Summary
Pulmonary hypertension (PH) is an increasingly recognized complication of premature birth and bronchopulmonary dysplasia (BPD), and is associated with increased morbidity and mortality. Extreme phenotypic variability exists among preterm infants of similar gestational ages, making it difficult to predict which infants are at increased risk for developing PH. Intrauterine growth retardation or drug exposures, postnatal therapy with prolonged positive pressure ventilation, cardiovascular shunts, poor postnatal lung and somatic growth, and genetic or epigenetic factors may all contribute to the development of PH in preterm infants with BPD. In addition to the variability of severity of PH, there is also qualitative variability seen in PH, such as the variable responses to vasoactive medications. To reduce the morbidity and mortality associated with PH, a multi-pronged approach is needed. First, improved screening for and increased recognition of PH may allow for earlier treatment and better clinical outcomes. Second, identification of both prenatal and postnatal risk factors for the development of PH may allow targeting of therapy and resources for those at highest risk. Third, understanding the pathophysiology of the preterm pulmonary vascular bed may help improve outcomes through recognizing pathways that are dysregulated in PH, identifying novel biomarkers, and testing novel treatments. Finally, the recognition of conditions and exposures that may exacerbate or lead to recurrent PH is needed to help with developing treatment guidelines and preventative strategies that can be used to reduce the burden of disease.
Keywords: pulmonary hypertension, bronchopulmonary dysplasia, prematurity, chronic lung disease
INTRODUCTION
Owing to advances in neonatal care and technology, survival rates for extremely low birth weight (ELBW) infants have increased dramatically over the past several decades. However, many ELBW survivors continue to develop bronchopulmonary dysplasia (BPD), a condition characterized by impaired alveolar growth and airway inflammation.1,2 Manifestations of BPD often include decreased pulmonary reserve (i.e., hypoxia, hypercarbia, and tachypnea) and airflow obstruction (i.e., wheezing and coughing). Although ELBW is associated with BPD,3 there is significant variability in the severity and course of BPD even among infants of similar gestational age. Even in adulthood, impairments in respiratory health have been shown to persist and to be greater in BPD infants compared to infants born at term.4
In preterm infants, pulmonary hypertension (PH) may develop as a consequence of BPD and contribute to the severity and persistence of BPD symptoms. PH has been shown to impose additional morbidity and mortality on already vulnerable preterm infants with BPD. Although PH in the BPD population usually resolves with time and catch-up lung growth, increased mortality rates are associated with PH ranging between 14% and 38% in retrospective studies5–9 and 12% in one prospective study.10 Understanding the relationship between BPD and PH may help minimize the detrimental impact of these conditions on health outcomes.
The diagnosis of PH after 2 months of age in preterm infants is often associated with severe BPD. However, not all infants with PH have severe BPD,5,6,11 which suggests that the impact of impaired alveolar growth on pulmonary vascular growth is variable. The development of PH is likely influenced by conditions that impact lung growth and function in utero and postnatally. Epigenetic, genetic, and environmental factors are also likely involved in the development of PH, but have not been extensively studied in this population. In this review we describe what is known about the burden, risk factors, pathophysiology, diagnosis, and management of PH in preterm infants with BPD as well as current gaps in our knowledge and future directions that should be explored (Table 1).
TABLE 1.
Future Directions and Gaps in Knowledge
| Burden of disease |
|
| Risk factors for disease |
|
| Pathophysiology of disease |
|
| Diagnosis of disease |
|
| Management of disease |
|
BURDEN OF DISEASE
Recently the tracking outcomes and practice in pediatric pulmonary hypertension (TOPP) registry reported that 12% of PH cases were associated with respiratory disease, with BPD being the most common cause.12 This may be an underestimate since most registry participants were diagnosed by catheterization, and most preterm infants are diagnosed via echocardiogram. Underestimation of the prevalence of PH due to BPD may also occur since no ICD-9 code exists that encompasses this diagnosis. In the absence of large prospective studies, the incidence of PH in infants and children with BPD is unknown.7,11 The only published prospective study from a single perinatal center found that 18% of ELBW infants were diagnosed with PH prior to discharge.10 Retrospective studies report that PH may occur in up to 17–43% of preterm infants with BPD.5–7,9 Prospective data on the natural course of disease, including time to resolution and rates of recrudescence, are also limited.11 Retrospective data suggest resolution may occur as early as 4–5 months of age on average.6 Large multicenter prospective studies are required to determine the incidence and course of disease, particularly as regional variability in BPD incidence and treatment strategies may exist.
It appears likely that PH adds additional healthcare costs for longer initial NICU stays, medical therapies and procedures. One retrospective study found that preterm infants with PH spent at least three additional weeks in the hospital.6 There are also no data on the quality of life in preterm infants with PH and their families, but it is likely to be affected by longer hospitalizations, more frequent outpatient encounters, medication burden, and the increased need for respiratory support. Data on long-term outcomes on these patients are lacking and it is unknown whether these infants will be at increased risk for the recrudescence of PH and/or chronic lung disease in later life.
RISK FACTORS FOR DISEASE
Risk factors for the development of PH are poorly understood.7 Birth weight and severity of BPD play a role, although the magnitude of their contributions is unclear as not every ELBW infant with severe BPD develops PH.5–7,11 Infants that are born small for gestational age (SGA) have been reported to be at higher risk for developing PH compared to non-SGA infants.5,7,10 However, it is not known whether the small pulmonary vascular bed in SGA infants, an SGA-associated in utero exposure (e.g., poor maternal nutrition, drug exposures including anti-depressants or secondhand smoke exposure), or some other factor common to SGA infants contributes to the development of PH.
The structural abnormalities and decreased surface area of the BPD lung along with functional abnormalities such as persistent ventilation-perfusion mismatch, intermittent hypoxia and/or hypercarbia,13 and poor airway clearance all may contribute to PH development. Any factor that hinders lung growth and recovery may increase the risk of the development of PH. Such factors may include positive pressure ventilation, dysphagia, gastro-esophageal reflux, respiratory infections, and suboptimal nutrition.6 In addition, concomitant lung disease such as surfactant disorders or interstitial lung diseases may also contribute to the development of PH. Further research is needed to identify infants with lung disease who are at the highest risk, including infants who are difficult to extubate, require frequent courses of systemic steroids, or have difficulty gaining weight.7
Maternal pre-eclampsia may also be a risk factor for PH in the preterm infant. Mothers with pre-eclampsia have been shown to have elevated serum and amniotic fluid levels of soluble VEGF receptor 1, also known as sFlt-1.14 sFlt-1 antagonizes the VEGF-VEGFR2 signaling pathway, thus interfering with endothelial maintenance and cell growth. Administration of sFlt-1 into amniotic fluid in rats has been shown to lead to decreased alveolar growth and pulmonary vessel density in the offspring.15 As demonstrated in a murine model, sFlt-1 may lead to vascular dysfunction and poor endothelial growth through an imbalance between the endothelial system and eNOS.16 Further understanding of these pathways in the preterm infant may help identify therapies that could potentially prevent PH development in preterm infants.
