Abstract
Highly active antiretroviral therapy (HAART) suppresses HIV-1 replication, transforming the outlook for infected patients. However, reservoirs of replication-competent forms of the virus persist during HAART, and when treatment is stopped, high rates of HIV-1 replication return. Recent insights into HIV-1 latency, as well as a report that HIV-1 infection was eradicated in one individual, have renewed interest in finding a cure for HIV-1 infection. Strategies for HIV-1 eradication include gene therapy and hematopoietic stem cell transplantation, stimulating host immunity to control HIV-1 replication, and targeting latent HIV-1 in resting memory CD4+ T cells. Future efforts should aim to provide better understanding of how to reconstitute the CD4+ T cell compartment with genetically engineered cells, exert immune control over HIV-1 replication, and identify and eliminate all viral reservoirs.
Keywords: reservoir, eradication, latency, HIV-1, cure
Renewed optimism for a cure
The past thirty years have witnessed remarkable advances in the struggle against HIV-1. Three key developments have renewed optimism that a cure may be possible. The first breakthrough came with the introduction of highly active antiretroviral therapy (HAART), which transformed HIV-1 infection from a death sentence into a manageable chronic illness. Ongoing refinements to HAART limit side effects and improve adherence, but high rates of HIV-1 replication return when HAART is interrupted. Although lifelong suppression of HIV-1 replication with HAART should be possible in adherent patients, the problems of access, cost, side effects, stigma, and the danger of resistance with non-adherence all contribute to the necessity of finding a cure. The second breakthrough involved the discovery of a long-lived latent reservoir for HIV-1 in resting CD4+ T cells that allows viral persistence despite HAART. This reservoir is widely recognized as a major obstacle to curing the infection [1–5]. Progress in delineating the mechanisms that contribute to HIV-1 latency (reviewed in [6, 7]) and in developing in vitro models (reviewed in [8]) and animal models [9, 10] of latency have allowed the design and testing of therapies that target the latent reservoir. The most recent breakthrough came unexpectedly in 2009 with the report of the possible cure of an HIV-1-infected patient who was treated for leukemia with a myeloablative regimen and a hematopoietic stem cell transplant (HSCT) from a donor with genetic resistance to HIV-1 [11].
The best approach to finding a cure is unclear. Latently infected cells are rare and difficult to target, and there may be additional reservoirs for the virus in other cell types. Thus the specific elimination of viral reservoirs, which is currently the most widely discussed approach, will be extremely challenging. Therefore, there has been interest in other approaches. One involves stimulating the immune response so that it can control viral replication in the absence of HAART to such a low level that immunodeficiency does not develop and transmission is blocked. In addition, the successful cure of a single patient by HSCT has stimulated research into other approaches for replacing the hematopoietic compartment with cells resistant to HIV-1 infection.
Two forms of cure for HIV-1 infection have been defined. A sterilizing cure is one in which the virus is completely eradicated, whereas a functional cure is the control of HIV-1 replication in the absence of HAART [12]. Here we discuss strategies for HIV-1 elimination, targeted therapies to eliminate HIV-1 in CD4 T cells and gene therapy approaches with or without HSCT. We also discuss data from studies of elite suppressor (ES), patients who control HIV-1 replication without treatment thus representing a naturally occurring functional cure.
Latent reservoirs and residual viremia: keys to a cure
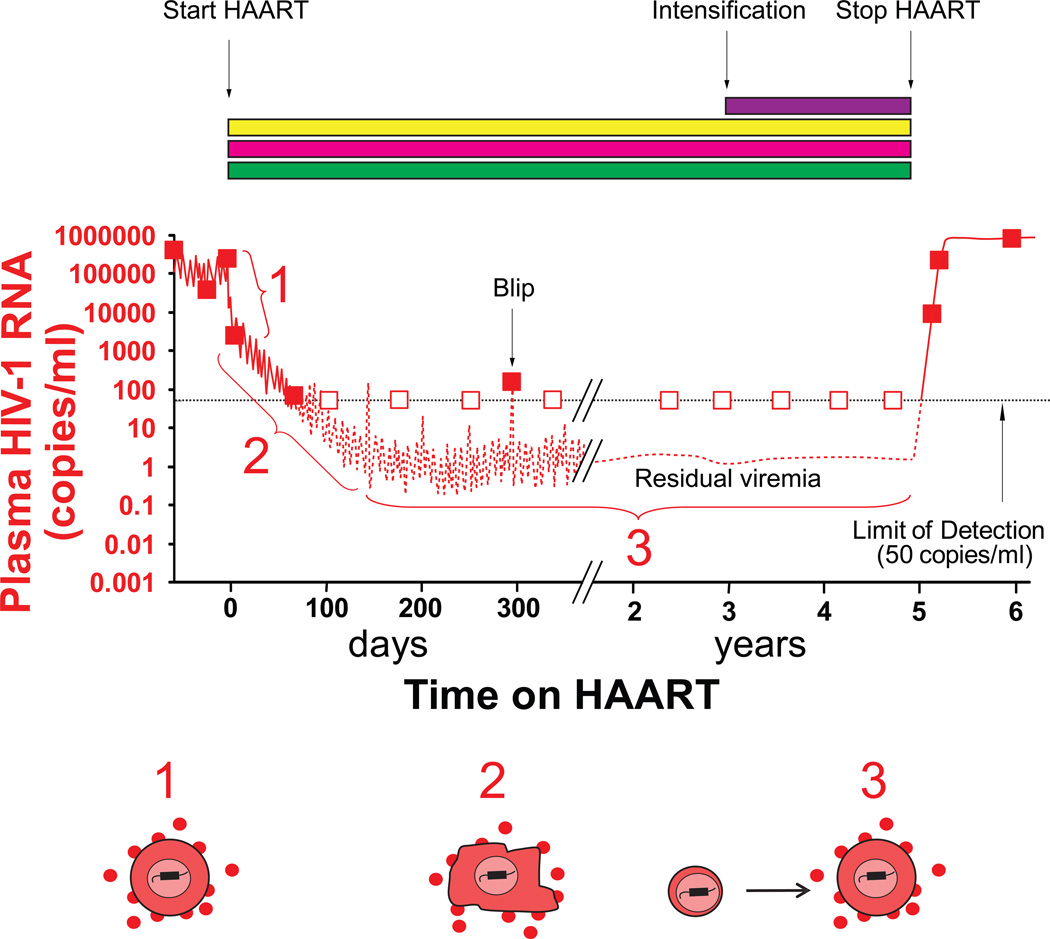
Untreated HIV-1 infection is characterized by continuous viral replication which drives CD4+ T cell loss and predicts disease progression [13]. Initial treatment efforts focused on nucleoside analogue reverse transcriptase inhibitors (NRTIs), which failed to control viral replication. HAART, first described in 1997, was made possible by the introduction two new classes of antiretroviral drugs, protease inhibitors (PIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs). Three drug combinations consisting of one of these drugs plus two NRTIs were able to reduce levels of HIV-1 below the limit of detection of clinical assays (50 copies of HIV-1 RNA/mL of plasma). As all of the drugs used in HAART regimens block new infection of susceptible cells, the fall in plasma HIV-1 RNA levels reveals the decay rates of various populations of virus-producing cells infected prior to the initiation of therapy [14, 15]. Analysis of viral decay kinetics [16] demonstrated a biphasic reduction in plasma HIV-1 after initiation of HAART (Figure 1). The first phase is rapid since most of the plasma virus is produced by activated CD4+ T cells which have a half-life of less than a day in the productively infected state [17]. The second phase reflects virus production by another population of infected cells with a half-life of 1–4 weeks. The cells responsible for this phase have not yet been definitively identified (18). Initial predictions that HAART could eradicate HIV-1 infection in treated individuals in 2–3 years were based on extrapolation of this second phase [16].
