Abstract
A novel class of 6-indolypyridine-3-carbonitrilile derivatives were synthesized and evaluated for antiproliferative activities to establish structure-activity relationship. The synthesis was carried out through one-pot multicomponent reaction of 3-acetylindole, aromatic aldehydes, ethyl cyanoacetate, and ammonium acetate in the presence of piperidine as a catalyst, using a microwave irradiation method or a traditional thermal method. This was followed by chlorination for compounds 13a–e and subsequent nucleophilic substitution of the chlorine group by ethylenediamine at C2 position of the pyridine ring. The antiproliferative activity of these new nicotinonitriles was evaluated against human ovarian adenocarcinoma (SK-OV-3), breast adenocarcinoma (MCF-7), and cervix adenocarcinoma (HeLa) cells. Among all compounds, 2-((2-aminoethyl)amino)-4-aryl-6-indolylnicotinonitriles series (15a, 15b, 15d, and 15e) exhibited higher antiproliferative activity cells with IC50 values of 4.1–13.4 μM.
Keywords: Antiproliferative agents, Indole, Indolyl carbonitriles, Microwave-assisted Synthesis, Multicomponent Reactions
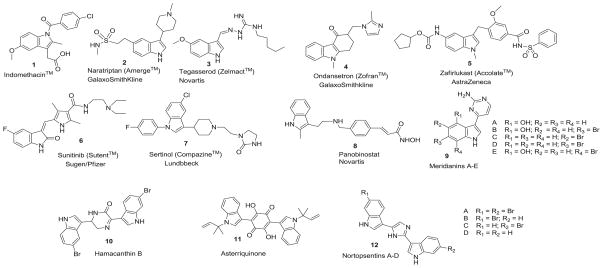
3-Substitued indolyl moiety is a basic constituent in numerous proteins, the neurotransmitter serotonin, and mammalian hormone melatonin as well as a large number of marketed available pharmaceutical drugs. For instance, indomethacin (1), naratriptan (2), tegaserod (3), ondansetron (4), zafirlukast (5), sunitinib (6), sertinole (7), and panobinostat (8) (Figure 1) contain 3-substituted indole scaffold in their chemical structures.1
Figure 1.
Common biologically active 3-substituted indolyl derivatives.
Additionally, several marine indole alkaloids have been isolated and evaluated for their anticancer, antiviral, and antiinflammatory activities. Meridianins A–E (9) were isolated from tunicate Aplidium Meridianm.2,3 Bisindolyl alkaloids spaced by five or six membered heterocyclic moieties, such as piperazinone (Hamacanthin B, 10), quinone (Asterriquinone, 11), or imidazole (Nortopsentins A–C, 12) (Figure 1) have exhibited modest to high anticancer activities against a wide range of human cell lines at micromolar concentrations.4–6
Furthermore, nicotinonitrile skeletons especially those with amino substituent at C2 and/or 4,6-diaryl-substitutent have demonstrated broad range of biological activities, such as antibacterial,8, antifungal9, antituberculosis10, antiviral11, antipyretic, analgesic, and antiinflammatory12–14 effects.
Furthermore, they have been used as inhibitors for protein kinase, topoisomerase15,16, phosphodiesterase17,18 as well as antiproliferative agents for the treatment of a number of human cancer cell lines.19–21
The application of one-pot multicomponent reactions (MCRs) and microwave-assisted have been demonstrated to offer smooth reaction conditions and higher overall yield when compared to classical synthesis methodologies.22–28 Bis(3′-indolyl)pyridine and pyrazolopyridine-indolyl derivatives have been previously synthesized through MCR and/or microwave assisted reactions29a.b according to the previously reported procedure29c.
In continuation of our efforts to synthesize and evaluate new indole derivatives as antiproliferative agents,30 we designed novel indole-3-cyanopyridine hybrid structures to determine the substituent effects at C2 and C4 on the cytotoxic potency of this scaffold. Although other heterocyclic indolyl derivatives have been previously synthesized,29 To the best of our knowledge this is the first microwave-assisted synthesis of hybrid indole and 3-cyano-4-arylsubstituted pyridine compounds and evaluation of their antiproliferative activities.
