Abstract
Interferon Response Factor 3 (IRF3) induces several NK-cell activating factors, is activated by poly-I:C, an experimental cancer therapeutic, but is suppressed during many viral infections. IRF3 Knockout (KO) mice exhibited enhanced B16 melanoma growth, impaired intratumoral NK cell infiltration, but not an impaired poly-I:C therapeutic effect due to direct suppression of B16 growth. IRF3 was responsible for poly-I:C decrease in TIM-3 expression by intratumoral dendritic cells, induction of NK-cell Granzyme B and IFN-γ, and induction of macrophage IL-12, IL-15, IL-6, and IRF3–dependent NK-activating molecule (INAM). Thus, IRF3 is a key factor controlling melanoma growth through NK-cell activities, especially during poly-I:C therapy.
Keywords: NK-cells, Melanoma, IRF3, poly-I:C, Cytokines
1. Introduction
Natural killer (NK) cells are lymphocytes of the innate immune system prohibited from attacking healthy cells through Class I MHC-binding inhibitory proteins, such as Ly49 [1], but which are licensed to kill cancer cells after activating receptor recognition of APC proteins such as IRF3–dependent NK-activating molecule (INAM) [2, 3]. NK cells are additionally stimulated by APC cytokines such as IL-12, IL-15, and IL-6 [4]. In comparison, CD8 T cells of the adaptive immune system are activated and differentiate to cytolytic T cells (CTL) that can kill cancer cells following recognition of tumor associated epitope peptides associated with Class I MHC proteins and stimulation by APC cytokines [5]. Following their activations, CTL and NK cells express GranzymeB (GrB), perforin, and Interferon-(IFN)-γ, which are involved in killing tumor cells [6]. Despite these responses, humans can develop cancerous and often fatal tumors. This is in part because the effector activity of NK cells against tumor cells that is rapidly initiated by INAM can be abruptly thwarted when NK cells encounter molecules like T cell immunoglobulin and mucin domain 3 (Tim-3) [7, 8] on APCs or when responding CD8 T cells encounter tumor infiltrating regulatory T cells [9], myeloid derived suppressor cells [10], or Tim-3 [8].
A more complete understanding of immune responses that control malignant melanomas is a major goal in cancer immunology. The mouse B16 melanoma implantation model has been used to gain a better understanding of innate and adaptive immune responses to melanoma [11]. In that model, subcutaneous injection of B16 melanoma cells into C57Bl/6 mice leads to primary tumors in about 7–9 days and spontaneous metastases in lungs. Development of B16 melanoma tumors is controlled by infiltration of activated NK cells expressing GrB, perforin, plus IFNγ [12, 13] and macrophages and dendritic cells (DCs) expressing ligands for NK cell activating receptors [14, 15], IL-12 [16], plus IL-15 [17]. In addition to NK cells, T cells are activated and differentiate when they recognize B16 melanoma antigenic epitopes [18], with Class I or Class II MHC and are stimulated by APC cytokines, such as IL-12 and IL-15 [4]. However, activation of NK cells and T cells have been shown to be disrupted by molecules such as Tim-3. Therefore the extent to which NK cells and T cells are activated by ligands for activating receptors and cytokines expressed by APCs determines the pace of melanoma growth.
IRF3 is expected to be involved in effective anti-melanoma immune responses because it participates in expression of IL-12 [19], IL-15 [20], and ligands for activating receptors such as INAM [14, 21]. The expression of ligands for activating receptors and cytokines occurs when APCs encounter nucleic acids from cancer cells, which bind to specific pattern recognition receptors (PRRs) that stimulate cell signaling pathways for activation of transcription factors, such as IRF3. Activation of the Toll-like receptor (TLR)-3 at APC cell membranes activates IRF3 [22–25]. Poly-I:C, which has been proposed as a cancer chemotherapeutic adjuvant, is an artificial TLR3 agonist that also activates IRF3 and stimulates expression of downstream IRF3-dependent genes associated with anti-tumor immune responses. Our hypothesis is that activation of NK cells during B16 melanoma growth and treatment with poly I:C requires IRF3 and plays a significant role in control of the tumor. The results show that growth of IRF3+/+ B16 melanomas was increased in IRF3 deficient mice, which was accompanied by decreased infiltration of NK cells into the tumors. Poly I:C treatment reduced growth of IRF3+/+ B16 melanomas, which was accompanied by an increased infiltration of intratumoral CD8 T cells and decreased expression of Tim-3 by intratumoral DCs in wild-type mice. In vitro treatment of splenic mononuclear cells with poly I:C resulted in significant activation of NK cells from wild-type but not IRF3 deficient mice as evidenced by increased GrB and IFN-γ expression. However poly I:C treatment of IRF3 deficient mice notwithstanding resulted in a significant decrease in IRF3+/+ B16 melanoma growth, suggesting that in addition to an effect on antitumor immune responses, poly I:C has a direct effect on tumor cell growth.
2. Materials and methods
2.1 Mice, cells, and reagents
C57BL/6 and SJL/J mice were obtained from Harlan Laboratories (Indianapolis, IN) and used at 6–8 weeks age. IRF3 deficient mice (IRF3KO) on the C57BL/6 background were offspring of breeder pairs obtained from Dr. Karen Mossman [26]. B16-F10 cells were obtained from Caliper Life Sciences (Hopkinton, MA) and maintained in DMEM with 10% FBS and 50 μg/ml gentamycin. Poly I:C was obtained from InvivoGen (San Diego, CA).