Figure 1.
Dynamics of viral replication and viremia after initiation of HAART. (A) When patients start a 3 drug HAART regimen (yellow, blue and green rectangles), plasma virus levels undergo triphasic decay, ultimately plateauing below the limit of detection of clinical assays. Boxes indicate clinical assays (filled boxes, detectable viremia; open boxes, viremia below 50 copies/ml). Fluctuations in the level of residual viremia can give rise to “blips” that are of no clinical significance. Intensification of HAART by addition of a fourth drug (purple rectangle) does not further reduce residual viremia because this viremia originates from cells infected prior to the initiation of HAART. Despite prolonged suppression of viremia on HAART, viral rebound occurs follow cessation of HAART. (B) The three phases reflect the decay rates of different populations of infected cells. The first phase has a half-life of 1 day and reflects the rapid decay of productively infected CD4+ T lymphoblasts. The cells responsible for the second phase, which has a half-life of about 14 days, have not been definitively identified. In the final phase, viral reservoirs are responsible for a very low but stable level of residual viremia. This residual viremia is partly derived from the activation of latently infected resting CD4+ T cells and partly from another unknown cell source.
The discovery of HIV-1 latency proved these predictions incorrect. Latency is defined as a silent or non-productive state of infection of individual cells that can be reversed. As early as 1995, it was shown that stably integrated but latent HIV-1 genomes were present in resting memory CD4+ T cells in vivo [1]. Resting CD4+ T cells are not readily infected by HIV-1, and thus one hypothesis about the origin of this latent reservoir is that occasionally activated CD4+ T cells, the major target cells for HIV-1 in vivo, become infected as they are transitioning back to a resting state that is non-permissive for virus gene expression. Evidence for this hypothesis comes from the finding that latent HIV-1 genomes are found in resting memory CD4+ T cells but not naïve CD4+ T cells [2]. Direct infection of resting memory CD4+ T cells may also occur [17]. The extremely long half-life of memory T cell populations provides a mechanism for HIV-1 persistence. Further studies showed that latently infected resting CD4+ T cells were present in all patients on HAART [3–5] and that after cellular activation, these cells could enter a state of productive infection. Despite ongoing effective therapy, the frequency of these HIV-1-infected memory cells remained constant over time [18, 19]. The process of homeostatic proliferation that maintains the pool of memory T cells appears to contribute to the stability of the latent reservoir [20].
In addition to latent HIV-1 in resting CD4+ T cells, trace amounts free virus can be detected in the plasma of nearly all patients on HAART using sensitive “single-copy” assays for HIV-1 RNA [21, 22]. The most likely source of this residual viremia is the release of HIV-1 from cells infected prior to initiation of HAART rather than ongoing HIV-1 replication. Several lines of evidence support this claim. First, extensive clinical experience has shown that adherent patients who maintain plasma viral loads less than 50 copies/mL do not develop resistance to antiretroviral drugs. Second, studies which evaluated the addition of potent antiretroviral drugs to the regimens of patients on standard HAART, often referred to as “intensification studies,” did not find any further decrease in the amount of residual viremia (Figure 1) [23–25]. Third, phylogenetic studies of residual plasma HIV-1 show no sequence evolution in patients on effective HAART even during transient “blips” in viremia between 50–200 copies/mL [26–28]. Despite this evidence, debate regarding ongoing HIV-1 replication continues. Some experts believe that persistent T cell activation and the detection of cell-associated 2 LTR circles (a proposed marker of recent infection) provide evidence that active HIV-1 replication continues despite HAART [29,30]. The significance of these indirect measures is controversial, and conflicting reports exist [31].