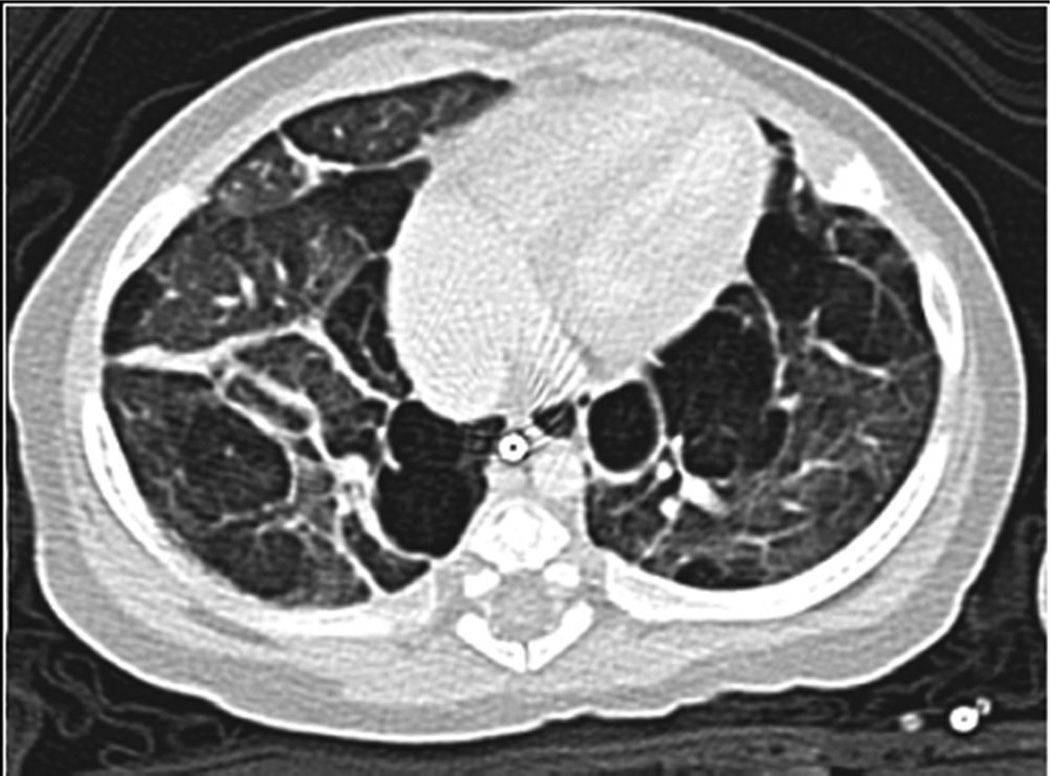
Associations between oligohydramnios and PH have also been reported in two retrospective studies of preterm infants with BPD.5,8 We have observed that pulmonary hypoplasia from premature rupture of membranes and prematurity appears to increase the risk of PH (Fig. 1). Other potential risk factors may include shunts that increase pulmonary blood flow (e.g., intracardiac shunts, large collateral vessels, or PDAs)6,17,18 and upper or lower airway anomalies that hamper effective gas exchange (e.g., subglottic stenosis, tracheomalacia, etc.).11,17 Other postnatal exposures which require additional study in the preterm population include postnatal secondhand smoke exposure19 and systemic inflammation.8
Fig. 1.
Chest CT scan of infant born at 26 weeks gestation with history of premature rupture of membranes at 22 weeks gestation. Although only briefly intubated in the NICU, the infant was found to have severe pulmonary hypertension and cystic emphysematous lung changes at 6 months of age.
PATHOPHYSIOLOGY OF DISEASE
Classification of Pediatric PH
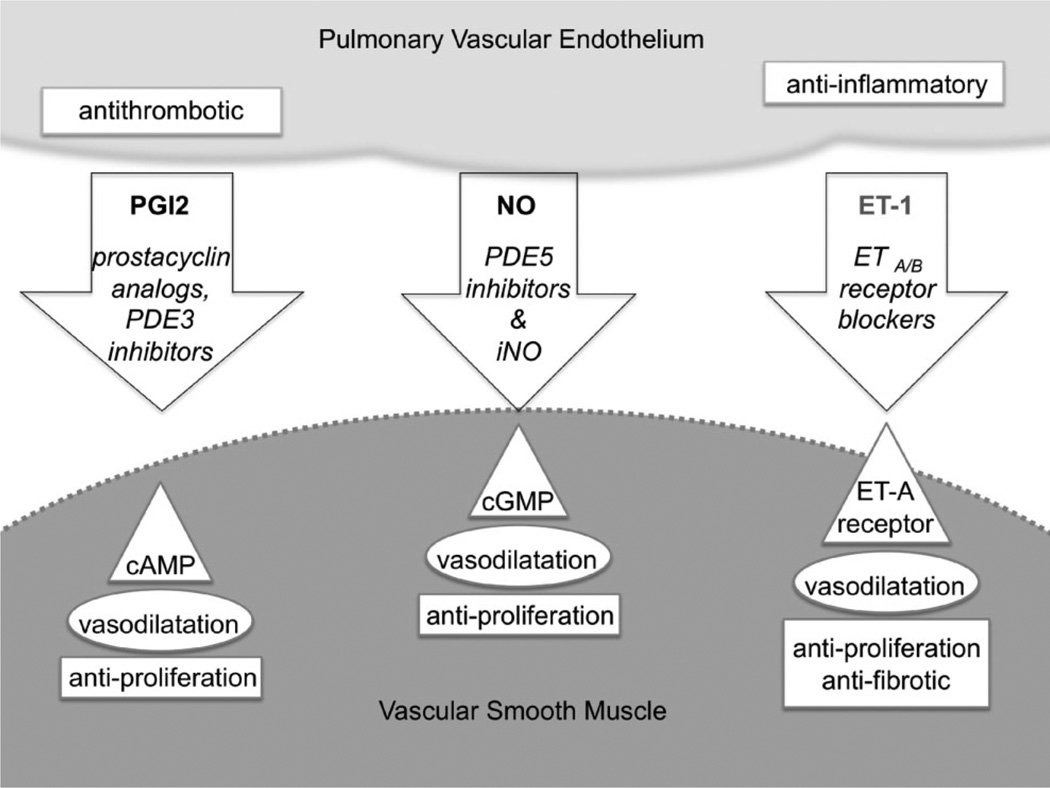
The variation in clinical presentation of PH is partially dependent on the contributions of different vascular elements, which is reflected in a recently proposed classification of PH within BPD.13 Lesions upstream of the pulmonary vascular bed, such as patent ductus arteriosus and left-to-right intracardiac shunts may contribute to PH in the preterm infant. Within the lungs, impaired lung growth is often associated with a small pulmonary vascular bed, giving a “fixed” component of PH. However, some of these children have a “responsive” component consisting of pulmonary arterioles that respond to vasodilator therapies such as nitric oxide, sildenafil, bosentan, and iloprost (Fig. 2).20 Lastly, downstream pulmonary venous lesions and left ventricular dysfunction may also contribute to PH as well. The physiological interplay between these components in the preterm infant is poorly understood. However, earlier gestation infants (<29 weeks gestation) may have more of a fixed component than later gestation infants as suggested by different response rates to nitric oxide therapy.8 Thus, recognizing the relative contribution of vascular components to PH will be crucial in optimizing management as an infant with predominantly “responsive” disease will respond to vasodilators, one with predominantly “fixed” disease may not, and one with a significant venous lesion may actually worsen with vasodilator therapy.
Fig. 2.
Pharmacotherapy for pulmonary hypertension. This schematic shows three major strategies (arrows) for both acute contraction state control and chronic remodeling of pulmonary vascular smooth muscle (SMC). All three strategies are derived from natural products of the pulmonary endothelium (PEC) that are shown in bold print at the tops of the arrows. Classes of therapeutic agents are shown in italics in each arrow. Principal molecular targets of each pharmacotherapeutic strategy are shown in triangles, acute effects are shown in ellipses, and subacute and longer term therapeutic consequences are shown in rectangles in either the vascular smooth muscle or pulmonary endothelial target tissues: PGI2, or prostacyclin, is a natural PEC product that relaxes SMC via increases in intracellular cAMP levels. Inhibitors of type 3 phosphodiesterases (PDE3), such as milrinone, stabilize the cAMP concentration; PEC also produce the gasotransmitter nitric oxide (NO) which dilates SMC by boosting cGMP levels, and these levels are buttressed by the PDE5 inhibitors, including sildenafil; The third class of PEC products that has inspired pharmacotherapy for PH is the endothelin receptor blocker group. ET-1 is a vasoconstrictor that is produced by PEC and broad-spectrum blockers of both ETA- and ETB-type receptors, such as bosentan, decrease SMC tone. Figure concept after Humbert et al.86
BPD and PH
Impaired alveolar development is associated with extreme prematurity, but postnatal factors such as neonatal infections, initiation of positive pressure ventilation, exposure to hyperoxia, and poor nutrition can also significantly impair lung growth. Even though significant alveolar catch up growth in the postnatal period is possible, many children with a history of prematurity and BPD have abnormal lung function and exercise intolerance in later life.21–23 Small airway disease is often a dominant clinical phenotype in both early and later life in the child with BPD; however, it is unknown whether small airway dysfunction is associated with or can exacerbate or worsen PH. Another concern is whether children with BPD are at greater risk for late-onset pulmonary vascular dysfunction. O’Reilly and colleagues found that exposure to neonatal hyperoxia caused pulmonary vascular disease and premature mortality in aged mice.24 Longitudinal studies are needed to determine if preterm infants are at increased risk for early onset cardiopulmonary disease in adult life.
