Abstract
The incidence of a cancer diagnosis in children and young adolescents is increasing. With better treatments, the number of young cancer survivors living through reproductive age is increasing. Fertility preservation of these men and women has become essential and needs to be discussed prior to the start of cancer treatment. Here we review the current guidelines for male oncofertility patients and highlight some of the important gonadotoxic effects of chemotherapy, radiotherapy and surgery. Options for fertility preservation are also discussed along with resources that should be made available to all patients.
Keywords: Cancer, fertility, fertility preservation, infertility
INTRODUCTION
It is estimated that one out of every two men will be diagnosed with cancer in his lifetime, of which 4% are under the age of 35.[1] Traditionally, the role of cancer treatment has focused on disease cure, however with advances in treatment efficacy and safety; there are a growing number of young adults who are long-term survivors of cancer. Patients under 15 years of age undergoing cancer treatment are projected to have a 75% five-year cancer survival rate.[2] Patients between the ages of 15-44 with a diagnosis of cancer are now projected to have a survival rate of 66%.[3] It has been estimated that 1 in 700 young adults is a cancer survivor[4] and by 2010, 1 in every 250 adults will be a childhood cancer survivor.[5] With the increasing incidence of this group of patients, fertility potential has emerged as a core survivorship concern.[6]
Fertility can be compromised both with cancer pathophysiology and treatment options.[7] While infertility may be reversible for some treatment regimens, persistent infertility may occur in 50-95% of malignancies, therefore a discussion surrounding potential fertility preservation options is paramount.[8,9] In addition, the desire for fatherhood later in life, the rise of divorce and second family offspring, and the unexpected death of spouses are factors that have influenced men to have offspring later in life.[10] Hence, health care professionals should not assume that older men have completed their families and must address the impact of disease and treatment on their reproductive health. In this review, we present a comprehensive overview of the impact of cancer treatment on male fertility and methods used to ensure adequate fertility preservation.
The effects of cancer therapies on male fertility
It is universally accepted that all cancer treatment options can negatively impact fertility. Normal spermatogenesis and sex hormone production may be disrupted by radiotherapy, chemotherapy, stem cell transplantation, and surgery. While each of these treatment options has different risks and benefits, patients should receive careful explanations of the impact, they may have on future fertility.[6]
The effects of radiation therapy on male fertility
Radiation therapy has been utilized in the treatment of many cancers including prostate, bladder, penile, testicular, and rectal cancer. Radiotherapy is one of the oldest forms of cancer treatment and its delivery has changed drastically from its inception by decreasing its associated morbidity. For example, radiation delivery has improved drastically in the treatment of prostate cancer. Initial modalities included conventional external beam radiotherapy and have since improved to lessen scatter radiation with 3-dimensional conformal radiotherapy and most recently, intensity-modulated radiotherapy.[11,12] Despite these advances, radiation therapy can still have irreversible effects on testicular function and fertility primarily. Similarly, even with a dose fractionation schedule, semen parameters may be affected.[13] Despite smaller single doses of radiation administered in multiple treatments, some authors have even reported worse semen parameters with fractionated radiation.[14]
The testis is one of the most sensitive organs in the body to radiation because of the rapidly dividing germinal epithelium. Immature spermatogonia are most sensitive to radiation injury, while Leydig cells are more resilient to doses as high as 20 Gy. When Leydig cell damage occurs, testosterone production decreases resulting in a concomitant increase in LH levels.[15] Radiation-induced testicular dysfunction occurs in a dose-dependent fashion.[13] Small doses as low as 0.1 Gy can affect the histological shape and number of spermatogonia, while exposure to 2-3 Gy leads to a significantly altered number of spermatids.[13] Similarly, a dose-dependent relationship is seen with regards to sperm concentration within the ejaculate. Radiation doses less than 0.8 Gy could lead to oligospermia, 0.8-2 Gy could lead to transient azoospermia and doses greater than 2 Gy could lead to irreversible azoospermia.[13] Damage to spermatogenesis could result either from direct radiation to the testis or scatter radiation used in the treatment of cancers below the diaphragm. The testes may receive as much as 18.7% of the administered radiation in pelvic cancers, with rectal cancer being amongst the highest scatter doses to the testes.[16] On average, return to pre-radiation semen parameters can be seen within 10-24 months; however, a prolonged recovery is seen with larger doses of radiation.
