Abstract
Neuroimaging, including PET, MRI, and MRS, is a powerful approach to the study of brain function. This article reviews neuroimaging findings related to alcohol and other drugs of abuse that have been published since 2011. Uses of neuroimaging are to characterize patients to determine who will fare better in treatment and to investigate the reasons underlying the effect on outcomes. Neuroimaging is also used to characterize the acute and chronic effects of substances on the brain and how those effects are related to dependence, relapse, and other drug effects. The data can be used to provide encouraging information for patients, as several studies have shown that long-term abstinence is associated with at least partial normalization of neurological abnormalities.
Keywords: PET, MRI, MRS, Brain, Ethanol, Alcoholism, Cocaine, Marijuana, Opioids, Heroin, Methamphetamine, Psychostimulants, Metabolism
Introduction
Neuroimaging traditionally has provided non-invasive anatomic views of the brain, but the applications of neuroimaging have expanded to include measurement of the concentrations of neurochemicals and specific proteins such as subtypes of neurotransmitter receptors or transporters, rates of metabolic pathways and blood flow, and the detection of functional or pharmacologic changes in the brain and evaluations of connections among brain regions. Over the past 3 years, the primary neuroimaging techniques used to study the effects of alcohol and other substances have been positron emission tomography (PET), magnetic resonance imaging (MRI), and magnetic resonance spectroscopy (MRS). Each approach has its own strengths and weaknesses, and there is the potential to utilize them synergistically. The following sections cover these methods in relation to specific substances.
Alcohol
A systematic review from 2011 identified more than 140 peer-reviewed articles in the alcohol neuroimaging literature [1]. In alcohol-dependent (AD) subjects, structural MRI, including diffusion tensor imaging (DTI), has identified diffuse atrophy in gray matter and white matter tracts with concomitant ventricular enlargement. PET, MRS, and fMRI have identified profound neurophysiological deficits in patients with alcohol use disorder (AUD). The interested reader is referred to the aforementioned systematic review for additional details, as here we will focus exclusively on neuroimaging results published since that review.
PET
PET has been used successfully to measure activity of neurotransmitter systems, including endogenous opioid systems, in subjects with AUD. For example, μ-opioid receptor antagonists provide effective harm reduction strategies in the treatment of AUD, but little is known about the system post-detoxification. AD subjects who, 5 days into abstinence, were imaged for μ- and Δ-opioid receptor availability with the ligands [11C]carfentanil and ([11C]methyl)naltrindole, respectively, showed greater μ-opioid receptor availability in multiple brain regions than controls [2]. The regions studied included the nucleus accumbens (NAcc)/ventral striatum (VS), where receptor availability was negatively correlated with craving, after correction for age, sex, and smoking. A study of μ-opioid and dopamine D2/D3 receptor availability in abstinent AD patients used [18F]-fallypride before and after administration of the μ-opioid receptor agonist remifentanil [3]. Although D2/D3 receptor availability did not change after remifentanil administration, NAcc/VS binding potential was correlated with AD severity.
To study GABA receptor availability, the α5 benzodiazepine receptor ligand [11C]Ro154513 was administered to AD men who were abstinent for at least 6 weeks. It revealed significantly less binding potential in limbic structures (NAcc/VS and hippocampus) [4]. In AD patients, but not controls, bilateral hippocampal α5 benzodiazepine receptor availability was positively correlated with delayed verbal memory performance.
Cannabinoid (CB1) receptors in recently abstinent AD subjects were imaged with [11C]OMAR, showing ~20 % lower receptor distribution volume in limbic and cortical areas [5]. In a separate study with the ligand [18F]FMPEP, AD patients were studied at two time points: 3 – 7 days after inpatient admission and after 2 – 4 weeks of abstinence. Over time, CB1 receptor binding fell by 20 – 30 % in all brain regions examined and was negatively correlated with years of abuse [6]. Additional studies are needed to validate these findings concerning CB1 receptor availability in AUD, especially as the endogenous cannabinoid system may be a therapeutic target.
MRI
MRI studies comparing AD patients to controls have revealed reduced cortical thickness in both hemispheres in AD patients; the effect is especially pronounced in women [7]. In cross-sectional studies examining this effect in humans, it is difficult to control for confounds like nutrition and comorbidities and attributing causality specifically to alcohol. Longitudinal imaging of macaques voluntarily consuming ≥3 g/kg alcohol (~12 standard drinks) daily provided clearer evidence of causality [8]. By 6 months after the initiation of drinking, the macaques had persistent cortical and subcortical volume reductions related to the number of standard drinks consumed.
DTI, an MRI technique that evaluates the diffusivity of water in tissue, has been applied to investigate alcohol-related brain damage, including Wernicke’s Syndrome, as described in an in-depth review that relates volume losses, white matter microstructure, and function [9]. The findings are consistent with the impaired attention and emotion processing seen with white matter fiber disruption [10]. Data show that patients can benefit from prolonged abstinence, with improvements in function and DTI-detectable white matter microstructure [11].
Functional MRI (fMRI) is frequently used to study cognitive function, including cue reactivity/craving and impulsivity/cognitive control. Several recent fMRI studies reported more alcohol cue-induced attentional bias [12] and changes in areas involved in self-control, memory, and reflective thinking [13] compared to non-dependent controls. A meta-analysis of 28 studies that included 679 patients and 174 controls assessed cue-induced reactivity in patients with AUDs [14]. Alcohol cues increased fMRI blood-oxygen-level dependent (BOLD) contrast in the ventromedial prefrontal cortex and limbic regions relative to controls. There was also greater contrast in the parietal cortex, temporal cortex, superior temporal gyrus and precuneus in subjects with AUD.
In a study of impulsivity in young adult heavy drinkers and low-to-moderate drinkers, fMRI showed a decreased BOLD signal in the right superior frontal gyrus and left caudate on risk-taking compared with risk aversion trials [15]. A longitudinal study of college students used alcohol-related cues on a go/no-go task at three times before and during their freshman year [16]. Increased BOLD was observed bilaterally in the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC) over time, which was interpreted as the recruitment of cognitive resources.
A recent development in the study of AUD is the use of imaging genetics. One such study linked the tachykinin receptor 1 (TACR1) gene to alcohol-cue related BOLD increases in DLPFC, medial PFC (mPFC), cingulate cortex, caudate/putamen, insula, and lentiform nucleus [17]. One of the polymorphisms in that study was associated with BOLD activation in mesocorticolimbic circuitry in response to gustatory alcohol cues, consistent with links between TACR1 and anti-drinking effects of TACR1-targeted medications.
The effects of treatment have also been a target in fMRI paradigms. Bilateral posterior cingulate cortex signal was increased in AD patients in response to alcohol cues, though these signals vanished after 2 weeks of inpatient interventions, including treatment with acamprosate [18]. However, there was no significant difference between the acamprosate and placebo groups in cue-induced response, suggesting that the medication’s effects are mediated by other factors (e.g., glutamatergic circuitry in the ACC) [19]. A study of injectable naltrexone in AD patients showed that, before treatment, alcohol-related cues increased fMRI contrast in the orbital, cingulate, inferior frontal and middle frontal cortices [20]. The effect abated in subjects who received long-acting injectable naltrexone.
