Abstract
Injuries to CNS axons result not only in Wallerian degeneration of the axon distal to the injury, but also in death or atrophy of the axotomized neurons, depending on injury location and neuron type. No method of permanently avoiding these changes has been found, despite extensive knowledge concerning mechanisms of secondary neuronal injury. The autonomous endoplasmic reticulum (ER) stress pathway in neurons has recently been implicated in retrograde neuronal degeneration. In addition to the emerging role of ER morphology in axon maintenance, we propose that ER stress is a common neuronal response to disturbances in axon integrity and a general mechanism for neurodegeneration. Thus manipulation of the ER stress pathway could have important therapeutic implications for neuroprotection.
Introduction
Injuries of central nervous system (CNS) axons often result in permanent loss of vital functions due to axon degeneration and retrograde neuronal cell atrophy or even death. Preventing neurodegeneration is, therefore, critical for minimizing the severe consequences of CNS injuries and preserving neuronal function. Although neuroprotectants have long been sought, none has been found either for acute neural injuries, such as stroke, traumatic brain injury (TBI) and spinal cord injury (SCI), or for chronic neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS) and glaucoma1, 2. The significant unmet clinical need for neuroprotectants is due to the lack of understanding of the key upstream signals that trigger the apoptotic cascade in injured neurons. Deciphering the mechanisms responsible for the retrograde death of axotomized and chronically degenerating neurons would allow us to identify molecular targets for the development of innovative and efficient neuroprotective treatments. Moreover, axonal degeneration is now understood to be an active process with a complex metabolic basis. This understanding opens the possibility of rescuing axons that have been injured either by trauma or diseases. The present review summarizes evidence that a key element in the response of neurons to injury of their axons is activation of neuronal endoplasmic reticulum (ER) stress. ER stress is a complex cascade of reactions that are normally activated when the ER, the organelle responsible for protein synthesis and proper folding, is overwhelmed by unfolded and misfolded proteins, a process that is called the unfolded protein response (UPR). We also consider the possibility that ER stress is initiated within the axon itself and thus could provide a target for axon protection after mechanical or metabolic insults.
Mechanisms of axotomy-induced neurodegeneration
Several hypotheses have been suggested to account for neurodegeneration after axon injury. Proposed mechanisms include: deprivation of retrogradely transported, target-derived neurotrophins; toxic influx of calcium ions through damaged axon membranes; and loss of synaptic connectivity and neuronal activity necessary for survival3. Excitotoxicity, oxidative stress and dysfunctional neuron-glia interactions may also contribute to neuronal cell death4. The available evidence, however, only partially supports any of these hypotheses. Because axon injury often is the initial pathology in both acute and chronic neurodegenerative diseases, and because axon degeneration often precedes neuronal cell body loss5-8, understanding the detrimental signals induced by axotomy is essential for effective neuroprotection.
Responses to axotomy differ among neuronal types. For example, cortico-spinal neurons, may atrophy but the vast majority survive after transection in the spinal cord9, 10, whereas most retinal ganglion cells (RGCs) die after optic nerve injury, even despite temporary rescue by delivery of exogenous trophic factors11. Several features of RGCs make them a particularly useful system for investigating the mechanisms responsible for neuronal death after axotomy. The optic nerve consists of unidirectionally projecting axons sent exclusively from RGCs. The unequivocal separation of optic nerve from RGC perikarya greatly simplifies interpretation of the specific responses of the isolated neuronal cell body to injury of their axons. Interestingly, the severity and time course of RGC death are directly correlated with the distance between the axon lesion and the neuronal perikaryon: the farther the lesion from the cell body, the fewer and more slowly the RGCs die12, 13. This correlation may explain why sectioning axons of the cortico-spinal tract (CST) in the spinal cord (far away from neuronal soma) does not induce significant short-latency cortical motoneuron death. Traditionally, this greater vulnerability of neurons to proximal axotomy has been attributed to dependence for survival on target-derived trophic factors provided by sustaining collaterals located along the axon. However, since there are no collateral branches along the course of the optic nerve, deprivation of target-derived neurotrophins cannot explain the increased vulnerability of RGCs to injuries close to their perikarya. An alternative hypothesis is that apoptosis is induced by signals derived from the tips of injured axons and transported retrogradely to the RGC perikaryon. The report that, after mild optic nerve crush, recovery of axonal transport induces additional RGC death, is consistent with the notion that an axon-derived death signal may be transferred retrogradely to the soma14. The report of reticulospinal neuron death in lampreys with a delay of 12 weeks or more after SCI15 raises the possibility that a very delayed form of corticospinal neuron death also might occur after SCI.
Recent studies from our laboratories have revealed that both acute and chronic optic nerve injuries induce ER stress in RCGs16 and that manipulating ER stress molecules exerts striking RGC-protection effects16. These findings support the emerging theme that axon injuries induce neuronal ER stress and that unresolved ER stress initiates neuron death. Consistent with this idea, spinal motoneurons showed signs of ER stress after axotomy17, 18 or ischemic injury19, 20. ER stress markers also have been detected in cortex after stroke21, 22 and in motoneurons in a mouse model of ALS23. Moreover, modulation of ER stress protected cortical neurons in models of ischemia and TBI24, decreased spinal motoneuron death17, 23 and improved functional recovery after SCI25-28. ER stress has been reviewed extensively in relation to chronic neurodegenerative diseases29-33. It has, for example, been suggested to be a critical mechanism for neurodegeneration in several hereditary motor neuron diseases, in which ER stress is precipitated by accumulation of the mutant ER-resident protein seipin within moroneurons34. Most aggregated proteins that are associated with chronic neurodegenerative diseases, however, are not confined to the ER, and whether ER stress is the cause or the consequence of the abnormal protein aggregation in the CNS remains unknown. ER stress has been documented in oligodendrocytes in demyelinating diseases35, thus it may contribute to neurodegeneration indirectly. Recent studies have demonstrated that oligodendrocytes play a fundamental metabolic role in axon survival36, and implicated oligodendrocyte dysfunction in the pathogenesis of ALS37.
