Abstract
These guidelines, written for clinicians, contains evidence-based recommendations for the prevention of hospital acquired infections Hospital acquired infections are a major cause of mortality and morbidity and provide challenge to clinicians. Measures of infection control include identifying patients at risk of nosocomial infections, observing hand hygiene, following standard precautions to reduce transmission and strategies to reduce VAP, CR-BSI, CAUTI. Environmental factors and architectural lay out also need to be emphasized upon. Infection prevention in special subsets of patients - burns patients, include identifying sources of organism, identification of organisms, isolation if required, antibiotic prophylaxis to be used selectively, early removal of necrotic tissue, prevention of tetanus, early nutrition and surveillance. Immunodeficient and Transplant recipients are at a higher risk of opportunistic infections. The post tranplant timetable is divided into three time periods for determining risk of infections. Room ventilation, cleaning and decontamination, protective clothing with care regarding food requires special consideration. Monitoring and Surveillance are prioritized depending upon the needs. Designated infection control teams should supervise the process and help in collection and compilation of data. Antibiotic Stewardship Recommendations include constituting a team, close coordination between teams, audit, formulary restriction, de-escalation, optimizing dosing, active use of information technology among other measure. The recommendations in these guidelines are intended to support, and not replace, good clinical judgment. The recommendations are rated by a letter that indicates the strength of the recommendation and a Roman numeral that indicates the quality of evidence supporting the recommendation, so that readers can ascertain how best to apply the recommendations in their practice environments.
Keywords: Hospital Acquired Infection prevention, Standard Precautions, Burns, Monitoring Surveillance, Antibiotic Stewardship
Introduction
Hospital acquired infections (HAIs) is a major safety concern for both health care providers and the patients. Considering morbidity, mortality, increased length of stay and the cost, efforts should be made to make the hospitals as safe as possible by preventing such infections.[1,2]
These guidelines have been developed for health care personnel involved in patient care in wards and critical care areas and for persons responsible for surveillance and control of infections in hospital.
The principles of the grading of recommendations assessment, development and evaluation (GRADE) system is used to guide assessment of quality of evidence from high (A) to very low (C) and to determine the strength of recommendations. Each recommendation is categorized on the basis of existing scientific data, theoretical rationale, applicability and economic impact. The GRADE system classifies recommendations as strong (grade 1) or weak (grade 2). The assignment of strong or weak is considered of greater clinical importance than a difference in letter level of quality of evidence.
The system for categorizing recommendations in this guideline is as follows.
Level of evidence
Evidence from at least one properly-designed randomized, controlled trial
Evidence from at least one well-designed clinical controlled analytic studies (preferably from more than one center), or from multiple time-series studies, or dramatic results from uncontrolled experiments
Evidence from opinions of respected authorities based on the clinical experience, descriptive studies, or reports of expert committees.
Strength of recommendation
Strong (we recommend)
Weak (we suggest).
General Measures of Infection Control
Isolation
-
Assess the need for isolation.[3] Screen all intensive care unit (ICU) patients for the following:
- Neutropenia and immunological disorder
- Diarrhea
- Skin rashes
- Known communicable disease
- Known carriers of an epidemic strain of bacterium.
-
Identify the type of isolation needed.
There are two types of isolation in the ICU:
- Protective isolation for neutropenic or other immunocompromised patients to reduce the chances of acquiring opportunistic infections
- Source isolation of colonized or infected patients to minimize potential transmission to other patients or staff.
Isolation rooms should have tight-fitting doors, glass partitions for observation and both negative-pressure (for source isolation) and positive-pressure (for protective isolation) ventilations.
Patient at risk of nosocomial infections
There are patient, therapy and environment related risk factors for the development of nosocomial infection.[3]
Age more than 70 years
Shock
Major trauma
Acute renal failure
Coma
Prior antibiotics
Mechanical ventilation
Drugs affecting the immune system (steroids, chemotherapy)
Indwelling catheters
Prolonged ICU stay (>3 days).
Observe hand hygiene
Hands are the most common vehicle for transmission of organisms and “hand hygiene” is the single most effective means of preventing the horizontal transmission of infections among hospital patients and health care personnel.[4]
When and why – follow World Health Organizations (WHO′s) five moments for hand hygiene [Figure 1]
Figure 1.
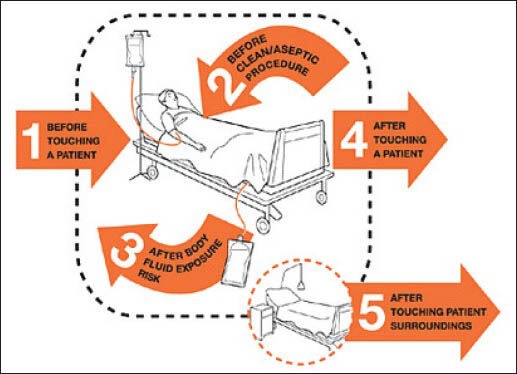
World Health Organization's five moments for hand hygiene
Before touching a patient (IB) – to protect the patient from harmful germs carried on your hands
Before aseptic procedures (IB) – to protect the patient against harmful germs, including the patient's own germs
After body fluid exposure/risk (IA) – to protect yourself and the health care environment from the harmful patient's germs
After touching the patient (IB) – to protect yourself and the health care environment from the harmful patient's germs
After touching the patient's surrounding (IB) – to protect yourself and the health care environment from the harmful patient's germs.
(Remember there are two moments before and three moments after touching the patient).
How
Wash hands with soap and water when they are soiled or visibly dirty with blood or other body fluids (IB). Wet your hands, apply soap and then scrub them vigorously for at least 15 s. Cover all surfaces of the hands and fingers, wash with water and then dry thoroughly using a disposable towel
-
Use an alcohol-based hand rub (IA) e.g. 0.5% chlorhexidine with 70% w/v ethanol, if hands are not visibly dirty. A combination of chlorhexidine and alcohol is ideal as they cover Gram-positive and Gram-negative organisms, viruses, mycobacteria and fungi. Chlorhexidine also has residual activity.
- During surgical hand preparation, all hand jewelries (e.g. rings, watches and bracelets) must be removed (2A)
- Finger nails should be trimmed to <0.5 cm (2A) with no nail polish or artificial nails (2A)
- Avoid wearing long sleeves, ties should be tucked in, house coats are discouraged and wearing scrubs is encouraged.
Follow standard precautions
Standard precautions include prudent preventive measures to be used at all times, regardless of a patient's infection status.[4]
Gloves
Sterile gloves should be worn after hand hygiene procedure while touching mucous membrane and non-intact skin and performing sterile procedures (2A) e.g. arterial, central line and Foley catheter insertion
Clean, non-sterile gloves are safe for touching blood, other body fluids, contaminated items and any other potentially infectious materials
Change gloves between tasks and procedures in the same patient especially when moving from a contaminated body area to a clean body area (1A)
Never wear the same pair of gloves for the care of more than one patient (1A)
Remove gloves after caring for a patient
Practice hand hygiene whenever gloves are removed.
Gown
Wear a gown to prevent soiling of clothing and skin during procedures that are likely to generate splashes of blood, body fluids, secretions or excretions (IB)
The sterile gown is required only for aseptic procedures and for the rest, a clean, non-sterile gown is sufficient (2A)
Remove the soiled gown as soon as possible, with care to avoid contamination.
