. Some authors erroneously equate virus production with viral replication. The mere detection of viral RNA in cells from patients on HAART does not demonstrate ongoing replication, nor does the presence of residual viremia. Both can be explained by the reactivation of latently infected cells (Figure 2). Virus production by these cells can occur without new rounds of infection and does not propagate the infection. We suggest that the term “replication” be used to mean new cycles of infection in which previously uninfected cells become infected and produce virus that goes on to infect additional cells.
Figure 2.
Distinguishing ongoing viral replication from virus production by latently infected cells that have been activated. A variety of parameters have been evaluated to determine whether a low degree of viral replication continues in patients on HAART. These are indicated in red and include expression of HIV-1 RNA, residual viremia, labile products of reverse transcription such as linear unintegrated HIV-1 DNA, LTR circles, and the accumulation of mutations (viral evolution). Depending on the HAART regimen, some of these parameters can also reflect the reactivation of latently infected cells without further replication (bottom panel). For example, in patients on regimens in which an integrase inhibitor is the most active drug, all of these features except evolution would be present. Only the accumulation of new mutations is uniquely characteristic of ongoing replication.
Reservoir
Viral reservoirs have been defined as cell types or anatomical sites in association with which replication-competent forms of the virus persist with more stable kinetic properties than the main pool of actively replicating virus (Blankson et al., 2002). There are two important elements to this definition. First, a reservoir allows persistence of some replication-competent form of the virus capable of replenishing the pool of infected cells in the future. Many HIV-1 genomes are defective, and thus the detection of proviral DNA by PCR does not establish that a viral reservoir is present. Second, there is a kinetic element to the definition. Classic studies of viral dynamics in HIV-1 infection (Ho et al., 1995; Perelson et al., 1996; Perelson et al., 1997; Wei et al., 1995; Zhang et al., 1999) established that most of the plasma virus is produced by activated CD4+ T cells, which turn over very quickly in the productively infected state (t1/2≈1 day). The mechanisms by which the cells are cleared have not yet been elucidated and may not involve direct lysis by CTL (Klatt et al., 2010; Wong et al., 2010). The rapid death of these cells causes the precipitous drop in viremia seen when new infection of susceptible cells is blocked by HAART (Figure 1).
By the definition given above, any infected cell with a half-life longer than 1 day could be considered a reservoir. Careful analysis of the rate of decay of viremia following the initiation of HAART revealed a minor population of virus-producing cells with a half-life of approximately two weeks (Perelson et al., 1997). The nature of these cells is controversial. Productively infected CD4+ T cells with a resting phenotype are observed in vivo in acute infection (Zhang et al., 1999), and in vitro studies suggest that chemokines can render resting CD4+ T cells susceptible to infection (Saleh et al., 2007). Macrophages are also infected in vivo, but whether either of these cell types is responsible for the second phase of decay remains unclear.
Eradication efforts will likely be attempted only in patients on HAART who have had sustained suppression of viral replication for years, and therefore the cells responsible for the second phase may not represent a barrier to eradication. Clinically significant reservoirs are more stable. This element of long-term stability is captured in an elegant genetic definition of a viral reservoir proposed by Nickle and colleagues (Nickle et al., 2003). Because sequences arising at different time points in the course of infection can be deposited and persist in a reservoir, the viral sequences in a reservoir will lack temporal structure and show less mean divergence from the most recent common ancestor (MRCA) than the contemporaneous pool of actively replicating virus. The latent reservoir in resting CD4+ T cells meets these criteria and can store the original wild type sequences as well as drug-resistant sequences arising due to suboptimal therapy (Noe et al., 2005; Persaud et al., 2000; Ruff et al., 2002). Extensive sequence analysis is required to define reservoirs in this way. We suggest that a simple and practical definition for an HIV-1 reservoir is an infected cell population that allows persistence of replication-competent HIV-1 in patients on optimal HAART regimens on a time scale of years. To date, the latent reservoir in resting CD4+ T cells is the only reservoir shown to fit this definition.
Sanctuaries and Compartments
There is also confusion over these terms. Both refer to anatomic sites in which the virus may be present. Sanctuary is typically used to refer to a site in which limited penetration of antiretroviral drugs allows persistent replication. Whether there are sanctuaries of this kind in patients on HAART is unclear. Most studies of pharmacologic sanctuaries measure the total drug concentration, including both protein-bound and unbound forms of the drug. However, according to the free drug hypothesis (Martin, 1965), only unbound forms distribute across membranes and determine the biological effect. An important recent study has shown that while the total concentration of the reverse transcriptase inhibitor efavirenz (EFV) is much higher in the blood than in the seminal plasma, the free EFV concentration is actually the same (Avery et al., 2011). Thus, the male genital tract is actually not a drug sanctuary for EFV despite having much a lower total EFV concentration. Similar arguments may apply to other putative drug sanctuaries such as the central nervous system (CNS), and it is critical that free drug concentrations be measured in these sites. Despite concerns about drug sanctuaries, there is no anatomical site where continued HIV-1 replication has been documented in the majority of patients.
Some antiretroviral drugs such as reverse transcriptase (RT) and integrase inhibitors act intracellularly. Nucleoside analogue RT inhibitors (NRTIs) must be phosphorylated by cellular enzymes to an active triphosphate form. Intracellular concentrations of NRTIs correlate significantly with virological efficacy, whereas plasma concentrations do not (for review, see Bazzoli et al., 2010). Because of the technical barriers to sampling intracellular drug concentrations in putative drug sanctuaries, other methods must be used to determine whether viral replication is ongoing, as is discussed below.
The term compartment is sometimes used to refer to an anatomical site in which the virus is present. More commonly, it refers to a site that has limited exchange of viral genetic information with other sites (Nickle et al., 2003). Compartments can be identified using phylogenetic criteria. Typically there are compartment-specific sequences – viral variants that evolve in a particular site and are not distributed to other sites. The extensive literature on compartmentalization of HIV-1 has been recently reviewed (Blackard, 2012). Many early studies of HIV-1 compartmentalization were flawed by PCR resampling (Jabara et al., 2011). Compartments can only be defined with adequate sampling of individual viral genomes in different sites. HIV-1 compartmentalization has been explored mainly in untreated patients, and in terms of eradication, the key issue is whether there are compartments that function as reservoirs.
