Abstract
Background:
The purpose of this study was to investigate the effects of the combination of vitex agnus castus extract, as a source of phytoestrogens, plus magnesium supplementation on osteogenic and angiogenic factors and callus formation in women with long bone fracture.
Material and Methods:
In a double-blind randomized placebo controlled trial, 64 women with long bone fracture, 20-45 years old, were randomly allocated to receive 1) one Agnugol tablet (4 mg dried fruit extract of vitex agnus castus) plus 250 mg magnesium oxide (VAC + Mg group (n = 10)), 2) one Agnugol tablet plus placebo (VAC group (n = 15)), 3) placebo plus 250 mg magnesium oxide (Mg group (n = 12)), or 4) placebo plus placebo (placebo group (n = 14)) per day for 8 weeks. At baseline and endpoint of the trial, serum alkaline phosphatase, osteocalcin, and vascular endothelial growth factor (VEGF) were measured together with radiological bone assessment.
Results:
There were no significant differences in the characteristic aspects of concern between the four groups at baseline. Despite the increased level of alkaline phosphatase in the VAC group (188.33 ± 16.27 to 240.40 ± 21.49, P = 0.05), administration of VAC + Mg could not increase alkaline phosphatase activity. However, treatment with VAC + Mg significantly enhanced the osteocalcin level. The serum concentration of VEGF was increased in the VAC group (269.04 ± 116.63 to 640.03 ± 240.16, P < 0.05). Callus formation in the VAC + Mg group was higher than the other groups but the differences between the four groups were not significant (P = 0.39). No relevant side effect was observed in patients in each group.
Conclusion:
Our results suggest that administration of vitex agnus castus plus magnesium may promote fracture healing. However, more studies need to further explore the roles of vitex agnus castus in fracture repair processes.
Keywords: Bone healing, magnesium, vitex agnus castus extract
“Study was carried out in Research Center for Bone & Joint Diseases, Chamran Hospital, Shiraz University of Medical Sciences, Shiraz, Iran”
INTRODUCTION
The occurrence rate of bone fracture reaches millions per year and 2% of the population experience at least one type of fracture annually.[1,2] The common cause of fractures is high-energy trauma,[3] but non-traumatic fractures can occur as a result of underlying bone disease including osteoporosis.[2,4] The healing of fracture immediately initiates after fracture occurrence, and bone repair continues to restore its structural integrity.[1] However, because of inadequate fracture healing in a high number of bone fractures, the healing of fracture requires further treatment.[5] The measurement of enzymatic activity of the osteoblasts including bone alkaline phosphatase, or bone matrix component such as osteocalcin can be investigated for the bone healing process.[6] Alkaline phosphatase (ALP) and osteocalcin (OCN), as serum osteogenic markers, represent growth and differentiation of osteoblasts and enhance bone mineralization.[7]
Other than pharmaceutical intervention, dietary supplementation and alternative medicine can be also useful in bone healing. Previous studies have demonstrated that phytoestrogens protect bone health through prevention of bone resorption, bone density augmentation, and promote fracture repair when used as bone graft material.[8,9] Due to vasodilatory effects of phytoestrogens, which are induced by nitric oxide production and also vascular endothelial growth factor (VEGF) expression, they can increase the vascular ingrowth and return the blood flow to fracture gap and facilitate the fracture healing process.[8,9] VEGF is an angiogenic factor which was evoked to the site of bone injury and stimulates angiogenesis, and thereby participates in new blood vessel formation.[10,11]
Chaste tree (vitex agnus castus (VAC)), known to be a good source of phytoestrogens,[12] is aboriginal to central Asia and Mediterranean Europe.[13] It has a long history of use in traditional medicine and today there has been an increasing interest in the role of VAC in managing disorders. Recently, studies have reported that VAC prevents osteopenia and promotes fracture healing.[9,12] But these studies were limited to animal models and, to the best of our knowledge, no data is available in humans.
On the other hand, nutritional treatment and supplementation with nutrients which incorporate in bone formation can be supportive in the fracture healing process. Magnesium (Mg), as a mineral which is involved in bone metabolism, mainly stores in bones.[14] Mg plays important roles in calcium and bone metabolism by regulating the release and action of parathyroid hormone and activation of vitamin D.[15] Moreover, it can directly affect bone mineralization.[15] In addition, a study has reported that serum level of Mg in elderly patients with long bone fracture is lower, compared to elderly patients without bone fracture.[16]
Given the phytoestrogenic content of VAC, which is capable of repairing the bone defect, and the limited number of studies on the effects of Mg supplementation in fracture healing, the aim of the present study was to evaluate the effects of VAC extract and Mg supplementation, alone and in combination, on osteogenic and angiogenic factors and callus formation in premenopausal women with long bone fracture.
