Abstract
This study examined concordance in heart rate (HR) and respiratory sinus arrhythmia (RSA) in a sample of 104 child-maltreating (CM) and nonCM mother–preschooler dyads (208 individuals). In a laboratory setting, mother and child cardiac physiology was simultaneously monitored via ECG in a 5-min resting period. Mothers ranged in age from 20 to 49 years; children ranged in age from 3 to 5 years. Significant within-dyad (WD) and between-dyad (BD) associations were observed for mother HR and both child HR and RSA, and the associations were moderated by CM status. Only CM dyads exhibited BD associations: Higher average maternal HR was associated with higher child HR and lower child RSA. By contrast, when the time interval was divided into 30 s epochs, nonCM dyads exhibited positive WD (dynamic) associations in mother and child HR, and both CM and nonCM dyads showed negative WD associations in mother HR and child RSA. Further, mothers’ mean HR levels moderated the extent of epoch-by-epoch WD concordance observed in mother and child, such that elevated average maternal HR was associated with lower levels of WD (dynamic) concordance. No BD or WD concordance in maternal and child RSA was observed. The findings suggest that measures of intraindividual variation provide useful, alternate perspectives in the study of dyadic processes in at-risk families.
Keywords: autonomic physiology, HR, dynamic concordance, parenting, child maltreatment
INTRODUCTION
Synchrony has been conceptualized as an overarching process that coordinates the on-going exchanges of sensory, hormonal, and physiological stimuli between parent and child during social interactions, providing critical inputs to child growth and development (Feldman, 2007). In mammals, studies show that synchrony depends on physiological mechanisms supporting bond formation, leading researchers to conceptualize synchrony in humans as biologically based (Feldman, 2007), and to investigate potentially informative patterns of matching or concordance in physiological states. Recent studies examining concordance in maternal–child physiology suggest that this may be moderated by various care-giving qualities such as maternal sensitivity, empathy, or depression, lending support to the idea that physiological concordance might be relevant to synchronous relationships. However, family systems theorists have described a process of being enmeshed or overinvolved in family relationships (e.g., Bowen, 1978; Minuchin, 1974), that might also be indicated by concordance rather than independence in emotional and physiological arousal. The implications of physiological concordance are further obscured by wide variation in how concordance is operationalized. To shed light on this in the present study, we examined two forms of concordance in autonomic function between mother–child dyads during a shared resting condition.
Studies of physiological concordance in autonomic measures have produced equivocal findings. Several have confirmed patterns of between-dyad (BD) associations in mother–child responses to challenge, where average maternal arousal is related to her child’s average arousal level, while none observed within-dyad (WD, or dynamic) concordance (where shifts in maternal arousal correspond to shifts in children’s arousal). For example, in a prospective study of 76 mother–infant dyads, Bornstein and Suess (2000) found no associations between mothers and children at 2 months or at 5 years in either resting heart rate (HR) or respiratory sinus arrhythmia (RSA) though RSA in response to challenge showed positive BD associations by age 5. Hill-Soderlund et al. (2008) found no dynamic concordance in autonomic functioning (as measured by salivary α-amylase and RSA) during the Strange Situation Paradigm, but HR concordance during the Strange Situation in mother–infant dyads has been reported (Donovan & Leavitt, 1985). Paradoxically, some dynamic concordance has been observed in mother and infant HPA axis functioning (indexed via cortisol reactivity in response to challenge), both in mother–infant dyads exposed to domestic violence and in dyads headed by more hostile and controlling mothers (Hibel, Granger, Blair, Cox, & The Family Life Project Investigators, 2009), as well as among more sensitive mothers and their infants (Van Bakel & Riksen-Walraven, 2008) and toddlers (Sethre-Hofstad, Stansbury, & Rice, 2002). In summary, whether or not physiological concordance is considered adaptive appears to depend on how it is operationalized (as BD or WD), the type of sample (i.e., normative or at-risk), the specific neurobiological process and parameter (e.g., autonomic, including HR and RSA) and the context in which it is assessed (i.e., at rest or in response to challenge). As such, it is unclear whether concordance in physiology reflects that type of synchrony thought to be critical to optimal development, or conversely, an inability to independently regulate one’s own physiological arousal, indicative of a deficit in differentiating oneself in one’s family or dyadic system.
Researchers have identified two key analytic approaches to developmental data that may help clarify the implications of physiological concordance. Variable-centered analytic models are frequently based on the assumption that the population is homogeneous with respect to how the predictors operate on the outcomes, and person-centered approaches describe differences among individuals in how variables are related to each other (Laursen & Hoff, 2006). Although these have been described as complementary rather than competing methodologies, studies tend to focus on variable-centered models whereby interindividual variation, or the effects of predictors on outcomes at the group level, is established. However, it is now appreciated that the application of interindividual findings to any one individual (i.e., generalizing from inter to intraindividual variation) is usually statistically invalid (except in rare cases where [i] the same statistical model applies to the data of all subjects in the population (homogeneity of the population) and [ii] the data has invariant statistical characteristics across time; for instance, a constant mean and variance; Molenaar & Campbell, 2009). As such, researchers have called for the application of both variable- and person-specific analyses to developmental datasets. This is particularly relevant to cases for which broad within-group variability in children’s outcomes have been documented, in addition to important group-level effects.