2.2. Tumor model
For B16-F10 implantation, mice were injected subcutaneously (s.c.) into the dorsal hind quarter with 5 × 105 B16-F10 cells in 100 μl of PBS. Palpable tumors were monitored with a micrometer caliper and tumor areas were calculated from perpendicular radii of tumors on individual mice from day 9 to day 15–16 post injection. A separate cohort of mice were injected s.c with B16-F10 melanoma cells and were also injected intraperitoneally (i. p.) with 250 μg poly I:C in 1 ml PBS on day 0 and day 5. In a separate experiment, 5 × 104 B16-F10 cells were grown in 2 ml of DMEM culture media with or without 50 μg/ml poly I:C. At 24, 48, and 72 h cells were detached with TrypLE, washed, resuspended in PBS, and counted with a hemacytometer using trypan blue.
2.2 Macrophage preparations and stimulation
Macrophages were elicited by intraperitoneal injection of 2 ml thioglycollate broth into mice. Four days later, the peritoneal cavities were flushed with 2 ml DMEM and the cells were incubated at 1 × 106 cells/2 ml of DMEM cell culture medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS) (Invitrogen), and 50 μg/ml gentamycin (Invitrogen). After 24 h, non-adherent cells were removed and 1 ml of culture medium added. Adherent cells were greater than 90% Mac-1+ as determined by FACS analysis. These macrophages were either untreated or treated with 50 μg/ml poly I:C and 8 or 24 h later RNA extracted for qRT-PCR.
2.3 Isolation of splenic mononuclear cells
Spleens were extracted from mice and placed into DMEM with 10% FBS and 50 μg/ml gentamycin. Cells were dispersed using 70-μm mesh screens; washed in Dulbecco’s PBS; treated with erythrocyte-lysing reagent containing 0.15 M NH4Cl, 1.0 M KHCO3, and 0.1 mM Na2EDTA; washed; and resuspended in cell culture medium. Cells were counted with a hemacytometer using trypan blue. 1 × 106 splenic mononuclear cells/2 ml of DMEM cell culture medium were either untreated or treated with 50 μg/ml poly I:C and 24 h later cells were analyzed by FACS.
2.4 RNA preparation and quantitative RT-PCR
RNA was extracted from macrophages the Purelink kit from Ambion/Invitrogen (Carlsbad, CA) according to the manufacturer’s specifications. One-hundred ng to one μg of RNA was reverse transcribed in 0.5 mM each of dATP, dGTP, dTTP, and dCTP, 20 U of RNAse inhibitor with Superscript II reverse transcriptase (Invitrogen) at 42°C for 1.5 h followed by 94°C for 5 min. One twenty-fifth of the cDNA sample was incubated with 0. 4 μM of the following primer pairs (Invitrogen): IL-15: sense 5’ TTAACTGAGGCTGGCATTCATG 3’ and antisense: 5’ ACCTACACT GACACAGCCCAAA 3’; IL-6 sense 5’-ATGAAGTTCCT CTCTGCAAGA GACT-3’ and antisense 5’-CACTAGGTTTGCCGAGTAGATCTC-3’; IL-12 p40: sense 5’-GACGTTTATG TTGTAGAGGT- 3’ and antisense 5’-TTCCAACGCCAGTTCAATGGGC-3’; INAM: sense 5’CAACTGCAATGCCACGCTA 3’ and antisense 5’ TCCAACCG AACACCTGAGACT 3’; GAPDH: sense 5’-TTGTCAGCAATGCATCC TGCAC-3’ and antisense 5’-ACAGCTTTCCA GAGGGGCCATC-3’. Quantitative PCR reactions were run on an ABI Prism 7000 thermal cycler at 50 °C for 2 min, 95 °C for 10 min, 45 cycles of 95 °C for 15 s/60 °C for 30 s. Relative levels of mRNA for each factor were normalized to GAPDH determined by using the Ct value and the formula: 2−ΔΔCt.
2.5 FACS analysis of intratumoral mononuclear cells and poly I:C stimulated splenic mononuclear cells
At day 16 after s.c. injection of B16-F10 cells, tumors were harvested into cold PBS, treated with collagenase, filtered through a 70 μM nylon filter, and then washed in PBS. Cells were stained for FACS analysis using PerCP anti-CD11b, FITC anti-CD11c plus PE anti-TIM3, or FITC anti-CD8 plus PerCP anti-CD4 (all from eBiosciences, Carlsbad, CA). Alternatively, cells were stained with FITC anti-CD8, PerCP CD4, plus PE anti-CD49b+, gated on CD4/CD8 double negative and FACS analyzed for PE-CD49+ cells. In a separate experiment splenic mononuclear cells were treated with or without 50 μg/ml poly I:C for 24 h and then cells were stained with FITC anti-CD49b- antibody fixed in 4% paraformaldehyde followed by intracellular staining with APC labeled anti-GrB antibody (Invitrogen) in 0.25% saponin (Sigma) buffer. For intracellular cytokine analysis, cells were treated with 5 μg/ml Brefeldin A (Sigma) and APC labeled anti-IFN-γ antibodies were added. Samples were analyzed using a Becton Dickinson FACSCalibur and data analyzed using FlowJo software (Treestar, Ashland, OR).
2.6 Statistical analyses
Statistical analyses were performed using GraphPad Prism Software. Student’s two-tailed unpaired t test or two-way ANOVA was used to determine the significance of differences between means; p < 0.05 was considered significant.