Cure
Definitions for two types of cures have been proposed (Dieffenbach and Fauci, 2011). In a sterilizing cure, there is complete eradication of the virus. In light of the high fraction of defective viral genomes, this definition could be further refined as follows: A sterilizing cure eliminates all replication-competent forms of the virus; no viral reservoirs remain. By this definition, a patient who retains some defective viral sequences would still be considered cured. In a functional cure, there is permanent control of viral replication without therapy. Elite suppressors (ES), patients who control viral replication without therapy, are often considered examples of a functional cure (Deeks and Walker, 2007). However, replication-competent virus can be isolated from ES (Blankson et al., 2007). In addition, it can be shown with sensitive assays (Pereyra et al., 2009) that these individuals have low-level viremia, analysis of which reveals evidence for viral evolution (Bailey et al., 2006b; O'Connell et al., 2010). Nevertheless, ES generally maintain stable CD4+ T cell counts and plasma HIV-1 levels below the limit of detection of clinical assays and are unlikely to transmit the virus. In this light, a functional cure could be an intervention that renders patients with progressive disease able to permanently control viral replication to below 50 copies/ml without therapy, thereby preventing clinical immunodeficiency and transmission.
The debate over ongoing virus replication in patients on HAART
A critical issue is whether HAART completely stops viral replication. This issue has been difficult to resolve because some indicators of viral replication can also reflect virus production from stable reservoirs (Figure 2). Certain virologic measures could provide conclusive evidence that HIV-1 replication is ongoing in patients on HAART. The first is the detection of labile products of reverse transcription (Zack et al., 1990). In patients on effective regimens composed of protease inhibitors, entry inhibitors, and/or RT inhibitors, labile products of reverse transcription should be absent. The final product of reverse transcription is a full-length, linear, double-stranded viral DNA which is inserted into host DNA by HIV-1 integrase. Pioneering studies by Mario Stevenson showed that linear unintegrated HIV-1 DNA is a prevalent species in resting CD4+ T cells in untreated patients (Bukrinsky et al., 1991). However, this form can be targeted by exonucleases and is labile until it is integrated into cellular DNA (Pierson et al., 2002b; Zhou et al., 2005). Thus, the detection of linear unintegrated HIV-1 DNA would indicate recent infection. Linear unintegrated HIV-1 DNA has not been conclusively demonstrated in patients on HAART. Circular forms of the viral genome, specifically one- and two-LTR circles, can arise particularly when integration is blocked (Bukrinsky et al., 1993; Farnet and Haseltine, 1991; Sharkey et al., 2000). These forms represent dead ends with respect to replication but could serve as indicators of recent infection if they are labile. However, the stability of these forms is controversial (Butler et al., 2002; Pierson et al., 2002a; Sharkey et al., 2005). In addition, as shown in Figure 2, these forms could be generated through the activation of latently infected cells in patients on regimens that rely on an integrase inhibitor to provide most of the antiviral effect. In summary, the analysis of unintegrated forms of the viral genome has not yet provided unequivocal evidence for ongoing replication in most patients on HAART.
Progressive evolutionary change is an inevitable characteristic of HIV-1 replication due the high error rate of RT (Mansky and Temin, 1995). In each infected individual, one or a small number of transmitted founder viruses evolve over time into a complex quasispecies (Keele et al., 2008; Wood et al., 2009). Thus ongoing evolution would also be a clear indication that HAART does not completely stop replication. However, most studies fail to detect evolution in the majority patients on HAART (Bailey et al., 2006a; Evering et al., 2012; Frenkel et al., 2003; Kieffer et al., 2004). Analysis is complicated by variable adherence in patient populations. In any population, there will be patients with poor adherence in whom viral replication occurs as a result of low drug levels. Thus, evolution in a small subset of patients cannot be taken as evidence that the virus continues to replicate in patients on optimal HAART regimens. There is no study documenting evolutionary change in patients known to have therapeutic levels of antiretroviral drugs. As discussed above, residual viremia in patients on HAART reflects ongoing virus production and might be expected to capture evolutionary change if new cycles of replication were occurring. Interestingly, detailed studies of residual viremia have shown that it is drug sensitive, archival in character, and non-evolving (Bailey et al., 2006a; Kieffer et al., 2004; Nettles et al., 2005; Persaud et al., 2004). This lack of evolution is also consistent the general clinical experience that adherent patients can maintain suppression of viremia on HAART indefinitely.
A final experimental test of the hypothesis that viral replication continues in patients on HAART would be the demonstration that intensification of HAART with additional antiretroviral drugs further reduces some measure of viral persistence. Numerous studies with several different intensification drugs have all shown that intensification has no effect on residual viremia (Dinoso et al., 2009; Gandhi et al., 2010; Yukl et al., 2010b). These results strongly suggest that residual viremia originates from stable reservoirs rather than ongoing replication. A single study suggests that intensification transiently increases the levels of 2-LTR circles in a subset of patients (Buzon et al., 2010), but in light of uncertainty about the half-life of these circles, the significance of this result remains unclear.
In summary, the weight of the current evidence suggests that HAART effectively halts ongoing viral replication as defined above. This conclusion is supported by recent pharmacodynamic studies that quantitate the inhibitory potential of HAART regimens (Jilek et al., 2012). Clinical concentrations of some antiretroviral drugs, specifically certain protease inhibitors, can produce 10 logs of inhibition of a single round of infection, and three-drug HAART regimens have an even higher combined inhibitory potential. Given that there are only 1012 lymphocytes in the entire body, only a small fraction of which are infected, this degree of inhibition should effectively block all new infection events in any drug-accessible region of the body. An interesting recent paper suggests that in the vicinity of a virus-producing cell, the exposure of adjacent cells to multiple infection events reduces the efficacy of antiretroviral drugs on a probabilistic basis (Sigal et al., 2011). However, cells with multiple proviruses are rare in vivo (Josefsson et al., 2011). Any replication that does continue in adherent patients on HAART is sufficiently limited that resistance does not evolve. The success of HAART at blocking replication has led to a shift in the HIV-1 treatment field away from the development of new antiretroviral drugs and towards the eradication of reservoirs (Richman et al., 2009).
The latent reservoir in resting CD4+ T cells
The mechanisms by which latent infection is established and maintained in resting CD4+ T cells have been recently reviewed (Karn, 2011; Richman et al., 2009; Siliciano and Greene, 2011) and will be only briefly summarized here. In resting cells, an interrelated set of changes in the transcriptional environment prevent viral gene expression. These include both the sequestration of critical host transcription factors and epigenetic modifications that inhibit transcription. The transcription factors NFκB and NFAT, both of which are involved in HIV-1 gene expression, are excluded from the nucleus in resting CD4+ T cells (Bohnlein et al., 1988; Nabel and Baltimore, 1987). In addition, in resting cells there is also sequestration of pTEFb, a complex of CDK9 and cyclin T1 that, in association with the HIV-1 protein Tat, promotes elongation of HIV-1 transcripts (Contreras et al., 2007; Kao et al., 1987; Peterlin and Price, 2006; Zhu et al., 1997). Even though HIV-1 proviruses are typically integrated within genes that are actively transcribed in resting CD4+ T cells (Han et al., 2004; Shan et al., 2011), repressive chromatin modifications of nucleosomes and DNA methylation at the HIV-1 LTR can interfere with expression of viral genes (Blazkova et al., 2009; Van Lint et al., 1996; Ylisastigui et al., 2004). Many of these mechanisms have been elucidated using transformed cell lines, and the relative importance of each mechanism in maintaining latency in vivo is unclear. The reversal of latency in vivo with agents designed to target these mechanisms may provide insight into this critical issue.