The present review focuses on the axon injury-induced neuronal ER stress and explores its critical role in neurodegeneration. Axon damage is an early manifestation of many neurodegenerative diseases and neuronal cell body loss can be secondary to primary axon damage1, 2, 38. Thus the ER stress detected in neurodegenerative diseases may be due to axon pathology rather than protein aggregation, and unresolved ER stress may be responsible for progressive neuronal death. Studies of experimental glaucoma have also shown that ER stress molecules are increased in glaucomatous RGCs16, 39, 40 and that appropriate manipulations of ER stress molecules can prevent their degeneration16. We will focus on the discrete, sequential responses of neurons as they progress from neuronal subcompartmental damage (axon injury) to cell body apoptosis. We consider the cellular and molecular evidence that the axonal ER network senses harmful signals from axon injuries, and propose that neuronal ER stress is the common neuronal response to disturbances in axon integrity and that it is a general underlying mechanism for neurodegeneration. We also consider the potential therapeutic strategies targeting neuronal ER stress. Finally, we highlight the important questions that remain to be answered and conclude with a working model of neuronal ER stress initiation and propagation.
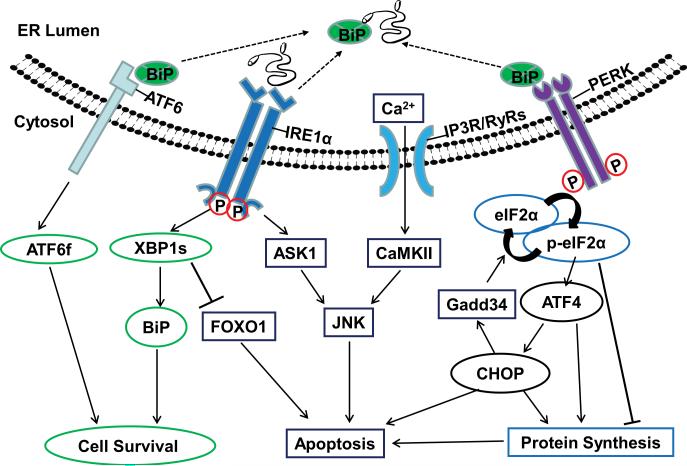
ER stress and the contradictory effects of UPR pathways on cell fate (Fig. 1)
Figure 1. The three UPR signaling pathways elicit both anti- and pro-apoptotic effects.
The release of BiP inhibition due to accumulation of unfolded or misfolded proteins activates three trans-ER membrane molecules, ATF6, IRE1α and PERK. 1) Upon ER stress, ATF6 is transported to the Golgi apparatus, where its cytosolic domain fragment (ATF6f) is released by enzymatic processing. ATF6f is a potent transcription factor and activates XBP-1 and other genes involved in recovery of ER function and cell survival. 2) IRE1α processes XBP-1 mRNA to generate the active transcription factor, spliced XBP-1 (XBP-1s). XBP-1s induces transcription of BiP and other genes involved in ER-associated degradation (ERAD), protein folding, lipogenesis, biogenesis and autophagy, and ultimate increase in ER mass and enhanced cell survival. Independent of its transcriptional function, XBP-1 directly interacts with and inhibits FOXO1 to block FOXO1-induced apoptosis. However, IRE1α also activates ASK1 and its downstream pro-apoptotic JNK. 3) PERK activation leads to phosphorylation of eIF2α. Phosphorylated eIF2α inhibits global protein synthesis but allows translation of ATF4, a transcription factor that induces CHOP expression, which leads to apoptosis. In addition, CHOP exerts feedback inhibition on phosphorylated eIF2α by inducing the expression of GADD34, which facilitates dephosphorylation of eIF2α. ATF4 and CHOP act together to promote apoptosis by increasing protein synthesis and inducing oxidative stress. 4) The release of calcium from the ER lumen through the calcium channels IP3R and RyRs could be either a cause or a consequence of ER stress. Elevation of the intracellular calcium level is a well-known mediator of apoptosis. Cytoplasmic Ca2+ triggers apoptosis through mitochondria and by activating CaMKII, which activates the apoptosis-inducing JNK.
ER is the cellular factory for biosynthesis of proteins, lipids and sterols. It exerts essential quality control functions in protein folding, maturation, trafficking and degradation. Moreover, ER is the major store of free calcium and contributes to critical cellular events by regulating intracellular calcium levels. The vital role of ER in the quality control of newly synthesized proteins makes it sensitive to the accumulation of unfolded or misfolded proteins. Elevations in the levels of these abnormal proteins beyond the handling capacity of the ER cause ER stress. This is revealed by the activation of characteristic signal transduction pathways that in aggregate are called the UPR41. The evolutionarily conserved UPR either counteracts the stress and enables the cell to survive, or introduces additional effects that lead to cell death. Thus the final outcome of UPR depends on the characteristics and levels of the downstream signaling molecules expressed, the dynamics of expression and the vulnerability of individual cell types: the cell may either achieve a new homeostasis of biosynthesis and intracellular calcium levels or initiate its own elimination.
The diverse stimuli that can trigger ER stress include hypoxia, nutrient deprivation, disruption of calcium homeostasis, high-fat diet and protein aggregates. All of these stimuli disturb ER functions and cause the accumulation of unfolded or misfolded proteins in the ER41. The ER senses the stress and initiates the canonical UPR through three ER-resident stress-sensing proteins: protein kinase RNA-like ER kinase (PERK), inositol-requiring protein-1 (IRE1α) and activating transcription factor-6 (ATF6) (Fig.1). The ER chaperone immunoglobulin-binding protein (BiP) binds to the three sensor molecules, which prevents their activation under homeostatic conditions. Increased unfolded or misfolded proteins recruit more BiP, which removes the inhibition and allows the dimerization and autophosphorylation of PERK and IRE1α, and translocation of ATF6 from ER to Golgi, thus leading to the activation of the UPR. ATF6 is cleaved sequentially by site-1 protease (S1P) and site-2 protease (S2P) in Golgi to generate an active transcription factor ATF6 fragment (ATF6f) 41. ATF6f is generally considered cytoprotective, although its downstream effectors have not been completely identified42, 43. We will focus on two other UPR signaling pathways, which employ distinct downstream effectors, either complementing or opposing each other, to promote cell survival or death. These pathways may also converge at certain integration nodal points, however, to determine the final fate of the stressed cell.