3. Results
3.1 IRF3 deficiency increases growth of B16 melanomas in vivo
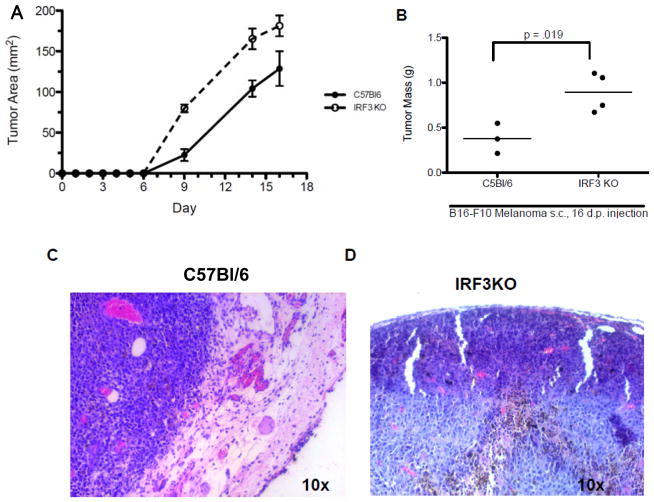
We and others have shown that IRF3 is essential to expression of IFN-β [27], IL-12 [19; 28], and IL-15 [20], which are cytokines involved in development, survival, and activation of NK cells [29, 30]. Therefore, IRF3 could have profound effects on anti-tumor NK cell activity. Since C57Bl/6-derived B16 melanoma cells are sensitive to NK cell cytotoxicity [31], we used B16-F10 cells to determine the role of IRF3 in immune control of tumor growth in vivo. B16-F10 cells were injected s. c. into C57Bl/6 (H-2b) and IRF3KO (H-2b) mice and primary tumor growth was monitored on subsequent days. The results indicate that solid B16 tumors were measurable in all mice starting at day 9 post injection and increased substantially in C57Bl/6 and IRF3KO mice through day 16 (Fig. 1A). However, from day 9 through day 16, tumor size was significantly greater in IRF3KO mice compared with C57Bl/6 mice. Likewise, at day 16 post injection tumor mass was significantly greater in IRF3KO mice compared with C57Bl/6 mice (Fig. 1B). Histological examination with H & E staining of day 16 tumors in C57Bl/6 mice revealed an intact epidermis overlaying the growing tumor (Fig. 1C), while the epidermis was impaired in IRF3KO mice. Therefore IRF3 deficiency increases susceptibility to melanoma growth.
Fig 1.
IRF3 deficiency increases growth of B16 melanomas. C57Bl/6 and IRF3KO mice were subcutaneously injected with 106 B16-F10 melanoma cells and tumor growth of individual mice was monitored for 16 days post injection. (A) Data are means of tumor areas + standard error, n=8–10; p<.0001 for differences between mouse strain between days 9–16 by two-way ANOVA (B) Data are means of tumor mass (g) of individual C57BL/6 or IRF3KO mice at day 16 post injection, n= 3–4. (C) H & E stains of representative B16-F10 melanomas in C57BL/6 or IRF3KO mice at day 16 post injection.
3.2 Poly I:C treatment decreases growth of B16 melanomas even in IRF3 deficient mice
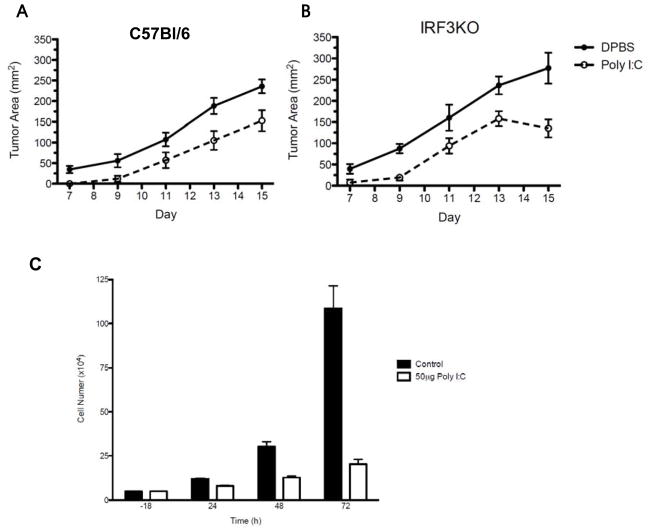
Because poly I:C is a TLR3 agonist that activates IRF3 to induce expression of cytokines such as IL-15 and NK activating ligands such as INAM, it has been used successfully as a potential therapeutic agent in treatment of experimental tumors [32]. However, poly I:C could work by enhancing anti-tumor immunity of NK cells [33] or work directly through TLR3 on tumor cells [34]. Therefore, we s.c injected B16-F10 melanoma cells, which express IRF3, into C57Bl/6 and IRF3KO mice. Tumor bearing mice were treated with i. p. PBS or 250 μg/ml poly I:C on day 0 and 5 and tumor growth was monitored on subsequent days. As expected, B16 melanoma growth was significantly decreased in tumor-bearing C57BL/6 mice treated with poly I:C (Fig. 2A). Unexpectedly, poly I:C treatment significantly decreased tumor growth in IRF3KO mice compared with PBS treated IRF3KO mice (Fig. 2B). Therefore, poly I:C is effective at reducing growth of melanomas that are expressing IRF3 even in the absence of IRF3 in the tumor-bearing host.
Fig 2.