Several approaches to curing HIV-1 infection have been proposed (reviewed in Durand et al., 2012a). One involves reactivation of latent virus while patients are on HAART. This could cause death of infected cells from viral cytopathic effects or allow the immune system to act against these cells. Classes of small molecules capable of reactivating latent HIV-1 include histone deacetylase inhibitors such as vorinostat (suberoylanilide hydroxamic acid, SAHA) (Archin et al., 2009; Contreras et al., 2009) and phorbol esters such as bryostatin and prostratin that induce transcription of HIV-1 through activation of the cellular protein kinase C pathway (Korin et al., 2002; Kulkosky et al., 2001; Mehla et al., 2010; Williams et al., 2004). One recent clinical study demonstrated increased HIV-1 RNA expression in resting CD4+ T cells of patients on HAART after a single oral dose of SAHA, suggesting that SAHA is capable of perturbing the latent reservoir in vivo (Archin et al., 2012). However, recent evidence indicates that simply reactivating latent HIV-1 may not be sufficient to induce immune-mediated purging of latent reservoirs (Shan et al., 2012). Ex vivo experiments have shown that priming of CD8+ T cells with HIV-1 gag peptides permits these cells to kill HIV-1-infected CD4+ T cells whose virus had been reactivated with SAHA, suggesting that a combination of immune priming and reactivation of latent HIV-1 may be necessary for a cure.
As approaches for targeting the latent reservoir are developed, it is important to understand how the reservoir is measured. The original in vivo measurements of the latent reservoir in resting CD4+ T cells were made using a viral outgrowth assay (Chun et al., 1997; Finzi et al., 1997). In this assay, serial dilutions of purified resting CD4+ T cells from an HIV-1 infected individual are activated with the mitogen phytohemagglutinin (PHA) and irradiated allogeneic peripheral blood mononuclear cells. This causes uniform T cell activation and thereby switches all of the cells carrying latent HIV-1 genomes into a state that is permissive for virus gene expression. CD4+ T lymphoblasts from healthy donors are added to amplify any virus released from the infected cells, and after a period of 2–3 weeks, the numbers of culture wells at each dilution that are positive for viral growth is determined by ELISA assay for HIV-1 p24 antigen. Using Poisson statistics, the number of positive wells can be used to calculate the number of latently infected cells per million resting CD4+ T cells (infectious units per million, IUPM). The advantage of this assay is that it quantifies the minimum number of latently infected cells harboring replication-competent virus. Disadvantages include cost and complexity and the fact that it does not distinguish between cells carrying integrated proviruses and recently infected cells carrying linear unintegrated HIV-1 DNA. However, because linear forms are labile, they may not confound the measurement in patients on HAART. The most serious problem is the dynamic range, which is limited by the low frequency of latently infected cells and the large amount of blood required to detect them. Eradication approaches that produce only small (1 log) reductions in the size of the latent reservoir may render the reservoir undetectable with this assay.
Alternative assays use PCR to quantify cells containing viral genomes. Some PCR assays are specific for integrated proviruses (Chun et al., 1995; Chun et al., 1997; O'Doherty et al., 2002). A problem with all PCR-based assays is that they do not differentiate between cells that can produce infectious virus and cells containing proviruses that have been either permanently silenced through epigenetic mechanisms or rendered non-infectious through APOBEC3G-mediated hypermutation or some other form of mutation (Chun et al., 1997; Kieffer et al., 2005). In patients on HAART, the frequency of resting CD4+ T cells carrying HIV-1 DNA is 100- to 300-fold greater than the frequency latently infected cells detected in the culture assay (J. Siliciano et al., unpublished data). Thus, although PCR assays are simpler, it is not yet clear that they will provide an effective way to monitor eradication efforts.
Other reservoirs
Although the existence of a stable latent reservoir in resting CD4+ T cells is clearly established, additional reservoirs could further complicate eradication efforts. Evidence for additional reservoirs comes from a detailed analysis of residual viremia. Although many viral sequences in the plasma of patients on HAART are identical to sequences found in the latent reservoir, the residual viremia in some patients is dominated by oligoclonal populations called predominant plasma clones (PPC) that are profoundly underrepresented among proviruses in circulating CD4+ T cells (Bailey et al., 2006a). Sophisticated genetic tests for population structure also suggest that some of the residual viremia originates from cells other than resting CD4+ T cells found in the circulation (Bailey et al., 2006a; Sahu et al., 2009). It is also possible that these clonal sequences originate from resting CD4+ T cells present only at low frequency in the circulation (Anderson et al., 2011). In any event, residual viremia provides an important window into the state of virologic suppression because it reveals ongoing virus production in patients on HAART and thus reports on the existence and persistence of viral reservoirs.
Macrophages
Among other cell types that could serve as HIV-1 reservoirs, macrophages are frequently mentioned. Early studies demonstrated HIV-1 infection of macrophages in vivo and showed that these cells are more resistant to viral cytopathic effects than are activated CD4+ T cells (Gartner et al., 1986; Koenig et al., 1986)). Macrophage-tropic HIV-1 variants utilize the CCR5 coreceptor (Alkhatib et al., 1996; Deng et al., 1996) but also have sequence changes in the Env protein that allow entry into cells that express low amounts of CD4, including macrophages (Schnell et al., 2011; Walter et al., 2005). Infection of macrophages is particularly prominent late in the course of infection when most of the CD4+ T cells have been lost (Igarashi et al., 2001). Although infected macrophages play a role in HIV-1 pathogenesis, it is not yet clear that these cells fit the definition for a reservoir proposed above.
Infection of macrophages should be viewed in the context of the normal differentiation of these cells (reviewed in Auffray et al., 2009; Shi and Pamer, 2011). Macrophages are derived from the pluripotent hematopoietic stem cells (HSC) that give rise to all cellular components of the blood. HSC give rise to hematopoietic progenitor cells (HPC) with more restricted differentiation potential including a common myeloid progenitor that gives rise to the granulocyte and monocyte-macrophage lineages through an integrated process of proliferation and differentiation occurring in the bone marrow (Iwasaki and Akashi, 2007). The bone marrow phase of macrophage differentiation lasts 2–5 days and ends when promonocytes leave the marrow and enter the circulation as monocytes. Monocytes can be divided into subsets based on expression of chemokine receptors and other surface proteins, trafficking patterns, and differentiation potential (Auffray et al., 2009; Shi and Pamer, 2011). After less than one day, monocytes leave the circulation and undergo further differentiation. This differentiation is tissue specific in the sense that it results in the generation of cells with different morphologies and functions depending on the tissue. These tissue forms include ordinary tissue macrophages, dendritic cells, Langerhans cells in the epidermis, alveolar macrophages in the lung, Kupffer cells in the liver, perivascular macrophages and microglial cells in the CNS, and osteoclasts in bone. The tissue half-lives of these cell types are not well studied. Importantly, there is evidence that Langerhans cells and microglial cells take up residence in their respective anatomic sites and persist with less need for continuous replacement by monocytes (Saijo and Glass, 2011).