Child maltreatment (CM) is one important context for which both inter- and intraindividual variation in outcomes has been observed. CM, most commonly in the form of harsh physical punishment or abuse, or neglect of a child’s basic physical needs, is known to contribute to impairments across many domains of children’s functioning (e.g., Cicchetti & Toth, 2005; Shonkoff, Garner, & The Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, Adoption, and Dependent Care, and Section on Developmental and Behavioral Pediatrics, 2012); however, not all children display such deficits, and the mechanisms accounting for individual differences are not well understood (Cicchetti & Valentino, 2006). Maltreating parents themselves have displayed deficits in physiological regulation. For example, CM parents show higher resting HR and greater HR activation (Bugental et al., 1993; Bugental, Lewis, Lin, Lyon, & Kopeikin, 1999; Frodi & Lamb, 1980), cortisol hypersecretion (Lin, Bugental, Turek, Martorell, & Olster, 2002), and declines in galvanic skin response (Bugental & Cortez, 1988) in response to neutral and child-specific stimuli, compared to nonCM parents. This constellation of cardiovascular and neuroendocrine hyper-responding indicates a pattern of “threat-sensitivity” (Bugental, 2009), and may compromise a parent’s ability to engage in mutual, consensually shared experiences with their child. To date, no study that we are aware of has examined physiological concordance in CM parent–child relationships. As CM is related to both group-level and intraindividual variation in developmental outcomes, it is possible that patterns of BD and WD physiological concordance differ between this at-risk group and normative mother–child relationships. Comparing patterns of concordance in CM and nonCM dyads may help shed light on the meaning of physiological concordance for synchrony within close dyadic relationships.
Therefore, we sought to determine whether BD and WD concordance in autonomic physiology would be observed among mothers and their children during resting conditions (i.e., during the absence of environmental challenge), and whether the pattern of associations would vary as a function of their risk status. We examined HR, a measure of autonomic function reflective of both sympathetic and parasympathetic nervous system influences, and RSA, a measure of the change in oscillatory dynamics of the heart across the respiration cycle that is considered to be a measure of parasympathetic influence (Berntson et al., 1997; Porges, 1995) and to be indicative of stress vulnerability (Porges, 2009). The sympathetic and parasympathetic divisions of the autonomic nervous system represent the principal channels of interaction between the brain and bodily organs, and according to Porges’s (1995) polyvagal theory, have complementary roles in the achievement of homeostasis and the regulation of physiological responses to emotional stimuli. Researchers have therefore hypothesized that concordance in autonomic function might underlie shared empathic experiences between mother and child (Ebisch et al., 2012). Divergent links between resting HR and adjustment have been observed depending on the context, with lower resting HR linked to emotion expression in preschoolers (Cole, Zahn-Waxler, Fox, Usher, & Welsh, 1996), and to externalizing problems in preadolescents (Dietrich et al., 2007). High resting RSA is thought to be critical to physiological selfregulation and appropriate engagement with the environment (Hofheimer, Wood, Porges, Pearson, & Lawson, 1995), with Porges (1995) describing how vagal tone, of which RSA is a measure, fosters physiological homeostasis during states of low environmental demand to promote growth and restoration.
Our aim in examining concordance in a resting context was to examine a form of concordance that is not influenced by variance in external cues. As this resting phase comprises a condition of minimal environmental stimulation, cardiac physiology levels are indicative of selfregulatory processes that function to maintain internal homeostasis. Observing concordance at rest may help clarify whether parent–child difficulties with regulation extend beyond overt interactions to nonstressful contexts, and might thus be more pervasive than studies examining stressful interactions suggest. Therefore, our analyses examine concordance in those processes regulating internal homeostasis (rather than regulation in response to external demands), and serve to illustrate differences in BD and WD concordance depending on risk status.
Two forms of concordance were considered. First, associations in the rank order status of a mother and her child’s HR and RSA were examined to determine the extent of BD physiological concordance (e.g., Do mothers who display higher average resting HR have children who also show higher average resting HR?). Second, dynamic concordance was defined as the extent of WD concordance in mother and child HR and RSA at each epoch across the duration of a phase. In other words, when a mother displayed lower HR/RSA in an epoch, relative to her usual level, did her child also display lower (or higher) HR/RSA in that same epoch? While some studies suggest that physiological concordance is indicative of synchrony (e.g., Van Bakel & Riksen-Walraven, 2008), others suggest it to indicate enmeshment, or failure to independently regulate one’s own arousal levels (e.g., Hibel et al., 2009), thus, we did not make specific predictions regarding which type of concordance would be observed in either group. Instead, our aim was to demonstrate that patterns of physiological concordance may differ between normative and at-risk groups depending on how this is operationalized. We therefore hypothesized that patterns of WD and BD concordance would differ as a function of CM status; that is, that CM group status would moderate BD and dynamic associations in mother–child resting HR and RSA.
We also examined the moderating effect of average maternal HR levels on dynamic WD concordance. We hypothesized that higher resting maternal HR would be associated either with lower dynamic concordance (if indicative of synchrony) or higher dynamic concordance (if indicative of enmeshment); therefore, we examined this moderator in an effort to shed light on the meaning (or interpretation) of observed patterns of concordance.
METHODS
Participants
The sample consisted of 52 CM and 52 nonCM mother–child dyads (N = 208 individuals) from low-income, rural communities. Children ranged in age between 3 and 5 years (M = 3.75, SD = .75), 53.8% were female, and 80.4% were Caucasian. Mothers ranged in age from 20 to 49 years (M = 29.20, SD = 5.96), averaged 13.0 years of education (SD = 2.10), and half (48.5%) were employed outside the home. Based on U.S. census income-to-needs ratios, the majority of families were classified as near (39.3%) or below (47.1%) the poverty line. Among the subsample of CM children, 14 were identified as having been previously physically abused and 38 as previously physically neglected, based on Child Protective Services documentation of mother’s status as a perpetrator toward the target child, and using the Maltreatment Classification System (MCS; Barnett, Manly, & Cicchetti, 1993). Physical abuse was coded when there was evidence of a caregiver-inflicted physical injury to the child by other than accidental means. Physical neglect was coded based on documentation that the caregiver failed to meet the child’s minimum physical needs. Comorbidity of subtypes (i.e., physical abuse with neglect and/or emotional abuse; physical neglect with emotional abuse) was observed in 48.1% of the CM group, consistent with other published findings (e.g., Belsky, 1993; Kaufman & Zigler, 1989). NonCM dyads were recruited from other public welfare agencies and a database maintained on birth announcements published in local newspapers. NonCM mothers consented to verification that their family was free of CPS preventive or protective service records.