MATERIALS AND METHODS
Study subjects
In a double-blind randomized placebo-controlled clinical trial, 64 women, 20 to 45 years old, with traumatic long bone fracture referred to Chamran or Rajaei Hospital at Shiraz University of Medical Sciences were enrolled in the study from April to December 2012. According to study of Oztürk et al,[9] the sample size was determined 16 for each group with a significant level of 0.05, power of 90%, μ1 = 4 ± 2.4, μ2 = 1.3 ± 1.89 and also with considering a drop-out rate of 31.25%.
All of the patients were treated by surgery (open reduction internal fixation). Exclusion criteria were pregnancy and lactation, postmenopausal women, patients with a history of chronic diseases, taking medication known to influence bone metabolism, hormonal drugs, herbal drugs containing phytoestrogen, diuretics, multivitamin/mineral supplementation, alcohol or tobacco abuse.
At the beginning of the study, patients were given an oral and written explanation of the study, including its procedure and benefits, and were asked to sign an informed consent document. This study was approved by the Ethics Committee of Human Experimentation of Shiraz University of Medical Sciences. Also, this trial was registered in Iranian Registry of Clinical Trials with ID number of IRCT2012092810955N1.
Intervention
The patients were randomly allocated to 1) VAC + Mg group, receiving 1 Agnugol tablet (4 mg dried fruit extract of vitex agnus castus) plus 250 mg magnesium oxide, 2) VAC group, receiving 1 Agnugol tablet plus placebo, 3) Mg group, receiving placebo plus 250 mg magnesium oxide, or 4) Placebo group, receiving placebo plus placebo, per day for 8 weeks. In order to perform a randomized study, we put 16 cards labeled different forms of A, B, C and D letters (for example ABCD, ACBD, ADBC, …) in a box. Then randomly picked up a card and allocated 4 patients according to the A, B, C and D ranking of the card. Agnugol and magnesium tablets were obtained from Goldaru Pharmacy Company (Isfahan, Iran) and Nature Made Company (USA), respectively. Two types of placebo which were identical in shape and color of both Agnugol and magnesium tablets, contained lactose, starch and maintaining substances and were obtained from laboratory of School of Pharmacy, Shiraz University of Medical Sciences. The Daily Value (DV) of magnesium supplement in this study was 62.5%. Researchers monitored the compliance of the supplements biweekly by phone call and patient who did not take tablets more than six days were excluded from the study. At the beginning of the study, a questionnaire including age, fracture etiology, and the history of previous fracture as well as anatomical distribution of the fracture was completed for each patient during an interview. Participants were categorized into 3 groups according to their physical activity level; light (doing household tasks, riding in a car, light activity while sitting), moderate (walking 3-5 km/h, light sport, occasional agronomic or husbandry) and severe (severe sport, agronomic or husbandry).[17]
Dietary intake
Nutritional intake was evaluated by a FFQ questionnaire that had been validated by the Department of Nutrition at School of Health and Nutrition, Shiraz University of Medical Sciences. The nutrient consumption was calculated by using the Nut-4 food processor software, modified by incorporating the Iranian food table. Phytoestrogen consumption was divided into 3 grades; high, moderate and low, which respectively represent more than 2 times/day, 1 time/day to 2-4 times/week, and 1 time/week to less than 1 time/month intake of dietary source of phytoestrogens.
Outcome assessment
At the beginning and the end of the study period for each patient, 5 cc fasting blood samples were collected to measure ALP, OCN and VEGF as well. Serum ALP was measured by spectrophotometry, and OCN and VEGF were measured by the ELISA technique.
A standard X-ray image was taken from the fracture site for each patient at weeks 0 and 8, in order to monitor callus formation as a radiological finding of fracture healing. Comparison of the X-ray images for each patient was confirmed by an orthopedic expert (third author).
