Abstract
Objectives:
This study aims to evaluate clinical predictors of nocturia in patients with obstructive sleep apnea (OSA).
Materials and Methods:
In retrospective manner, a total of 200 patients with OSA were randomly included. Group I contained 100 patients with OSA and no nocturia, and Group II included 100 patients with OSA and nocturia. Bivariate logistic analyses were used to identify variables most likely to contribute to nocturia. Multivariate logistic regression of age, waist circumference, STOP score (Snore, Tired, Obstruction and Pressure), and Apnea–Hypopnea Index (AHI) was performed to evaluate predictors of nocturia. Statistical significance was defined as P < 0.05.
Results:
Median nocturia episodes were 2.2 in Group II. Patients were younger in Group I, with a mean age of 45 vs 50 years (P = 0.008). Mean BMI of 30 was similar in both groups, but there were more overweight patients in Group II (28% vs 18%). AHI approached significance between groups—18 vs 23 in group I and II, respectively (P = 0.071). In multivariate analysis, age over 70 years and moderate AHI were statistically significant predictors of nocturia (coefficients 0.6 and –0.2 with P = 0.003 and 0.03, respectively).
Conclusions:
This study identifies age and AHI score as predictors of nocturia in patients with OSA. This may indicate the usefulness of incorporating nocturia in the screening of patients with OSA. Future studies are needed to further evaluate mechanism of action, clinical significance, and effect of treatment for nocturia in patients with OSA.
Keywords: Clinical predictors, nocturia, obstructive sleep apnea
INTRODUCTION
Nocturia is a key feature of lower urinary tract symptoms (LUTS) that are often attributable to an underlying Benign Prostatic Hyperplasia (BPH). However, nocturia can signify possible contribution of numerous systematic health-related disorders, including Obstructive sleep apnea (OSA).
Definitions for nocturia have been previously published by the Standardization Committee of the International Continence Society (ICS) as complaint that the individual has to wake at night one or more times to void. The sign of nocturia is the number of voids recorded during a night's sleep: Each void is preceded and followed by sleep. The first morning void after a night's sleep is counted toward daytime (diurnal) frequency rather than nocturia, as it is not followed by sleep.
The causes of nocturia in patients with sleep apnea are not well understood; however, it has been hypothesized as a function of Atrial Natriuretic Peptide (ANP).[1] OSA causes intermittent occlusion of the airway during sleep which leads to a gasping respiratory pattern associated with episodic, potentially severe hypoxia. The gasping attempts to draw in a breath against the obstructed airway leads to substantial fluctuation in the intrathoracic pressure, with reflexive hypoxic pulmonary vasoconstriction. This ultimately causes the right atrium to secrete ANP, a powerful natural vasodilator polypeptide, secreted by the atrial myocytes in response to high blood pressure and/or hypoxia. ANP acts upon the kidneys to cause vasodilatation of the afferent glomerular arterioles, precipitating an increased glomerular filtration rate which leads to increased urinary output through natriuresis.[2] Effectively, ANP acts on specific renal, cardiac, and vascular receptors to promote natriuresis to reduce the intravascular circulatory volume, reducing blood pressure.[1,2]
Recent published evidence has linked nocturia as a predictive symptom of OSA.[3,4,5,6,7,8,9,10,11] Moreover, clinical work has clearly established the effective role of the Continuous Positive Airway Pressure (CPAP) on nocturia symptoms in patients with OSA.[12] In this single institutional study, we sought to systematically evaluate clinical predictors of nocturia in patients with OSA.
MATERIALS AND METHODS
Between January 2009 and January 2012, total of 200 study patients were randomly identified through a retrospective review of our institution's electronic health records. Approval was obtained through the Institutional Review Board (IRB). All patients were over the age of 18 years, underwent polysomnography, were diagnosed with OSA, and subsequently followed up in outpatient otolaryngology sleep clinics at University of California San Diego.