Demographic Variables
CM and nonCM children did not differ on dimensions of child age as indicated in Table 1, and there was no difference in distribution of boys and girls between groups (24 boys in either group). CM and nonCM mothers did not significantly differ on employment status; t(101) = −.88, p = .38; or age t(102) = −1.99, p = .05. However, mothers from the CM group reported fewer total years of education, t(100) = 3.08, p < .003 (M = 13.60 vs. M = 12.40), and lower household income, t(100) = 5.33, p < .0001, than nonCM mothers. Ninety-six per cent of CM families reported incomes below $50,000 per year, as contrasted with 76.5% of nonCM families reporting income below $50,000 per year.
Table 1.
Descriptive Statistics and Correlations Among Mother and Child Resting Heart Rate (HR) and Respiratory Sinus Arrhythmia (RSA)
| Variable | M | SD | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|
| CM dyads (n = 52)† | ||||||||
| 1. Child age | 3.73 | .77 | — | |||||
| 2. Child sex | — | — | .07 | — | ||||
| 3. Mother education | 12.41* | 2.04 | .28* | −.15 | — | |||
| 4. Mother HRa | 82.60* | 9.94 | −.11 | −.18 | .01 | — | ||
| 5. Mother RSAa | 5.51 | 1.28 | −.08 | .13 | −.21 | −.69** | — | |
| 6. Child HRa | 103.27 | 11.59 | −.13 | −.24 | .01 | .48** | −.35* | — |
| 7. Child RSAa | 5.91 | 1.13 | −.03 | .21 | −.04 | −.32* | .24 | −.82** |
| NonCM dyads (n = 52) | ||||||||
| 1. Child age | 3.77 | .73 | — | |||||
| 2. Child sex | — | — | .03 | — | ||||
| 3. Mother education | 13.63* | 1.95 | .01 | −.10 | — | |||
| 4. Mother HRa | 77.21* | 11.74 | .11 | .14 | −.22 | — | ||
| 5. Mother RSAa | 6.04 | 1.17 | −.09 | −.22 | .11 | −.62** | — | |
| 6. Child HRa | 99.85 | 9.65 | −.40** | −.20 | −.07 | −.01 | .10 | — |
| 7. Child RSAa | 6.29 | 1.16 | .27 | −.08 | .13 | −.08 | .06 | −.66** |
Mean value reflects average taken across all 30-s epochs. In the case of M’s and SD’s, significance tests assess CM group differences.
p < .05.
p < .01.
We conducted a series of independent samples t-tests to compare CM subtypes on cardiac variables. Only one significant difference between abuse (PA) and neglect (PN) groups was observed; child RSA was marginally higher for PA children in comparison with PN children (M = 5.99 vs. M = 5.70; p = .045). No significant differences on any demographic variables were observed.
Procedures
All procedures employed in this study were approved and monitored by the Office for Research Protection. Mother–child dyads were invited to participate if the mother was 18 years of age or older, spoke fluent English, and was living with her preschool child. Dyads completed a three-visit protocol over a 2- to 3-week period conducted by a team of two trained interviewers, comprising two home visits for psychosocial and cognitive assessments, and a laboratory session: they were paid $150, and provided transportation, snacks, and children’s small toys/gifts. During the laboratory session, dyads participated in a 5-min resting condition in which mother and child dyads were seated closely together on a couch with lights dimmed, and instructed to rest quietly while viewing a low-action animated videotape.
Physiological Assessment
To monitor mother and child cardiac physiology, disposable pregelled Ag/AgCl electrodes were placed in a modified Lead II placement on the distal end of the right clavicle, lower left rib cage chest, and the lower abdomen. Data were acquired via Mindware Technologies© (Gahanna, OH) ambulatory electrocardiograph MW1000A, transmitted via wireless signal to a computer and monitored by a research assistant. Heart rate data were quantified by taking the ECG signals and passing them through an A/D converter with ECG sampled at a rate of 500 Hz. Electrocardiograph data were processed offline using Mindware Technologies HRV 3.0.10 analysis program, and epochs were visually inspected by trained research assistants. Erroneously identified, or missing heart beats were manually deleted or inserted as appropriate. The resulting interbeat interval time series was subjected to a fast-Fourier transformation, and power in the respiratory frequency band was derived from the spectral density function. The RSA frequency band was set between .24 and 1.04 for children (e.g., Calkins & Keane, 2004), and .12–.40 for mothers. Heart rate and RSA were calculated in 30-s epochs across the 5-min joint resting phase, and overall HR and RSA means for the task were calculated for mothers and children. Dynamic changes in mothers’ and children’s HR and RSA were operationalized by calculating concurrent concordance in centered HR or RSA values for each epoch across the ten 30-s epochs (i.e., we examined concurrent covariation in mother and child epoch by epoch physiology within the resting condition, rather than cross-epoch or lead-lag associations).
Analyses
Mother and child HR and RSA data were collected simultaneously and longitudinally nested within dyads. Across 104 mother–child dyads comprising 208 individuals, HR data were collected for a total of 985 epochs for children and 992 epochs for mothers, and RSA data were collected from 930 epochs for children, and 949 epochs from mothers. We used SAS PROC MIXED 9.3 to model BD associations in overall mean HR or mean RSA, and dynamic WD concurrent associations between mother and child HR and RSA over the course of the resting period (e.g., Saxbe & Repetti, 2010; Sliwinski, Smyth, Hofer, & Stawski, 2006).