Inflammation and oxidative stress are believed to be important contributors to BPD severity,25 which may be mediated through exposure to hyperoxia.26–29 Although increased levels of inflammatory cytokines have been reported in preterm infants who subsequently developed BPD,2 it is unknown whether inflammatory cytokines independently contribute to the development of PH. Similarly, the impact of oxidative stress on the pulmonary vasculature of the preterm infant has not been extensively studied. Endothelial cells are susceptible to oxidative stress and increased oxidative stress can induce vascular pathology in many adult diseases.30 Compounding the effect of increased oxidative stress, preterm infants are often deficient in antioxidant reserves, thus increasing their risk of oxidative stress induced injury. It has been suggested that supplementation with both enzymatic and non-enzymatic antioxidants may be helpful in reducing oxidative stress in the preterm infant.31 A study in mice found that activation of the transcriptional factor Nrf2 protected neonatal mice exposed to hyperoxia through the induction of antioxidant pathway genes.32 Along those lines, further study into interventions that induce activation of antioxidant pathways prior to an oxidative stress, may be warranted.
Molecular Pathophysiology
In vitro work has demonstrated that nitric oxide is necessary for normal growth, proliferation, migration, and tube formation of fetal pulmonary artery endothelial cells.33 Although routine use of inhaled nitric oxide in preterm infants is currently not recommended,34,35 selected use of nitric oxide on preterm infants with PH or at high risk of developing PH may still be of benefit.8 Arginases I and II compete with nitric oxide synthase (NOS) for l-arginine and specific inhibition of arginase in human endothelium has been shown to increase nitric oxide production.36 An imbalance between arginase II and NOS in the preterm lung may partially account for the development of PH in the preterm infant. Supplementation with l-citrulline may be a potential therapy to consider, as it has been shown to decrease arginase activity, increase arginine and nitric oxide levels, and rescue a BPD phenotype in a neonatal rat model.37 Further work on the nitric oxide pathway and other pathways identified by genetic or biomarker screening is needed to elucidate the molecular pathophysiology of PH in the BPD infant.
Genetic and Epigenetic Factors in PH
The contribution of genetic and/or epigenetic factors to the development and severity of PH in infants with BPD is currently unknown. It is plausible that such mechanisms contribute to the development of PH, as susceptibility to developing BPD has a strong heritable component based on twin studies.38,39 Identifying causal or modifier genes of BPD and PH may eventually serve as a tool for identifying infants at risk of developing PH. A number of loci have been implicated in BPD susceptibility through candidate gene association studies, including the genes encoding surfactant proteins B and D, TNF-α, mannose binding lectin, angiotensin converting enzyme,40 and TLR5,41 but no single locus has been consistently associated with BPD in general or PH specifically. Recently, SPOCK2 gene polymorphisms were found to be associated with BPD in a genome-wide association study.42 The function of this protein is unknown, but its expression pattern during development and within the lung supports a potential role in BPD pathogenesis. Specific lung diseases known to have a genetic basis, such as surfactant dysfunction disorders and alveolar capillary dysplasia, should be considered in infants with unresponsive PH, particularly those in which severity of lung disease seems out of proportion to what might be expected based upon their gestational age and postnatal course. Future research should include identifying modifier genes through exome and genome-wide analyses, candidate SNP studies of genes involved in familial pulmonary arterial hypertension (e.g., BMPR2, ENG, and ALK1),20 and gene sequencing for loss of function mutations in the evaluation of specific candidate genes.
Studies linking the development of PH with epigenetic changes in the neonate are limited. A study by Koukoura et al.,43 has shown an association between DNA methylation and decreased expression of IGF2, a gene involved in placental and fetal growth. A study in chronically hypoxic hypertensive calves also demonstrated a link between epigenetic changes and vascular remodeling.44 They found increased class I histone deacetylases activity associated with increased inflammatory gene expression in adventitial fibroblasts isolated from hypoxic hypertensive calves compared to controls. A better understanding of the genetic basis of PH in the BPD infant may allow for the identification of biomarkers that could be used in diagnosing and guiding therapy.
DIAGNOSIS OF DISEASE
PH may manifest through physical findings such as parasternal lift, quality of the second heart sound, or evidence of right heart failure. Chest radiography findings can include cardiomegaly, right atrial, and right ventricular dilatation and enlarged central arteries with electrocardiogram findings of right axis deviation or right ventricular hypertrophy. However, these findings may be non-specific and often subtle in the small infant, leading to the need for better diagnostic modalities.6,17,45 Non-invasive studies for PH diagnosis should first be considered to minimize procedural complications that can occur with cardiac catheterization.
Echocardiograms in Diagnosis of PH
In practice, echocardiograms (echo) are frequently used to both screen and follow PH in children with BPD; however no guidelines exist for either screening selection or follow-up intervals in this population.7,17,18 Based on our experience and others, evaluation by echo should be considered in all preterm children with BPD who require supplemental oxygen and/or need positive pressure ventilation beyond 2 months of age; special consideration should also be given to those who were SGA and are growing poorly. Children on vasodilator therapy for PH should undergo echo evaluation before weaning therapy or with pulmonary exacerbations. An echo should also be considered in children with BPD and a history of PH who fail to improve during an acute pulmonary exacerbation. Commonly used methods of assessing PH via echocardiography include quantitative methods such as tricuspid regurgitant jet velocity (TRJV), ventricular septal defect or patent ductus arteriosus velocity, pulmonary regurgitation velocity to estimate pulmonary artery end diastolic pressure, and qualitative measures such as interventricular septal position in systole, right ventricular hypertrophy, dilation, and/or dysfunction.17 Of these methods, TRJV is the mainstay of pediatric right ventricular systolic pressure (RVSP) evaluation in clinical practice. Using the TRJV, the systolic pressure gradient between the right atrium and right ventricle (and hence the RVSP and inferred systolic PA pressure) can be estimated using the modified Bernoulli equation of RVSP = 4v2 (where v, velocity of the TR jet) plus the right atrial pressure.
Simultaneous measurements of echo TRJV (and calculated RVSP) with directly measured RVSP during cardiac catheterization in sedated adults demonstrated an excellent correlation (r = 0.96).46 However, the strength of this correlation has not been borne out in pediatric data. Hill et al.,47 recently assessed pediatric patients undergoing cardiac catheterization for a suspected diagnosis of PH (n = 127) within the Mid-Atlantic Group of Interventional Cardiology (MAGIC) registry and found a poor correlation (R2 = 0.36, P < 0.01) between transthoracic echocardiographic estimates of RVSP based on TRJV and cardiac catheterization. However, the echo and catheterization data were not simultaneously obtained, and the majority of these patients were under general anesthesia. Mourani et al.48 retrospectively analyzed the correlation between systolic PAP via nonsimultaneous echo TRJV and cardiac catheterization in 25 patients <2 years of age with chronic lung disease, and also found a poor correlation (r = 0.19, P = 0.43); however despite this poor correlation, echo correctly diagnosed the presence or absence of PH in 79% of the studies, giving a sensitivity of 88% and specificity of 33%. A major limitation of echocardiographic evaluation of TRJV is that it relies on adequately analyzeable TR regurgitant jet, which is not present in all patients. Mourani et al. described 61% of studies with adequate TR jet for analysis; Currie et al.46 reported analyzeable TR in 80% of adults with elevated right ventricular pressure, and 57% of adult patients with normal right ventricular pressure.
