Abstract
Luteinizing hormone (LH) in synergy with follicle stimulating hormone (FSH) stimulates normal follicular growth and ovulation. FSH is frequently used in assisted reproductive technology (ART). Recent studies have facilitated better understanding on the complementary role of the LH to FSH in regulation of the follicle; however, role of LH in stimulation of follicle, optimal dosage of LH in stimulation and its importance in advanced aged patients has been a topic of discussion among medical fraternity. Though the administration of exogenous LH with FSH is obligatory for controlled ovarian stimulation in patients with hypogonadotropic hypogonadism, there is still a paucity of information of its usage in other patient population. In this review we looked in to the multiple roles that LH plays complementary to FSH to better understand the LH requirement in patients undergoing ART.
KEY WORDS: Assisted reproductive techniques, follicle stimulating hormone, luteinizing hormone, ovarian hyper-stimulation
INTRODUCTION
Luteinizing hormone (LH) plays a key role in gonadal function. LH in synergy with follicle stimulating hormone (FSH) stimulates follicular growth and ovulation. Thus, normal follicular growth is the result of complementary action of FSH and LH.
FSH is frequently used in assisted reproductive technology (ART). The most commonly used protocol in ART consists of controlled ovarian hyper-stimulation (COH) with daily injections of recombinant human FSH (r-hFSH) to induce multiple follicle growth in the ovaries. To prevent premature LH surge and premature ovulation, gonadotropin-releasing hormone (GnRH) agonist or antagonist is injected daily. The pituitary down-regulation (endogenous pituitary suppression) that is achieved with GnRH analogs creates an environment where LH is deficient or very low and which may be detrimental to the development of normal healthy follicles. It has been shown that growing follicles become increasingly sensitive to and ultimately dependent on, the presence of LH for their development.[1] Documented results associate poorer outcomes with patients whose LH concentration was low, after pituitary suppression was achieved with GnRH analog treatment.[2,3]
The availability of recombinant human LH (r-hLH) has paved a way for supplementation of LH in down-regulated IVF cycles. Several recent studies have evlauated the role of r-LH in women undergoing GnRH analog/r-hFSH therapy and IVF and observed variable results. One such study observed that supplementation with r-hLH showed lower levels of cumulus cell apoptosis than treatment with FSH alone, possibly indicating improved oocyte quality in LH-supplemented cycles.[4] Reduction in apoptosis of cumulus cells in the r-hLH group might be the result of lower levels of follicular fluid vascular endothelial growth factor (FF VEGF-marker of maturity and quality of occytes) that is produced by granulosa and theca cells in response to FSH, LH, human chorionic gonadotropin (hCG) and proliferative and apoptotic factors.[4,5] All these studies point that LH may be crucial in COH. The poor outcome of COH includes increased age (above 35 years), poor ovarian reserve, poor response to previous ART cycles, genetic variations and hormonal status majorly LH, FSH, estradiol and anti-Mullerian hormone (AMH).[6] Overall, these studies suggest that LH supplementation could be beneficial for a particular sub-population, including older patients and poor responders. This might be due to the better ooctye quality resulting from a restored follicle at the end of stimulation in these ART patients.[7] These findings reinforce that the use of the r-hLH in ART should be guided by a rationale that is based on the need of the patient.
Although recent researches have facilitated better understanding of supplementation of LH with FSH hormone and effect on fertilization and implantation, there is still a paucity of information on its usage in ART patients. In this review, we looked into the multiple roles that LH plays complementary to FSH to better understand the LH requirement in patients undergoing ART.
ROLE OF LH IN PHYSIOLOGY: THE PHYSIOLOGICAL HORMONAL INTERPLAY
“Two-cell, two-gonadotropin” theory
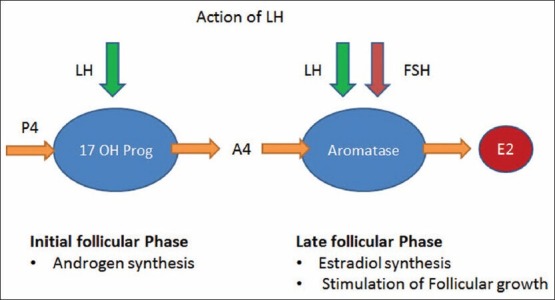
The ovary comprises of two cellular components, which are stimulated independently by LH and FSH, leading to the production of ovarian steroids.[8,9] Androgen production from cholesterol and release during folliculogenesis is dependent on the stimulation of the theca cells by LH and FSH [Figure 1]. This is universally recognized as the key driver of ovarian follicle growth and maturation.[10]
Figure 1.