First, we estimated associations between mothers’ overall average (BD) and dynamic (WD) HR on one hand, and children’s HR on the other, and tested whether maternal average HR moderated the extent of mother–child dynamic HR concordance. Covariates included child sex, age, and maternal education. Next, we examined CM group differences in concordance between mother’s mean HR (BD) and dynamic HR (WD) and child HR. The WD associations across the 10 epoch resting phase are modeled in Level 1, where HR for child in dyad j at epoch i is shown as a function of β0j, the intercept for child j, β1j is the expected rate of change in child HR associated with concurrent variation in mother’s person-mean-centered HR over time, and the residual term, εij, reflects within dyad error. BD variability in the Level 1 intercept and slope is modeled in the Level 2 equations. Thus, BD differences in child j’s HR were a function of γ00, the average intercept (i.e., grand mean for child HR at mothers’ grand mean centered HR scores), and the random intercept, γ01. BD variability in child j’s HR slope, β1j, was based on the average BD HR slope, γ10, and the strength of epoch-by-epoch associations with mother’s HR, or random HR slope, γ11. To test the moderating role of CM status on these associations, we added a dummy-coded CM group status variable at Level 2. Mothers were assigned to the CM group based on documented perpetration of child abuse/neglect (i.e., CM group), or to the nonCM group which served as the reference group. The covariates child sex, age, and maternal education were entered as additional Level 2 predictors in all models. Models were re-specified to test associations between maternal and child RSA, and finally to examine associations between maternal HR and child RSA. As the findings for concordance in maternal RSA and child HR broadly corroborated the other models, we have focused on child RSA as the outcome variable in this case.
- Within-Dyad
- Between-Dyad
RESULTS
Descriptive Statistics
Table 1 shows the means, standard deviations, and zero-order correlations for mother and child mean resting physiology by CM status. There were significant associations between mother and child mean HR and mother mean HR and child mean RSA measures in the CM group, but not for the nonCM group. Age effects were observed in children’s resting HR and RSA levels (for the nonCM children only). Younger nonCM children displayed higher resting HR, r(52) = −.40, p < .01, and lower RSA levels, r(50) = .27, p = .06, relative to older nonCM children. Sex differences were observed in children’s HR, t(104) = 2.21, p = .03, with girls posting higher average resting HR (M = 103.7, SD = 10.60) than boys (M = 99.10, SD = 10.50); children resting RSA levels did not differ by sex, t(100) = −.64, p = .53. In light of the socio-demographic stratification by CM status, maternal education was included as a covariate in the major analyses, along with children’s age and sex.
HR and RSA by CM Group Status
Independent t-tests assessed differences between mother and child mean resting physiology by CM status. CM mothers showed significantly higher resting HR (M = 82.60, SD = 9.94) and lower resting RSA (M = 5.51, SD = 1.28) than did nonCM mothers [HR: M = 77.21, SD = 11.74; RSA: M = 6.04, SD = 1.17, t(102) = 2.52, p = .01 and t(102) = −2.21, p = .03, respectively]. As indicated in Table 1, no CM group differences were observed in children’s resting HR and RSA.
Testing Within- and Between-Dyad-Physiological Concordance
Dyadic associations in mother and child HR levels over time were analyzed using MLM procedures. In Model 1a (Table 2), the Level 1 (WD) predictor was mother’s mean-centered HR during each of the 30 sec epochs (i.e., mother’s HR at each epoch, centered around mother’s task mean (Maternal HR_pmc), and the Level 2 (BD) predictors were mother’s overall mean HR (i.e., average HR over the 10 epochs; Maternal HR_mnij), and the covariates of maternal education, children’s age and sex. Age (β = −3.43, p < .01) and child sex (β = −4.90, p < .05) were significantly associated with child HR, such that younger children showed higher HR levels, and girls posted higher HR relative to boys. Maternal education was not significantly associated with child HR (β = .09, p = .86). Mothers with higher average resting HR had children who also posted higher resting HR, β = .28, p < .01. Dynamic (WD) associations were also observed as mother and child HR showed positive concurrent associations over the 5-min resting condition (β = .33, p < .0001). Further, the dyadic WD HR concordance was moderated by differences in mothers’ average HR (β = −.02, p < .05), indicating that dyads for which mothers showed greater HR elevations, displayed lower dynamic HR concordance over time. Conversely, dyads for which mothers showed lower average HR, displayed greater dynamic concordance in mother and child HR. However, as the magnitude of the WD effect was .33, and this was lowered by .015 (see Tab. 2) for every beat above maternal mean HR, this interaction effect is relevant only to those mothers with a mean HR of less than 22 beats above the mean. As such, this interaction effect is somewhat small.
Table 2.
Multilevel Model Parameter Estimates of the Within- and Between-Dyadic Associations in Mother and Children Physiology by CM Status
| Variable | Children’s HR
|
Children’s RSA
|
||||||
|---|---|---|---|---|---|---|---|---|
| Model 1a
|
Model 2a
|
Model 1b
|
Model 2b
|
|||||
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Fixed effects | ||||||||
| Intercept | 115.41 | 7.50** | 112.26 | 7.73** | 5.17 | .89** | 5.46 | .95** |
| Covariates | ||||||||
| Maternal education | .09 | .48 | .09 | .49 | .01 | .06 | .01 | .06 |
| Age | −3.43 | 1.29** | −2.99 | 1.25* | .19 | .15 | .17 | .15 |
| Sex | −4.90 | 1.94* | −3.94 | 1.90* | .12 | .23 | .06 | .24 |
| Maternal HR_mn | .28 | .09** | .06 | .11 | −.02 | .01* | −.01 | .01 |
| Maternal HR_pmc | .33 | .07** | .45 | .09** | −.04 | .01** | −.04 | .01** |
| Maternal HR_mn × HR_pmc | −.015 | .006* | −.012 | .006 | .002 | .0007* | .0015 | .0007* |
| CM status | .69 | 2.02 | −.15 | .25 | ||||
| CM Status × maternal HR_mn | .51 | .18** | −.03 | .02 | ||||
| CM Status × maternal HR_pmc | −.26 | .13* | .015 | .015 | ||||
| Variance components | ||||||||
| Between dyad | ||||||||
| Intercept | 91.40 | 13.53** | 85.18 | 12.76** | 1.23 | .19** | 1.21 | .19** |
| Within dyad | ||||||||
| Residual | 15.07 | .78 | 15.02 | .78 | .52 | .03 | .51 | .03 |
| Maternal HR_pmc | .15 | .06** | .14 | .05** | — | — | — | — |
| −2LL | 5522.6 | 5510.4 | 2286.4 | 2296.6 | ||||
| AIC | 5530.6 | 5518.4 | 2290.4 | 2300.3 | ||||
Notes: Unstandardized estimates and standard errors. Slopes or rates of HR change are scaled in 30-s epochs. Model is based on 10 occasions nested within 104 mother–child dyads. AIC, Akaike Information Criterion; −2LL = −2 log likelihood, relative model fit statistics.
p < .05.
p < .01.
