Abstract
Estrogen action is mediated through several types of receptors (ERs), such as ERα, ERβ and putative membrane ERs. Oxytocin receptor (OTR) and ER expression levels in the rat uterus are regulated by estrogen; however, which types of ERs are involved has not been elucidated. This study examined OTR, ERα and ERβ levels in ovariectomized rats treated with 17β-estradiol (E2), an ERα agonist (PPT), an ERβ agonist (DPN) or estren (Es). E2 and PPT increased OTR mRNA levels and decreased ERα and ERβ mRNA levels 3 and 6 h posttreatment. DPN decreased ERα and ERβ mRNA levels at 3 and 6 h, while OTR mRNA levels increased at 3 h and decreased at 6 h. OTR mRNA levels increased 3 h after the Es treatment and then declined until 6 h. ERα and ERβ mRNA levels decreased by 3 h and remained low until 6 h posttreatment with Es. The ER antagonist ICI182,780 (ICI) suppressed the increases in OTR mRNA levels induced 3 h after the Es treatment. However, ICI and tamoxifen (Tam) had no significant effect on ERα and ERβ mRNA levels in the Es-treated or vehicle-treated group. In intact rats, proestrus-associated increases in OTR mRNA levels were antagonized by both ICI and Tam. However, decreases in ERα and ERβ mRNA levels were not antagonized by Tam and ICI, respectively. Therefore, uterine OTR gene expression is upregulated by estrogen through the classical nuclear (or non-nuclear) ERs, ERα and ERβ, while the levels of these ERs are downregulated by estrogen through multiple pathways including Es-sensitive nonclassical ERs.
Keywords: Estren, Estrogen, Estrogen receptor, Oxytocin receptor, Rats
Oxytocin was initially isolated as a neurohypophysial hormone that stimulates contraction of the myometrium and myoepithelium to facilitate parturition and milk ejection, respectively. It has also been shown to play a role in various reproductive functions in the mammary gland, ovary, brain and uterus. In the uterus, the near-term myometrium is extremely sensitive to oxytocin. This increased uterine responsiveness to oxytocin was shown to occur in parallel with an increase in the number of uterine oxytocin binding sites in rats [1, 2], humans [3], rabbits [4, 5] and cows [6]. Corresponding increases in uterine oxytocin receptor (OTR) mRNA expression in late pregnancy and parturition have also been reported in cows [7], rats [8,9,10], humans [11] and sheep [12, 13].
Estrogen stimulates an increase in both the number of uterine oxytocin binding sites [1, 14, 15] and OTR mRNA expression in ovariectomized (OVX) virgin rats [8, 9]. However, an injection of estrogen did not stimulate OTR mRNA expression in late pregnant rats or in progesterone-primed OVX virgin rats and was only effective following ovariectomy and the removal of progesterone, respectively [16]. These results suggest that, in addition to increases in serum estrogen levels in near-term rats, the regulation of uterine responsiveness to estrogen is an important part of the mechanism of action of estrogen in the uterus.
The actions of estrogen are mediated through several types of receptors, including two transcription-regulating intracellular estrogen receptors (ERs), ERα and ERβ, which have been cloned and characterized from the human uterus [17] and rat prostate [18], respectively. ERα and ERβ were shown to be encoded by the ESR1 and ESR2 genes, respectively, and are widely distributed in various tissues [19], including the endometrium and myometrium in several animal species [20, 21]. In addition to these classical nuclear receptors, some actions of estrogen have been reported to be mediated through putative membrane receptors [22], including G-protein-coupled receptor 30 (GPR30) [23, 24] and ERα localized at the membrane [25,26,27]. However, although several actions of specific ER types have been observed, the ERs involved in regulating expression of OTR and ER genes in the uterus have not yet been identified.
The synthetic compound 4-estren-3α, 17β-diol (estren, Es) has been reported to increase bone mass and strength in ovariectomized mice without affecting transcriptional activity or reproductive organ function [28, 29], which suggested that Es functions through membrane ERs. Es has recently been used as a nonclassical ER analogue [30, 31]. On the other hand, Es was shown to affect transcriptional activities in several cell types including murine uterine cells [32,33,34]. Thus, the effect of Es on uterine function is controversial. Therefore, it is important to confirm whether Es has any effect on uterine function, including the regulation of OTR. If Es affects OTR expression, it may be a good analogue to further characterize ER pathways involved in the dynamics of OTR gene regulation, including membrane ERs, in the uterus.
To identify the ERs involved in regulating OTR gene expression by estrogen, the effects of 17β-estradiol (E2), an ERα agonist (PPT), an ERβ agonist (DPN) and Es were examined in OVX rats in the present study. The effects of the ER antagonists, ICI182,780 (ICI) and tamoxifen (Tam), on uterine OTR and ER mRNA levels were also examined to further characterize the types of ERs involved in regulating the actions of estrogen. The effects of these antagonists were investigated in both Es-treated OVX rats and intact rats during the proestrus phase of the estrous cycle, in which OTR mRNA levels have been shown to increase and ERα and β mRNA levels have been shown to decrease [35].
Materials and Methods
Animals
Adult female Wistar rats (body weight 180–220 g) were obtained from Japan SLC (SLC, Hamamatsu, Japan), or from the Institute for Animal Reproduction (IAR, Kasumigaura, Japan). They were kept in an environmentally controlled room (temperature 23 ± 3 C; lights on 0800–2000 h) with free access to tap water and pelleted rat food (NMF; Oriental Yeast, Tokyo, Japan). Virgin Wistar rats (SLC) were ovariectomized under ether anesthesia 2 weeks before the steroid treatment. Virgin Wistar-Imamichi rats (IAR) were monitored during the estrous cycle by taking vaginal smears each morning (0900–1000 h). Those that showed regular 4-day cycles were used. The rats were euthanized, and the uteri were collected, frozen and stored at –70 C until RNA extraction. Animal care, maintenance and surgery were approved by the Animal Care and Use Committee and were conducted according to the Guidelines for Animal Experiments at University of Fukui.
Exp. 1. Effects of 17β-estradiol on OTR, ERα and ERβ mRNA levels in the uteri of OVX rats.
Ovariectomized (OVX) rats (SLC) were given a subcutaneous injection of 17β-estradiol (E2, 0.5, 2.5 or 12.5 μg, Nacalai Tesque, Kyoto, Japan) dissolved in 0.2 ml of sesame oil at 1100–1120 h and were euthanized at 1100–1200 h the next day. OVX rats also were given a subcutaneous injection of E2 (12.5 μg) and euthanized 1, 2, 3, 4, 5, 6, 7, 8 or 9 h after the injection.
