Abstract
Aim:
To report safety and efficacy of chemotherapy incorporating the combination of paclitaxel and platinum in patients with advanced penile carcinoma.
Materials and Methods:
Retrospective analysis of patient with advanced penile carcinoma undergoing palliative chemotherapy with paclitaxel and platinum combination. The demographic profile, indication of treatment, chemotherapy details, toxicity and survival outcome were noted. Statistical analysis was done for estimation of progression free survival and overall survival. Factors affecting these outcomes were sought for.
Results:
Eighteen patients with a median age of 47.5 years (31-68 years) were offered palliative intent chemotherapy over a period of 2.5 years. ECOG performance was 1 in 12 patients (66.7%) and 2 in 6 patients (33.3%). The grade of tumor was poorly differentiated in 8 patients (44.4%), moderately differentiated in 5 (27.8%) and we1l differentiated in 5 patients (27.8%). Twelve patients had previous surgical treatment (66.7%), with 2 of them having received groin radiation in past. The indication for treatment was metastatic disease in 7 patients (38.9%) and locally advanced disease in 11 patients (61.1%). Out of 18 patients 13 received chemotherapy. Paclitaxel and carboplatin combination was given in 10 patients (76.9%) while paclitaxel and cisplatin was received by 3 patients (23.1%). The median numbers of cycles received were 3 (1-6 cycles). Response rate was 30.8%. The median estimated progression free survival (PFS) and overall survival (OS) for patients receiving atleast one cycle of chemotherapy (n = 13) were 96 days and 246 days respectively. Among tested variables the median OS in patients who had received 2 or more cycles was 351 days versus 55 days in those who received less than 2 cycles (P = 0.025). However, after applying Bonferroni correction, the difference was no longer significant. There was no toxicity related death or life threatening complication.
Conclusion:
Our institutional protocol of platinum-based doublet with paclitaxel is effective, well-tolerated and has the advantage being delivered on an outpatient basis alone. Overall, we believe that paclitaxel-platinum is an effective regimen that needs to be investigated further in larger studies.
Keywords: Chemotherapy, metastatic penile carcinoma, palliative
INTRODUCTION
Squamous cell carcinoma of the penis commonly presents in a locally advanced stage.[1] While the incidence of stage IV disease at presentation varies from 6% to 13.4% across various studies, systemic spread occurs in locally advanced penile cancers in around 1.9% to 7% of cases.[2,3,4]
In stage IV disease and in locally advanced penile cancers which are not amenable for local treatment, chemotheray remains an option.
A number of chemotherapeutic agents including cisplatin, fluoropyrimidines, gemcitabine, paclitaxel, bleomycin, methotrexate and irinotectan have been used in different combinations and schedules with similar outcomes in patients with metastatic disease and unresectable locally advanced disease.[4] Though cisplatin-based chemotherapy is currently accepted as the standard treatment, the survival of these patients continues to be dismal. There is urgent unmet need for an effective and safe protocol for this group of patients.
Paclitaxel has been used in combination with platinum agents for adjuvant and as palliative chemotherapy in head and neck, ovarian and lung neoplasm's.[5,6,7] This is a well-tolerated protocol with defined toxicity profile and demonstatable activity in squamous cell carcinoma. However, aside from a single case report, there is a paucity of data regarding the use of taxanes in penile carcinoma.[8,9]
We report our experience of using the combination of paclitaxel and platinum compounds in patients with advanced and metastatic penile carcinoma.
MATERIALS AND METHODS
We conducted a retrospective analysis of patients with biopsy proven squamous cell carcinomas of penis, who were not amenable to loco-regional treatment. The cohort included patients who had relapsed after previous therapy and those patients with metastases at presentation. However, all patients were chemotherapy-naive. These patients were planned for chemotherapy with palliative intent after multidisciplinary clinic review.
Paclitaxel was administered in a dose of 175 mg/m2 given as an infusion over 3 hours. Cisplatin was administered over 3 days with adequate hydration at a dose of 25 mg/m2 per day in an infusion over 1-2 hours. If the creatinine clearance was less than 60 ml/min, patients received carboplatin at a dose of 5 times the AUC (area under curve). Standard premedication's were administered.