While alterations in semen analysis secondary to radiation therapy are proven, the impact of DNA damage from radiation is less concrete. There is an increase in DNA fragmentation in men with testicular carcinoma receiving adjuvant radiation up to 2 years after treatment, but the clinical impact of these results is unclear.[17]
The effects of chemotherapy on male fertility
Chemotherapeutic agents have deleterious effects on spermatogenesis. Similar to radiation therapy, Leydig cells may incur damage following chemotherapy resulting in subsequent hypogonadism.[18] However, with advances in chemotherapy delivery, side effects have been minimized using synergistic agents at lower toxic doses but a risk of infertility is still present. The extent of gonadal damage is largely dependent on the type, the age of the patient, and the extent of the chemotherapeutic agent administered. Table 1 highlights some common chemotherapeutic agents and their effect on spermatogenesis.
Table 1.
The impact of common chemotherapeutics on male fertility
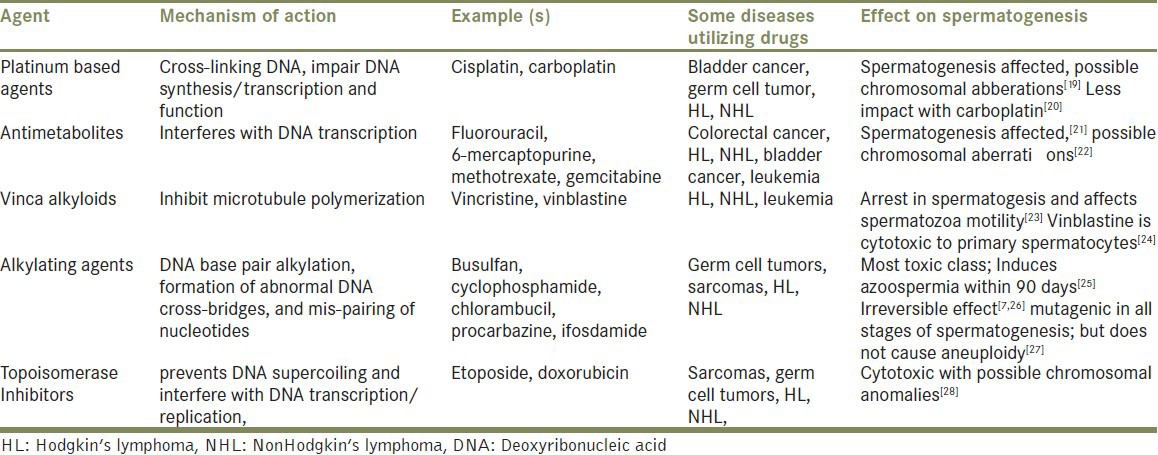
Combination chemotherapies
For certain cancers, combination chemotherapies are the mainstay of treatment; for instance, common regimens include MOPP (mechlorethamine, oncovin/vincristine, procarbazine and prednisone) for Hodgkin's disease, MVAC (methotrexate, vinblastine, adriamycin and cisplatin) for bladder cancer, and R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) for non-Hodgkin's lymphoma. MOPP causes azoospermia in 90% of men up to 4 years after their treatment regimen as well as increased rates of aneuploidy.[29] The combination of bleomycin, etoposide, and cisplatin (BEP) commonly used in testicular cancer was found to have increased sperm chromosomal anomalies[30] and sperm aneuploidy was seen with NOVP (novantrone/mitoxantrone, oncovin/vincristine, vinblastine and prednisone) chemotherapy.[31] The combination of chemotherapy while working synergistically on cancer cells also has a detrimental impact on the rapidly dividing germinal epithelium of the testis and patients must be counseled on the risks of permanent azoospermia following treatment.