In vivo and in vitro effect of treatment with poly I:C on B16-F10 melanoma growth. C57Bl/6 and IRF3KO mice were subcutaneously injected with 106 B16-F10 melanoma cells on day 0, intraperitoneally injected with PBS or 250 μg poly I:C on day 0 and then again on day 5, and tumor growth of individual mice was monitored for 16 days post injection or tumors were extracted on day 19 and weighed individually. (A, B) Data are means of tumor areas + standard error, n= 6–7. P<.0001 for differences between PBS and poly I:C treatment for C57BL/6 mice (A) and IRF3KO mice (B) by two-way ANOVA analysis. (C) B16-F10 (5 × 104) melanoma cells were grown in cell culture media with or without 50 μg/ml poly I:C and viable cells were enumerated after 24, 48, and 72 h using trypan blue and hemacytometer microscopy. Data are means + standard error, n=3. P<.0001 for differences between control and poly I:C treated B16 cells by two-way ANOVA analysis.
It has been presumed that poly I:C is an effective adjuvant in slowing tumor growth due to its effect on the immune system of tumor bearing experimental animals. The previous experiment indicates that even when IRF3 is ablated in tumor-bearing animals, poly I:C affects tumor growth, presumably through activation of tumor cell IRF3. To test this, B16-F10 cells were treated with 50 μg/ml poly I:C in vitro and cell growth was monitored for 72 h. The data confirm that poly I:C directly slows the growth of B16-F10 melanoma cells (Fig. 2C).
3.3 IRF3 deficiency impairs infiltration of intratumoral NK cells
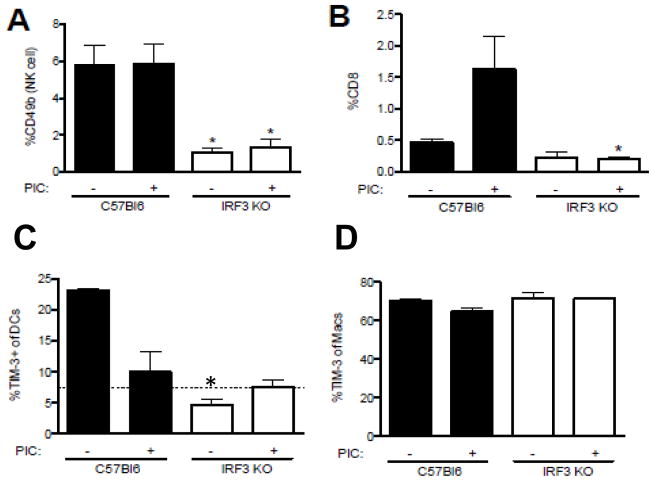
IRF3 has been shown to contribute to the development of anti-tumor CD8 T cells by contributing to expression of IL-15, IL-12, and IL-6 [35] and activation of NK cells by inducing INAM [3]. To determine if IRF3 deficiency affects the infiltration of NK cells into growing tumors, B16-F10 cells were injected into C57BL/6 and IRF3KO mice. At day 16, tumors were extracted and mononuclear cells were isolated and FACS analyzed with antibodies to CD49b (NK cell marker) or CD8. The results show that IRF3 deficiency significantly impaired the infiltration of intratumoral NK cells (Fig. 3A) but not infiltration of CD8 T cells (Fig. 3B). Treatment of tumor bearing mice with poly I:C did not increase intratumoral NK cells in either C57BL/6 or IRF3KO mice (Fig. 3A). However, poly I:C treatment significantly increased CD8 T cell infiltration into tumors of C57BL/6 mice (Fig. 3B), but did not increase CD8 T cell infiltration into tumors of IRF3KO mice. These data suggest that IRF3 is required both for NK cell infiltration into tumors and poly I:C stimulation of CD8 T cell infiltration into melanomas.
Fig 3.
Effects of IRF3 and poly I:C on phenotype of tumor infiltrating mononuclear cells. Day 16 B16-F10 melanomas were excised, dissociated and mononuclear cells stained with antibodies to cell surface CD49b (A), CD8 (B), CD11c (C), CD11b (D), and TIM-3 (C, D). Data are means of % of total mononuclear cells, or % of gated NK cells (CD49b), CD8 T cells, or DCs (CD11c), and macrophages (CD11b) expressing TIM-3 for individual mice. Means were analyzed by Student t test. *P<.05 compared to C57Bl/6.
3.4 IRF3 is required for TIM-3 expression by DC and for poly I:C repression of TIM-3 expression by intratumoral DCs
In contrast to INAM expression by tumor-associated DCs and macrophages, which brings about anti-tumor effects through NK cells, TIM-3 expression by tumor-associated DCs and macrophages suppresses anti-tumor immune responses [8]. Therefore, we determined TIM-3 expression by FACS analysis of intratumoral CD11c and CD11b cells at day 16 of tumor growth with or without poly I:C treatment. The results show that TIM-3 expression by CD11c DCs was greater in tumor-bearing C57Bl/6 mice compared with IRF3KO mice. Also, treatment with poly I:C significantly decreased TIM-3 expression by intratumoral DCs (Fig. 3C). In contrast to DCs, TIM-3 expression by CD11b intratumoral macrophages was high in both tumor-bearing C57Bl/6 and IRF3KO mice, however poly I:C treatment had little effect on expression of TIM-3 by intratumoral macrophages (Fig. 3D). Therefore, IRF3 contributes to TIM-3 expression by DCs but also contributes to its repression by poly I:C.