Exp. 2. Effects of the ERα or ERβ agonist on OTR, ERα and ERβ mRNA levels in the uteri of OVX rats
OVX rats were given a subcutaneous injection of the ERα-selective ligand 4,4’,4’’-(4-propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (PPT, 200 μg, Tocris Biosciences, Ellisville, MS, USA) or the ERβ selective ligand 2,3-bis (4-hydroxyphenyl)-propionitrile (DPN, 200 μg, Tocris Biosciences), both of which were dissolved in 0.25 ml of sesame oil and administered at 1100–1120 h. Rats were euthanized 3 h or 6 h after the treatment.
Exp. 3. Effects of estren (Es) on OTR, ERα and ERβ mRNA levels in the uteri of OVX rats
OVX rats were given a subcutaneous injection of Es (800 μg, Tocris Biosciences) dissolved in 0.2 ml of sesame oil at 1100–1120 h and were euthanized 1, 3 and 6 h after injection. The ER antagonists ICI (250 μg, Tocris Biosciences) and Tam (250 μg, Sigma, St Louis, MO, USA) were dissolved in 0.25 ml of sesame oil and injected 30 min prior to the Es injection (800 μg). Rats were euthanized 3 h after the Es treatment. To exclude the possibility that the effect of Es was mediated through androgen receptors, testosterone (T, 500 μg, Wako, Osaka, Japan) or dihydrotestosterone (DHT, 500 μg, Wako) dissolved in 0.5 ml of sesame oil were also injected into OVX rats, which were euthanized 3 h after the treatment.
Exp. 4. Effects of ER antagonists on OTR, ERα and ERβ mRNA levels in the uterus during the estrous cycle
The ER antagonists ICI (250 μg) or TAM (250 μg), dissolved in 0.25 ml of sesame oil, were subcutaneously injected during diestrus (1100–1130 h) into rats (IAR), and rats were euthanized the next day (proestrus) (1030–1200 h).
Complementary DNA synthesis
Complementary DNA (cDNA) synthesis was performed as described previously [9]. Briefly, uterine tissue (50–100 mg) was homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA samples were prepared according to the acid guanidinium thiocyanate-phenol-chloroform extraction method and treated with RNase-free DNase I (Invitrogen) to exclude genomic DNA. The quantity of total RNA was assessed using a spectrophotometer at a wavelength of 260 nm. Total RNA samples (1 μg) were reverse transcribed using 200 U of SuperScript II reverse transcriptase (Invitrogen) and 10 pmol of a 9-mer random primer.
Real-time PCR analysis
Real-time PCR was performed using SYBR Green master mix and an ABI PRISM 7000 sequence detector (Applied Biosystems, Foster City, CA, USA). Previously described reaction protocols and primers (OTR, 5’-CGATTGCTGGGCGGTCTT-3’ and 5’-CCGCCGCTGCCGTCTTGA-3’ [9]; ERα, 5’-TGACCAACC TGGCAGACAGG-3’ and 5’-GCCTTTGTTACTCATGTGCC-3’ [36]; ERβ, 5’-AG AGAGACACTGAAGAGGAAG-3’ and 5’-GCCAGGAGCATGTCAAAGATT-3’ [37]; β-actin, 5’-GTCACCCACACTGTGCCCATCT-3’, 5’-ACAGAGTACTTGCGCTCAGGAG-3’ [38]) were used for each PCR assay [33]. OTR, ERα and ERβ mRNA levels were standardized by dividing by the value for β-actin in the same sample.
Statistical analysis
Data were expressed as relative amounts (%) by dividing the value of each sample by the mean value for the corresponding control group. Data were expressed as the means ± SEM and were evaluated statistically using Tukey’s or Dunnett’s multiple comparison test.
Results
Exp. 1. Effects of 17β-estradiol on OTR, ERα and ERβ mRNA levels in the uteri of steroid-treated OVX virgin rats
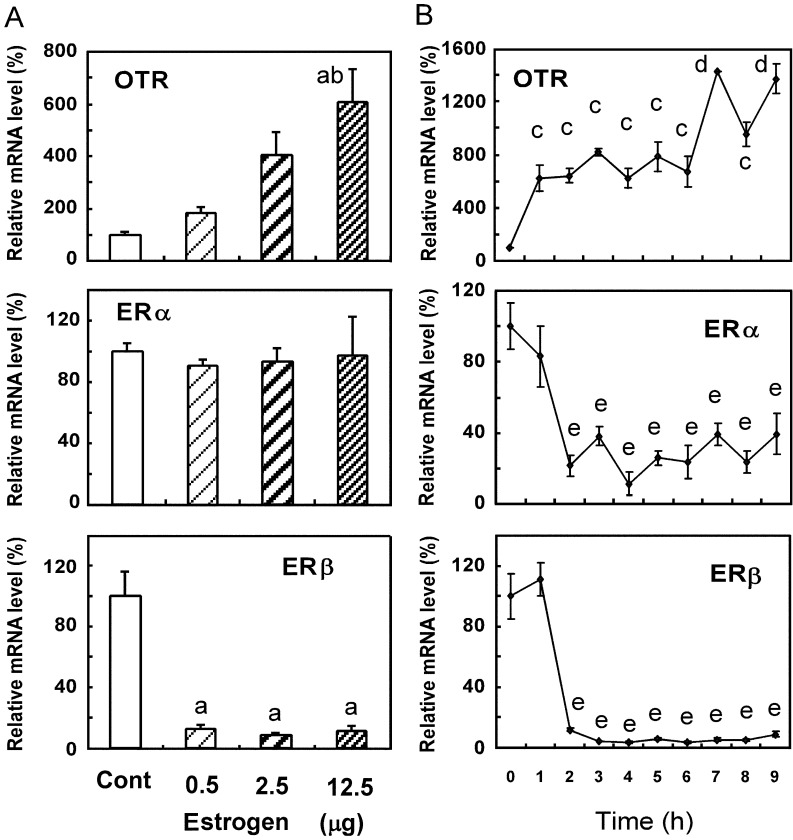
A single injection of E2 (12.5 μg) significantly increased OTR mRNA levels 24 h after treatment, but the injection of E2 (0.5 and 2.5 μg) did not (Fig. 1A). There was no significant change in ERα mRNA levels 24 h after E2 injection at the three doses tested (Fig. 1A). However, the three E2 doses decreased ERβ mRNA levels 24 h after injection (Fig. 1A). The injection of E2 (12.5 μg) increased OTR mRNA levels starting 1 h after injection, and these levels were sustained until 6 h postinjection, followed by additional increases at 7 h and 9 h (Fig. 1B). However, ERα and ERβ mRNA levels decreased within 2 h and were sustained at lower levels than those at 0 h until 9 h postinjection (Fig. 1B).