MRS
1H MRS showed lower glutamate and increased glutamine concentrations in the bilateral ACC in subjects with a current or past AUD compared to controls [21], so glutamatergic alterations may predate AUD onset, or, if affected by alcohol itself, persist during abstinence, and potentially contribute to relapse. 1H MRS in recently abstinent AD subjects showed a higher glutamate concentration in two critical nodes of central reward circuitry (NAcc/VS and ACC), with the concentration correlated with craving, suggesting that higher glutamate concentrations in early abstinence may predict relapse [22]. A study of the neurochemical impact of polysubstance use [23] showed that people who drink and also use at least one other substance have greater changes in the concentration of myoinositol (believed to serve as an osmotic regulator) and N-acetylaspartate (NAA, believed to reflect neuronal health) than those who have only AUD. A hypothesized mechanism in acute alcohol withdrawal is an imbalance between glutamatergic and GABAergic neurotransmission, which theoretically increases the risk for alcohol withdrawal-related seizures. A novel translational study showed a high brain glutamate concentration in human ACC and rodent mPFC during acute withdrawal [24].
1H MRS has also been used to study acute ethanol effects before and during a 1-hour intravenous alcohol administration. GABA concentration decreased 12 % within minutes of the infusion start and stayed constant, while NAA levels decreased gradually by 8 % [25]. Glutamate decreased (p= 0.019, but non-significant after Bonferroni correction), so possibly the elevations seen with chronic exposure reflect adaptations to ethanol’s acute effects to decrease glutamate concentrations.
Energetics
PET measurements with substrates labeled with 11C or 18F, and MRS with the non-radioactive isotope 13C have been applied to study brain metabolism. Acetate, a metabolic byproduct of ethanol produced in the liver, reaches 1 – 2 mM in the blood and is readily oxidized by the brain. Moderate alcohol consumption reduced brain glucose uptake detected by PET [26], and PET showed increased uptake of [1-11C]acetate with acute alcohol administration, with heavy drinkers trending toward higher uptake than light drinkers [27••]. Heavy drinkers have chronically elevated acetate concentrations, and 13C MRS shows that their capacity to transport acetate into the brain and oxidize it is twofold greater than light drinkers [28••], possibly due to chronic exposure to elevated monocarboxylic acids. Rats exposed chronically to ethanol also show increased oxidation of acetate [29]. The PET and MRS results are consistent with a report that elevations of blood ethanol and acetate decrease the rates of glucose oxidation and phosphorylation in rats, in proportion to the concentration of plasma acetate [30]. An additional factor may be intracerebral oxidation of ethanol itself. 13C MRS in rats showed that for a blood ethanol concentration of ~130 mg/dL, ethanol supplies 12 % of glial oxidation, and after 3 weeks of vapor exposure, it rises to 20%, comprising about 3%of total oxidative brain metabolism [31••]. Intracerebral oxidation of ethanol is significant in the promotion of oxidative stress and the formation of acetaldehyde in the brain, which has been shown to be rewarding [32, 33]. To summarize, cerebral energetic studies show that the brain consumes both alcohol and alcohol-derived acetate, with glucose consumption decreasing proportionately.
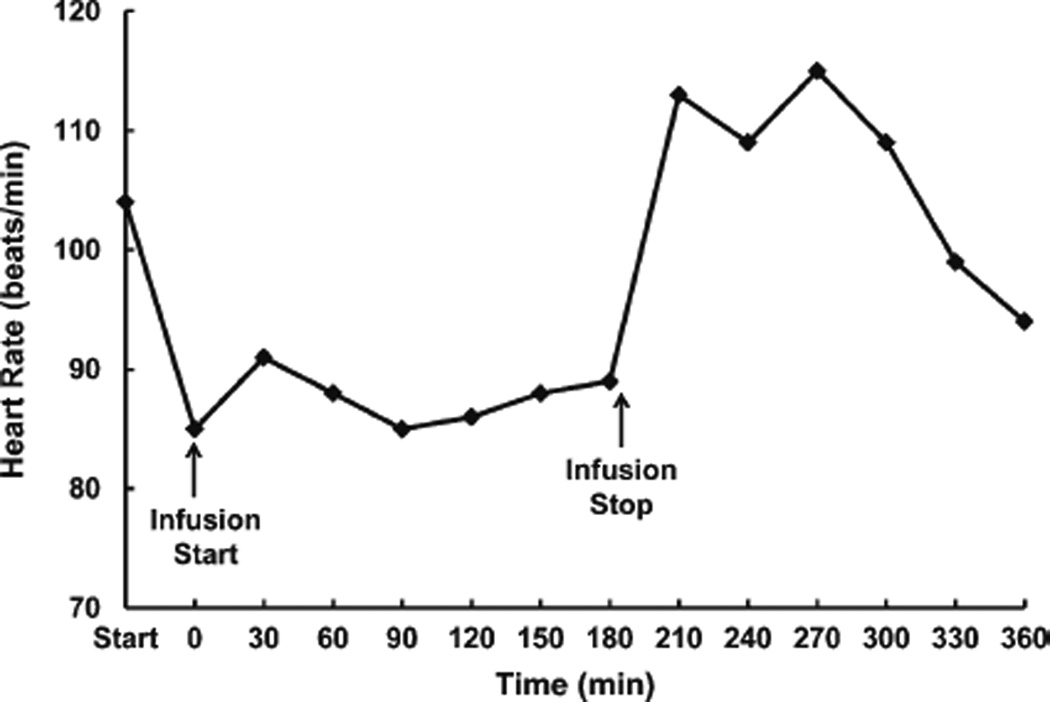
Based on the above and other preclinical studies [34, 35], our group hypothesized that intravenous administration of acetate would decrease alcohol withdrawal symptoms. In an open-label pilot test of intravenous sodium acetate (0.6 g/kg) in a 42-year-old AD man during acute alcohol withdrawal, Clinical Institute of Withdrawal Assessment-Alcohol Revised (CIWA-Ar) scores were obtained during the infusion and up to 3 hours post-infusion. The CIWA score rose slightly from an initial value of 5 to 8 during the infusion, peaked afterward when the subject stood up and smoked, and then dropped to 0. His heart rate dropped sharply with the onset of the infusion (Fig. 1). The subject reported feeling sedated during the infusion, which was confirmed by the research team. Although initially skeptical of the efficacy of the procedure, afterward the subject reported surprise at how much better he felt 2 hours later. In this single patient, intravenous acetate was well-tolerated without long-term adverse consequences. Further testing for safety, tolerability, and efficacy is required.
Fig. 1.