Mask, eye protection/face shield
Wear a mask and adequate eye protection (eyeglasses are not enough), or a face shield to protect mucous membranes of the eyes, nose and mouth during procedures and patient care activities that are likely to generate splashes/sprays of blood and body fluids, etc., (2B)
Patients, relatives and health care workers (HCWs) presenting with respiratory symptoms should also use masks (e.g. cough) (2A).
Shoe and head coverings
They are not required for routine care (2B).
Patient-care equipment
Used patient-care equipment soiled with blood, body fluids, secretions, or excretions should be handled carefully to prevent skin and mucous membrane exposures, contamination of clothing and transfer of microorganisms to HCWs, other patients or the environment (1B)
Ensure that reusable equipment is not used for the care of another patient until it has been cleaned and sterilized appropriately (2A)
Ensure that single use items and sharps are discarded properly (1A).
Follow transmission-based precautions
In addition to standard precautions, the following should be observed in those patients known or suspected to have airborne, contact or droplet infections:[4]
Airborne precautions
Disease-causing microorganisms may be suspended in the air as small particles, aerosols, or dust and remain infective over time and distance, for example, Mycobacterium tuberculosis (pulmonary/laryngeal), varicella zoster virus (chickenpox), herpes zoster (shingles), rubella virus and measles
Isolate with negative-pressure ventilation (2B)
Respiratory protection must be employed when entering the isolation room (1B)
Use the disposable N-95 respirator mask, which fits tightly around the nose and mouth to protect against both large and small droplets. This should be worn by all persons entering the room, including visitors (1B).
Contact precautions
Infections can be spread by usual direct or indirect contact with an infected person, the surfaces or patient care items in the room, for example, parainfluenza virus infection, respiratory syncytial virus infection, varicella (chickenpox), herpes zoster, hepatitis A and rotavirus infections.[4]
Isolation is required (1B)
Non-critical patient-care equipment should preferably be of single use. If unavoidable, then clean and disinfect them adequately before using to another patient (2B)
Limit transport of the patient (2B).
Droplet precautions
Microorganisms are also transmitted by droplets (large particles >5 μm in size) generated during coughing, sneezing and talking, or a short-distance travelling, for example, influenza virus, Bordetella pertussis, Hemophilus influenzae (meningitis, pneumonia), Neisseria meningitidis (meningitis, pneumonia and bacteremia), Mycoplasma pneumoniae, Severe acute respiratory syndrome-associated coronavirus, Group A Streptococcus, adenovirus and rhinovirus[4]
Isolation is required (1A)
Respiratory protection must be employed when entering the isolation room or within 6-10 ft of the patient. Use the disposable N-95 respirator mask, which fits tightly around the nose and mouth to protect against both large and small droplets. This should be worn by all persons entering the room, including visitors (1B)
Limit transport of the patient (2B).
Use specific strategies focused on prevention of specific nosocomial infections
In addition to the standard and transmission-based precautions, there are several strategies focused on prevention of specific nosocomial infections in critically ill patients. Of these, ventilator-associated pneumonia (VAP), catheter-related bloodstream infection (CRBSI) and urinary tract infection (UTI) are the most important.
Strategies to reduce VAP
Consider noninvasive ventilation whenever possible (2B)
Prefer oral intubations to nasal unless contraindicated (2B)
Keep head elevated at 30-45° in the semi-recumbent body position (IA)
Daily oral care with chlorhexidine solution of strength 0.12% (IA)
Daily sedation vacation if feasible and assessment of readiness to extubate (IA)
Avoid re intubation whenever possible (2B)
Routine change of ventilator circuits is not required (2B)
Monitor endotracheal tube cuff pressure (keep it >20 cm H2 O) to avoid air leaks around the cuff, which can allow entry of bacterial pathogens into the lower respiratory tract (2B)
Prefer endotracheal tubes with a subglottic suction port to prevent pooling of secretions around the cuff leading to microaspiration (2A)
The heat moisture exchanger may be better than the heated humidifier (2B)
Closed endotracheal suction systems may be better than the open suction (2B)
Periodically drain and discard any condensate that collects in the tubing of a mechanical ventilator (2B).
Strategies to reduce CRBSI
Prefer the upper extremity for catheter insertion. Avoid femoral route for central venous cannulation (CVC) (IA)[8]
If the catheter is inserted in a lower extremity site, replace to an upper extremity site as soon as possible (2A)
Use maximal sterile barrier precautions (cap, mask, sterile gown and sterile gloves) and a sterile full-body drape while inserting CVCs, peripherally inserted central catheters, or guidewire exchange (IA)
Clean skin with more than 0.5% chlorhexidine preparation with alcohol (usually 2% chlorhexidine with 70% w/v ethanol) before CVC, arterial catheter insertion, etc., (IA)
Use chlorhexidine/silver sulfadiazine or minocycline/rifampin-impregnated CVCs when the catheter is expected to remain in place for more than 5 days and only if the bloodstream infection rates are high in the unit despite successful implementation of measures to reduce CRBSI (2A)
Use ultrasound-guided insertion if technology and expertise are available (IB)
Use either sterile gauze or sterile, transparent, semipermeable dressing to cover the catheter site (IA). Replace the catheter site dressing only when the dressing becomes damp, loosened, or visibly soiled
Evaluate the catheter insertion site daily and check if a transparent dressing is present and palpate through the dressing for any tenderness (1B)
Insertion date should be put on all vascular access devices (2B)
Use 2% chlorhexidine wash daily for skin cleansing to reduce CRBSI (2B)
Use needleless intravascular catheter access systems (2B) and avoid stopcocks. Closed catheter access systems should be preferred to open systems (2A)
Clean injection ports with an appropriate antiseptic (chlorhexidine, povidone-iodine, an iodophor, or 70% alcohol), accessing the port only with sterile devices. Cap stopcocks when not in use (2A)
Assess the need for the intravascular catheter daily and remove when not required (IA)
Peripheral lines should not be replaced more frequently than 72-96 h. Routine replacement of CVCs is not required (2A)
Replace administration sets, including secondary sets and add-on devices, every day in patients receiving blood, blood products, or fat emulsions (2A)
If other intravenous fluids are used, change no <96-h intervals and at least every 7 days (IA)
Needleless connectors should be changed frequently (every 72 h) (2A)
Replace disposable or reusable transducers at 96-h intervals (2A).
Strategies to reduce UTI
Insert catheters only for appropriate indications (2A)[9]
Follow aseptic insertion of the urinary catheter (IB)
Maintain a closed drainage system (IB)
Maintain unobstructed urine flow. At all times the urinary catheter should be placed and taped above the thigh and the urinary bag should hang below the level of the bladder (2B)
The urinary bag should never have floor contact (2B)
Changing indwelling catheters or drainage bags at fixed intervals is not recommended. Change only if there are clinical indications such as infection or obstruction, or when the closed system is compromised (2B)
Remove the catheter when it is no longer needed (2A).
Consider environmental factors
Cleaning and disinfection
High-quality cleaning and disinfection of all patient-care areas is important, especially surfaces close to the patient (e.g. bedrails, bedside tables, doorknobs and equipment)[10]
Some pathogens can survive for long periods in the environment, particularly methicillin-resistant Sataphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), Acinetobacter species, Clostridium difficile and norovirus
EPA-registered disinfectants or detergents that best meet the overall needs of the ICU should be used for routine cleaning and disinfection
Frequency of cleaning should be as follows: Surface cleaning (walls) twice weekly, floor cleaning 2-3 times/day and terminal cleaning (patient bed area) after discharge or death (2B).