RSA Concordance
We then specified the same model substituting maternal and child RSA for maternal and child HR. None of the three covariates were significant predictors of child RSA (β = .19, p = .21 for age; β = .15, p = .51 for sex, and β = .03, p = .54 for maternal education). There were no BD associations between overall mother RSA and child RSA (β = .06, p = .11); nor were dynamic WD associations in mother and child RSA observed (β = .17, p = .06). In addition, the cross-level interaction testing whether differences in mothers’ average RSA might moderate dynamic concordance in RSA was not significant (β = .02, p = .41).
Mother HR and Child RSA Concordance
Next, we respecified the model to test concordance in mother HR and child RSA. As shown in Table 2, none of the covariates were associated with child RSA scores in Model 1b. A significant BD effect was observed whereby mothers with lower average resting HR had children who posted higher resting RSA scores (β = −.03, p < .0001). Dynamic WD associations also were observed in mother HR and child RSA over time (β = −.02, p < .05) such that lower maternal HR was associated with higher concurrent RSA in her child over the course of the 5-min resting phase. Further, a significant cross-level interaction was observed (β = .002, p < .05), such that dyads with higher resting maternal HR displayed lower dynamic concordance in mother HR and child RSA.
Differences in Dyadic Physiological Concordance by CM Status
HR Concordance by CM status
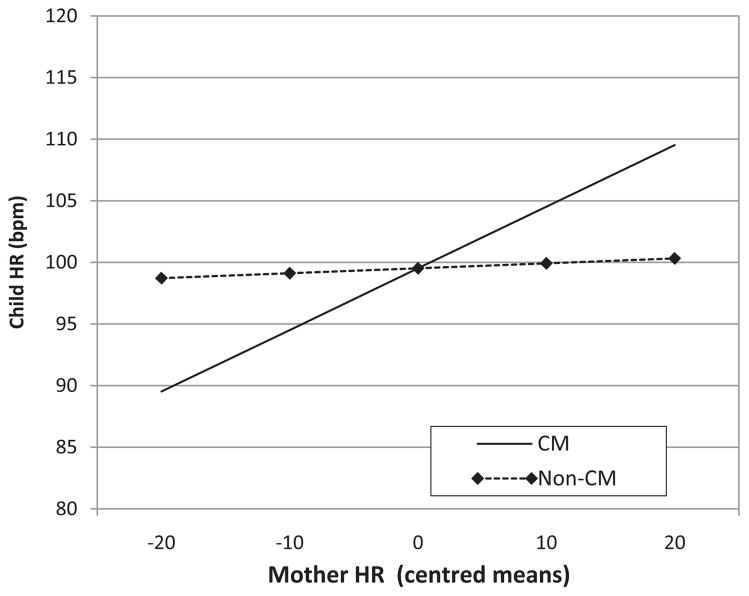
In Model 2a, interactions the effects of Level 2 between CM group status and both were considered in addition to our covariates child age, sex, and maternal education. As shown in Table 2, Model 2a, though children’s mean resting HR did not differ by CM status as shown in Table 2, BD and WD associations in mother and child HR were moderated substantially by CM status. Specifically, a significant BD effect for mother and child HR was observed for the CM dyads only (β = .56, p < .0001); not for nonCM dyads (β = .06, p = .60). This difference between CM and nonCM groups was statistically significant (β = .51, p < .01), indicating that only CM mothers and children displayed significant positive associations in their average resting HR. In contrast, significant WD (dynamic) associations in mother and child HR were present only among the nonCM dyads (β = .45, p < .01), and not among CM dyads (β = .14, p = .05). The findings indicate that over time, nonCM mothers, and children showed positive, concurrent associations in their HR. Thus-when CM a mother displayed lower HR in an epoch, relative to her usual level, her child also displayed lower HR in that same epoch. Conversely, higher maternal HR in an epoch was associated with higher child HR in that same epoch, only in nonCM dyads. These BD and WD associations for child HR are depicted graphically in Figures 1 and 2.
FIGURE 1.
Graphical depiction of association between mother and child HR by CM status, with the stronger association evident in the maltreating group.
FIGURE 2.
Histograms depicting the mother–child within-dyad concordance across the ten epochs (based on correlational associations for illustrative purposes only, reflecting multilevel statistical analyses). For the nonmaltreating dyads, these correlations indicating concordance are, on average, significantly more positive.
RSA Concordance by CM status
Although no significant WD or BD concordance in maternal and child RSA was observed, we examined CM status as a potential moderator. There was no main effect of CM status (β = −.19, p = .45) Associations between average maternal RSA and child RSA were not significant for the CM dyads (β = .19, p = .16), or for nonCM dyads (β = .07, p = .60). Nor was a significant difference in BD associations between CM and nonCM groups observed, β = .13, SE = .19, p = .51. In addition, no WD dynamic associations in mother and child RSA were present for the CM dyads (β = .03, SE = .05, p = .55), or for the nonCM dyads (β = .08, SE = .06, p = .19). There was no significant difference between CM and nonCM WD associations (β = −.06, SE = .07, p = .45); nor were any covariates significant in this model.