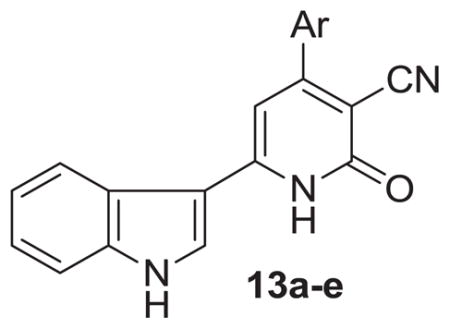
Considering the advantages of MCRs approach and microwave irradiation, 3-acetyl indole ketone reacted with ethyl cyanoacetate and a series of aromatic aldehydes with an excess of ammonium acetate under microwave irradiation for 15–20 min affording novel 4-aryl-6-indolyl-nicotinonitrile-2-one derivatives (13a–e) (Scheme 1). All compounds were characterized by mass, and NMR spectroscopy (Supplementary Material).
Scheme 1.
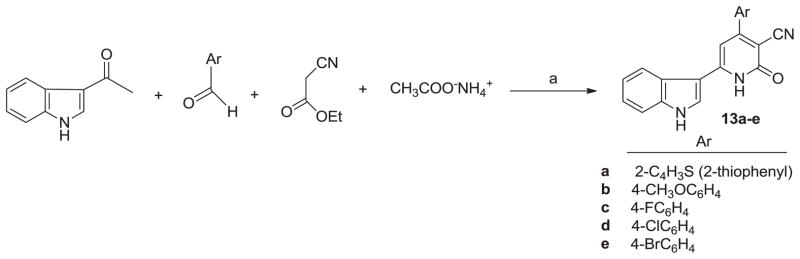
Synthesis of indolylnicotinonitriles (13a–e). Reagents and conditions: (a) piperidine (1 mL), ethylene glycol (1 mL), MW irradiation (W 250 and T 150 °C).
Ethylene glycol and piperidine were used as a solvent and a catalyst, respectively, during the microwave reaction at a power of 250 W and 150 °C for a given time. The reactions were successful in achieving the benefits of both utilizing microwave irradiation and the one-pot MCRs. Compared to the traditional thermal method, the reaction time was shortened from hours to minutes with improvement in both the target product purity and overall product yield (77–87%) in case of microwave method. The results for each entry are summarized in (Table 1).
Table 1.
Comparative synthesis of 2-oxo-1,2-dihydropyridine-3-carbonitrile derivatives (13a–e) by microwave irradiation and thermal heating.
| ||||||
|---|---|---|---|---|---|---|
| product | Ar | Time | Yieldc (%) | mp (°C) | ||
|
| ||||||
| MWa (min) | Thb (h) | MWa | Thb | |||
| 13a | 2-C4H3S | 20 | 17 | 77 | 44 | >300 |
| 13b | 4-OCH3C6H4 | 20 | 18 | 79 | 45 | >300 |
| 13c | 4-FC6H4 | 15 | 10 | 87 | 56 | >300 |
| 13d | 4-ClC6H4 | 17 | 15 | 82 | 63 | >300 |
| 13e | 4-BrC6H4 | 17 | 14 | 83 | 61 | >300 |
The reaction was carried out by microwave irradiation at 250 W and 150 °C;
The reaction was carried out by thermal heating at 150 °C in oil bath;
Isolated yields.
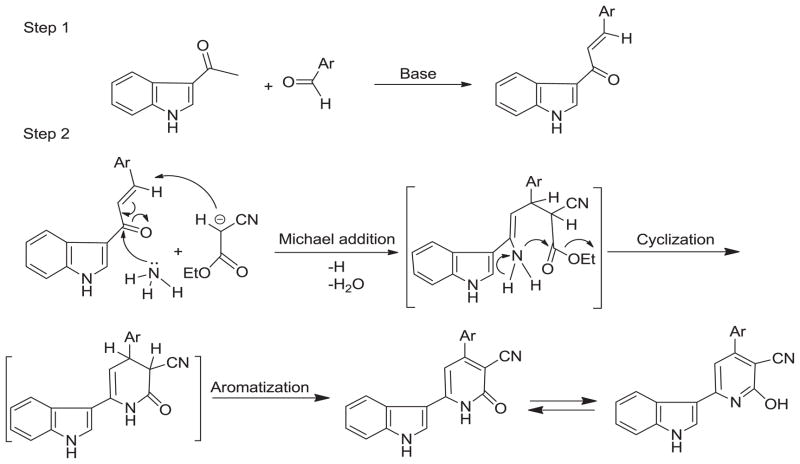
The mechanism of one pot syntheses of nicotinonitrile derivatives is known to be through the formation of α,β-unsaturated ketones intermediate via Claisen-Schimdt reaction between active methylene containing ketones and aromatic aldehydes using catalytic amount (10%) of strong bases like sodium hydroxide, triethylamine, or piperidine. This reaction is followed by condensation with nitrile containing active methylene compounds (e.g. ethyl cyanoacetate or malononitrile) through Michael addition reaction in the presence of ammonium acetate, cyclization, and aromatization to afford the corresponding 4-aryl-2-oxo-1H-pyridine-3-carbonitrile derivatives31 (Scheme 2).