The effects of surgery on male fertility
As part of many initial cancer treatment protocols, surgery plays an important role in achieving cancer cure. However, like radiation therapy or chemotherapy, surgery has its associated risks with respect to infertility. For aggressive testicular cancer, a retroperitoneal lymphadenectomy may be performed. Such an operation may cause an ejaculation or retrograde ejaculation due to disruption of the lumbar sympathetic plexus and hypogastic plexus.[32] To best prevent these consequences, modified nerve-sparing procedures are performed with a reported ejaculatory preservation of 50-85% in men.[33]
Disruption of the genitourinary system is unavoidable in either cystectomy or prostatectomy. Sperm production is not impaired in such procedures, only the genital ducts that transport sperm into the prostatic urethra. In addition, erectile function may be compromised. However, with avoidance of nerve fibers in the neurovascular bundles that innervate the corpora cavernosa, 70% of men who underwent either a “nerve-sparing” radical prostatectomy or radical cystoprostatectomy maintained erectile function.[34] A discussion with patients surrounding the potential loss of erectile function, and more importantly the permanent loss of fertility, should routinely be done.
Strategies for Fertility Preservation
Onco-fertility: A new paradigm in survivorship
It has been well documented that there is a reluctance to discuss the effects of cancer therapies on fertility, especially when the patient is a single male in his adolescence or young adulthood.[35] Over the past decade, there has been substantial advocacy for fertility preservation in this young group of patients. In 2004, the Presidents’ Cancer Panel published a series of guidelines urging all physicians to speak to their patients about the effects of cancer treatment strategies on their reproductive potential. In 2006, the American Society of Clinical Oncology (ASCO) echoed this recommendation with an additional report advocating for an early dialogue regarding fertility preservation during an initial discussion about a new cancer diagnosis; if there is interest, these patients should be referred to a fertility preservation specialist.[36]
Fortunately, over the last several years, many groups advocating for fertility preservation have emerged. Among them is the Oncofertility Consortium, which is a national, interdisciplinary initiative designed to explore the reproductive future of cancer survivors.[37] This international network consists of physicians, scientists, and scholars who are providing awareness and resources to all patients battling with cancer and interested in future fertility. Additional national organizations that have been instrumental in shaping the paradigm for fertility preservation include the Livestrong Foundation, Fertile Hope, The American Society for Reproductive Medicine, and The Endocrine Society.
Despite these national initiatives, barriers continue to exist on a daily basis, further advocating for an awareness of fertility preservation at a local level. Reassuringly, a greater number of institutions are developing their own programs to target this concern and provide ample resources for patients and providers. Specific initiatives that have been shown to amplify access to care include creation of a “patient navigator” position or having certain skilled personnel readily available to provide information to patients and families on sperm cryopreservation.[38] At Northwestern University, we recently demonstrated how a formalized male fertility preservation program led to a 2.4- and 2.7-fold annual increase in the number of men who underwent formal fertility preservation consultation and sperm cryopreservation.[39] Anecdotally, we have also noticed that we have been referred male cancer patients more consistently across all ages and malignancy subtypes.
Methods of sperm collection preceding initiation of cancer therapies
For most patients seeking cryopreservation, the semen sample is collected via masturbation. The patient should be allowed ample time and privacy, and he may collect his sample with a sterile specimen cup. It is important to avoid the use of lubricants as these substances are often spermatotoxic. This is the preferred method as it provides the best quality sperm at the lowest financial cost.[32] Should no ejaculate be achieved or if the patient has a history of retrograde ejaculation, a post-ejaculate urinalysis should be done to assess for sperm in the urine. If sperm are present in the urine sample, a trial of alpha agonists may be administered to direct ejaculate forwards. If this is unsuccessful, alkalization of the urine followed by urine collection and processing of the post-ejaculate urine specimen can be done to isolate viable sperm.