3.5 IRF3 deficiency precludes GrB and IFN-γ expression by NK cells in response to poly I:C
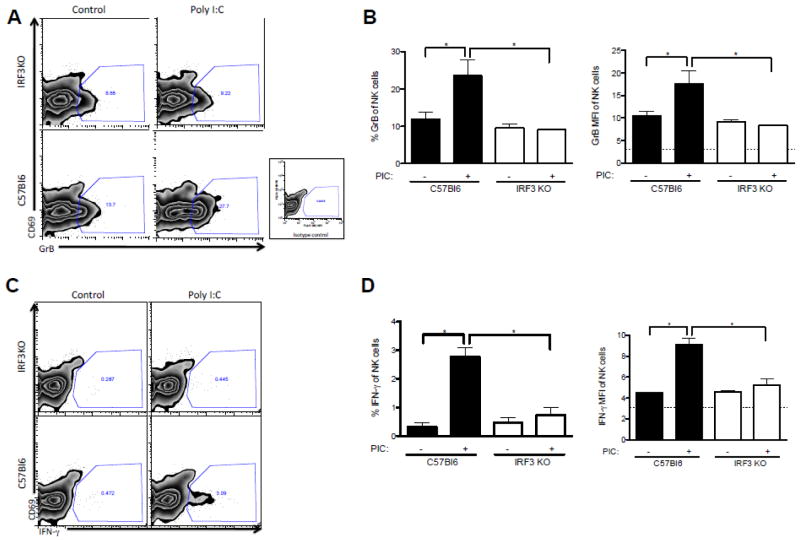
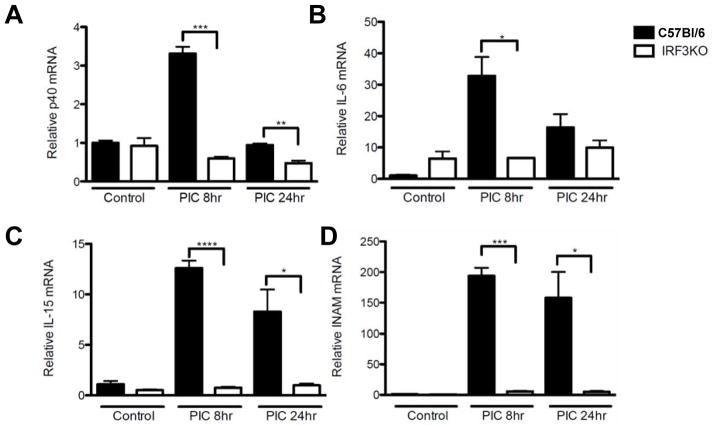
Data from several labs indicate that expression of GrB [36] and IFN-γ [37] are critical factors in the anti-tumor effect of activated NK cells. Therefore, splenic mononuclear cells from C57Bl/6 and IRF3KO mice were treated with poly I:C in vitro and after 24 h, CD49b+ NK cells were examined by FACS analysis for intracellular GrB and IFN-γ. Poly I:C treatment significantly increased expression of GrB and IFN-γ in CD49b+ NK cells of C57Bl/6 mice but did not increase expression of GrB (Fig. 4 A,B) or IFN-γ (Fig. 4 C,D) from those of IRF3KO mice. Poly I:C may in part control tumor growth by activating macrophage IRF3, which induces expression of NK cell-promoting cytokines, such as IL-12, IL-6, and IL-15, or NK cell-activating molecules, such as INAM. To determine if IRF3 deficiency had an impact on expression of poly I:C induced cytokines and NK cell-activating molecules from APCs that contribute to induction of GrB and IFN-γ, macrophages from C57Bl/6 and IRF3KO mice were treated with poly I:C for 8 and 24 h and IL-12 p40, IL-15, IL-6, and INAM expression was evaluated by qRT-PCR. The results indicate that IRF3 deficiency significantly impaired poly I:C induced expression of IL-12 p40, IL15, IL-6, and INAM (Fig. 5). Therefore, IRF3 likely contributes to activation and intratumoral infiltration of NK cells expressing GrB and IFN-γ, in part through expression of cytokines and NK-cell activating molecules by inflammatory macrophages.
Fig 4.
In vitro effect of poly I:C on activation of NK cells. Splenic mononuclear cells of C57Bl/6 and IRF3KO mice were treated with poly I:C (50 μg/ml) for 24 h and then stained with fluorescent antibodies to cell surface CD49b and fluorescent antibodies to intracellular GrB and IFN-γ. Data are representative FACS plots (A, C) and means + standard error (B, D) of % NK cells (CD49b) expressing GrB or IFN-γ or of mean fluorescent intensity (MFI) for individual mice. *P<.05 compared to C57Bl/6.
Fig 5.
Key macrophage factors that contribute to NK cell activity are impaired in IRF3 deficiency. Peritoneal inflammatory macrophages, from C57Bl/6 or IRF3KO mice, at 1 × 106/ml of culture media were treated with or without 50 μg/ml poly I:C for 8 or 24 h after which cells were lysed, RNA extracted, and analyzed for expression of (A) IL-12 p40, (B) IL-6, (C) IL-15, and (D) INAM by qRT-PCR. Means + standard error of relative expression normalized to GAPDH expression were analyzed by Student t test. *P<.05, **P<.01, ***P<.005 compared to C57Bl/6.
4. Discussion
The data herein, using the B16 melanoma mouse model, point to IRF3 as a critical factor leading to responses controlling tumor growth. We found that IRF3 has direct and indirect effects on growth of B16 melanomas. Specifically, IRF3 was found to indirectly affect the growth of B16 melanomas by contributing to the infiltration of NK cells into the tumor. This suggests that IRF3 promotes expression of chemokines for NK cell intratumoral infiltration. IRF3 has been shown to be responsible for expression of several chemokines NK cells including Rantes and IP-10 [38,39] and activated NK cells express receptors for these chemokines [40]. Therefore, IRF3 likely contributes to intratumoral infiltration of NK cells seen in this report through its well-known role in expression of Rantes and IP-10.