Two-cell, two-gonadotropin theory
Ovarian steroidogenesis in the preovulatory follicle takes place through LH receptors on theca and FSH (possibly plus LH) receptors on granulosa cells.[11] The steroidogenic acute regulatory protein (StAR protein) is the primary regulator of production of androstenedione, which subsequently diffuses into granulosa cells to serve as an estrogen precursor. In the preovulatory follicle, cholesterol in theca cells arises from circulating lipoproteins and de novo biosynthesis.[12,13]
FSH is responsible for follicular growth and estrogen formation. FSH may be crucial at an earlier stage of follicular development, perhaps earlier in the follicular phase, to induce the aromatase enzyme that converts androgen to estradiol.[14] During the later stages of follicular growth [Figure 1], activins and estradiol, the predominant estrogen in humans, enhance the actions of FSH.[15]
Concept of follicle stimulating hormone threshold and role of luteinizing hormone
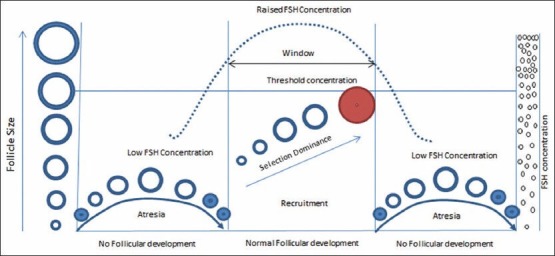
The concept of the FSH “threshold” proposed by Brown postulated that in gonadotropin therapy, the ovary has a minimum requirement level (threshold requirement) for FSH below which follicular development does not occur.[16] More recent studies also confirm that follicular growth does not occur below the threshold levels.
Following optimum FSH stimulation, there is follicular recruitment, growth, selection and dominance. Subsequent development of this cohort during the follicular phase becomes dependent on continued stimulation by gonadotropins. Increasing FSH concentrations should surpass the threshold level to initiate the final gonadotropin-dependent phase of follicular growth [Figure 2].[17]
Figure 2.
Follicle stimulating hormone threshold and recruitment window
There is a secretion of increasing amounts of estradiol during this phase. The peripheral estradiol levels are increased with feedback inhibition of FSH secretion. The maturing follicle inhibits FSH secretion leading to a fall in its levels below threshold, thus stopping less mature follicles from maturing.[18]
Further, it has been shown that FSH threshold is not fixed for any given follicle, but depends on the developmental stage and varies over time.[17,18] The follicles exhibit different degrees of FSH sensitivity at the time of recruitment; highest need for FSH is at the early antral stage and declines in the late antral stage. The follicle with the highest sensitivity will benefit most from increasing FSH levels and will subsequently gain dominance.[18]
The suggested reasons for the response of ovarian follicles to certain FSH level than to a specific dose are fluctuating levels of the endogenous production of gonadotropin,[16] and up-regulation of its receptors due to FSH administration.[17]
Although FSH can induce follicular growth even without LH, there is evidence that the follicles may have developmental deficiencies like abnormally reduced estradiol production and lack of ability to luteinize and rupture, following hCG stimulus.[19] Hence, a certain amount of LH exposure is necessary for optimal follicular development.
Another possibility is that FSH stimulates the production of progesterone by driving cholesterol conversion into the steroid pathway.[20,21,22,23,24] Early increased exposure to progesterone can advance the endometrium, leading to asynchrony of embryo development to endometrial development and the reduction of implantation. LH stimulates the conversion of progesterone into androgens, which can be further aromatized to estrogens. The addition of LH may benefit the endometrium by decreasing the risk of a premature progesterone increase and therefore improve the likelihood of implantation and clinical pregnancy.[23,24]
Concept of luteinizing hormone therapeutic window
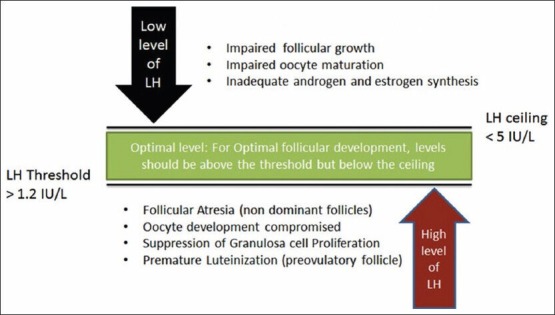
The concept of the LH therapeutic window has been explained in brief in Figure 3. Though studies support the use of r-hLH in addition to r-hFSH in GnRH antagonist protocols in ovarian follicular development, these studies are fewer in number. There is also no clear cut guideline regarding the optimum levels of serum LH and timing of its supplementation are fewer in number. This is an area that warrants further research.[6] Studies have shown that serum LH levels should be between 1.2 IU/L and 5.0 IU/L,[8] for optimal development follicle in cycles where endogenous LH is suppressed.[6,19]
Figure 3.