The chemotherapy was administered once every 21 days. The next cycle was administered after an adequate recovery of hematological parameters (absolute neutrophil count more than 1,500/cu.mm and platelet count more than 100,000/cu.mm). Response assessment with abdominal contrast-enhanced computer-assisted tomography was done after 2 cycles. The patients who had clinical improvement and stable disease or reduction in size of mass radiologically underwent further 2 cycles. Abdominal imaging was repeated after 4 cycles and chemotherapy was continued for 2 more cycles for all patients with non-progression radiologically or clinically. After 6 cycles, no further chemotherapy was given and patients were kept on regular observation.
The demographic details, indication of chemotherapy, radiological response to chemotherapy, toxicity in accordance with CTCAE version 4.02, date of progression and status at last follow-up was acquired from the prospectively filled database in our out-patient department. The data was checked for its accuracy from the individual case record files and electronic medical record system.
Statistical analysis
SPSS version 16 was used for analysis. Descriptive statistics have been performed. The Kaplan Meier method was used for estimation of survival. Progression-free survival (PFS) was calculated from the date of biopsy to date of progression. Overall survival (OS) was calculated from the date of biopsy to date of death. Log rank test was used to identify factors which would affect PFS and OS. Among the tested variables were age (above or below 50 years), performance status (PS 1 versus PS 2), grade (poorly differentiated versus others), previous treatment received, site of disease (locoregional versus metastasis) and number of cycles of chemotherapy received (one or more than 1. As multiple variables were tested, Bonferroni correction was applied for multiplicity. The significant post correction alpha value calculated was 0.008.[10]
RESULTS
Eighteen patients were identified between January 2008 and July 2011 [Figure 1]. All patients had squamous cell carcinoma. Patient baseline details prior to start of chemotherapy are shown in Table 1
Figure 1.
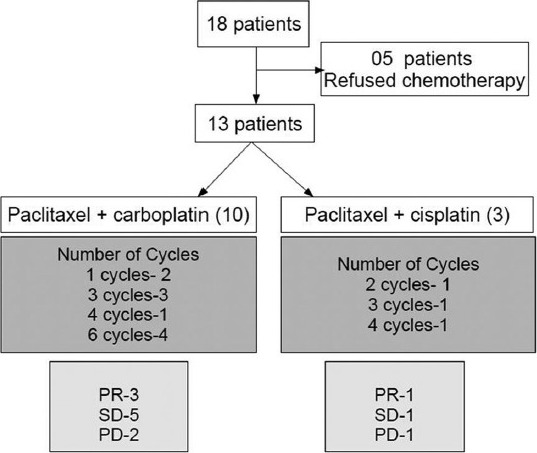
Patients showing the chemotherapy received, number of cycles and response
Table 1.
Baseline details of patients prior to start of palliative chemotherapy
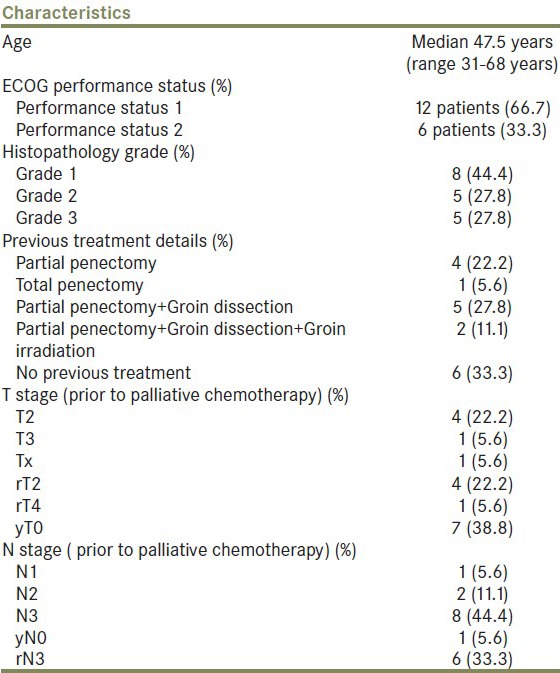
Twelve patients had previous treatment (66.7%) of these patients, 8 relapsed with only local unresectable recurrence, 1 had only metastatic recurrence (lung) and 3 had relapsed at both sites. The median disease free period from these previous treatments was 5.5 month (2-36 month). The indication for chemotherapy was metastatic disease in 7 patients (38.9%) and locally advanced disease not amenable to local treatment in 11 patients (61.1%). In these 11 patients, two patients had already undergone groin dissection and irradiation so were not eligible for any local modality, rest 9 patients were those who had extensive inguinal and pelvic lymph nodes were deemed unresectable by surgeons while there target volumes were considered to big to be safely encompassed in radiation portals. All of these decisions were done in a multidisciplinary joint clinic. The site of metastasis was the lung in 6 patients and the bones (pelvis) in 1 patient
