Abstract
Background:
Hepatitis B with its complications has become one of the universal problems. Injection drug use is one of the most important risk factors in the transmission of hepatitis B. Therefore, we assessed hepatitis B virus prevalence among cases with a history of intravenous drug use (IVDU) as the first announcement-based study in this regard.
Materials and Methods:
The announcement-based detection of hepatitis B seroprevalence in volunteers with a history of intravenous drug use was conducted in the Isfahan province. A comprehensive community announcement was made in all the public places and to all physicians, in all the regions. One thousand five hundred and eighty-eight volunteers were invited to the Isfahan reference laboratories and serum samples were tested for HBs-Ag, HBc Ab, and HBs-Ab, using the enzyme-linked immunosorbent assay (ELISA) method.
Results:
In this study, 1588 individuals volunteered, who were estimated to be 50% of all the expected intravenous drug users in the community. HBs Ag was detected in 4.2% of them. HBc Ab and HBs Ab were detected in order in 11.4 and 17.3%, respectively.
Conclusion:
We estimated that the seroprevalence of hepatitis B positivity in intravenous drug users was moderate to high. Therefore, it was suggested that this group be encouraged to prevent acquiring infection by vaccination, education, counseling for risk reduction, and treatment of substance abuse, and finally hepatitis B virus (HBV) screening.
Keywords: Hepatitis B, history of IVDU, Iran
INTRODUCTION
Hepatitis B and its critical consequences and complications have become one of the universal problems.[1,2] Intravenous (IV) drug abuse is considered to be one of the most important risk factors in the transmission of hepatitis B. According to new evidence, there are about 200 million addicts worldwide, from which 13.2 million are intravenous drug users (IDUs) and more than 78% of these IDUs live in developing countries.[3,4,5]
In Iran, because of various reasons, including Afghan refugees, the prevalence of IDUs has increased.[6,7,8]
Hepatitis B is one of the most significant health problems worldwide, because of its progression to chronic hepatitis, cirrhosis, and hepatocellular carcinoma. In addition to that, its related morbidity, mortality, and costs are very high.
The worldwide prevalence of HBV infections is beyond 2,000,000 individuals, of whom 45% are Asians.[9]
The most important mode of transmission of the hepatitis B virus is through the blood and its products. Also it can transmit with uncontrolled sexual contact and from mother to her fetus prenatally.[10]
Intravenous drug use is a major contributor and one of the most prevalent modes of transmission and IDUs are one of the high-risk groups for hepatitis B infection.[11]
As most patients with hepatitis B are asymptomatic and unaware of their infection, they can spread the infection among their family members and general population with risky parenteral and non-parenteral exposures such as common syringe use and sexual contacts.
It seems that screening of high-risk groups for HBV infection is very useful, both for the infected patients and society.
Studies show that treatment of hepatitis B in the early course of infection may prevent liver injury and its related complications.[12]
In order to assess an effective program for controlling HBV infection and reducing injury, screening for HBV infection in high-risk groups, especially IDUs, is needed.[13]
The studies in Iran and other countries for determining the prevalence of HBV infection have been conducted in particular groups, such as prisoners, although an up-to-date community-based study for hepatitis B screening among IDUs or people with a background of IDU has not been conducted.[14,15] As the prisoner may be at increased risk of Hepatitis B, this study was performed to determine the seroepidemiology of hepatitis B markers is persons with an IVDU history, through a community announcement–based study, for the first time in Iran.
MATERIALS AND METHODS
In this cross-sectional study, volunteers from the Isfahan province, with a history of intravenous drug use, enrolled. The protocol was approved by the Institutional Review Board of the Isfahan Infectious and Tropical Disease Research Center and Medical Ethics Committee of the Isfahan University of Medical Sciences.
At the first step, six focus groups were formed to determine the methods to identify the IDUs and to assess the hepatitis B seroprevalence among them.
The method of choice for this purpose was determined by using content analysis and member check methods. After that, the protocol of the study was designed and two pilot studies were carried out in two cities (Tiran and Golpayegan) of the Isfahan province to design the main study.
According to the protocol, all the IDUs and those with a background of IDU were invited to refer to the reference laboratory of each city for HBV serology tests.
The Laboratory staff in all the cities was trained for the appropriate contact with the referees.
Written informed consent was obtained from each volunteer, with the assurance that all obtained information would be just for research purposes and would remain confidential.
Five milliliters of venous blood samples were obtained from each person and centrifuged and the sera were separated and transferred to sterile tubes having the corresponding codes.
The extracted sera were stored at-20°C, until the specimen collection time was completed. After that period, all the stored sera were transferred to the laboratory of the Isfahan Infectious and Tropical Diseases Research Center for laboratory processing.
HBs-Ag, HBc-Ag, and HBs-Ab were detected using the ELISA method with the Diapro kit predicted in Italy (DIA.PRO, Italy).