Luteinizing hormone therapeutic window
Some of the recent studies suggest that the indicators for adding LH to an ART cycle are mid follicular (day 6) hypo-response to long GnRH agonist, no follicles > 10 mm, E2 < 200 pg/ml, endometrial thickness < 6 mm and baseline serum LH < 1.2 IU/ml on day 6.[8,25]
A recent meta-analysis of seven randomized controlled trials (RCTs) done by Hill et al. on the use of LH in ART in advanced patient age group concluded that five RCTs were in favor of adding LH in ART therapy in patients of advanced age group.[26] However, it is critical that add-back LH is administered in appropriate patients as an excess of LH can cause suppression of granulosa cells and follicular atresia.[6,26]
Pharmacogenomics and ovarian stimulation
Follicle stimulating hormone polymorphism
The FSH receptor (FSHR) gene is thought to play a significant role in the success of ovarian stimulation and can be used as a marker to predict differences in FSHR function and ovarian response to FSH. Patients with unfavorable genotypes are reported to require higher doses of r-hFSH to overcome relative ovarian insensitivity. The FSHR gene contains two important single nucleotide polymorphisms (SNPs) in exon 10, which are in linkage disequilibrium and change two amino acids at positions 307 and 680. Women with the 307 Ala and 680 Ser SNPs are associated with reduced COH outcomes, the 680 SNP Series specifically associated with lower clinical pregnancy. These patients when undergoing ART are characterized by higher basal FSH serum concentrations, higher administered amounts of FSH required and higher risks of hypo- or hyper-responses. Up to 35% of patients requiring ART are detected with alternatively spliced FSHR products. Genotyping the FSHR Asn680Ser SNP, together with some additional novel markers (e.g. transcript levels), may therefore provide a means of identifying a group of poor responders before infertility treatment is initiated.[27,28]
Luteinizing hormone polymorphism
The LH receptor gene is known to carry as many as 282 SNPs.[29] In 1991, Pettersson and Söderholm identified a common genetic LHβ variant or v-βLH owing to the alterations in two polymorphic base changes in the β subunit gene leading to changes in the amino acid sequence, Trp8Arg and Ile15Thr. They had initially suggested this discovery as an immunological anomalous LH form.[30,31]
The short half-life of v-βLH may be linked to the presence of extra glycosylation signal into the β subunit that could lead to an addition of the second oligosaccharide to Asn13 of the β protein. It has been found that there is more potency of the overall LH activity of v-βLH at the receptor site; however, its duration is shorter in vivo.[32] Previous clinical trials conducted to determine the impact of this variant on reproductive health reported its association with ovulatory disorders, premature ovarian failure, hyperprolactinemia, luteal insufficiency, menstrual disorders, endometriosis and infertility.[33] An observational study noted low response in some women following ovarian stimulation, resulting in a greater need for r-hFSH (>2500 IU).[32] In another preliminary study, the total r-hFSH consumption was elevated during ovarian stimulation due to the presence of v-βLH.[31] Based on the findings, the researchers indicated the potential of v-βLH as a marker of ovarian responsiveness to r-hFSH. This role of v-βLH, if validated by further research, could thus facilitate clinicians in identifying patients requiring exogenous LH addition during ovarian stimulation.[32]
Optimizing follicle stimulating hormone dosing
Various studies suggest four parameters of FSH administration management involved in the risk of multifollicular development: (a) the choice of the FSH starting dose,[34,35](b) the duration of the starting, dose before stepping up or stepping down,[34,36](c) the rate of increase in FSH dose at each increment[37] and (d) the reduction of the FSH dose once a follicle has been selected.[38]
In an attempt to prevent the risks of overstimulation and multiple pregnancies, it is crucial to use a low starting dose of FSH,[37] and to use small increments in the daily dosage.[34,36,37]
Exogenous luteinizing hormone supplementation
LH is important in regulating steroidogenesis throughout follicular development; adequate LH is particularly important for oocyte maturation.[39] Most of the Asian assisted reproduction practitioners make use of both long agonist and antagonist protocols for ovarian stimulation; majority using the former approach. Published literature on the beneficial effects of exogenous LH in patients with previous suboptimal response or low baseline serum LH concentrations is more extensive in long agonist protocols.[5,40] Documented results associate poorer outcomes with patients whose LH concentration was low after GnRH agonist treatment.[3,40]
The Asia Pacific Fertility Advisory Group[6] in 2011 strongly recommended r-hLH co-treatment with r-hFSH in patients with a history of poor response as in:
-
Suboptimal response on day 6 in long agonist cycles
- absence of >10 mm follicles
- endometrial thickness of <6 mm
- estradiol levels <200 pg/mL
r-hLH may also be beneficial in women aged >35 years undergoing ovarian stimulation with long agonist or antagonist protocols.[6]
Poor responders and low ovarian reserve
Many factors are linked to a decreased ovarian response and hence, it is difficult to identify poor responders. Although several tests have been suggested, none can indicate it accurately.[41]
Some putative biomarkers to identify poor responders include (i) LH concentrations either at baseline or day 6 midfollicular (ii) AMH levels and (iii) antral follicle count (AFC). Wong et al. recommended that further research is needed in patients with suboptimal response based on the following biomarkers: (i) AFC < 6 in both ovaries; (ii) AMH concentration <1.5 ng/mL; and (iii) LH polymorphisms.[6]