Fig. 1.
Effects of 17β-estradiol (E2) on OTR, ERα and ERβ mRNA levels in the uteri of OVX rats. Rats were given a subcutaneous injection of 17β-estradiol (0.5, 2.5 or 12.5 μg) or vehicle (cont) (A) and 17β-estradiol (12.5 μg) (B) at 1100–1120 h and were euthanized 24 h (A) and 1, 2, 3, 4, 5, 6, 7, 8 and 9 h (B) after the injection. Uteri were extracted, and the gene expression levels of OTR, ERα and ERβ were determined by real-time PCR. Data are expressed as means ± SEM (n=5). The value in the vehicle-treated control group was defined as 100%. a, vs. vehicle-treated group (cont); b, vs. group treated with 0.5 μg E2; c, vs. groups at 0 h, 7 h and 9 h; d, vs. groups at 0–6 h and 8 h; e, vs. groups at 0 h and 1 h; P < 0.05, by Tukey’s test.
Exp. 2. Effects of PPT or DPN on OTR, ERα and ERβ mRNA levels in the uteri of OVX rats
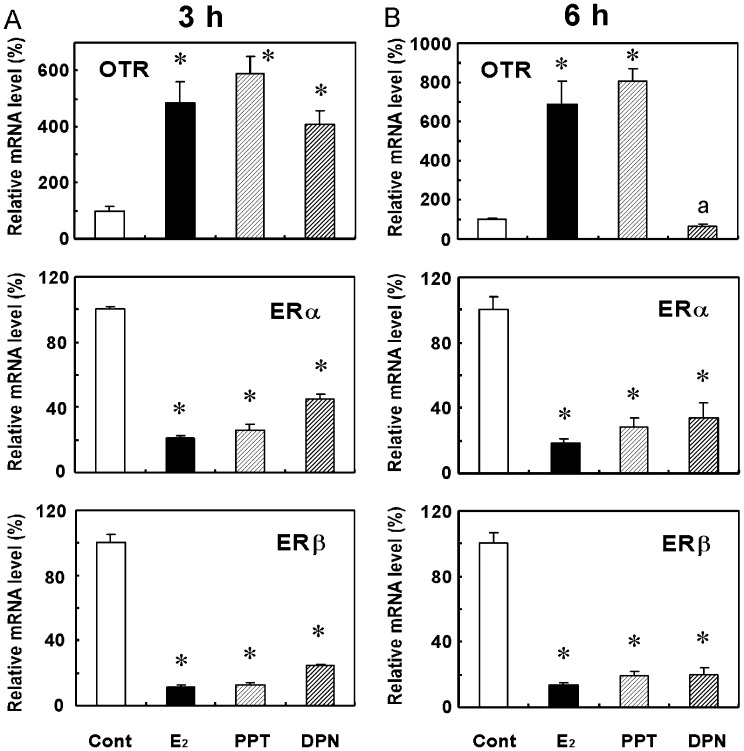
Because OTR and ER mRNA levels changed within 2 h after the E2 injection, their expression levels were examined 3 h and 6 h after treatments in later experiments. OTR mRNA levels were higher and ERα and ERβ mRNA levels were lower 3 h (Fig. 2A) and 6 h (Fig. 2B) after the PPT (an ERα agonist) treatment than those of the corresponding vehicle-treated control group. Treatment with DPN (an ERβ agonist) also decreased ERα and ERβ mRNA levels at 3 h (Fig. 2A) and 6 h (Fig. 2B). However, OTR mRNA levels only increased 3 h after the DPN treatment (Fig. 2A) and decreased at 6 h to a level that was similar to that observed in control animals (Fig. 2B).
Fig. 2.
Effects of PPT and DPN on OTR, ERα, and ERβ mRNA levels in the uteri of OVX rats. Rats were given a subcutaneous injection of PPT (200 μg) or DPN (200 μg) at 1100–1200 h and were euthanized 3 h (A) and 6 h (B) after the injection. Uteri were extracted, and the gene expression levels of OTR, ERα and ERβ were determined by real-time PCR. Data are expressed as means ± SEM (n=5). The value in the vehicle-treated control group was defined as 100%. * vs. vehicle-treated group (cont); P < 0.05, by Dunnett’s test.