Because cytotoxic chemotherapies target rapidly proliferating cells, it has been postulated that altering hypothalamic-pituitary axis with gonadotropin releasing hormone antagonists may prevent long-term infertility. However, it has been shown that hormonal therapy is not successful when men receive high doses of chemotherapy.[40,41] Furthermore, hormonal therapy does not speed up the recovery of spermatogenesis.[42]
If the patient is unable to ejaculate, then other techniques can be performed. These include vibratory stimulation and electro-ejaculation in an effort to induce ejaculation.[32] For azoospermic men, more invasive surgical techniques for sperm extraction such as microsurgical testicular sperm extraction (microTESE) or microsurgical epididymal sperm aspiration (MESA) are performed prior to cancer therapy. Prior to chemotherapy, patients may be rendered azoospermic as a result of their testicular tumor burden. These men may undergo “Onco-TESE” (Oncological Testicular Sperm Extraction). Schrader et al. first described this procedure using microsurgical dissection and extraction of the seminiferous tubules with selected tubules examined on a wet prep slide under microscopy for viable sperm.[43] This technique was shown to yield viable sperm in 6 of 14 men with testicular germ cell tumors and 8 of 17 men with malignant lymphoma.[43] In another recent cohort, four out of six men undergoing an onco-TESE had sperm in their testicular tissue and two were able to father a child following IVF with this sperm extracted during onco-TESE.[44]
For prepubertal boys, the options for fertility preservation are currently investigatory.[45] Research focusing on stem cell transplantation is under review with experimental work on in vitro generation of sperm from harvested spermatogonial stem cells and preservation of immature testicular tissue.[6,46]
Sperm preservation and fertilization methods
After successful collection, patients have the option of sperm cryopreservation prior to chemotherapy, radiotherapy, and/or surgery that may potentially affect reproductive organs. This induces a “suspended animation state” for storage as long as 15 years.[34] Unfortunately, prior literature demonstrating low pregnancy rates with cryopreserved sperm using intrauterine insemination (IUI) has tainted much of clinical decision-making.[47,48] With the advent of IVF and intracytoplasmic sperm injection (ICSI), sperm cryopreservation allows achievable pregnancy using testicular or epididymal sperm, or those with less optimal motility and/or morphology.
While these techniques have success rates up to 40%, men may have several reasons for not proceeding with cyropreservation. For example, there may be no time to bank because of many other pressing health issues, or in rare instances there may be no viable sperm for freezing. Moreover, the patient may be on a fertility-friendly protocol and may not be recommended for banking. Finally, survival from cancer may be the focus of care rather than the potential outcomes after treatment. Ultimately, it is the patient's decision whether to pursue fertility options; however, it remains prudent for the provider to explain the aforementioned collection and preservation options, and provide resources for the patient interested in his future fertility.
CONCLUSION
Male factor infertility is a known side effect of cancer therapies. While the precise impact of cancer therapy on fertility is dependent largely on the therapeutic regimen, most cancer treatment modalities will undeniably have a detrimental effect on male reproductive potential. Because men are living longer following a cancer diagnosis due to improved cancer diagnostics and therapeutics, the option for fertility preservation must be discussed at the time of diagnosis. All patients should be thoroughly educated about the impact of treatment on their reproductive capacity and provided with ample resources regarding preserving their future fertility potential. Interested patients should be directed to a specialist in fertility preservation.
Footnotes
Source of Support: Nil
Conflict of Interest: None
REFERENCES
- 1.Institute, N.C. Surveillance epidemiology and end results. Cancer Statistics Review. 2009. [Last cited on 2012 Dec 21]. Available from: http://www.seer.cancer.gov/statfacts .
