Abstract
Objective
To measure bone age (BA) in patients with Cushing syndrome (CS) before and 1-year after transsphenoidal surgery or adrenalectomy, and to correlate BA with hormonal and other measurements.
Study design
Case series at the National Institutes of Health Clinical Center including 93 children with Cushing Disease (CD) (43 F, 12.3±2.9 yr.) and 31 children with ACTH-independent CS (AICS) (22 F, 10.3±4.5 yr.). BA was obtained prior to surgery and at follow-up. Outcome measures were comparison of BA in CD versus AICS; and analysis of the effect of hypercortisolism, insulin excess, BMI and androgen excess on BA.
Results
26 out of 124 (21.0%) children with CS had advanced BA, compared with the expected general population prevalence of 2.5% (p<0.0001). Only 4/124 (3.2%) had delayed BA. The majority of patients (76%) had normal bone age. Average BA Z-score (BAZ) was similar in patients with CD and AICS (0.6±1.4 vs. 0.5±1.8, p=0.8865). BMI SDS, and normalized values of DHEA, DHEA-S, androsteonedione, estradiol and testosterone were all significantly higher in the patients with advanced bone age versus those with normal or delayed bone age. 59 cured patients had follow-up BA 1.2±0.3 years after TSS, with decrease of BAZ (1.0±1.6 vs. 0.3±1.4, p<0.0001).
Conclusions
Contrary to common belief, endogenous CS in children appears to be associated with normal or even advanced skeletal maturation. When present, bone age advancement in CS is related to obesity, insulin resistance and elevated levels of adrenal androgens (and their aromatization). This finding may have significant implications for treatment decisions and final height predictions in these patients.
Keywords: growth plate, hypercortisolism, hyperandrogenism
One of the hallmarks of Cushing syndrome (CS) in pediatrics is growth failure 1-4. However, only a limited number of reports describe the status of skeletal maturation at diagnosis of Cushing Disease (CD), and none describe bone age (BA) in ACTH-independent CS (AICS). Glucocorticoids impair somatic growth by directly inhibiting the development of epiphyseal cartilage in growing long bones 5. Androgens, via aromatization to estrogens, induce epiphyseal closure 6. CD is associated with elevated ACTH, excessive virilization, and increased adrenal androgens 7.
Prolonged exposure to exogenous corticosteroids is associated with growth inhibition and delayed skeletal maturation. In 1965, a study of 36 children with adrenocortical virilism, hypopituitarism, Addison disease, and allergy demonstrated that administration of 35-50 mg of hydrocortisone per square meter per day tended to reduce rates of growth and skeletal maturation below normal8. Skeletal maturation is delayed in children with chronic disease who receive long term exogenous glucocorticoid therapy 9. The effects of corticosteroid treatment on growth and skeletal maturation has been evaluated in children with severe asthma; however, poor growth and pubertal delay due to underlying disease may have independent effects on bone maturation. Interestingly, suppression of BA advancement has been shown to be more profound in boys with asthma exposed to glucocorticoids as compared with girls 10. Exogenous steroid inhibition of growth and skeletal maturation has been shown to be dose-dependent 11. Additional evidence of the effect of steroids on inhibition of skeletal maturation comes from the observation that after suspension of steroid treatment, bone maturation was equivalent to that of healthy children 11. More pronounced BA delay in patients versus controls has also been noted in children with steroid-dependent nephrotic syndrome 12.
Smaller studies of BA specifically in pediatric patients with CS have yielded conflicting results, with some studies reporting delayed BA in the majority of patients, and others report BA consistent with chronological age in the majority of patients 13-15. Ours is the largest report of BA in children with CS thus far. In addition, it is the first report comparing BA in children with CD versus AICS, and the first longitudinal BA analysis in children with CS. Based on the fact that in CD, patients have elevated ACTH and elevated levels of adrenal steroids that in turn are aromatized to estrogens, we hypothesized that children with CD might have more advanced BA Z-scores (BAZ) compared with patients with AICS. We also hypothesized that BMI, androgen excess, insulin resistance, and IGF-1 excess may correlate with BA. As a secondary objective, we aimed to see how BAZ changed after surgical cure of CS.
Methods
Children with the diagnosis of CD (n=93) and children with AICS (n=31) were included in the study. Criteria included having had a preoperative BA performed and completion of diagnostic TSS or adrenalectomy at the NIH between January 1994 and January 2012. Of these individuals, 64 had a follow-up BA film performed at the 1-year follow-up, 5 of whom had recurrence of their CD and were excluded from the follow-up BA analysis. All studies were conducted under clinical protocol 97-CH0076 that was approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. Informed consent from the patients' parents (and assent from older children) was obtained for all patients. Diagnosis of CD was confirmed, as previously described 16. A single radiologist read the BA blinded to the diagnosis using the Greulich and Pyle atlas 17. Advanced and delayed BA was defined as BAZ of ≥2 and ≤−2, respectively. Pre-surgical bone age and hormonal measurements were taken a mean of 1.2 ± 2 months prior to the date of surgery. Testicular volume was measured using the Prader orchidometer.