We found that poly I:C, a TLR3 agonist that activates IRF3 and proposed as an adjuvant in cancer therapy, decreased tumor growth in IRF3KO mice. These data suggest that activation of IRF3 directly affects growth of B16 tumor cell, which do express IRF3. Indeed overexpression of IRF3 in B16 melanoma cells has previously been found to decrease tumor growth when the tumor cells were implanted into mice [38]. This was attributed to IRF3 induced expression of Rantes and IP-10 from B16 melanoma cells. Therefore, poly I:C induction of Rantes and IP-10 from the implanted B16 melanoma cells could increase NK cell infiltration. However, others have shown that IRF3 decreases cell growth by increasing p53 and p21 activity in cells [41] through induction of promyelocytic leukemia (PML) protein[42]. Moreover IRF3 is involved in induction of apoptosis through induction of Noxa [43] and by dimerization with Bax and translocation to mitochondria [44]. Recently, poly I:C through TLR3 has been shown to induce necroptosis in an IRF3 independent but RIP-3 dependent fashion [45]. Our data suggest that, in addition to its IRF3-dependent effect on NK cells, poly I:C directly contributes to reduced proliferation of B16 melanoma cells. The observed direct effect of poly I:C could be due to IRF3-dependent cell cycle arrest and apoptosis or IRF3-independent necrosis.
In addition to its effect on NK cell infiltration into B16 melanomas IRF3 also has an impact on poly I:C induction of NK cell cytolytic potential. One of the main anti-tumor effects of poly I:C is its ability to enhance NK cell activity [33]. We showed that IRF3 deficiency impaired poly I:C-induced NK cell expression of GrB and IFN-γ. Similarly, we have seen recently that IRF3 deficiency impairs GrB and IFN-γ expression in recall responses of CTL from virus infected mice (data not shown). Because of these effects on NK cells, poly I:C is viewed as a promising adjuvant in vaccine development and in therapy of cancers [46]. However, evidence so far indicates that the effect of poly I:C on NK cell activity is through its ability to induce the expression of NK cell-enhancing cytokines, such as IL-12 and IL-15 from macrophages and DCs or ligands of NK activating receptors, such as INAM. We show here that expression of poly I:C-induced IL-12 p40, IL-15, IL-6, and INAM is impaired in macrophages of IRF3KO mice, which strongly suggests that reduced poly I:C induced GrB and IFN-γ expression is due to deficient expression of IL-12, IL-15, or INAM from macrophages. It is not known if poly I:C directly induces expression of GrB or IFN-γ by NK cells.
The data herein concerning the role of IRF3 in NK cell activity during tumor growth is significant due to the fact that virus infection often target IRF3 to decrease its activity so as to establish the cellular environment for viral replication. The influenza virus NS1 protein [47], rotavirus NSP1 protein [48], and Theiler’s virus L-protein [49] inhibit IRF3 activity. In addition, following viral infection and nuclear localization of IRF3 for transcriptional purposes, IRF3 exports from the nucleus, is polyubiquinated, and degraded at the proteasome [50]. Therefore virus infection either directly or indirectly contributes to intermittent decreases in IRF3 activity. Our data herein suggest that any decrease in IRF3 activity will impair NK cell activity and contribute to accelerated tumor growth.
Acknowledgments
The authors wish to thank Marian Schmid for her excellent animal care and animal technical services. This work was supported by funding from the University of Nebraska Medical Center College of Dentistry and University of Nebraska Lincoln, School of Biological Sciences, and supported by Award Number P30GM10350903 and P20GM103489 from the National Institute of General Medicine, a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Conflict of Interest
The authors of this paper have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakamura MC, Niemi EnC, Fisher MJ, Shultz LD, Seaman WE, Ryan JC. Mouse Ly-49A Interrupts Early Signaling Events in Natural Killer Cell Cytotoxicity and Functionally Associates with the SHP-1 Tyrosine Phosphatase. The J Exp Med. 1997;185:673–684. doi: 10.1084/jem.185.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerwenka A, Bakker ABH, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic Acid Early Inducible Genes Define a Ligand Family for the Activating NKG2D Receptor in Mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 3.Ebihara T, Azuma M, Oshiumi H, Kasamatsu J, Iwabuchi K, Matsumoto K, Saito H, Taniguchi T, Matsumoto M, Seya T. Identification of a polyI:C-inducible membrane protein that participates in dendritic cell-mediated natural killer cell activation. J Exp Med. 2010;207:2675–2687. doi: 10.1084/jem.20091573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, Biron CA. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279–87. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 5.Harada M, Matsuzaki G, Shinomiya Y, Kurosawa S, Ito O, Okamoto T, Takenoyama M, Sumitika H, Nishimura Y, Nomoto K. Generation of tumor-specific cytotoxic T lymphocytes in vivo by combined treatment with inactivated tumor cells and recombinant interleukin-2. Cancer Immunol Immunother. 