Decreased sensitivity in assessing PH severity is a major limitation to using echo.48 This may be due to chest hyperinflation, lack of cooperation, and small chest size.11 In addition, pulmonary venous stenosis can be difficult to diagnose by echo.49 Future studies in children using tricuspid annular plane systolic excursion (TAPSE), speckle tracking of the right ventricle, and 3D echo to evaluate right ventricular size and function, as well as inferior vena cava dilation ratio may provide additional insight into the diagnosis of PH and assessment of severity.
Cardiac Catheterization
Cardiac catheterization, offers the advantage of definitive and comprehensive information about cardiopulmonary hemodynamics and therapeutic drug testing. However, due to higher complication rates in pediatric patients, cardiac catheterization should only be performed in tertiary care centers that have cardiologists and anesthesiologists with experience in the care of these children.50 There are no widely accepted guidelines that indicate which patients with BPD and PH should be catheterized. However, catheterization should be considered when the severity of PH is uncertain despite noninvasive assessment, when acute responsiveness to pulmonary vasodilators must be assessed, when severe PH is not satisfactorily responding to therapy, and when vascular narrowings or shunt lesions must be assessed and/or treated. In one series, 31% of formerly premature infants with BPD and PH underwent cardiac catheterization.7
The catheterization WHO diagnostic criteria for PH are presently mean pulmonary artery pressure >25 mmHg [normal 14–16 mmHg], mean pulmonary capillary wedge pressure <15 mmHg, and pulmonary vascular resistance >3 mmHg/L/min (Wood units). The WHO criteria are based on adults and have been adapted to children, however as the systemic blood pressure is remarkably lower in neonates, the WHO criteria results in a proportionally higher right ventricular to left ventricular pressure ratio in infants than adults.
Acute hemodynamic responses to pulmonary vasodilators can be assessed and can inform decision-making about long-term use of such agents. Typically, baseline hemodynamic measurements are made while the patient is breathing air or no more than the patient’s usual supplemental FiO2. The FiO2 is then increased to 100%, and the hemodynamic measurements are repeated. Nitric oxide in concentrations that may range between 20 and 80 parts per million is then added to the inspired oxygen, and hemodynamic measurements are repeated again. Responses to other agents such as intravenous or inspired prostacyclin analogues or enteral sildenafil can also be assessed in similar fashion. Responsiveness in pediatric patients is presently defined as a decrease in mean pulmonary artery pressure ≥20%, an increase or no change or only slight decrease in cardiac output, and decrease or no change in the ratio between pulmonary vascular resistance and systemic vascular resistance.51
The presence and degree of left ventricular dysfunction (systolic and diastolic) can be demonstrated and further guide medical management.52 Precise information about pulmonary arterial and venous anatomy can be obtained using angiography. Peripheral pulmonary artery stenoses can contribute to right ventricular hypertension in these infants, and these can be assessed angiographically as well as hemodynamically. Rare disorders, which cause PH in infants, such as alveolar capillary dysplasia and primary pulmonary vein stenosis, can be identified. Information obtained during cardiac catheterization can guide decision making for surgical or transcatheter management of pulmonary arterial and venous narrowings and closure of shunt lesions: atrial septal defects, patent ductus arteriosus, or aortopulmonary collateral arteries. Such shunt lesions may be found in as many as 58% of patients undergoing cardiac catheterization.48 Cardiac catheterization in children with BPD is labor-intensive, time-consuming, and expensive. There is also significant anesthetic risk associated with the procedure. Although one registry study showed that cardiac catheterization can be performed for pediatric PH patients with few (mostly technical) adverse events and zero mortality,47 another retrospective study from a single center showed that resuscitation or death occurred in 6% of cases.53 Thus, discretion is essential in selecting patients for referral for cardiac catheterization.
Biomarkers and Other Non-Invasive Testing for PH
Given the invasive nature of cardiac catheterization, there is a need to identify non-invasive modalities for diagnosis and follow-up, which may include circulating biomarkers. Although no biomarkers for PH in preterm infants have been identified at this time, strategies for identifying promising biomarkers may include testing adult biomarkers, such as osteopontin,54 and novel discovery projects. The best studied PH biomarker is the cardiac hormone brain natriuretic protein (BNP) and its prohormone N-terminal pro-BNP (NT-proBNP). Levels of BNP have been shown to correlate with disease severity and prognosis in adults with PH.55–57 However, BNP and NT-proBNP have been challenged as biomarkers in the infant with BPD as BNP is a cardiac protein, and thus an indirect measure of pH associated with lung disease. BNP is developmentally regulated in the heart with levels peaking in the neonate and declining after birth reaching a more stable adult equivalent level after 2 years of age.58,59 In normal children the median NT-proBNP (pg/ml) was 3,183 at 0–2 days of age, 2,210 at 3–11 days and 141 at 1–12 months.58 Steady state adult range levels were not achieved until >2 years of age. Additional confusion with the use of BNP is the heterogeneity and lack of acceptance of a standard assay platform, resulting in widely divergent blood concentrations, making it challenging to compare studies from multiple institutions. Assays for NT-proBNP alone vary significantly as the reported mean difference between the Roche and Biomedica assays is 1,649 pg/ml.59 Even with these caveats,58,60 BNP or NT-proBNP levels may be useful in following response to treatment in infants older than 1 month of age and may support diagnosis of PH in neonates without congenital heart disease.17 Discovery projects (e.g., proteomic screening) focused more on the lung and children may yield novel circulating biomarkers with improved diagnostic/prognostic efficacy of disease progression.
In addition, other studies may be useful in assessing factors contributing to PH severity. High resolution CT and CT angiograms performed in conjunction with an experienced pediatric radiologist can be used to assess parenchymal lung injury, pulmonary venous occlusive disease, and pulmonary vein stenosis. Abdominal ultrasound can be used to rule out abdominal malformations and portal vein obstruction that can be associated with PH.50 In addition, as hypoxia and hypercarbia may worsen PH severity, assessing gas exchange in infants with PH and BPD prior to weaning supplemental oxygen may be helpful.11 Office-based pulse-oximetry may not be adequate for all patients; we recently demonstrated that infants with BPD with normal awake oxygen saturations commonly had intermittent oxygen desaturations on overnight polysomnography.61 Further study is needed to assess the risk of intermittent hypoxia/hypercarbia for PH before recommending overnight polysomnography as a more sensitive means of assessment.
MANAGEMENT OF DISEASE
There are no clinical guidelines for the treatment of PH in infants and children with BPD. Basic questions regarding PH management remain unanswered such as whether aggressive pulmonary management and vasodilator therapy attenuate morbidity and mortality. Contributing to the lack of guidelines are the limited number of clinical outcomes studies and the variable response of preterm infants to PH treatments. Over the last several years, there has been increasing off-label use of pulmonary vasodilators in the treatment of PH with little evidence to support their use.18 When pulmonary vasodilator therapies are considered, we believe that they should be administered in consultation with a pediatric cardiologist. Sildenafil has been used in preterm infants with BPD and PH.62–66 A recent study describing the use of sildenafil in infants with BPD and suspected PH reported an improvement in estimated right ventricular peak systolic pressure by echo, but no significant improvement in respiratory scores in the first 48 hr after initiation of sildenafil.63 Another retrospective study of 25 children <2 years of age with chronic lung disease and PH found that 88% had hemodynamic improvement after a median of 40 days of treatment.62
Adequate management of respiratory symptoms is the mainstay of care for all BPD children including those with PH. Supplemental oxygen to maintain baseline oxygen saturations at 95% should be considered in preterm infants with PH and BPD, once the retinal vasculature is mature.7,17,45,67 It is unknown whether BPD therapies such as diuretics, inhaled corticosteroids, chest physiotherapy, and bronchodilators can improve outcomes in BPD children with PH, however it is recommended that ventilation perfusion mismatch be minimized to avoid episodes of hypoxia and hypercarbia. Lastly, good nutrition is needed for lung growth, which may help optimize pulmonary vascular bed growth. In patients with BPD and PH who do not improve with supportive measures, cardiac catheterization should be considered.11,68–75 Cardiac catheterization can help direct medical therapy and it can be used to evaluate and close intracardiac shunts and collateral vessels that can worsen PH in the preterm infant.18
Other management strategies include ruling out or diagnosing conditions that can exacerbate PH in the BPD infant such as upper airway obstructive lesions or lower airway malacia.18 Lower airway symptoms may exacerbate chronic PH; thus causes of lower airway inflammation should be aggressively managed. This includes appropriate immunoprophylaxis against respiratory syncytial virus, influenza, and pertussis.11 An immunological evaluation should be considered in patients with recurrent fever and those exposed to prolonged steroid courses during infancy.76 Aspiration of oral feeds or stomach contents can lead to chronic lung inflammation, which may lead to more severe PH; thus swallowing studies, upper gastrointestinal series, trials of nasogastric or nasoduodenal tube feeding, and antireflux medications should be considered.18 In some cases gastrostomy tube placement and/or Nissen fundoplication may be required. Further research is needed to determine the contribution of orapharyngeal dysfunction to PH severity.