Hormonal Assays
Androstenedione, dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEAS) were measured by high performance liquid chromatography/ tandem mass spectrometry (LC-MS/MS) from 2006 to present and by radioimmunoassay prior to 2005 at Mayo Medical Labs, Rochester, MN. Testosterone and insulin-like growth factor-1 (IGF-1) were measured by chemiluminescence immunoassay on Siemens Immulite 2500 analyzer. IGF-1 Z scores were calculated using the age-specific normal ranges provided by the NIH Clinical Center laboratory. Estradiol (E2) and fasting insulin were measured via electrochemiluminescence immunoassay on Roche Cobas e601 analyzer. Adrenocorticotropic hormone (ACTH) was measured from 2005-2012 on the Siemens Immulite 2500 analyzer, whereas prior specimens were measured via Nichols Advantage Immunochemiluminometric assay (ICMA). Diurnal plasma cortisol was obtained by placing an IV catheter at least 2 hours before the test; cortisol levels were drawn at 2330 and 2400, and the patient was asleep to determine midnight cortisol values. Plasma cortisol was measured by chemiluminescence immunoassay. Twenty-four-hour urinary free cortisol (UFC) was averaged from two separate preoperative measurements and was measured by LC-MS/MS. In order to account for known differences in androgen levels between sexes and at different ages, values of DHEA-S, DHEA, androsteonedione,estradiol and testosterone were normalized and expressed as a ratio of the patients value to the mean value for age and sex18, 19.
Statistical analyses
Simple descriptive statistics and frequency distributions described the data, which are reported as mean ± standard deviation (SD) or median (inter-quartile range (IQR): 25th percentile, 75th percentile), and frequency (count). Comparisons of continuous data between groups (CD vs. AICS, males vs. females, advanced BA vs. delayed/normal VA) were done by t-tests, or Wilcoxon rank-sum tests, as appropriate. Where necessary, certain data were log-transformed for comparisons. Fisher exact tests compared categorical data between groups. A one-sample binomial test compared the prevalence of advanced BA in children with CS to the expected prevalence of 2.5% in the general population. Analysis of covariance (ANCOVA) considered the role of sex in the comparison of certain clinical features, such as testosterone and estradiol. Logistic regression modeling and correlation analyses were carried out for assessing the relations among clinical features and BA. Initial and 1-year follow-up data were compared by paired t-test and McNemar's test. A p-value ≤0.05 was considered statistically significant. Data were analyzed using SAS v9.2 (SAS Institute, Inc, Cary, NC).
Results
Data from 93 children with CD (43 F, mean age12.3±2.9 yr.) and 31 children with AICS (22 F, mean age 10.3±4.5 yr.) were retrospectively analyzed. The date of the pre-surgical bone age and laboratory evaluation was 1.2±2 months prior of the date of surgery. These and other demographics are presented in Table I. As ACTH-independent CS is known to be more common in females, it follows that our population had statistically significantly more females in the AICS group than in the CD group. When we compared characteristics of the children with CD to those with AICS (Table I), the children with CD were older at the time of surgery and their duration of symptoms was longer. As expected, individuals with CD had elevated ACTH compared with those with AICS. They also had higher DHEA and DHEA-S levels than those with AICS, similar results were found when these androgens were normalized and expressed as a ratio of the patient's value over the mean value for normal individuals of the same age and sex. Testosterone was not significantly different between the patients with CD and those with AICS, after normalized for age and sex. No statistically significant differences in Tanner stages, height SDS scores, IGF-1 Z-scores, androstenedione levels, estradiol levels, UFC, or midnight cortisol levels were noted when comparing patients with CD to those with AICS.
Table 1.
Baseline characteristics of children with CD and AICS1.