Statistical analysis
Data processing and analysis were performed using SPSS software (ver. 16 for Windows; SPSS Inc., Chicago, USA). Distribution of variables was not normal, so we used non-parametric tests. Differences between the four groups were analyzed by the Kruskal-Wallis or Jonckheere-Terpstra tests. The Mann-Whitney U test and Wilcoxon signed-rank test were used for post hoc between-group and within-group analysis, respectively. The differences in frequency were tested using Pearson chi-square analysis. P values <0.05 were considered statistically significant in all statistical tests.
RESULTS
Sixty four patients contributed in the trial, of them 51 completed the study and 6 were excluded in the VAC + Mg group (two because they did not use the tablets, three did not come to follow up and one did not come back because lived too far away from the study site), one were excluded in the VAC group because the patient did not come to follow up, four were excluded in the Mg group (two because they did not use the tablets, one did not come to follow up and one did not come back because of personal reasons) and two were excluded in placebo group (one because the patient did not use the tablets and one did not come back because lived too far away from the study site). The compliance rate of the medication was 95.45% in our study.
Mean ± standard deviation (SD) of the age of the participants was 39.0 ± 7.3, 34.8 ± 8.3, 35.2 ± 8.1 and 38.3 ± 9.2 years old in VAC + Mg, VAC, Mg and placebo groups, respectively. The etiology of fracture was road accident (62.7%) and fall (37.3%). The fracture rate was 52.9% in tibia, 25.5% in femur, 13.7% in humerus and 7.8% in radius and ulna. Moreover, 21.6% of patients had previous history of fractures. The results of physical activity levels and dietary analysis are represented in Table 1.
Table 1.
Comparison of physical activity and dietary intake between the four groups at baseline
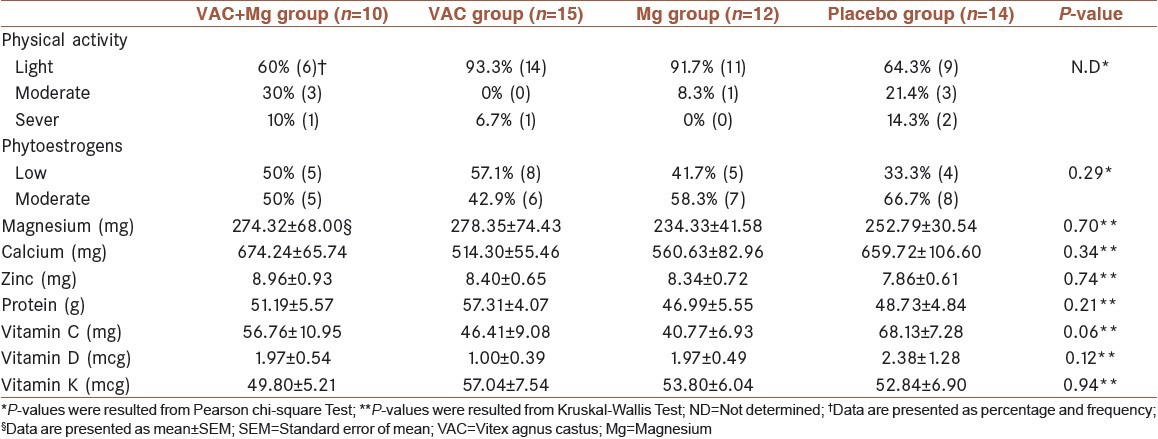
There were no significant differences in age, fracture etiology, anatomical distribution of fractures, intake of dietary sources of phytoestrogens and mean intake of Mg between the four study groups. Moreover, as shown in Table 1, the dietary intake of nutrients which affected bone healing was not statistically significant between the four study groups.
The mean ± SEM of biochemical parameters in the four groups of the study are shown in Table 2. No significant differences were observed in the variables between the four groups at the baseline.
Table 2.