The progress of the project in each city was supervised by the Hepatitis Committee of each city and reported to the Disease Control Unit of the Isfahan Province Health Center.
The obtained data was analyzed using the SPSS (version 15, SPSS Inc, Chicago, IL) software.
The tests conducted on the persons involved in the project was collected and analyzed by content analysis.
RESULTS
In this study, 1,588 individuals, who were estimated to be 50% of all the expected intravenous drug users in the community presented themselves.
HBs Ag was detected in 4.2% of the volunteers. The prevalence of total positive HBc Ab and HBs Ab was in the order of 11.4 and 17.3%, respectively. Volunteers with positive HBs Ag and positive HBc Ag were referred to the Diseases Control Unit of the Isfahan Province Health Center for complementary tests, education, and maybe treatment, if needed.
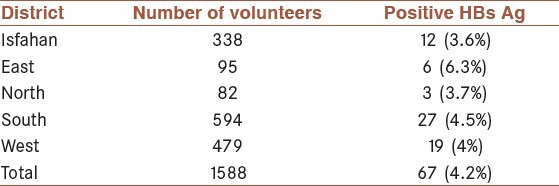
The prevalence of HBs Ag in IDUs or those people with a background history of IDU was more common in the east district of the Isfahan province (6.3%). The results with regard to the prevalence of HBs Ag in IDUs or in those people with a background history of IDU, in Isfahan city and the four districts of the Isfahan province have been shown in Table 1.
Table 1.
The prevalence of HBs Ag in Intravenous drug users (IDUs) or in those people with a background history of IDU in Isfahan city and the four districts of the Isfahan province
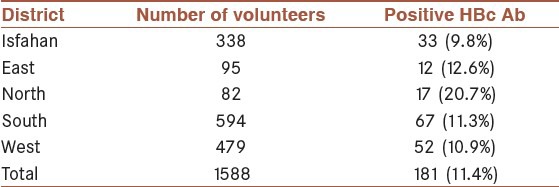
Positive HBc Ab was more common in the IDUs who lived in the southern district of the Isfahan province (11.3%) [Table 2].
Table 2.
The prevalence of HBc Ab in the total Intravenous drug users (IDUs) or in those people with a background history of IDU in Isfahan city and the four districts of the Isfahan province
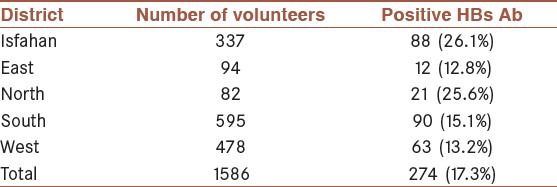
The prevalence of HBs Ab was also high in the southern district of the Isfahan province (15.1%) [Table 3].
Table 3.
The prevalence of HBs Ab in the total Intravenous drug users (IDUs) or in those people with background history of IDU in Isfahan city and the four districts of the Isfahan province
DISCUSSION
In this community announcement — based study, the seroprevalence of hepatitis B was assessed among IDUs or among those people with a background of IDU, in the city of Isfahan, in Iran. The results indicated that 4.2% of the studies population was HBs Ag positive and 11.4% of them had positive HBc Ab. HBs Ab was detected in 17.3%. All cases were asymptomatic.
It is estimated that 3,400 IDUs or those people with a background of IDU are living in the Isfahan province, except for those identified in prisons, drop-in centers (DIC), and high-risk centers.
Fifty percent of the estimated IDUs were studied. Several reports exist on the seroprevalence of hepatitis B among IDUs in the different cities of Iran and different countries worldwide. However this study was the first community announcement — based study in Iran. Therefore, the results of this study would be helpful in designing further nationwide studies.
Alavi et al., in Ahvaz, investigated the seroprevalence of HBV among hospitalized intravenous drug users.[16] According to their results, out of a total of 333 IDUs, 12 patients (3.6%) had HBV infection. It was associated with sharing of injection equipment, long duration of drug usage, long duration of the prison stage, HIV co-infection, and history of surgery, blood, and blood product transfusion.
Camoni et al., in Italy, showed that the prevalence of HBV among injecting drug users treated within the public drug treatment area was 70.4%.[17]
Stark et al., in Berlin, showed that in prisoners with a history of syringe sharing, of all the IDUs, 62% had serologic evidence of HBV infection and only 5% had serologic evidence of HBV vaccination; 7% were HBs Ag positive, and 18 of these were also HBe Ag positive.[18]
In our study 32.9% had serologic evidence of HBV infection, only 4.2% were HBs Ag positive but 11.4% of them had positive HBc Ab. However, it was not clear if these HBc Ab positive patients were in fact low-level hepatitis B carriers or if it was the evidence of hepatitis B from the remote past; 17.3% of all IDUs were HBs Ab positive. It was also not clear if it was because of immunization with HBs Ag (after vaccination) or if it was evidence of hepatitis B from the remote past. Further studies might be needed in this regard.