| CD (n=93) | AICS (n=31) | P-value | |
|---|---|---|---|
| Chronologic age at time of pre-surgery bone age | 12.3 ± 2.9 | 10.3 ± 4.5 | 0.0223* |
| Females(%)/males(%) | 43(46.2)/50(53.8) | 22(71.0)/9(29.0) | 0.0221* |
| 0.7981 | |||
| Race (%) | 4 (4.3) | 2 (6.5) | |
| Asian | 6 (6.4) | 3 (9.7) | |
| Black | 61 (65.6) | 21 (67.7) | |
| White Other/unknown |
22 (23.7) | 5 (16.1) | |
| 0.3301 | |||
| Ethnicity (%) | |||
| Latino or Hispanic | 24 (25.8) | 5 (16.1) | |
| 66 (71.0) | 26 (83.9) | ||
| Not Latino or Hispanic Unknown | 3 (3.2) | 0 | |
| Duration of symptoms until surgery (months) | 32.5 ± 21.0 | 26.2 ± 25.1 | 0.0178* |
| BMI Z-score | 2.3 ± 0.6 | 2.1 ± 0.7 | 0.0742 |
| Height SDS | −1.1 ±1.2 | −1.1 ± 1.6 | 0.9872 |
| Breast tanner stage (females) | 3.0 (3.0, 5.0) | 2.5 (1.0, 4.5) | 0.0741 |
| Testicular volume by Prader orchidometer, cc (males) | 8.0 (5.4, 10.0) | 4.5 (2.5, 15.0) | 0.1663 |
| Bone age, yr | 12.8 ± 2.9 | 10.7 ± 4.6 | 0.0312* |
| Bone age - Chronological age (difference in yr) | 0.5 ± 1.3 | 0.4 ± 1.4 | 0.4861 |
| Bone age Z-score | 0.6 ± 1.4 | 0.5 ± 1.8 | 0.8865 |
| ACTH, pg/mL | 53 ± 44 | 6 ± 3 | <0.0001* |
| IGF-1 Z-score | −0.4 ± 1.6 | 0.1 ± 1.8 | 0.1495 |
| DHEA, ng/dL | 595 ± 502 | 156±253 | <0.0001* |
| Normalized ratio of mean for age and gender DHEA, | 2.2 ± 2.0 | 1.0 ± 1.0 | <0.0001* |
| DHEA-S, μg/ dL | 236 ± 203 | 63 ± 83 | <0.0001* |
| Normalized ratio of mean for age and gender DHEA-S | 2.2 ± 2.0 | 0.9 ± 0.8 | <0.0001* |
| Androstenedione, ng/dL | 142±118 | 146±101 | 0.7952 |
| Normalized ratio of mean for age and gender Androstenedione, | 2.6 ± 2.3 | 4.4 ± 8.2 | 0.2031 |
| Estradiol, pg/mL | 23 ± 31 | 31 ± 44 | 0.2376 |
| Normalized ratio of mean for age and gender estradiol | 1.4±1.2 | 1.7±1.4 | 0.31 |
| Testosterone, ng/dL | 74 ± 137 | 40 ± 70 | 0.0471* |
| Normalized ratio of mean for age and gender Testosterone | 1.2± 1.2 | 1.4 ± 1.2 | 0.1869 |
| UFC, μg/24 hr | 322 ± 353 | 286 ± 259 | 0.2548 |
| Midnight cortisol, μg/dL | 19.7 ± 15.0 | 20.5 ± 13.6 | 0.7329 |
| Fasting insulin mcU/mL | 34.2 ± 33.6 | 25.4 ± 13.9 | 0.4611 |
| BMI Z score | 2.1 ± 0.7 | 2.3 ± 0.7 | 0.196 |
statistically significant difference
Data are reported as median (inter-quartile range: 25th percentile, 75th percentile) if they required a non-parametric test for comparisons. Otherwise, they are mean ± standard deviation.
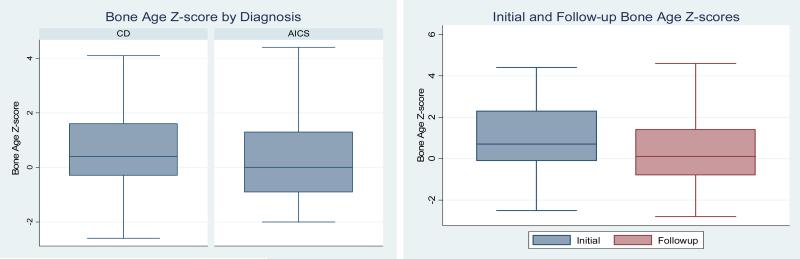
Average BAZ in patients with CD was not statistically different from patients with AICS. The median BAZ for individuals with CD and are shown in the Figure, A. Twenty-six out of 124 (21.0%) children with CS had advanced BA, compared with the expected prevalence of 2.5%. A nearly identical percentage of patients in both diagnostic groups (CD vs. AICS) had advanced BA, 19.4% (6 out of 31) AICS had advanced BA. Only 4 out of 124 of the entire patient cohort (3.2%) had delayed BA.
Figure.
(A) Box plot comparing baseline bone age Z scores in patients with Cushing disease versus those with ACTH-independent Cushing syndrome, 0.6± 1.4 vs. 0.5 ± 1.8, p = 0.8865), (B): Box plot comparing initial BAZ scores with BAZ scores at the one-year follow-up after surgical cure of CS (1.0±1.6 vs. 0.3±1.4, p<0.0001).
There were 65 females and 59 males in the cohort. Females were equally likely as males to have advanced BAZ.