Although lung transplantation can be considered for irreversible PH in patients with BPD, lung transplantation during infancy is associated with increased mortality in the first year post-transplant compared to other pediatric age groups.77 Exciting work however, is currently being done to evaluate the utility of prenatal interventions to attenuate PH. A recent study demonstrated improvement of PH in a rat model of congenital diaphragmatic hernia with the administration of antenatal sildenafil.78 Another study of preterm infants found antenatal steroids to be protective against PH.9
Management of Pulmonary Hypertensive Crises
Pulmonary hypertensive crises may present with increased respiratory effort, increased hypoxemia, and circulatory or respiratory failure, or cardio-pulmonary arrest. These episodes can occur acutely and may require ICU-level care. Triggers most commonly include aspiration, infectious respiratory illnesses,11 or anesthesia for cardiac catheterization or surgery.79 A drop in alveolar oxygen tension may be the common denominator linking these inciting scenarios.80 The ICU management of PH crises requires an integrative approach that focuses on the treatment of increased pulmonary vascular resistance and its impact on right ventricular function. In addition, when present and treatable, intercurrent infectious processes must be addressed as primary inciting causes of life-threatening deterioration. Successful sedation strategies in this setting are essential to control sympathetic outflow and avoid agitation that may interrupt mechanical ventilation during regular care and essential pulmonary toilet. Approaches that balance narcotic analgesia and dexmedetomedine infusions for sedation may be advantageous,81,82 whereas boluses of benzodiazepines and the use of barbiturates may suddenly drop cardiac output and/or systemic vascular resistance. Neuromuscular blockade may be a helpful adjunct. Optimal mechanical ventilation incorporates PEEP and inspiratory times that reestablish and maintain functional residual capacity without regional overdistention that could elevate pulmonary vascular resistance. Mean airway pressures should be kept modest to maximize systemic venous return and preload delivery to the challenged right ventricle. There are limited data on the use of pulmonary vasodilator therapies with acute PH crises in infants with BPD, but agents such as inhaled prostacyclin and nitric oxide, and enteral sildenafil may provide avenues for modulating pulmonary vascular resistance (Fig. 2). Inhaled prostacyclin has been documented to result in a 30% mean drop in the oxygenation index of neonates with PH, albeit in the setting of meconium aspiration.83 In another series, nebulized iloprost was found to be useful in preterm infants with respiratory failure and PH.84 Airway reactivity however may limit the utility of inhaled iloprost in the BPD population. In a study by Ivy et al.,85 22 children with PH were transitioned from IV prostanoids to inhaled iloprost. They found that 38% of children had a 15% drop or greater in FEF25–75% with inhaled iloprost. Bosentan is another vasodilator used to treat PH however its longer length of time to achieve therapeutic levels, may limit its use during an acute hypertensive crisis.80,86 Recently, though a randomized double-blind placebo-controlled study in infants with persistent PH of the newborn reported significant improvement in oxygenation within 6 hr of initiation of bosentan.87 Inotropic support with milrinone may be useful for a patient with right- or bi-ventricular failure following PH crises and/or cardiac arrest that complicates chronic dysfunction of a pressure-loaded right ventricle. In addition, milrinone optimizes cyclic AMP concentrations in the smooth muscle cells of pulmonary resistance vessels by inhibiting type 3 phosphodiesterases.88,89 The use of milrinone may also obviate or minimize the need for adrenergic agents whose deleterious consequences include chronotropic stimulation that may compromise filling time in the setting of a stiff right ventricle, and elevation of both systemic and pulmonary vascular resistance. Use of ECMO may be considered in a subset of cases where there is an identifiable and reversible proximate cause for the acute crisis.83
Anesthesia and the BPD Infant With PH
Infants with BPD with PH are at increased risk for PH crises while under anesthesia or sedation. The incidence of PH-related complications may be higher during cardiac catheterization than during non-cardiac procedures.79 Anesthetic induction, procedural stimulation and emergence from anesthesia may all increase the risk of PH crisis due to physiological changes that can cause hypercarbia, hypoxia, increased catecholamine release, and acidosis.90,91 The overall risk of cardiac arrest in children undergoing anesthesia has been estimated at ~1.4 per 10,000 cases92 and a retrospective review in Denver showed a nearly sixfold increase in risk of perioperative/periprocedural cardiac arrest in children with PH.79 This report further detailed the perioperative risks associated with PH including a 1.3% mortality rate and a 4.5% risk of major complications. Most complications occurred in patients that had suprasystemic pulmonary artery pressures documented preoperatively. Another review of over 100,000 anesthetic cases at a quarternary referral pediatric center reported that fully 50% of anesthetic-related deaths occurred in patients with PH, primarily in infants.91 With careful collaborative preoperative planning that integrates surgical, cardiology, and cardiac anesthesiology expertise, even cardiac catheterization for children with PH can be relatively safe.47 Judicious preoperative sedative premedication can decrease the risk of sympathetic stimulation during anesthetic induction and continuation of vasodilator therapies such as sildenafil and nitric oxide may prevent a PH crisis in the operating room. Management considerations during the critical induction period include the placement of all standard monitors, maintenance of adequate alveolar and arterial oxygenation, ample minute ventilation, protection of systemic blood pressure by careful administration of IV fluids and slow infusion of vasoactive induction agents, and the availability of adequate vascular access and inotropic support.91 For optimal anesthetic maintenance in PH, a balanced technique incorporating inhalational agents, narcotics, and neuromuscular blockade is recommended. Controlled ventilation is indicated in order to reduce hypoventilation and the resultant hypoxia that may occur during spontaneous ventilation, however, adequate anesthetic depth must be ensured prior to placement of an endotracheal tube. Emergence from anesthesia is also challenging, and deep extubation of the trachea may be considered in order to decrease airway stimulation during emergence. Finally, postoperative care should incorporate PH therapy that is compatible with needs for continued NPO status, and some success with pre-emptive perioperative use of inhaled iloprost has been reported.93
CONCLUSIONS
Our understanding of the pathophysiology and management of PH in preterm infants is rapidly evolving. The prognosis remains difficult to predict and given that retrospective studies have demonstrated an increased risk of mortality, frank discussions of the risks associated with PH with families are warranted. At this juncture, comprehensive research strategies are needed to elucidate the pathophysiology of PH and develop guidelines for management. Due to the complexity of these children, we recommend a multidisciplinary approach to help improve outcomes. Equally important is the ongoing education of healthcare providers to recognize infants at risk for developing PH and management strategies for preterm infants with PH.
Acknowledgments
Funding source: none reported
Footnotes
Conflict of interest: None.