- 2.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. 1. [DOI] [PubMed] [Google Scholar]
- 3.Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, et al. EUROCARE-3: Survival of cancer patients diagnosed 1990-94--results and commentary. Ann Oncol. 2003;14(Suppl 5):v61–118. doi: 10.1093/annonc/mdg754. [DOI] [PubMed] [Google Scholar]
- 4.Muller J. Impact of cancer therapy on the reproductive axis. Horm Res. 2003;59(Suppl 1):12–20. doi: 10.1159/000067835. [DOI] [PubMed] [Google Scholar]
- 5.Blatt J. Pregnancy outcome in long-term survivors of childhood cancer. Med Pediatr Oncol. 1999;33:29–33. doi: 10.1002/(sici)1096-911x(199907)33:1<29::aid-mpo6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Holoch P, Wald M. Current options for preservation of fertility in the male. Fertil Steril. 2011;96:286–90. doi: 10.1016/j.fertnstert.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: Damage and recovery. J Natl Cancer Inst Monogr. 2005;34:12–7. doi: 10.1093/jncimonographs/lgi003. [DOI] [PubMed] [Google Scholar]
- 8.Hendry WF, Stedronska J, Jones CR, Blackmore CA, Barrett A, Peckham MJ. Semen analysis in testicular cancer and Hodgkin's disease: Pre- and post-treatment findings and implications for cryopreservation. Br J Urol. 1983;55:769–73. doi: 10.1111/j.1464-410x.1983.tb03423.x. [DOI] [PubMed] [Google Scholar]
- 9.Selby P, Brada M, Horwich A, Wiltshaw E, McElwain TJ, Lindsay KS. Semen cryopreservation for patients surviving malignant disease: Implications of proposed legislation. Lancet. 1988;2:1197. doi: 10.1016/s0140-6736(88)90266-8. [DOI] [PubMed] [Google Scholar]
- 10.Ruggles S. The rise of divorce and separation in the United States, 1880-1990. Demography. 1997;34:455–79. doi: 10.2307/3038300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheets NC, Goldin GH, Meyer AM, Wu Y, Chang Y, Stürmer T, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–20. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bekelman JE, Mitra N, Efstathiou J, Liao K, Sunderland R, Yeboa DN, et al. Outcomes after intensity-modulated versus conformal radiotherapy in older men with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81:e325–34. doi: 10.1016/j.ijrobp.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shalet SM. Effect of irradiation treatment on gonadal function in men treated for germ cell cancer. Eur Urol. 1993;23:148–52. doi: 10.1159/000474584. [DOI] [PubMed] [Google Scholar]
- 14.Ash P. The influence of radiation on fertility in man. Br J Radiol. 1980;53:271–8. doi: 10.1259/0007-1285-53-628-271. [DOI] [PubMed] [Google Scholar]
- 15.Giwercman A, von der Maase H, Berthelsen JG, Rørth M, Bertelsen A, Skakkebaek NE. Localized irradiation of testes with carcinoma in situ: Effects on Leydig cell function and eradication of malignant germ cells in 20 patients. J Clin Endocrinol Metab. 1991;73:596–603. doi: 10.1210/jcem-73-3-596. [DOI] [PubMed] [Google Scholar]
- 16.Hermann RM, Henkel K, Christiansen H, Vorwerk H, Hille A, Hess CF, et al. Testicular dose and hormonal changes after radiotherapy of rectal cancer. Radiother Oncol. 2005;75:83–8. doi: 10.1016/j.radonc.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Ståhl O, Eberhard J, Jepson K, Spano M, Cwikiel M, Cavallin-Ståhl E, et al. The impact of testicular carcinoma and its treatment on sperm DNA integrity. Cancer. 2004;100:1137–44. doi: 10.1002/cncr.20068. [DOI] [PubMed] [Google Scholar]