1994;38:332–8. doi: 10.1007/BF01525512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohkawa T, Seki S, Dobashi H, Koike Y, Habu Y, Ami K, Hiraide H, Sekine I. Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells. Immunology. 2001;103:281–90. doi: 10.1046/j.1365-2567.2001.01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D, Liu Y, Zhu F, Zhang L, Sun W, Liang X, Gao L, Ma C. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol. 2010;52:322–9. doi: 10.1016/j.jhep.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, Hirashima M, Uede T, Takaoka A, Yagita H, Jinushi M. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13:832–42. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 10.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazumder A, Rosenberg SA. Successful immunotherapy of natural killer-resistant established pulmonary melanoma metastases by the intravenous adoptive transfer of syngeneic lymphocytes activated in vitro by interleukin 2. J Exp Med. 1984;159:495–507. doi: 10.1084/jem.159.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–70. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 13.Shresta S, Heusel JW, Macivor DM, Wesselschmidt RL, Russell JH, Ley TJ. Granzyme B plays a critical role in cytotoxic lymphocyte-induced apoptosis. Immunol Rev. 1995;146:211–21. doi: 10.1111/j.1600-065x.1995.tb00690.x. [DOI] [PubMed] [Google Scholar]
- 14.Hamerman JA, Ogasawara K, Lanier LL. Cutting Edge: Toll-Like Receptor Signaling in Macrophages Induces Ligands for the NKG2D Receptor. J Immunol. 2004;172:2001–2005. doi: 10.4049/jimmunol.172.4.2001. [DOI] [PubMed] [Google Scholar]
- 15.Carayannopoulos LN, Naidenko OV, Kinder J, Ho EL, Fremont DH, Yokoyama WM. Ligands for murine NKG2D display heterogeneous binding behavior. Eur J Immunol. 2002;32:597–605. doi: 10.1002/1521-4141(200203)32:3<597::aid-immu597>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Kodama T, Takeda K, Shimozato O, Hayakawa Y, Atsuta M, Kobayashi K, Ito M, Yagita H, Okumura K. Perforin-dependent NK cell cytotoxicity is sufficient for anti-metastatic effect of IL-12. Eur J Immunol. 1999;29:1390–6. doi: 10.1002/(SICI)1521-4141(199904)29:04<1390::AID-IMMU1390>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 17.Yajima T, Nishimura H, Wajjwalku W, Harada M, Kuwano H, Yoshikai Y. Overexpression of interleukin-15 in vivo enhances antitumor activity against MHC class I-negative and -positive malignant melanoma through augmented NK activity and cytotoxic T-cell response. Int J Cancer. 2002;99:573–8. doi: 10.1002/ijc.10395. [DOI] [PubMed] [Google Scholar]
- 18.Lupetti R, Pisarra P, Verrecchia A, Farina C, Nicolini G, Anichini A, Bordignon C, Sensi M, Parmiani G, Traversari C. Translation of a Retained Intron in Tyrosinase-related Protein (TRP) 2 mRNA Generates a New Cytotoxic T Lymphocyte (CTL)-defined and Shared Human Melanoma Antigen Not Expressed in Normal Cells of the Melanocytic Lineage. J Exp Med. 1998;188:1005–1016. doi: 10.1084/jem.188.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahlberg A, Auble MR, Petro TM. Reduced expression of IL-12 p35 by SJL/J macrophages responding to Theiler's virus infection is associated with constitutive activation of IRF-3. Virology. 2006;353:422–432. doi: 10.1016/j.virol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 20.Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory Monocytes Activate Memory CD8(+) T and Innate NK Lymphocytes Independent of Cognate Antigen during Microbial Pathogen Invasion. Immunity. 2012;37:549–62. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thaxton JE, Nevers T, Lippe EO, Blois SM, Saito S, Sharma S. NKG2D Blockade Inhibits Poly(I:C)-Triggered Fetal Loss in Wild Type but Not in IL-10-/- Mice. J Immunol. 2013;190:3639–3647. doi: 10.4049/jimmunol.1203488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heil F, Ahmad-Nejad P, Hemmi H, Hochrein H, Ampenberger F, Gellert T, Dietrich H, Lipford G, Takeda K, Akira S, Wagner H, Bauer S. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur J Immunol. 2003;33:2987–97. doi: 10.1002/eji.200324238. [DOI] [PubMed] [Google Scholar]
- 23.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–31. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 24.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–8. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 25.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–33. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 26.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–48. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 27.Wathelet MG, Lin CH, Parekh BS, Ronco LV, Howley PM, Maniatis T. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol Cell. 1998;1:507–18. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- 28.Goriely S, Molle C, Nguyen M, Albarani V, Haddou NO, Lin R, De Wit D, Flamand V, Willems F, Goldman M. Interferon regulatory factor 3 is involved in Toll-like receptor 4 (TLR4)- and TLR3-induced IL-12p35 gene activation. Blood. 2006;107:1078–84. doi: 10.1182/blood-2005-06-2416. [DOI] [PubMed] [Google Scholar]
- 29.Trinchieri G, Wysocka M, D'Andrea A, Rengaraju M, Aste-Amezaga M, Kubin M, Valiante NM, Chehimi J. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog Growth Factor Res. 1992;4:355–68. doi: 10.1016/0955-2235(92)90016-b. [DOI] [PubMed] [Google Scholar]