We compared subject characteristics in the category of patients with advanced BA versus the population with normal or delayed BA (Table II). Patients with advanced BAZ were significantly younger than those with normal or delayed BAZ. IGF-1 Z-score was significantly higher in the group of patients with CS and advanced BA than those with normal/delayed BA. Height SDS score was also higher in the individuals with advanced BA compared with the normal/delayed group. When comparing the patients with advanced bone age versus those with normal or delayed bone age, the normalized ratio for sex and age appropriate values of DHEA, DHEA-S, androsteonedione, estradiol and testosterone were all significantly higher in the patients with advanced bone age versus those with normal or delayed bone age (Table II). Estradiol is known to be one of the most important hormones responsible for skeletal maturation. When corrected for age and sex, the ratio to the mean estradiol value for age and sex was higher in the individuals with advanced bone age (1.8 times the mean) versus those with delayed bone age (1.3 times the mean). Mean fasting insulin levels were higher in the group with advanced BA as compared with those with normal/delayed BA, but the difference did not reach statistical significance; BMI Z score was higher in the patients with advanced BA as compared to those with delayed/normal BA (Table II). In addition, there was a positive correlation between BAZ and each of the three variables individually: log-normal ratio of mean DHEA-S, log-normal ratio of mean D4A, and log-normal ratio of mean DHEA-S. Elevated IGF-1 Z-score was correlated with advanced BAZ, as was height SDS scores with BAZ. However because IGF-I SDS in adolescence can be biased by abnormalities in pubertal onset20, 21, we performed a regression analysis of IGF-1 SDS score and BAZ score adjusted for Tanner stage, separately in males and females. In both males and females, once we adjusted for Tanner stage, the relationship between IGF-1 Z score and BAZ was no longer significant. BMI SDS did have a positive correlation with BA Z score, (rp= 0.23, p= 0.0108). Also, fasting insulin did have a positive correlation with BA Z score (rp= 0.31, p= 0.0172)
Table 2.
Clinical characteristics of CS patients with advanced or normal/delayed bone age.
| Advanced Bone age Z-score (n=26) | Normal/Delayed Bone age Z-score (n=98) | P-value | |
|---|---|---|---|
| Chronologic age at time of pre-surgery bone age | 9.9 ± 2.5 | 12.3 ± 3.5 | 0.0001* |
| Duration of symptoms (months) | 36.7 ± 25.5 | 29.4 ± 21.1 | 0.1150 |
| Height SDS | −0.3 ± 1.1 | −1.3 ± 1.3 | 0.0005* |
| Breast tanner stage (females) | 3.0 (1.0, 4.0) | 3.0 (2.0, 5.0) | 0.0679 |
| Testicular volume by Prader orchidometer (males) | 6.5 (6.0, 8.0) | 8.5 (4.5, 20.0) | 0.3327 |
| ACTH, pg/mL | 52 ± 69 | 39 ± 33 | 0.5367 |
| IGF-1 Z-score | 0.7 ± 1.8 | −0.6 ± 1.5 | 0.0004* |
| DHEA-S, μ g/ dL | 206±212 | 189 ± 192 | 0.5117 |
| Normalized ratio of mean for age and sex DHEA-S | 3.2±2.8 | 1.5±1.3 | 0.0001* |
| DHEA, ng/dL | 566 ± 622 | 457 ± 445 | 0.5973 |
| Normalized ratio of mean for age and sex DHEA | 2.9±2.6 | 1.6±1.5 | 0.0054* |
| Androstenedione, ng/dL | 140 ± 95 | 144±119 | 0.8382 |
| Normalized ratio of mean for age and sex Androstenedione | 4.2±3.0 | 2.8±4.9 | 0.0010* |
| Estradiol, pg/mL | 25 ± 42 | 24 ± 32 | 0.4574 |
| Normalized ratio of mean for age and sex Estradiol | 1.8±1.1 | 1.3±1.1 | 0.016* |
| Testosterone, ng/dL | 32 ± 36 | 76 ± 139 | 0.0553 |
| Normalized ratio of mean for age and sex Testosterone | 1.8±1.4 | 1.1±1.1 | 0.0256* |
| UFC, μ g/24 hr | 206±201 | 343 ± 355 | 0.0598 |
| Midnight cortisol, μ g/dL | 17.3 ± 10.9 | 20.6 ± 15.5 | 0.2623 |
| Fasting insulin mcU/mL | 47.0±53.2 | 28.5±19.6 | 0.0651 |
| BMI Z score | 2.4±0.5 | 2.0±0.7 | 0.0146* |
statistically significant difference
We did find that individuals with advanced BAZ had higher UFCs than the individuals with normal or delayed BAZ, yet this did not reach statistical significance. To further explore this, we compared UFC in the individuals with advanced BAZ to a group restricted to only the 4 individuals with delayed BAZ, and this did in fact show that median UFC was higher in the individuals with delayed BAZ (325.5 mcg/24 hr vs 149.1 mcg/24 hr, p = 0.0185), suggesting that elevated UFC is associated with BA delay, yet the small numbers of patients in this group needs to be taken into account.