Mother HR–Child RSA Concordance by CM Status
For Model 2b shown in Table 2, though no CM group differences were observed for child’s resting RSA, BD, and WD associations in mother HR and child RSA were significantly moderated by CM status. Specifically, BD associations between average maternal HR and child RSA were significant for the CM dyads only (β = −.04, p < .05 for CM; β = −.01, p = .48 for nonCM), indicating that higher average HR among CM mothers was associated with lower child RSA scores. In contrast, dynamic (WD) associations in mother HR and child RSA were present for the CM dyads (β = −.02, p = .04), and the nonCM dyads (β = −.04, p < .0001), with no significant group difference in the strength of the effects (β = .02, SE = .02, p = .35). Across both groups then, when a mother displayed lower HR in an epoch, relative to her usual level, her child displayed higher RSA in that same epoch. Likewise, higher maternal HR in an epoch was associated with lower child RSA in that same epoch (i.e., concurrent concordance).
DISCUSSION
The present study examined BD and dynamic WD concordance in resting HR and RSA in CM and nonCM mother–child dyads. The findings indicate that evidence of physiological concordance, shown in previous work to be important to synchrony, varies depending on whether person-centered or variable-centered approaches are utilized, and depending on the autonomic measures assessed. In the present study, BD concordance was observed only for CM dyads, whereby higher average maternal HR was associated with higher child HR and lower child RSA. Dynamic (WD) concordance was observed among nonCM dyads for maternal HR with child HR and RSA measures. Further, WD concordance in maternal HR and child RSA was also observed among CM dyads. Only maternal HR levels were associated with children’s HR and RSA scores.
Mothers’ average resting HR also moderated the extent of dynamic physiological concordance with children’s resting HR and RSA levels. Specifically, mother–child dyads for which mothers had lower average resting HR showed greater dynamic concordance over the course of the resting period. Here, greater positive dynamic concordance was observed in mother and child HR levels and greater negative (inverse) concordance emerged in mother HR and child RSA levels. Conversely, dyads headed by mothers showing higher resting HR exhibited weaker physiological concordance—that is, weaker positive dynamic concordance in mother–child HR levels and weaker inverse concordance in mother HR and child RSA levels. As such, elevated resting HR appears to impede dynamic concordance between maternal and child autonomic physiology. As CM mothers tend to exhibit higher resting HR than did nonCM mothers both in this study and in others (e.g., Frodi & Lamb, 1980), mother–child dyads characterized by CM appear more likely to display patterns of elevated physiological function that may impede dynamic concordance in autonomic physiology.
Further, BD concordance was observed only for CM dyads across maternal HR and child HR and RSA. This pattern of BD concordance observed only among CM dyads suggests that the highest risk mothers and children exhibit corresponding traitlike elevations in autonomic function that are not present among the lower risk, nonCM dyads. In contrast, evidence of dynamic WD concordance was observed across the sample. Notably, nonCM mothers’ HR showed concurrent WD associations both with their children’s HR and RSA scores, whereas CM mothers’ HR showed concurrent WD associations only with child RSA. Taken together with the finding that elevated maternal HR attenuated dyadic WD concordance in maternal HR and child HR and RSA, these data tentatively suggest that WD concordance might signify a more adaptive form of synchrony at rest. From a family systems perspective, this WD concordance in resting maternal HR and child HR and RSA may be indicative of lower emotional reactivity, and adaptive synchrony (e.g., Feldman, Magori-Cohen, Galili, Singer, & Louzoun, 2011). In other words, greater consistency of dynamic associations observed in nonCM mother and child physiology during the neutral context suggests a greater capacity to experience a novel situation (i.e., a low stimuli video presented during the resting period) similarly, with mutual awareness and immediacy in their individual experience of context. However, as dynamic concordance in maternal HR and child RSA was observed in both groups, it appears that CM dyads also have the capacity to demonstrate WD concordance that might indicate synchrony, albeit less consistently than the nonCM group. Although we have not reported on analyses of concordance in maternal RSA–child HR here, these revealed BD concordance for the CM group only, with no other effects. This is consistent with the other analyses indicating BD concordance only for the CM group, though no WD concordance was observed.
In sum, the pattern of findings suggests that the extent of BD rather than WD concordance may better differentiate between normative and CM dyads. Although no external measures of child and family function were analyzed, as CM is a potent predictor of negative outcomes, it seems possible that the CM group differences observed here may be indicative of adaptive and mal-adaptive family functioning.
Participants in both the CM and nonCM groups came from low-income families, thus, the comparison group may also be characterized as socioeconomically at-risk for a number of adverse developmental outcomes. Given that CM status was not confounded with SES in this sample, it is likely that CM exposure, rather than low SES generally, is the key adverse environment pertinent to the findings. From a family systems perspective (e.g., Bowen, 1978; Kerr, 2008; Kerr & Bowen, 1988; Minuchin, 1974), in less differentiated family systems such as those in which child abuse and neglect are known to occur (Skowron, Kozlowski, & Pincus, 2010), parent–child exchanges are thought to take on an anxious automaticity that extends into the physiological domain of functioning. These maltreating parents and their children may display matching levels of heightened resting autonomic levels simply as a function of risk status. However, maltreatment can involve a host of negative parenting practices that may influence the degree to which physiological concordance is observed or disrupted. Numerous studies have implied that threat-biased adults (such as CM parents) appear to be more vigilant in parenting contexts (Bugental, 2009), report more negative emotions and attributions in the context of parenting, and display elevated physiological arousal (Bugental, 2009). Likewise CM children also evidence threat-biased perceptions, and show greater chronic elevations in biologically based stress reactivity (Gunnar, Frenn, Wewerka, & Van Ryzin, 2009; Pollak, 2008), than nonCM children. These and other findings (e.g., Casanova, Domanic, McCanne, & Milner, 1992) suggest that CM mothers may experience the parenting context, on a neurobiological level, as more threatening than their low-risk peers. According to Porges’s (2011) concept of neuroception, the nervous system engages in ongoing evaluation of risk in the environment. To effectively shift from a defensive stance to positive relational engagement, one must first perceive the environment to be safe, and only then does one proceed to inhibit a sympathetic response. The CM mothers and children in this study may be less capable in this regard, thus accounting for the observed BD associations in this group. The greater HR arousal experienced by CM mothers may also hinder them from displaying modest fluctuations in their level of HR arousal to the resting state stimuli that show concurrent associations with their child’s HR and parasympathetic responses to those same stimuli. The possibility of a ceiling effect suggested by polyvagal theory, whereby mothers with high resting HR are limited in their ability to further modulate their HR, must be considered. Alternatively, CM mothers and children may be less able to tune in to the immediate context and experience it in moment-by-moment ways, because their resting physiology is more influenced by chronic arousal that characterizes a defensive or alert stance.