Architecture and layout, especially while designing a new ICU
The unit may be situated close to the operating theater and emergency department for easy accessibility, but should be away from the main ward areas (2B)[11]
Central air-conditioning systems are designed in such a way that recirculated air must pass through appropriate filters (1B)[11]
It is recommended that all air should be filtered to 99% efficiency down to 5 μm (1A)[11]
Suitable and safe air quality must be maintained at all times. Air movement should always be from clean to dirty areas (1A)[11]
It is recommended to have a minimum of six total air changes per room per hour, with two air changes per hour composed of outside air (1B)[11]
Isolation facility should be with both negative- and positive-pressure ventilations (2B)[11]
Clearly demarcated routes of traffic flow through the ICU are required (2B)[11]
Adequate space around beds is ideally 2.5-3 m (2B)
Electricity, air, vacuum outlets/connections should not hamper access around the bed (2B)[11]
Adequate number of washbasins should be installed (2B)[11]
Alcohol gel dispensers are required at the ICU entry, exits, every bed space and every workstation (1B)[11]
There should be separate medication preparation area (1B)[11]
There should be separate areas for clean storage and soiled and waste storage and disposal (1B)[11]
Adequate toilet facilities should be provided (1B)[11]
Organizational and administrative measures[4,11]
Work with hospital administration for better patient to nurse ratio in the ICU (1B)[4,11]
Policies for controlling traffic flow to and from the unit to reduce sources of contamination from visitors, staff and equipment (1B)
Waste and sharp disposal policy (1A)
Education and training for ICU staff about prevention of nosocomial infections (1A)
ICU protocols for prevention of nosocomial infections (1A)
Audit and surveillance of infections and infection control practices (IB)
Infection control team (multidisciplinary approach) (1B)
Antibiotic stewardship (1B)
Vaccination of health care personnel (1A).
Guidelines for Infection Prevention in Burns Patients
Burn wounds can provide optimal conditions for colonization, infection and transmission of pathogens; infection acquired by burn patients is a frequent cause of morbidity and mortality.
Epidemiology of infection
The development of infection depends on the presence of three conditions, a source of organisms; a mode of transmission; and the susceptibility of the patient.
Source of organisms
Sources of organisms are found in the patient's own endogenous (normal) flora, from exogenous sources in the environment and from health care personnel. Although burn wound surfaces are sterile immediately following thermal injury, these wounds eventually become colonized with microorganisms.[12] Gram-positive bacteria that survive the thermal insult, such as staphylococci located deep within sweat glands and hair follicles, heavily colonize the wound surface within the first 48 h unless topical antimicrobial agents are used.[13] Eventually (after an average of 5-7 days), these wounds are subsequently colonized with other microbes, including Gram-positive bacteria, Gram-negative bacteria and yeasts derived from the host's normal gastrointestinal and upper respiratory flora and/or from the hospital environment or that are transferred via a HCW hands.[12,14]
Mode of transmission
In burn patients, the primary mode is direct or indirect contact-either through the hands of the personnel caring for the patient or from contact with inappropriately decontaminated equipment.[15] Burn patients are unique in their susceptibility to colonization from organisms in the environment as well as in their propensity to disperse organisms into the surrounding environment. In general, the larger the burn injury, the greater the volume of organisms that will be dispersed into the environment from the patient.
Hydrotherapy equipment is an important environmental reservoir of Gram-negative organisms (2A).
Patient susceptibility
The patient has three principal defense against infection: Physical defenses, nonspecific immune responses and specific immune responses. Changes in these defenses determine the patient's susceptibility to infection. Invasive devices, such as endotracheal tubes, intravascular catheters and urinary catheters, bypass the body's normal defense mechanisms.[16,17]
Culturing and surveillance
Burn wound flora and antibiotic susceptibility patterns change during the course of the patient's hospitalization so that the purposes of obtaining routine surveillance cultures are:
To provide early identification of organisms colonizing the wound
To monitor the effectiveness of current wound treatment
To guide perioperative or empiric antibiotic therapy
To detect any cross-colonizations, which occur quickly so that further transmission can be prevented
Routine surveillance wound cultures should be obtained when the patient is admitted and at least weekly until the wound is closed. Surveillance of infection has been shown to diminish the rate of nosocomial infection (2A).[18]
Isolation guidelines
Standard precautions should be followed when caring for all patients with burn injury. The effectiveness of simple protective barrier precautions reduces nosocomial colonization and infection (1A)[19]
It is recommended that patients with larger burn injuries be isolated in private rooms or other enclosed bed spaces to ensure physical separation from other patients on the unit. Such isolation has been associated with a decrease in cross transmission of organisms (IB).[20,21]
Patients with >30% TBSA burn injuries are more immunocompromised, due to the larger size of their injury. This, in combination with their loss of physical defenses and need for invasive devices, significantly increases their risk of infection. These patients also represent a significant risk for contamination of their surrounding environment with organisms, which may then be spread to other patients on the unit.
Environmental issues
Plants and flowers should not be allowed in units with burn patients because they harbor Gram-negative organisms, such as Pseudomonas species, other enteric Gram-negative organisms and fungi. Many of these organisms are intrinsically resistant to multiple antibiotics, which may serve as reservoirs to colonize the burn wound[22] (2A)
Routine cleaning, disposal of waste and gathering of soiled linen is required to reduce the bioload of organisms, which are present and ensure that the unit is as clean as possible[22] (2A)
Routine environmental surveillance culturing is not generally recommended on units with burn patients[22] (2A).
Antibiotic prophylaxis
-
Several studies have demonstrated the role of topical antimicrobials in decreasing morbidity and mortality in patients with major burn injuries (partial-or full-thickness skin involvement), particularly before early excision[23] (1B)
Topical antibiotic agents should first be applied directly to the patient's dressings before application to the burn wound to prevent contamination of the agent's container by burn wound flora
Studies of the clinical benefit of prophylactic courses of systemic antibiotics in burn patients in decreasing the occurrence of burn wound infections have not demonstrated improved outcome compared to the use of topical therapy along with surgical excision
-
Systemic antibiotic administration in burn patients should therefore only be used selectively and for a short period of time. Due to the secondary bacteremia, burn wound manipulation and/or excision, prophylactic systemic antibiotic therapy may be given immediately before, during and for one or two doses after the procedure, particularly in burn patients with extensive injury (e.g. 40% TBSA) (2A).[24,25]
Early burn wound excision now occurs within the first few days after burn injury and has resulted in improved survival. The primary aims of early excision are removal of the dead tissue that stimulates an overwhelming systemic inflammatory response syndrome and prevention of infection by temporary or permanent closure of the burn wound.[25] Furthermore, shortening the period of wound inflammation, which in turn reduces the development of hypertrophic scarring, may optimize the outcome in terms of function and appearance.[25] This is achieved by early removal of necrotic tissue (e.g. eschar) and wound closure with autograft, allograft, or skin substitutes in selected patients.
Early nutrition
Early enteral feeding diminished the incidence of wound colonization and infection by bowel flora and sepsis (IB).[26]
Early enteral feeding is likely effective because it increases circulation to the bowel, thereby decreasing ischemia post-injury and the translocation of bowel flora.
Prevention of tetanus
Burn centers routinely administer human tetanus immunoglobulin (250-500 IU) to provide immediate passive immunization regardless of the patient's active immunization status. Active immunization with tetanus toxoid is also given (0.5 ml intramuscularly) to burn patients who have not received a complete primary immunizing series or who have not received a tetanus toxoid booster within the past 10 years[27] (2A).