Poor ovarian reserve is estimated to occur in about 9-26% of the ART procedures. Evidence indicates that r-hLH and r-hFSH co-administration in these patients may help in improving ongoing pregnancy rates in poor responders and women of advanced age.[7,26,42,43] However, further studies are needed in this regard as some studies report that the available evidence is not enough to validate the effectiveness of r-hLH in subjects with poor response undergoing ART.[44,45]
Advanced reproductive aged patients
A recent systemic review and meta-analysis concluded that the inclusion of r-hLH to FSH stimulation enhanced the clinical pregnancy and implantation rates in ART cycles in patients aged ≥35 years.[42] Similar results were reported in many other randomized trials.[7,26] Similarly, a Cochrane review reiterated the usefulness of r-hLH in poor responders and advanced aged women at risk of spontaneous miscarriage.[46]
An open-label randomized controlled study found that r-hLH is beneficial in improving the implantation rate in women aged 36-39 years, but not so in those younger than 36 years of age.[7] This might be due to the fact that the serum androgen levels decline steeply with age, as does the response to FSH stimulation. LH administration enhances follicular androgen production followed by its aromatization to estrogen. It also controls progesterone production by granulosa cells, which is also FSH dependent. Several studies correlated the occurrence of apoptosis in granulosa cells with the IVF outcome. The incidence of apoptosis was lower in granulosa cells of follicles aspirated from patients who became pregnant after ivf cycle compared with granulosa cells of follicles aspirated from patients who are non-pregnant.[47,48] Bencomo et al. reported that, the percentage of apoptotic cells was significantly less in younger age group (<38 years) compared with older age group (>38 years) and further suggested that apoptosis may be a marker for ovarian age or reserve as granulosa cells of older women are more susceptible to apoptosis.[49] In a study by Ruvolo et al. shown that the r-LH administration resulted in a reduction in the apoptosis observed in the cumulus cells of the patients whose clinical pregnancy rate and implantation rate was significantly high compared with the non-r-LH administered group.[4] The beneficial effect of LH was attributed to its direct action on cumulus and granulosa cells, or by the paracrine effect mediated by secreting factors in the theca and oocyte cells viz. by inducing the expression of epidermal growth factor in the theca cell, which has a reported antiapoptotic activity. Recently Gatta et al. studied the gene expression profiles of cumulus cells obtained from r-LH treated patients and found that 84 genes were up regulated with the following cellular function: gene expression, cell-to-cell signaling and interaction, cellular growth and proliferation, cell cycle, morphology and death, inflammatory response and molecular transport.[50] Data from the above recent studies indicated the significance of LH at cellular and molecular pathways. Thus, LH supplementation seems appropriate for aged patients and poor responders where it restores the follicular and endometrial milieu and improves the cycle outcome.[39,51]
Another retrospective observational study evaluating ART patients undergoing stimulation with an antagonist procedure reported clinical pregnancy success of 36% for patients aged 38 years treated with r-hFSH and r-hLH compared with 19.1% (P = 0.048) for those stimulated with r-hFSH and human menopausal gonadotrophin (hMG).[52] Conversely there were two studies, Fabregues et al. and Nyboeandersen et al. who found no benefit in supplementing rLH in the GnRH agonist long protocol.
Role of luteinizing hormone in polycystic ovary syndrome (PCOS)
The detrimental impact of endocrinological disorder, which is linked to hyper-secretion of LH and ovulatory dysfunction, is attributed to increased LH levels. Studies have found that such women are associated with poor fertilization, oocyte quality and embryo quality, which could be due to underlying mechanisms such as androgen excess induced by LH. However, contrary to previous belief, it was later demonstrated that hyper-insulinemia and not LH hyper-secretion plays a vital role in PCOS pathogenesis.[53] Adding LH in this scenario would lead to OHSS and hence LH should be avoided.
Role of luteinizing hormone
LH supplementation is important in older and poor-responding patients because they usually receive higher FSH doses for COS, show higher progesterone levels at the end of stimulation and subsequently, their endometrium receptivity diminishes.[7] Previous studies have shown the benefical effects of LH supplementation in older patients.[6,7]
Dosing of luteinizing hormone
In 1998, the European Study Group conducted the first randomized efficacy clinical study to investigate the safety and tolerability of r-hLH supplementation in hypogonadotropic hypogonadal women (WHO group 1 anovulation). The researchers also aimed to assess the minimal effective dose for this patient population. The patients (n = 38) randomly received daily injections of 0 IU, 25 IU, 75 IU, or 225 IU of r-hLH in conjunction with 150 IU r-hFSH/day for up to 20 days. The results were showed that r-hLH helped in:
Promoting dose-associated increase in the secretion of estradiol and androstenedione by r-hFSH-induced follicles.
Enhancing ovarian sensitivity to FSH as observed in the number of patients who developed follicles following FSH administration.
Increasing the successful luteinization of follicles on exposure to hCG.
It was observed that 75 IU r-hLH promoted adequate follicular development and steriodogenesis in 46% of the treatment cycles, with sufficient secretion of estrogen and progesterone in 75-80% of the cycles. Based on the findings, the researchers recommended that 75 IU r-hLH is effective in most of the women by facilitating maximal endometrial growth and optimal follicular development, which is defined as:
≥1 follicle of ≥17 mm.
Estradiol levels of ≥400 pmol/L.
Mid-luteal phase progesterone level of ≥25 nmol/L.