Resting CD4+ T cells are considered one source of residual viremia. Phylogenetic studies show that in some cases, residual plasma HIV-1 sequences are identical to HIV-1 sequences in resting CD4+ T cells [32, 33]. However, in about half of patients, residual viremia is dominated by invariant HIV-1 clones that persist over time. These predominant plasma clones are rarely found in circulating resting CD4+ T cells [33–36]. This phenomenon has suggested the existence of a second reservoir for HIV-1, in a cell-type capable of proliferating after infection. Cellular proliferation allows viral genomes to be distributed without error into progeny cells. Hematopoietic progenitor cells (HPCs) have been proposed as a second reservoir. A recent study suggested HIV-1 could establish latent infection in HPCs [37]. However, the purity of HPCs in this analysis ranged from 30–90%, and new studies of HPCs in patients on HAART which exclude contaminating lymphocytes have not confirmed latent infection of HPCs [38, 39]. There is clear evidence for infection of tissue macrophages [17], but whether infected macrophages or related cells such microglial cells in the central nervous system can persist on a time scale of years in patients on optimal HAART is not yet clear. In any event, determining the source of these persistent oligoclonal HIV-1 populations is critical to eradication strategies. If the predominant plasma clones are produced by a different cell type, additional interventions may be required because current eradication strategies focus on latently infected CD4+ T cells as the primary barrier to HIV-1 eradication.
Gene therapy and hematopoietic stem cell transplantation: recapitulating the Berlin patient
Gene therapy was first proposed as a treatment for HIV-1 infection in 1988 when David Baltimore coined the term “intracellular immunization” [40]. Broadly speaking, gene therapy involves genetically manipulating cells (most commonly HPCs or T cells) to produce an anti-HIV-1 effect. Multiple approaches exist, including transplanting HIV-1-resistant cells, modifying cells to interfere with HIV-1 production, and potentiating immune responses to HIV-1. An expanding array of tools is available including RNA-based strategies (ribozymes, antisense RNA, small-interfering RNA) or protein-based strategies such as zinc-finger nucleases (ZFNs) (reviewed in [41]). Recently published clinical trials of gene therapy in HIV-1-infected adults will be discussed here and are summarized in Table 1.
Table 1.
Components of curative gene therapy and HSCT approaches in clinical studies.
| Study | |||||
|---|---|---|---|---|---|
| Berlin patient | OZ-1 vector Tat-vpr ribozyme |
Multiple anti-HIV-1 RNAs |
NCT00842634a ZFN for CCR5 |
NCT01044654a ZFN for CCR5 |
|
| Source of engineered/modified cells | HSCT from HLA matched, CCR5Δ32 homozygous unrelated donor | Autologous peripheral blood mobilized CD34+ HPCs | Autologous peripheral blood mobilized CD34+ HPCs | Autologous CD4+ T cells | Autologous CD4+ T cells |
| Modifications | Natural genetic polymorphism | Tat-vpr ribozyme | Tat/rev shRNA, TAR decoy, CCR5 ribozyme | ZFN (SB-728-T) for CCR5 | ZFN (SB-728-T) for CCR5 |
| Cytotoxic regimen | Amsacrine, fludarabine, cytarabine, cyclophosphamide, antithymocyte globulin, total body irradiation | None | Bischloronitrosourea, etoposide, cyclophosphamide | None | None |
| HAART | Stopped at HSCT | 4-week interruption to select for modificied cells, analytic 8 week interruption | Maintained | Cohort 1: maintained Cohort 2: 10-week interruption | Maintained |
| Ablation of reservoirs | Confirmed | Unknown | Unknown | Ongoing study | Ongoing study |
| Cytotoxic therapy | Potential contribution | Not applicable | Potential contribution | Not applicable | Not applicable |
| Graft vs reservoir effect | Potential Contribution | Not applicable | Not applicable | Not applicable | Not applicable |
| Reconstitution with resistant cells | Confirmed | Low level .01–.38% cells | Low level 0.1–0.15% cells | Ongoing | Ongoing |
| Outcome | Definite functional cure Possible sterilizing cure | Primary endpoint, mean plasma HIV-1 RNA after second interruption, no difference | Not intended to test effect on HIV-1 viral replication or reservoirs | Ongoing | Ongoing |
| References | 11, 50, 51 | 52 | 53 | 56 | 56 |
Abbreviation NCT: National clinical trial
After the discovery that the chemokine receptor CCR5 was the major co-receptor for HIV-1 [42–46], it was found that a 32 base-pair deletion in at least one copy of this gene naturally occurs in 5–15% of Europeans [47]. Individuals homozygous for this CCR5Δ32 mutation are resistant to acquiring HIV-1 infection because their cells lack surface CCR5 [48, 49]. Heterozygotes may become infected but demonstrate delayed disease progression [50, 51]. Knowledge of a natural form of resistance to HIV-1 infection gave rise to hopes that gene therapy aimed at CCR5 might constitute effective treatment for HIV-1 infection. However, progress on this front has been modest over the past 15 years due to the technical challenges of gene therapy.
A type of targeted gene therapy in the form of hematopoietic stem cell transplantation (HSCT) has achieved historic results in one patient. In 2007, an HIV-1-infected patient with acute myelogenous leukemia was treated with chemotherapy, total body irradiation, and HSCT from a donor who was homozygous for CCR5Δ32 [11]. This patient, who has since become known as the "Berlin patient," stopped HAART the day prior to transplant and over five years later has not had a rebound in viremia or other indications of ongoing viral replication [52]. This unique case has renewed hope that replacement of recipient cells with donor cells or engineered cells that lack CCR5 expression may be curative. However, some aspects of this case may not be explained by the CCR5 deletion alone (Table 1). After stopping HAART at the time of HSCT, the patient had no rebound of HIV-1 viremia despite the fact that host-derived, CCR5-expresssing myeloid cells remained detectable for months. In addition, based on sequencing of the HIV-1 envelope gene prior to HSCT, the patient’s viral quasispecies included a small percentage of HIV-1 variants predicted to use the chemokine receptor CXCR4 for cellular entry. After HSCT, in vitro studies demonstrated that the donor-derived cells were susceptible to infection by X4 virus, but X4 variants have not emerged in vivo [53]. Further studies of HIV-1-infected patients undergoing cytotoxic chemotherapy and HSCT may clarify whether the myeloablative regimen or graft-versus-host (GVH) effects contributed to cure in this unique case.