The anti- and pro-apoptotic effects of the PERK-eIF2α-ATF4-CHOP pathway
This branch of UPR promotes both cell survival and cell death. PERK is a Ser/Thr protein kinase that phosphorylates eukaryotic translation initiation factor 2α (eIF2α), upon which cap-dependent mRNA translation is inhibited. Therefore PERK-phosphorylated eIF2α lowers ER stress by reducing the protein workload of ER. Translation attenuation by the PERK- eIF2α pathway is required for pancreatic β cell survival through translation attenuation44; this process also enables other cell types to adapt to ER stress30. A small molecule, Salubrinal, inhibits the dephosphorylation of eIF2α, thus attenuating protein synthesis and promoting motor neuron survival in mouse models of chronic degenerative illnesses23, 45. Suppression of protein translation by phosphorylated eIF2α is not always protective, however, since it may block the expression of cell survival proteins46.
The phosphorylated eIF2α allows selective translation of ATF4 through the internal ribosomal entry site (IRES)41. ATF4 induces the pro-apoptotic C/EBP homologous protein (CHOP)41. CHOP is believed to function as the major mediator of ER stress for apoptosis by regulating B-cell leukemia-2 (Bcl-2) family molecules, the principal intracellular regulators of cell survival and death. CHOP down-regulates anti-apoptotic Bcl-247 and up-regulates pro-apoptotic BH-3-only molecules Bim48 and PUMA49. CHOP also promotes oxidative stress through activation of ER oxidase 1α (ERO1α)50, 51. ERO1α may also stimulate calcium release from ER through activation of calcium channel inositol (1,4,5)-trisphosphate receptors (IP3R)52. CHOP-induced calcium release from ER further activates calcium/calmodulin-dependent protein kinase II (CaMKII), which in turn induces death molecule Fas through activation of c-Jun N-terminal kinase (JNK)53. JNK can also be activated by the IRE1α pathway (see details below), and thus may act as an integration point for both of these pathways. In addition, CHOP exerts feedback inhibition on phosphorylated eIF2α by inducing the expression of growth arrest and DNA damage-inducible protein-34 (GADD34), which facilitates dephosphorylation of eIF2α41. ATF4 and CHOP have recently been shown to act synergistically to promote protein synthesis and induce oxidative stress, which results in apoptosis54. Importantly, superoxide induced by axon injury plays a critical role in RGC death55, 56. Since ER stress is metabolically convergent with oxidative stress, it suggests that oxidative stress mediates ER stress –induced apoptosis30.
The anti-apoptotic IRE1α-XBP-1 pathway vs the pro-apoptotic IRE1α-RIDD and IRE1α-JNK pathways
IRE1α is a bi-functional enzyme that contains both a Ser/Thr kinase domain and an endoribonuclease (RNase) domain. BiP release and subsequent occupation with misfolded protein allow the oligomerization and activation of IRE1α57. IRE1α RNase activity cleaves XBP-1 mRNA unconventionally to generate the spliced form of XBP-1 (XBP-1s). XBP-1s is a potent transcription factor that induces various genes to restore ER homeostasis and promote cell survival by increasing ER biogenesis as well as promoting protein degradation through ER-associated degradation complexes (ERAD)58. In addition to its transcriptional function, XBP-1 directly interacts with Forkhead box O1 (FOXO1) to assist its degradation through the proteasome system59, which blocks FOXO-dependent apoptosis.
On the other hand, IRE1α also leads to cell death. Regulated IRE1-dependent decay (RIDD) is an XBP-1-independent signaling event downstream of IRE1α RNase activity. IRE1α degrades a spectrum of ER-located mRNAs by RIDD, which may contribute to apoptosis due to lost translation of cell survival genes60, 61. IRE1α binds to an adaptor molecule, TNF-receptor-associated factor 2 (TRAF2) and activates apoptosis signal-regulating kinase 1 (ASK1), which in turn activates JNK62. JNK mediates both extrinsic death receptor-initiated and intrinsic mitochondria-originated apoptotic signaling pathways63. ER-localized pro-apoptotic Bim and PUMA selectively activate the IRE1α-TRAF2-JNK pathway, but not XBP-1 splicing64, indicating the causative link between the TRAF2-JNK branch of the IRE1α pathway and apoptosis. However, it is not known what determines whether the XBP1 splicing or JNK activation predominates, and therefore whether the IRE1α enhances cell survival or apoptosis.
How is ER stress initiated in axotomized neurons?
Our results indicate that the UPR is an intrinsic neuronal reaction to disrupted axonal integrity and that it could be a general upstream mechanism for neurodegeneration. It is, however, unlikely due to upregulation of unfolded or misfolded proteins in the ER. ER stress in the perikaryon might be activated indirectly by a retrograde injury signal. Alternatively, ER stress might be initiated locally in the axon and subsequently translocated to the cell body. At least three forms of retrograde signaling could be triggered by axon injury. These include rapid propagation of action potentials, interruption of target-derived neurotrophins, and slower dynein/microtubule-dependent transportation of locally synthesized signaling molecules65, 66. The action potential triggers calcium influx both in the axon and the soma, which has been implicated in RGC death after optic nerve injury67. The critical role of calcium in ER function, signaling and apoptosis32 makes it the leading candidate as the axonal damage signal that induces ER stress and apoptosis (Fig. 2). Smooth ER (sER) is the major intracellular calcium store and maintains a much higher calcium concentration (10-100uM) than cytoplasm (100-300nM) due to the activity of SERCA (smooth ER Ca2+ ATPase) in the ER membrane68. Thapsigargin, a SERCA inhibitor, is the most commonly used experimental ER stress inducer, because of the sensitivity of ER to calcium homeostasis. Increase in axoplasmic calcium is an early neuronal response to axon injury and plays key roles in the cytoskeletal breakdown of Wallerian degeneration38, 69 and in neural excitotoxicity and apoptosis68. The release of Ca2+ from ER through ryanodine receptors (RyRs) and IP3Rs contributes significantly to the intra-axonal Ca2+ influx38, 68, as does extracellular Ca2+ influx through ion-channels on axon membrane. The wave of intra-axonal Ca2+ elevation propagates retrogradely to the cell body, where it may disturb the calcium homeostasis and induce soma ER stress. Moreover, there is intimate physical interaction and signaling crosstalk between ER and mitochondria69-71. Uptake by mitochondria of the Ca2+ released from ER results in opening of the mitochondrial permeability transmembrane pore (mPTP), energetic failure, outer membrane fragmentation, release of cytochrome C and finally apoptosis. Interestingly, mutations of presenilin 1 and 2 (PS1,2), which are located primarily in ER and involved in calcium release from ER, are linked with early onset of familial AD, indicating the possibility that the malfunction of the ER contributes to pathogenesis of AD72. In addition to calcium, an ever-increasing number of molecules have been identified as the retrograde injury signals from the axon to the cell body in peripheral neurons. These signals are mediated by the nuclear transport factors importin, RanGTPase and vimentin and the dynein complex, including members of the mitogen-activated protein kinase family (ERK, JNK and DLK1)65, 66 and transcription factors such as STAT373. It remains to be determined whether CNS neurons employ similar molecules and mechanism as retrograde injury signals. It will also be critical to determine whether and how these signaling molecules induce ER stress in the cell body.