Furthermore, they suggested that a small percentage of women may require up to 225 IU of r-hLH/day subcutaneously, but emphasized that the high dose of r-hLH was also found to be immunogenic and well tolerated.[54] To achieve an optimal benefit Ramu et al. suggested a dose of 75 IU/day of r-hLH for supplementation with r-HFSH.[25]
The widely used dosage is a ratio of 2:1 for FSH: LH, i.e., 150 IU: 75 IU starting on day 1 or 6 of stimulation, especially in hypo-hypo patients.[6] A study carried out by Lisi et al., shown that the administration of r-hLH (75 IU/day for 4 days), 1 day before the beginning r-hFSH stimulation, offers some benefits in terms of clinical pregnancies when compared with the patients undergoing stimulation with r-hFSH alone.[55] Though starting patients with r-hLH on day 1 maximizes the benefit of increased ovarian androgen production triggered due to the presence of the exogenous LH, it acts synergistically with FSH to promote FSH receptor mRNA expression, follicular development and steroidogenesis.[51]
Numerous studies have demonstrated that r-hLH in combination with FSH is better than hMG with FSH. This might be due to excessive or inconsistent LH activity from the hCG component in hMG may affect ocyte maturation in the latter half of the ovarian stimulation cycle, giving rise to the differences in numbers of oocytes retrieved and success of pregnancy.[56,57]
CONCLUSION
Optimal follicle development with subsequent ovulation requires the complex interaction of FSH, LH and their complementary activities. Low endogenous LH production may lead to a poor outcome of ART. Exogenous LH specifically in patients with hypogonadotrophic hypogonadism and patients >35 years may result in improved assisted reproduction outcomes. However, the dosage of LH is critical as elevated LH might have detrimental effects on ART. Thus, ART outcome can be improved with optimization of FSH dose in various patient populations and supplementation of LH in various subgroups discussed above. Biomarkers to ascertain women who are in need of exogenous LH need to be sought. With the increasing evidence of pharmacogenetic approaches, it is likely that the choice of ART regimen will be also guided by patient's genetic makeup. We suggest that before deciding on use of exogenous LH, it is crucial to identify patients who would benefit the most from LH supplementation and assess the cost-benefit ratio in the use of exogenous LH. Further research is needed to arrive at a clear and uniform consensus on dosage, timing and patient population who would benefit the most with LH supplementation.
ACKNOWLEDGMENTS
The authors are grateful to acknowledge Knowledge Isotopes (www.knowledgeisotopes.com) for editing support.
Footnotes
Source of Support: Advisory board meetings supported by Merck Specialties Private Limited
Conflict of Interest: This review article is the result of a series of advisory board meetings supported by Merck Specialties Private Limited.
REFERENCES
- 1.Shoham Z. The clinical therapeutic window for luteinizing hormone in controlled ovarian stimulation. Fertil Steril. 2002;77:1170–7. doi: 10.1016/s0015-0282(02)03157-6. [DOI] [PubMed] [Google Scholar]
- 2.Humaidan P, Bungum L, Bungum M, Andersen CY. Ovarian response and pregnancy outcome related to mid-follicular LH levels in women undergoing assisted reproduction with GnRH agonist down-regulation and recombinant FSH stimulation. Hum Reprod. 2002;17:2016–21. doi: 10.1093/humrep/17.8.2016. [DOI] [PubMed] [Google Scholar]
- 3.Lahoud R, Al-Jefout M, Tyler J, Ryan J, Driscoll G. A relative reduction in mid-follicular LH concentrations during GnRH agonist IVF/ICSI cycles leads to lower live birth rates. Hum Reprod. 2006;21:2645–9. doi: 10.1093/humrep/del219. [DOI] [PubMed] [Google Scholar]
- 4.Ruvolo G, Bosco L, Pane A, Morici G, Cittadini E, Roccheri MC. Lower apoptosis rate in human cumulus cells after administration of recombinant luteinizing hormone to women undergoing ovarian stimulation for in vitro fertilization procedures. Fertil Steril. 2007;87:542–6. doi: 10.1016/j.fertnstert.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 5.Pezzuto A, Ferrari B, Coppola F, Nardelli GB. LH supplementation in down-regulated women undergoing assisted reproduction with baseline low serum LH levels. Gynecol Endocrinol. 2010;26:118–24. doi: 10.3109/09513590903215516. [DOI] [PubMed] [Google Scholar]