Comparison of the mean values of biochemical parameters between the study groups at baseline and endpoint
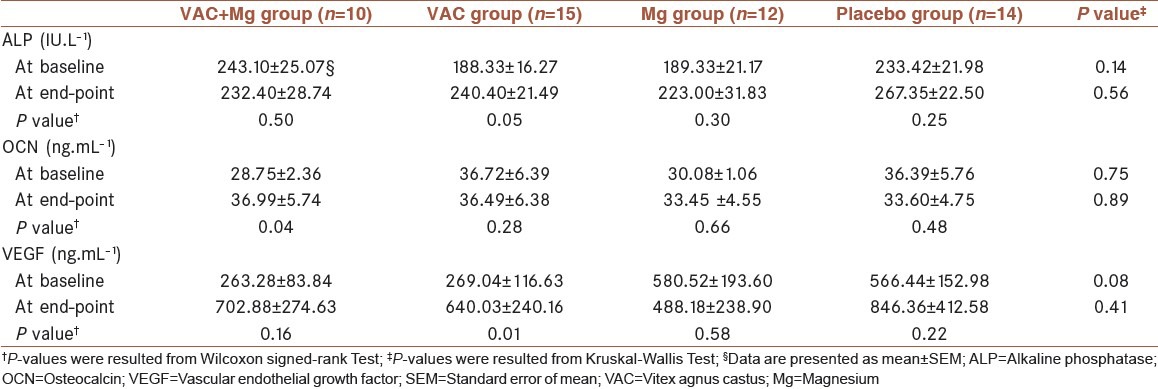
Comparison of the mean differences of ALP levels at the beginning and endpoint of the intervention was not statistically significant between the four interventional groups [Table 3]. Changes in the level of ALP during the study revealed that increase in ALP was considerable only in the VAC group (P = 0.05).
Table 3.
Comparison of the mean differences of ALP, OCN and VEGF levels at the baseline and endpoint of intervention between the four groups
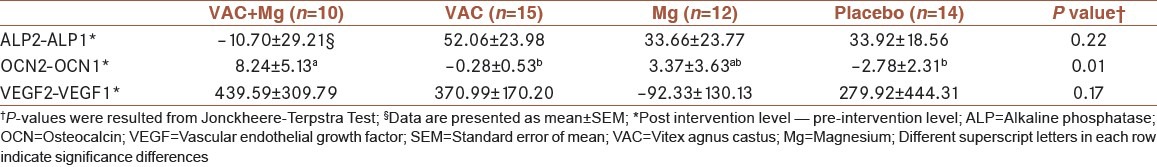
Comparison of the mean value of OCN variation between the study groups showed significant differences [Table 3]. The increased OCN level in VAC + Mg group was significant compared to either VAC or placebo group, using post hoc analysis (P = 0.01).
Again, no significant differences were observed in mean values of VEGF variation between the study groups due to the intervention. However, there was a significant increase in the level of VEGF in the VAC treated group (P = 0.01).
Bone radiography assessment depicted that the percentage of callus formation in VAC + Mg, VAC, Mg and placebo groups was 80.0%, 71.4%, 50.0% and 53.8%, respectively. However, there were no significant differences in callus formation percentages between all interventional groups.
The possible side effects of “vitex agnus castus” were reported in previous studies is mild and reversible and include nausea, mild gastrointestinal complaints, fatigue, menstrual disorders, dry mouth, acne, pruritus and erythematous rash. In this study, no relevant adverse effects were reported with patients in each group.
DISCUSSION
The findings of our study showed that treatment with the combination of VAC extract and Mg supplementation enhance the serum concentration of osteogenic and angiogenic factors and increase callus formation during fracture healing. However, only the change in serum concentration of OCN was observed to be statistically significant.
The present evidences have revealed that serum markers of bone formation, including ALP and OCN, are increased after fracture and reflect osteoblast activity as well as callus integrity during bone repair.[6,18] Moreover, numerous studies have demonstrated that phytoestrogens affect both osteoblastic and osteoclastic cells. Genistein enhanced ALP activity[19] and naringin, a flavonoid, induced bone cell activity and also in high concentration could enhance the ALP activity.[9]
Although we observed a considerable increase in ALP activity in the VAC treated group, the ALP activity did not change by treatment with VAC + Mg. Previous studies have indicated various effects of phytoestrogens on serum ALP levels. A study by Oztürk et al,[9] indicated that injection of 0.75 mg/day VAC extract for 3 weeks had no increasing effect on serum ALP activity in an experimental model of tibia fracture in rabbits. In contrast, Huh et al,[8] reported a significant increase in the expression of mesenchymal progenitor including ALP after a two-week formononetin administration in rats with femur fracture. Another study[20] revealed a significant induced osteoblast MG-63 cell activity as well as increased ALP activity by quercetin and kaempefrol induction. As a result of the present study and previous reports, phytoestrogens promote ALP activity but this effect may be dose dependent.
Mg is a mineral with a noticeable role in bone formation and several studies have reported that serum and bone concentration of ALP reduced in Mg deficiency.[14,21] However, our results have shown that serum ALP did not change in the Mg treated group. Our result may be due in part to the dosage of Mg supplement.