All 18 patients were offered chemotherapy. However, 5 patients refused chemotherapy due to unwillingness and logistic issues. The remaining 13 patients received chemotherapy. Paclitaxel and carboplatin combination was given to 10 patients (76.9%) while paclitaxel and cisplatin was given to 3 patients (23.1%), depending on the serum creatinine clearance
The median number of cycles received was 3 (1-6 cycles). The number of chemotherapy cycles delivered was 1 in 2 patients, 2 in 1, 3 in 4, 4 in 2 and 6 in 4 patients. The best response achieved post chemotherapy and the distribution of patients according to the chemotherapy received is shown in Table 1. The response rate was 30.8%.
The cause of discontinuation of chemotherapy was progression of disease (5 patients), noncompliance of patients (3 patients) and intolerable side effects (1 patient). Among the noncompliant patients, one had regression in tumor size; however, he discontinued after 4 cycles of chemotherapy as he was not willing for further chemotherapy. The other two patients had stable disease and they discontinued after 3 cycles of chemotherapy; this was due to social and familial reasons. One patient had grade 3 nausea and vomiting in the first cycle of chemotherapy, leading to dehydration and hospital admission. This patient's disease had slightly increased clinically in size (10%), he withdrew consent for further chemotherapy and opted for best supportive care only.
The median estimated progression free survival (PFS) and overall survival (OS) for the whole cohort (n = 18) was 94 days and 203 days respectively. Factors tested for significance in univariate analysis included age (above or below 50 years), performance status (PS 1 versus PS 2, grade (poorly differentiated versus others), previous treatment received, number of cycles chemotherapy received (1 vs. more than 1). All 18 patients were included in this analysis. There was a trend towards improved median PFS in patients without poorly differentiated tumors and in those who had received 2 or more chemotherapy cycles. However, these differences were not statistically significant. There was a trend towards increased median OS in patients without poorly differentiated tumors and in those who had received 2 or more chemotherapy cycle. The median OS in patients who had received 2 or more cycles was 351 days versus 55 days in those who received less than 2 cycles (P = 0.025) [Figure 2]. However, after applying Bonferroni correction, this difference was no longer significant.
Figure 2.
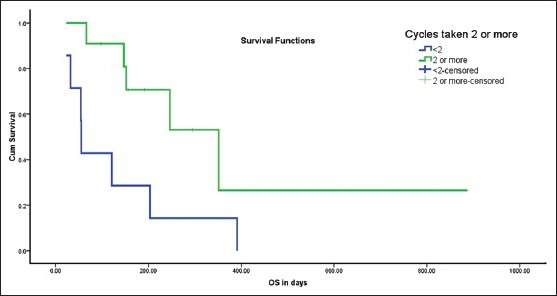
Survival difference between patients receiving more than 2 cycles versus those receiving less than 2 cycles
The chemotherapy toxicity has been shown in Table 2. There was no toxicity related death or life threatening complication. Only one patient discontinued chemotherapy due to toxicity.
Table 2.