- 6.Wong PC, Qiao J, Ho C, Ramaraju GA, Wiweko B, Takehara Y, et al. Current opinion on use of luteinizing hormone supplementation in assisted reproduction therapy: An Asian perspective. Reprod Biomed Online. 2011;23:81–90. doi: 10.1016/j.rbmo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Pellicer A. Impact of luteinizing hormone administration on gonadotropin-releasing hormone antagonist cycles: An age-adjusted analysis. Fertil Steril. 2011;95:1031–6. doi: 10.1016/j.fertnstert.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 8.O'Dea L, O'Brien F, Currie K, Hemsey G. Follicular development induced by recombinant luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in anovulatory women with LH and FSH deficiency: Evidence of a threshold effect. Curr Med Res Opin. 2008;24:2785–93. doi: 10.1185/03007990802374815. [DOI] [PubMed] [Google Scholar]
- 9.Vaskivuo T. Regulation of Apoptosis in the Female Reproductive System. PhD [dissertation] Oulu, Finland: University of Oulu; 2002. [Google Scholar]
- 10.Brown JB. Pituitary control of ovarian function - Concepts derived from gonadotrophin therapy. Aust N Z J Obstet Gynaecol. 1978;18:46–54. doi: 10.1111/j.1479-828x.1978.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 11.Gonçalves PB, Portela VM, Ferreira R, Gasperin BG. Role of angiotensin II on follicle development and ovulation. Anim Reprod. 2010;7:140–5. [Google Scholar]
- 12.Kronenberg HM, Memed S, Polonsky KS, Larsen PR. Williams Textbook of Endocrinology. 11th ed. Philadelphia: Saunders Elsevier; 2007. [Google Scholar]
- 13.Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed. PA: Saunders Elsevier; 2011. [Google Scholar]
- 14.Fortune JE, Quirk SM. Regulation of steroidogenesis in bovine preovulatory follicles. J Anim Sci. 1988;66(Suppl 2):1–8. [Google Scholar]
- 15.Richards JS, Pangas SA. The ovary: Basic biology and clinical implications. J Clin Invest. 2010;120:963–72. doi: 10.1172/JCI41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Souza MJ, Miller BE, Loucks AB, Luciano AA, Pescatello LS, Campbell CG, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: Blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220–32. doi: 10.1210/jcem.83.12.5334. [DOI] [PubMed] [Google Scholar]
- 17.Fauser BC, Van Heusden AM. Manipulation of human ovarian function: Physiological concepts and clinical consequences. Endocr Rev. 1997;18:71–106. doi: 10.1210/edrv.18.1.0290. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan MW, Stewart-Akers A, Krasnow JS, Berga SL, Zeleznik AJ. Ovarian responses in women to recombinant follicle-stimulating hormone and luteinizing hormone (LH): A role for LH in the final stages of follicular maturation. J Clin Endocrinol Metab. 1999;84:228–32. doi: 10.1210/jcem.84.1.5389. [DOI] [PubMed] [Google Scholar]
- 19.Loumaye E, Engrand P, Shoham Z, Hillier SG, Baird DT. Clinical evidence for an LH ‘ceiling’ effect induced by administration of recombinant human LH during the late follicular phase of stimulated cycles in World Health Organization type I and type II anovulation. Hum Reprod. 2003;18:314–22. doi: 10.1093/humrep/deg066. [DOI] [PubMed] [Google Scholar]
- 20.Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: Analysis of over 4000 cycles. Hum Reprod. 2010;25:2092–100. doi: 10.1093/humrep/deq125. [DOI] [PubMed] [Google Scholar]
- 21.Bosch E, Valencia I, Escudero E, Crespo J, Simón C, Remohí J, et al. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril. 2003;80:1444–9. doi: 10.1016/j.fertnstert.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Filicori M, Cognigni GE, Pocognoli P, Tabarelli C, Spettoli D, Taraborrelli S, et al. Modulation of folliculogenesis and steroidogenesis in women by graded menotrophin administration. Hum Reprod. 2002;17:2009–15. doi: 10.1093/humrep/17.8.2009. [DOI] [PubMed] [Google Scholar]
- 23.Palermo R. Differential actions of FSH and LH during folliculogenesis. Reprod Biomed Online. 2007;15:326–37. doi: 10.1016/s1472-6483(10)60347-1. [DOI] [PubMed] [Google Scholar]
- 24.Voutilainen R, Tapanainen J, Chung BC, Matteson KJ, Miller WL. Hormonal regulation of P450scc (20,22-desmolase) and P450c17 (17 alpha-hydroxylase/17,20-lyase) in cultured human granulosa cells. J Clin Endocrinol Metab. 1986;63:202–7. doi: 10.1210/jcem-63-1-202. [DOI] [PubMed] [Google Scholar]