Scheme 2.
Mechanistic illustration for the formation of 2-oxo-1,2-dihydropyridine-3-carbonitrile system.
Results in Table 1 showed that, the electronic effect and the nature of the substituent on the aromatic aldehyde ring played a critical effect in terms of reaction time and product yield under smilar reaction conditions. When aromatic aldehydes bearing a strong electron withdrawing group (e.g. 4-fluorine, 4-chloro, 4-bromo) in para positions was used, the yield of the products was increased in a shorter reaction time compared to those carrying electron donating groups (e.g. 4-methoxy group) in para position under a similar reaction condition.
Moreover, the reaction of compounds 13a–e with phosphoryl chloride for 18–24 h afforded the corresponding 2-chloropyridine derivatives (14a–e) after thermal heating at 80 °C as shown in Scheme 3. 2-Chloropyridine derivatives (14a–e) were used as precursors for nucleophilic substitution reaction with ethylenediamine under a reflux condition in ethanol to afford the corresponding 2-aminoethylenamino 6-indolylnicotinonitrile derivatives (15a–e), The chemical structures of these novel compounds 14a–e and 15a–e were elucidated by IR, mass, and NMR spectroscopy (see Supplementary Material).
Scheme 3.
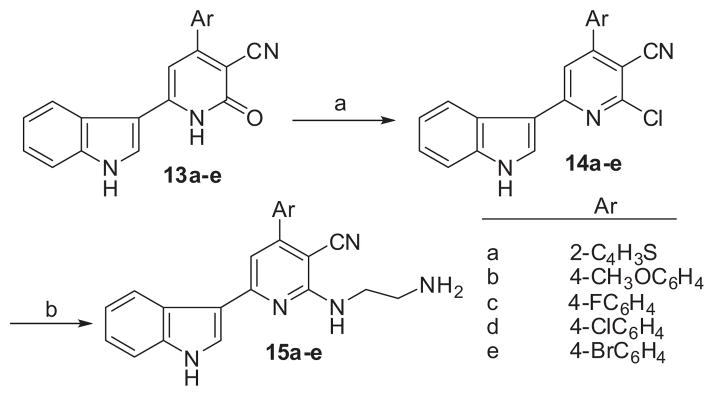
Reagents and conditions: (a) POCl3, reflux, 80 °C for 18–24 h; (b) ethylenediamine, ethanol, reflux, 36–48 h.
The antiproliferative activities of all synthesized compounds in a panel of cancer cell lines including human ovarian adenocarcinoma (SK-OV-3), breast adenocarcinoma (MCF-7), and cervix adenocarcinoma (HeLa) cells were evaluated. All compounds (50 μM) were tested for their anticancer potency after 72 h incubation. DMSO (3%) and doxorubicin (Dox 10 μM) were used as negative and positive controls for the assay.
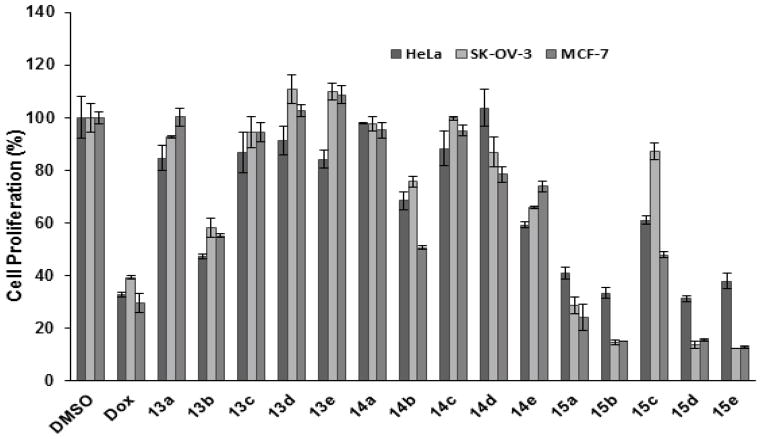
As it is shown in Figure 2, compounds 13a, 13c, 13d, 13e, 14a, 14c, and 14d did not show any significant antiproliferative activity against HeLa, SK-OV-3, and MCF-7 cells. Among all derivatives, compounds 13b, 14b, and 15a–e showed modest to high antiproliferative potency. However, compounds 15b, 15d, and 15e showed comparable potency with that of Dox in HeLa cells and significantly higher potency in SK-OV-3 and MCF-7 cells versus Dox. For example, compounds 15b, 15d, and 15e inhibited the proliferation of HeLa, SK-OV, and MCF-7 cells by 62–67%, 85–88%, and 84–87%. Interestingly, these three compounds inhibited the cell proliferation of SK-OV-3 and MCF-7 cells with higher potency compared to that of HeLa cells, indicating that their activity was cell-specific.