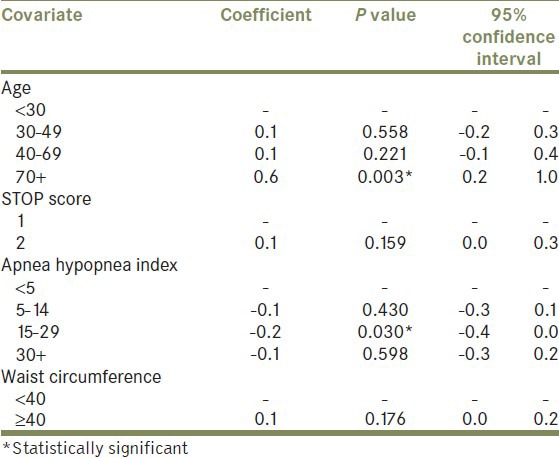
Group I contained 100 patients with OSA and no symptoms of nocturia, and Group II included 100 patients with OSA and nocturia. Careful attention was taken to exclude patients with prior LUTS by comprehensive review of their current electronic medical records. Patient demographics and clinical characteristics were evaluated. Standard methods were used to calculate mean values, and unpaired Students t-tests were used to compare the two groups [Table 1]. A bivariate logistic analysis with the outcome of nocturia >1 was carried out for each variable, to screen for factors most likely to contribute to nocturia. Multivariate logistic regression with outcome of nocturia events >1 was subsequently performed with those variables found to be most contributory toward this outcome based on bivariate methods. These included age, STOP score (utilizes snore, tired, obstruction, and pressure symptoms), apnea–hypopnea index (AHI), and waist circumference [Table 2]. All analyses were performed using Stata SE 64-bit, version 11.1. Statistical significance was assessed using 2-sided tests, with statistical significance defined as P < 0.05.
Table 1.
Bivariate logistic analyses of OSA variables most likely to contribute to nocturia
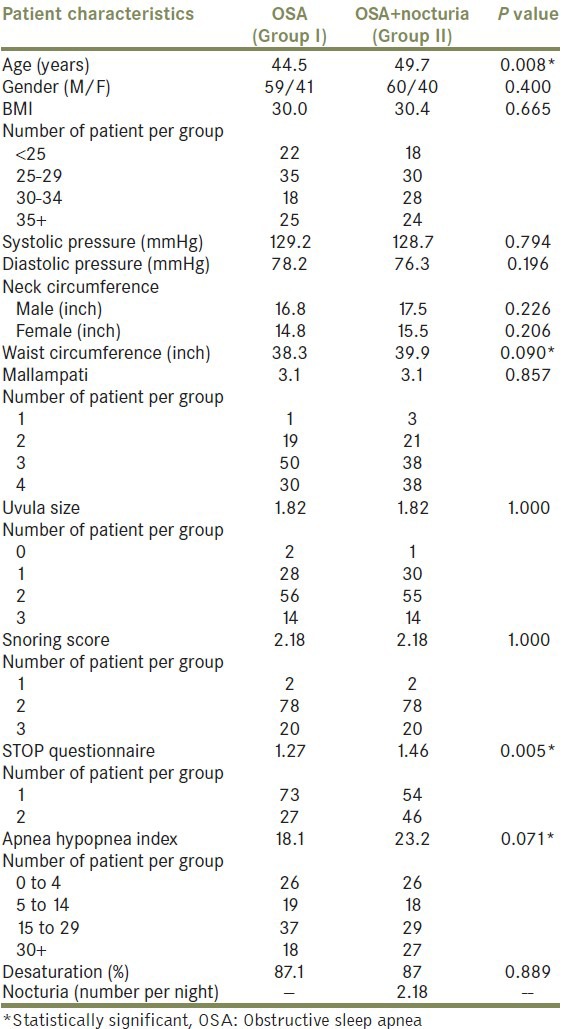
Table 2.
Multivariate logistic regression of age, waist circumference, STOP questionnaire, and apnea hypopnea index
RESULTS
Median age was 45 years in Group I and 50 years in Group II (P = 0.008), with a male to female ratio of 2:1 in both [Table 1]. There were more obese patients (BMI, 30-34) in Group II, but the overall mean BMI of 30 was similar between groups. Median systolic and diastolic pressures did not differ between groups.
Median neck circumference was higher among males in both groups, but neck size did not differ between the two groups when stratified by sex. Waist circumference did not differ significantly (38 vs 40 inch respectively, P = 0.09). There were no differences in Mallampati, uvula size, snoring score, or oxygen desaturation nadir during sleep testing.
Group II, with OSA and nocturia, had significantly higher STOP scores (1.46 vs 1.27 in Group I, P = 0.005), and a trend toward higher AHI approached statistical significance (P = 0.071). Median nocturia episodes were 2.18 in Group II.
In multivariate analysis, nocturia was positively predicted in patients over the age of 70 years with a coefficient of 0.6 (P = 0.003). Nocturia was found to be negatively predicted in patients with a moderate AHI of 15-29 (coefficient – 0.2, P = 0.03). No other covariates of age, STOP score, AHI, or waist circumference approached significance [Table 2].
