Abstract
Background:
Acute kidney injury (AKI) is common in patients in the pediatric intensive care unit (PICU) and is associated with poor outcome. We conducted the present study to determine the incidence, risk factors and outcomes of AKI in the PICU.
Materials and Methods:
We collected data retrospectively from case records of children admitted to the PICU during one year. We defined and classified AKI according to modified pRIFLE criteria. We used multivariate logistic regression to determine risk factors of AKI and association of AKI with mortality and morbidity.
Results:
Of the 252 children included in the study, 103 (40.9%) children developed AKI. Of these 103 patients with AKI, 39 (37.9%) patients reached pRIFLE max of Risk, 37 (35.9%) patients reached Injury, and 27 (26.2%) had Failure. Mean Pediatric Risk of Mortality (PRISM III) score at admission was higher in patients with AKI than in controls (P < 0.001).
Keywords: Acute kidney injury, pediatric intensive care unit, pRIFLE, PRISM III, sepsis
Introduction
Acute kidney injury (AKI) is a clinical condition that commonly occurs in critically ill patients in the pediatric intensive care unit (PICU). Studies have shown that AKI is independently associated with poor outcome.[1] Published data about AKI in Indian children are limited;[2,3,4,5] most data available are from developed countries.[6,7,8,9] Very few Indian studies provide incidence of AKI in pediatric ICU. The incidence and risk factors of AKI in developing countries may be different from those of developed countries. Therefore, extrapolation of results from studies from the developed countries to children in developing countries may not be valid. Information on overall incidence, risk factors and outcome of AKI could help in developing strategies for prevention and treatment of AKI.
The primary aim of this study was to determine the incidence of AKI and classify patients with AKI using pRIFLE criteria. The secondary aim was to identify risk factors associated with AKI in the PICU patients, and to determine the short-term clinical outcome in these children.
Materials and Methods
This retrospective, observational study was conducted over a one-year period from 1 January to 31 December 2011 at a Level III PICU attached to a medical college, which caters to children with both medical and surgical illnesses.
Study population
All children admitted to the PICU during this period were included in the study, unless they met the exclusion criteria. They were classified as AKI cases if AKI was diagnosed based on pRIFLE criteria[10] either at admission or subsequently during PICU stay; patients who did not develop AKI served as control. Maximum pRIFLE stage reached during the PICU stay was recorded. We used only the creatinine clearance criteria to classify AKI as this was a retrospective study and accurate details of urine output could not be obtained for many patients. Baseline estimated creatinine clearance (eCCL) was calculated using the Schwartz equation from serum creatinine measured within 3 months before current ICU admission, and if this was unavailable, the patients were assigned to a baseline eCCL of 120 ml/min/1.73 m2 (normal).[11,12]
Exclusion criteria
Patients younger than 1 month or greater than 18 years of age as well as those with end-stage renal disease on renal replacement therapy were excluded.
Clinical data were collected retrospectively from case records, and baseline characteristics including age and gender of all the patients were noted. The Pediatric Risk of Mortality (PRISM III) was calculated for all patients[13] to describe disease severity on admission. Assessment of organ dysfunction was done. Each patient was evaluated for risk factors known to cause AKI. A risk factor was considered to be present only if it occurred before development of AKI. Potential risk factors considered were age, gender, PRISM III score as well as those which developed during the PICU stay like hypotension, hypoxia, multiorgan dysfunction, infection, sepsis and coagulopathy. Hypoxia was defined as pulse oximetry saturation of < 90% or PaO2 < 60 mmHg, irrespective of level of FiO2 (Fraction of inspired oxygen concentration). Hypotension was defined as systolic blood pressure below two standard deviations of the normal value for the patient's height, gender and age. Multiorgan dysfunction syndrome was defined as dysfunction of two or more systems excluding AKI. Sepsis was defined as systemic inflammatory response syndrome in the presence of suspected or proven infection. Nephrotoxic drugs considered were aminoglycosides and vancomycin. Other details like length of mechanical ventilation, length of PICU stay, total hospital stay and PICU mortality were noted.
Calculations and Statistics
The incidence of AKI was described as the number of AKI episodes per 100 patients. We used univariate and multivariate logistic regression to determine association of AKI with PICU mortality. The multivariate model was adjusted for age, number of organs failed and need for mechanical ventilation. We compared difference in length of PICU stay and length of hospital stay between AKI group and non-AKI group by Mann-Whitney U test. We used multivariate logistic regression to determine predictors of AKI. Results were considered statistically significant if two-sided P value was less than 0.05.