Sixty-four of the initial 124 (51.6%) of the patients had a follow-up BA examination performed at the 1-year follow- up. Of these, five with CD were excluded from the follow-up BA analysis due to recurrence of CD. Therefore, BA follow-up data were analyzed for 10 (32.3%) patients with AICS and 49 (57.0%) patients with CD total 59 (50.4%). The average time elapsed between the initial follow-up BA was as 1.2±0.3 years. Average BAZ significantly decreased from the pre-op BA to the 1-year follow-up (Figure, B). At follow-up, there were only 2 (3.4%) patients with delayed BA and 6 (10.2%) patients with advanced BA. All 6 with advanced BA at follow-up had advanced BA on the earlier examination; twelve of the patients who had advanced BA initially normalized at the follow-up exam. The growth velocity in the 1 year post TSS was no different in the group with advanced vs normal/delayed BA, annual growth velocity was 7.6 ± 3.2 cm/year in the group with advanced BA compared with 6.6 ± 3.5 cm/year in the group with delayed / normal BA.
Discussion
Our findings show that CS is associated with BA advancement; normalization occurred following surgical cure in 50% of the patients. This is the first study to compare the BA in individuals with CS from either a pituitary tumor or an adrenal tumor. Contrary to our hypothesis, there was no difference in the proportion of individuals with advanced BA in either group, although we found both elevated ACTH and elevated adrenal androgens in the CD group as compared with the CS group. BMI Z score as well as normalized levels of DHEAS, DHEA, androstenedione, estradiol, testosterone, were all significantly higher in individuals with advanced bone age, illustrating the impact of obesity and elevated androgens at increasing skeletal maturation. BAZ significantly decreased by an average of −0.6±1.0 Z-scores from the pre-operative to the one-year follow-up BA.
Our population as a whole had more patients with advanced BA than would be expected in a normal distribution, which is in contrast to the results of the previously published much smaller studies on BA in children with CS 1, 13-15, 22. The results of previous studies reporting bone age in pediatric CS over the years are presented in Table III. We found that individuals with advanced BAZ were younger as a group than those with normal or delayed BAZ. As advanced bone age may lead to premature fusion of the growth plates and resultant short stature, early diagnosis and treatment of these children is imperative in order to ensure their proper growth and development. Of note, this finding may be a function of how older children are not as severely affected as they have already had a larger percentage of their bone age maturation take place prior to developing CS. In the study by Magiakou et al13, published from the NIH clinical center in 1994, skeletal maturation was evaluated in 59 children and adolescents with CS; 81% had BA consistent with chronological age, 8% had an accelerated BA, and only 11% had delayed BA. Magiakou et al reported that the mean ± SD of BAZ for children with CD (n=50) was −0.2±1.4 and that of children with AICS (n=6) was −0.3±1.1; in addition, BA delay clinically correlated with delayed sexual development, and advancement correlated with early sexual development. The authors concluded that the BA of patients with CS reflects the combined effects of cortisol, which has an inhibitory effect, and adrenal androgens and gonadal steroids, which have a stimulatory effect.
Table 3.
Reported bone age in pediatric patients with Cushing syndrome over the years
| Reference | Age Median (range) | Sample size | Diagnostic Method | Definition of advanced BA | Definition of delayed BA | Bone Age % (n/N) | ||
|---|---|---|---|---|---|---|---|---|
| Normal | Delayed | Advanced | ||||||
| Hauffa et al 1984 (18) | 14.6 (10.4-18.8) | 10 | Greulich and Pyle | BA-CA>1 yr | BA-CA<-1 yr | 20% (2/10) | 80% (8/10) | 0 |
| Magiakou 1994 (13) | 14 (4-20) | 37 | Greulich and Pyle | BA Z score ≥ 2SD | BA Z score ≤-2SD | 81% (30/37) | 11% (4/37) | 8% (3/37) |
| Lebrethon 2000 (2) | 13.7 (6.8-17.6) | 10 | TW2RUS | BA-CA>1 yr | BA-CA<-1 yr | |||
| Davies 2005 (1) | 12.2 (6.4-16.6) | 14 | TW2RS | BA-CA>1 yr | BA-CA<-1yr | 57% (8/14) | 43% (6/14) | 0 |
| Peters et al 2007 (15) | 12.1 (5.8-17.4) | 17 | TW3 RUS | BA-CA >0 | BA-CA <0 | 0 | 88% (15/17) | (12%) 2/17 |
| BA-CA>1 yr | BA-CA<-1 yr | 29% (5/17) | 71% (12/17) | 0 | ||||
| Achayra et al 2010 (14) | 14.8 (9-19) | 48 | Greulich and Pyle | BA-CA>0 | BA-CA<0 | 27% (13/48) | 73% (35/48) | 0 |
| BA-CA>1 yr | BA-CA<-1 yr | 46% (22/48) | 54% (26/48) | 0 | ||||
| Present study 2013 | 12 (2-18) | 124 | Greulich and Pyle | BA-CA>1 yr | BA-CA<-1 yr | 65% (81/124) | 9% (11/124) | 26% (32/124) |