DISCUSSION
Indeed, nocturia is one of the most common reasons for patient referral to urology. Nocturia is a highly prevalent symptom, particularly in the patients over 60 years of age.[13,14,15,16] In one northern European study, the prevalence of nocturia in a Danish cohort of patients reached up to 77%.[15] Likewise, epidemiological prevalence studies have generally indicated that nocturia increase with ageing.[16,17] Nocturia is traditionally defined as storage-phase dysfunction that eventually leads to LUTS. The underlying etiology of nocturia is potentially caused by reduced nocturnal bladder capacity, and a large urine volume produced during the night, and/or sleep problems.[15,19]
Despite variable pathogenesis, nocturia is primarily caused by underlying BPH.[15] Moreover, nocturia can signify possible contribution of systemic disease; renal, endocrine, neurological, or cardiovascular malfunction, making treatment a challenge. Medications such as diuretics, selective serotonin re-uptake inhibitors (SSRIs), calcium channel blockers, tetracycline, and lithium can also result in increased diuresis.[14,16]
The multivariate analysis reported herein identifies two risk factors for nocturia in a population with OSA. Held alone, age is a well-known positive risk factor for nocturia. In our study, age over 70 years held up as such, even when controlling for other covariates. Interestingly, moderate AHI of 15-29 showed negative predictive value which means that patients in this category were less likely to have nocturia. From what is known both anecdotally and based on pathophysiologic mechanisms, AHI would be predicted to positively correlate with nocturia. That is to say, the higher the AHI, the higher the likelihood of having nocturia.
The mixed, counterintuitive results demonstrating moderate AHI as a negative predictor for nocturia could signify several things. Nocturia may be a multi-factorial component of some patients OSA disease process, but not necessarily a surrogate for OSA severity. The most likely explanation is that other unmeasured confounders exist in this equation. If captured in future analyses, these additional confounders could be turned into covariates and a positive predictive value for nocturia might exist for increasing AHI scores.
Recent studies have highlighted the relationship between sleep disturbance and nocturia. However, a detailed understanding of the underlying mechanistic effects is yet to be determined. In a large northern European sleep study of 1 485 patients, Middelkoop et al. reported that the most frequent subjective cause of disturbed sleep maintenance was nocturia (68%).[18] Bliwise et al. reported that nocturia was an independent predictor both of self-reported insomnia (75% increased risk) and reduced sleep quality (71% increased risk). Adding to this, nocturia was found to be frequently overlooked cause of poor sleep in the elderly and may warrant targeted interventions.[19]
Several epidemiological studies have shown a correlation of insomnia/poor sleep to nocturia.[8,9,20,21] Along with sleep quality, duration of sleep is also associated with the number of nocturia episodes.[22] The OSA to nocturia association has been confirmed by the fact that intervention for OSA, such as CPAP treatment, decreases nocturia episodes.[23,24,25] More recently, it has been suggested that nocturia can be used as a predictive symptom of OSA.[6] Severity of OSA in the elderly population may be proportional to the degree of nocturia.[23,24,26,27] In a large United States population sleep study of 1 007 adult patients, Romero et al. have demonstrated that nocturia is comparable to snoring as a screening tool for OSA. In Romero's study, the positive predictive value was 85% and 81% in snoring and nocturia, respectively. With linear regression, patient-reported nocturia frequency predicted AHI above and beyond BMI, sex, age, and self-reported snoring (P < 0.0001).[7] Our study identifies statistically significant correlation between OSA patients with nocturia and advanced age (>70 years) and moderate AHI score (P = 0.003 and P = 0.03, respectively). Furthermore, there was strong correlation between OSA patients with nocturia and wide waist circumference and higher STOP score. This may potentially indicate the usefulness of incorporating nocturia in the evaluation of OSA.
In addition, systematic literature review has shown paucity of data in urological literature detailing nocturia and OSA.[28,29] This study aimed to increase awareness of nocturia as a potentially complex comorbidity when treating patients with OSA. Particularly, practicing urologists should actively think about sleep apnea when presented by old- or young-aged patients who are complaining of nocturia. Hence, this prompts sleep specialist referral to avoid potential complications of sleep apnea including nocturia.
The authors recommend that patients seen for nocturia should be screened for OSA with the STOP score. If there is high risk for OSA, a sleep study is warranted with reflexive specialist referral for positive tests or confusing sleep disturbance symptoms. The converse is equally important. Patients with sleep apnea should be assessed for LUTS/BPH and nocturia symptoms, as they may require specialist evaluation for such. The inter-related disease processes of OSA and nocturia are only beginning to be understood, and a multidisciplinary, team-based approach is critical for the optimal care of these patients.