Results
Total admissions in the PICU during the year were 288, of which records could be obtained in 259 (89.9%) children. Seven patients with end-stage renal disease and on maintenance dialysis were excluded from the study. Of the remaining 252 patients, 103 (40.9%) patients developed AKI as defined by modified RIFLE criteria [Figure 1].
Figure 1.

Diagram of stratification of patients in the study
Patients were classified into 2 groups i.e., AKI and non-AKI and baseline characteristics in both groups were compared as shown in Table 1.
Table 1.
Comparison of baseline characteristics of patients in the AKI and non-AKI groups

The mean (SD) age of the entire cohort was 3.30 (3.80) years (range 1 month to 16 years). Mean (SD) age of children in the AKI group was 2.35 (3.24) years, whereas in the non-AKI group was 3.95 (4.02) yrs (P = 0.002).
Mean PRISM III score of the entire cohort at PICU admission was 4.84 (4.79); it was 6.86 (5.23) in those who developed AKI and this was significantly higher than the PRISM score of 3.45 (3.91) in those who did not develop AKI (P < 0.001).
Table 2 shows the comparison of admission diagnosis in AKI and non-AKI patients. Sepsis, gastroenteritis, status epilepticus, bronchopneumonia and central nervous system infections were significantly more common in patients with AKI.
Table 2.
Comparison of diagnosis at admission of patients in the AKI and non-AKI groups

Clinical course of AKI
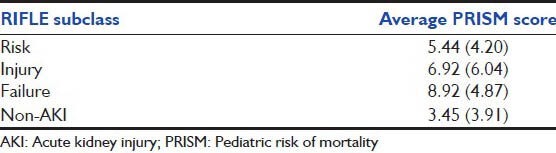
Of the 103 patients diagnosed to have AKI as defined by modified RIFLE criteria, 39 (37.9%) patients reached pRIFLE max of Risk, 37 (35.9%) patients reached Injury, and 27 (26.2%) patients had Failure. A comparison of PRISM score as per RIFLE subclasses showed that patients with a more severe pRIFLE subclass had higher PRISM III score [Table 3]. Mean (SD) PRISM III scores for non-AKI, R, I, and F classes were 3.45 (3.91), 5.44 (4.20), 6.92 (6.04) and 8.92 (4.87) respectively.
Table 3.
RIFLE subclass and average PRISM score of patients with AKI
Onset of AKI
Ninety of 103 patients (90.2%) had AKI at admission while 99% patients developed AKI within 72 h. The maximum time to development of AKI was 10 days.
RISK factors for AKI
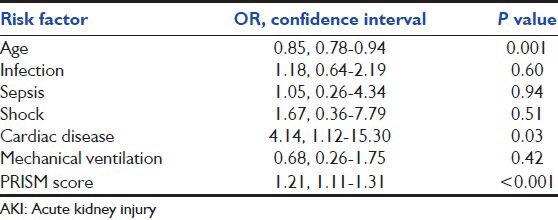
Univariate analysis was done to identify risk factors for AKI. Age, presence of infection, sepsis, shock, cardiac disease, mechanical ventilation PRISM score, hypoxia and coagulopathy were significant predictors of AKI in univariate analysis, whereas gender was not significantly associated [Table 4]. In multivariate analysis, only lower age (OR 0.85, 95% CI 0.78 - 0.94, P = 0.001), higher PRISM score (OR 1.21, 95% CI 1.11-1.31, P < 0.001) and presence of cardiac disease (OR 4.14, 95% CI 1.12-15.3, P = 0.03) were found to be independent risk factors for AKI [Table 5].
Table 4.
Risk factors for developing AKI (obtained by univariate analysis)
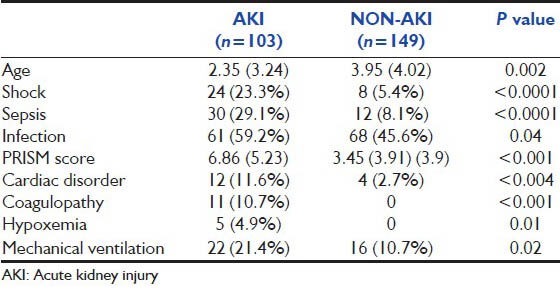
Table 5.
Independent risk factors of AKI (multivariate regression analysis)
Dialysis
Renal replacement therapy (RRT) was required in 5.8% patients. RRT was initiated for specific indications like electrolyte imbalances, acidosis, and severe fluid overload, and was not dependent on the class of AKI. All the patients underwent peritoneal dialysis. Mortality in patients requiring renal replacement was 66%.