Macrophages could in principle be infected at three different stages: during differentiation in the marrow, in the blood as monocytes, or in the tissues as mature macrophages. Infection of HPC in the marrow is discussed below. Little is known about infection of bone marrow progenitor cells committed to the monocyte-macrophage lineage, but if it does occur, it must be rare. The number of circulating monocytes carrying HIV-1 DNA is extremely low, so low in fact that contaminating CD4+ T cells cannot be excluded as a source of the signal (Zhu et al., 2002). Monocytes appear relatively resistant to in vitro infection with HIV-1, due to inefficient entry and delays in early steps in the life cycle (Arfi et al., 2008). In addition, the recently identified host restriction factor SAMHD1 may protect these cells from infection (Laguette et al., 2011). Neither monocytes nor any of the committed progenitors in the marrow can be considered reservoirs by the definition proposed in Table 1 because these are transient stages of differentiation lasting only a matter of days before further differentiation occurs. In contrast, macrophages could serve as a reservoir if they can persist in an infected state on a time scale of years in patients on HAART. Most studies documenting HIV-1 infection of macrophages in various tissues were from the pre-HAART era (Hufert et al., 1993; Koenig et al., 1986; McIlroy et al., 1996; Wiley et al., 1986). Proving that macrophages function as a reservoir will require definitive demonstration that these cells harbor replication-competent HIV-1 in patients who have had prolonged suppression of viremia on HAART.
Hematopoietic progenitor cells (HPCs). HPCs express low amounts of CD4 and the co-receptors required for HIV-1 entry, CCR5 and CXCR4. Most studies have concluded that these cells do not harbor HIV-1 in vivo (reviewed in McNamara and Collins, 2011)). Recently, Carter et al. reported HIV-1 infection in CD34+ HPCs in 4 of 9 patients on HAART at a frequency of 2.5–40 copies of HIV-1 DNA/104 cells (Carter et al., 2010). Interestingly, the infection remained latent until the cells were driven to differentiate. A follow-up study indicated that CD133+ cord-blood-derived cells were susceptible to in vitro infection, but only with X4-tropic virus (Carter et al., 2011). Other more recent studies in patients on HAART have used more rigorous purification of CD34+ cells and have not detected HIV-1 DNA by PCR (Durand et al., 2012b). It is therefore not clear that hematopoietic stem and progenitor cells constitute a reservoir.
Anatomic sites of HIV-1 persistence
CNS
The CNS is an anatomic site in which viral replication has unique pathophysiologic consequences. In the pre-HAART era, HIV-1-associated dementia was a devastating consequence of the disease (reviewed in Gonzalez-Scarano and Martin-Garcia, 2005; McArthur et al., 2010). HIV-1 has been detected in several areas of the brain as early as 15 days after infection (Davis et al., 1992). Within the CNS, infection is detected principally in perivascular macrophages and microglial cells (Wiley et al., 1986). There is strong evidence for compartmentalization of HIV-1 in the CNS and concern that the CNS is a pharmacologic sanctuary. CNS-specific viral variants can be demonstrated in untreated individuals and may be associated with dementia (Schnell et al., 2010; Schnell et al., 2011). With respect to the sanctuary issue, drug concentrations in brain parenchyma are hard to assess, but for some drugs, concentrations in the cerebrospinal fluid (CSF) are substantially lower than in the blood. A sophisticated index for the CNS penetration and effectiveness of antiretroviral drugs has been developed (Letendre et al., 2008), but the best predictor of neurocognitive disorders is the pretreatment CD4+ T cell count nadir (Heaton et al., 2010). Studies of drug concentrationsin the CSF are subject to the same caveats mentioned above for the male genital tract. Antiretroviral drugs are substrates for a variety of transporters that could potentially affect drug levels (reviewed in Varatharajan and Thomas, 2009). Arguing against the sanctuary concept is the general finding that initiation of HAART rapidly reduces virus concentrations in the CSF, generally to undetectable levels, and prevents or improves many of the neurocognitive problems seen in untreated HIV-1 infection (Price and Spudich, 2008). However, there are rare patients in whom replication appears to continue, as evidenced by detectable free virus in the CSF (Price and Spudich, 2008). Given the prevalence of asymptomatic neurocognitive impairment in patients on HAART (25–50% in some studies; Heaton et al., 2010), this is an extremely important area for future research.
Compelling evidence for viral persistence in the CNS comes from an elegant model of HIV-1-associated neurologic disease in SIV-infected macaques (Zink and Clements, 2002). In this model, infection with a neurovirulent SIV inoculum consistently produces neurologic disease within months of infection in untreated animals. Infection of microglial cells is particularly prominent (Babas et al., 2003). Antiretroviral drugs rapidly reduce plasma and CSF levels of virus, and viral RNA in the brain parenchyma (Zink et al., 2010). Interestingly, levels of SIV DNA remain constant. Because of the evidence from murine studies that microglial cells turn over less rapidly than other monocyte-derived cells (Saijo and Glass, 2011), there is concern that these cells may serve as an important HIV-1 reservoir. The SIV model affords the best opportunity to establish whether infected microglial cells can persist on a time scale of years in on the setting of HAART.