Exp. 3. Effects of Es on OTR, ERα and ERβ mRNA levels in the uteri of OVX rats
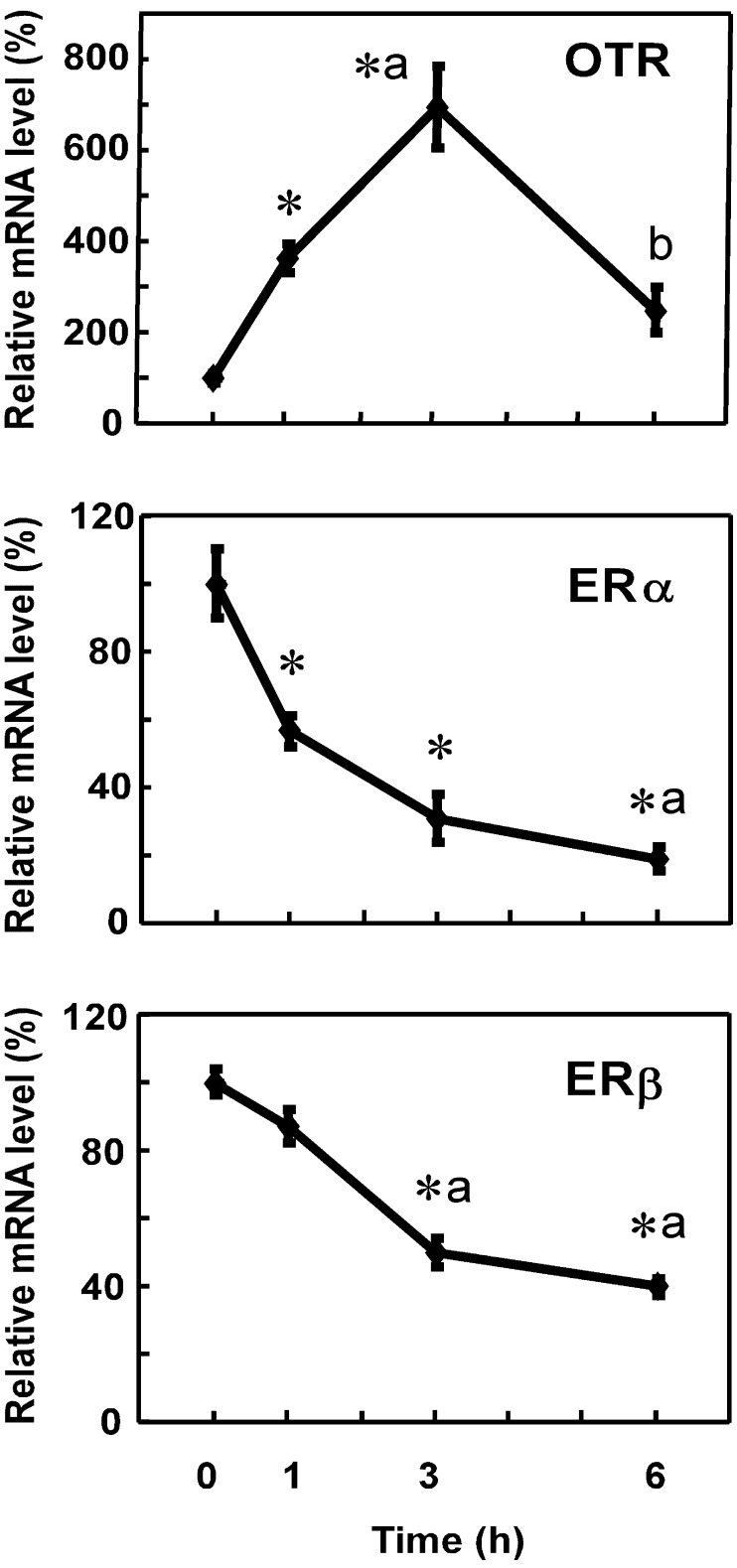
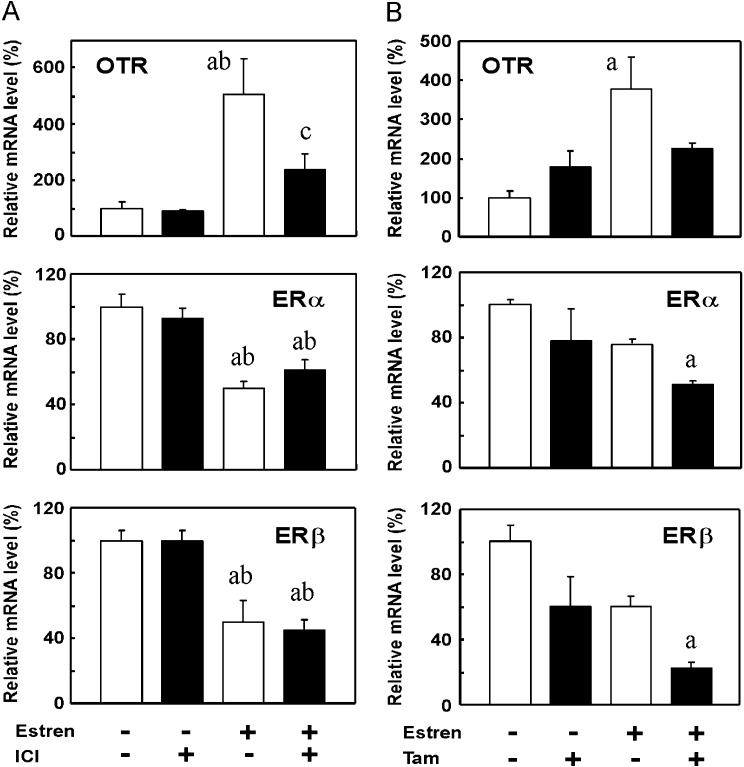
OTR mRNA levels increased 1 h after the Es treatment, reached a peak at 3 h and decreased until 6 h (Fig. 3). Meanwhile, ERα and ERβ mRNA levels showed a sustained decrease for 6 h, which began 1 h and 3 h following the Es injection in the case of ERα and ERβ, respectively (Fig. 3). The effects of the ER antagonists, ICI and Tam, on Es-induced changes in OTR, ERα and ERβ mRNA levels were then examined. Es significantly increased the OTR mRNA level and decreased ERα and ERβ mRNA levels at 3 h. ICI significantly suppressed the Es-mediated increase in the OTR mRNA level. On the other hand, the Es-induced decrease in ERα and ERβ mRNA levels was not affected by ICI. The basal expression levels of OTR and ER mRNA in the uterus were not influenced by ICI (Fig. 4A). In another set of experiments, the effects of Tam on the receptor mRNA levels were examined (Fig. 4B). The effects of Es on OTR (increase) and ER mRNA levels (decrease) were confirmed, although the decrease in ERs was not statistically significant partly due to the limited number of animals used. In the presence of Tam, Es showed no stimulatory effect on OTR expression, while ER expression was slightly decreased by Es. Treatment with Tam alone also tended to downregulate the ER mRNA expression, and the combination of Es and Tam significantly decreased ER mRNA levels when compared to the vehicle-treated control.
Fig. 3.
Effects of estren (Es) on ERα and ERβ mRNA levels in the uteri of OVX rats. Rats were given a subcutaneous injection of Es (800 μg) at 1100–1120 h and were euthanized 1, 3 and 6 h after the injection. Uteri were extracted, and the gene expression levels of OTR, ERα and ERβ were determined by real-time PCR. Data are expressed as means ± SEM (n=5). The value in the group at 0 h was defined as 100%. * vs. group at 0 h; a, vs. group at 1 h; b, vs. group at 3 h; P < 0.05, by Tukey’s test.
Fig. 4.
Effects of ER antagonists on Es-induced changes in OTR, ERα and ERβ mRNA levels in the uteri of OVX rats. Rats were given a subcutaneous injection of ICI (250 μg) (A) or Tam (250 μg) (B) 30 min prior to an injection of Es (800 μg). Es was injected at 1100–1120 h, and rats were euthanized 3 h after the injection. Uteri were extracted, and the gene expression levels of OTR, ERα and ERβ were determined by real-time PCR. Data are expressed as means ± SEM (n=4). The value in the vehicle-treated control group was defined as 100%. a, vs. vehicle- and vehicle-treated group; b, vs. vehicle- and ICI-treated group; c, vehicle- and Es-treated group; P < 0.05, by Tukey’s test.