Heart rate (beats per minute) during an intravenous infusion of sodium acetate in a 42-year-old alcohol-dependent Caucasian male. Compared to the start of the protocol, the heart rate dropped and remained consistent during the infusion, returning to its pre-infusion level after the infusion ended
Tobacco and Nicotine
PET
Nicotine affects many neurotransmitter systems and brain regions. In a within-subject PET study, smokers were scanned on two different days, either after smoking or while being idle and not smoking, and their craving and withdrawal symptoms were measured [36]. The subjects were injected with the radiochemical [11C]PHNO, which binds primarily to D3 receptors, and dopamine released by smoking competes with PHNO, thereby displacing the radiotracer. The authors found that smoking increased dopamine release in D3-rich areas in association with reductions in craving and withdrawal symptoms. This extends recent previous work with the D2/D3-binding radiotracer [11C] raclopride, which showed that smoking-induced dopamine release in the NAcc/VS was correlated with improved mood [37]. However, smoking cues are insufficient to release dopamine in smokers after an hour’s abstinence, although overnight abstinence might have a measurable effect [38]. Smoking even denicotinized cigarettes causes dopamine release in the NAcc/VS, though less than with regular cigarettes [39], which could be explained by findings from a PET study of nicotinic acetylcholine receptors (nAChRs). When smokers consume standard cigarettes (1 – 2 mg nicotine), nAChRs are ~80 % occupied, but after denicotinized cigarettes, the nAChRs showed ~25 % occupancy, which is consistent with the 0.05 mg of nicotine that remains in denicotinized cigarettes [40]. The authors concluded that nicotine is responsible for essentially all nAChR binding [40], so perhaps the small amount of remaining nicotine could promote dopamine release. A potentially related and intriguing finding is that second-hand smoke leads to ~20 % nAChR occupancy in smokers and non-smokers alike [41].
Other recent developments in PET include the creation of a new tracer, [18F] AZAN, a nAChR ligand with high specificity and more rapid kinetics, thereby allowing shorter scan times [42]. A study with [18F] AZAN showed reduced α2β4 receptor availability in smokers and also after treatment with varenicline, which binds to nAChR [43]. A novel target in the treatment of nicotine dependence is metabotropic glutamate receptors. Less availability of mGluR5-containing receptors is seen in smokers and ex-smokers [44], and lower mGluR5 binding is related to the duration of abstinence, with less binding in people who stopped smoking more recently [45].
MRI
MRI studies have shown both the structural and functional effects of acute and chronic smoking. An MRI study of 116 current smokers showed smoking cues increased BOLD response more than food cues in the orbitofrontal cortex (OFC) and supplementary motor cortex (SMC) [46]. Increased connectivity between the OFC and the SMC, lateral inferior parietal lobe, somatosensory cortex, right insula, and the striatum was also reported. The authors concluded that smokers’ attentional networks are engaged more during smoking cues, while the sensorimotor and motor preparation circuits are associated with habitual behavior.
fMRI studies of nicotine, smoking, abstinence, and craving generally relate behavioral or cognitive effects to changes in BOLD contrast. Smokers abstinent for 24 hours displayed less accuracy and slower responses in working memory tests, compared to when they had smoked shortly before the scan; their BOLD response was also decreased [47]. However, the Stroop test revealed greater BOLD contrast after abstinence than after smoking [48]. Another study reported that nicotine patch administration increased BOLD contrast in the dorsal striatum in response to an anticipated monetary award [49]. After a period of abstinence of 16 weeks, aided with the nicotinic agonist varenicline, subjects who succeeded in their abstinence had greater BOLD activation in a distributed network of alertness, learning, and memory [50]. It appears that smoking and abstinence affect diverse cognitive activities, and different routes of nicotine administration, duration of abstinence, and abstinence-focused therapies may have differential effects.
DTI has generally been applied to chronic conditions, but recently DTI showed acute nicotine-related decreases in fractional anisotropy (FA) in relation to sustained attention performance [51]. An extensive, multimodal study used structural MRI, DTI, and MRS to evaluate interactions of alcohol abstinence and smoking [52]. In this study, smokers and nonsmokers displayed distinct patterns of white matter abnormalities in diffusivity, structure, and NAA levels, also differing regionally with recovery: smokers recovered more frontal white matter volume with alcohol abstinence than did non-smokers.
Psychostimulants (Cocaine, Methamphetamine)
PET
Dopaminergic signaling has been a target for the treatment of stimulant use disorders and has been studied using neuroimaging. An [11C]raclopride study showed that methamphetamine abusers (MPHAs) (≤6 months abstinence) had less D2/D3 binding than controls [53]. The same patients showed less dopamine release than controls in response to methylphenidate administration. When the MPHAs were stratified by abstinence/relapse (10 abstinent/six relapsed) after 9 months, those who relapsed had less dopamine receptor availability and less dopamine release than controls or abstinent patients. The abstainers, however, showed no difference from controls. Thus, dopamine receptor availability and dopamine release might be useful to predict abstinence [53]. Consistent with dopamine receptor deficits, administration of methamphetamine to monkeys led to less dopamine receptor binding [54] and dopamine transporter availability, which was associated with increased gray matter in the putamen and altered responses to reward stimuli [55]. However, the effect of low dopaminergic activity is not clear. A study of polydrug-using MPHAs, employing the D3-preferring ligand [11C]PHNO, showed that they had increased D3 binding in the globus pallidus and substantia nigra and slightly decreased binding in the striatum compared to controls. The authors concluded that D3 receptors may be upregulated in the MPHAs and contribute to their drug use and vulnerability to relapse [56].
Dopamine receptor binding was also investigated recently in the context of cocaine and serotonin effects, using tryptophan depletion to decrease serotonin [57]. This study of non-dependent cocaine users showed that reductions of serotonin via tryptophan depletion was associated with increased drug craving and striatal dopamine release, which suggests that individuals with low serotoninergic activity may be at greater risk for abuse and relapse. A study of rats placed the D2/D3 receptor issue in a larger context by demonstrating that receptor availability was negatively correlated with preferences for cocaine and food, and binding was predictive of future weight gain [58]. A recent metabolic study using FDG-PET to study responses to cocaine cues found that the cues reduced global glucose utilization by 9 % in women but increased it by 5.5 % in men [59]. In women, the decrements occurred primarily in frontal, cingulate and parietal cortices, thalamus and midbrain, while in men the increase was in the right inferior frontal cortex. The authors noted that, based on these findings, gender-specific interventions may be beneficial in cocaine dependence.
A study of mGluR5 binding with [11C]ABP688 showed 20 % less binding in the striatum in cocaine dependent (CD) patients. This study was unique in its inclusion of 1H MRS of glutamate and glutamine in the same individuals, revealing no differences in those metabolite concentrations [60]. The relationships between glutamatergic function and 1H MRS measures of glutamate and glutamine levels remain to be clarified, likely differing due to diverse influences, including substance abuse.
MRI
Structural findings in cocaine and methamphetamine users, reviewed recently [61], show consistent reductions of frontal cortex, particularly the ventral mPFC and insula, and enlarged striatum. MRI has also been used to investigate functional differences, and sometimes the regionality of fMRI contrast points to particular neurotransmitter systems. A study of macaques showed that cocaine self-administration decreased blood volumes in the basal ganglia and motor/pre-motor cortex [62], which the authors hypothesized reflected a hypofunction of D2 receptors that blunted cocaine-induced functional responses.