REFERENCES
- 1.Viscardi RM. Perinatal inflammation and lung injury. Semin Fetal Neonatal Med. 2012;17:30–35. doi: 10.1016/j.siny.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kallapur SG, Jobe AH. Contribution of inflammation to lung injury and development. Arch Dis Child Fetal Neonatal Ed. 2006;91:F132–F135. doi: 10.1136/adc.2004.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K. Validation of the national institutes of health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 4.Gough A, Spence D, Linden M, Halliday HL, McGarvey L. General and respiratory health outcomes in adult survivors of bronchopulmonary dysplasia: a systematic review. Chest. 2012;141:1554–1567. doi: 10.1378/chest.11-1306. [DOI] [PubMed] [Google Scholar]
- 5.Kim DH, Kim HS, Choi CW, Kim EK, Kim BI, Choi JH. Risk factors for pulmonary artery hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Neonatology. 2012;101:40–46. doi: 10.1159/000327891. [DOI] [PubMed] [Google Scholar]
- 6.An HS, Bae EJ, Kim GB, Kwon BS, Beak JS, Kim EK, Kim HS, Choi JH, Noh CI, Yun YS. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J. 2010;40:131–136. doi: 10.4070/kcj.2010.40.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 8.Kumar VH, Hutchison AA, Lakshminrusimha S, Morin FC, III, Wynn RJ, Ryan RM. Characteristics of pulmonary hypertension in preterm neonates. J Perinatol. 2007;27:214–219. doi: 10.1038/sj.jp.7211673. [DOI] [PubMed] [Google Scholar]
- 9.Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol. 2011;31:635–640. doi: 10.1038/jp.2010.213. [DOI] [PubMed] [Google Scholar]
- 10.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics. 2012;129:e682–e689. doi: 10.1542/peds.2011-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farquhar M, Fitzgerald DA. Pulmonary hypertension in chronic neonatal lung disease. Paediatr Respir Rev. 2010;11:149–153. doi: 10.1016/j.prrv.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Berger RM, Beghetti M, Humpl T, Raskob GE, Ivy DD, Jing ZC, Bonnet D, Schulze-Neick I, Barst RJ. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537–546. doi: 10.1016/S0140-6736(11)61621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerro MJ, Abman S, Diaz G, Freudenthal AH, Freudenthal F, Harikrishnan S, Haworth SG, Ivy D, Lopes AA, Raj JU, et al. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: report from the PVRI pediatric taskforce, Panama 2011. Pulm Circ. 2011;1:286–298. doi: 10.4103/2045-8932.83456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lapaire O, Shennan A, Stepan H. The preeclampsia biomarkers soluble fms-like tyrosine kinase-1 and placental growth factor: current knowledge, clinical implications and future application. Eur J Obstet Gynecol Reprod Biol. 2010;151:122–129. doi: 10.1016/j.ejogrb.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Tang JR, Karumanchi SA, Seedorf G, Markham N, Abman SH. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012;302:L36–L46. doi: 10.1152/ajplung.00294.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li F, Hagaman JR, Kim HS, Maeda N, Jennette JC, Faber JE, Karumanchi SA, Smithies O, Takahashi N. eNOS deficiency acts through endothelin to aggravate sFlt-1-induced pre-eclampsia-like phenotype. J Am Soc Nephrol. 2012;23:652–660. doi: 10.1681/ASN.2011040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim GB. Pulmonary hypertension in infants with bronchopulmonary dysplasia. Korean J Pediatr. 2010;53:688–693. doi: 10.3345/kjp.2010.53.6.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mourani PM, Mullen M, Abman SH. Pulmonary hypertension in bronchopulmonary dysplasia. Progress in Pediatric Cardiology. 2009;27:43–48. [Google Scholar]
- 19.Barst RJ. Classification of pediatric pulmonary hypertensive vascular disease: does it need to be different from the adult classification? Pulm Circ. 2011;1:134–137. doi: 10.4103/2045-8932.83443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abman SH, Ivy DD. Recent progress in understanding pediatric pulmonary hypertension. Curr Opin Pediatr. 2011;23:298–304. doi: 10.1097/MOP.0b013e3283464a52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lum S, Kirkby J, Welsh L, Marlow N, Hennessy E, Stocks J. Nature and severity of lung function abnormalities in extremely pre-term children at 11 years of age. Eur Respir J. 2011;37:1199–1207. doi: 10.1183/09031936.00071110. [DOI] [PubMed] [Google Scholar]
- 22.Baraldi E, Carraro S, Filippone M. Bronchopulmonary dysplasia: definitions and long-term respiratory outcome. Early Hum Dev. 2009;85:S1–S3. doi: 10.1016/j.earlhumdev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Northway WH, Jr, Moss RB, Carlisle KB, Parker BR, Popp RL, Pitlick PT, Eichler I, Lamm RL, Brown BW., Jr Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med. 1990;323:1793–1799. doi: 10.1056/NEJM199012273232603. [DOI] [PubMed] [Google Scholar]
- 24.Yee M, White RJ, Awad HA, Bates WA, McGrath-Morrow SA, O’Reilly MA. Neonatal hyperoxia causes pulmonary vascular disease and shortens life span in aging mice. Am J Pathol. 2011;178:2601–2610. doi: 10.1016/j.ajpath.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jobe AH. Blood cytokines and BPD. J Pediatr. 2009;154:A2. doi: 10.1016/j.jpeds.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Speer CP. New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol Neonate. 2001;79:205–209. doi: 10.1159/000047092. [DOI] [PubMed] [Google Scholar]
- 27.Speer CP. Inflammation and bronchopulmonary dysplasia. Semin Neonatol. 2003;8:29–38. doi: 10.1016/s1084-2756(02)00190-2. [DOI] [PubMed] [Google Scholar]
- 28.Speer CP. Pulmonary inflammation and bronchopulmonary dysplasia. J Perinatol. 2006;26:S57–S62. doi: 10.1038/sj.jp.7211476. [DOI] [PubMed] [Google Scholar]
- 29.Bagchi A, Viscardi RM, Taciak V, Ensor JE, McCrea KA, Hasday JD. Increased activity of interleukin-6 but not tumor necrosis factor-alpha in lung lavage of premature infants is associated with the development of bronchopulmonary dysplasia. Pediatr Res. 1994;36:244–252. doi: 10.1203/00006450-199408000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Case J, Ingram DA, Haneline LS. Oxidative stress impairs endothelial progenitor cell function. Antioxid Redox Signal. 2008;10:1895–1907. doi: 10.1089/ars.2008.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JW, Davis JM. Future applications of antioxidants in premature infants. Curr Opin Pediatr. 2011;23:161–166. doi: 10.1097/MOP.0b013e3283423e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGrath-Morrow S, Lauer T, Yee M, Neptune E, Podowski M, Thimmulappa RK, O’Reilly M, Biswal S. Nrf2 increases survival and attenuates alveolar growth inhibition in neonatal mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2009;296:L565–L573. doi: 10.1152/ajplung.90487.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balasubramaniam V, Maxey AM, Fouty BW, Abman SH. Nitric oxide augments fetal pulmonary artery endothelial cell angiogenesis in vitro. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1111–L1116. doi: 10.1152/ajplung.00431.2005. [DOI] [PubMed] [Google Scholar]
- 34.Askie LM, Ballard RA, Cutter GR, Dani C, Elbourne D, Field D, Hascoet JM, Hibbs AM, Kinsella JP, Mercier JC, et al. Inhaled nitric oxide in preterm infants: an individual-patient data meta-analysis of randomized trials. Pediatrics. 2011;128:729–739. doi: 10.1542/peds.2010-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donohue PK, Gilmore MM, Cristofalo E, Wilson RF, Weiner JZ, Lau BD, Robinson KA, Allen MC. Inhaled nitric oxide in preterm infants: a systematic review. Pediatrics. 2011;127:e414–e422. doi: 10.1542/peds.2010-3428. [DOI] [PubMed] [Google Scholar]