Figure 2.
Antiproliferative activity of 13a–e, 14a–e, and 15a–e.
All synthesized compounds have a common scaffold of conjugated substituted 6-indolyl pyridine ring. Compounds 15a–e also have an ethylene-1,2-diamine moiety attached to the substituted pyridine ring. Changing the substation at C2 from oxo (compounds 13a–e) to ethylene-1,2-diamine (compounds 15a–e) showed that, an ethylene-1,2-diamine moiety plays a significant role in elevating the anti-proliferative activity. However, among indolyl nicotinonitrile (15a–e), compound 15c with p-fluorophenyl substituent at C4 did not show similar potency when compared with the other compounds in this series, suggesting that the presence of a strong electron withdrawing fluorine group is not productive. On the other hand, the presence of a heterocyclic ring as in compound 15a or an electron donating group like p-methoxy group as in compound 15b resulted in higher antiproliferative activity. Thus, electronic effect of the substituent of the phenyl group substituent appears to have a direct effect on antiproliferative activity.
Based on the results from the preliminary screening, compounds 15a, 15b, 15d, and 15e were selected for further IC50 evaluation. IC50 is the concentration that causes 50% inhibition of cancer cell growth. The IC50 values of 15a, 15b, 15d, and 15e derivatives were tested in HeLa, SK-OV-3, and MCF-7 cells (Table 2). As it is shown in the IC50 graphs (Figure S1, Supplementary Material), all these four derivatives showed high potency in the inhibition of the proliferation of different cancer cells. The IC50 values of compounds 15a, 15b, 15d, and 15e were in the range of 4.1–13.4 μM, 6.5–8.1 μM, 5.9–7.1 μM, and 5.8–8.8 μM, respectively, in HeLa, SK-OV-3, and MCF-7 cells. Compounds 15a and 15e showed slightly lower IC50 values in MCF-7 and SK-OV-3 cells compared to the other compounds. The partition coefficient (Log P) of all the synthesized compounds were calculated by using ChemDraw 10.0 (Supplementary information, Table S1). The data revealed that the compounds 15a–e with moderate Log P values of 3.23–4.19 showed significantly higher antiproliferative activity compared to other compounds possibly because of higher cellular uptake of these compounds).32 Compounds 13a–e with low Log P values (2.43–3.28) did not show high antiproliferative activity, while compounds 14a–e with high lipophilicity (Log P = 4.82–5.77) showed moderate activity. These data indicate that there is a correlation between the partition coefficient and antiproliferative activity of these compounds, and an optimal Log P is required for generating maximum activity.
Table 2.
IC50 values of four selected compounds in SK-OV-3, MCF-7, and HeLa cells.
| Entry | HeLaa | SK-OV-3 | MCF-7 |
|---|---|---|---|
| 15a | 13.4 | 4.7 | 4.1 |
| 15b | 7.2 | 6.5 | 8.1 |
| 15d | 6.8 | 5.9 | 7.1 |
| 15e | 8.8 | 5.8 | 6.8 |
| Dox | 0.1533a | 3.2 | 7.533 b |
The IC50 values of compounds were calculated in μM. The data are average of triplicate experiments.
In conclusion, we have demonstrated a facile and efficient method for the preparation of a new series of 6-indolypyridine-3-carbonitrile derivatives via the one-pot MCR with the microwave-assisted irradiation affording high yields, short reaction times, and the easy workup procedure. Among all compounds, 2-((2-aminoethyl)amino)-4-aryl-6-indolylnicotinonitrile series (15a, 15b, 15d, and 15e) exhibited higher antiproliferative activity than Dox against SK-OV-3, MCF-7, and HeLa cells. These data suggest that indolylnicotinonitriles chemical scaffold can be used as a template for further structure optimization for generating compounds with higher antiproliferative activity.