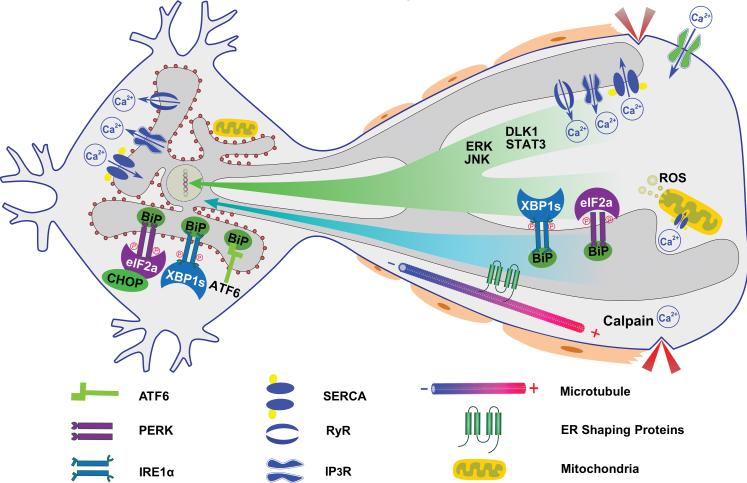
Figure 2. The initiation and propagation of neuronal ER stress after axotomy.
The neuronal ER is a continuous membrane system that is comprised of the nuclear envelope, the sheet-like rER decorated with ribosomes in perikaryon, and the tubular sER, which is distributed throughout the axon as a continuous tubular network. ER stress may be induced in the cell body by retrograde signals including: 1) Injury-associated signaling such as Ca2+, ERK, JNK, DLK1 and STAT3. 2) ER stress molecules, such as XBP-1s, phosphorylated eIF2α and BiP, which are induced locally in axon. 3) Morphological changes in the axonal tubular sER.
Ca2+ is pumped into the ER lumen by the SERCA and released through RyRs and IP3Rs to contribute to the increase of intra-axonal Ca2+ level early after axon injury. The wave of intraaxonal Ca2+ increase could propagate retrogradely to disturbant the calcium homeostasis and induce ER stress in the cell body. The uptake of the Ca2+ released from ER by mitochondria results in opening of mPTP, energetic failure, ROS production, outer membrane fragmentation, release of cytochrome C and finally apoptosis. Ca2+ activated calpain breaks down axonal cytoskeleton in Wallerian degeneration, which may subsequently change the shape and location of tubular sER. The morphology of the axonal sER is critical for axon maintenance, and relies on the interaction between microtubules and the ER-shaping proteins spastin, atlastin-1, REEP1 and reticulons. Alterations of these proteins cause axon abnormalities during development and disease, which may be due to impairing ER morphology and distribution. Axon injury disrupts the ER network which may cause ER stress directly. In addition, morphological changes of the axonal tubular sER itself or other locally activated ER stress signals may propagate along the ER endomembrane system to the cell body, where ER stress is activated.
It is possible that axon injury induces ER stress in the cell body and axon independently and nearly simultaneously. If so, then the earlier degeneration of the axon might be due to its greater sensitivity to ER stress. There are few studies of axonal ER stress, which might also be induced locally by calcium influx into the axon. It has been shown that XBP-1 mRNA splicing occurs in neurites and that the spliced form of XBP-1 protein is transported back to the neuronal soma after BDNF stimulation74. LPA treatment also induces axonal eIF2α phosphorylation and BiP expression locally75. These findings suggest that the UPR pathways can be activated in axons. If so, then this would raise the intriguing question of how ER stress is translocated retrogradely to the neuronal soma. Possible mechanisms include the retrograde transport of activated UPR molecules to the perikaryon, where they could induce cell death (Fig. 2). However, the unique distribution and morphology of the neuronal ER network may represent an even more intriguing mechanism for ER stress initiation and propagation as discussed below.
The neuronal ER network is a continuous membrane system that comprises the nuclear envelope; sheet-like rough ER (rER) decorated with polyribosomes, which is present predominantly in neuronal perikarya and proximal dendrites; and tubular sER, which is distributed throughout the axons and distal dendrites as a continuous tubular network76-78. It is the major component of the cellular endomembrane system, which physically interacts with, and is functionally coupled with, other cellular organelles. Due to its function in biosynthesis, ER may support the massive membrane expansion that occurs during axon and dendrite formation, extension and maintenance79; recent evidence demonstrates that the preservation of ER morphology in axons is important for normal neuronal development and maintenance77, 80. The shape and proper position of the tubular sER network in axons is coordinated by interaction between spastin and other ER-shaping proteins, including atlastin-1, receptor expression enhancing protein 1 (REEP1) and reticulon-177, 80. Autosomal dominant mutations in these genes are responsible for the most common forms of hereditary spastic paraplegias (HSPs), a class of neurological disorders caused by degeneration of upper motoneurons and their axons in the CST80. Remarkably, alteration of ER shape and distribution in axons appears to be a novel pathologic mechanism underlying both HSPs and ALS77, 80. This association receives support from recent discoveries of the mutations of another ER-shaping protein reticulon-2 in a less common form of HSP81 and the ER-resident VAPB protein (vesicle-associated membrane protein-associated protein B) in a familial form of ALS82. However, the mechanisms by which changes in ER shape lead to neurodegeneration are unknown. One possibility is that damage of the tubular ER in the axon causes dysfunction of ER, which in turn triggers ER stress that can move along the endomembrane system of ER back to the cell body (Fig. 2). If so, then ER stress may also be involved in axon degeneration.