Cellular engineering strategies presumably carry less risk than treatment with cytotoxic therapy and HSCT. However, they have met with more limited success. The largest human study to date was a randomized trial which included 74 patients. HPCs from participants in the treatment arm were transduced ex vivo with OZ1, a retroviral vector carrying a gene for a ribozyme targeting viral RNA encoding the proteins Tat and Vpr. HAART was interrupted to provide selective pressure for survival of the modified cells [54]. The trial demonstrated the safety and feasibility of this gene transfer approach; however, there was no significant difference between groups in the primary endpoint, mean plasma HIV-1 RNA levels eight weeks after a second treatment interruption. The lack of efficacy may have been due to the transient nature and low levels of engraftment of the modified cells. A significant problem with the strategy of introducing cells engineered to resist HIV-1 infection is how to eliminate all of the infected cells that persist in the patient. Neither the ablative therapy and GVH effects used in the Berlin patient nor the use of uncontrolled HIV-1 replication to select for HIV-1-resistant cells represent attractive approaches for general use.
HIV-1-infected patients with hematologic malignancy provide a unique opportunity to study gene therapy. Lymphoma treatments often include cytotoxic chemotherapy followed by autologous HSCT. Diguisto et al. combined the cellular engineering and HSCT approaches in a study involving patients with lymphoma. Following harvest and before reinfusion, autologous stem cells may be manipulated and engineered ex vivo. In this small study of four patients, HPCs were transfected with a vector that delivers a ribozyme targeting the CCR5 coreceptor, a short-interfering RNA targeting expression of the HIV-1 proteins Tat and Rev, and an RNA decoy to Tat. In addition to safety and feasibility, this trial demonstrated long-lived engraftment and multi-lineage hematopoiesis of vector-expressing cells but again engraftment was low (< 0.2% of cells). There was no HAART interruption by trial design, and effects on HIV-1 disease could not be assessed [55].
An approach that has generated substantial excitement in the field is the use of zinc finger nucleases (ZFN) or similar engineered, sequence-specific nucleases to produce HIV-1-resistant cells. ZFNs are engineered proteins that contain two domains: a zinc finger domain with arrays of zinc fingers that each target three or four base pairs of DNA and an endonuclease domain which, when dimerized, makes a double-stranded cut in the target DNA that can be subsequently repaired with non-homologous end joining. This error-prone cellular DNA repair mechanism frequently introduces mutations that render the targeted gene product non-functional. ZFNs have been designed to disrupt expression of CCR5 and CXCR4; they can be delivered by adenoviral or retroviral vectors or with non-vector methods such as nucleofection. Disruption of CCR5 in CD4+ T cells [56] and HPCs [57] has been shown to result in lower HIV-1 viral loads and higher levels of CD4+ T cells in humanized mouse models of HIV-1 infection. Currently, a clinical trial of this approach is being conducted in persons with HIV-1 infection. In this study, large numbers of CD4+ T cells are collected by pheresis. A CCR5-specific ZFN is delivered to these cells ex vivo with an adenovirus vector, and the genetically-modified cells are re-infused into the individual [58]. A potential limitation of this strategy is that the CCR5-specific ZFN does not directly eliminate latently infected cells. To address this issue, some of the trials combine the gene therapy approach with an intentional HAART interruption; in theory, this would lead to active HIV-1 replication during which genetically modified cells will preferentially survive and proliferate. However, this strategy exposes individuals to the morbidity and mortality risks associated with HAART interruption [59].
Elite suppressors: models for a functional cure
Elite suppressors (ES) are HIV-1-infected patients who maintain viral loads below the limit of detection of commercial assays without HAART (reviewed in [60]). HIV-1 proviral DNA can be amplified from the CD4+ T cells of virtually all ES [61–65], and more importantly, replication-competent virus can be cultured from these cells in many ES [66–68]. While infection with attenuated virus can lead to ES status in some patients [69], it is unlikely that all ES are infected with defective virus. Full genome sequence analysis of replication-competent virus from ES has generally not revealed large deletions or signature inactivating mutations [66, 70], and phenotypic analyses have suggested that some isolates can replicate as efficiently in vitro as reference isolates [66, 70]. Studies of trace levels of free virus in the plasma of ES have provided evidence for evolution, suggesting that there is ongoing viral replication in vivo [71–73]. Furthermore, transmission of virus from patients with progressive disease to patients who became ES has been documented [74, 75]. Therefore it appears that in some cases, fully replication-competent virus can be controlled by host elements. Thus, ES may be viewed as models of functional cure. They have not completely eradicated the virus, but they are able to effectively control viral replication in the absence of treatment.
Functional cures will probably be easier to achieve than complete eradication. In fact several studies have shown that early treatment with HAART can lead to the subsequent control of HIV-1 replication in some patients [76–79]. While the vast majority of these patients eventually develop breakthrough viremia [80], these studies serve as an important proof of concept. Early control of viral replication may be critical; ES have low peak viral loads during primary infection and usually achieve undetectable viral loads within a few months of seroconversion [81–83]. This may explain the low frequency of latently infected cells seen in these patients [66]. Early control of viral replication may also serve to preserve an effective HIV-1-specific immune response that could potentially be boosted through therapeutic vaccination.
The mechanisms involved in elite control are not fully understood, but many studies have shown that certain class I HLA alleles such as HLA-B*27, B*57 and B*5801 are associated with elite control [84–86]. These protective HLA alleles are thought to present conserved immunodominant epitopes leading to effective cytotoxic T lymphocyte (CTL) responses [87–90]. The question then becomes whether it will be possible to induce CTL responses to conserved epitopes in patients who lack the protective HLA alleles? This would require the design of vaccines that elicit CTL responses to critical immunodominant epitopes that can be presented by many different HLA alleles. Of note, not all ES have these protective HLA alleles or readily measurable CTL responses [91, 92], and identifying the mechanisms of control in these patients may also inform vaccine design. Because of the immunosuppressive effects of unchecked viral replication [87, 89, 90], it is likely that therapeutic vaccine strategies would be employed in patients who already have suppression of viremia on HAART, with an eventual interruption of HAART once HIV-1-specific CTL responses have been enhanced (Figure 2). If therapeutic vaccination allows control of viral replication to below 50 copies/mL after HAART is stopped, then a functional cure can be achieved, because at this level of viremia, immunosuppression is reversed and transmission is unlikely.