Because Es has affinity for androgen receptors [28, 39], the effect of an androgen was examined. Treatment with T (500 μg) and DHT (500 μg) did not cause significant changes in OTR, ERα or ERβ mRNA levels. The OTR mRNA levels in vehicle-treated, T-treated and DHT-treated rats were 100 ± 7.4, 102.6 ± 10.1 and 100.4 ± 16.7, respectively; the ERα mRNA levels were 100 ± 7.6, 95.5 ± 3.9 and 91.7 ± 4.4, respectively; and the ERβ mRNA levels were 100 ± 12.9, 85.4 ± 2.9 and 84.6 ± 4.9, respectively (n=4).
Exp. 4. Effects of ER antagonists on OTR, ERα and ERβ mRNA levels in the uterus during the estrous cycle
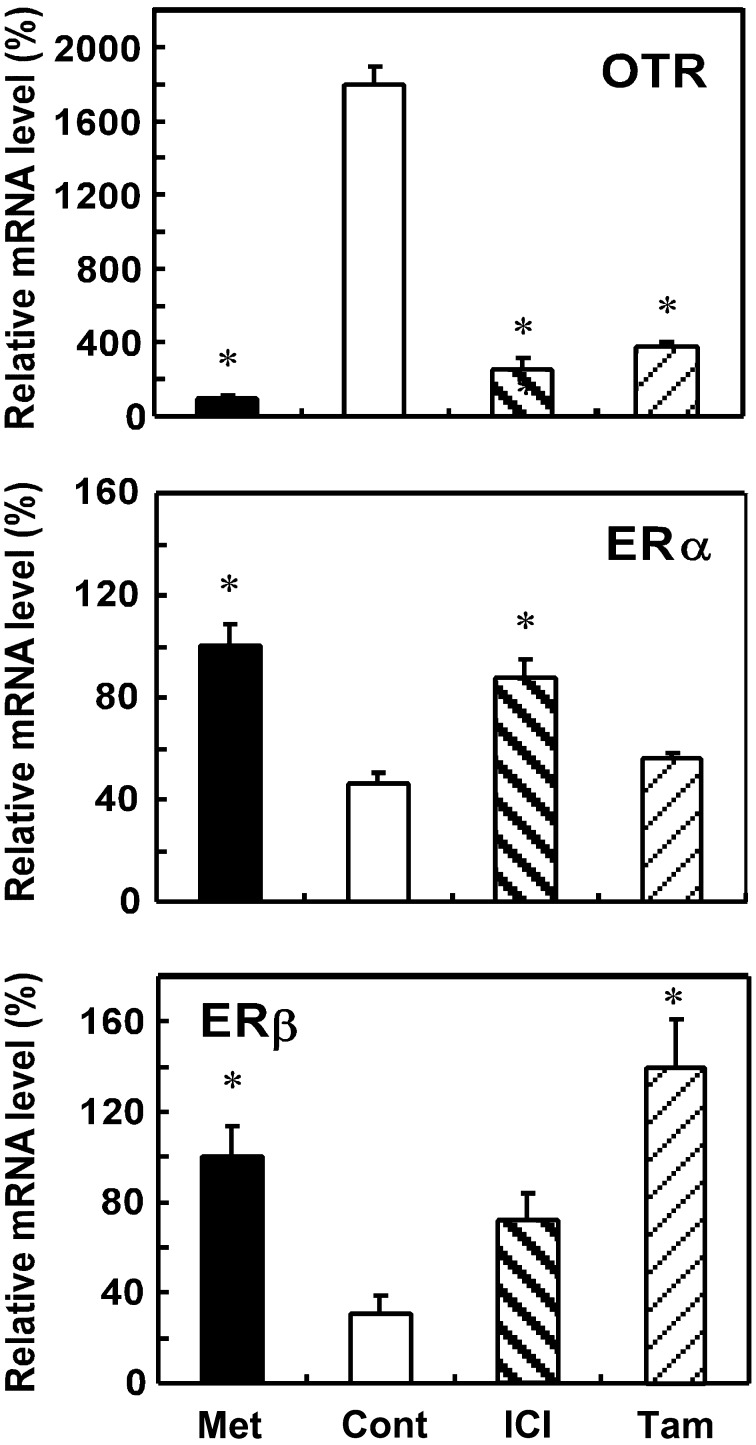
OTR mRNA levels were higher in proestrus during the estrous cycle than in metestrus, and this upregulation was suppressed by treatment with either ICI or Tam (Fig. 5). Although decreases in both ERα and ERβ mRNA levels were observed during proestrus, only the former decrease was blocked by the ICI treatment, but not by Tam (Fig. 5). In contrast, Tam prevented decreases in ERβ mRNA levels during proestrus, whereas ICI did not increase ERβ mRNA levels to significantly higher than those of vehicle-treated controls (Fig. 5).
Fig. 5.
Effects of ER antagonists on OTR, ERα and ERβ mRNA levels in the uterus of rats in proestrus. Rats were given a subcutaneous injection of ICI (250 μg) or Tam (250 μg) during diestrus (1100–1130 h) and were euthanized at 1030–1200 h the next day (proestrus). Uteri were extracted, and the gene expression levels of OTR, ERα and ERβ were determined by real-time PCR. Data are expressed as means ± SEM (n=4). The value of intact rats at metestrus (Met) was defined as 100%. * vs. the vehicle-treated control group; P < 0.05, by Dunnett’s test.
Discussion
This study showed that although estrogen had a predictable effect on OTR, ERα and ERβ mRNA levels within 2 h of the treatments examined, it had a differential pattern of effects 24 h later. For example, OTR mRNA levels increased 24 h after treatment with E2, while those of ERβ decreased and those of ERα were unchanged. These results suggest that E2 continues to affect the regulation of OTR and ERβ but not that of ERα 24 h after its initial administration and indicate that recovery from the E2-mediated downregulation of ERα mRNA levels was faster than that from the E2-mediated downregulation of ERβ. Microarray analysis of gene expression in the uteri of OVX mice [40] and immature rats [41] previously revealed clusters of genes that were both positively and negatively regulated by estrogen within 2 h of treatment. Estrogen was also shown to induce changes in the expression of at least 3867 genes in rat uteri, and approximately 3.0% (116–124 genes) of these were changed within 2 h of the estrogen treatment [41]. Our study identified three genes, OTR, ERα and ERβ, as additional members of the genes that were induced by estrogen in the early phase in the uterus. Furthermore, Hewitt et al. described distinct clusters of genes regulated by estrogen in the early or late phases in the mouse uterus, as well as clusters of genes regulated at both times [40]. According to this categorization, the findings of our study shown in Fig. 1 suggest that OTR and ERβ genes belong to a group of genes induced/suppressed in both phases, whereas the ERα gene belongs to a group of genes influenced in the early phase only.