In this study, the serum concentration of OCN significantly increased following an 8-week intervention with the combination of VAC extract and Mg compared to the baseline. This increase was also significantly higher than that of both VAC and placebo groups. Presumably, Mg has a substantial role in OCN improvement.
Osteocalcin, as a protein of extracellular matrix, participates in bone formation.[21] Parallel to the expression of the OCN gene in the third stage of growth and differentiation of osteoblasts, the minerals accumulate in bone.[22] Therefore, we assume that OCN level and subsequently, bone mineralization are increased after Mg intake. In accordance, Aydin et al.[23] have shown that short-term oral Mg supplementation caused a significant increase in OCN levels on the 10th day of the intervention in postmenopausal women when compared with the controls and stayed significantly high at the end of 30 days.
Besides, an in vitro study[24] has shown that formononetin, a phytoestrogen isolated from Astragalus membranaceus, markedly increases the expression of ALP and OCN in human normal osteoblasts. Furthermore, Icariin enhanced OCN secretion in a dose-dependent manner in mesenchymal stem cells.[25] Although our results in the VAC group did not show a consistent change in OCN level in contrast to those of the above-mentioned studies, However, administration of VAC + Mg synergistically and significantly elevated the OCN level.
Treatment with VAC + Mg and also single VAC raised the serum level of VEGF. It has been revealed that a positive correlation between phytoestrogen and VEGF exists. Our findings were consistent with this evidence that phytoestrogens, through enhancing VEGF expression, induce vascular invasion in fracture gap and enhance angiogenesis.[9,26] Angiogenesis with a main role in endochondral ossification and forming vascular osseous tissue can be crucial in repairing and regeneration of bone.[8,27] In this regard, Huh et al.[8] designed an experimental model of femur fracture, evaluated the effects of formononetin, and reported a significant increase in the number of vessels, expression of VEGF and its receptor (VEGF-R2) in the early stage of chondrogenesis. In another study, Chen et al,[27] established an ulnar bone defect model in rabbits and concluded that implanted scaffold containing Icaritin phytomolecule (an intestinal metabolite of epimedium-derived flavonoids) demonstrated new vessel ingrowth in bone defect at the second week of intervention and even more new vessels at the end of the trial.
Our study and other related work suggest that in a short-period of treatment, phytoestrogens exert an appropriate effect in vascular ingrowth and thereby promote early stage of fracture healing. In the present study, VEGF was significantly increased in the VAC group compared with the baseline. Despite the greater increase in the variation of mean VEGF in the VAC + Mg group, this elevation was not significant. This was likely due to outliers.
Our results did not show a considerable effect of Mg in serum concentration of VEGF. We also could not find any documented evidence about this effect.
Radiological appearance results showed that callus formation was 80% in the VAC + Mg group. A similar study by Oztürk et al[9] revealed that VAC extract significantly accelerates fracture healing on the seventh day after surgery of tibia fracture in rabbits. This observation is consistent with the results of the present study, which shows 71.4% callus formation in the VAC-treated group compared with placebo (53.8%). However, this difference was not significant.
Several studies have reported a positive correlation between dietary or supplemental Mg intake and bone mineral density. A significantly increased Mg concentration has also been reported in tibias of rats fed a diet with 10-fold increased Mg content than standard.[28] Another study by Janning et al.[29] revealed that implantation of compressed cylinders of pure Mg (OH)2 into rabbit femur condyles increased bone volume at the end of a 4-week intervention. In the study of Janning et al., direct presence of Mg created an alkaline environment near the bone defect and facilitated bone formation.
In the present study, single Mg treatment could not change the percentage of callus formation compared with placebo. However, Mg in combination with VAC extract had a synergistic positive effect in bone repair. In our study, the dose of the administered Mg supplement was lower than the recommended dietary allowance. Increasing the Mg dosage or continuing supplementation to late stages of healing may reveal better outcome and exhibit the absolute effect of Mg.
In this study, there were no significant differences between the four study groups in callus formation. Possibly, the statistical test could not show significant differences due to low sample size.
The limitations of our study were high number of patients lost to follow up, so unequal and small sample size in subgroups affected the power of recognition of significant differences between groups. Also, the dose of magnesium in this study was lower than RDA, so we recommend the higher dose of magnesium supplementation in other studies.
CONCLUSION
In summary, administration of VAC + Mg may synergistically improve fracture healing by increased OCN and VEGF levels and callus formation. We hypothesize that even a single treatment of phytoestrogens in a short time after fracture may facilitate the process of bone repair, however, further studies need to investigate the role of single treatment of Mg in bone healing.