Targeting cognitive function in MPHAs, fMRI has been used to relate performance on the Stroop task to PFC BOLD contrast [63]. Compared to controls, MPHAs demonstrated less BOLD in the right inferior frontal cortex, ACC, and anterior insula during task-incongruent conditions of the Stroop task [64], leading the authors to conclude that hypofunction underlies cognitive control deficits in MPHAs. fMRI also has prognostic potential for treatment, demonstrated by a study of treatment status and motivation to quit in CD patients [65]. Treatment-seeking, motivated CD patients had less BOLD contrast in many cortical regions in response to cocaine cues than non-treatment-seeking and less motivated participants.
MRS
A cross-sectional 1H MRS study of MPHAs abstinent for 1 – 5 years compared to those abstinent ≤6 months and control subjects, showed low NAA in short-term abstinence, but normal levels in those abstinent for 1 – 5 years [66]. Although that study could not differentiate recovery of NAA with abstinence from selection bias (i.e., perhaps normal NAA is a characteristic of patients who will succeed with long-term abstinence), a longitudinal study of rhesus monkeys showed neurochemical normalization with abstinence [67]. MPHAs have also been shown recently to have lower levels of phosphocreatine than non-using controls [68], and adolescents who use marijuana and methamphetamine have lower NAA than controls, but those who used methamphetamine alone had normal NAA [69]. Adolescent MPHAs who do not use marijuana may more effectively maintain NAA levels than adult MPHAs.
Marijuana
PET
Marijuana users (MUs) studied with [11C]raclopride did not differ from controls in D2/D3 receptor binding, but receptor availability was negatively correlated with urine cannabinoid metabolite levels and self-reported recent intake [70]. [11C]PHNO showed D3 binding in cannabis users to be greater in the striatum [71], the opposite of what was reported for D3 binding in MPHAs [56]. CB1 receptor density in MUs showed differences outside the striatum and other regions that are often implicated in drug abuse. [18F]FMPEP demonstrated that in MUs most of the cortex has low CB1 density, which normalized after 1 month of abstinence [72]. However, gender and psychopathology can affect results: in post-traumatic stress disorder, [11C]OMAR binding, which reflects CB1 density, was abnormally high, with women more affected than men [73].
MRI
DTI has shown less FA in MUs than in controls, and those who began using marijuana consistently before age 16 years showed a negative correlation between FA and impulsivity [74]. However, white matter volumes were recently reported not to differ in heavy MUs, while gray matter volumes differed heterogeneously–greater in the anterior cerebellum and lower in the amygdala and hippocampus, according to the amount of use [75].
Using fMRI to study response to visual cues, MUs had slower motor reaction times than non-using controls, and BOLD contrast was more commonly located in regions associated with executive function than with automated function [76]. Healthy volunteers given delta-9-tetrahydrocannabinol orally showed less BOLD bilaterally in the temporal cortex during auditory processing, and some visual areas showed more BOLD and others less, with findings possibly related to induced psychotic symptoms [77]. Consistent with the effects of many other drugs of abuse, marijuana has repeatedly been associated with poorer cognitive performance. Incremental increases in marijuana consumption have also been positively correlated with go/no-go task BOLD activation in regions believed to subserve response inhibition [78]. Overlapping regions were reported to differ in MUs with respect to negative consequences in decision-making tasks [79]. In sum, BOLD contrast is altered in MUs with respect to sensory processing, decision-making, and cognitive performance.
MRS
A recent review addressed MRS studies of MUs [80]. A recent study showed lower levels of myoinositol globally in MUs than in controls [81], and left thalamic myoinositol reductions in particular were associated with greater impulsivity [82].
Opioids
PET
In heroin-dependent subjects (HDs), [11C]raclopride before and after a challenge with methylphenidate revealed lower D2/D3 availability and dopamine release, although deficits did not predict heroin self-administration [83]. Brain energetics were investigated with FDG-PET in HDs and across many other substances, including cocaine, marijuana, stimulants, and ecstasy, showing that users of these substances have lower glucose metabolism in the DLPFC and temporal cortex than controls [84]. With comorbid AUD, there were also deficits in the frontal premotor cortex and putamen.
MRI
A DTI study in HDs with an average of 9 years of drug use (including polysubstance users and those stable on methadone or buprenorphine) showed that opioid use is associated with white matter damage [85]. A longitudinal study suggests that such microstructural abnormalities develop over time, based on the finding of more severe abnormalities in individuals with 10 – 20 years of use than those using <10 years [86]. fMRI in HDs showed that acute heroin administration decreased BOLD contrast in the right inferior frontal cortex, which was associated with attention allocation [87].
MRS
1H MRS measures in the ACC of HDs maintained on methadone or buprenorphine showed a positive association between glutamate levels and the number of previous withdrawal episodes but no difference in ACC glutamate relative to controls [88]. Also noted were abnormally high levels of choline in frontal white matter. Another study of HDs showed high levels of myoinositol associated with maintenance on buprenorphine or low-dose methadone (5–15 mg/day), but normal myoinositol for patients on higher doses of methadone [89]. Methadone was also associated with normal NAA and Glx (combined 1HMRS-detectable glutamate and glutamine). 1H MRS has also shown an environmental effect in opioid addiction: a significant negative correlation between NAA and social discrimination [90].
Cerebral Perfusion and BOLD
fMRI studies of smoking, drinking, methamphetamine use, and abuse of other substances should be planned carefully and interpreted with caution. For example, acute nicotine increases cerebral blood flow (CBF) in the thalamus, pons, occipital cortex, and the cerebellum, and it decreases CBF in the ACC, NAcc/VS, amygdala, and hippocampus, with some dependence on plasma nicotine concentrations [91–93]. The effects are greater after the first cigarette of the day compared to later smoking [93]. Heart rate is also significantly correlated with plasma nicotine concentrations [91] and can confound fMRI studies if not considered in data acquisition and analysis. Smoking chronically has long been known to reduce CBF [94], while acute smoking increases global CBF without changing oxygen extraction [95]. Acute alcohol administration alters CBF and blood volume globally, with regional variations [96–100], as does chronic heavy alcohol consumption [101–103] and alcohol withdrawal [104–106]. Amphetamines, both acutely and chronically, alter cerebral perfusion [107–110]. Changes in cerebral perfusion and vascular responses signify alterations in the mechanistic links between neuronal activity and CBF (and, hence, BOLD signal) that are used as surrogate markers of neuronal activity. A recent study focused precisely on this issue in rats exposed to cocaine [111••]. For studies that seek synchrony in BOLD response, such as that evaluated for functional connectivity and mode networks, there exist additional confounds: with breathing and pulsatile blood flow, the transit times for oxygenated blood, local volume expansions and rates of flow will lead to some brain regions matching others, producing BOLD synchrony among those regions, independent of neuronal activity. Medications, emotional and cognitive tasks, age, and other factors that alter heart rate, vascular elasticity, and blood volume, sometimes in spatially heterogeneous ways, will change that synchrony. An opportunity exists to investigate the links between fMRI contrasts and neuronal activity in conditions such as substance abuse and such studies will be important for the accurate interpretation of fMRI.