- 30.Naume B, Gately M, Espevik T. A comparative study of IL-12 (cytotoxic lymphocyte maturation factor)-, IL-2-, and IL-7-induced effects on immunomagnetically purified CD56+ NK cells. J Immunol. 1992;148:2429–36. [PubMed] [Google Scholar]
- 31.Talmadge JE, Meyers KM, Prieur DJ, Starkey JR. Role of natural killer cells in tumor growth and metastasis: C57BL/6 normal and beige mice. J Natl Cancer Inst. 1980;65:929–35. [PubMed] [Google Scholar]
- 32.Akazawa T, Ebihara T, Okuno M, Okuda Y, Shingai M, Tsujimura K, Takahashi T, Ikawa M, Okabe M, Inoue N, Okamoto-Tanaka M, Ishizaki H, Miyoshi J, Matsumoto M, Seya T. Antitumor NK activation induced by the Toll-like receptor 3-TICAM-1 (TRIF) pathway in myeloid dendritic cells. Proc Natl Acad Sci USA. 2007;104:252–257. doi: 10.1073/pnas.0605978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyake T, Kumagai Y, Kato H, Guo Z, Matsushita K, Satoh T, Kawagoe T, Kumar H, Jang MH, Kawai T, Tani T, Takeuchi O, Akira S. Poly I:C-Induced Activation of NK Cells by CD8 Dendritic Cells via the IPS-1 and TRIF-Dependent Pathways. J Immunol. 2009;183:2522–2528. doi: 10.4049/jimmunol.0901500. [DOI] [PubMed] [Google Scholar]
- 34.Chuang HC, Huang CC, Chien CY, Chuang JH. Toll-like receptor 3-mediated tumor invasion in head and neck cancer. Oral Oncology. 2012;48:226–232. doi: 10.1016/j.oraloncology.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Wang C, Zhuang Y, Zhang Y, Luo Z, Gao N, Li P, Pan H, Cai L, Ma Y. Toll-like receptor 3 agonist complexed with cationic liposome augments vaccine-elicited antitumor immunity by enhancing TLR3-IRF3 signaling and type I interferons in dendritic cells. Vaccine. 2012;30:4790–9. doi: 10.1016/j.vaccine.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 36.Shi L, Mai S, Israels S, Browne K, Trapani JA, Greenberg AH. Granzyme B (GraB) autonomously crosses the cell membrane and perforin initiates apoptosis and GraB nuclear localization. J Exp Med. 1997;185:855–66. doi: 10.1084/jem.185.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoover RG, Gullickson G, Kornbluth J. Impaired NK cytolytic activity and enhanced tumor growth in NK lytic-associated molecule-deficient mice. J Immunol. 2009;183:6913–21. doi: 10.4049/jimmunol.0901679. [DOI] [PubMed] [Google Scholar]
- 38.Maghazachi AA, al-Aoukaty A, Schall TJ. C-C chemokines induce the chemotaxis of NK and IL-2-activated NK cells. Role for G proteins. J Immunol. 1994;153:4969–77. [PubMed] [Google Scholar]
- 39.Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155:3877–88. [PubMed] [Google Scholar]
- 40.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:173–83. [PubMed] [Google Scholar]
- 41.Kim TK, Lee JS, Jung JE, Oh SY, Kwak S, Jin X, Lee SY, Lee JB, Chung YG, Choi YK, You S, Kim H. Interferon regulatory factor 3 activates p53-dependent cell growth inhibition. Cancer Letters. 2006;242:215–221. doi: 10.1016/j.canlet.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Kim T-K, Lee J-S, Oh S-Y, Jin X, Choi Y-J, Lee T-H, Lee Eh, Choi Y-K, You S, Chung YG, Lee J-B, DePinho RA, Chin L, Kim H. Direct Transcriptional Activation of Promyelocytic Leukemia Protein by IFN Regulatory Factor 3 Induces the p53-Dependent Growth Inhibition of Cancer Cells. Cancer Res. 2007;67:11133–11140. doi: 10.1158/0008-5472.CAN-07-1342. [DOI] [PubMed] [Google Scholar]
- 43.Goubau D, Romieu-Mourez R, Solis M, Hernandez E, Mesplede T, Lin R, Leaman D, Hiscott J. Transcriptional re-programming of primary macrophages reveals distinct apoptotic and anti-tumoral functions of IRF-3 and IRF-7. Eur J Immunol. 2009;39:527–40. doi: 10.1002/eji.200838832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chattopadhyay S, Marques JT, Yamashita M, Peters KL, Smith K, Desai A, Williams BRG, Sen GC. Viral apoptosis is induced by IRF-3-mediated activation of Bax. Embo J. 2010;29:1762–73. doi: 10.1038/emboj.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like Receptor 3-mediated necrosis via TRIF, RIP3 and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jasani B, Navabi H, Adams M. Ampligen: a potential toll-like 3 receptor adjuvant for immunotherapy of cancer. Vaccine. 2009;27:3401–4. doi: 10.1016/j.vaccine.2009.01.071. [DOI] [PubMed] [Google Scholar]
- 47.Talon J, Horvath CM, Polley R, Basler CF, Muster T, Palese P, Garcia-Sastre A. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J Virol. 2000;74:7989–96. doi: 10.1128/jvi.74.17.7989-7996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graff JW, Mitzel DN, Weisend CM, Flenniken ML, Hardy ME. Interferon regulatory factor 3 is a cellular partner of rotavirus NSP1. J Virol. 2002;76:9545–50. doi: 10.1128/JVI.76.18.9545-9550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ricour C, Delhaye S, Hato SV, Olenyik TD, Michel B, van Kuppeveld FJM, Gustin KE, Michiels T. Inhibition of mRNA export and dimerization of interferon regulatory factor 3 by Theiler's virus leader protein. J Gen Virol. 2009;90:177–186. doi: 10.1099/vir.0.005678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saitoh T, Tun-Kyi A, Ryo A, Yamamoto M, Finn G, Fujita T, Akira S, Yamamoto N, Lu KP, Yamaoka S. Negative regulation of interferon-regulatory factor 3-dependent innate antiviral response by the prolyl isomerase Pin1. Nat Immunol. 2006;7:598–605. doi: 10.1038/ni1347. [DOI] [PubMed] [Google Scholar]