That axon degeneration is an actively regulated process is suggested by a mouse mutant in which Wallerian degeneration is delayed83. The chimeric mutant protein slow Wallerian degeneration protein (Wlds) contains the full-length NAD+-synthetic enzyme nicotinamide mononucleotide adenylyltransferase 1 (Nmnat1) at its C-terminal region, while the N-terminal region is required for translocation of Wlds from the nucleus to the axon. Both of these features are necessary for axon protection84, indicating that axonal NAD+ production is critical for axon survival. Another NAD+-synthetic enzyme, Nmnat2, is constantly transported to the axon to maintain axonal integrity85. Nmnat2 is highly enriched in CNS neurons and is associated primarily with Golgi and ER, whereas Nmnat3 is located in mitochondria86, 87. Since general elevation of cytosolic NAD+ level fails to protect injured axons, it has been suggested that the regulation of NAD+ levels in axonal ER and mitochondria is critical for axon survival, presumably due to its role in regulating cytosolic levels of calcium and reactive oxygen species (ROS)69. Importantly, the elevation of calcium and ROS levels induces the mPTP, which links mitochondria dysfunction to axon degeneration88, 89. Due to the close relationship between ER and mitochondria and their co-regulation of calcium and ROS30, 89, it suggests that ER stress also plays a role in axon degeneration. Thus it would be very interesting to determine the relationship between the activities of Nmnats/NAD+ levels and ER function in axons after injury, which could indicate the molecular mechanism for axon degeneration.
Therapeutic strategies targeting ER stress for neuroprotection
If UPR activation is designed to help cells to cope with ER stress, why does it seem only to cause neuron death after axon injury? One possibility is preponderant pro-apoptotic UPR activation after axonal insults. Studies of cultured non-neuronal cells, for example, led to the proposal that the duration of IRE1/XBP-1 activation correlates with its protective effects90. Consistent with this idea, we found that axotomy triggers differential activation of diverse UPR pathways in RGCs: whereas CHOP is robustly and persistently activated, XBP-1 is activated only transiently and modestly16. Differences in these responses might account for the prominent pro-apoptotic effect of ER stress in injured neurons. In addition, the unique polarization characteristics of the axonal compartments in neurons might favor retrograde transportation of specific signaling molecules which may also contribute to the differential activation of UPR.
Considerable experimental evidence shows the therapeutic potential of targeting ER stress in CNS injuries and suggests that better understanding of the responsible mechanisms is likely to generate even more effective treatments. For example, inhibition of ER stress is associated with neuroprotection in models of both ischemia and TBI24. In addition, modulation of ER stress molecules BiP, CHOP, XBP-1 and ATF4 decreased motoneuron death17 and improved functional recovery after SCI25-28. Our own work with axotomized RGCs has shown that manipulating CHOP and XBP-1 in opposite directions, by deleting CHOP or by overexpressing XBP-1s, exerts striking RGC-protection effects16. Since XBP-1 regulates numerous genes that are involved in multiple cellular functions, it is important to define the downstream mechanism by which XBP-1 contributes to neuroprotection. In a Drosophila model of AD, XBP-1 exerts a neuroprotective effect that is due to downregulation of RyR3, with consequent inhibition of calcium release from ER91. XBP-1 also could be deleterious to motoneurons, however, since neural specific deletion of XBP-1 delays the onset of ALS in SOD1 transgenic mice, potentially through upregulation of autophagy and clearance of SOD1 aggregates92. It remains to be determined why XBP-1 behaves differently in diverse neuronal populations. CHOP deficiency also protects neurons in several pathological conditions29-33. We have found that the combination of increasing XBP-1 and deleting CHOP together exerts a significantly more potent neuroprotective effect on injured RGCs than targeting either pathway alone, suggesting the potential benefits of treatments that can act synergistically by targeting multiple UPR pathways16.
JNK, a toxic signal that is induced by axon injury93, 94, is another promising molecular target for promoting neuroprotection, although JNK inhibition failed to slow PD in a clinical trial95. JNK deficiency rescues RGCs after optic nerve crush96, and ASK1, the upstream kinase of JNK, plays a key role in SOD1 induced ER stress and motoneuron loss97. In addition, JNK inhibition modestly delays axonal Wallerian degeneration93. JNK could be the mediator of apoptosis downstream of both PERK-CHOP-Calcium -CaMKII and IRE1α-TRAF2-ASK1 pathways and a nodal point that integrates two pathways that lead to neuron death.
Some obvious strategies based on recent advances in our understanding of ER stress await more extensive trials in vivo. For example, IRE1α activation can lead to cell death or survival, depending on the balance between its kinase and RNase activities. Its RNase activity is primarily directed to XBP-1 splicing and RIDD. IRE1α kinase activity is involved in JNK activation. It is now possible to block IRE1α kinase activity, thus inhibiting JNK activation and at the same time to fine tune IRE1α kinase activity to bias its RNase activity towards XBP-1 splicing but not RIDD60, 98. It would be important to determine whether applying these strategies to modulate ER stress in vivo will enhance neuron survival.