Conclusions
Observed as a set, the neuroimaging studies reviewed here reflect several themes on the effects of alcohol and drugs of abuse that have been investigated. One is a trend toward the characterization of individual patients or groups, with the goal of predicting their prognosis or personalizing treatment. Next, there is a trend toward more acute and dynamic studies of drug or medication effects, or longer-term studies extending over hours, days, or years to study chronic factors. Third, degradation of white matter structure is seen across a number of substances, and has in several cases been associated with cognitive deficits that could, in turn, make abstinence more difficult to achieve and maintain. It is important to identify risk factors for cognitive deficits and structural and neurochemical abnormalities so that the research community can seek ways to ameliorate their impact. A final practical conclusion is the need to consider specific factors when studying any of these substances. Environmental factors like social discrimination and stress are likely to affect both research results and clinical outcomes, as will the number of previous withdrawal episodes in dependent patients. Finally, the data presented here offer some encouraging news: multiple studies of diverse substances present the neuroimaging correlates of recovery, although with a caveat that subtle abnormalities may persist in some patients and increase both short- and long-term vulnerability to relapse.
Acknowledgments
Graeme Mason is a consultant for UCB Pharma and received a grant from the NIH.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by the author.
Conflict of Interest Mark Niciu has no conflicts.
Contributor Information
Mark J. Niciu, Email: mark.niciu@nih.gov, National Institutes of Health and Department of Health and Human Services, Experimental Therapeutics & Pathophysiology Branch, National Institute of Mental Health, 10 Center Dr., Building 10/CRC, Room 7-5545, Bethesda, MD 20892, USA.
Graeme F. Mason, Email: graeme.mason@yale.edu, Yale University Department of Diagnostic Radiology and Psychiatry, New Haven, CT, USA; N-141 TAC-MRRC, Yale University School of Medicine, 300 Cedar Street, P.O. Box 208043, New Haven, CT 06520-8043, USA.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1.Buhler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res. 2011;35:1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- 2.Weerts EM, Wand GS, Kuwabara H, et al. Positron emission tomography imaging of mu- and delta-opioid receptor binding in alcohol-dependent and healthy control subjects. Alcohol Clin Exp Res. 2011;35:2162–2173. doi: 10.1111/j.1530-0277.2011.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spreckelmeyer KN, Paulzen M, Raptis M, et al. Opiate-induced dopamine release is modulated by severity of alcohol dependence: an [(18)F]fallypride positron emission tomography study. Biol Psychiatry. 2011;70:770–776. doi: 10.1016/j.biopsych.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 4.Lingford-Hughes A, Reid AG, Myers J, et al. A [11C] Ro15 4513 PET study suggests that alcohol dependence in man is associated with reduced alpha5 benzodiazepine receptors in limbic regions. J Psychopharmacol. 2012;26:273–281. doi: 10.1177/0269881110379509. [DOI] [PubMed] [Google Scholar]
- 5.Neumeister A, Normandin MD, Murrough JW, et al. Positron emission tomography shows elevated cannabinoid CB1 receptor binding in men with alcohol dependence. Alcohol Clin Exp Res. 2012;36:2104–2109. doi: 10.1111/j.1530-0277.2012.01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 6.Hirvonen J, Zanotti-Fregonara P, Umhau JC, et al. Reduced cannabinoid CB1 receptor binding in alcohol dependence measured with positron emission tomography. Mol Psychiatry. 2013;18:916–921. doi: 10.1038/mp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Momenan R, Steckler LE, Saad ZS, et al. Effects of alcohol dependence on cortical thickness as determined by magnetic resonance imaging. Psychiatry Res. 2012;204:101–111. doi: 10.1016/j.pscychresns.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Kroenke CD, Rohlfing T, Park B, et al. Monkeys that voluntarily and chronically drink alcohol damage their brains: a longitudinal MRI study. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zahr NM, Kaufman KL, Harper CG. Clinical and pathological features of alcohol-related brain damage. Nat Rev Neurol. 2011;7:284–294. doi: 10.1038/nrneurol.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulte T, Müller-Oehring E, Sullivan E, et al. White matter fiber compromise contributes differentially to attention and emotion processing impairment in alcoholism, HIV-infection, and their comorbidity. Neuropsychologia. 2012;50(12):2812–2822. doi: 10.1016/j.neuropsychologia.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alhassoon OM, Sorg SF, Taylor MJ, et al. Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcohol Clin Exp Res. 2012;36:1922–1931. doi: 10.1111/j.1530-0277.2012.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vollstädt-Klein S, Loeber S, Richter A, et al. Validating incentive salience with functional magnetic resonance imaging: association between mesolimbic cue reactivity and attentional bias in alcohol-dependent patients. Addict Biol. 2012;17:807–816. doi: 10.1111/j.1369-1600.2011.00352.x. [DOI] [PubMed] [Google Scholar]
- 13.Krienke UJ, Nikesch F, Spiegelhalder K, et al. Impact of alcohol-related video sequences on functional MRI in abstinent alcoholics. Eur Addict Res. 2013;20:33–40. doi: 10.1159/000349909. [DOI] [PubMed] [Google Scholar]
- 14.Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bednarski SR, Erdman E, Luo X, et al. Neural processes of an indirect analog of risk taking in young nondependent adult alcohol drinkers-an FMRI study of the stop signal task. Alcohol Clin Exp Res. 2012;36:768–779. doi: 10.1111/j.1530-0277.2011.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beltz AM, Gates KM, Engels AS, et al. Changes in alcohol-related brain networks across the first year of college: a prospective pilot study using fMRI effective connectivity mapping. Addict Behav. 2013;38:2052–2059. doi: 10.1016/j.addbeh.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blaine S, Claus E, Harlaar N, et al. TACR1 genotypes predict fMRI response to alcohol cues and level of alcohol dependence. Alcohol Clin Exp Res. 2013;37(Suppl 1):E125–E130. doi: 10.1111/j.1530-0277.2012.01923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langosch JM, Spiegelhalder K, Jahnke K, et al. The impact of acamprosate on cue reactivity in alcohol dependent individuals: a functional magnetic resonance imaging study. J Clin Psychopharmacol. 2012;32:661–665. doi: 10.1097/JCP.0b013e318267b586. [DOI] [PubMed] [Google Scholar]
- 19.Umhau JC, Momenan R, Schwandt ML, et al. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry. 2010;67:1069–1077. doi: 10.1001/archgenpsychiatry.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukas SE, Lowen SB, Lindsey KP, et al. Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: a bold FMRI study. Neuroimage. 2013;78:176–185. doi: 10.1016/j.neuroimage.2013.03.055. [DOI] [PubMed] [Google Scholar]
- 21.Thoma R, Mullins P, Ruhl D, et al. Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology. 2011;36:1359–1365. doi: 10.1038/npp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer J, Pedersen A, Scherbaum N, et al. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology. 2013;38:1401–1408. doi: 10.1038/npp.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abé C, Mon A, Hoefer ME, et al. Metabolic abnormalities in lobar and subcortical brain regions of abstinent polysubstance users: Magnetic Resonance Spectroscopic Imaging. Alcohol Alcohol. 2013;48:543–551. doi: 10.1093/alcalc/agt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermann D, Weber-Fahr W, Sartorius A, et al. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry. 2012;71:1015–1021. doi: 10.1016/j.biopsych.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 25.Gomez R, Behar KL, Watzl J, et al. Intravenous ethanol infusion decreases human cortical γ-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 Tesla. Biol Psychiatry. 2012;71:239–246. doi: 10.1016/j.biopsych.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volkow ND, Wang GJ, Franceschi D, et al. Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage. 2006;29:295–301. doi: 10.1016/j.neuroimage.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Volkow ND, Kim SW, Wang GJ, et al. Acute alcohol intoxication decreases glucose metabolism but increases acetate uptake in the human brain. Neuroimage. 2013;64:277–283. doi: 10.1016/j.neuroimage.2012.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiang L, Gulanski BI, De Feyter HM, et al. Increased brain uptake and oxidation of acetate in heavy drinkers. J Clin Invest. 2013;123:1605–1614. doi: 10.1172/JCI65153. These references together demonstrate that heavy drinkers possess a greater ability to oxidize acetate than light drinkers do. It may be that chronic heavy drinking leads the brain to become dependent on acetate, either for its energy or for metabolic byproducts of its oxidation, like adenosine.