The findings of the current study indicated that there were also asymptomatic cases of HBV infection among the general population. The seroprevalence of hepatitis B positivity in intravenous drug users was moderate to high. Therefore, it was suggested that this group be encouraged to prevent acquiring infection, by vaccination, education, counseling for risk reduction, treatment of substance abuse, and finally HBV screening.
Limitations of the study
Lack of information about the vaccination history and length of IVDU may have been a limitation for better analysis of the data.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Kalantari H, Davari M, Akbari M, Hejazi SM, Kalantari M, Zakerin S, et al. The Estimation of Direct Medical Costs of Treating Patients with Chronic Hepatitis B and C in Iran. Int J Prev Med. 2012;3:191–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Alavian SM, Tabatabaei SV, Ghadimi T, Beedrapour F, Kafiabad SA, Gharehbaghian A, et al. Seroprevalence of HBV Infection and Its Risk Factors in the West of Iran: A Population-based Study. Int J Prev Med. 2012;3:770–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Des J, Semaan S. HIv prevention for injecting drug users: The first 25 years and counting. Psychosom Med. 2008;70:606–1. doi: 10.1097/PSY.0b013e3181772157. [DOI] [PubMed] [Google Scholar]
- 4.Jarlais Des DC, Seman S. HIV prevention in injection drug users: 25 years and counting. Psychosom Med. 2008;70:606–11. doi: 10.1097/PSY.0b013e3181772157. [DOI] [PubMed] [Google Scholar]
- 5.Nobari RF, Meshkati M, Ataei B, Yazdani MR, Hiedari K, Kassaian N, et al. Identification of patients with hepatitis C Virus Infections in persons. With background of intravenous drug use: The first Community Announcement-Based Study form Iran. Indian J Pathol Microbiol. 2012;(Special Issue):170–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Mojtahedzadeh V, Razami N, Malekinejad M, Vazirian M, Shoaee S, Saberi Zafarghandi MB, et al. Injection drug use in Rural Iran: Integrating HIV prevention into iran's rural primary health care system. AIDS Behav. 2008;12(4 Suppl):S7–12. doi: 10.1007/s10461-008-9408-y. [DOI] [PubMed] [Google Scholar]
- 7.Razzaghi EM, Movaghar AR, Green TC, Khoshnood K. Profiles of risk: A qualitative study of injecting drug users in Tehran, Iran. Harm Reduct J. 2006;3:12. doi: 10.1186/1477-7517-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Drug Report. Vienna: UNODC; 2005. United Nations Office in Drugs and Crime. [Google Scholar]
- 9.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–45. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 10.Nair S, Perillo R. Hepatitis B virus epidemiology, diseases burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 11.Robotin MC, Percher A, Mednger M, Drers J. Hepatitis B prevention and Control: Lessons from the east and the west. World J Hepatol. 2011;3:31–7. doi: 10.4254/wjh.v3.i2.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sydnor ER, Perl TM. Hospiral epidemiology and infection control in acute care settings. Clin Microbiol Rev. 2011;24:141–73. doi: 10.1128/CMR.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirahmadizadeh AR, Kadivar MR, Hemmati AR, Javadi A. Infections with HIV and hepatitis C and B viruses among injecting drug users in Shiraz, Southern Iran. Int Conf AIDS. 2004;15:16. [Google Scholar]
- 14.Rowhani Rahbar A, Rooholamini S, Khoshnood K. Prevalence of HIV infection and other blood borne infections in incarcerated and non incarcerated injecting drug users (IDUs) in Mashhad, Iran. Intraven J Drug Pol. 2004;15:151–5. [Google Scholar]
- 15.Imani R, Karimi A, Rouzbahani R, Rouzbahani A. Seroprevalence of HBV, HCV and HIV infection among intravenous drug users in Shahrekord, Islamic Republic of Iran. East Mediterr Health J. 2008;14:1136–41. [PubMed] [Google Scholar]
- 16.Alavi SM, Behdad F. Seroprevalence Study of Hepatitis B virus among Hospitalized Intravenous Drug Users in Ahvaz, Iran (2002-2006) Hepat Mon. 2010;10:101–4. [PMC free article] [PubMed] [Google Scholar]
- 17.Camoni L, Regine V, Salfa MC, Nicoletti C, Conuzzi P, Magliocchetti N, et al. Continued high prevalence of HIV, HBV and HCV among injecting and noninjecting drug users in Italy. Ann Isf Super Sanita. 2010;46:59–65. doi: 10.4415/ANN_10_01_08. [DOI] [PubMed] [Google Scholar]
- 18.Stark K, Bienzle U, Vonk R, Guggenmoos – Holzmonn I. History of syringe sharing in Prison and Risk of Hepatitis B virus, Hepatitis C virus, and Human Immunodeficiency Virus Infection among Injecting Drug Users in Berlin. Int J Epidemiol. 1995;26:1356–66. doi: 10.1093/ije/26.6.1359. [DOI] [PubMed] [Google Scholar]