Concluding remarks
Axon injury-induced ER stress reveals a new link between axon pathology and retrograde neurodegeneration. Because these sequential events may be a general phenomenon in both acute neural trauma and chronic neurodegenerative diseases, modulation of neuronal ER stress represents a novel and promising strategy for neuroprotection. The development of neuroprotective agents has proven to be challenging and complex. A more thorough understanding of how ER stress is activated in neurons, and how UPR pathways crosstalk with each other to determine cell fate, is a prerequisite for advances in the treatment of neurodegeneration. The correlative evidence described in this review suggests that local ER stress at the site of injury may elicit a retrograde signal that can induce cell death. What that signal is and how the transmission might function are unanswered questions. Since the ER network is continuous from nucleus to the most distal portions of the axon, it is possible that some signals of the UPR are transported within the ER membrane system. Finally, compelling evidence supports that neuron cell body death and axon degeneration are autonomous effects of injury and independent of each other. It is obvious, however, that these two sequential events must be linked in some fashion. Might ER stress be the common upstream mechanism responsible for neuron and axon degeneration in neurological diseases? More importantly, the complex and often contradictory characteristics of the diverse ER stress signaling molecules will demand systematic analysis of their definitive roles simultaneously in a broad spectrum of diseases to develop optimal combinatory neuroprotective strategies.
Acknowledgements
We apologize that we could not cite all of the relevant research that contributed to our understanding of ER function and neurodegeneration due to space restrictions. We thank Alan Tessler for critically reading the manuscript. This work was supported by grants from Shriners Hospitals for Children, National Institutes of Health and BrightFocus Foundation.
References
- 1.Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002 May 3;296(5569):868–71. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- 2.Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002 Oct;25(10):532–7. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- 3.Tuszynski MH, Kordower JH. CNS regeneration : basic science and clinical advances. Academic Press; San Diego: 1999. [Google Scholar]
- 4.Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012 Mar;31(2):152–81. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Adalbert R, Nogradi A, Babetto E, et al. Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain. 2009 Feb;132(Pt 2):402–16. doi: 10.1093/brain/awn312. [DOI] [PubMed] [Google Scholar]
- 6.Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010 Feb;13(2):190–6. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke RE, O'Malley K. Axon degeneration in Parkinson's disease. Exp Neurol. 2012 Jan 18; doi: 10.1016/j.expneurol.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitmore AV, Libby RT, John SW. Glaucoma: thinking in new ways-a role for autonomous axonal self-destruction and other compartmentalised processes? Prog Retin Eye Res. 2005 Nov;24(6):639–62. doi: 10.1016/j.preteyeres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Nielson JL, Sears-Kraxberger I, Strong MK, Wong JK, Willenberg R, Steward O. Unexpected survival of neurons of origin of the pyramidal tract after spinal cord injury. J Neurosci. 2010 Aug 25;30(34):11516–28. doi: 10.1523/JNEUROSCI.1433-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielson JL, Strong MK, Steward O. A reassessment of whether cortical motor neurons die following spinal cord injury. The Journal of comparative neurology. 2011 Oct 1;519(14):2852–69. doi: 10.1002/cne.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1632–6. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994 Jul;14(7):4368–74. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villegas-Perez MP, Vidal-Sanz M, Rasminsky M, Bray GM, Aguayo AJ. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol. 1993 Jan;24(1):23–36. doi: 10.1002/neu.480240103. [DOI] [PubMed] [Google Scholar]
- 14.Prilloff S, Henrich-Noack P, Sabel BA. Recovery of axonal transport after partial optic nerve damage is associated with secondary retinal ganglion cell death in vivo. Invest Ophthalmol Vis Sci. 2012 Mar;53(3):1460–6. doi: 10.1167/iovs.11-8306. [DOI] [PubMed] [Google Scholar]
- 15.Shifman MI, Zhang G, Selzer ME. Delayed death of identified reticulospinal neurons after spinal cord injury in lampreys. The Journal of comparative neurology. 2008 Sep 20;510(3):269–82. doi: 10.1002/cne.21789. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Park KK, Yang L, et al. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron. 2012 Feb 9;73(3):445–52. doi: 10.1016/j.neuron.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penas C, Font-Nieves M, Fores J, et al. Autophagy, and BiP level decrease are early key events in retrograde degeneration of motoneurons. Cell Death Differ. 2011 Oct;18(10):1617–27. doi: 10.1038/cdd.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penas C, Guzman MS, Verdu E, Fores J, Navarro X, Casas C. Spinal cord injury induces endoplasmic reticulum stress with different cell-type dependent response. J Neurochem. 2007 Aug;102(4):1242–55. doi: 10.1111/j.1471-4159.2007.04671.x. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi T, Sakurai M, Abe K, Matsumiya G, Sawa Y. Impact of the endoplasmic reticulum stress response in spinal cord after transient ischemia. Brain Res. 2007 Sep 12;1169:24–33. doi: 10.1016/j.brainres.2007.06.093. [DOI] [PubMed] [Google Scholar]
- 20.Mizukami T, Orihashi K, Herlambang B, et al. Sodium 4-phenylbutyrate protects against spinal cord ischemia by inhibition of endoplasmic reticulum stress. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2010 Dec;52(6):1580–6. doi: 10.1016/j.jvs.2010.06.172. [DOI] [PubMed] [Google Scholar]
- 21.Nakka VP, Gusain A, Raghubir R. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotoxicity research. 2010 Feb;17(2):189–202. doi: 10.1007/s12640-009-9110-5. [DOI] [PubMed] [Google Scholar]