In Fig. 2, the involvement of ERα and ERβ in the regulation of OTR, ERα and ERβ mRNA levels was examined using ER agonists. PPT is known to have a 410-fold greater binding affinity for ERα than ERβ [42], while DPN has a 70-fold greater binding affinity for ERβ than ERα [43]. In this study, both PPT and DPN decreased ERα and ERβ mRNA levels 3 h and 6 h after the treatments examined, an effect that was similar to that achieved with the E2 treatment. These results suggest that the E2-induced downregulation of ERα and ERβ may be mediated through both ERα and ERβ receptors, which could account for its effect, at least within 6 h of administration. The PPT treatment increased OTR mRNA levels at both 3 h and 6 h, whereas DPN only transiently increased these levels at 3 h, which returned to levels that were similar to those in the controls at 6 h. This suggests that different pathways are involved in the regulation of OTR through ERα and ERβ.
Another finding of the present study was that the Es treatment decreased ERα and ERβ mRNA levels 3 h and 6 h after treatment (Fig. 3, lower panels), which was similar to the changes observed after the E2 treatment. However, the effect of Es on OTR mRNA levels differed from that of E2. For example, while the Es treatment increased OTR mRNA expression at 3 h, its influence waned 6 h after administration, as evidenced by similar OTR mRNA levels in the control and 6 h postinjection groups (Fig. 3, upper panel). This response of OTR mRNA levels to the Es treatment was similar to that observed with DPN in Fig. 2, provided that changes were determined within 3 h and 6 h after the treatment. Therefore, it is conceivable that Es may exert a functional influence on pathways involving ERβ mediation; verification of this proposition awaits further investigation. Nevertheless, it should be noted that short-term transient cellular transactions (lasting less than 6 h) may be involved in induction of the OTR gene by estrogen and is mediated at least in part through ERβ.
Previous studies have shown that treatment with Es induced transcriptional activity in human embryonic kidney 293 cells expressing ERα and ERβ [32] and changed gene expression in mouse uteri [33, 34]. These effects of Es were abolished in ERα/ERβ [32] or ERα [34] knockout mice. Additionally, Es induced ERK1/2 and Akt phosphorylation in transduced HeLa cells expressing the ligand-binding domain of ERα localized to the cell membrane [33]. Thus, the target ER for Es may be ERα or ERβ localized close to the membrane. For example, using ICI to specifically bind ERα and ERβ also blocked the E2-mediated activation of both ERα and ERβ in ERα- or ERβ-transfected COS1 cells, respectively [44]. Since the action of Es through ERα or ERβ was blocked by ICI [32], the effect of Es on OTR expression observed in Figs. 3 and 4 was considered to be mediated by ERα or ERβ and more plausibly by ERβ for the reason mentioned above, although whether it belongs to a classical nuclear or non-nuclear type could not be confirmed in this study. On the other hand, Es-induced changes in ERα and ERβ were not antagonized by ICI. Thus, the action of Es on the expression of ERα and ERβ does not appear to involve ERα or ERβ. This raises the possibility that membrane ERs may be responsible for the observed effects of Es. One membrane ER, GPR30, is a potential candidate for the downstream mediation of an Es-induced mechanism of ER action. It should be noted that both Tam and ICI are strong agonists of GPR30. For example, ICI increased cAMP in GPR30-transfected HEK293 cells [45] and increased intracellular calcium oscillations in cultured primate neurons that responded to the GPR30 agonist [46], while Tam activated PI3K activities and c-fos induction in GPR30-transfected COS7 [24] and HeLa cells [47], respectively. Based on the findings that ICI and Tam did not have any significant effect on ERα and ERβ expressions in vehicle-treated groups, the involvement of GPR30 in this Es-sensitive ER action in ERα and ERβ regulations appears to be weak. Concerning another type of membrane ER, ICI affected neither basal nor BSA-conjugated E2-stimulated PKC activity in cultured chondrocytes from female rats [48], which indicated the presence of one or more ICI-unresponsive membrane ERs different from GPR30. These nonclassical membrane ERs may be involved in the action of Es on ERα and ERβ expression in the rat uterus. Therefore, taken together, uterine OTR gene expression is upregulated by estrogen through the classical nuclear (or non-nuclear) ERs, ERα and ERβ, while the levels of these ERs are downregulated by estrogen through multiple pathways including Es-sensitive nonclassical ERs.
Although Es has been shown to bind to androgen receptors [28, 39], the possibility that Es induced the changes in OTR, ERα and ERβ mRNA levels observed in the present study through androgen receptors is remote because testosterone alone did not cause any significant changes in the expression levels of those genes.
OTR and ER mRNA levels are known to undergo dynamic changes during the estrous cycle. Significant increases in OTR mRNA levels and decreases in ER mRNA levels have been reported in the proestrus phase in the rat uterus [35]. In the present study, these changes were shown to be induced by estrogen because they could be abolished by ER antagonism. However, while changes in OTR mRNA levels were abolished by an injection with either ICI or Tam, changes in ERα and ERβ mRNA expression were not abolished by Tam and ICI, respectively. These results suggest that classical ERs, which are antagonized by both ICI and Tam, are involved in the regulation of OTR and that the regulation of ERα and ERβ may involve nonclassical types of ERs. However, because the effects of estrogen may have occurred in combination with multiple types of ERs expressed in all kinds of target tissues in vivo, the involvement of complex mechanisms cannot be excluded. Furthermore, other factors, such as progesterone, which affects the gene expression of OTR and ERα [16, 49], may need to be considered for studies on the physiological state, such as during the estrous cycle, pregnancy and labor.