Gut-associated lymphoid tissue (GALT). A substantial fraction of the total lymphocytes in the body are in specialized sites along the gastrointestinal tract. The GALT includes the Peyer’s patches, organized lymphoid structures in the small intestine that function as inductive sites. In addition, there are activated T cells in the lamina propria layer of the intestinal mucosa, beneath the epithelium. There are also lymphocytes within the epithelium. Pioneering studies by Veazey and colleagues in the SIV model (Veazey et al., 1998) indicated that a dramatic loss of lamina propria CD4+ T cells occurs early in infection, much earlier than the loss of CD4+ T cells in other sites. The same is true for patients with HIV-1 infection (Brenchley et al., 2004b; Guadalupe et al., 2003). This appears to reflect the high frequency of lamina propria CD4+ T cells that express the HIV-1 coreceptor CCR5 and that are in an activated state that is optimal for HIV-1 infection. The loss of CD4+ T cells from the lamina propria is not rapidly reversed by HAART (Chun et al., 2008; Guadalupe et al., 2003; Mehandru et al., 2006). In patients on HAART, the fraction of CD4+ T cells in the lamina propria that carry HIV-1 DNA is substantially higher than in other sites (Chun et al., 2008; Yukl et al., 2010a). Chun and colleagues reported an average of approximately 5000 HIV-1 DNA copies/106 CD4+ T cells in CD8-depleted biopsy samples from the terminal ileum of patients on HAART, roughly 5 times greater than the HIV-1 DNA levels in resting CD4+ T cells from the peripheral blood. These findings raise the issue of whether CD4+ T cells in the GALT provide a unique reservoir for HIV-1. However, there are unresolved issues. Because many CD4+ T cells in the gut are in an activated state, it is important to know whether the infected cells in this site are expressing viral RNA, and if so, whether ongoing viral replication in this site contributes to the high level of proviruses observed. A recent report describes a striking lack of evolution in the proviral sequences from recto-sigmoid biopsies (Evering et al., 2012). These results are inconsistent with continuous viral replication in this site. It is also important to determine the half-life of these infected cells and whether they contribute to residual viremia and viral rebound following treatment interruption.
Conclusions
Recent developments have contributed to renewed optimism that HIV-1 infection can be cured, but the discussion of this issue has been hampered by lack of agreement regarding the meaning of important terms. This review suggests definitions to facilitate the discussion. The weight of evidence suggests that HIV-1 persistence in patients on HAART is due to long-lived infected cells that function as a reservoir rather than viral replication that continues despite HAART or in drug sanctuaries. Although previous studies have proposed the existence of multiple reservoirs for HIV-1, only the latent reservoir in resting CD4+ T cells meets the practical definition proposed here, a cell type in which replication-competent virus can persist on a time scale of years in patients on optimal HAART regimens. Macrophages are clearly infected in vivo, but it is not yet clear that infected macrophages in tissue sites survive on a time-scale of years in patients on optimal HAART regimens. It will be particularly important to understand viral persistence in compartments such as the CNS, where long-lived microglial cells may represent a reservoir, and in the GALT, which is a major site of HIV-1 replication in untreated individuals.
Acknowledgements
This work was supported by the National Institutes of Health, the Foundation for AIDS Research, and the Howard Hughes Medical Institute. We thank J. Siliciano for a critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- Anderson JA, Archin NM, Ince W, Parker D, Wiegand A, Coffin JM, Kuruc J, Eron J, Swanstrom R, Margolis DM. Clonal sequences recovered from plasma from patients with residual HIV-1 viremia and on intensified antiretroviral therapy are identical to replicating viral RNAs recovered from circulating resting CD4+ T cells. J. Virol. 2011;85:5220–5223. doi: 10.1128/JVI.00284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Espeseth A, Parker D, Cheema M, Hazuda D, Margolis DM. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res. Hum. Retroviruses. 2009;25:207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfi V, Riviere L, Jarrosson-Wuilleme L, Goujon C, Rigal D, Darlix JL, Cimarelli A. Characterization of the early steps of infection of primary blood monocytes by human immunodeficiency virus type 1. J. Virol. 2008;82:6557–6565. doi: 10.1128/JVI.02321-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- Avery LB, Bakshi RP, Cao YJ, Hendrix CW. The male genital tract is not a pharmacological sanctuary from efavirenz. Clin. Pharmacol. Ther. 2011;90:151–156. doi: 10.1038/clpt.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babas T, Munoz D, Mankowski JL, Tarwater PM, Clements JE, Zink MC. Role of microglial cells in selective replication of simian immunodeficiency virus genotypes in the brain. J. Virol. 2003;77:208–216. doi: 10.1128/JVI.77.1.208-216.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JR, Sedaghat AR, Kieffer T, Brennan T, Lee PK, Wind-Rotolo M, Haggerty CM, Kamireddi AR, Liu Y, Lee J, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J. Virol. 2006a;80:6441–6457. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JR, Williams TM, Siliciano RF, Blankson JN. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J. Exp. Med. 2006b;203:1357–1369. doi: 10.1084/jem.20052319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoli C, Jullien V, Le Tiec C, Rey E, Mentre F, Taburet AM. Intracellular Pharmacokinetics of Antiretroviral Drugs in HIV-Infected Patients, and their Correlation with Drug Action. Clin. Pharmacokinet. 2010;49:17–45. doi: 10.2165/11318110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Blackard JT. HIV compartmentalization: a review on a clinically important phenomenon. Curr. HIV. Res. 2012;10:133–142. doi: 10.2174/157016212799937245. [DOI] [PubMed] [Google Scholar]
- Blankson JN, Bailey JR, Thayil S, Yang HC, Lassen K, Lai J, Gandhi SK, Siliciano JD, Williams TM, Siliciano RF. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J. Virol. 2007;81:2508–2518. doi: 10.1128/JVI.02165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 2002;53:557–593. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- Blazkova J, Trejbalova K, Gondois-Rey F, Halfon P, Philibert P, Guiguen A, Verdin E, Olive D, Van Lint C, Hejnar J, Hirsch I. CpG methylation controls reactivation of HIV from latency. PLoS Pathog. 2009;5:e1000554. doi: 10.1371/journal.ppat.1000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnlein E, Lowenthal JW, Siekevitz M, Ballard DW, Franza BR, Greene WC. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell. 1988;53:827–836. doi: 10.1016/0092-8674(88)90099-2. [DOI] [PubMed] [Google Scholar]
- Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, Nelson JA, Gairin JE, Hahn BH, Oldstone MB, Shaw GM. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, Casazza JP, Kuruppu J, Yazdani J, Migueles SA, Connors M, et al. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J. Virol. 2004a;78:1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 2004b;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky M, Sharova N, Stevenson M. Human immunodeficiency virus type 1 2-LTR circles reside in a nucleoprotein complex which is different from the preintegration complex. J. Virol. 1993;67:6863–6865. doi: 10.1128/jvi.67.11.6863-6865.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SL, Johnson EP, Bushman FD. Human immunodeficiency virus cDNA metabolism: notable stability of two-long terminal repeat circles. J. Virol. 2002;76:3739–3747. doi: 10.1128/JVI.76.8.3739-3747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzon MJ, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, Gatell JM, Domingo P, Paredes R, Sharkey M, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat. Med. 2010;16:460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- Carter CC, McNamara LA, Onafuwa-Nuga A, Shackleton M, Riddell J, 4th, Bixby D, Savona MR, Morrison SJ, Collins KL. HIV-1 utilizes the CXCR4 chemokine receptor to infect multipotent hematopoietic stem and progenitor cells. Cell. Host Microbe. 2011;9:223–234. doi: 10.1016/j.chom.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell J, 4th, Bixby D, Savona MR, Collins KL. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat. Med. 2010;16:446–451. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat. Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, Hallahan CW, Kottilil S, Moir S, Mican JM, Mullins JI, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J. Infect. Dis. 2008;197:714–720. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras X, Barboric M, Lenasi T, Peterlin BM. HMBA releases P-TEFb from HEXIM1 and 7SK snRNA via PI3K/Akt and activates HIV transcription. PLoS Pathog. 2007;3:1459–1469. doi: 10.1371/journal.ppat.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras X, Schweneker M, Chen CS, McCune JM, Deeks SG, Martin J, Peterlin BM. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J. Biol. Chem. 2009;284:6782–6789. doi: 10.1074/jbc.M807898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey RT, Jr, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl. Acad. Sci. U. S. A. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Dieffenbach CW, Fauci AS. Thirty years of HIV and AIDS: future challenges and opportunities. Ann. Intern. Med. 2011;154:766–771. doi: 10.7326/0003-4819-154-11-201106070-00345. [DOI] [PubMed] [Google Scholar]
- Dinoso JB, Kim SY, Wiegand AM, Palmer SE, Gange SJ, Cranmer L, O'Shea A, Callender M, Spivak A, Brennan T, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9403–9408. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornadula G, Zhang H, VanUitert B, Stern J, Livornese L, Jr, Ingerman MJ, Witek J, Kedanis RJ, Natkin J, DeSimone J, Pomerantz RJ. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA. 1999;282:1627–1632. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- Durand CM, Blankson JN, Siliciano RF. Developing strategies for HIV-1 eradication. Trends Immunol. 2012a doi: 10.1016/j.it.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Ghiaur G, Siliciano JD, Rabi SA, Eisele EE, Salgado M, Shan L, Lai JF, Zhang H, Margolick J, et al. HIV-1 DNA Is Detected in Bone Marrow Populations Containing CD4+ T Cells but Is not Found in Purified CD34+ Hematopoietic Progenitor Cells in Most Patients on Antiretroviral Therapy. J. Infect. Dis. 2012b;205:1014–1018. doi: 10.1093/infdis/jir884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evering TH, Mehandru S, Racz P, Tenner-Racz K, Poles MA, Figueroa A, Mohri H, Markowitz M. Absence of HIV-1 evolution in the gut-associated lymphoid tissue from patients on combination antiviral therapy initiated during primary infection. PLoS Pathog. 2012;8:e1002506. doi: 10.1371/journal.ppat.1002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnet CM, Haseltine WA. Circularization of human immunodeficiency virus type 1 DNA in vitro. J. Virol. 1991;65:6942–6952. doi: 10.1128/jvi.65.12.6942-6952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- Frenkel LM, Wang Y, Learn GH, McKernan JL, Ellis GM, Mohan KM, Holte SE, De Vange SM, Pawluk DM, Melvin AJ, et al. Multiple viral genetic analyses detect low-level human immunodeficiency virus type 1 replication during effective highly active antiretroviral therapy. J. Virol. 2003;77:5721–5730. doi: 10.1128/JVI.77.10.5721-5730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi RT, Zheng L, Bosch RJ, Chan ES, Margolis DM, Read S, Kallungal B, Palmer S, Medvik K, Lederman MM, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7:e1000321. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat. Rev. Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Lassen K, Monie D, Sedaghat AR, Shimoji S, Liu X, Pierson TC, Margolick JB, Siliciano RF, Siliciano JD. Resting CD4+ T cells from human immunodeficiency virus type 1 (HIV-1)-infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. J. Virol. 2004;78:6122–6133. doi: 10.1128/JVI.78.12.6122-6133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Hufert FT, Schmitz J, Schreiber M, Schmitz H, Racz P, von Laer DD. Human Kupffer cells infected with HIV-1 in vivo. J. Acquir. Immune Defic. Syndr. 1993;6:772–777. [PubMed] [Google Scholar]
- Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- Igarashi T, Brown CR, Endo Y, Buckler-White A, Plishka R, Bischofberger N, Hirsch V, Martin MA. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): Implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. U. S. A. 2001;98:658–663. doi: 10.1073/pnas.021551798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H, Akashi K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity. 2007;26:726–740. doi: 10.1016/j.immuni.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Jabara CB, Jones CD, Roach J, Anderson JA, Swanstrom R. Accurate sampling and deep sequencing of the HIV-1 protease gene using a Primer ID. Proc. Natl. Acad. Sci. U. S. A. 2011;108:20166–20171. doi: 10.1073/pnas.1110064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilek BL, Zarr M, Sampah ME, Rabi SA, Bullen CK, Lai J, Shen L, Siliciano RF. A quantitative basis for antiretroviral therapy for HIV-1 infection. Nat. Med. 2012 doi: 10.1038/nm.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos B, Fischer M, Kuster H, Pillai SK, Wong JK, Boni J, Hirschel B, Weber R, Trkola A, Gunthard HF Swiss HIV Cohort Study. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16725–16730. doi: 10.1073/pnas.0804192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson L, King MS, Makitalo B, Brannstrom J, Shao W, Maldarelli F, Kearney MF, Hu WS, Chen J, Gaines H, et al. Majority of CD4+ T cells from peripheral blood of HIV-1-infected individuals contain only one HIV DNA molecule. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11199–11204. doi: 10.1073/pnas.1107729108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao SY, Calman AF, Luciw PA, Peterlin BM. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987;330:489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- Karn J. The molecular biology of HIV latency: breaking and restoring the Tat-dependent transcriptional circuit. Curr. Opin. HIV. AIDS. 2011;6:4–11. doi: 10.1097/COH.0b013e328340ffbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer TL, Finucane MM, Nettles RE, Quinn TC, Broman KW, Ray SC, Persaud D, Siliciano RF. Genotypic analysis of HIV-1 drug resistance at the limit of detection: virus production without evolution in treated adults with undetectable HIV loads. J. Infect. Dis. 2004;189:1452–1465. doi: 10.1086/382488. [DOI] [PubMed] [Google Scholar]