Chemotherapy toxicity (in accordance with CTCAE version 4.02)
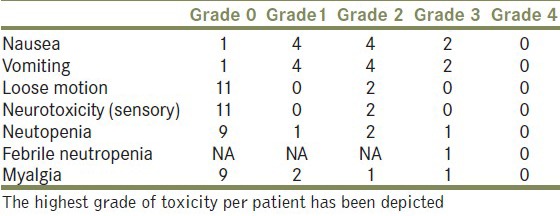
The number shown is the actual number of patients. The worst toxicity in each patient has been depicted. None of the responding patients become eligible for surgery. And the response to chemotherapy was consolidated by radiation in 3 patients
DISCUSSION
Previous studies have demonstrated poor survival ranging from 3 to 11 months in patients with penile carcinoma with metastatic disease at presentation or recurrent unresectable disease. These patients have been treated with various chemotherapy agents and schedules.[11,12,13,14,15,16,17,18] The heterogeneity in these studies makes it difficult to compare the different protocols. Consequently, there is no single standard regimen for the treatment of metastatic and recurrent disease. Though the need of the hour is well-conducted randomized trials, the relative rarity of the malignancy makes it practically difficult and we have to depend upon individual institutional experiences. There is thus an urgent unmet need for more effective regimens.[18]
Pettaway et al. in a recent review concluded that platinum compounds (especially cisplatin) are the most active agents in penile cancers. Bleomycin containing regimens have been found to be toxic and add little benefit in palliative setting.[4] Recently, paclitaxel (175 mg/2) has been shown to have activity in this disease in several case reports and a single case series by Di lorenzo et al.[8]
Based on the efficacy, safety and experience in squamous carcinoma at other sites, we used a protocol containing paclitaxel and platinum. These drugs can be administered on outpatient basis and do not require an indwelling catheter as is the case with infusional protocols. The drugs were administered on a single day as has been the practice in recent trials.[19] These logistical considerations also help in decreasing the treatment-associated costs which is an important consideration especially in the economically unprivileged countries where this disease is prevalent
The patients in the present study were an unselected heterogeneous group that included patients with poor performance status (ECOG > 1) and short time to recurrence. These patients have traditionally been considered to have poor prognosis and our study confirms the short survival periods for this group.
The combination was well tolerated without any episodes of life threatening hematological toxicity and no toxicity related death. This is in sharp contrast to the toxicity reported with earlier bleomycin based combinations.[11,13,14] Table 3 shows a comparison of the toxicity profile of our institutional protocol compared with protocols reported in case series with at least 10 patients.[11,13,16,20,21]
Table 3.
Comparison of clinically significant serious toxicity
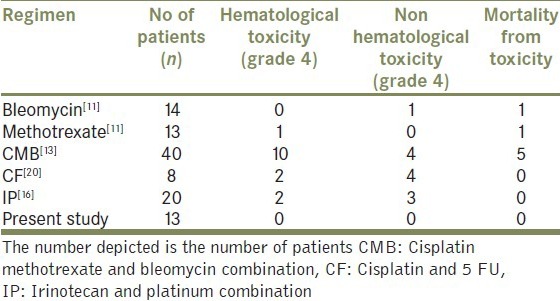
Table 4 shows the comparison of the overall response rate and estimated median overall survival in our study with the reported literature. Due to rarity of disease and heterogeneous patient populations included in the different observational studies, it is difficult to draw any firm conclusions regarding the optimum dose and schedule of chemotherapy. However the results in our study appear similar to the reported literature
Table 4.
Comparison of RR (response rates to chemotherapy) and OS (overall survival) with other studies
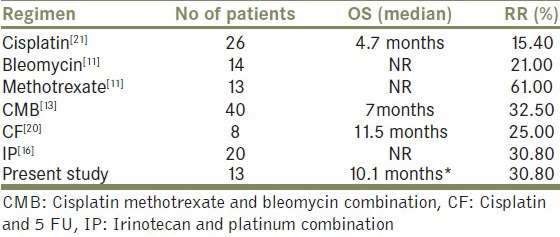
According to Pettaway et al., chemotherapy which is used in the palliative setting in carcinoma penis should be considered effective if it is associated with overall response rate of at least 30% and does not cause any life-threatening toxicity.[4] By these criteria, the regimen used in our report might be considered as effective and should be investigated further in larger studies. To the best of our knowledge this is the first case series highlighting the use of tisane and platinum in advanced squamous cell carcinoma of penis
Our study highlights the social and familial barriers to treatment of advanced disease, especially in economically disadvantaged sections of the society. This has been also highlighted in other solid tumors were socioeconomic status predicts survival.[22,23] As many as 5 out of 18 patients were unwilling for chemotherapy due to lack of social and familial support. Another patient discontinued chemotherapy after 3 cycles for similar reasons. A further 2 patients withdrew consent for treatment despite repeated counseling from doctors and counselors. Poor awareness and the general perception of incurability of disease are impediments to compliance to treatment
CONCLUSION
The role of the available chemotherapy in penile carincoma remains to be questionable as the toxicity rates are high and the survival rates under chemotherapy are not promising. Our results, although having a small group of patients, showed a similar response rates to other regimens published in literature but with a lower toxicity rates and added advantage of been delivered on outpatient basis.