- 18.Howell SJ, Radford JA, Ryder WD, Shalet SM. Testicular function after cytotoxic chemotherapy: Evidence of Leydig cell insufficiency. J Clin Oncol. 1999;17:1493–8. doi: 10.1200/JCO.1999.17.5.1493. [DOI] [PubMed] [Google Scholar]
- 19.Choudhury RC, Jagdale MB, Misra S. Potential transmission of the cytogenetic effects of cisplatin in the male germline cells of Swiss mice. J Chemother. 2000;12:352–9. doi: 10.1179/joc.2000.12.4.352. [DOI] [PubMed] [Google Scholar]
- 20.Lampe H, Horwich A, Norman A, Nicholls J, Dearnaley DP. Fertility after chemotherapy for testicular germ cell cancers. J Clin Oncol. 1997;15:239–45. doi: 10.1200/JCO.1997.15.1.239. [DOI] [PubMed] [Google Scholar]
- 21.D’Souza UJ. Re: 5-Fluorouracil-induced sperm shape abnormalities in rats. Asian J Androl. 2003;5:353. [PubMed] [Google Scholar]
- 22.Bokemeyer C, Schmoll HJ, van Rhee J, Kuczyk M, Schuppert F, Poliwoda H. Long-term gonadal toxicity after therapy for Hodgkin's and non-Hodgkin's lymphoma. Ann Hematol. 1994;68:105–10. doi: 10.1007/BF01727413. [DOI] [PubMed] [Google Scholar]
- 23.Arnon J, Meirow D, Lewis-Roness H, Ornoy A. Genetic and teratogenic effects of cancer treatments on gametes and embryos. Human Reprod Update. 2001;7:394–403. doi: 10.1093/humupd/7.4.394. [DOI] [PubMed] [Google Scholar]
- 24.Sjoblom T, Parvinen M, Lahdetie J. Stage-specific DNA synthesis of rat spermatogenesis as an indicator of genotoxic effects of vinblastine, mitomycin C and ionizing radiation on rat spermatogonia and spermatocytes. Mutat Res. 1995;331:181–90. doi: 10.1016/0027-5107(95)00067-s. [DOI] [PubMed] [Google Scholar]
- 25.Byrne J, Mulvihill JJ, Myers MH, Connelly RR, Naughton MD, Krauss MR, et al. Effects of treatment on fertility in long-term survivors of childhood or adolescent cancer. N Engl J Med. 1987;317:1315–21. doi: 10.1056/NEJM198711193172104. [DOI] [PubMed] [Google Scholar]
- 26.Buchanan JD, Fairley KF, Barrie JU. Return of spermatogenesis after stopping cyclophosphamide therapy. Lancet. 1975;2:156–7. doi: 10.1016/s0140-6736(75)90059-8. [DOI] [PubMed] [Google Scholar]
- 27.Witt KL, Bishop JB. Mutagenicity of anticancer drugs in mammalian germ cells. Mutat Res. 1996;355:209–34. doi: 10.1016/0027-5107(96)00029-2. [DOI] [PubMed] [Google Scholar]
- 28.van Buul PP, Goudzwaard JH. Bleomycin-induced structural chromosomal aberrations in spermatogonia and bone-marrow cells of mice. Mutat Res. 1980;69:319–24. doi: 10.1016/0027-5107(80)90096-2. [DOI] [PubMed] [Google Scholar]
- 29.Genescà A, Caballín MR, Miró R, Benet J, Bonfill X, Egozcue J. Human sperm chromosomes. Long-term effect of cancer treatment. Cancer Genet Cytogenet. 1990;46:251–60. doi: 10.1016/0165-4608(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 30.Martin RH, Ernst S, Rademaker A, Barclay L, Ko E, Summers N. Analysis of sperm chromosome complements before, during, and after chemotherapy. Cancer Genet Cytogenet. 1999;108:133–6. doi: 10.1016/s0165-4608(98)00125-3. [DOI] [PubMed] [Google Scholar]
- 31.Robbins WA, Meistrich ML, Moore D, Hagemeister FB, Weier HU, Cassel MJ, et al. Chemotherapy induces transient sex chromosomal and autosomal aneuploidy in human sperm. Nat Genet. 1997;16:74–8. doi: 10.1038/ng0597-74. [DOI] [PubMed] [Google Scholar]
- 32.Stahl PJ, Stember DS, Mulhall JP. Options for fertility preservation in men and boys with cancer. Adv Exp Med Biol. 2012;732:29–39. doi: 10.1007/978-94-007-2492-1_3. [DOI] [PubMed] [Google Scholar]
- 33.Maltaris T, Koelbl H, Seufert R, Kiesewetter F, Beckmann MW, Mueller A, et al. Gonadal damage and options for fertility preservation in female and male cancer survivors. Asian J Androl. 2006;8:515–33. doi: 10.1111/j.1745-7262.2006.00206.x. [DOI] [PubMed] [Google Scholar]