- 36.Ryoo S, Lemmon CA, Soucy KG, Gupta G, White AR, Nyhan D, Shoukas A, Romer LH, Berkowitz DE. Oxidized low-density lipoprotein-dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circ Res. 2006;99:951–960. doi: 10.1161/01.RES.0000247034.24662.b4. [DOI] [PubMed] [Google Scholar]
- 37.Vadivel A, Aschner JL, Rey-Parra GJ, Magarik J, Zeng H, Summar M, Eaton F, Thébaud B. L-citrulline attenuates arrested alveolar growth and pulmonary hypertension in oxygen-induced lung injury in newborn rats. Pediatr Res. 2010;68:519–525. doi: 10.1203/PDR.0b013e3181f90278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, Ment LR, Gruen JR Neonatal Genetics Study Group. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006;117:1901–1906. doi: 10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 39.Lavoie PM, Pham C, Jang KL. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics. 2008;122:479–485. doi: 10.1542/peds.2007-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryckman KK, Dagle JM, Kelsey K, Momany AM, Murray JC. Genetic associations of surfactant protein D and angiotensin-converting enzyme with lung disease in preterm neonates. J Perinatol. 2011;32:349–355. doi: 10.1038/jp.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sampath V, Garland JS, Le M, Patel AL, Konduri GG, Cohen JD, Simpson PM, Hines RN. A TLR5 (g.1174C>T) variant that encodes a stop codon (R392X) is associated with bronchopulmonary dysplasia. Pediatr Pulmonol. 2011;117:1901–1906. doi: 10.1002/ppul.21568. [DOI] [PubMed] [Google Scholar]
- 42.Hadchouel A, Durrmeyer X, Bouzigon E, Incitti R, Huusko J, Jarreau PH, Lenclen R, Demenais F, Franco-Montoya ML, Layouni I, et al. Identification of SPOCK2 as a susceptibility gene for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2011;184:1164–1170. doi: 10.1164/rccm.201103-0548OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koukoura O, Sifakis S, Spandidos DA. DNA methylation in the human placenta and fetal growth (Review) Mol Med Report. 2012;5:883–889. doi: 10.3892/mmr.2012.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M, Riddle SR, Frid MG, El Kasmi KC, McKinsey TA, Sokol RJ, Strassheim D, Meyrick B, Yeager ME, Flockton AR, et al. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J Immunol. 2011;187:2711–2722. doi: 10.4049/jimmunol.1100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhillon R. The management of neonatal pulmonary hypertension. Arch Dis Child Fetal Neonatal Ed. 2012;97:F223–F228. doi: 10.1136/adc.2009.180091. [DOI] [PubMed] [Google Scholar]
- 46.Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, Reeder GS, Nishimura RA, Tajik AJ. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6:750–756. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 47.Hill KD, Lim DS, Everett AD, Ivy DD, Moore JD. Assessment of pulmonary hypertension in the pediatric catheterization laboratory: current insights from the magic registry. Catheter Cardiovasc Interv. 2010;76:865–873. doi: 10.1002/ccd.22693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008;121:317–325. doi: 10.1542/peds.2007-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drossner DM, Kim DW, Maher KO, Mahle WT. Pulmonary vein stenosis: prematurity and associated conditions. Pediatrics. 2008;122:e656–e661. doi: 10.1542/peds.2008-0075. [DOI] [PubMed] [Google Scholar]
- 50.Mullen MP. Diagnostic strategies for acute presentation of pulmonary hypertension in children: particular focus on use of echocardiography, cardiac catheterization, magnetic resonance imaging, chest computed tomography, and lung biopsy. Pediatr Crit Care Med. 2010;11:S23–S26. doi: 10.1097/PCC.0b013e3181c7683a. [DOI] [PubMed] [Google Scholar]
- 51.Barst RJ, McGoon MD, Elliott CG, Foreman AJ, Miller DP, Ivy DD. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation. 2012;125:113–122. doi: 10.1161/CIRCULATIONAHA.111.026591. [DOI] [PubMed] [Google Scholar]
- 52.Mourani PM, Mullen M, Abman SH. Pulmonary hypertension in bronchopulmonary dysplasia. Prog Pediatr Cardiol. 2009;27:43–48. [Google Scholar]
- 53.Taylor CJ, Derrick G, McEwan A, Haworth SG, Sury MR. Risk of cardiac catheterization under anaesthesia in children with pulmonary hypertension. Br J Anaesth. 2007;98:657–661. doi: 10.1093/bja/aem059. [DOI] [PubMed] [Google Scholar]
- 54.Lorenzen JM, Nickel N, Kramer R, Golpon H, Westerkamp V, Olsson KM, Haller H, Hoeper MM. Osteopontin in patients with idiopathic pulmonary hypertension. Chest. 2011;139:1010–1017. doi: 10.1378/chest.10-1146. [DOI] [PubMed] [Google Scholar]
- 55.Goto K, Arai M, Watanabe A, Hasegawa A, Nakano A, Kurabayashi M. Utility of echocardiography versus BNP level for the prediction of pulmonary arterial pressure in patients with pulmonary arterial hypertension. Int Heart J. 2010;51:343–347. doi: 10.1536/ihj.51.343. [DOI] [PubMed] [Google Scholar]
- 56.Andreassen AK, Wergeland R, Simonsen S, Geiran O, Guevara C, Ueland T. N-terminal pro-B-type natriuretic peptide as an indicator of disease severity in a heterogeneous group of patients with chronic precapillary pulmonary hypertension. Am J Cardiol. 2006;98:525–529. doi: 10.1016/j.amjcard.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 57.Mauritz GJ, Rizopoulos D, Groepenhoff H, Tiede H, Felix J, Eilers P, Bosboom J, Postmus PE, Westerhof N, Vonk-Noordegraaf A. Usefulness of serial N-terminal pro-B-type natriuretic peptide measurements for determining prognosis in patients with pulmonary arterial hypertension. Am J Cardiol. 2011;108:1645–1650. doi: 10.1016/j.amjcard.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 58.Nir A, Lindinger A, Rauh M, Bar-Oz B, Laer S, Schwachtgen L, Koch A, Falkenberg J, Mir TS. NT-pro-B-type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr Cardiol. 2009;30:3–8. doi: 10.1007/s00246-008-9258-4. [DOI] [PubMed] [Google Scholar]
- 59.Albers S, Mir TS, Haddad M, Laer S. N-Terminal pro-brain natriuretic peptide: normal ranges in the pediatric population including method comparison and interlaboratory variability. Clin Chem Lab Med. 2006;44:80–85. doi: 10.1515/CCLM.2006.016. [DOI] [PubMed] [Google Scholar]
- 60.Joseph L, Nir A, Hammerman C, Goldberg S, Ben SE, Picard E. N-terminal pro-B-type natriuretic peptide as a marker of bronchopulmonary dysplasia in premature infants. Am J Perinatol. 2010;27:381–386. doi: 10.1055/s-0029-1243312. [DOI] [PubMed] [Google Scholar]
- 61.McGrath-Morrow SA, Ryan T, McGinley BM, Okelo SO, Sterni LM, Collaco JM. Polysomnography in preterm infants and children with chronic lung disease. Pediatr Pulmonol. 2012;47:172–179. doi: 10.1002/ppul.21522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of longterm sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr. 2009;154:379–384. doi: 10.1016/j.jpeds.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nyp M, Sandritter T, Poppinga N, Simon C, Truog WE. Sildenafil citrate, bronchopulmonary dysplasia and disordered pulmonary gas exchange: any benefits? J Perinatol. 2012;32:64–69. doi: 10.1038/jp.2011.131. [DOI] [PubMed] [Google Scholar]