Furthermore, the associative role of ANP and OSA has been previously examined with conflicting results. In a community-based observational study, Patwardhan et al. have hypothesized that alterations in cardiac hemodynamics associated with OSA would be reflected in higher ANP levels. However, Patwardhan's study did not establish statistically significant relationships between OSA indices such as AHI and ANP.[30] More recently, the ANP role in the OSA patients treated with CPAP therapy was re-examined in a prospective study by Hu et al. in 23 OSA patients. Hu's study demonstrated that CPAP can effectively reverse the nocturia in the OSA patients by decreasing the nocturnal excretion of ANP.[26]
The elucidation of ANP's role in the development of nocturia in patients with OSA clearly demands additional inquiry. Furthermore, prospective studies and/or systematic clinical trial are needed to fully characterize the effect of OSA treatment with CPAP on symptoms of nocturia.
CONCLUSION
This study aims to increase awareness among clinicians caring for patients with both nocturia and OSA disease processes. This study highlights advanced age and moderate AHI score as predictors for nocturia symptoms. These findings suggest the importance of incorporating nocturia in the evaluation of OSA, and vice versa. Future work into the mechanistic role of ANP, and the long-term nocturia outcomes in optimally treated OSA, will be welcomed additions to this investigation.
Footnotes
Source of Support: Nil
Conflict of Interest: None
REFERENCES
- 1.de Bold AJ. Atrial natriuretic factor: A hormone produced by the heart. Science. 1985;230:767–70. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- 2.Yalkut D, Lee LY, Grider J, Jorgensen M, Jackson B, Ott C, et al. Mechanism of atrial natriuretic peptide release with increased inspiratory resistance. J Lab Clin Med. 1996;128:322–8. doi: 10.1016/s0022-2143(96)90034-7. [DOI] [PubMed] [Google Scholar]
- 3.Davidson TM. The Great Leap Forward: The anatomic basis for the acquisition of speech and obstructive sleep apnea. Sleep Med. 2003;237:185–94. doi: 10.1016/s1389-9457(02)00237-x. [DOI] [PubMed] [Google Scholar]
- 4.Nashi N, Kang S, Barkdull G, Lucas J, Davidson TM. Lingual Fat at Autopsy. Laryngoscope. 2007;117:1467–73. doi: 10.1097/MLG.0b013e318068b566. [DOI] [PubMed] [Google Scholar]
- 5.Davidson TM, Patel M. Waist, circumference and sleep disordered breathing. Laryngoscope. 2008;118:339–47. doi: 10.1097/MLG.0b013e3181587d7c. [DOI] [PubMed] [Google Scholar]
- 6.Barkdull G, Kohl C, Davidson T. Computed tomography imaging of patients with obstructive sleep apnea. Laryngoscope. 2008;118:1486–92. doi: 10.1097/MLG.0b013e3181782706. [DOI] [PubMed] [Google Scholar]
- 7.Romero E, Krakow B, Haynes P, Ulibarri V. Nocturia and snoring: Predictive symptoms for obstructive sleep apnea. Sleep Breath. 2010;14:337–43. doi: 10.1007/s11325-009-0310-2. [DOI] [PubMed] [Google Scholar]
- 8.Tsujimura A, Takao T, Miyagawa Y, Yamamoto K, Fukuhara S, Nakayama J, et al. Urgency is an independent factor for sleep disturbance in men with obstructive sleep apnea. Urology. 2010;76:967–70. doi: 10.1016/j.urology.2010.01.070. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura K, Oka Y, Kamoto T, Yoshimura K, Ogawa O. Differences and associations between nocturnal voiding/nocturia and sleep disorders. BJU Int. 2010;106:232–7. doi: 10.1111/j.1464-410X.2009.09045.x. [DOI] [PubMed] [Google Scholar]
- 10.Gopal M, Sammel MD, Pien G, Gracia C, Freeman EW, Lin H, et al. Investigating the associations between nocturia and sleep disorders in perimenopausal women. J Urol. 2008;180:2063–7. doi: 10.1016/j.juro.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moriyama Y, Miwa K, Tanaka H, Fujihiro S, Nishino Y, Deguchi T, et al. Nocturia in men less than 50 years of age may be associated with obstructive sleep apnea syndrome. Urology. 2008;71:1096–8. doi: 10.1016/j.urology.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 12.Margel D, Shochat T, Getzler O, Livne PM, Pillar G. Continuous positive airway pressure reduces nocturia in patients with obstructive sleep apnea. Urology. 2006;67:974–7. doi: 10.1016/j.urology.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 13.Barry MJ, Fowler FJ, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 14.Grunfeld JP, Rossier BC. Lithium nephrotoxicity revisited. Nat Rev Nephrol. 2009;5:270–6. doi: 10.1038/nrneph.2009.43. [DOI] [PubMed] [Google Scholar]