- 34.Puscheck E, Philip PA, Jeyendran RS. Male fertility preservation and cancer treatment. Cancer Treat Rev. 2004;30:173–80. doi: 10.1016/j.ctrv.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Redig AJ, Brannigan R, Stryker SJ, Woodruff TK, Jeruss JS. Incorporating fertility preservation into the care of young oncology patients. Cancer. 2011;117:4–10. doi: 10.1002/cncr.25398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 37.Woodruff TK. The oncofertility consortium--addressing fertility in young people with cancer. Nat Rev Clin Oncol. 2010;7:466–75. doi: 10.1038/nrclinonc.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott-Trainer J. The role of a patient navigator in fertility preservation. Cancer Treat Res. 2010;156:469–70. doi: 10.1007/978-1-4419-6518-9_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheth KR, Sharma V, Helfand BT, Cashy J, Smith K, Hedges JC, et al. Improved fertility preservation care for male patients with cancer after establishment of formalized oncofertility program. J Urol. 2012;187:979–86. doi: 10.1016/j.juro.2011.10.154. [DOI] [PubMed] [Google Scholar]
- 40.Johnson DH, Linde R, Hainsworth JD, Vale W, Rivier J, Stein R, et al. Effect of a luteinizing hormone releasing hormone agonist given during combination chemotherapy on posttherapy fertility in male patients with lymphoma: Preliminary observations. Blood. 1985;65:832–6. [PubMed] [Google Scholar]
- 41.Waxman JH, Ahmed R, Smith D, Wrigley PF, Gregory W, Shalet S, et al. Failure to preserve fertility in patients with Hodgkin's disease. Cancer Chemother Pharmacol. 1987;19:159–62. doi: 10.1007/BF00254570. [DOI] [PubMed] [Google Scholar]
- 42.Brennemann W, Brensing KA, Leipner N, Boldt I, Klingmüller D. Attempted protection of spermatogenesis from irradiation in patients with seminoma by D-Tryptophan-6 luteinizing hormone releasing hormone. Clin Investig. 1994;72:838–42. doi: 10.1007/BF00190737. [DOI] [PubMed] [Google Scholar]
- 43.Schrader M, Müller M, Sofikitis N, Straub B, Krause H, Miller K. “Onco-tese”: Testicular sperm extraction in azoospermic cancer patients before chemotherapy-new guidelines? Urology. 2003;61:421–5. doi: 10.1016/s0090-4295(02)02264-1. [DOI] [PubMed] [Google Scholar]
- 44.Furuhashi K, Ishikawa T, Hashimoto H, Yamada S, Ogata S, Mizusawa Y, et al. Onco-testicular sperm extraction: Testicular sperm extraction in azoospermic and very severely oligozoospermic cancer patients. Andrologia. 2013;45:107–10. doi: 10.1111/j.1439-0272.2012.01319.x. [DOI] [PubMed] [Google Scholar]
- 45.Stensvold E, Magelssen H, Oskam IC. Fertility-preserving measures for boys and young men with cancer. Tidsskr Nor Laegeforen. 2011;131:1433–5. doi: 10.4045/tidsskr.11.0426. [DOI] [PubMed] [Google Scholar]
- 46.Wyns C, Curaba M, Vanabelle B, Van Langendonckt A, Donnez J. Options for fertility preservation in prepubertal boys. Hum Reprod Update. 2010;16:312–28. doi: 10.1093/humupd/dmp054. [DOI] [PubMed] [Google Scholar]
- 47.Sanger WG, Armitage JO, Schmidt MA. Feasibility of semen cryopreservation in patients with malignant disease. JAMA. 1980;244:789–90. [PubMed] [Google Scholar]
- 48.Rosenlund B, Westlander G, Wood M, Lundin K, Reismer E, Hillensjö T. Sperm retrieval and fertilization in repeated percutaneous epididymal sperm aspiration. Hum Reprod. 1998;13:2805–7. doi: 10.1093/humrep/13.10.2805. [DOI] [PubMed] [Google Scholar]