Figure 2.
Use of therapeutic vaccination to achieve a functional cure. Vaccination of patients on a 3 drug HAART regimen (yellow, blue and green rectangles) may allow the subsequent control of viremia after cessation of HAART to levels seen in ES. Because the mechanisms for control of HIV-1 replication in ES are not completely clear, the nature of the therapeutic vaccine is still uncertain. However, the stimulation of HIV-1- specific CTL responses is likely to be important.
Can the immune response be used to eradicate the latent reservoir? This seems unlikely if latently infected cells are not actively making viral proteins. Interestingly, in ES with protective HLA alleles, HIV-1 clones amplified from resting CD4+ T cells do not contain escape mutations in immunodominant epitopes [62, 93]. These cells should therefore be susceptible to CTL if they are activated and begin to express viral genes. As is discussed below, strategies that incorporate therapeutic vaccination with the selective activation of latently infected cells could theoretically lead to complete eradication of the virus.
Targeting latently-infected CD4+ T cells
The most widely discussed approach to eradicating HIV-1 involves reactivating latent HIV-1 genomes in resting CD4+ T cells. Early studies demonstrated that the use of global T cell activators to upregulate HIV-1 gene expression induced substantial toxicity and was ineffective (reviewed in [94]). Newer approaches seek to reactivate HIV-1 gene expression more specifically by targeting particular mechanisms of latency rather than activating all resting CD4+ T cells.
Multiple mechanisms involved in the establishment and maintenance of HIV-1 latency have been the subject of several recent reviews [6, 7, 95]. Viral transcription is blocked due to epigenetic modifications such as histone deacetylation and DNA methylation and the sequestration of critical host transcriptional factors, including NFκB. In addition, there are post-transcriptional mechanisms including restricted nuclear export of HIV-1 RNAs and inhibition by host microRNAs. Thus, there is no single mechanism of HIV-1 latency. This can be understood by considering the fundamental nature of HIV-1 latency. In untreated patients, there is continuous replication of the virus which is readily evident in the form of detectable viremia (Figure 1). The virus evades the immune system through rapid evolution [96], not through latency. Rather, for HIV-1, latency can be viewed as an accidental consequence of viral tropism for activated CD4+ T cells that occasionally become infected as they are transitioning back to a non-permissive resting state.
The complicated balance of host and viral factors regulating latency initially suggested that a combination of drugs targeting multiple pathways might be required to efficiently reactivate HIV-1, and it was not clear that any single agent would be capable of reactivating latent HIV-1 without inducing global T cell activation. The development of improved primary cell models to study HIV-1 latency in vitro has greatly facilitated the search for compounds with this activity [97–101]. Recent studies have identified individual compounds that are capable of reversing latency without T cell activation [97, 102, 103]. These include the histone deacetylase (HDAC) inhibitor vorinostat (suberoylanilide hydroxamic acid or SAHA) [104, 105] and the alcoholism drug disulfiram [100]. Several lines of evidence (reviewed in [106]) indicate that HDACs are recruited to the HIV-1 LTR in latently infected cells [6, 92, 103, 104]. Thus HDAC inhibitors may contribute to reversing latency, although an alternative mechanism has been proposed for SAHA [104]. The mechanism by which disulfiram reverses latency is under investigation. Clinical trials of these agents are underway.
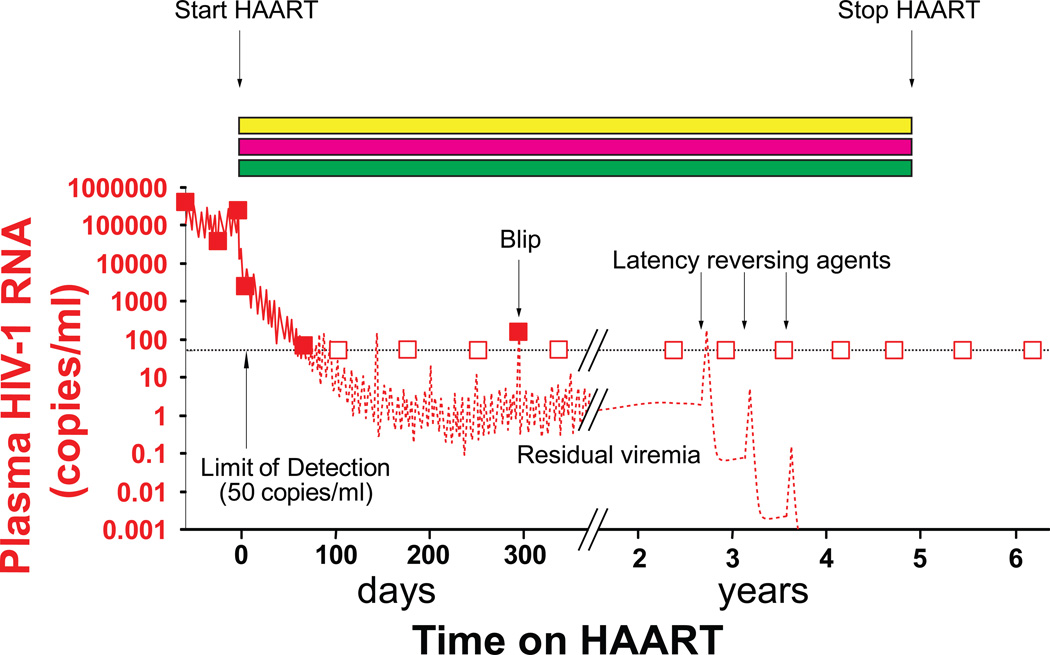
Clinical trials of latency reversing agents are carried out in patients who have had prolonged suppression of viremia on HAART (Figure 3). The reversal of latency may lead to transient increases in the level of viremia, but it is unlikely that the released viruses will be able to infect new cells because of the extraordinary potential of HAART to suppress replication [107]. It was anticipated that the infected cells in which virus gene expression had been induced would die quickly from viral cytopathic effects or be lysed by host CTL, leading to a decrease in the latent pool (Figure 3). However, a recent study has shown that reversal of latency by agents that do not induce global T cell activation does not lead to the death of the infected cells [108]. The lower levels of HIV-1 gene expression in resting cells and differences in susceptibility to cell death pathways may protect these cells from viral cytopathic effects. In addition, in vitro studies suggest that HIV-1-specific CD8+ T cells from most patients on HAART are relatively ineffective at killing these cells following reversal of latency [108]. Thus some form of therapeutic vaccination may be needed here as well.