Footnotes
Source of Support: Nil
Conflict of Interest: None
REFERENCES
- 1.Misra S, Chaturvedi A, Misra NC. Penile carcinoma: A challenge for the developing world. Lancet Oncol. 2004;5:240–7. doi: 10.1016/S1470-2045(04)01427-5. [DOI] [PubMed] [Google Scholar]
- 2.Leijte JA, Gallee M, Antonini N, Horenblas S. Evaluation of current TNM classification of penile carcinoma. J Urol. 2008;180:933–8. doi: 10.1016/j.juro.2008.05.011. discussion 938. [DOI] [PubMed] [Google Scholar]
- 3.Pandey D, Mahajan V, Kannan RR. Prognostic factors in node-positive carcinoma of the penis. J Surg Oncol. 2006;93:133–8. doi: 10.1002/jso.20414. [DOI] [PubMed] [Google Scholar]
- 4.Pettaway CA, Pagliaro L, Theodore C, Haas G. Treatment of visceral, unresectable, or bulky/unresectable regional metastases of penile cancer. Urology. 2010;76:S58–65. doi: 10.1016/j.urology.2010.03.082. [DOI] [PubMed] [Google Scholar]
- 5.Isonishi S, Yasuda M, Takahashi F, Katsumata N, Kimura E, Aoki D, et al. Randomized phase III trial of conventional paclitaxel and carboplatin (c-TC) versus dose dense weekly paclitaxel and carboplatin (dd-TC) in women with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer: Japanese gynecologic oncology. J Clin Oncol (Meeting Abstracts) 2008;26(15 suppl):5506. [Google Scholar]
- 6.Adamo V, Ferraro G, Pergolizzi S, Sergi C, Laudani A, Settineri N, et al. Paclitaxel and cisplatin in patients with recurrent and metastatic head and neck squamous cell carcinoma. Oral Oncol. 2004;40:525–31. doi: 10.1016/j.oraloncology.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Grossi F, Aita M, Defferrari C, Rosetti F, Brianti A, Fasola G, et al. Impact of third-generation drugs on the activity of first-line chemotherapy in advanced non-small cell lung cancer: A meta-analytical approach. Oncologist. 2009;14:497–510. doi: 10.1634/theoncologist.2008-0260. [DOI] [PubMed] [Google Scholar]
- 8.Di Lorenzo G, Cartenì G, Autorino R, Gonnella A, Perdonà S, Ferro M, et al. Activity and toxicity of paclitaxel in pretreated metastatic penile cancer patients. Anticancer Drugs. 2009;20:277–80. doi: 10.1097/CAD.0b013e328329a293. [DOI] [PubMed] [Google Scholar]
- 9.Joerger M, Warzinek T, Klaeser B, Kluckert JT, Schmid HP, Gillessen S. Major tumor regression after paclitaxel and carboplatin polychemotherapy in a patient with advanced penile cancer. Urology. 2004;63:778–80. doi: 10.1016/j.urology.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–8. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed T, Sklaroff R, Yagoda A. Sequential trials of methotrexate, cisplatin and bleomycin for penile cancer. J Urol. 1984;132:465–8. doi: 10.1016/s0022-5347(17)49693-5. [DOI] [PubMed] [Google Scholar]
- 12.Corral DA, Sella A, Pettaway CA, Amato RJ, Jones DM, Ellerhorst J. Combination chemotherapy for metastatic or locally advanced genitourinary squamous cell carcinoma: A phase II study of methotrexate, cisplatin and bleomycin. J Urol. 1998;160:1770–4. [PubMed] [Google Scholar]