- 15.Bing MH, Moller LA, Jennum P, Mortensen S, Skovgaard LT, Lose G. Prevalence and bother of nocturia, and causes of sleep interruption in a Danish population of men and women aged 60-80 years. BJU Int. 2006;98:599–604. doi: 10.1111/j.1464-410X.2006.06390.x. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald MP, Litman HJ, Link CL, McKinlay JB BACH Survey Investigators. The association of nocturia with cardiac disease, diabetes, body mass index, age and diuretic use: Results from the BACH survey. J Urol. 2007;177:1385–9. doi: 10.1016/j.juro.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 17.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatr Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 18.Middelkoop HA, Smilde-van den Doel DA, Neven AK, Kamphuisen HA, Springer CP. Subjective sleep characteristics of 1,485 males and females aged 50-93: Effects of sex and age, and factors related to self-evaluated quality of life. J Gerontol A Biol Sci Med Sci. 1996;51A:M108–15. doi: 10.1093/gerona/51a.3.m108. [DOI] [PubMed] [Google Scholar]
- 19.Bliwise DL, Foley DJ, Vitiello MV, Ansari FP, Ancoli-Israel S, Walsh JK. Nocturia and disturbed sleep in the elderly. Sleep Med. 2009;10:540–8. doi: 10.1016/j.sleep.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asplund R. Nocturia in relation to sleep, somatic diseases and medical treatment in the elderly. BJU Int. 2002;90:533–6. doi: 10.1046/j.1464-410x.2002.02975.x. [DOI] [PubMed] [Google Scholar]
- 21.Samuelsson E, Victor A, Tibbin G. A population study of urinary incontinence and nocturia among women aged 20–59 years. Prevalence, well-being and wish for treatment. Acta Obstet Gynecol Scand. 1997;76:74–80. doi: 10.3109/00016349709047789. [DOI] [PubMed] [Google Scholar]
- 22.Coyne KS, Zhou Z, Bhattacharyya SK, Thompson CL, Dhawan R, Versi E. The prevalence of nocturia and its effect on health-related quality of life and sleep in a community sample in the USA. BJU Int. 2003;92:948–54. doi: 10.1111/j.1464-410x.2003.04527.x. [DOI] [PubMed] [Google Scholar]
- 23.Endeshaw YW, Johnson TM, Kutner MH, Ouslander JG, Bliwise DL. Sleep disordered breathing and nocturia in older adults. J Am Geriatr Soc. 2004;52:957–60. doi: 10.1111/j.1532-5415.2004.52264.x. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald MP, Mulligan M, Parthasarathy S. Nocturic frequency is related to severity of obstructive sleep apnea, improves with continuous positive airways treatment. Am J Obstet Gynecol. 2006;194:1399–403. doi: 10.1016/j.ajog.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 25.Weaver TE, Chasens ER. Continuous positive airway pressure treatment for sleep apnea in older adults. Sleep Med Rev. 2007;11:99–111. doi: 10.1016/j.smrv.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu K, Tu ZS, Lü SQ, Li QQ, Chen XQ. Urodynamic changes in patients with obstructive sleep apnea-hypopnea syndrome and nocturnal polyuria. Zhonghua Jie He He Hu Xi Za Zhi. 2011;34:182–6. doi: 10.3760/cma.j.issn1001-0939.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Tandeter H, Gendler S, Dreiher J, Tarasiuk A. Nocturic episodes in patients with benign prostatic enlargement may suggest the presence of obstructive sleep apnea. J Am Board Fam Med. 2011;24:146–51. doi: 10.3122/jabfm.2011.02.100110. [DOI] [PubMed] [Google Scholar]
- 28.Gulur DM, Mevcha AM, Drake MJ. Nocturia as a manifestation of systemic disease. BJU Int. 2011;107:702–13. doi: 10.1111/j.1464-410X.2010.09763.x. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura K. Correlates for nocturia: A review of epidemiological studies. Int J Urol. 2012;19:317–29. doi: 10.1111/j.1442-2042.2011.02956.x. [DOI] [PubMed] [Google Scholar]
- 30.Patwardhan AA, Larson MG, Levy D, Benjamin EJ, Leip EP, Keyes MJ, et al. Obstructive sleep apnea and plasma natriuretic peptide levels in a community-based sample. Sleep. 2006;29:1301–6. doi: 10.1093/sleep/29.10.1301. [DOI] [PubMed] [Google Scholar]