- 22.Yuan Y, Guo Q, Ye Z, Pingping X, Wang N, Song Z. Ischemic postconditioning protects brain from ischemia/reperfusion injury by attenuating endoplasmic reticulum stress-induced apoptosis through PI3K-Akt pathway. Brain Res. 2011 Jan 7;1367:85–93. doi: 10.1016/j.brainres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Saxena S, Cabuy E, Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci. 2009 May;12(5):627–36. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- 24.Krajewska M, Xu L, Xu W, et al. Endoplasmic reticulum protein BI-1 modulates unfolded protein response signaling and protects against stroke and traumatic brain injury. Brain Res. 2011 Jan 25;1370:227–37. doi: 10.1016/j.brainres.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penas C, Verdu E, Asensio-Pinilla E, et al. Valproate reduces CHOP levels and preserves oligodendrocytes and axons after spinal cord injury. Neuroscience. 2011 Mar 31;178:33–44. doi: 10.1016/j.neuroscience.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Ohri SS, Maddie MA, Zhao Y, Qiu MS, Hetman M, Whittemore SR. Attenuating the endoplasmic reticulum stress response improves functional recovery after spinal cord injury. Glia. 2011 Oct;59(10):1489–502. doi: 10.1002/glia.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valenzuela V, Collyer E, Armentano D, Parsons GB, Court FA, Hetz C. Activation of the unfolded protein response enhances motor recovery after spinal cord injury. Cell death & disease. 2012;3:e272. doi: 10.1038/cddis.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong Z, Hong H, Chen H, Wang Z, Hong D. Protective effects of erythropoietin in experimental spinal cord injury by reducing the C/EBP-homologous protein expression. Neurological research. 2012 Jan;34(1):85–90. doi: 10.1179/1743132811Y.0000000026. [DOI] [PubMed] [Google Scholar]
- 29.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006 Mar;13(3):385–92. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- 30.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxidants & redox signaling. 2007 Dec;9(12):2277–93. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 31.Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008 Dec;7(12):1013–30. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 33.Doyle KM, Kennedy D, Gorman AM, Gupta S, Healy SJ, Samali A. Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. Journal of cellular and molecular medicine. 2011 Oct;15(10):2025–39. doi: 10.1111/j.1582-4934.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito D, Suzuki N. Seipinopathy: a novel endoplasmic reticulum stress-associated disease. Brain. 2009 Jan;132(Pt 1):8–15. doi: 10.1093/brain/awn216. [DOI] [PubMed] [Google Scholar]
- 35.Lin W, Popko B. Endoplasmic reticulum stress in disorders of myelinating cells. Nat Neurosci. 2009 Apr;12(4):379–85. doi: 10.1038/nn.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Y, Morrison BM, Li Y, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012 Jul 26;487(7408):443–8. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang SH, Li Y, Fukaya M, et al. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci. 2013 May;16(5):571–9. doi: 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stirling DP, Stys PK. Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends in molecular medicine. 2010 Apr;16(4):160–70. doi: 10.1016/j.molmed.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doh SH, Kim JH, Lee KM, Park HY, Park CK. Retinal ganglion cell death induced by endoplasmic reticulum stress in a chronic glaucoma model. Brain Res. 2010 Jan 13;1308:158–66. doi: 10.1016/j.brainres.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 40.Shimazawa M, Inokuchi Y, Ito Y, et al. Involvement of ER stress in retinal cell death. Mol Vis. 2007;13:578–87. [PMC free article] [PubMed] [Google Scholar]
- 41.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007 Jul;8(7):519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 42.Lynch JM, Maillet M, Vanhoutte D, et al. A Thrombospondin-Dependent Pathway for a Protective ER Stress Response. Cell. 2012 Jun 8;149(6):1257–68. doi: 10.1016/j.cell.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J, Rutkowski DT, Dubois M, et al. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007 Sep;13(3):351–64. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Back SH, Scheuner D, Han J, et al. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell metabolism. 2009 Jul;10(1):13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Y, Fenik P, Zhan G, Sanfillipo-Cohn B, Naidoo N, Veasey SC. Eif-2a protects brainstem motoneurons in a murine model of sleep apnea. J Neurosci. 2008 Feb 27;28(9):2168–78. doi: 10.1523/JNEUROSCI.5232-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allagnat F, Cunha D, Moore F, Vanderwinden JM, Eizirik DL, Cardozo AK. Mcl-1 downregulation by pro-inflammatory cytokines and palmitate is an early event contributing to beta-cell apoptosis. Cell Death Differ. 2011 Feb;18(2):328–37. doi: 10.1038/cdd.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001 Feb;21(4):1249–59. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puthalakath H, O'Reilly LA, Gunn P, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007 Jun 29;129(7):1337–49. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 49.Galehdar Z, Swan P, Fuerth B, Callaghan SM, Park DS, Cregan SP. Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J Neurosci. 2010 Dec 15;30(50):16938–48. doi: 10.1523/JNEUROSCI.1598-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marciniak SJ, Yun CY, Oyadomari S, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004 Dec 15;18(24):3066–77. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008 Oct;118(10):3378–89. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li G, Mongillo M, Chin KT, et al. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009 Sep 21;186(6):783–92. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Timmins JM, Ozcan L, Seimon TA, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009 Oct;119(10):2925–41. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han J, Back SH, Hur J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013 May;15(5):481–90. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanamori A, Catrinescu MM, Kanamori N, Mears KA, Beaubien R, Levin LA. Superoxide is an associated signal for apoptosis in axonal injury. Brain. 2010 Sep;133(9):2612–25. doi: 10.1093/brain/awq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghaffarieh A, Levin LA. Optic nerve disease and axon pathophysiology. International review of neurobiology. 2012;105:1–17. doi: 10.1016/B978-0-12-398309-1.00002-0. [DOI] [PubMed] [Google Scholar]
- 57.Gardner BM, Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011 Sep 30;333(6051):1891–4. doi: 10.1126/science.1209126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: integrating stress signals through the stress sensor IRE1alpha. Physiological reviews. 2011 Oct;91(4):1219–43. doi: 10.1152/physrev.00001.2011. [DOI] [PubMed] [Google Scholar]
- 59.Zhou Y, Lee J, Reno CM, et al. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat Med. 2011 Mar;17(3):356–65. doi: 10.1038/nm.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han D, Lerner AG, Vande Walle L, et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009 Aug 7;138(3):562–75. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009 Aug 10;186(3):323–31. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000 Jan 28;287(5453):664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 63.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008 Oct 20;27(48):6245–51. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klee M, Pallauf K, Alcala S, Fleischer A, Pimentel-Muinos FX. Mitochondrial apoptosis induced by BH3-only molecules in the exclusive presence of endoplasmic reticular Bak. The EMBO journal. 2009 Jun 17;28(12):1757–68. doi: 10.1038/emboj.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abe N, Cavalli V. Nerve injury signaling. Curr Opin Neurobiol. 2008 Jun;18(3):276–83. doi: 10.1016/j.conb.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rishal I, Fainzilber M. Retrograde signaling in axonal regeneration. Exp Neurol. 2010 May;223(1):5–10. doi: 10.1016/j.expneurol.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 67.Prilloff S, Noblejas MI, Chedhomme V, Sabel BA. Two faces of calcium activation after optic nerve trauma: life or death of retinal ganglion cells in vivo depends on calcium dynamics. The European journal of neuroscience. 2007 Jun;25(11):3339–46. doi: 10.1111/j.1460-9568.2007.05550.x. [DOI] [PubMed] [Google Scholar]