ACKNOWLEDGMENT
The present article was extracted from the thesis written by Zahra Hassanzadeh Rostami and was financially supported by Shiraz University of Medical Sciences grants No. 91-6235. We thank Bone and Joint Research Center at Shiraz University of Medical Sciences for co-operating this project and the Research Consultation Center of the University for editorial advice. Also, we are grateful to the personnel of Chamran and Rajaei Teaching Hospitals, Shiraz University of Medical Sciences and participating patients.
Footnotes
Source of Support: Shiraz University of Medical Sciences (grants No. 91-6235)
Conflict of Interest: None declared
REFERENCES
- 1.Malizos KN, Hantes ME, Protopappas V, Papachristos A. Low-intensity pulsed ultrasound for bone healing: An overview. Injury. 2006;37(Suppl 1):S56–62. doi: 10.1016/j.injury.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 2.Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci USA. 2011;108:1585–90. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannoudis PV, Mushtaq S, Harwood P, Kambhampati S, Dimoutsos M, Stavrou Z, et al. Accelerated bone healing and excessive callus formation in patients with femoral fracture and head injury. Injury. 2006;37(Suppl 3):S18–24. doi: 10.1016/j.injury.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Shuid AN, Mohamad S, Mohamed N, Fadzilah FM, Mokhtar SA, Abdullah S, et al. Effects of calcium supplements on fracture healing in a rat osteoporotic model. J Orthop Res. 2010;28:1651–6. doi: 10.1002/jor.21180. [DOI] [PubMed] [Google Scholar]
- 5.Keramaris NC, Calori GM, Nikolaou VS, Schemitsch EH, Giannoudis PV. Fracture vascularity and bone healing: A systematic review of the role of VEGF. Injury. 2008;39(Suppl 2):S45–57. doi: 10.1016/S0020-1383(08)70015-9. [DOI] [PubMed] [Google Scholar]
- 6.Yu-Yahiro JA, Michael RH, Dubin NH, Fox KM, Sachs M, Hawkes WG, et al. Serum and urine markers of bone metabolism during the year after hip fracture. J Am Geriatr Soc. 2001;49:877–83. doi: 10.1046/j.1532-5415.2001.49177.x. [DOI] [PubMed] [Google Scholar]
- 7.Unsworth J, Kaneez S, Harris S, Ridgway J, Fenwick S, Chenery D, et al. Pulsed low intensity ultrasound enhances mineralisation in preosteoblast cells. Ultrasound Med Biol. 2007;33:1468–74. doi: 10.1016/j.ultrasmedbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Huh JE, Kwon NH, Baek YH, Lee JD, Choi DY, Jingushi S, et al. Formononetin promotes early fracture healing through stimulating angiogenesis by up-regulating VEGFR-2/Flk-1 in a rat fracture model. Int Immunopharmacol. 2009;9:1357–65. doi: 10.1016/j.intimp.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Oztürk A, Ilman AA, Sağlam H, Yalçinkaya U, Aykut S, Akgöz S, et al. The effects of phytoestrogens on fracture healing: Experimental research in New Zealand white rabbits. Ulus Travma Acil Cerrahi Derg. 2008:14s21–7. [PubMed] [Google Scholar]
- 10.Hankenson KD, Dishowitz M, Gray C, Schenker M. Angiogenesis in bone regeneration. Injury. 2011;42:556–61. doi: 10.1016/j.injury.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarahrudi K, Thomas A, Braunsteiner T, Wolf H, Vécsei V, Aharinejad S. VEGF serum concentrations in patients with long bone fractures: A comparison between impaired and normal fracture healing. J Orthop Res. 2009;27:1293–7. doi: 10.1002/jor.20906. [DOI] [PubMed] [Google Scholar]
- 12.Sehmisch S, Boeckhoff J, Wille J, Seidlova-Wuttke D, Rack T, Tezval M, et al. Vitex agnus castus as prophylaxis for osteopenia after orchidectomy in rats compared with estradiol and testosterone supplementation. Phytother Res. 2009;23:851–8. doi: 10.1002/ptr.2711. [DOI] [PubMed] [Google Scholar]