Figure 3.
Use of latency reversing strategies to achieve a sterilizing cure. Reversal of latency in patients on a 3 drug HAART regimen (yellow, blue and green rectangles) may lead to a transient increase in viremia followed by a decrease in the size of the latent reservoir. Latency could be reversed with agents such as the histone deacetylase inhibitor SAHA. This decrease will be difficult to detect with current assays. To ensure that infected cells die after reversal of latency, it may be necessary to combine latency reversing strategies with therapeutic vaccination.
Clinical trials of latency reversing agents have raised the critical issue of how eradication efforts will be monitored. As discussed above, residual viremia appears to reflect the release of virus from stable reservoirs, but levels of residual viremia are already below the limit of detection of standard assays (Figure 3). Direct quantitation of latently infected cells would be ideal, but latently infected cells are very rare in vivo, with a frequency of less than 1 in 106 resting CD4+ T cells in the peripheral blood. They probably do not produce viral proteins and are essentially indistinguishable from uninfected cells. This has made study of latently-infected cells in vivo difficult. Currently, the gold standard assay for measuring the size of the latent reservoir is a limiting dilution virus culture assay [109]. In this assay, highly purified populations of resting CD4+ T cells are isolated from patients on HAART who have clinically undetectable levels of HIV-1 plasma RNA. These cells are plated in a limiting dilution format and then stimulated ex vivo to induce activation of all cells. Uninfected CD4+ T lymphoblasts are added to amplify virus over a 2 week period, and viral production is measured using an ELISA assay for HIV-1 p24 antigen. The assay is specific for replication-competent virus, but it is time-consuming, and the sensitivity is limited by the number of resting CD4+ T cells that can be isolated from a given patient. Simpler PCR based assays also detect replication-defective forms of the virus, which greatly outnumber replication-competent forms [2]. Stopping HAART, which is of course the ultimate goal of eradication efforts, cannot be easily used to evaluate eradication efforts because of the adverse effects associated with treatment interruption [59]. Thus improved assays to follow eradication efforts are urgently needed.
Concluding remarks
Remarkable progress in the treatment of HIV-1 infection has been made over the past three decades, but a practical, general strategy for curing patients remains elusive. Novel eradication strategies that hold some promise include gene therapy, therapeutic vaccination, and pharmacologic reactivation of latent HIV-1. However, the global scale of the epidemic necessitates that a successful strategy must allow large numbers of patients to be cured, even in resource-limited settings. Thus immunologic and pharmacologic approaches may be more relevant than gene therapy. Improved assays for detecting viral persistence are needed so that the efficacy of eradication strategies can be monitored through laboratory measurements rather than potentially harmful treatment interruptions. Additional mechanistic studies of HIV-1 latency will identify novel targets for pharmacologic approaches to reactivate latent virus, and a better understanding of the specific factors responsible for control of HIV-1 infection in ES will aid in the design of therapeutic vaccines. A hopeful development is the recent recognition of the need for a concerted collaborative effort to solve this problem [108]. Open collaborations between scientists studying molecular mechanisms and animal models of HIV-1 persistence, physicians conducting clinical trials, and pharmaceutical companies interested in the problem will clearly speed the discovery of a cure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chun TW, et al. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat. Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 2.Chun TW, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 3.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 4.Wong JK, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 5.Chun TW, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. U.S.A. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakre S, et al. Epigenetic regulation of HIV latency. Curr. Opin. HIV. AIDS. 2011;6:19–24. doi: 10.1097/COH.0b013e3283412384. [DOI] [PubMed] [Google Scholar]
- 7.Karn J. The molecular biology of HIV latency: breaking and restoring the Tat-dependent transcriptional circuit. Curr. Opin. HIV. AIDS. 2011;6:4–11. doi: 10.1097/COH.0b013e328340ffbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang HC. Primary cell models of HIV latency. Curr. Opin. HIV. AIDS. 2011;6:62–67. doi: 10.1097/COH.0b013e3283412568. [DOI] [PubMed] [Google Scholar]
- 9.Clements JE, et al. A simian immunodeficiency virus macaque model of highly active antiretroviral treatment: viral latency in the periphery and the central nervous system. Curr. Opin. HIV. AIDS. 2011;6:37–42. doi: 10.1097/COH.0b013e3283412413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deere JD, et al. Simian immunodeficiency virus macaque models of HIV latency. Curr. Opin. HIV. AIDS. 2011;6:57–61. doi: 10.1097/COH.0b013e32834086ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutter G, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 12.Dieffenbach CW, Fauci AS. Thirty years of HIV and AIDS: future challenges and opportunities. Ann. Intern. Med. 2011;154:766–771. doi: 10.7326/0003-4819-154-11-201106070-00345. [DOI] [PubMed] [Google Scholar]
- 13.Mellors JW, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 14.Wei X, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 15.Ho DD, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 16.Perelson AS, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 18.Finzi D, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 19.Siliciano JD, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 20.Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dornadula G, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA. 1999;282:1627–1632. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- 22.Palmer S, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J. Clin. Microbiol. 2003;41:4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinoso JB, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc. Natl. Acad. Sci. U.S.A. 2009;106:9403–9408. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandhi RT, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7:e1000321. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Besson GJ, et al. Short-course Raltegravir Intensification Does Not Increase 2 Long Terminal Repeat Episomal HIV-1 DNA in Patients on Effective Antiretroviral Therapy. Clin. Infect. Dis. 2011 doi: 10.1093/cid/cir721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermankova M, et al. HIV-1 drug resistance profiles in children and adults with viral load of <50 copies/ml receiving combination therapy. JAMA. 2001;286:196–207. doi: 10.1001/jama.286.2.196. [DOI] [PubMed] [Google Scholar]