We previously reported changes in ERα mRNA levels concomitant with those of OTR around parturition, which suggested that ERα is an important ER for OTR regulation during parturition. The present study demonstrated that the ERα agonist mimicked the long-lasting E2 effect on OTR mRNA levels, which supported the proposal that ERα is a key ER for OTR regulation. On the other hand, changes in OTR mRNA levels within 3 h of the estrogen treatment were regulated by a complicated mechanism involving ERβ, in addition to ERα. Regarding Es-responsive receptors, the present study showed that two types of Es-responsive receptors exist based on their sensitivity to ER antagonists. One type consists of one or more ICI/Tam-sensitive classical ERs that mediate OTR expression, and the other type consists of one or more ICI/Tam-unresponsive ERs that mediate the downregulation of ERα and ERβ expression. Therefore, controversial reports concerning the transcriptional activity of Es in the uterus [28, 29, 32,33,34] may be explained by the existence of these multiple ERs. Although further studies are needed to prove this hypothesis, the early phase of estrogen action is a potentially good experimental model for investigating multiple types of ERs as well as physiological phenomena, such as changes associated with the estrous cycle and the initiation and progression of parturition.
Acknowledgments
We thank Dr. Chuma O. Okere for reviewing this manuscript and for critical comments. This work was supported by JSPS KAKENHI Grant Number 21590253).
References
- 1.Fuchs A-R, Periyasamy S, Alexandrroa M, Soloff MS. Correlation between oxytocin receptor concentration and responsiveness to oxytocin in pregnant rat myometrium: Effects of ovarian steroids. Endocrinology 1983; 113: 742–749 [DOI] [PubMed] [Google Scholar]
- 2.Soloff MS, Alexandrova M, Fernstrom MJ. Oxytocin receptors: triggers for parturition and lactation? Science 1979; 204: 1313–1315 [DOI] [PubMed] [Google Scholar]
- 3.Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol 1984; 150: 734–741 [DOI] [PubMed] [Google Scholar]
- 4.Maggi M, Genazzani AD, Giannini S, Torrisi C, Baldi E, di Tomaso M, Munson PJ, Rodbard D, Serio M. Vasopressin and oxytocin receptors in vagina, myometrium, and oviduct of rabbits. Endocrinology 1988; 122: 2970–2980 [DOI] [PubMed] [Google Scholar]
- 5.Maggi M, Peri A, Giannini S, Fantoni G, Guardabasso V, Serio M. Oxytocin and V1 vasopressin receptors in rabbit endometrium during pregnancy. J Reprod Fertil 1991; 91: 575–581 [DOI] [PubMed] [Google Scholar]
- 6.Fuchs AR, Helmer H, Behrens O, Liu HC, Antonian L, Chang SM, Fields MJ. Oxytocin and bovine parturition: a steep rise in endometrial oxytocin receptors precedes onset of labor. Biol Reprod 1992; 47: 937–944 [DOI] [PubMed] [Google Scholar]
- 7.Ivell R, Rust W, Einspanier A, Hartung S, Fields M, Fuchs A-R. Oxytocin and oxytocin receptor gene expression in the reproductive tract of the pregnant cow: rescue of luteal oxytocin production at term. Biol Reprod 1995; 53: 553–560 [DOI] [PubMed] [Google Scholar]
- 8.Larcher A, Neculcea J, Breton C, Arslan A, Rozen F, Russo C, Zingg HH. Oxytocin receptor gene expression in the rat uterus during pregnancy and the estrous cycle and in response to gonadal steroid treatment. Endocrinology 1995; 136: 5350–5356 [DOI] [PubMed] [Google Scholar]
- 9.Liu C-X, Takahashi S, Murata T, Hashimoto K, Agatsuma T, Matsukawa S, Higuchi T. Changes in oxytocin receptor mRNA in the rat uterus measured by competitive reverse transcription-polymerase chain reaction. J Endocrinol 1996; 150: 479–486 [DOI] [PubMed] [Google Scholar]
- 10.Ou C-W, Chen Z-Q, Qi S, Lye SJ. Increased expression of the rat myometrial oxytocin receptor messenger ribonucleic acid during labor requires both mechanical and hormonal signals. Biol Reprod 1998; 59: 1055–1061 [DOI] [PubMed] [Google Scholar]
- 11.Kimura T, Takemura M, Nomura S, Nobunaga T, Kubota Y, Inoue T, Hashimoto K, Kumazawa I, Ito Y, Ohashi K, Koyama M, Azuma C, Kitamura Y, Saji F. Expression of oxytocin receptor in human pregnant myometrium. Endocrinology 1996; 137: 780–785 [DOI] [PubMed] [Google Scholar]
- 12.Wathes DC, Smith HF, Leung ST, Stevenson KR, Meier S, Jenkin G. Oxytocin receptor development in ovine uterus and cervix throughout pregnancy and at parturition as determined by in situ hybridization analysis. J Reprod Fertil 1996; 106: 23–31 [DOI] [PubMed] [Google Scholar]
- 13.Wu WX, Verbalis JG, Hoffman GE, Derks JB, Nathanielsz PW. Characterization of oxytocin receptor expression and distribution in the pregnant sheep uterus. Endocrinology 1996; 137: 722–728 [DOI] [PubMed] [Google Scholar]
- 14.Soloff MS. Uterine receptor for oxytocin: effects of oestrogen. Biochem Biophys Res Commun 1975; 65: 205–212 [DOI] [PubMed] [Google Scholar]
- 15.Soloff MS, Fernstrom MA, Periyasamy S, Soloff S, Baldwin S, Wieder M. Regulation of oxytocin receptor concentration in rat uterine explants by oestrogen and progesterone. Can J Biochem Cell Biol 1983; 61: 625–630 [DOI] [PubMed] [Google Scholar]
- 16.Murata T, Murata E, Liu C-X, Narita K, Honda K, Higuchi T. Oxytocin receptor gene expression in rat uterus: regulation by ovarian steroids. J Endocrinol 2000; 166: 45–52 [DOI] [PubMed] [Google Scholar]
- 17.Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science 1986; 231: 1150–1154 [DOI] [PubMed] [Google Scholar]
- 18.Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-Å. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 1996; 93: 5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson J-Å. Comparison of the ligand binding specificity and transcript tissue distribution of oestrogen receptors α and β. Endocrinology 1997; 138: 863–870 [DOI] [PubMed] [Google Scholar]
- 20.Saunders PTK, Maguire SM, Gaughan J, Millar MR. Expression of oestrogen receptor beta (ERβ) in multiple rat tissues visualized by immunohistochemistry. J Endocrinol 1997; 154: R13–R16 [DOI] [PubMed] [Google Scholar]