- 13.Dugoua JJ, Seely D, Perri D, Koren G, Mills E. Safety and efficacy of chastetree (Vitex agnus-castus) during pregnancy and lactation. Can J Clin Pharmacol. 2008;15:e74–9. [PubMed] [Google Scholar]
- 14.Martini LA. Magnesium supplementation and bone turnover. Nutr Rev. 1999;57:227–9. doi: 10.1111/j.1753-4887.1999.tb06948.x. [DOI] [PubMed] [Google Scholar]
- 15.Ryder KM, Shorr RI, Bush AJ, Kritchevsky SB, Harris T, Stone K, et al. Magnesium intake from food and supplements is associated with bone mineral density in healthy older white subjects. J Am Geriatr Soc. 2005;53:1875–80. doi: 10.1111/j.1532-5415.2005.53561.x. [DOI] [PubMed] [Google Scholar]
- 16.Saito N, Tabata N, Saito S, Andou Y, Onaga Y, Iwamitsu A, et al. Bone mineral density, serum albumin and serum magnesium. J Am Coll Nutr. 2004;23:701S–3. doi: 10.1080/07315724.2004.10719412. [DOI] [PubMed] [Google Scholar]
- 17.Ireton Jones CS. Intake: Energy. In: Mahan LK, Escott-Stump S, Raymond J, editors. Krause's food and the nutrition care process. 13th ed. Missouri: Elsevier; 2012. p. 25. [Google Scholar]
- 18.Seebeck P, Bail HJ, Exner C, Schell H, Michel R, Amthauer H, et al. Do serological tissue turnover markers represent callus formation during fracture healing? Bone. 2005;37:669–77. doi: 10.1016/j.bone.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Branca F. Dietary phyto-oestrogens and bone health. Proc Nutr Soc. 2003;62:877–87. doi: 10.1079/PNS2003309. [DOI] [PubMed] [Google Scholar]
- 20.Prouillet C, Mazière JC, Mazière C, Wattel A, Brazier M, Kamel S. Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochem Pharmacol. 2004;67:1307–13. doi: 10.1016/j.bcp.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Lee AJ, Hodges S, Eastell R. Measurement of osteocalcin. Ann Clin Biochem. 2000;37:432–46. doi: 10.1177/000456320003700402. [DOI] [PubMed] [Google Scholar]
- 22.Lammens J, Liu Z, Aerssens J, Dequeker J, Fabry G. Distraction bone healing versus osteotomy healing: A comparative biochemical analysis. J Bone Miner Res. 1998;13:279–86. doi: 10.1359/jbmr.1998.13.2.279. [DOI] [PubMed] [Google Scholar]
- 23.Aydin H, Deyneli O, Yavuz D, Gözü H, Mutlu N, Kaygusuz I, et al. Short-term oral magnesium supplementation suppresses bone turnover in postmenopausal osteoporotic women. Biol Trace Elem Res. 2010;133:136–43. doi: 10.1007/s12011-009-8416-8. [DOI] [PubMed] [Google Scholar]
- 24.Huh JE, Seo DM, Baek YH, Choi DY, Park DS, Lee JD. Biphasic positive effect of formononetin on metabolic activity of human normal and osteoarthritic subchondral osteoblasts. Int Immunopharmacol. 2010;10:500–7. doi: 10.1016/j.intimp.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Wang XL, Xie XH, Zhang G, Chen SH, Yao D, He K, et al. Exogenous phytoestrogenic molecule icaritin incorporated into a porous scaffold for enhancing bone defect repair. J Orthop Res. 2013;31:164–72. doi: 10.1002/jor.22188. [DOI] [PubMed] [Google Scholar]
- 26.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–61. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen SH, Lei M, Xie XH, Zheng LZ, Yao D, Wang XL, et al. PLGA/TCP composite scaffold incorporating bioactive phytomolecule icaritin for enhancement of bone defect repair in rabbits. Acta Biomater. 2013;9:6711–22. doi: 10.1016/j.actbio.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Takeda R, Nakamura T. Effects of high magnesium intake on bone mineral status and lipid metabolism in rats. J Nutr Sci Vitaminol (Tokyo) 2008;54:66–75. doi: 10.3177/jnsv.54.66. [DOI] [PubMed] [Google Scholar]
- 29.Janning C, Willbold E, Vogt C, Nellesen J, Meyer-Lindenberg A, Windhagen H, et al. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling. Acta Biomater. 2010;6:1861–8. doi: 10.1016/j.actbio.2009.12.037. [DOI] [PubMed] [Google Scholar]