- 25.Raju GA, Teng SC, Kavitha P, Lakshmi BK, Ravikrishna C. Combination of recombinant follicle stimulating hormone with human menopausal gonadotrophin or recombinant luteinizing hormone in a long gonadotrophin-releasing hormone agonist protocol: a retrospective study. Reprod Med Biol. 2012;11:129–33. doi: 10.1007/s12522-012-0120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill MJ, Levy G, Levens ED. Does exogenous LH in ovarian stimulation improve assisted reproduction success. An appraisal of the literature? Reprod Biomed Online. 2012;24:261–71. doi: 10.1016/j.rbmo.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Simoni M, Tempfer CB, Destenaves B, Fauser BC. Functional genetic polymorphisms and female reproductive disorders: Part I: Polycystic ovary syndrome and ovarian response. Hum Reprod Update. 2008;14:459–84. doi: 10.1093/humupd/dmn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simoni M, Tüttelmann F, Michel C, Böckenfeld Y, Nieschlag E, Gromoll J. Polymorphisms of the luteinizing hormone/chorionic gonadotropin receptor gene: Association with maldescended testes and male infertility. Pharmacogenet Genomics. 2008;18:193–200. doi: 10.1097/FPC.0b013e3282f4e98c. [DOI] [PubMed] [Google Scholar]
- 29.Themmen AP. An update of the pathophysiology of human gonadotrophin subunit and receptor gene mutations and polymorphisms. Reproduction. 2005;130:263–74. doi: 10.1530/rep.1.00663. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson CH, Kaleva M, Virtanen H, Haavisto AM, Pettersson K, Huhtaniemi IT. Disparate response of wild-type and variant forms of LH to GnRH stimulation in individuals heterozygous for the LHbeta variant allele. Hum Reprod. 2001;16:230–5. doi: 10.1093/humrep/16.2.230. [DOI] [PubMed] [Google Scholar]
- 31.Pettersson KS, Söderholm JR. Individual differences in lutropin immunoreactivity revealed by monoclonal antibodies. Clin Chem. 1991;37:333–40. [PubMed] [Google Scholar]
- 32.Alviggi C, Clarizia R, Pettersson K, Mollo A, Humaidan P, Strina I, et al. Suboptimal response to GnRHa long protocol is associated with a common LH polymorphism. Reprod Biomed Online. 2009;18:9–14. doi: 10.1016/s1472-6483(10)60418-x. [DOI] [PubMed] [Google Scholar]
- 33.Mafra FA, Bianco B, Christofolini DM, Souza AM, Zulli K, Barbosa CP. Luteinizing hormone beta-subunit gene (LHbeta) polymorphism in infertility and endometriosis-associated infertility. Eur J Obstet Gynecol Reprod Biol. 2010;151:66–9. doi: 10.1016/j.ejogrb.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton-Fairley D, Kiddy D, Watson H, Sagle M, Franks S. Low-dose gonadotrophin therapy for induction of ovulation in 100 women with polycystic ovary syndrome. Hum Reprod. 1991;6:1095–9. doi: 10.1093/oxfordjournals.humrep.a137491. [DOI] [PubMed] [Google Scholar]
- 35.Shoham Z, Patel A, Jacobs HS. Polycystic ovarian syndrome: Safety and effectiveness of stepwise and low-dose administration of purified follicle-stimulating hormone. Fertil Steril. 1991;55:1051–6. doi: 10.1016/s0015-0282(16)54351-9. [DOI] [PubMed] [Google Scholar]
- 36.Hedon B, Hugues JN, Emperaire JC, Chabaud JJ, Barbereau D, Boujenah A, et al. A comparative prospective study of a chronic low dose versus a conventional ovulation stimulation regimen using recombinant human follicle stimulating hormone in anovulatory infertile women. Hum Reprod. 1998;13:2688–92. doi: 10.1093/humrep/13.10.2688. [DOI] [PubMed] [Google Scholar]
- 37.Leader A. Monofollicular Ovulation Induction Study Group. Improved monofollicular ovulation in anovulatory or oligo-ovulatory women after a low-dose step-up protocol with weekly increments of 25 international units of follicle-stimulating hormone. Fertil Steril. 2006;85:1766–73. doi: 10.1016/j.fertnstert.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 38.Hugues JN, Cédrin-Durnerin I, Avril C, Bulwa S, Hervé F, Uzan M. Sequential step-up and step-down dose regimen: An alternative method for ovulation induction with follicle-stimulating hormone in polycystic ovarian syndrome. Hum Reprod. 1996;11:2581–4. doi: 10.1093/oxfordjournals.humrep.a019173. [DOI] [PubMed] [Google Scholar]
- 39.Hillier SG, Ross GT. Effects of exogenous testosterone on ovarian weight, follicular morphology and intraovarian progesterone concentration in estrogen-primed hypophysectomized immature female rats. Biol Reprod. 1979;20:261–8. doi: 10.1095/biolreprod20.2.261. [DOI] [PubMed] [Google Scholar]
- 40.De Placido G, Alviggi C, Perino A, Strina I, Lisi F, Fasolino A, et al. Recombinant human LH supplementation versus recombinant human FSH (rFSH) step-up protocol during controlled ovarian stimulation in normogonadotrophic women with initial inadequate ovarian response to rFSH. A multicentre, prospective, randomized controlled trial. Hum Reprod. 2005;20:390–6. doi: 10.1093/humrep/deh625. [DOI] [PubMed] [Google Scholar]
- 41.Ubaldi FM, Rienzi L, Ferrero S, Baroni E, Sapienza F, Cobellis L, et al. Management of poor responders in IVF. Reprod Biomed Online. 2005;10:235–46. doi: 10.1016/s1472-6483(10)60946-7. [DOI] [PubMed] [Google Scholar]