| BA Z score ≥ 2SD | BA Z score ≤-2SD | 76% (94/124) | 3% (4/124) | 21% (26/124) | ||||
Two prior publications reported specifically on BA and factors affecting skeletal maturation at diagnosis of pediatric CD 14, 15. However, both papers overestimated the prevalence of delayed BA; they defined “delayed BA” as a BA value less than CA value (ie, any child with a BA-CA of −1 to 0 would be considered delayed by this definition, even hough these individuals would be within normal limits). Using this definition, Acharya et. al. analyzed 48 patients with a mean age of 14.8 years and found BA delay present in 35/48 patients (mean delay 1.6 years, range 0.5-5 years). Our methodology of using BAZ to define delayed versus advanced BA takes into account the different standard deviations of BA as a child grows. In our study, using the definition of a BAZ ≤−2, only 4/124 (3%) had delayed BA, however using the definition provided by these authors, 37/124 would have delayed BA. Peters et. al. analyzed BA in 17 patents with CD, median age 12.1, and found BA delay present in 15/17 patients (mean delay 2.0 years, range −0.5-4.1 years). In a study by Davies et. al. of 14 children with CD, BA was consistent with chronological age (as defined by CA ±1 year) in 8 patients and delayed (BA-CA<−1 year) in the remaining 6 patients 1. Lebrethon et al published BA results in 10 patients mean age of 12.9 years with CD, BA was consistent with CA (CA ±1 year) in 5 and was delayed (BA-CA<−1 year) in 5 patients. Of note, Peters et al, Lebrethon et al and Davies et al used the Tanner and Whitehouse method to assess BA, and we used the Greulich and Pyle atlas.
The most clinically significant finding of our study is that a large proportion of the patients with CS had premature epiphyseal maturation. This is supported by the elevated normalized androgen and estrogen levels in the individuals with advanced BA. Our group recently reported a positive correlation between fasting insulin levels and waist circumference z- score in a cohort of children with CS 23. Advancement in skeletal maturation or inappropriately normal BA in the face of hypercortisolism may be accounted for in part by the extreme adiposity of children with CS. Indeed, BAZ scores correlated with BMI Z scores and fasting insulin levels in this study. Obesity is known to be associated with advanced skeletal maturity in childhood. A study of skeletal maturation in 252 children showed that BA-CA and BA/CA were significantly correlated with lean body mass, BMI, BMI SDS, and DXA fat mass (all r >0.46, p<0.001) 24. A longitudinal study of skeletal age in 521 subjects from birth to age 18 showed that among those individuals who developed a BMI of >25 kg/m2 in young adulthood, those who were overweight or obese were more skeletally advanced throughout childhood 25. Skeletal maturity differences between controls and individuals who became overweight were at their peak at chronological age of 12 in boys and 14 in girls (p<0.001) with obesity at that age associated with approximately one year of BA advancement as compared with the normal weight population 25.
There is a multitude of features of the underlying disease in CS that likely influence BA, including obesity, hyperandrogenism, hyperinsulinism, and endogenous hypercortisolism. We know about the opposing effects of glucocorticoid exposure and androgen excess to BA advancement and growth outcomes from experience with congenital adrenal hyperplasia 26 When normalized for age and sex, the younger patients with more advanced BA had higher androgen levels for age, and their epiphyseal plates were more sensitive for these high levels than the older children. In fact, the degree of androgen excesss correlaed with BA advancement, as did BMI Z score and fasting insulin levels. All of these factors contribute to the “inappropriately normal” BA in patients with hypercortisolism, and this may be part of the reason that these patients have compromised growth potential.
The likelihood of “catch up” growth after the cure of CS is further impaired by BA advancement at the time of diagnosis, and final height in patients with CS presenting in childhood is known to be compromised 27. Although it would be expected that a loss in the ratio of height age to BA would result in a tendency to decreased ultimate stature, unfortunately one limitation of our study is lack of available data on the adult height of these patients. In addition, it is impossible to measure the biologically active estradiol at the growth plate, and our current assays for estradiol fail to accurately measure levels at the low end of the normal range 28.