- 64.Farrow KN, Steinhorn RH. Phosphodiesterases: emerging therapeutic targets for neonatal pulmonary hypertension. Handb Exp Pharmacol. 2011;204:251–277. doi: 10.1007/978-3-642-17969-3_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caputo S, Furcolo G, Rabuano R, Basilicata AM, Pilla LM, De Simone A, Pasquariello B, Ciampi Q, Vetrano G, Villari B. Severe pulmonary arterial hypertension in a very premature baby with bronchopulmonary dysplasia: normalization with long-term sildenafil. J Cardiovasc Med (Hagerstown) 2010;11:704–706. doi: 10.2459/JCM.0b013e328332e745. [DOI] [PubMed] [Google Scholar]
- 66.Hon KL, Cheung KL, Siu KL, Leung TF, Yam MC, Fok TF, Ng PC. Oral sildenafil for treatment of severe pulmonary hypertension in an infant. Biol Neonate. 2005;88:109–112. doi: 10.1159/000085646. [DOI] [PubMed] [Google Scholar]
- 67.Abman SH. Monitoring cardiovascular function in infants with chronic lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. 2002;87:F15–F18. doi: 10.1136/fn.87.1.F15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bell SG. Bosentan. Neonatal Netw. 2011;30:249–251. doi: 10.1891/0730-0832.30.4.249. [DOI] [PubMed] [Google Scholar]
- 69.Eifinger F, Sreeram N, Mehler K, Huenseler C, Kribs A, Roth B. Aerosolized iloprost in the treatment of pulmonary hypertension in extremely preterm infants: a pilot study. Klin Padiatr. 2008;220:66–69. doi: 10.1055/s-2007-984370. [DOI] [PubMed] [Google Scholar]
- 70.Gurakan B, Kayiran P, Ozturk N, Kayiran SM, Dindar A. Therapeutic combination of sildenafil and iloprost in a preterm neonate with pulmonary hypertension. Pediatr Pulmonol. 2011;46:617–620. doi: 10.1002/ppul.21415. [DOI] [PubMed] [Google Scholar]
- 71.Hwang SK, YC O, Kim NS, Park HK, Yum MK. Use of inhaled iloprost in an infant with bronchopulmonary dysplasia and pulmonary artery hypertension. Korean Circ J. 2009;39:343–345. doi: 10.4070/kcj.2009.39.8.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krishnan U, Krishnan S, Gewitz M. Treatment of pulmonary hypertension in children with chronic lung disease with newer oral therapies. Pediatr Cardiol. 2008;29:1082–1086. doi: 10.1007/s00246-008-9260-x. [DOI] [PubMed] [Google Scholar]
- 73.Radicioni M, Bruni A, Camerini P. Combination therapy for life-threatening pulmonary hypertension in a premature infant: first report on bosentan use. Eur J Pediatr. 2011;170:1075–1078. doi: 10.1007/s00431-011-1422-9. [DOI] [PubMed] [Google Scholar]
- 74.Rosati E, Butera G, Bossone E, De FC, Latini G. Inhaled nitric oxide and oral nifedipine in a preterm infant with bronchopulmonary dysplasia and pulmonary hypertension. Eur J Pediatr. 2007;166:737–738. doi: 10.1007/s00431-006-0292-z. [DOI] [PubMed] [Google Scholar]
- 75.Zaidi AN, Dettorre MD, Ceneviva GD, Thomas NJ. Epoprostenol and home mechanical ventilation for pulmonary hypertension associated with chronic lung disease. Pediatr Pulmonol. 2005;40:265–269. doi: 10.1002/ppul.20238. [DOI] [PubMed] [Google Scholar]
- 76.Lederman HM, Metz SJ, Zuckerberg AL, Loughlin GM. Antibody deficiency complicating severe bronchopulmonary dysplasia. Pediatr Pulmonol. 1989;7:52–54. doi: 10.1002/ppul.1950070112. [DOI] [PubMed] [Google Scholar]
- 77.Benden C, Aurora P, Edwards LB, Kucheryavaya AY, Christie JD, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI. the registry of the international society for heart and lung transplantation: fourteenth pediatric lung and heart–lung transplantation report—2011. J Heart Lung Transplant. 2011;30:1123–1132. doi: 10.1016/j.healun.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 78.Luong C, Rey-Perra J, Vadivel A, Gilmour G, Sauve Y, Koonen D, Walker D, Todd KG, Gressens P, Kassiri Z, et al. Antenatal sildenafil treatment attenuates pulmonary hypertension in experimental congenital diaphragmatic hernia. Circulation. 2011;123:2120–2131. doi: 10.1161/CIRCULATIONAHA.108.845909. [DOI] [PubMed] [Google Scholar]
- 79.Carmosino MJ, Friesen RH, Doran A, Ivy DD. Perioperative complications in children with pulmonary hypertension undergoing noncardiac surgery or cardiac catheterization. Anesth Analg. 2007;104:521–527. doi: 10.1213/01.ane.0000255732.16057.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Friesen RH, Williams GD. Anesthetic management of children with pulmonary arterial hypertension. Paediatr Anaesth. 2008;18:208–216. doi: 10.1111/j.1460-9592.2008.02419.x. [DOI] [PubMed] [Google Scholar]
- 81.Diaz LK, Jones L. Sedating the child with congenital heart disease. Anesthesiol Clin. 2009;27:301–319. doi: 10.1016/j.anclin.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 82.Tobias JD, Gupta P, Naguib A, Yates AR. Dexmedetomidine: applications for the pediatric patient with congenital heart disease. Pediatr Cardiol. 2011;32:1075–1087. doi: 10.1007/s00246-011-0092-8. [DOI] [PubMed] [Google Scholar]
- 83.Brown AT, Gillespie JV, Miquel-Verges F, Holmes K, Ravekes W, Spevak P, Brady K, Easley RB, Golden WC, McNamara L, et al. Inhaled epoprostenol therapy for pulmonary hypertension: improves oxygenation index more consistently in neonates than in older children. Pulmonary Circulation. 2012;2:61–66. doi: 10.4103/2045-8932.94835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piastra M, De LD, De Carolis MP, Tempera A, Stival E, Caliandro F, Pietrini D, Conti G, De Rosa G. Nebulized iloprost and noninvasive respiratory support for impending hypoxaemic respiratory failure in formerly preterm infants: a case series. Pediatr Pulmonol. 2011 doi: 10.1002/ppul.21619. [DOI] [PubMed] [Google Scholar]
- 85.Ivy DD, Doran AK, Smith KJ, Mallory GB, Jr, Beghetti M, Barst RJ, Brady D, Law Y, Parker D, Claussen L, et al. Short-and long-term effects of inhaled iloprost therapy in children with pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:161–169. doi: 10.1016/j.jacc.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Humbert M, Barst RJ, Robbins IM, Channick RN, Galiè N, Boonstra A, Rubin LJ, Horn EM, Manes A, Simonneau G. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J. 2004;24:353–359. doi: 10.1183/09031936.04.00028404. [DOI] [PubMed] [Google Scholar]
- 87.Mohamed WA, Ismail M. A randomized, double-blind, placebo-controlled, prospective study of bosentan for the treatment of persistent pulmonary hypertension of the newborn. J Perinatol. 2011 doi: 10.1038/jp.2011.157. [DOI] [PubMed] [Google Scholar]
- 88.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sehgal A, Athikarisamy SE, Adamopoulos M. Global myocardial function is compromised in infants with pulmonary hypertension. Acta Paediatr. 2012;101:410–413. doi: 10.1111/j.1651-2227.2011.02572.x. [DOI] [PubMed] [Google Scholar]
- 90.Shukla AC, Almodovar MC. Anesthesia considerations for children with pulmonary hypertension. Pediatr Crit Care Med. 2010;11:S70–S73. doi: 10.1097/PCC.0b013e3181c76c6e. [DOI] [PubMed] [Google Scholar]
- 91.van der Griend BF, Lister NA, McKenzie IM, Martin N, Ragg PG, Sheppard SJ, Davidson AJ. Postoperative mortality in children after 101,885 anesthetics at a tertiary pediatric hospital. Anesth Analg. 2011;112:1440–1447. doi: 10.1213/ANE.0b013e318213be52. [DOI] [PubMed] [Google Scholar]
- 92.Morray JP, Geiduschek JM, Ramamoorthy C, Haberkern CM, Hackel A, Caplan RA, Domino KB, Posner K, Cheney FW. Anesthesia-related cardiac arrest in children: initial findings of the pediatric perioperative cardiac arrest (POCA) registry. Anesthesiology. 2000;93:6–14. doi: 10.1097/00000542-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 93.Gorenflo M, Gu H, Xu Z. Peri-operative pulmonary hypertension in paediatric patients: current strategies in children with congenital heart disease. Cardiology. 2010;116:10–17. doi: 10.1159/000313864. [DOI] [PubMed] [Google Scholar]