Days of PICU and hospital stay
Total days of PICU and hospital stay were higher in patients with AKI, compared to non-AKI patients (P < 0.001 and P = 0.009 respectively). Though patients with AKI were more likely to need mechanical ventilation (P = 0.02), among the patients who needed mechanical ventilation, duration of ventilation was similar between AKI and non-AKI groups (P = 0.49) [Table 6].
Table 6.
Duration of mechanical ventilation, length of PICU stay, length of hospitalization and mortality in AKI and Non-AKI groups

Renal recovery
All the patients who had left the PICU with abnormal serum creatinine were followed up in the pediatric ward. Sixty-three patients out of 103 (61%) patients with AKI had complete renal recovery before discharge. Amongst the patients with persistent renal dysfunction, 16 died in the PICU and 2 developed chronic kidney disease and 2 patients had normalized serum creatinine at follow-up.
Hospital mortality and survival rates
The overall mortality among patients studied was 11.5% (29/252). Crude mortality in the AKI group tended to be higher than that in the non-AKI group. Mortality in the AKI group was15.5% (16/103) whereas that in the non-AKI group was 8.7% (13/149) (P = 0.10). When mortality in patients with AKI was compared between different RIFLE classes, there was a progressive and significant increase in the mortality rate associated with increasing RIFLE class i.e. 5.1% for risk, 16.2% for injury, and 29.6% for failure patients (P = 0.04 for trend) [Table 7]. We found that in univariate analysis, presence of number of organs failed and use of mechanical ventilator were significantly associated with increased risk of mortality, whereas age, sex and AKI were not significantly associated with mortality. In multivariate analysis, presence of AKI was not associated with mortality. Need for mechanical ventilation and multi-organ dysfunction were independent risk factors for increased mortality [Table 8].
Table 7.
Correlation of RIFLE class with length of PICU stay, length of hospitalization and mortality
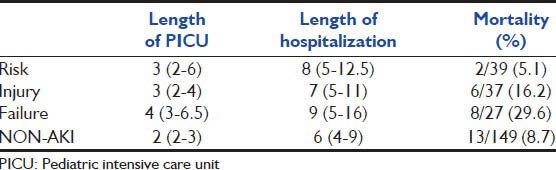
Table 8.
Predictors of mortality: Multivariate logistic regression

Discussion
Our study was a single-centre study with an objective to determine the incidence and risk factors of AKI and classify patients with AKI using pRIFLE criteria. One hundred and three patients of the study population of 252 developed AKI, an incidence of 40.9%. We found that lower age, higher PRISM score and presence of cardiac disease were independent risk factors for development of AKI. Though AKI was not an independent predictor of mortality, patients with AKI had significantly longer PICU stay and hospital stay compared to patients without AKI.
Various studies of AKI in the PICU have reported incidence ranging widely from 4.5-70%.[1,6,14] In a recent paper from northern India, Mehta et al.,[5] reported a 36.1% incidence of AKI in the critically ill children included in their study. Different definitions of AKI account for some of the reported differences in the incidence of AKI. Zappitelli[12] showed that using baseline estimated creatinine clearance, more patients were diagnosed as having AKI compared to using change in creatinine as the defining criteria for diagnosis of AKI. They also showed that assuming a baseline eCCL of 120 ml/min was also associated with higher incidence of AKI compared to assuming 100 ml/min as baseline eCCL. We used the pRIFLE classification scheme using change-estimated creatinine clearance as defining criteria and assumed a baseline eCCL of 120 ml/min. This may have lead to a relatively higher incidence of AKI in the present study.
In our study, 90.2% patients had AKI at admission while nearly all developed AKI within 72 h, similar to the study by Bailey et al., where ARF was present at entry in 84%.[6] Schneider et al., also reported that almost 50% patients developed their maximum RIFLE score within 24 h of ICU admission and about 75% achieved it by the 7th day of PICU stay.[15] These data also support previous pediatric studies demonstrating that children develop their maximum number of organ failures early in the intensive care unit (ICU) course, unlike the observation in adult patients who develop organ dysfunction late.[16]
Various studies have attempted to identify risk factors for development of AKI in critically ill children. Mehta et al.,[5] found that younger age, shock, sepsis and need for mechanical ventilation were independent risk factors for AKI in their cohort. Bailey et al.,[6] found that thrombocytopenia, higher age, hypotension, hypoxemia and coagulopathy were independent predictors for development of AKI. Bailey's study was different from our study and Mehta's study in that Bailey defined AKI as doubling of baseline serum creatinine.