- Kieffer TL, Kwon P, Nettles RE, Han Y, Ray SC, Siliciano RF. G-->A hypermutation in protease and reverse transcriptase regions of human immunodeficiency virus type 1 residing in resting CD4+ T cells in vivo. J. Virol. 2005;79:1975–1980. doi: 10.1128/JVI.79.3.1975-1980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt NR, Shudo E, Ortiz AM, Engram JC, Paiardini M, Lawson B, Miller MD, Else J, Pandrea I, Estes JD, et al. CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog. 2010;6:e1000747. doi: 10.1371/journal.ppat.1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Korin YD, Brooks DG, Brown S, Korotzer A, Zack JA. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J. Virol. 2002;76:8118–8123. doi: 10.1128/JVI.76.16.8118-8123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkosky J, Culnan DM, Roman J, Dornadula G, Schnell M, Boyd MR, Pomerantz RJ. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood. 2001;98:3006–3015. doi: 10.1182/blood.v98.10.3006. [DOI] [PubMed] [Google Scholar]
- Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch. Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansky LM, Temin HM. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J. Virol. 1995;69:5087–5094. doi: 10.1128/jvi.69.8.5087-5094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BK. Potential effect of the plasma on drug distribution. Nature. 1965;207:274–276. doi: 10.1038/207274a0. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann. Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- McIlroy D, Autran B, Cheynier R, Clauvel JP, Oksenhendler E, Debre P, Hosmalin A. Low infection frequency of macrophages in the spleens of HIV+ patients. Res. Virol. 1996;147:115–121. doi: 10.1016/0923-2516(96)80225-1. [DOI] [PubMed] [Google Scholar]
- McNamara LA, Collins KL. Hematopoietic stem/precursor cells as HIV reservoirs. Curr. Opin. HIV. AIDS. 2011;6:43–48. doi: 10.1097/COH.0b013e32834086b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehandru S, Poles MA, Tenner-Racz K, Jean-Pierre P, Manuelli V, Lopez P, Shet A, Low A, Mohri H, Boden D, Racz P, Markowitz M. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 2006;3:e484. doi: 10.1371/journal.pmed.0030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehla R, Bivalkar-Mehla S, Zhang R, Handy I, Albrecht H, Giri S, Nagarkatti P, Nagarkatti M, Chauhan A. Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner. PLoS One. 2010;5:e11160. doi: 10.1371/journal.pone.0011160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, Cooper CL, Nachega JB, Dybul M, Hogg RS. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann. Intern. Med. 2011;155:209–216. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Nettles RE, Kieffer TL, Kwon P, Monie D, Han Y, Parsons T, Cofrancesco J, Jr, Gallant JE, Quinn TC, Jackson B, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005;293:817–829. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- Nickle DC, Jensen MA, Shriner D, Brodie SJ, Frenkel LM, Mittler JE, Mullins JI. Evolutionary indicators of human immunodeficiency virus type 1 reservoirs and compartments. J. Virol. 2003;77:5540–5546. doi: 10.1128/JVI.77.9.5540-5546.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe A, Plum J, Verhofstede C. The latent HIV-1 reservoir in patients undergoing HAART: an archive of pre-HAART drug resistance. J. Antimicrob. Chemother. 2005;55:410–412. doi: 10.1093/jac/dki038. [DOI] [PubMed] [Google Scholar]
- O'Connell KA, Brennan TP, Bailey JR, Ray SC, Siliciano RF, Blankson JN. Control of HIV-1 in elite suppressors despite ongoing replication and evolution in plasma virus. J. Virol. 2010;84:7018–7028. doi: 10.1128/JVI.00548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty U, Swiggard WJ, Jeyakumar D, McGain D, Malim MH. A sensitive, quantitative assay for human immunodeficiency virus type 1 integration. J. Virol. 2002;76:10942–10950. doi: 10.1128/JVI.76.21.10942-10950.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N. Engl. J. Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, Kempf DJ, Mellors JW, Coffin JM, King MS. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JM, Polis M, Dewar RL, Planta A, Liu S, Metcalf JA, Mellors JW, Coffin JM. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J. Clin. Microbiol. 2003;41:4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho DD. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- Pereyra F, Palmer S, Miura T, Block BL, Wiegand A, Rothchild AC, Baker B, Rosenberg R, Cutrell E, Seaman MS, Coffin JM, Walker BD. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J. Infect. Dis. 2009;200:984–990. doi: 10.1086/605446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng GC, Jones C. Towards an understanding of the herpes simplex virus type 1 latency-reactivation cycle. Interdiscip. Perspect. Infect. Dis. 2010;2010:262415. doi: 10.1155/2010/262415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud D, Pierson T, Ruff C, Finzi D, Chadwick KR, Margolick JB, Ruff A, Hutton N, Ray S, Siliciano RF. A stable latent reservoir for HIV-1 in resting CD4(+) T lymphocytes in infected children. J. Clin. Invest. 2000;105:995–1003. doi: 10.1172/JCI9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud D, Siberry GK, Ahonkhai A, Kajdas J, Monie D, Hutton N, Watson DC, Quinn TC, Ray SC, Siliciano RF. Continued production of drug-sensitive human immunodeficiency virus type 1 in children on combination antiretroviral therapy who have undetectable viral loads. J. Virol. 2004;78:968–979. doi: 10.1128/JVI.78.2.968-979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin BM, Price DH. Controlling the elongation phase of transcription with P-TEFb. Mol. Cell. 2006;23:297–305. doi: 10.1016/j.molcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Piatak M, Jr, Saag MS, Yang LC, Clark SJ, Kappes JC, Luk KC, Hahn BH, Shaw GM, Lifson JD. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993;259:1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- Pierson T, Hoffman TL, Blankson J, Finzi D, Chadwick K, Margolick JB, Buck C, Siliciano JD, Doms RW, Siliciano RF. Characterization of chemokine receptor utilization of viruses in the latent reservoir for human immunodeficiency virus type 1. J. Virol. 2000;74:7824–7833. doi: 10.1128/jvi.74.17.7824-7833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson TC, Kieffer TL, Ruff CT, Buck C, Gange SJ, Siliciano RF. Intrinsic stability of episomal circles formed during human immunodeficiency virus type 1 replication. J. Virol. 2002a;76:4138–4144. doi: 10.1128/JVI.76.8.4138-4144.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson TC, Zhou Y, Kieffer TL, Ruff CT, Buck C, Siliciano RF. Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J. Virol. 2002b;76:8518–8531. doi: 10.1128/JVI.76.17.8518-8531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RW, Spudich S. Antiretroviral therapy and central nervous system HIV type 1 infection. J. Infect. Dis. 2008;197(Suppl 3):S294–S306. doi: 10.1086/533419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramratnam B, Bonhoeffer S, Binley J, Hurley A, Zhang L, Mittler JE, Markowitz M, Moore JP, Perelson AS, Ho DD. Rapid production and clearance of HIV-1 and hepatitis C virus assessed by large volume plasma apheresis. Lancet. 1999;354:1782–1785. doi: 10.1016/S0140-6736(99)02035-8. [DOI] [PubMed] [Google Scholar]