- 27.Kieffer TL, et al. Genotypic analysis of HIV-1 drug resistance at the limit of detection: virus production without evolution in treated adults with undetectable HIV loads. J. Infect. Dis. 2004;189:1452–1465. doi: 10.1086/382488. [DOI] [PubMed] [Google Scholar]
- 28.Nettles RE, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005;293:817–829. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- 29.Buzon MJ, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat. Med. 2010;16:460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 30.Sharkey ME, et al. Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nat. Med. 2000;6:76–81. doi: 10.1038/71569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandhi RT, et al. No effect of raltegravir intensification on viral replication markers in the blood of HIV-1-infected patients receiving antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2012;59:229–235. doi: 10.1097/QAI.0b013e31823fd1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobin NH, et al. Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J. Virol. 2005;79:9625–9634. doi: 10.1128/JVI.79.15.9625-9634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bailey JR, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J. Virol. 2006;80:6441–6457. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brennan TP, et al. Analysis of human immunodeficiency virus type 1 viremia and provirus in resting CD4+ T cells reveals a novel source of residual viremia in patients on antiretroviral therapy. J. Virol. 2009;83:8470–8481. doi: 10.1128/JVI.02568-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahu GK, et al. Low-level plasma HIVs in patients on prolonged suppressive highly active antiretroviral therapy are produced mostly by cells other than CD4 T-cells. J. Med. Virol. 2009;81:9–15. doi: 10.1002/jmv.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson JA, et al. Clonal sequences recovered from plasma from patients with residual HIV-1 viremia and on intensified antiretroviral therapy are identical to replicating viral RNAs recovered from circulating resting CD4+ T cells. J. Virol. 2011;85:5220–5223. doi: 10.1128/JVI.00284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter CC, et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat. Med. 2010;16:446–451. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durand CM, et al. HIV-1 DNA Is Detected in Bone Marrow Populations Containing CD4+ T Cells but Is not Found in Purified CD34+ Hematopoietic Progenitor Cells in Most Patients on Antiretroviral Therapy. J. Infect. Dis. 2012 doi: 10.1093/infdis/jir884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Josefsson L, et al. Hematopoietic Precursor Cells Isolated from Patients on Long Term Suppressive HIV Therapy Did Not Contain HIV-1 DNA. J. Infect. Dis. 2012 doi: 10.1093/infdis/jis301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baltimore D. Gene therapy. Intracellular immunization. Nature. 1988;335:395–396. doi: 10.1038/335395a0. [DOI] [PubMed] [Google Scholar]
- 41.Rossi JJ, et al. Genetic therapies against HIV. Nat. Biotechnol. 2007;25:1444–1454. doi: 10.1038/nbt1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dragic T, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 43.Deng H, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 44.Choe H, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 45.Doranz BJ, et al. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 46.Alkhatib G, et al. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 47.Libert F, et al. The deltaccr5 mutation conferring protection against HIV-1 in Caucasian populations has a single and recent origin in Northeastern Europe. Hum. Mol. Genet. 1998;7:399–406. doi: 10.1093/hmg/7.3.399. [DOI] [PubMed] [Google Scholar]
- 48.Liu R, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 49.Samson M, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 50.Dean M, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 51.Huang Y, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 52.Hutter G, Ganepola S. Eradication of HIV by transplantation of CCR5-deficient hematopoietic stem cells. ScientificWorldJournal. 2011;11:1068–1076. doi: 10.1100/tsw.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allers K, et al. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood. 2011;117:2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 54.Mitsuyasu RT, et al. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat. Med. 2009;15:285–292. doi: 10.1038/nm.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DiGiusto DL, et al. RNA-based gene therapy for HIV with lentiviral vector-modified CD34(+) cells in patients undergoing transplantation for AIDS-related lymphoma. Sci. Transl. Med. 2010;2:36ra43. doi: 10.1126/scitranslmed.3000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez EE, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 2008;26:808–816. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holt N, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat. Biotechnol. 2010;28:839–847. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tebas P, et al. Disruption of CCR5 in Zinc Finger Nuclease-treated CD4 T Cells: Phase I Trials; Conference on Retroviruses and Opportunistic Infections; 2011. [Google Scholar]
- 59.Seminari E, et al. CD4+ guided antiretroviral treatment interruption in HIV infection: a meta-analysis. AIDS. Rev. 2008;10:236–244. [PubMed] [Google Scholar]
- 60.O'Connell KA, et al. Elucidating the elite: mechanisms of control in HIV-1 infection. Trends Pharmacol. Sci. 2009;30:631–637. doi: 10.1016/j.tips.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Lambotte O, et al. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin. Infect. Dis. 2005;41:1053–1056. doi: 10.1086/433188. [DOI] [PubMed] [Google Scholar]
- 62.Bailey JR, et al. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J. Exp. Med. 2006;203:1357–1369. doi: 10.1084/jem.20052319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatano H, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J. Virol. 2009;83:329–335. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Julg B, et al. Infrequent recovery of HIV from but robust exogenous infection of activated CD4(+) T cells in HIV elite controllers. Clin. Infect. Dis. 2010;51:233–238. doi: 10.1086/653677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Graf EH, et al. Elite suppressors harbor low levels of integrated HIV DNA and high levels of 2-LTR circular HIV DNA compared to HIV+ patients on and off HAART. PLoS Pathog. 2011;7:e1001300. doi: 10.1371/journal.ppat.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blankson JN, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J. Virol. 2007;81:2508–2518. doi: 10.1128/JVI.02165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lamine A, et al. Replication-competent HIV strains infect HIV controllers despite undetectable viremia (ANRS EP36 study) AIDS. 2007;21:1043–1045. doi: 10.1097/QAD.0b013e3280d5a7ac. [DOI] [PubMed] [Google Scholar]