Supplementary Material
Acknowledgments
We thank the financial support from the American Cancer Society Grant # RSG-07-290-01-CDD, National Research Cancer (Dokki, Giza, Egypt), and the Cultural Affairs and Mission Sector, Ministry of Higher Education, Egypt) for the financial support. We also thank the National Center for Research Resources, NIH, and Grant Number 8 P20 GM103430-12 for sponsoring the core facility. The authors would like to thank Dr. Brenton DeBoef for providing the microwave facility.
Footnotes
Supplementary data associated with this article can be found in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Wu Y-J. Top Heterocycl Chem. Vol. 26. Springer-Verlag; Berlin Heidelberg: 2010. pp. 1–29. [Google Scholar]
- 2.Franco LH, Joffe EBK, Puricelli L, Tatian M, Seldes, Palermo AM, JA J Nat Prod. 1998;61:1130–2. doi: 10.1021/np970493u. [DOI] [PubMed] [Google Scholar]
- 3.Radwan MAA, El-Sherbiny M. Bioorg Med Chem. 2007;14:1206–11. doi: 10.1016/j.bmc.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Sakemi S, Sun HH. J Org Chem. 1991;56:4304–7. [Google Scholar]
- 5.Gunasekera SP, McCarthy PJ, Borges MK. J Nat Prod. 1994;57:1437–41. doi: 10.1021/np50112a014. [DOI] [PubMed] [Google Scholar]
- 6.Wijeratne EMK, Turbyville TJ, Zhang Z, Bigelow D, Pierson LS, VanEtten HD, Whitesell L, Canfield LM, Gunatilaka AAL. J Nat Prod. 2003;66:1567–73. doi: 10.1021/np030266u. [DOI] [PubMed] [Google Scholar]
- 7.Bao B, Sun Q, Yao X, Hong J, Lee CO, Sim CJ, Im KS, Jung JH. J Nat Prod. 2005;68:711–5. doi: 10.1021/np049577a. [DOI] [PubMed] [Google Scholar]
- 8.Khidre RE, Abu-Hashem AA, El-Shazly M. Eur J Med Chem. 2011;46:5057–64. doi: 10.1016/j.ejmech.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Gholap AR, Toti KS, Shirazi FR, Kumari MK, Bhat MV, Deshpande, Srinivasan KV. Bioorg Med Chem. 2007;15:6705–15. doi: 10.1016/j.bmc.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Manikannan R, Muthusubramanian S, Yogeeswari P, Sriram D. Bioorg Med Chem Lett. 2010;20:3352–5. doi: 10.1016/j.bmcl.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim ES, Elgemeie GEH, Abbasi MM, Abbas YA, Elbadawi MA, Attia AME. Nucleosides and Nucleotides. 1995;14:1415–23. [Google Scholar]
- 12.Manna F, Chimenti F, Bolasco A, Filippelli A, Palla A, Filippelli W, Lampa E, Mercantin R. Eur J Med Chem. 1992;27:627–32. [Google Scholar]
- 13.Hamdy NA, Gamal-Eldeen AM. Eur J Med Chem. 2009;44:4547–56. doi: 10.1016/j.ejmech.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Murata T, Shimada M, Sakakibara S, Yoshino T, Masuda T, Shintani T, Sato H, Koriyama Y, Fukushima K, Nunami N, Yamauchi, Fuchikami M, Komura KH, Watanabe A, Ziegelbauer KB, Bacon KB, Lowinger TB. Bioorg Med Chem Lett. 2004;14:4019–22. doi: 10.1016/j.bmcl.2004.05.041. [DOI] [PubMed] [Google Scholar]
- 15.Zhao L-X, Moon Y-S, Kim AB, Ek, Jahng Y, Park JG, Jeong TC, Cho W-J, Choi SU, Lee CO, Lee SY, Lee CS, Lee ES. Bioorg Med Chem Lett. 2004;14:1333–7. doi: 10.1016/j.bmcl.2003.11.084. [DOI] [PubMed] [Google Scholar]