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CT, Ray SC, Kwon P, Zinn R, Pendleton A, Hutton N, Ashworth R, Gange S, Quinn TC, Siliciano RF, Persaud D. Persistence of wild-type virus and lack of temporal structure in the latent reservoir for human immunodeficiency virus type 1 in pediatric patients with extensive antiretroviral exposure. J. Virol. 2002;76:9481–9492. doi: 10.1128/JVI.76.18.9481-9492.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu GK, Paar D, Frost SD, Smith MM, Weaver S, Cloyd MW. Low-level plasma HIVs in patients on prolonged suppressive highly active antiretroviral therapy are produced mostly by cells other than CD4 T-cells. J. Med. Virol. 2009;81:9–15. doi: 10.1002/jmv.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu GK, Sarria JC, Cloyd MW. Recovery of replication-competent residual HIV-1 from plasma of a patient receiving prolonged, suppressive highly active antiretroviral therapy. J. Virol. 2010;84:8348–8352. doi: 10.1128/JVI.00362-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- Saleh S, Solomon A, Wightman F, Xhilaga M, Cameron PU, Lewin SR. CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood. 2007;110:4161–4164. doi: 10.1182/blood-2007-06-097907. [DOI] [PubMed] [Google Scholar]
- Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R. HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog. 2011;7:e1002286. doi: 10.1371/journal.ppat.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. J. Virol. 2010;84:2395–2407. doi: 10.1128/JVI.01863-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF. Stimulation of HIV-1-Specific Cytolytic T Lymphocytes Facilitates Elimination of Latent Viral Reservoir after Virus Reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L, Yang HC, Rabi SA, Bravo HC, Shroff NS, Irizarry RA, Zhang H, Margolick JB, Siliciano JD, Siliciano RF. Influence of host gene transcription level and orientation on HIV-1 latency in a primary-cell model. J. Virol. 2011;85:5384–5393. doi: 10.1128/JVI.02536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey M, Triques K, Kuritzkes DR, Stevenson M. In vivo evidence for instability of episomal human immunodeficiency virus type 1 cDNA. J. Virol. 2005;79:5203–5210. doi: 10.1128/JVI.79.8.5203-5210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey ME, Teo I, Greenough T, Sharova N, Luzuriaga K, Sullivan JL, Bucy RP, Kostrikis LG, Haase A, Veryard C, et al. Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nat. Med. 2000;6:76–81. doi: 10.1038/71569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, Milo R, Baltimore D. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011;477:95–98. doi: 10.1038/nature10347. [DOI] [PubMed] [Google Scholar]
- Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- Siliciano RF, Greene WC. HIV Latency. Cold Spring Harb Perspect. Med. 2011;1:a007096. doi: 10.1101/cshperspect.a007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain MC, Gunthard HF, Havlir DV, Ignacio CC, Smith DM, Leigh-Brown AJ, Macaranas TR, Lam RY, Daly OA, Fischer M, et al. Heterogeneous clearance rates of long-lived lymphocytes infected with HIV: intrinsic stability predicts lifelong persistence. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4819–4824. doi: 10.1073/pnas.0736332100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint C, Emiliani S, Ott M, Verdin E. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO. J. 1996;15:1112–1120. [PMC free article] [PubMed] [Google Scholar]
- Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009;82:A99–A109. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- Walensky RP, Paltiel AD, Losina E, Mercincavage LM, Schackman BR, Sax PE, Weinstein MC, Freedberg KA. The survival benefits of AIDS treatment in the United States. J. Infect. Dis. 2006;194:11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- Walter BL, Wehrly K, Swanstrom R, Platt E, Kabat D, Chesebro B. Role of low CD4 levels in the influence of human immunodeficiency virus type 1 envelope V1 and V2 regions on entry and spread in macrophages. J. Virol. 2005;79:4828–4837. doi: 10.1128/JVI.79.8.4828-4837.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Ghosh SK, Taylor ME, Johnson VA, Emini EA, Deutsch P, Lifson JD, Bonhoeffer S, Nowak MA, Hahn BH. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc. Natl. Acad. Sci. U. S. A. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Chen LF, Kwon H, Fenard D, Bisgrove D, Verdin E, Greene WC. Prostratin antagonizes HIV latency by activating NF-kappaB. J. Biol. Chem. 2004;279:42008–42017. doi: 10.1074/jbc.M402124200. [DOI] [PubMed] [Google Scholar]
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- Wong JK, Strain MC, Porrata R, Reay E, Sankaran-Walters S, Ignacio CC, Russell T, Pillai SK, Looney DJ, Dandekar S. In vivo CD8+ T-cell suppression of siv viremia is not mediated by CTL clearance of productively infected cells. PLoS Pathog. 2010;6:e1000748. doi: 10.1371/journal.ppat.1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood N, Bhattacharya T, Keele BF, Giorgi E, Liu M, Gaschen B, Daniels M, Ferrari G, Haynes BF, McMichael A, et al. HIV evolution in early infection: selection pressures, patterns of insertion and deletion, and the impact of APOBEC. PLoS Pathog. 2009;5:e1000414. doi: 10.1371/journal.ppat.1000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylisastigui L, Archin NM, Lehrman G, Bosch RJ, Margolis DM. Coaxing HIV-1 from resting CD4 T cells: histone deacetylase inhibition allows latent viral expression. AIDS. 2004;18:1101–1108. doi: 10.1097/00002030-200405210-00003. [DOI] [PubMed] [Google Scholar]
- Yukl SA, Gianella S, Sinclair E, Epling L, Li Q, Duan L, Choi AL, Girling V, Ho T, Li P, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J. Infect. Dis. 2010a;202:1553–1561. doi: 10.1086/656722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukl SA, Shergill AK, McQuaid K, Gianella S, Lampiris H, Hare CB, Pandori M, Sinclair E, Gunthard HF, Fischer M, Wong JK, Havlir DV. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS. 2010b doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhang H, Siliciano JD, Siliciano RF. Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ T cells. J. Virol. 2005;79:2199–2210. doi: 10.1128/JVI.79.4.2199-2210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Muthui D, Holte S, Nickle D, Feng F, Brodie S, Hwangbo Y, Mullins JI, Corey L. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J. Virol. 2002;76:707–716. doi: 10.1128/JVI.76.2.707-716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Pe'ery T, Peng J, Ramanathan Y, Marshall N, Marshall T, Amendt B, Mathews MB, Price DH. Transcription elongation factor P-TEFb is required for HIV-1 tat transactivation in vitro. Genes Dev. 1997;11:2622–2632. doi: 10.1101/gad.11.20.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Brice AK, Kelly KM, Queen SE, Gama L, Li M, Adams RJ, Bartizal C, Varrone J, Rabi SA, et al. Simian immunodeficiency virus-infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J. Infect. Dis. 2010;202:161–170. doi: 10.1086/653213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Clements JE. A novel simian immunodeficiency virus model that provides insight into mechanisms of human immunodeficiency virus central nervous system disease. J. Neurovirol. 2002;8(Suppl 2):42–48. doi: 10.1080/13550280290101076. [DOI] [PubMed] [Google Scholar]