Bloodstream and intravascular catheter infection
Whenever possible, catheters should be placed through unburned skin, preferably at a sufficient distance from the wound to prevent contamination of the insertion site. This is not always feasible in patients with large burn injuries, requiring long-term vascular access[28] (2B).
Pneumonia
Burn patients with severe inhalation injury requiring prolonged intubation are also at risk for developing VAP.
Prevention should also include vigorous chest physiotherapy, turning, coughing, deep breathing and suctioning[29] (2B).
UTI
Patients usually develop significant bacteriuria after 72 h of urinary catheter insertion, so these devices should be removed after the initial period of fluid resuscitation and output monitoring[15] (2B).
Guidelines for Infection Control in the Special Subsets - Immunocompromised and Transplant Patients
Immunocompromised patients are those patients whose immune mechanisms are deficient because of immunologic disorders (e.g. human immunodeficiency virus (HIV) infection or congenital immune deficiency syndrome), chronic diseases (e.g. diabetes, cancer, emphysema, or cardiac failure), or immunosuppressive therapy (e.g. radiation, cytotoxic chemotherapy anti-rejection medication, or steroids). Immunocompromised patients who are identified as high-risk patients have the greatest risk of infection caused by airborne or waterborne microorganisms. Patients in this subset include persons who are severely neutropenic for prolonged periods of time (i.e. an absolute neutrophil count (ANC) of <500 cells/mL), allogeneic HSCT patients and those who have received the most intensive chemotherapy (e.g. childhood acute myelogenous leukemia patients).[30]
In general, opportunistic infections result from at least 1 of 3 basic mechanisms: (1) Exogenous acquisition of a particularly virulent pathogen (e.g. meningococcal meningitis or pneumococcal pneumonia), (2) reactivation of an endogenous latent organism (e.g. herpes simplex virus, herpes zoster virus [HZV or shingles], or tuberculosis and (3) endogenous invasion of a normally commensal or saprophytic organism (e.g. bacteria, viruses, fungi, or protozoa/parasites).
The post-transplant timetable can be divided into three time periods:[31]
During the 1st month after transplantation: >95% of the infections are due to bacterial or candida infection of the surgical wound, vascular access, endotracheal tube, or drainage catheters. These infections are comparable to those observed in non-immunosuppressed patients undergoing similar surgery
During the period 1-6 months after transplantation: Two classes of infection are observed: Infections caused by immunomodulatory viruses and infections caused by opportunistic pathogens such as Pneumocystis carinii, Listeria monocytogenes and Aspergillus species
In the late period: >6 months after transplantation, the patient population can be divided into three subgroups: more than two-thirds of transplant patients have had a good result from transplantation and are primarily at risk from community-acquired respiratory viruses. On an average 10-15% of transplant patients suffer from chronic viral infection, such as infection with hepatitis B or C virus, which progresses inexorably to end-stage organ dysfunction and/or cancer unless effective antiviral therapy can be administered. Finally, 5-10% are who have relatively poor allograft function and who have received excessive amounts of immunosuppression. These patients are the subgroup at greatest risk of opportunistic infection, particularly with such organisms as Cryptococcus neoformans, P. carinii and L. monocytogenes.
Hand hygiene
The most important intervention is hand hygiene. When hands are visibly dirty, contaminated with proteinaceous material, or visibly soiled with blood or body fluids, wash hands with either a non-antimicrobial soap and water or an antimicrobial soap and water[32] (IA)
If hands are not visibly soiled, or after removing visible material with non-antimicrobial soap and water, the preferred method of hand decontamination is with an alcohol-based hand rub[32,33,34,35] (1B).
Isolation
Simple protective isolation offered no advantage over routine care for most immunocompromised patients (2B)
Immunocompromised individuals should never be placed in the same room or adjacent to people with a known infection (1A)
Isolation of potentially contagious patients within the ICU should be attempted if practical to reduce the chances of cross infection. Although isolation is recommended for control of airborne spread of pathogens, cross-colonization with organisms predominantly spread by contact (such as MRSA), infection may only be reduced by changing behavior of staff. In the absence of adequate isolation rooms, barrier precautions with gloves and gown combined with good hand hygiene is paramount[36] (1B).
Room ventilation
Patient should be placed in rooms with >12 air exchanges/h and point-of-use HEPA filters that are capable of removing particles >0.3 μm in diameter (2A)
Inspection and preventive maintenance of duct and filter systems should occur on a routine, scheduled basis (2A)
Patient room should have positive room air pressure when compared with any adjoining hallways, toilets and anterooms, if present (2A)
The use of single rooms with HEPA filtration may reduce the risk of hospital-acquired infection by airborne fungi, in particular the Aspergillus genus. This is especially true where refurbishment, building or demolition are in progress in the hospital or nearby[37] (IA).
Cleaning
Rooms should be cleaned >1 times/day with special attention to dust control (1A)
Exhaust vents, window sills and all horizontal surfaces should be cleaned with cloths and mop heads that have been premoistened with disinfectant (2B).
Prohibit exposures of patients to such activities as vacuuming or other floor or carpet vacuuming that could cause aerosolization of fungal spores (e.g. Aspergillus species) (1B)
Moisture problems (i.e. rainwater or plumbing leaks) should be promptly reported and repaired (1B)
Organic materials that become moist must be dried or removed within 24-48 h to prevent fungal growth (1B).
Medical devices and other equipment should be decontaminated according to existing guidance (2B). The role of cleaning and decontamination should not be underestimated. At times of local epidemics or outbreaks, the closure of part of or the whole unit should be considered to allow thorough cleaning. Hydrogen peroxide vapor decontamination has been shown to be superior to conventional cleaning but can only be used in enclosed rooms as it is toxic to humans. Interestingly, MRSA may not be completely cleared with a conventional solution containing 5-15% non-ionic surfactant and 5-15% cationic surfactant, diluted 1:500.[11]
Guidelines exist for floor space in ICUs (ESICM and SCCM references). The transmission of micro-organisms will occur more readily in cramped conditions. There are recommendations for the number of isolation cubicles that should be available for patients with resistant organisms and for immunocompromised patients.[37,38]
Protective clothing
Food and drink
Hospital food is normally very safe. However, the immunocompromised patient is at increased risk of food-borne illness and the acquisition of harmful micro-organisms from some food and drink. Therefore immunocompromised individuals are advised to avoid certain high-risk foods, for example soft cheeses and foods made with raw egg, such as mayonnaise[3,39] (1B)
All drinking water for immunocompromised patients (including bottled water) should be chemically sterilized or boiled (1B)
Visitors who might have communicable infectious diseases (e.g. upper respiratory tract infections (URTIs), flu-like illnesses, recent exposure to communicable diseases, an active shingles rash whether covered or not, a VZV-like rash within 6 weeks of receiving a live-attenuated VZV vaccine, or a history of receiving an oral polio vaccine within the previous 3-6 weeks) should not be allowed to visit immunocompromised patients[40,41] (1B)
Vaccination in Immunocompromised patients should be done in accordance with the published guidelines.[42,43]
Pharmacological prophylaxis against diseases in transplant patients may be done as per recommendations in the references mentioned.[40,43,44]
Monitoring of Infection Control
What should be monitored? Monitoring includes various aspects of infection control practices [Table 1]. Simultaneous monitoring of all the aspects might not be possible therefore prioritization must be done by the infection control team depending upon the need and situation. Monitoring of process compliance is most important to reduce incidence of HAI, preventing multidrug resistance to antimicrobials and protecting HCWs from getting infection. Methodology of monitoring should be adopted as per the institutional policy.[45,46,47] Environmental monitoring along with microbiological surveillance has been claimed to reduce infection rate. Adherence to hand hygiene is being considered as one of the most important preventive action.[47] Observed adherence to hand hygiene protocol ranges from 5% to 89% (38.7%) among the HCWs.