- 16.Thapa P, Choi RK, Choi HJH, Yun M, Jeong BS, Jung MJ, Nam JM, Na Y, Cho WJ, Kwon Y, Lee ES. Bioorg Med Chem. 2010;18:2245–54. doi: 10.1016/j.bmc.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 17.Abadi AH, Ibrahim TM, Abouzid KM, Lehmann J, Tinsley HN, Gary BD, Piazza GA. Bioorg Med Chem. 2009;17:5974–82. doi: 10.1016/j.bmc.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serry AM, Luik S, Laufer S, Abadi AH. J Comb Chem. 2010;12:559–65. doi: 10.1021/cc1000488. [DOI] [PubMed] [Google Scholar]
- 19.Onnis V, Cocco MT, Fadda R, Congiu C. Bioorg Med Chem. 2009;17:6158–65. doi: 10.1016/j.bmc.2009.07.066. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Fan, Zhao Yanfang, Sun Li, Ding Lu, Gu Yucheng, Gong Ping. Synthesis and anti-tumor activity of 2-amino-3-cyano-6-(1H-indol-3-yl)-4-phenylpyridine derivatives in vitro. Eur J Med Chem. 2011;46:3149–57. doi: 10.1016/j.ejmech.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 21.Elzahabi HSA. Eur J Med Chem. 2011;46:4025–34. doi: 10.1016/j.ejmech.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 22.Cotterill IC, Usyatinsky AY, Arnold JM, Douglas SC, Dordick JS, Michels PC, Khmelnitsky YL. Tet Lett. 1998;39:1117–20. [Google Scholar]
- 23.Kappe CO, Dallinge D. Nat Rev Drug Discovery. 2006;5:51–63. doi: 10.1038/nrd1926. [DOI] [PubMed] [Google Scholar]
- 24.Tu S, Li C, Li G, Cao L, Shao Q, Zhou D, JB, Zhou J, Xi M. J Comb Chem. 2007;9:1144–48. doi: 10.1021/cc700124g. [DOI] [PubMed] [Google Scholar]
- 25.Feng J, Zhou, Song Y-Z, Lv J-S, Gong G-X, Tu S. Synth Commun. 2009;39:1443–50. [Google Scholar]
- 26.Poerwono H, Sasaki S, Hattori Y, Higashiyama K. Bioorg Med Chem Lett. 2010;20:2086–73. doi: 10.1016/j.bmcl.2010.02.068. [DOI] [PubMed] [Google Scholar]
- 27.Mentese MY, Bayrak H, Uygun Y, Mermer A, Ulker S, Karaoglu SA, Demirbas N. Eur J Med Chem. 2013;67:230–42. doi: 10.1016/j.ejmech.2013.06.045. [DOI] [PubMed] [Google Scholar]
- 28.Jordão AK, Novais J, Leal B, Escobar AC, Júnior HMdS, Castro HC, Ferreira VF. Eur J Med Chem. 2013;63:196–201. doi: 10.1016/j.ejmech.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 29.(a) Zhu SL, Ji SJ, Su XM, Sun C, Liu Y. Tet Lett. 2008;49:1777–1781. [Google Scholar]; (b) Zhu SL, Ji SJ, Zhao K, Liu Y. Tet Lett. 2008;49:2578–2582. [Google Scholar]; (c) El-Zahor MI. Al-Azhar Bulletin of Science. 1992;3:735–41. [Google Scholar]
- 30.(a) Nasrolahi Shirazi A, Tiwari RK, Brown A, Mandal D, Sun G, Parang K. Bioorg Med Chem Lett. 2013;23:3230–4. doi: 10.1016/j.bmcl.2013.03.124. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rao VK, Chhikara BS, Nasrolahi Shirazi A, Tiwari R, Parang K, Kumar A. Bioorg Med Chem Lett. 2011;21:3511–4. doi: 10.1016/j.bmcl.2011.05.010. [DOI] [PubMed] [Google Scholar]; (c) Rao VK, Chhikara BS, Tiwari R, Nasrolahi Shirazi A, Parang K, Kumar A. Bioorg Med Chem Lett. 2012;22:410–4. doi: 10.1016/j.bmcl.2011.10.124. [DOI] [PubMed] [Google Scholar]
- 31.Rong L, Wang H, Shi J, Yang F, Yao H, Tu S, Sh D. J Heterocycl Chem. 2007;44:1505–08. [Google Scholar]
- 32.Waring MJ. Expert Opin Drug Discov. 2010;5:235–248. doi: 10.1517/17460441003605098. [DOI] [PubMed] [Google Scholar]
- 33.(a) Réthy B, Kovács A, Zupkó I, Forgo P, Vasas A, Falkay G, Hohmann J. Planta Med. 2006;72:767–70. doi: 10.1055/s-2006-941505. [DOI] [PubMed] [Google Scholar]; (b) Zeng X, Morgenstern R, Nyström AM. Biomaterials. 2014;35:1227–39. doi: 10.1016/j.biomaterials.2013.10.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.