- 13.Haas GP, Blumenstein BA, Gagliano RG, Russell CA, Rivkin SE, Culkin DJ, et al. Cisplatin, methotrexate and bleomycin for the treatment of carcinoma of the penis: A Southwest Oncology Group study. J Urol. 1999;161:1823–5. [PubMed] [Google Scholar]
- 14.Hakenberg OW, Nippgen JB, Froehner M, Zastrow S, Wirth MP. Cisplatin, methotrexate and bleomycin for treating advanced penile carcinoma. BJU Int. 2006;98:1225–7. doi: 10.1111/j.1464-410X.2006.06496.x. [DOI] [PubMed] [Google Scholar]
- 15.Vokes EE, Schilsky RL, Weichselbaum RR, Kozloff MF, Panje WR. Induction chemotherapy with cisplatin, fluorouracil, and high-dose leucovorin for locally advanced head and neck cancer: A clinical and pharmacologic analysis. J Clin Oncol. 1990;8:241–7. doi: 10.1200/JCO.1990.8.2.241. [DOI] [PubMed] [Google Scholar]
- 16.Theodore C, Skoneczna I, Bodrogi I, Leahy M, Kerst JM, Collette L, et al. A phase II multicentre study of irinotecan (CPT 11) in combination with cisplatin (CDDP) in metastatic or locally advanced penile carcinoma (EORTC PROTOCOL 30992) Ann Oncol. 2008;19:1304–7. doi: 10.1093/annonc/mdn149. [DOI] [PubMed] [Google Scholar]
- 17.Pagliaro LC, Williams DL, Daliani D, Williams MB, Osai W, Kincaid M, et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: A phase II study. J Clin Oncol. 2010;28:3851–7. doi: 10.1200/JCO.2010.29.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Protzel C, Ruppin S, Milerski S, Klebingat KJ, Hakenberg OW. The current state of the art of chemotherapy of penile cancer: Results of a nationwide survey of German clinics. Urologe A. 2009;48:1495–8. doi: 10.1007/s00120-009-2108-z. [DOI] [PubMed] [Google Scholar]
- 19.Mannel RS, Brady MF, Kohn EC, Hanjani P, Hiura M, Lee R, et al. A randomized phase III trial of IV carboplatin and paclitaxel×3 courses followed by observation versus weekly maintenance low-dose paclitaxel in patients with early-stage ovarian carcinoma: A Gynecologic Oncology Group Study. Gynecol Oncol. 2011;122:89–94. doi: 10.1016/j.ygyno.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shammas FV, Ous S, Fossa SD. Cisplatin and 5-fluorouracil in advanced cancer of the penis. J Urol. 1992;147:630–2. doi: 10.1016/s0022-5347(17)37327-5. [DOI] [PubMed] [Google Scholar]
- 21.Gagliano RG, Blumenstein BA, Crawford ED, Stephens RL, Coltman CA, Jr, Costanzi JJ. cis-Diamminedichloroplatinum in the treatment of advanced epidermoid carcinoma of the penis: A Southwest Oncology Group Study. J Urol. 1989;141:66–7. doi: 10.1016/s0022-5347(17)40590-8. [DOI] [PubMed] [Google Scholar]
- 22.Soares A, Biasoli I, Scheliga A, Luiz RR, Costa MA, Land M, et al. Socioeconomic inequality and short-term outcome in Hodgkin's lymphoma. Int J Cancer. 2007;120:875–9. doi: 10.1002/ijc.22417. [DOI] [PubMed] [Google Scholar]
- 23.Munro AJ, Bentley AH. Deprivation, comorbidity and survival in a cohort of patients with colorectal cancer. Eur J Cancer Care (Engl) 2004;13:254–62. doi: 10.1111/j.1365-2354.2004.00480.x. [DOI] [PubMed] [Google Scholar]