- 21.Tessier C, Deb S, Prigent-Tessier A, Ferguson-Gottschall S, Gibori GB, Shiu RP, Gibori G. Oestrogen receptors α and β in rat decidua cells: cells specific expression and differential regulation by steroid hormones and prolactin. Endocrinology 2000; 141: 3842–3851 [DOI] [PubMed] [Google Scholar]
- 22.Hammes SR, Levin ER. Minireview: Recent advances in extranuclear steroid receptor actions. Endocrinology 2011; 152: 4489–4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr.Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 2000; 14: 1649–1660 [DOI] [PubMed] [Google Scholar]
- 24.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005; 307: 1625–1630 [DOI] [PubMed] [Google Scholar]
- 25.Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol Cell Biol 2003; 23: 1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem 2007; 282: 22278–22288 [DOI] [PubMed] [Google Scholar]
- 27.Pedram A, Razandi M, Kim JK, O’Mahony F, Lee EY, Luderer U, Levin ER. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. J Biol Chem 2009; 284: 3488–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O’Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science 2002; 298: 843–846 [DOI] [PubMed] [Google Scholar]
- 29.Kousteni S, Almeida M, Han L, Bellido T, Jilka RL, Manolagas SC. Induction of osteoblast differentiation by selective activation of kinase-mediated actions of the estrogen receptor. Mol Cell Biol 2007; 27: 1516–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zárate S, Jaita G, Zaldivar V, Radl DB, Eijo G, Ferraris J, Pisera D, Seilicovich A. Estrogens exert a rapid apoptotic action in anterior pituitary cells. Am J Physiol Endocrinol Metab 2009; 296: E664–E671 [DOI] [PubMed] [Google Scholar]
- 31.Koszegi Z, Szego ÉM, Cheong RY, Tolod-Kemp E, Ábrahám IM. Postlesion estradiol treatment increases cortical cholinergic innervations via estrogen receptor-α dependent nonclassical estrogen signaling in vivo. Endocrinology 2011; 152: 3471–3482 [DOI] [PubMed] [Google Scholar]
- 32.Movérare S, Dahllund J, Andersson N, Islander U, Carlsten H, Gustafsson JA, Nilsson S, Ohlsson C. Estren is a selective estrogen receptor modulator with transcriptional activity. Mol Pharmacol 2003; 64: 1428–1433 [DOI] [PubMed] [Google Scholar]
- 33.Almeida M, Han L, O’brien CA, Kousteni S, Manolagas SC. Classical genotropic versus kinase-initiated regulation of gene transcription by the estrogen receptor alpha. Endocrinology 2006; 147: 1986–1996 [DOI] [PubMed] [Google Scholar]
- 34.Hewitt SC, Collins J, Grissom S, Hamilton K, Korach KS. Estren behaves as a weak estrogen rather than a nongenomic selective activator in the mouse uterus. Endocrinology 2006; 147: 2203–2214 [DOI] [PubMed] [Google Scholar]
- 35.Murata T, Narita K, Honda K, Higuchi T. Changes of receptor mRNAs for oxytocin and estrogen during the estrous cycle in rat uterus. J Vet Med Sci 2003; 65: 707–712 [DOI] [PubMed] [Google Scholar]
- 36.Knauthe R, Diel P, Hegele-Hartung C, Engelhaupt A, Fritzemeier K-H. Sexual dimorphism of steroid hormone receptor messenger ribonucleic acid expression and hormonal regulation in rat vascular tissue. Endocrinology 1996; 137: 3220–3227 [DOI] [PubMed] [Google Scholar]
- 37.Price RH, Jr, Lorenzon N, Handa RJ. Differential expression of oestrogen receptor beta splice variants in rat brain: identification and characterization of a novel variant missing exon 4. Mol Brain Res 2000; 80: 260–268 [DOI] [PubMed] [Google Scholar]
- 38.Murata T, Takezawa T, Funaba M, Fujimura H, Murata E, Torii K. Quantitation of mouse and rat β-actin mRNA by competitive polymerase chain reaction using capillary electrophoresis. Anal Biochem 1997; 244: 172–174 [DOI] [PubMed] [Google Scholar]
- 39.Windahl SH, Galien R, Chiusaroli R, Clément-Lacroix P, Morvan F, Lepescheux L, Nique F, Horne WC, Resche-Rigon M, Baron R. Bone protection by estrens occurs through non-tissue-selective activation of the androgen receptor. J Clin Invest 2006; 116: 2500–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol 2003; 17: 2070–2083 [DOI] [PubMed] [Google Scholar]
- 41.Naciff JM, Overmann GJ, Torontali SM, Carr GJ, Khambatta ZS, Tiesman JP, Richardson BD, Daston GP. Uterine temporal response to acute exposure to 17alpha-ethinyl estradiol in the immature rat. Toxicol Sci 2007; 97: 467–490 [DOI] [PubMed] [Google Scholar]
- 42.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem 2000; 43: 4934–4947 [DOI] [PubMed] [Google Scholar]
- 43.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 2001; 44: 4230–4251 [DOI] [PubMed] [Google Scholar]
- 44.Tremblay A, Tremblay GB, Labrie C, Labrie F, Giguère V. EM-800, a novel antiestrogen, acts as a pure antagonist of the transcriptional functions of estrogen receptors alpha and beta. Endocrinology 1998; 139: 111–118 [DOI] [PubMed] [Google Scholar]
- 45.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 2005; 146: 624–632 [DOI] [PubMed] [Google Scholar]
- 46.Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol 2009; 23: 349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Andò S, Maggiolini M. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol 2006; 20: 631–646 [DOI] [PubMed] [Google Scholar]
- 48.Sylvia VL, Walton J, Lopez D, Dean DD, Boyan BD, Schwartz Z. 17 beta-estradiol-BSA conjugates and 17 beta-estradiol regulate growth plate chondrocytes by common membrane associated mechanisms involving PKC dependent and independent signal transduction. J Cell Biochem 2001; 81: 413–429 [DOI] [PubMed] [Google Scholar]
- 49.Murata T, Narita K, Honda K, Matsukawa S, Higuchi T. Differential regulation of estrogen receptor alpha and beta mRNAs in the rat uterus during pregnancy and labor: possible involvement of estrogen receptors in oxytocin receptor regulation. Endocr J 2003; 50: 579–587 [DOI] [PubMed] [Google Scholar]