Table 1.
Monitoring of Infection Control
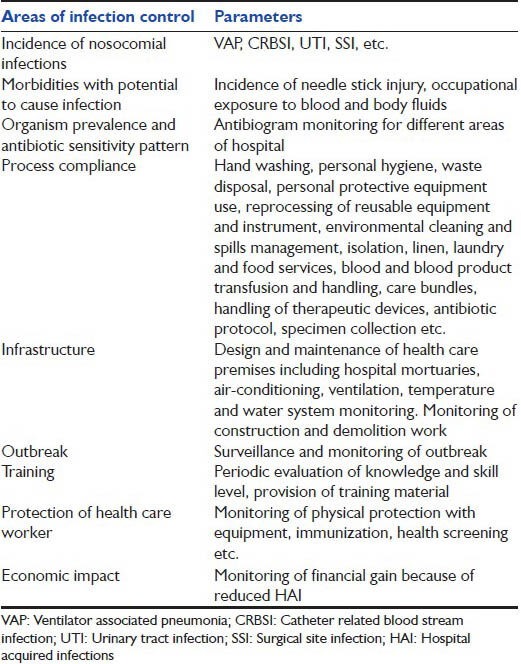
Process related recommendations
Hand washing: This is the single most effective hygiene practice for minimizing health care associated infections. HCWs must wash their hands before and after every significant patient contact. Hand basins with hot and cold water supplies, non-touch taps with antisplash devices, supplies of liquid handwash (preferably in non-refillable disposable containers) and disposable paper towels or single-use, clean, cloth towels are recommended to facilitate hand hygiene. CDC guidelines[48] and the WHO guidelines[4] recommend that HCWs wash their hands with soap and water when visibly soiled and exposure to potential spore-forming pathogens is strongly suspected or proven, including outbreaks of C. difficile (1B). Otherwise, hand rubbing with an alcohol based agent is recommended (1A) for all other opportunities as it is faster, more effective and better tolerated by the skin. Exposure of hands to body fluid in presence of intact skin deserves decontamination (recommendation level 1B) but it is particularly vital when skin is not intact or mucous membrane has been exposed (recommendation level 1A).[5] Compliance monitoring for hand hygiene among HCWs therefore had been given a higher recommendation level (1A).[4,48,49]
-
Recommendations for use of gloves:[50]
- Sterile gloves – for procedures requiring a sterile field, involving normally sterile areas of the body
- Non-sterile gloves – for procedures other than the above
- General purpose utility gloves – for housekeeping and cleaning
- Use of gloves had been advocated when it can be reasonably anticipated that contact of blood or other potentially infectious materials with, mucous membranes, or non-intact skin will occur (IC).[4]
-
Recommendations for changing gloves:[50]
- After contact with each patient and when performing separate procedures on the same patient if cross-contamination is possible
- as soon as gloves are damaged (torn or punctured);
- After completion of any task not involving patients but requiring the use of gloves
- Before answering telephones or recording patient notes
- Remove gloves after caring for a patient. Do not wear the same pair of gloves for the care of more than one patient (IB).[4]
Recommendations for eyewear or face-shields: During procedures where there is potential for splashing, splattering or spraying of blood or other body substances[50]
Waste disposal and spillage: Disposal protocol should be followed differently for general waste (concern is not more than household waste), cytotoxic waste, pharmaceutical waste, chemical waste and radioactive waste. For blood spillage in ward or operation theater, cleaning should be done at the earliest with paper towels followed by water and detergents. Sodium hypochlorite is not necessary for such cleaning. Laboratory spillage should be absorbed on to paper towels and disposed of as clinical waste. The contaminated surfaces should be treated with 2.0-2.5% sodium hypochlorite, left for 1 h and cleaned again with paper towels that are disposed of as clinical waste. In suspected creutzfeldt-jackob disease spillage of brain tissue or spinal fluid is treated in the similar manner but the paper towels are incinerated.[50] It has been observed that HBV and HCV in dry blood remain infectious even when exposed to external environment for up to a week and 16 h respectively. Implications remain the same even if blood is invisible or not present in sufficient quantity.[51] Considering this glucometers should be cleaned and disinfected as per CDC recommendation after every use to avoid contamination[11,52]
-
6.
Compliance monitoring of care bundles should be done in a very stringent manner. Non-compliance to a single measure should be interpreted as failure to comply with the whole bundle. Therefore, a higher target of > 95% compliance had been advocated[47]
-
7.
Training: Effectiveness of staff education on HAI prevention is controversial but as a part of comprehensive infection control program it's value had been appreciated.[53] Designated infection control personnel can help in imparting prescheduled training, maintaining record, monitoring the knowledge base and bed side application of the knowledge.[54] Ongoing staff education is also a regulatory need.[53] Education of HCW and periodic assessment of adherence to insertion and maintenance of intravascular catheters protocol had been given higher level of recommendation (Category IA).[55] New employee orientation to infection control program and making the employees responsible for infection prevention had been recommended by Joint Commission on Accreditation of Healthcare Organizations (JCAHOs)[53]
-
8.
To prevent airborne transmission negative pressure single rooms (1/100 beds) with anterooms and fresh air at 100% (that is, no recirculating air) is recommended to achieve most effective dilution of airborne microorganisms[50]
-
9.
Periodic safety and operational inspection should be done by engineering and building services department
-
10.
Mortuary guidelines:[50]
- Body storage at an internal temperature of 4°C
- Longer-term storage in a freezer maintained at −20°C
- Mortuary must not be used except storage of bodies
- All refrigerators/freezers should be monitored and fitted with alarms that operate 24 h a day
- Mortuary design should minimize manual handling of bodies
- Where autopsies are performed, dedicated room(s) should be used with negative air pressure ventilation.
Who should monitor: Designated infection control nurses should supervise the process and help in collection and compilation of data. However, for case controlled study of an outbreak, the primary investigating team should include the hospital epidemiologist, the director of employee health, the infection-control team and microbiologist. External consultants might be necessary in some cases.[54]
How to monitor: Following methods had been advocated for monitoring HAI data.[56]
Observations
Interviews
Surveys and inspections
Quality assurance activities.