Notably, regardless of CM group status, maternal RSA did not demonstrate BD or WD concordance with child RSA, suggesting that resting RSA concordance neither characterized nor differentiated between normative and CM dyads. While a mother’s RSA at rest may not be associated with her child’s physiology at rest, other studies have shown links between maternal RSA and indicators of parenting quality (e.g., Moore et al., 2009; Perlman, Camras, & Pelphrey, 2008). Subsequent investigations are needed to explore these patterns of WD and BD concordance by risk group, at rest and in the context of challenge, and in conjunction with alternate measures of child and family function to further clarify our understanding of their impact for adaptive parent–child relationships and children’s outcomes.
The limitations of the current study include its restricted sampling of physiological measures, which were gathered from an intensive repeated-measures design during a single research session. However, as access to high-risk cohorts can be difficult to obtain, the current findings are particularly valuable in highlighting how cardiovascular regulation might offer potentially important insights in this vulnerable group, and in particular the utility of examining both within-person and between-person variations. Future research with larger sample size is needed to further elucidate the nature of CM subtype effects (i.e., physical abuse, sexual abuse, or neglect) on physiological concordance between parents and young children. In addition, the degree of physical contact between dyads may be relevant to this concordance (Feldman et al., 2011). Although the dyads in our study were instructed not to interact during this resting phase, mothers and children were seated together on a couch while measurements were taken, thus additional work might examine physical proximity as a factor relevant to concordance.
Future research is also needed to examine dyadic influences on cardiovascular physiology over a longer time-frame, its effects on children’s developmental trajectories, and whether behavioral or affective synchrony, along with other individual differences further moderate the patterns of cardiovascular concordance observed here. The present study illustrates the importance of employing both variable-focused and person-and dyad-specific approaches to the study of early adversity and the intersection of biology and experience in understanding physiological regulation processes in normative and high-risk families.
Acknowledgments
We thank members of the Family Systems Lab for their assistance with data collection, transcription, and coding.
This project was supported by a Fulbright Fellowship to the National University of Ireland at Galway and National Institutes of Health Research Grant R01 MH079328 to Elizabeth A. Skowron, funded by the National Institute of Mental Health and Administration for Children and Families/Children’s Bureau of the Administration on Children, Youth and Families as part of the Federal Child Neglect Research Consortium.
References
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Child abuse, child development, and social policy. Norwood, NJ: Ablex; 1993. pp. 7–73. [Google Scholar]
- Belsky J. Etiology of child maltreatment: A developmental-ecological analysis. Psychological Bulletin. 1993;114:413–434. doi: 10.1037/0033-2909.114.3.413. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufman PG. Heart rate variability: Origins, methods, and interpretative caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Suess PE. Child and mother cardiac vagal tone: Continuity, stability, and concordance across the first 5 years. Developmental Psychology. 2000;36:54–65. [PubMed] [Google Scholar]
- Bowen M. Family therapy in clinical practice. New York: Jason Aronson; 1978. [Google Scholar]
- Bugental DB. Predicting and preventing child maltreatment: A biocognitive transactional approach. In: Sameroff AJ, editor. The transactional model of development: How children and contexts shape each other. Washington, DC: APA; 2009. pp. 97–115. [Google Scholar]
- Bugental DB, Blue J, Cortez V, Fleck K, Kopeikin H, Lewis JC, Lyon J. Social cognitions as organizers of autonomic and affective responses to social challenge. Journal of Personality and Social Psychology. 1993;64:94–103. doi: 10.1037//0022-3514.64.1.94. [DOI] [PubMed] [Google Scholar]
- Bugental DB, Cortez VL. Physiological reactivity to responsive and unresponsive children as moderated by perceived control. Child Development. 1988;59:686–693. [PubMed] [Google Scholar]
- Bugental DB, Lewis JC, Lin E, Lyon J, Kopeikin H. In charge but not in control: The management of teaching relationships by adults with low perceived power. Developmental Psychology. 1999;35:1367–1378. doi: 10.1037//0012-1649.35.6.1367. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology. 2004;45:101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Casanova GM, Domanic J, McCanne TR, Milner J. Physiological responses to non-child-related stressors in mothers at risk for child abuse. Child Abuse and Neglect. 1992;16:31–44. doi: 10.1016/0145-2134(92)90006-d. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. Child maltreatment. Annual Review of Clinical Psychology. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Valentino K. An ecological transactional perspective on child maltreatment: Failure of the average expectable environment and its influence upon child development. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology (2nd ed.): Risk, Disorder, and Adaptation. Vol. 3. New York: Wiley; 2006. pp. 129–201. [Google Scholar]
- Cole PM, Zahn-Waxler C, Fox NA, Usher BA, Welsh JD. Individual differences in emotion regulation and behavior problems in preschool children. Journal of Abnormal Psychology. 1996;105:518–529. [PubMed] [Google Scholar]
- Dietrich A, Riese H, Sondeijker FE, Greaves-Lord K, van Roon AM, Ormel J, Rosmalen JG. Externalizing and internalizing problems in relation to autonomic function: A population-based study in preadolescents. Journal of the American Academy for Child and Adolescent Psychiatry. 2007;46:378–386. doi: 10.1097/CHI.0b013e31802b91ea. [DOI] [PubMed] [Google Scholar]