We conclude that BA advancement in children with CS is more common than previously reported. Delayed diagnosis of CS may be associated with further advancement of BA and loss of potential for catch-up growth. There are multifactorial contributions to BA in these patients; suppressed growth hormone secretion, pubertal delay and elevated cortisol levels likely contribute to BA delay, and elevated adrenal androgen levels, obesity, and insulin resistance contribute to BA advancement 3. Early recognition and proper management of CS in childhood is critical; individualization of the management of each case based on the data provided in this study may be needed for optimal final height outcome.
Acknowledgments
Supported by the Intramural program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
List of abbreviations
- ACTH
Adrenocorticotropic hormone
- AICS
Adrenocorticotropic hormone - independent Cushing Syndrome
- ANCOVA
Analysis of covariance
- BA
Bone age
- BAZ
Bone Age Z-score
- CD
Cushing Disease
- CS
Cushing syndrome
- DHEA
Dehydroepiandrosterone
- DHEAS
Dehydroepiandrosterone sulfate
- E2
Estradiol
- LC-MS/MS
High performance liquid chromatography/ tandem mass spectrometry
- IQR
Inter-quartile range
- ICMA
Immunochemiluminometric assay
- IGF-1
Insulin-like growth factor-1
- SD
Standard deviation
- UFC
Urinary free cortisol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Registered with ClinicalTrials.gov: NCT00001595
The authors declare no conflicts of interest.
References
- 1.Davies JH, Storr HL, Davies K, Monson JP, Besser GM, Afshar F, et al. Final adult height and body mass index after cure of paediatric Cushing's disease. Clinical endocrinology. 2005;62:466–72. doi: 10.1111/j.1365-2265.2005.02244.x. [DOI] [PubMed] [Google Scholar]
- 2.Lebrethon MC, Grossman AB, Afshar F, Plowman PN, Besser GM, Savage MO. Linear growth and final height after treatment for Cushing's disease in childhood. The Journal of clinical endocrinology and metabolism. 2000;85:3262–5. doi: 10.1210/jcem.85.9.6817. [DOI] [PubMed] [Google Scholar]
- 3.Magiakou MA, Mastorakos G, Gomez MT, Rose SR, Chrousos GP. Suppressed spontaneous and stimulated growth hormone secretion in patients with Cushing's disease before and after surgical cure. The Journal of clinical endocrinology and metabolism. 1994;78:131–7. doi: 10.1210/jcem.78.1.7507118. [DOI] [PubMed] [Google Scholar]
- 4.Strickland AL, Underwood LE, Voina SJ, French FS, Van Wyk JJ. Growth retardation in Cushing's syndrome. Am J Dis Child. 1972;123:207–13. doi: 10.1001/archpedi.1972.02110090077007. [DOI] [PubMed] [Google Scholar]
- 5.Koedam JA, Smink JJ, van Buul-Offers SC. Glucocorticoids inhibit vascular endothelial growth factor expression in growth plate chondrocytes. Molecular and cellular endocrinology. 2002;197:35–44. doi: 10.1016/s0303-7207(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 6.Lindberg MK, Vandenput L, Moverare Skrtic S, Vanderschueren D, Boonen S, Bouillon R, et al. Androgens and the skeleton. Minerva endocrinologica. 2005;30:15–25. [PubMed] [Google Scholar]
- 7.Dupuis CC, Storr HL, Perry LA, Ho JT, Ahmed L, Ong KK, et al. Abnormal puberty in paediatric Cushing's disease: relationship with adrenal androgen, sex hormone binding globulin and gonadotrophin concentrations. Clinical endocrinology. 2007;66:838–43. doi: 10.1111/j.1365-2265.2007.02822.x. [DOI] [PubMed] [Google Scholar]
- 8.Blodgett FM, Burgin L, Iezzoni D, Gribetz D, Talbot NB. Effects of prolonged cortisone therapy on the statural growth, skeletal maturation and metabolic status of children. The New England journal of medicine. 1956;254:636–41. doi: 10.1056/NEJM195604052541402. [DOI] [PubMed] [Google Scholar]
- 9.Allen DB. Growth suppression by glucocorticoid therapy. Endocrinology and metabolism clinics of North America. 1996;25:699–717. doi: 10.1016/s0889-8529(05)70348-0. [DOI] [PubMed] [Google Scholar]
- 10.Morris HG. Growth and skeletal maturation in asthmatic children: effect of corticosteroid treatment. Pediatric research. 1975;9:579–83. doi: 10.1203/00006450-197507000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Aicardi G, Benso L, Vignolo M, Terragna A, Verrina E, Cordone G, et al. Dose-dependent effects of deflazacort and prednisone on growth and skeletal maturation. British journal of rheumatology. 1993;32(Suppl 2):39–43. doi: 10.1093/rheumatology/32.suppl_2.39. [DOI] [PubMed] [Google Scholar]