- 29.Wang J, Du H, Ma X, et al. Metabolic products of [2-13C] ethanol in the rat brain after chronic ethanol exposure. J Neurochem. 2013;127:353–364. doi: 10.1111/jnc.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pawlosky RJ, Kashiwaya Y, Srivastava S, et al. Alterations in brain glucose utilization accompanying elevations in blood ethanol and acetate concentrations in the rat. Alcohol Clin Exp Res. 2010;34:375–381. doi: 10.1111/j.1530-0277.2009.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang J, Du H, Jiang L, et al. Oxidation of ethanol in the rat brain and effects associated with chronic ethanol exposure. Proc Natl Acad Sci. 2013;110:14444–14449. doi: 10.1073/pnas.1306011110. The demonstration that living, intact brain oxidizes ethanol provides feasibility data for the concept of ethanol’s breakdown product, acetaldehyde, serving as an intracerebral reward.
- 32.Deehan GA, Engleman EA, Ding ZM, et al. Microinjections of acetaldehyde or salsolinol into the posterior ventral tegmental area increase dopamine release in the nucleus accumbens shell. Alcohol Clin Exp Res. 2012;37:722–729. doi: 10.1111/acer.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karahanian E, Quintanilla ME, Tampier L, et al. Ethanol as a prodrug: brain metabolism of ethanol mediates its reinforcing effects. Alcohol Clin Exp Res. 2011;35:606–612. doi: 10.1111/j.1530-0277.2011.01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derr RF, Draves K, Derr M. Abatement by acetate of an ethanol withdrawal syndrome. Life Sci. 1981;29:1787–1790. doi: 10.1016/0024-3205(81)90189-2. [DOI] [PubMed] [Google Scholar]
- 35.Carmichael FJ, Israel Y, Crawford M, et al. Central nervous system effects of acetate: contribution to the central effects of ethanol. J Pharmacol Exp Ther. 1991;259:403–408. [PubMed] [Google Scholar]
- 36.Le Foll B, Guranda M, Wilson AA, et al. Elevation of dopamine induced by cigarette smoking: novel insights from a [11C]-(+)-PHNO PET study in humans. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brody AL, Mandelkern MA, Olmstead RE, et al. Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology. 2009;34:282–289. doi: 10.1038/npp.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiuccariello L, Boileau I, Guranda M, et al. Presentation of smoking-associated cues does not elicit dopamine release after one-hour smoking abstinence: a [11C]-(+)-PHNO PET study. PLoS One. 2013;8:e60382. doi: 10.1371/journal.pone.0060382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Domino EF, Ni L, Domino JS, et al. Denicotinized versus average nicotine tobacco cigarette smoking differentially releases striatal dopamine. Nicotine Tobacco Res. 2013;15:11–21. doi: 10.1093/ntr/nts029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brody AL, Mandelkern MA, Costello MR, et al. Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. Int J Neuropsychopharmacol. 2009;12:305–316. doi: 10.1017/S146114570800922X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brody AL, Mandelkern MA, London ED, et al. Effect of second-hand smoke on occupancy of nicotinic acetylcholine receptors in brain. Arch Gen Psychiatry. 2011;68:953–960. doi: 10.1001/archgenpsychiatry.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuwabara H, Wong DF, Gao Y, et al. PET imaging of nicotinic acetylcholine receptors in baboons with 18 F-AZAN, a radioligand with improved brain kinetics. J Nucl Med. 2012;53:121–129. doi: 10.2967/jnumed.111.092338. [DOI] [PubMed] [Google Scholar]
- 43.Wong DF, Kuwabara H, Kim J, et al. PET imaging of high-affinity α4β2 nicotinic acetylcholine receptors in humans with 18 F-AZAN, a radioligand with optimal brain kinetics. J Nucl Med. 2013;54:1308–1314. doi: 10.2967/jnumed.112.108001. [DOI] [PubMed] [Google Scholar]
- 44.Akkus F, Ametamey SM, Treyer V, et al. Marked global reduction in mGluR5 receptor binding in smokers and ex-smokers determined by [11C] ABP688 positron emission tomography. Proc Natl Acad Sci U S A. 2013;110:737–742. doi: 10.1073/pnas.1210984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hulka L, Treyer V, Scheidegger M, et al. Smoking but not cocaine use is associated with lower cerebral metabotropic glutamate receptor 5 density in humans. Molecular psychiatry. 2013 doi: 10.1038/mp.2013.51. [DOI] [PubMed] [Google Scholar]
- 46.Claus ED, Blaine SK, Filbey FM, et al. Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology. 2013;38(12):2363–2372. doi: 10.1038/npp.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falcone M, Wileyto EP, Ruparel K, et al. Age-related differences in working memory deficits during nicotine withdrawal. Addiction Biology. 2013 doi: 10.1111/adb.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Froeliger B, Modlin L, Wang L, et al. Nicotine withdrawal modulates frontal brain function during an affective Stroop task. Psychopharmacology (Berl) 2012;220:707–718. doi: 10.1007/s00213-011-2522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rose EJ, Ross TJ, Salmeron BJ, et al. Acute nicotine differentially impacts anticipatory valence- and magnitude-related striatal activity. Biol Psychiatry. 2013;73:280–288. doi: 10.1016/j.biopsych.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartwell KJ, Lematty T, McRae-Clark AL, et al. Resisting the urge to smoke and craving during a smoking quit attempt on varenicline: results from a pilot fMRI study. Am J Drug Alcohol Abuse. 2013;39:92–98. doi: 10.3109/00952990.2012.750665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kochunov P, Du X, Moran LV, et al. Acute nicotine administration effects on fractional anisotropy of cerebral white matter and associated attention performance. Front Parmacology. 2013;4:117. doi: 10.3389/fphar.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gazdzinski S, Durazzo TC, Mon A, et al. Cerebral white matter recovery in abstinent alcoholics—a multimodality magnetic resonance study. Brain. 2010;133:1043–1053. doi: 10.1093/brain/awp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang GJ, Smith L, Volkow ND, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2011;17:918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Groman SM, Lee B, Seu E, et al. Dysregulation of D2-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci. 2012;32:5843–5852. doi: 10.1523/JNEUROSCI.0029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Groman SM, Morales AM, Lee B, et al. Methamphetamine-induced increases in putamen gray matter associate with inhibitory control. Psychopharmacology. 2013:1–12. doi: 10.1007/s00213-013-3159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boileau I, Payer D, Houle S, et al. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydronaphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci. 2012;32:1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cox SM, Benkelfat C, Dagher A, et al. Effects of lowered serotonin transmission on cocaine-induced striatal dopamine response: PET [11C] raclopride study in humans. Br J Psychiatry. 2011;199:391–397. doi: 10.1192/bjp.bp.110.084178. [DOI] [PubMed] [Google Scholar]