- Donovan WL, Leavitt LA. Cardiac responses of mothers and infants in Ainsworth’s strange-situation. In: Reite M, Field T, editors. The psychobiology of attachment. New York: Academic Press; 1985. pp. 130–142. [Google Scholar]
- Ebisch SJ, Aureli T, Bafunno D, Cardone D, Romani GL, Merla A. Mother and child in synchrony: Thermal facial imprints of autonomic contagion. Biological Psychology. 2012;89:123–129. doi: 10.1016/j.biopsycho.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Feldman R. Parent–infant synchrony: Biological foundations and developmental outcomes. Current Directions in Psychological Science. 2007;16:340–345. [Google Scholar]
- Feldman R, Magori-Cohen R, Galili G, Singer M, Louzoun Y. Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behavior and Development. 2011;34:569–577. doi: 10.1016/j.infbeh.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Frodi AM, Lamb ME. Child abusers’ responses to infant smiles and cries. Child Development. 1980;51:238–241. [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10- to 12-year old children. Psychoneuroendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Blair C, Cox M The Family Life Project Investigators. Intimate partner violence moderates the relationship between mother-infant adrenocortical responses to an emotional challenge. Journal of Family Psychology. 2009;23:615–625. doi: 10.1037/a0016323. [DOI] [PubMed] [Google Scholar]
- Hill-Soderlund AL, Mills-Koonce WR, Propper C, Calkins S, Granger DA, Moore GA, Cox MJ. Parasympathetic and sympathetic responses to the Strange Situation in infants and mothers from avoidant and securely attached dyads. Developmental Psychobiology. 2008;50:361–376. doi: 10.1002/dev.20302. [DOI] [PubMed] [Google Scholar]
- Hofheimer JA, Wood BR, Porges SW, Pearson E, Lawson EE. Respiratory sinus arrhythmia and social interaction patterns in preterm newborns. Infant Behavior and Development. 1995;18:233–245. [Google Scholar]
- Kaufman J, Zigler E. The intergenerational transmission of child abuse. In: Cicchetti D, Carlson V, editors. Child maltreatment: Theory and research on the causes and consequences of child abuse and neglect. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Kerr ME. Why do siblings often turn out very differently? In: Fogel A, King BJ, Shanker SG, editors. Human development in the twenty first century. London: Cambridge University Press; 2008. pp. 206–215. [Google Scholar]
- Kerr ME, Bowen M. Family evaluation. New York: Norton; 1988. [Google Scholar]
- Laursen BP, Hoff E. Person-centered and variable-centered approaches to longitudinal data. Merrill-Palmer Quarterly. 2006;52(3):377–389. [Google Scholar]
- Lin EK, Bugental DB, Turek V, Martorell GA, Olster DH. Children’s vocal properties as mobilizers of stress-related physiological responses in adults. Personality and Social Psychology Bulletin. 2002;28:346–357. [Google Scholar]
- Minuchin S. Families and family therapy. Oxford, England: Harvard University Press; 1974. [Google Scholar]
- Molenaar PCM, Campbell CG. The new person-specific paradigm in psychology. Current Directions in Psychology. 2009;18(2):112–117. [Google Scholar]
- Moore GA, Hill AL, Propper CB, Calkins SD, Mills-Koonce WR, Cox MJ. Mother-infant vagal regulation in the face-to-face still-face paradigm is moderated by maternal sensitivity. Child Development. 2009;80:209–223. doi: 10.1111/j.1467-8624.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- Perlman SB, Camras LA, Pelphrey KA. Physiology and functioning: Parents’ vagal tone, emotion socialization, and children’s emotion knowledge. Journal of Experimental Child Psychology. 2008;100:308–315. doi: 10.1016/j.jecp.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Pollak SD. Mechanisms linking early experience and the emergence of emotions: Illustrations from the study of maltreated children. Current Directions in Psychological Science. 2008;17:370–375. doi: 10.1111/j.1467-8721.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. Cardiac vagal tone: A physiological index of stress. Neuroscience and Biobehavioral Reviews. 1995;19:225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: New insights into adaptive reactions of the autonomic nervous system. Cleveland Clinic Journal of Medicine. 2009;76(Suppl 2):S86–S90. doi: 10.3949/ccjm.76.s2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: Neurophysiological foundations of emotions, attachment, communication, and self-regulation. New York: Norton & Co; 2011. [Google Scholar]
- Saxbe D, Repetti RL. For better or worse? Coregulation of couples’ cortisol levels and mood states. Journal of Personality and Social Psychology. 2010;98:92–103. doi: 10.1037/a0016959. [DOI] [PubMed] [Google Scholar]
- Sethre-Hofstad L, Stansbury K, Rice MA. Attunement of maternal and child adrenocortical response to child challenge. Psychoneuroendocrinology. 2002;27:731–747. doi: 10.1016/s0306-4530(01)00077-4. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS The Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, Adoption, and Dependent Care, and Section on Developmental and Behavioral Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:2011–2663. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Skowron EA, Kozlowski JM, Pincus A. Differentiation, self-other representations, and rupture-repair processes: Predicting child maltreatment risk. Journal of Counseling Psychology. 2010;57:304–316. doi: 10.1037/a0020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinski MJ, Smyth JM, Hofer SM, Stawski RS. Intraindividual coupling of daily stress and cognition. Psychology and Aging. 2006;21:545–557. doi: 10.1037/0882-7974.21.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bakel HJA, Riksen-Walraven JMA. Behavioral and adrenocortical attunement in parent-infant dyads. A replication in one-year-olds. Developmental Psychobiology. 2008;50:196–201. doi: 10.1002/dev.20281. [DOI] [PubMed] [Google Scholar]