Several studies have shown that AKI is associated with a significant increase in mortality.[6,10,17] Similarly, AKI was not found to be an independent predictor of mortality in some studies.[5] Mehta et al.,[5] found that although crude mortality was higher in patients with AKI than those without AKI (37% vs. 8.7%), AKI was not an independent predictor of mortality in multivariate analysis. Mehta found that shock was the only independent predictor of mortality. We also found that though there was a significant trend of higher mortality with higher AKI stage, AKI as a whole was not an independent predictor of mortality. Need for mechanical ventilation and presence of multi-organ failure were the only independent risk factors of mortality in our cohort of critically ill children admitted in the PICU. This suggests that increased mortality seen in patients with AKI is largely because patients with AKI are more sick with more organ failure and increased need for mechanical ventilation.
Our study has some limitations. Firstly, it was a single-centre retrospective study using existing medical records, which caused some difficulty in the assessment of AKI. We did not use urine output criterion, as exact documentation of urine output was not available from records. Secondly, we classified patients by pRIFLE criteria, which used a change in eCCL, and if baseline creatinine was unavailable, the patients were assigned to a normal baseline eCCL of 120 ml/min/1.73 m2. More recently, the Acute Kidney Injury Network (AKIN) group proposed refinements to the RIFLE classification which use change in serum creatinine instead of change in eCCL.[18] Further studies may be required to compare AKIN and pRIFLE to determine which classification system is more consistent and is a better predictor of important outcomes.
Conclusion
AKI is common in pediatric critically ill patients. Most of those who develop AKI do so within the first 72 h. Lower age, higher PRISM score and cardiac disease were independent risk factors for AKI. Though AKI was not associated with higher mortality, it was associated with significantly longer ICU and hospital stay, making it a major burden on the healthcare system.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Plotz FB, Bouma AB, Van Wijk JA, Kneyber MC, Bokenkamp A. Pediatric acute kidney injury in the ICU: An independent evaluation of pRIFLE criteria. Intensive Care Med. 2008;34:1713–17. doi: 10.1007/s00134-008-1176-7. [DOI] [PubMed] [Google Scholar]
- 2.Nasir SA, Bhat MA, Hijaz SW, Charoo BA, Sheikh BA. Profile of acute renal failure in children in Kashmir. Indian Pediatr. 2011;48:491–2. [PubMed] [Google Scholar]
- 3.Agarwal I, Kirubakaran C, Markandeyulu V. Clinical profile and outcome of acute renal failure in South Indian children. J Indian Med Assoc. 2004;102:353–6. [PubMed] [Google Scholar]
- 4.Kandoth PW, Agarwal GJ, Dharnidharka VR. Acute renal failure in children requiring dialysis therapy. Indian Pediatr. 1994;31:305–9. [PubMed] [Google Scholar]
- 5.Mehta P, Sinha A, Sami A. Incidence of acute kidney injury in hospitalized children. Indian Pediatr. 2012;49:537–42. doi: 10.1007/s13312-012-0121-6. [DOI] [PubMed] [Google Scholar]
- 6.Bailey D, Phan V, Litalien C. Risk factors of acute renal failure in critically ill children: A prospective descriptive epidemiological study. Pediatr Crit Care Med. 2007;8:29–35. doi: 10.1097/01.pcc.0000256612.40265.67. [DOI] [PubMed] [Google Scholar]
- 7.Williams DM, Sreedhar SS, Mickell JJ, Chan JC. Acute kidney failure: A pediatric experience over 20 years. Arch Pediatr Adolesc Med. 2002;156:893–900. doi: 10.1001/archpedi.156.9.893. [DOI] [PubMed] [Google Scholar]
- 8.Andreoli SP. Acute renal failure. Curr Opin Pediatr. 2002;14:183–8. doi: 10.1097/00008480-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Chan JC, Williams DM, Roth KS. Kidney failure in infants and children. Pediatr Rev. 2002;23:47–60. doi: 10.1542/pir.23-2-47. [DOI] [PubMed] [Google Scholar]
- 10.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028–35. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–90. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 12.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3:948–54. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated pediatric risk of mortality score. Crit Care Med. 1996;24:743–52. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Liano F, Pascual J. Epidemiology of acute renal failure: A prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50:811–8. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 15.Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38:933–9. doi: 10.1097/CCM.0b013e3181cd12e1. [DOI] [PubMed] [Google Scholar]
- 16.Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier M. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. 1996;109:1033–7. doi: 10.1378/chest.109.4.1033. [DOI] [PubMed] [Google Scholar]
- 17.Park WY, Hwang EA, Jang MH, Park SB, Kim HC. The risk factors and outcome of acute kidney injury in the intensive care units. Korean J Intern Med. 2010;25:181–7. doi: 10.3904/kjim.2010.25.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]