Observation
Direct observation is the gold standard to monitor compliance with optimal hand hygiene practice. Monthly monitoring of hand hygiene product consumption (Soap, alcohol rub) had also been advocated as an indirect measure of hand hygiene but this needs further validation. Number of dispenser filled with alcohol rub and number of dispenser working when compared to total number of dispensers available are also simple monitoring parameters.[57]
Predesigned format for survey, inspection and interview
Data collection and compliance monitoring are facilitated if a format is available for doing so.[58] For full, partial and minimal compliance >85%, 76-84% and <75% respective scores had been suggested.[59]
Surveillance
Surveillance is defined as ‘′ongoing, systematic collection, analysis and interpretation of health data essential to the planning, implementation and evaluation of public health care practice. It is closely integrated with timely dissemination of these data to those who need to know.′’[58,60]
Periodicity of data collection
Frequency of data collection, analysis and generation of report for evaluation and taking corrective measures should also be predefined.[54] If data is being collected intermittently then, it should be done at least for four continuous weeks in each time period under study.[58] Authenticity of data remains a matter of concern therefore source of data should always be mentioned and its must be verified before relying on the same for decision making. Data can be presented as pooled mean, median and percentile manner. Team should further analyze and investigate extremely higher or lower rate or ratio (>90th percentile, ≤10th percentile).[61]
Automated monitoring
Automated monitoring helps in avoiding errors and variability related to manual collection of data. Hospitals with sophisticated information systems are in a position to streamline surveillance process through computer-based algorithms that identifies patients at highest risk of HAI.[54] Computerized surveillance helps in better implementation of preventive strategies, but lower infection rates had not been proven conclusively.[62,63] The use of sinks and hand rub dispensers had also been used for electronic monitoring of hand hygiene practice.[4,57]
Endemic rate of infection
Surveillance of endemic infection is the focal point of infection control activity. It helps in generating a data base and identifying epidemic when incidence crosses the endemic threshold.[47] The first aim of surveillance is to determine endemic rate of infection.[54] The base line data of HAI helps in taking improvement initiatives. It has been claimed that infection rate can be reduced by 31-44% with the implementation of effective surveillance system.[18,64]
Outbreak investigation
Investigation for outbreak should be considered when monthly incidence of a particular type of infection is more than 95% of the confidence interval of same infection in the same month in the previous year.[54] Analysis of all isolates, patients’ demographic data and exposure to potential risk factors (medication, procedure and contact) are necessary for such investigation. This should be followed by analysis of time interval between infection and exposure to potential risk factors. Based on the generated data, case controlled study is recommended according to age, gender and exposure to potential risk factors. This exercise helps in developing a hypothesis regarding source and transmission of infection. Temporary infection control measures are implemented based on the hypothesis formed. Molecular typing is advocated if such facilities are available.[54]
Coding of clinical indicators, trend analysis and bench marking
Use of ICD-10-AM codes for clinical indicators of infection control is desirable as it helps in data collating and benchmarking between health care institutions. However, all indicators do not have their ICD code.[58] Interhospital comparison of HAI demands standardization of definitions, data collection and analysis.[65] Therefore comparison of data might not be justified always due to differences in the infrastructure, quantitative and qualitative gap in human resource, compliance level, variation in the practices and case mix. Such exercise is further constrained because tools needed to compare infection rates for a given case mix is provided by very few systems.[65] Trend monitoring of unit's own data over a period of time therefore is more appropriate.[66]
Organism prevalence, sensitivity pattern and antimicrobial use
Tracking of organism prevalence, sensitivity pattern and antimicrobial use should be part of epidemiology program. Community acquired and HAI should be separated while analyzing the data.[54] Active and aggressive surveillance of all isolates and patients has been advocated on admission and weekly basis provided higher risk for carrying MRSA and VRE is anticipated. More frequent culture had been advised if >50% isolates are MRSA positive.[67] But this approach has been challenged.[68]
Recommendations for Antimicrobial Stewardship
Core members of a multidisciplinary antimicrobial stewardship program should include an infectious disease physician and a clinical pharmacist with infectious disease training (1B)[69,70,71,72,73]
Other members of the team may include clinical microbiologist, an information system specialist, an infection control professional and hospital epidemiologist (1C)
In resource limited setting a physician (hospital based practitioner preferable) with interest in infectious disease should lead the program along with the hospital microbiologist (1C)
Close collaboration between the antimicrobial stewardship team, microbiology lab, hospital pharmacy and infection control team should be maintained (1C)
Involvement of the administration with their buy in to the program is essential for the success of any stewardship program (1C)
It is desirable that antimicrobial stewardship programs function under the auspices of quality assurance and patient safety department (1C)
Prospective audit of antimicrobial use with direct interaction and feedback to the prescriber by senior members of antimicrobial stewardship team can result in reduced inappropriate use of antibiotics (1A)
This is the preferable mode of antimicrobial stewardship in an “open” prescription writing setting as prevalent in India (1C)
Formulary restriction and preauthorization requirements can lead to significant reductions in antimicrobial use (1B)
Formulary restriction may also help in decreasing nosocomial outbreak of resistant infection (2B)
Formulary restriction and preauthorization from a logistic point of view may not be universally applicable in an “open” prescription writing prevalent health care delivery system as is existing in India (1C)
Continuing education of all the stakeholders should be done to provide a foundation of knowledge that will enhance and increase the acceptance of stewardship strategies (1C)
Education alone without active intervention like audit and feedback do not have a sustained impact on prescribing behavior of physicians (2B)
Guidelines and clinical pathways based on evidence and incorporating local microbiology and resistance pattern can improve antibiotic utilization (1A)
Antimicrobial cycling to decrease antibiotic resistance has not been found to be useful and is logistically difficult in Indian setting (2B)
Antimicrobial order form has been given a weak recommendation (2B) in IDSA guidelines of North America, but this may be a readily implementable, documentable and a useful tool for stewardship program in India (1C)
There are insufficient data to recommend the routine use of combination therapy to prevent the emergence of resistance (2B)
De-escalation of antibiotic once culture results are back is an essential ingredient of any stewardship program and should be practiced (1B)
De-escalation is poorly practiced in India and an audit of de-escalation practices and education on its proper implementation should be an important ingredient of any antibiotic stewardship program in India (1C)
Optimizing antibiotic dose taking into consideration pk/pd characteristic should be universally practiced (1B)
As under dosing may be prevalent in resource limited setting a close vigilance on the appropriate dosing and a hospital information system and warning mechanism should be incorporated (1C)
An early switch from parenteral to oral antibiotics is highly desirable specially in resource limited setting to decrease cost of therapy and should be actively implemented (1 C)
Decreasing duration of antibiotic use as per clinical guideline to decrease the cost of therapy, antibiotic consumption and reduce side effects of drugs should be actively incorporated in the antibiotic stewardship program (1C)
Active use of information technologies such as electronic medical record, hospital information system, computerized physician order entry and clinical decision support facilitates delivery of the stewardship program more effectively (1B)
In resource limited setting an effort should be made customizing use of existing information technology and using indigenous innovation to utilize the existing resources to achieve similar objective (1C)
Optimal use of microbiology lab is an essential ingredient of any stewardship program (1C).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Plowman R, Graves N, Griffin M, Roberts JA, Swan AV, Cookson B, Taylor L. The socioeconomic burden of hospital acquired infection. London Public health laboratory service and the London school of hygiene and tropical Medicine. 1999 [Google Scholar]
- 2.Wenzel RP. The Lowbury Lecture. The economics of nosocomial infections. J Hosp Infect. 1995;31:79–87. doi: 10.1016/0195-6701(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 3.Guideline for isolation precautions: Preventing transmission of infectious agents in healthcare settings. 2014. Mar 10, Available from: http://www.cdc.gov/hicpac/pdf/isolation/Isolation2007.pdf . [DOI] [PMC free article] [PubMed]
- 4.WHO guidelines on hand hygiene in health care: A summary. 2014. Mar 10, Available from: http://www.whqlibdoc.who.int/hq/2009/WHO_IER_PSP_2009.07_eng.pdf .
- 5.Maselli DJ, Restrepo MI. Strategies in the prevention of ventilator-associated pneumonia. Ther Adv Respir Dis. 2011;5:131–41. doi: 10.1177/1753465810395655. [DOI] [PubMed] [Google Scholar]
- 6.Coffin SE, Klompas M, Classen D, Arias KM, Podgorny K, Anderson DJ, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S31–40. doi: 10.1086/591062. [DOI] [PubMed] [Google Scholar]
- 7.Lorente L, Blot S, Rello J. Evidence on measures for the prevention of ventilator-associated pneumonia. Eur Respir J. 2007;30:1193–207. doi: 10.1183/09031936.00048507. [DOI] [PubMed] [Google Scholar]
- 8.Guidelines for the prevention of intravascular catheter-related infections. Available from http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf .
- 9.Guidelines for prevention of catheter-associated urinary tract infections. Available from http://www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf . [DOI] [PubMed]
- 10.Circulation for disinfection and sterilization in healthcare facilities. 2008. Available from: http://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf .
- 11.Guidelines for environmental infection control in health care facilities. Available from: http://www.cdc.gov/hicpac/pdf/guidelines/eic_in_HCF_03.pdf .
- 12.Wysocki AB. Evaluating and managing open skin wounds: Colonization versus infection. AACN Clin Issues. 2002;13:382–97. doi: 10.1097/00044067-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Erol S, Altoparlak U, Akcay MN, Celebi F, Parlak M. Changes of microbial flora and wound colonization in burned patients. Burns. 2004;30:357–61. doi: 10.1016/j.burns.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 14.LeVoyer T, Cioffi WG, Jr, Pratt L, Shippee R, McManus WF, Mason AD, Jr, et al. Alterations in intestinal permeability after thermal injury. Arch Surg. 1992;127:26–9. doi: 10.1001/archsurg.1992.01420010032005. [DOI] [PubMed] [Google Scholar]
- 15.Wurtz R, Karajovic M, Dacumos E, Jovanovic B, Hanumadass M. Nosocomial infections in a burn intensive care unit. Burns. 1995;21:181–4. doi: 10.1016/0305-4179(95)80005-9. [DOI] [PubMed] [Google Scholar]
- 16.Goldmann DA, Pier GB. Pathogenesis of infections related to intravascular catheterization. Clin Microbiol Rev. 1993;6:176–92. doi: 10.1128/cmr.6.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker WK, Cioffi WG, Jr, McManus AT, Kim SH, McManus WF, Mason AD, et al. Fungal burn wound infection. A 10-year experience. Arch Surg. 1991;126:44–8. doi: 10.1001/archsurg.1991.01410250048008. [DOI] [PubMed] [Google Scholar]
- 18.Haley RW, Culver DH, White JW, Morgan WM, Emori TG, Munn VP, et al. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol. 1985;121:182–205. doi: 10.1093/oxfordjournals.aje.a113990. [DOI] [PubMed] [Google Scholar]
- 19.Klein BS, Perloff WH, Maki DG. Reduction of nosocomial infection during pediatric intensive care by protective isolation. N Engl J Med. 1989;320:1714–21. doi: 10.1056/NEJM198906293202603. [DOI] [PubMed] [Google Scholar]
- 20.Burke JF, Quinby WC, Bondoc CC, Sheehy EM, Moreno HC. The contribution of a bacterially isolated environment to the prevention of infection in seriously burned patients. Ann Surg. 1977;186:377–87. doi: 10.1097/00000658-197709000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McManus AT, McManus WF, Mason AD, Jr, Aitcheson AR, Pruitt BA., Jr Microbial colonization in a new intensive care burn unit. A prospective cohort study. Arch Surg. 1985;120:217–23. doi: 10.1001/archsurg.1985.01390260077011. [DOI] [PubMed] [Google Scholar]
- 22.Kates SG, McGinley KJ, Larson EL, Leyden JJ. Indigenous multiresistant bacteria from flowers in hospital and nonhospital environments. Am J Infect Control. 1991;19:156–61. doi: 10.1016/0196-6553(91)90022-5. [DOI] [PubMed] [Google Scholar]
- 23.Monafo WW, West MA. Current treatment recommendations for topical burn therapy. Drugs. 1990;40:364–73. doi: 10.2165/00003495-199040030-00004. [DOI] [PubMed] [Google Scholar]
- 24.Mozingo DW, McManus AT, Kim SH, Pruitt BA., Jr Incidence of bacteremia after burn wound manipulation in the early postburn period. J Trauma. 1997;42:1006–10. doi: 10.1097/00005373-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403–34. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart DW, Wolf SE, Chinkes DL, Beauford RB, Mlcak RP, Heggers JP, et al. Effects of early excision and aggressive enteral feeding on hypermetabolism, catabolism, and sepsis after severe burn. J Trauma. 2003;54:755–61. doi: 10.1097/01.TA.0000060260.61478.A7. [DOI] [PubMed] [Google Scholar]
- 27.Edlich RF, Hill LG, Mahler CA, Cox MJ, Becker DG, Horowitz JH, et al. Management and prevention of tetanus. J Long Term Eff Med Implants. 2003;13:139–54. [PubMed] [Google Scholar]
- 28.Lesseva M. Central venous catheter-related bacteraemia in burn patients. Scand J Infect Dis. 1998;30:585–9. doi: 10.1080/00365549850161142. [DOI] [PubMed] [Google Scholar]
- 29.Sheridan RL, Kacmarek RM, McEttrick MM, Weber JM, Ryan CM, Doody DP, et al. Permissive hypercapnia as a ventilatory strategy in burned children: Effect on barotrauma, pneumonia, and mortality. J Trauma. 1995;39:854–9. doi: 10.1097/00005373-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Linden PK. Approach to the immunocompromised host with infection in the intensive care unit. Infect Dis Clin North Am. 2009;23:535–56. doi: 10.1016/j.idc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Fishman JA, Issa NC. Infection in organ transplantation: Risk factors and evolving patterns of infection. Infect Dis Clin North Am. 2010;24:273–83. doi: 10.1016/j.idc.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Dykewicz DA, Jaffe H, Spira T, et al. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients: recommendations of CDC, the Infectious Disease Society of America, and the American Society of Blood and Marrow Transplantation. [Last accessed date March 10, 2014];MMWR Morb Mortal Wkly Rep. 2000 49:1–128. [Google Scholar]
- 33.Boyce JM, Pittet D Healthcare Infection Control Practices Advisory Committee; HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. [Last accessed date March 10, 2014]. Available at http://www.cdc.gov/mmwr/pdf/rr/rr5116.pdf .
- 34.Nishimura S, Kagehira M, Kono F, Nishimura M, Taenaka N. Handwashing before entering the intensive care unit: What we learned from continuous video-camera surveillance. [Last accessed date March 10, 2014];Am J Infect Control. 1999 27:367–9. doi: 10.1016/s0196-6553(99)70058-1. [DOI] [PubMed] [Google Scholar]
- 35.Pittet D. Hand hygiene: Improved standards and practice for hospital care. Curr Opin Infect Dis. 2003;16:327–35. doi: 10.1097/00001432-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 36.French GL, Otter JA, Shannon KP, Adams NM, Watling D, Parks MJ. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): A comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J Hosp Infect. 2004;57:31–7. doi: 10.1016/j.jhin.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Guidelines for intensive care unit design. Guidelines/Practice Parameters Committee of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 1995;23:582–8. [PubMed] [Google Scholar]
- 38.Ferdinande P. Recommendations on minimal requirements for Intensive Care Departments. Members of the Task Force of the European Society of Intensive Care Medicine. Intensive Care Med. 1997;23:226–32. doi: 10.1007/s001340050321. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention, Infectious Disease Society of America, American Society of Blood and Marrow Transplantation. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm Rep. 2000;49:1–125. CE1. [PubMed] [Google Scholar]
- 40.Green M, Avery R, Preiksaitis J. Guidelines for the prevention and management of infectious complications of solid organ transplantation. (57-109).Am J Transplant. 2004;4(Suppl 10):10–7. 160-3. [Google Scholar]
- 41.Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep. 2001;50:1–43. [PubMed] [Google Scholar]
- 42.Advisory Committee on Immunization Practices. Recommended adult immunization schedule: United States, 2010. Ann Intern Med. 2010;152:36–9. doi: 10.7326/0003-4819-152-1-201001050-00008. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. 2009 Apr 10; [PubMed] [Google Scholar]
- 44.Grabe M, Bishop MC, Bjerklund-Johansen TE, Botto H, Çek M, Lobel B, Naber KG, Palou J, Tenke P, Wagenlehner F, editors. Guidelines on urological infections. Arnhem, The Netherlands: European Association of Urology (EAU); 2009. Mar, UTIs in renal insufficiency, transplant recipients, diabetes mellitus and immunosuppression; pp. 52–63. Available at: http://www.guideline.gov/content.aspx?id=14806 . [Google Scholar]
- 45.Yokoe DS, Mermel LA, Deverick AJ, Kathleen AM, Burstin H, David CP, et al. Executive Summary: A Compendium of Strategies to Prevent Healthcare-Associated Infections in Acute Care Hospitals. Infection Control and Hospital Epidemiology. 2008;29:S12–S21. doi: 10.1086/591060. [DOI] [PubMed] [Google Scholar]
- 46.Starling B. Postnote July 2005 Number 247 Infection control in healthcare settings. [Last accessed date March 10, 2014]. Available from: http://www.parliamentuk/parliamentary_offices/post/pubs2005.cfm .