- 12.Polito C, Greco N, Opallo A, Cimmaruta E, La Manna A. Alternate-day steroids affect carpal maturation more than radius, ulna and short bones. Pediatr Nephrol. 1994;8:480–2. doi: 10.1007/BF00856538. [DOI] [PubMed] [Google Scholar]
- 13.Magiakou MA, Mastorakos G, Oldfield EH, Gomez MT, Doppman JL, Cutler GB, Jr., et al. Cushing's syndrome in children and adolescents. Presentation, diagnosis, and therapy. The New England journal of medicine. 1994;331:629–36. doi: 10.1056/NEJM199409083311002. [DOI] [PubMed] [Google Scholar]
- 14.Acharya SV, Gopal RA, Lila A, Menon PS, Bandgar TR, Shah NS. Bone age and factors affecting skeletal maturation at diagnosis of paediatric Cushing's disease. Pituitary. 2010;13:355–60. doi: 10.1007/s11102-010-0246-3. [DOI] [PubMed] [Google Scholar]
- 15.Peters CJ, Ahmed ML, Storr HL, Davies KM, Martin LJ, Allgrove J, et al. Factors influencing skeletal maturation at diagnosis of paediatric Cushing's disease. Hormone research. 2007;68:231–5. doi: 10.1159/000101336. [DOI] [PubMed] [Google Scholar]
- 16.Batista DL, Riar J, Keil M, Stratakis CA. Diagnostic tests for children who are referred for the investigation of Cushing syndrome. Pediatrics. 2007;120:e575–86. doi: 10.1542/peds.2006-2402. [DOI] [PubMed] [Google Scholar]
- 17.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford University Press; Stanford, Calif: 1959. [Google Scholar]
- 18.Kushnir MM, Blamires T, Rockwood AL, Roberts WL, Yue B, Erdogan E, et al. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clinical chemistry. 2010;56:1138–47. doi: 10.1373/clinchem.2010.143222. [DOI] [PubMed] [Google Scholar]
- 19.Elmlinger MW, Kuhnel W, Ranke MB. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2002;40:1151–60. doi: 10.1515/CCLM.2002.202. [DOI] [PubMed] [Google Scholar]
- 20.Lofqvist C, Andersson E, Gelander L, Rosberg S, Blum WF, Albertsson Wikland K. Reference values for IGF-I throughout childhood and adolescence: a model that accounts simultaneously for the effect of gender, age, and puberty. The Journal of clinical endocrinology and metabolism. 2001;86:5870–6. doi: 10.1210/jcem.86.12.8117. [DOI] [PubMed] [Google Scholar]
- 21.Kanbur NO, Derman O, Kinik E. The relationships between pubertal development, IGF-1 axis, and bone formation in healthy adolescents. Journal of bone and mineral metabolism. 2005;23:76–83. doi: 10.1007/s00774-004-0544-9. [DOI] [PubMed] [Google Scholar]
- 22.Hauffa BP, Kaplan SL, Grumbach MM. Dissociation between plasma adrenal androgens and cortisol in Cushing's disease and ectopic ACTH-producing tumour: relation to adrenarche. Lancet. 1984;1:1373–6. doi: 10.1016/s0140-6736(84)91873-7. [DOI] [PubMed] [Google Scholar]
- 23.Keil MF, Graf J, Gokarn N, Stratakis CA. Anthropometric measures and fasting insulin levels in children before and after cure of Cushing syndrome. Clin Nutr. 2012;31:359–63. doi: 10.1016/j.clnu.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell DL, Keil MF, Bonat SH, Uwaifo GI, Nicholson JC, McDuffie JR, et al. The relation between skeletal maturation and adiposity in African American and Caucasian children. The Journal of pediatrics. 2001;139:844–8. doi: 10.1067/mpd.2001.119446. [DOI] [PubMed] [Google Scholar]
- 25.Johnson W, Stovitz SD, Choh AC, Czerwinski SA, Towne B, Demerath EW. Patterns of linear growth and skeletal maturation from birth to 18 years of age in overweight young adults. Int J Obes (Lond) 2012;36:535–41. doi: 10.1038/ijo.2011.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.New MI. Factors determining final height in congenital adrenal hyperplasia. Journal of pediatric endocrinology & metabolism : JPEM. 2001;14(Suppl 2):933–7. doi: 10.1515/jpem-2001-s204. [DOI] [PubMed] [Google Scholar]
- 27.Magiakou MA, Mastorakos G, Chrousos GP. Final stature in patients with endogenous Cushing's syndrome. The Journal of clinical endocrinology and metabolism. 1994;79:1082–5. doi: 10.1210/jcem.79.4.7962277. [DOI] [PubMed] [Google Scholar]
- 28.Cao Z, Swift TA, West CA, Rosano TG, Rej R. Immunoassay of estradiol: unanticipated suppression by unconjugated estriol. Clinical chemistry. 2004;50:160–5. doi: 10.1373/clinchem.2003.023325. [DOI] [PubMed] [Google Scholar]