- 58.Michaelides M, Thanos PK, Kim R, et al. PET imaging predicts future body weight and cocaine preference. Neuroimage. 2012;59:1508–1513. doi: 10.1016/j.neuroimage.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Volkow ND, Tomasi D, Gene-Jack W, et al. Reduced metabolism in brain “control networks” following cocaine-cues exposure in female cocaine abusers. PLoS ONE. 2011;6:e16573. doi: 10.1371/journal.pone.0016573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martínez D, Slifstein M, Nabulsi N, et al. Imaging glutamate homeostasis in cocaine addiction with the metabotropic glutamate receptor 5 positron emission tomography radiotracer [11C]ABP688 and magnetic resonance spectroscopy. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.06.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mackey S, Paulus M. Are there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants? Neurosci Biobehavl Rev. 2012;37:300–316. doi: 10.1016/j.neubiorev.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mandeville JB, Choi J-K, Jarraya B, et al. FMRI of cocaine self-administration in macaques reveals functional inhibition of basal ganglia. Neuropsychopharmacology. 2011;36:1187–1198. doi: 10.1038/npp.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salo R, Fassbender C, Buonocore MH, et al. Behavioral regulation in methamphetamine abusers: an fMRI study. Psychiatry Res Neuroimaging. 2012 doi: 10.1016/j.pscychresns.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nestor LJ, Ghahremani DG, Monterosso J, et al. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res Neuroimaging. 2011;194:287–295. doi: 10.1016/j.pscychresns.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prisciandaro JJ, McRae-Clark AL, Myrick H, et al. Brain activation to cocaine cues and motivation/treatment status. Addiction Biology. 2012 doi: 10.1111/j.1369-1600.2012.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salo R, Buonocore MH, Leamon M, et al. Extended findings of brain metabolite normalization in MA-dependent subjects across sustained abstinence: A proton MRS study. Drug Alcohol Dep. 2011;113:133–138. doi: 10.1016/j.drugalcdep.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang S, Belcher AM, Chefer S, et al. Withdrawal from long-term methamphetamine self-administration ‘normalizes’ neurometabolites in rhesus monkeys: a 1H MR spectroscopy study. Addict Biol. 2013;17(1):100–102. doi: 10.1111/adb.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sung Y-H, Yurgelun-Todd DA, Shi X-F, et al. Decreased frontal lobe phosphocreatine levels in methamphetamine users. Drug Alcohol Dep. 2012;129:102–109. doi: 10.1016/j.drugalcdep.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sung Y-H, Carey PD, Stein DJ, et al. Decreased frontal N-acetylaspartate levels in adolescents concurrently using both methamphetamine and marijuana. Behav Brain Res. 2013;6C:80–86. doi: 10.1016/j.bbr.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Albrecht DS, Skosnik PD, Vollmer JM, et al. Striatal D2/D3 receptor availability is inversely correlated with cannabis consumption in chronic marijuana users. Drug Alcohol Dep. 2012;128:52–57. doi: 10.1016/j.drugalcdep.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mizrahi R, Suridjan I, Kenk M, et al. Dopamine response to psychosocial stress in chronic cannabis users: a PET study with [11C]-(+)-PHNO. Neuropsychopharmacology. 2012;38:673–682. doi: 10.1038/npp.2012.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirvonen J, Goodwin R, Li C, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2011;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neumeister A, Normandin M, Pietrzak R, et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Molecular Psychiatry. 2013 doi: 10.1038/mp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gruber SA, Dahlgren MK, Sagar KA, et al. Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology. 2013 doi: 10.1007/s00213-013-3326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cousijn J, Wiers RW, Ridderinkhof KR, et al. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 2012;59:3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 76.King GR, Ernst T, Deng W, et al. Altered brain activation during visuomotor integration in chronic active cannabis users: relationship to cortisol levels. J Neurosci. 2011;31:17923–17931. doi: 10.1523/JNEUROSCI.4148-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winton-Brown TT, Allen P, Bhattacharrya S, et al. Modulation of auditory and visual processing by delta-9-tetrahydrocannabinol and cannabidiol: an FMRI study. Neuropsychopharmacology. 2011;36:1340–1348. doi: 10.1038/npp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith AM, Zunini RAL, Anderson CD, et al. Impact of marijuana on response inhibition: an fMRI study in young adults. J Behav Brain Sci. 2011;1:124–133. [Google Scholar]
- 79.Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res Neuroimaging. 2011;191:51–59. doi: 10.1016/j.pscychresns.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sneider J, Mashhoon Y, Silveri M. A review of magnetic resonance spectroscopy studies in marijuana using adolescents and adults. J Addict Res Ther S. 2013;4:2. doi: 10.4172/2155-6105.S4-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Silveri MM, Jensen JE, Rosso IM, et al. Preliminary evidence for white matter metabolite differences in marijuana-dependent young men using 2D J-resolved magnetic resonance spectroscopic imaging at 4 Tesla. Psychiatry Res Neuroimaging. 2011;191:201–211. doi: 10.1016/j.pscychresns.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mashhoon Y, Jensen J, Sneider J, et al. Lower left thalamic myoinositol levels associated with greater cognitive impulsivity in marijuana-dependent young men: preliminary spectroscopic evidence at 4 T. J Addict Res Ther S. 2013;4:2. doi: 10.4172/2155-6105.S4-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martinez D, Saccone PA, Liu F, et al. Deficits in dopamine D2 receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry. 2012;71:192–198. doi: 10.1016/j.biopsych.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moreno-López L, Stamatakis EA, Fernández-Serrano MJ, et al. Neural correlates of the severity of cocaine, heroin, alcohol, MDMA and cannabis use in polysubstance abusers: a resting-PET brain metabolism study. PLoS One. 2012;7:e39830. doi: 10.1371/journal.pone.0039830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bora E, Yücel M, Fornito A, et al. White matter microstructure in opiate addiction. Addict Biol. 2013;17:141–148. doi: 10.1111/j.1369-1600.2010.00266.x. [DOI] [PubMed] [Google Scholar]