- 68.Mattson MP, LaFerla FM, Chan SL, Leissring MA, Shepel PN, Geiger JD. Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2000 May;23(5):222–9. doi: 10.1016/s0166-2236(00)01548-4. [DOI] [PubMed] [Google Scholar]
- 69.Wang JT, Medress ZA, Barres BA. Axon degeneration: molecular mechanisms of a self-destruction pathway. J Cell Biol. 2012 Jan 9;196(1):7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008 Oct 27;27(50):6407–18. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grimm S. The ER-mitochondria interface: the social network of cell death. Biochim Biophys Acta. 2012 Feb;1823(2):327–34. doi: 10.1016/j.bbamcr.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 72.Tu H, Nelson O, Bezprozvanny A, et al. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006 Sep 8;126(5):981–93. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ben-Yaakov K, Dagan SY, Segal-Ruder Y, et al. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. The EMBO journal. 2012 Mar 21;31(6):1350–63. doi: 10.1038/emboj.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hayashi A, Kasahara T, Iwamoto K, et al. The role of brain-derived neurotrophic factor (BDNF)-induced XBP1 splicing during brain development. J Biol Chem. 2007 Nov 23;282(47):34525–34. doi: 10.1074/jbc.M704300200. [DOI] [PubMed] [Google Scholar]
- 75.Vuppalanchi D, Merianda TT, Donnelly C, et al. Lysophosphatidic acid differentially regulates axonal mRNA translation through 5'UTR elements. Molecular and cellular neurosciences. 2012 Jun;50(2):136–46. doi: 10.1016/j.mcn.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsukita S, Ishikawa H. Three-dimensional distribution of smooth endoplasmic reticulum in myelinated axons. Journal of electron microscopy. 1976;25(3):141–9. [PubMed] [Google Scholar]
- 77.Ramirez OA, Couve A. The endoplasmic reticulum and protein trafficking in dendrites and axons. Trends in cell biology. 2011 Apr;21(4):219–27. doi: 10.1016/j.tcb.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 78.Broadwell RD, Cataldo AM. The neuronal endoplasmic reticulum: its cytochemistry and contribution to the endomembrane system. II. Axons and terminals. The Journal of comparative neurology. 1984 Dec 1;230(2):231–48. doi: 10.1002/cne.902300208. [DOI] [PubMed] [Google Scholar]
- 79.Pfenninger KH. Plasma membrane expansion: a neuron's Herculean task. Nat Rev Neurosci. 2009 Apr;10(4):251–61. doi: 10.1038/nrn2593. [DOI] [PubMed] [Google Scholar]
- 80.Blackstone C. Cellular pathways of hereditary spastic paraplegia. Annu Rev Neurosci. 2012;35:25–47. doi: 10.1146/annurev-neuro-062111-150400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Montenegro G, Rebelo AP, Connell J, et al. Mutations in the ER-shaping protein reticulon 2 cause the axon-degenerative disorder hereditary spastic paraplegia type 12. J Clin Invest. 2012 Feb 1;122(2):538–44. doi: 10.1172/JCI60560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fasana E, Fossati M, Ruggiano A, et al. A VAPB mutant linked to amyotrophic lateral sclerosis generates a novel form of organized smooth endoplasmic reticulum. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010 May;24(5):1419–30. doi: 10.1096/fj.09-147850. [DOI] [PubMed] [Google Scholar]
- 83.Mack TG, Reiner M, Beirowski B, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001 Dec;4(12):1199–206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- 84.Conforti L, Wilbrey A, Morreale G, et al. Wld S protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. J Cell Biol. 2009 Feb 23;184(4):491–500. doi: 10.1083/jcb.200807175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS biology. 2010 Jan;8(1):e1000300. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem. 2005 Oct 28;280(43):36334–41. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- 87.Cahoy JD, Emery B, Kaushal A, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008 Jan 2;28(1):264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barrientos SA, Martinez NW, Yoo S, et al. Axonal degeneration is mediated by the mitochondrial permeability transition pore. J Neurosci. 2011 Jan 19;31(3):966–78. doi: 10.1523/JNEUROSCI.4065-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Court FA, Coleman MP. Mitochondria as a central sensor for axonal degenerative stimuli. Trends Neurosci. 2012 Jun;35(6):364–72. doi: 10.1016/j.tins.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 90.Lin JH, Li H, Yasumura D, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007 Nov 9;318(5852):944–9. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Casas-Tinto S, Zhang Y, Sanchez-Garcia J, Gomez-Velazquez M, Rincon-Limas DE, Fernandez-Funez P. The ER stress factor XBP1s prevents amyloid-beta neurotoxicity. Hum Mol Genet. 2011 Jun 1;20(11):2144–60. doi: 10.1093/hmg/ddr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hetz C, Thielen P, Matus S, et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009 Oct 1;23(19):2294–306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, DiAntonio A. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci. 2009 Apr;12(4):387–9. doi: 10.1038/nn.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ghosh AS, Wang B, Pozniak CD, Chen M, Watts RJ, Lewcock JW. DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. J Cell Biol. 2011 Sep 5;194(5):751–64. doi: 10.1083/jcb.201103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology. 2007 Oct 9;69(15):1480–90. doi: 10.1212/01.wnl.0000277648.63931.c0. [DOI] [PubMed] [Google Scholar]
- 96.Fernandes KA, Harder JM, Fornarola LB, et al. JNK2 and JNK3 are major regulators of axonal injury-induced retinal ganglion cell death. Neurobiology of disease. 2012 May;46(2):393–401. doi: 10.1016/j.nbd.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nishitoh H, Kadowaki H, Nagai A, et al. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 2008 Jun 1;22(11):1451–64. doi: 10.1101/gad.1640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bouchecareilh M, Higa A, Fribourg S, Moenner M, Chevet E. Peptides derived from the bifunctional kinase/RNase enzyme IRE1alpha modulate IRE1alpha activity and protect cells from endoplasmic reticulum stress. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011 Sep;25(9):3115–29. doi: 10.1096/fj.11-182931. [DOI] [PubMed] [Google Scholar]