- 47.Chastre J, Luyt CE. Ventilator-associated pneumonia. In: Mason RJ, Broaddus VC, Martin TR, et al., editors. Murray& Nadel's Textbook of Respiratory Medicine. 5th ed. chap 33. Philadelphia, PA: Elsevier Saunders; 2010. [Google Scholar]
- 48.Boyce JM, Pittet D. Guideline for Hand Hygiene in Health-Care Settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Morbid Mortal Wkly Rep. 2002;51:1–45. [Google Scholar]
- 49.Mody L, McNeil SA, Sun R, Bradley SE, Kauffman CA. Introduction of a waterless alcohol-based hand rub in a long-term-care facility. Infect Control Hosp Epidemiol. 2003;24:165–71. doi: 10.1086/502185. [DOI] [PubMed] [Google Scholar]
- 50.Australian Government Department of Health and ageing; 2008. [Last accessed date March 10, 2014]. Infection control guidelines for the prevention of transmission of infectious diseases in the health care setting. Available from: http://www.health.gov.au/internet/main/./icg-guidelines-index.htm . [Google Scholar]
- 51.Perz JF, Thompson ND, Schaefer MK, Patel PR. US outbreak investigations highlight the need for safe injection practices and basic infection control. [Last accessed date March 10, 2014];Clin Liver Dis. 2010 14:137–51. doi: 10.1016/j.cld.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention (CDC). Transmission of hepatitis B virus among persons undergoing blood glucose monitoring in long-term-care facilities – Mississippi, North Carolina, and Los Angeles County, California, 2003-2004. MMWR Morb Mortal Wkly Rep. 2005;54:220–3. [PubMed] [Google Scholar]
- 53.Mody L. Infection control issues in older adults. Clin Geriatr Med. 2007;23:499–514. doi: 10.1016/j.cger.2007.02.001. vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edmond MB, Wenzel RP. Organization for infection control. In: Mandel GL, Bennet JE, Dolin R, editors. Principles and practice of infectious diseases. 5th ed. Philadelphia: Churchill Livingston; 2009. pp. 2988–2995. [Google Scholar]
- 55.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Review of outcomes data analysis HLTIN403B-Implement and monitor infection control policies and procedures. Available from: http://www.riskresponse.com. au/training/units-of-competency/hlt07-health/hltin403b-implement-and-monitor-infection-control-policies-and -procedures .
- 57.Hargreaves S. UK team adopts unique approach to hospital infection control. The Lancet. 2008;8(12):747. [Google Scholar]
- 58.Infection control program quality monitoring indicators. Vs 2. NSW Department of Health. 2005. Available at www.health.nsw.gov.au/pubs/2005/pdf/infection_ctrl_manual.pdf .
- 59.Department of Health U.K; 2005. ICNA Audit Tools for Monitoring ICNA Audit Tools for Monitoring Infection Control Guidelines within the Community Setting; pp. 1–48. Available at: http://www.ips.uk.net/icna/Admin/uploads/AuditTools2005.pdf . [Google Scholar]
- 60.Do AN, Ray BJ, Banerjee SN, Illian AF, Barnett BJ, Pham MH, et al. Bloodstream infection associated with needleless device use and the importance of infection-control practices in the home health care setting. J Infect Dis. 1999;179:442–8. doi: 10.1086/314592. [DOI] [PubMed] [Google Scholar]
- 61.Edwards JR, Peterson KD, Mu Y, Banerjee S, Allen-Bridson K, Morrell G, et al. National Healthcare Safety Network (NHSN) report: Data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37:783–805. doi: 10.1016/j.ajic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 62.Boughton B. Computerized infection monitoring and rapid control measures benefit patients and hospitals. From Medscape Medical News. Available from: http://www.medscape.com/viewarticle/725013 .
- 63.Pittet D, Allegranzi B, Sax H, Bertinato L, Concia E, Cookson B, et al. Considerations for a WHO European strategy on health-care-associated infection, surveillance, and control. Lancet Infect Dis. 2005;5:242–50. doi: 10.1016/S1473-3099(05)70055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention (CDC). Monitoring hospital-acquired infections to promote patient safety – United States, 1990-1999. MMWR Morb Mortal Wkly Rep. 2000;49:149–53. [PubMed] [Google Scholar]
- 65.Coffin SE, Zaoutis TE. Infection control, hospital epidemiology, and patient safety. Infect Dis Clin North Am. 2005;19:647–65. doi: 10.1016/j.idc.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 66.Ray B, Samaddar D.P, Todi S.K, Ramakrishnan N, George John, Suresh Ramasubban. Quality indicators for ICU: ISCCM guidelines for ICUs in India. Indian J Crit Care Med. 2009 Oct-Dec;13(4):173–206. [PMC free article] [PubMed] [Google Scholar]
- 67.Muto CA, Jernigan JA, Ostrowsky BE, Richet HM, Jarvis WR, Boyce JM, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol. 2003;24:362–86. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 68.Lautenbach E. Expanding the universe of methicillin-resistant staphylococcus aureus prevention. Ann Intern Med. 2008;148:474–6. doi: 10.7326/0003-4819-148-6-200803180-00009. [DOI] [PubMed] [Google Scholar]
- 69.Fishman N. Antimicrobial stewardship. Am J Med. 2006;119(6 Suppl 1):S53–61. doi: 10.1016/j.amjmed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 70.Dellit TH, Owens RC, McGowan JE, Jr, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–77. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 71.Drew RH. Antimicrobial stewardship programs: How to start and steer a successful program. J Manag Care Pharm. 2009;15:S18–23. doi: 10.18553/jmcp.2009.15.s2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shah RC, Shah P. Antimicrobial stewardship in institutions and office practices. Indian J Pediatr. 2008;75:815–20. doi: 10.1007/s12098-008-0153-z. [DOI] [PubMed] [Google Scholar]
- 73.Raghunath D. Emerging antibiotic resistance in bacteria with special reference to India. J Biosci. 2008;33:593–603. doi: 10.1007/s12038-008-0077-9. [DOI] [PubMed] [Google Scholar]