- 42.Hill MJ, Levens ED, Levy G, Ryan ME, Csokmay JM, DeCherney AH, et al. The use of recombinant luteinizing hormone in patients undergoing assisted reproductive techniques with advanced reproductive age: A systematic review and meta-analysis. Fertil Steril. 2012;97:1108–14. doi: 10.1016/j.fertnstert.2012.01.130. [DOI] [PubMed] [Google Scholar]
- 43.Musters AM, van Wely M, Mastenbroek S, Kaaijk EM, Repping S, van der Veen F, et al. The effect of recombinant LH on embryo quality: A randomized controlled trial in women with poor ovarian reserve. Hum Reprod. 2012;27:244–50. doi: 10.1093/humrep/der371. [DOI] [PubMed] [Google Scholar]
- 44.Barrenetxea G, Agirregoikoa JA, Jiménez MR, de Larruzea AL, Ganzabal T, Carbonero K. Ovarian response and pregnancy outcome in poor-responder women: A randomized controlled trial on the effect of luteinizing hormone supplementation on in vitro fertilization cycles. Fertil Steril. 2008;89:546–53. doi: 10.1016/j.fertnstert.2007.03.088. [DOI] [PubMed] [Google Scholar]
- 45.Bosdou JK, Venetis CA, Kolibianakis EM, Toulis KA, Goulis DG, Zepiridis L, et al. The use of androgens or androgen-modulating agents in poor responders undergoing in vitro fertilization: A systematic review and meta-analysis. Hum Reprod Update. 2012;18:127–45. doi: 10.1093/humupd/dmr051. [DOI] [PubMed] [Google Scholar]
- 46.Mochtar MH, Van der Veen, Ziech M, van Wely M. Recombinant Luteinizing Hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles. Cochrane Database Syst Rev. 2007;2:CD005070. doi: 10.1002/14651858.CD005070.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Nakahara K, Saito H, Saito T, Ito M, Ohta N, Sakai N, et al. Incidence of apoptotic bodies in membrana granulosa of the patients participating in an in vitro fertilization program. Fertil Steril. 1997;67:302–8. doi: 10.1016/S0015-0282(97)81915-2. [DOI] [PubMed] [Google Scholar]
- 48.Oosterhuis GJ, Michgelsen HW, Lambalk CB, Schoemaker J, Vermes I. Apoptotic cell death in human granulosa-lutein cells: A possible indicator of in vitro fertilization outcome. Fertil Steril. 1998;70:747–9. doi: 10.1016/s0015-0282(98)00266-0. [DOI] [PubMed] [Google Scholar]
- 49.Bencomo E, Pérez R, Arteaga MF, Acosta E, Peña O, Lopez L, et al. Apoptosis of cultured granulosa-lutein cells is reduced by insulin-like growth factor I and may correlate with embryo fragmentation and pregnancy rate. Fertil Steril. 2006;85:474–80. doi: 10.1016/j.fertnstert.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Gatta V, Tatone C, Ciriminna R, Vento M, Franchi S, d'Aurora M, et al. Gene expression profiles of cumulus cells obtained from women treated with recombinant human luteinizing hormone+recombinant human follicle-stimulating hormone or highly purified human menopausal gonadotropin versus recombinant human follicle-stimulating hormone alone. Fertil Steril. 2013;99:2000–81. doi: 10.1016/j.fertnstert.2013.01.150. [DOI] [PubMed] [Google Scholar]
- 51.Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84:2951–6. doi: 10.1210/jcem.84.8.5929. [DOI] [PubMed] [Google Scholar]
- 52.Thornton K, Alper MM, Ryley D, Ezcurra D. Outcomes of GnRH antagonist IVF cycles using LH supplementation for COH: FSH/rhLH versus FSH/hMG. Fertil Steril. 2006;86:S411. [Google Scholar]
- 53.Rekha P, Jirge S. Is LH necessary in ovulation induction? In: Desai S, Parihar M, Allahabadia G, editors. Infertility: Principles and Practice. India: BI Publications Pvt. Ltd; 2004. p. 5. [Google Scholar]
- 54.Recombinant human luteinizing hormone (LH) to support recombinant human follicle-stimulating hormone (FSH)-induced follicular development in LH- and FSH-deficient anovulatory women: A dose-finding study. The European Recombinant Human LH Study Group. J Clin Endocrinol Metab. 1998;83:1507–14. doi: 10.1210/jcem.83.5.4770. [DOI] [PubMed] [Google Scholar]
- 55.Lisi F, Caserta D, Montanino M, Berlinghieri V, Bielli W, Carfagna P, et al. Recombinant luteinizing hormone priming in multiple follicular stimulation for in-vitro fertilization in downregulated patients. Gynecol Endocrinol. 2012;28:674–7. doi: 10.3109/09513590.2011.652716. [DOI] [PubMed] [Google Scholar]
- 56.Carone D, Vizziello G, Vitti A, Chiappetta R. Clinical outcomes of ovulation induction in WHO Group I anovulatory women using r-hFSH .r-hLH in a 2:1 ratio compared to hMG? Hum Reprod. 2010;25:285–321. [Google Scholar]
- 57.Ferraretti AP, Gianaroli L, Magli MC, D'angelo A, Farfalli V, Montanaro N. Exogenous luteinizing hormone in controlled ovarian hyperstimulation for assisted reproduction techniques. Fertil Steril. 2004;82:1521–6. doi: 10.1016/j.fertnstert.2004.06.041. [DOI] [PubMed] [Google Scholar]