- 68.Julg B, et al. Infrequent recovery of HIV from but robust exogenous infection of activated CD4(+) T cells in HIV elite controllers. Clin. Infect. Dis. 2010;51:233–238. doi: 10.1086/653677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deacon NJ, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 70.Gaillard S, et al. Sustained elite suppression of replication competent HIV-1 in a patient treated with rituximab based chemotherapy. J. Clin. Virol. 2011;51:195–198. doi: 10.1016/j.jcv.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O'Connell KA, et al. Control of HIV-1 in elite suppressors despite ongoing replication and evolution in plasma virus. J. Virol. 2010;84:7018–7028. doi: 10.1128/JVI.00548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mens H, et al. HIV-1 continues to replicate and evolve in patients with natural control of HIV infection. J. Virol. 2010;84:12971–12981. doi: 10.1128/JVI.00387-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salgado M, et al. Evolution of the HIV-1 nef gene in HLA-B*57 positive elite suppressors. Retrovirology. 2010;7:94. doi: 10.1186/1742-4690-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bailey JR, et al. Transmission of human immunodeficiency virus type 1 from a patient who developed AIDS to an elite suppressor. J. Virol. 2008;82:7395–7410. doi: 10.1128/JVI.00800-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buckheit RW, 3rd, et al. Host factors dictate control of viral replication in two HIV-1 controller/chronic progressor transmission pairs. Nat. Commun. 2012;3:716. doi: 10.1038/ncomms1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosenberg ES, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 77.Margolick JB, et al. Prolonged viral suppression without therapy in an HIV-1 seroconverter following early antiretroviral therapy and daily interleukin-2. AIDS. 2010;24:932–935. doi: 10.1097/QAD.0b013e328333adfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hocqueloux L, et al. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS. 2010;24:1598–1601. doi: 10.1097/qad.0b013e32833b61ba. [DOI] [PubMed] [Google Scholar]
- 79.Salgado M, et al. Prolonged control of replication-competent dual- tropic human immunodeficiency virus-1 following cessation of highly active antiretroviral therapy. Retrovirology. 2011;8:97. doi: 10.1186/1742-4690-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaufmann DE, et al. Limited durability of viral control following treated acute HIV infection. PLoS Med. 2004;1:e36. doi: 10.1371/journal.pmed.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Altfeld M, et al. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS. 2003;17:2581–2591. doi: 10.1097/00002030-200312050-00005. [DOI] [PubMed] [Google Scholar]
- 82.Goujard C, et al. Spontaneous control of viral replication during primary HIV infection: when is "HIV controller" status established? Clin. Infect. Dis. 2009;49:982–986. doi: 10.1086/605504. [DOI] [PubMed] [Google Scholar]
- 83.Okulicz JF, et al. Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J. Infect. Dis. 2009;200:1714–1723. doi: 10.1086/646609. [DOI] [PubMed] [Google Scholar]
- 84.Migueles SA, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fellay J, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.International HIV Controllers Study et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330:1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Migueles SA, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 2002;3:1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 88.Saez-Cirion A, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. U.S.A. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Migueles SA, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hersperger AR, et al. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 2010;6:e1000917. doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pereyra F, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 2008;197:563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 92.Emu B, et al. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J. Virol. 2008;82:5398–5407. doi: 10.1128/JVI.02176-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bailey JR, et al. Evidence of CD8+ T-cell-mediated selective pressure on human immunodeficiency virus type 1 nef in HLA-B*57+ elite suppressors. J. Virol. 2009;83:88–97. doi: 10.1128/JVI.01958-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Archin NM, Margolis DM. Attacking latent HIV provirus: from mechanism to therapeutic strategies. Curr. Opin. HIV. AIDS. 2006;1:134–140. doi: 10.1097/01.COH.0000203837.47092.fd. [DOI] [PubMed] [Google Scholar]
- 95.Chan JK, Greene WC. NF-kappaB/Rel: agonist and antagonist roles in HIV-1 latency. Curr. Opin. HIV. AIDS. 2011;6:12–18. doi: 10.1097/COH.0b013e32834124fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coffin JM. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 97.Yang HC, et al. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J. Clin. Invest. 2009;119:3473–3486. doi: 10.1172/JCI39199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sahu GK, et al. A novel in vitro system to generate and study latently HIV-infected long-lived normal CD4+ T-lymphocytes. Virology. 2006;355:127–137. doi: 10.1016/j.virol.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 99.Marini A, et al. An in vitro system to model the establishment and reactivation of HIV-1 latency. J. Immunol. 2008;181:7713–7720. doi: 10.4049/jimmunol.181.11.7713. [DOI] [PubMed] [Google Scholar]
- 100.Bosque A, Planelles V. Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood. 2009;113:58–65. doi: 10.1182/blood-2008-07-168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tyagi M, et al. Establishment of HIV latency in primary CD4+ cells is due to epigenetic transcriptional silencing and P-TEFb restriction. J. Virol. 2010;84:6425–6437. doi: 10.1128/JVI.01519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xing S, et al. Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. J. Virol. 2011;85:6060–6064. doi: 10.1128/JVI.02033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xing S, et al. Novel structurally related compounds reactivate latent HIV-1 in a bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. J. Antimicrob. Chemother. 2012;67:398–403. doi: 10.1093/jac/dkr496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Contreras X, et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J. Biol. Chem. 2009;284:6782–6789. doi: 10.1074/jbc.M807898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Archin NM, et al. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res. Hum. Retroviruses. 2009;25:207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Margolis DM. Histone deacetylase inhibitors and HIV latency. Curr. Opin. HIV. AIDS. 2011;6:25–29. doi: 10.1097/COH.0b013e328341242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jilek BL, et al. A quantitative basis for antiretroviral therapy for HIV-1 infection. Nat. Med. 2012;18:446–451. doi: 10.1038/nm.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shan L, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol. Biol. 2005;304:3–15. doi: 10.1385/1-59259-907-9:003. [DOI] [PubMed] [Google Scholar]