- 86.Qiu Y, Jiang G, Su H, et al. Progressive white matter microstructure damage in male chronic heroin dependent individuals: a DTI and TBSS study. PLoS One. 2013;8:e63212. doi: 10.1371/journal.pone.0063212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmidt A, Walter M, Gerber H, et al. Inferior frontal cortex modulation with an acute dose of heroin during cognitive control. Neuropsychopharmacology. 2013;38(11):2231–2239. doi: 10.1038/npp.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hermann D, Frischknecht U, Heinrich M, et al. MR spectroscopy in opiate maintenance therapy: association of glutamate with the number of previous withdrawals in the anterior cingulate cortex. Addict Biol. 2011;17:659–667. doi: 10.1111/j.1369-1600.2010.00290.x. [DOI] [PubMed] [Google Scholar]
- 89.Verdejo-García A, Lubman DI, Roffel K, et al. Cingulate bio-chemistry in heroin users on substitution pharmacotherapy. Aust N Z J Psychiatry. 2013;47:244–249. doi: 10.1177/0004867412463088. [DOI] [PubMed] [Google Scholar]
- 90.Frischknecht U, Hermann D, Heinrich M, et al. Experience of social discrimination correlates with neurometabolism: a pilot study in heroin addicts. Eur Arch Psyc Clin Neurosci. 2012;263:197–203. doi: 10.1007/s00406-012-0319-6. [DOI] [PubMed] [Google Scholar]
- 91.Domino EF, Minoshima S, Guthrie S, et al. Nicotine effects on regional cerebral blood flow in awake, resting tobacco smokers. Synapse. 2000;38:313–321. doi: 10.1002/1098-2396(20001201)38:3<313::AID-SYN10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 92.Zubieta J-K, Lombardi U, Minoshima S, et al. Regional cerebral blood flow effects of nicotine in overnight abstinent smokers. Biol Psychiatry. 2001;49:906–913. doi: 10.1016/s0006-3223(00)01070-2. [DOI] [PubMed] [Google Scholar]
- 93.Domino EF, Ni L, Xu Y, et al. Regional cerebral blood flow and plasma nicotine after smoking tobacco cigarettes. Prog NeuroPsychopharm Biol Psychiatry. 2004;28:319–327. doi: 10.1016/j.pnpbp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 94.Kubota K, Yamaguchi T, Abe Y, et al. Effects of smoking on regional cerebral blood flow in neurologically normal subjects. Stroke. 1983;14:720–724. doi: 10.1161/01.str.14.5.720. [DOI] [PubMed] [Google Scholar]
- 95.Skinhϕj E, Olesen J, Paulson O. Influence of smoking and nicotine on cerebral blood flow and metabolic rate of oxygen in man. J App Physiology. 1973;35:820–822. doi: 10.1152/jappl.1973.35.6.820. [DOI] [PubMed] [Google Scholar]
- 96.Gundersen H, van Wageningen H, Gruner R. Alcohol-induced changes in cerebral blood flow and cerebral blood volume in social drinkers. Alcohol Alcohol. 2013;48:160–165. doi: 10.1093/alcalc/ags121. [DOI] [PubMed] [Google Scholar]
- 97.Volkow ND, Mullani N, Gould L, et al. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res. 1988;24:201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- 98.Sano M, Wendt PE, Wirsén A, et al. Acute effects of alcohol on regional cerebral blood flow in man. J Stud Alcohol Drugs. 1993;54:369. doi: 10.15288/jsa.1993.54.369. [DOI] [PubMed] [Google Scholar]
- 99.Schwartz JA, Speed NM, Gross MD, et al. Acute effects of alcohol administration on regional cerebral blood flow: the role of acetate. Alcohol Clin Exp Res. 1993;17:1119–1123. doi: 10.1111/j.1530-0277.1993.tb05217.x. [DOI] [PubMed] [Google Scholar]
- 100.Mathew R, Wilson W. Regional cerebral blood flow changes associated with ethanol intoxication. Stroke. 1986;17:1156–1159. doi: 10.1161/01.str.17.6.1156. [DOI] [PubMed] [Google Scholar]
- 101.Lotfi J, Meyer J. Cerebral hemodynamic and metabolic effects of chronic alcoholism. Cerebrovasc Brain Metab Rev. 1989;1:2–25. [PubMed] [Google Scholar]
- 102.Christie IC, Price J, Edwards L, et al. Alcohol consumption and cerebral blood flow among older adults. Alcohol. 2008;42:269–275. doi: 10.1016/j.alcohol.2008.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Melgaard B, Henriksen L, Ahlgren P, et al. Regional cerebral blood flow in chronic alcoholics measured by single photon emission computerized tomography. Acta Neurol Scand. 1990;82:87–93. doi: 10.1111/j.1600-0404.1990.tb01594.x. [DOI] [PubMed] [Google Scholar]
- 104.Berglund M, Risberg J. Regional cerebral blood flow during alcohol withdrawal. Arch Gen Psychiatry. 1981;38:351–355. doi: 10.1001/archpsyc.1981.01780280119014. [DOI] [PubMed] [Google Scholar]
- 105.Caspari D, Trabert W, Heinz G, et al. The pattern of regional cerebral blood flow during alcohol withdrawal-a single photon emission tomography study with 99mTc-HMPAO. Acta Psychiatr Scand. 1993;87:414–417. doi: 10.1111/j.1600-0447.1993.tb03397.x. [DOI] [PubMed] [Google Scholar]
- 106.Tutus A, Kuğu N, Sofuoğlu S, et al. Transient frontal hypoperfusion in Tc-99 m hexamethylpropyleneamineoxime single photon emission computed tomography imaging during alcohol withdrawal. Biol Psychiatry. 1998;43:923–928. doi: 10.1016/s0006-3223(97)00322-3. [DOI] [PubMed] [Google Scholar]
- 107.Ances BM, Vaida F, Cherner M, et al. HIV and chronic methamphetamine dependence affect cerebral blood flow. J Neuroimmune Pharmacol. 2011;6:409–419. doi: 10.1007/s11481-011-9270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Broadley KJ. The vascular effects of trace amines and amphetamines. Pharmacol Ther. 2010;125:363–375. doi: 10.1016/j.pharmthera.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 109.Chung YA, Peterson BS, Yoon SJ, et al. In vivo evidence for long-term CNS toxicity, associated with chronic binge use of methamphetamine. Drug Alcohol Dep. 2010;111:155–160. doi: 10.1016/j.drugalcdep.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 110.Polesskaya O, Silva J, Sanfilippo C, et al. Methamphetamine causes sustained depression in cerebral blood flow. Brain Res. 2011;1373:91–100. doi: 10.1016/j.brainres.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yuan Z, Luo Z, Volkow ND, et al. Imaging separation of neuronal from vascular effects of cocaine on rat cortical brain in vivo. Neuroimage. 2011;54:1130–1139. doi: 10.1016/j.neuroimage.2010.08.045. Neuronal and vascular effects can be difficult to deconvolve with neuroimaging and is likely to confound results in many cases. This study demonstrates the importance of that notion.