Abstract
Introduction:
Obstructive azoospermia (OA) is characterized by normal spermatogenesis and the absence of sperm in the ejaculate. Variable success rates have been reported using in-vitro fertilization (IVF) combined with PESA in cases of men with OA.
Aims:
To determine fertilization and pregnancy outcomes from PESA-derived spermatozoa and to ascertain whether frozen spermatozoa yields similar outcomes compared to fresh specimens.
Materials and Methods:
The charts of 68 consecutive couples undergoing 68 cycles of sperm retrieval for OA over eight years (2002-2010) were retrospectively reviewed. Patients requiring testicular intervention were excluded (n = 17).
Results:
Viable sperms were identified in 100% of men, and fresh spermatozoa were obtained in 40 patients (78.4%) simultaneously with female egg retrieval. The average fertilization rate was 77.7% with five embryos not surviving to transfer (12.5%). Pregnancies were confirmed in 48.6% (17/35). Twin gestations occurred in 11.8% (2/17) of cases. Frozen-thawed spermatozoa were used in 11 patients (21.6%). In this subgroup, the average fertilization rate was 73.6% with pregnancies confirmed in 54.5% (6/11). No multiple gestations were generated, and no complications occurred. The use of fresh spermatozoa for PESA provided no significant improvements in outcomes over frozen specimens.
Summary:
PESA is a very effective, simple, and safe method of obtaining spermatozoa for IVF. Outcomes obtained using fresh and frozen PESA-derived spermatozoa were similar and as such, either could be used during the IVF process.
Keywords: Infertility, obstructive azoospermia, percutaneous epididymal sperm aspiration
INTRODUCTION
Azoospermia, defined as the complete absence of sperm in ejaculate, is found in up to 15% of men seeking evaluation for infertility.[1] Over 40% of cases are obstructive in nature, resulting from an impediment somewhere along the ductal system.[2] Common obstructive causes include genitourinary infections, congenital anomalies, iatrogenic injuries, or previous vasectomy.[3] Clinically, obstructive azoospermia (OA) is characterized by normal spermatogenesis in conjunction with normal testicular volume and hormone profile.[4,5] Patients with OA may be managed with microsurgical reconstruction of the genital tract via vasovasostomy or vasoepididymostomy.[6] In patients where surgical reconstruction is not possible, treatment options include the use of donor sperm.[7]
The advent of intracytoplasmic sperm injection (ICSI) technology has necessitated the development of accurate, reliable, and convenient methods of sperm retrieval. First described in 1994, percutaneous epididymal sperm aspiration (PESA) is a minimally invasive technique for retrieval of sperm directly from the epididymis.[8,9] Coupled with ICSI and in-vitro fertilization (IVF), PESA is an effective mean of establishing both fertilization and pregnancy; however, it is not frequently used given the small quantities of spermatozoa obtained and the risks of hematoma, spermatic cord injury, and scarring.[10,11]
We sought to determine the fertilization and pregnancy outcomes from PESA-derived spermatozoa from a single center. We also investigated whether frozen spermatozoa yielded similar outcomes to freshly-obtained specimens.
MATERIALS AND METHODS
The charts of 68 consecutive couples undergoing PESA for ICSI/IVF from 2002 to 2010 were retrospectively reviewed. A total of 17 patients required testicular intervention, and were excluded from this study. The remaining 51 couples were analyzed for fertility outcomes.
Percutaneous epididymal sperm aspiration has been described previously; however, subtle modifications exist, and their impacts cannot be discounted.[8,9] In brief, the patient was laid supine on the examination table, and the genital region was shaved with a straight razor to allow for better access and visualization of the epididymis. The area was prepared with iodine cleansing solution, and sterile drapes were placed over-top. A local cord block anesthetic of 15 cc 2% plain Lidocaine was injected and allowed to settle for five minutes. The epididymis was then grasped between the thumb and fore-finger. A 21 gauge needle attached to a 3 cc syringe was then inserted into the epididymis. Suction was applied following insertion, and multiple passes were made without removing the needle from an initial site of insertion. The spermatozoa obtained were then placed into a sperm buffer and transported to an IVF laboratory. Epididymal spermatozoa were either obtained at the same time as oocyte retrieval and used immediately for ICSI or cryopreserved. Once the mature oocytes were retrieved from the female patient, the fresh or frozen-thawed motile epididymal spermatozoa were microinjected into the mature oocytes.
Fertilization was confirmed microscopically by the presence of two pro-nuclei and two polar bodies following ICSI. Subsequently, embryo transfer occurred three to five days after fertilization. Clinical pregnancy was confirmed by ultrasound examination documenting fetal heart rate. Chi-squared analysis was used to compare the fertilization rates, embryo transfer rates, and pregnancy rates between groups. All appropriate consents and approvals were obtained prior to commencing this study.
RESULTS
A total of 51 couples underwent PESA followed by cycles of ISCI/IVF. All male patients had OA while no men had non-obstructive azoospermia. Viable sperm were obtained from all 51 men (100%) who underwent PESA.
Fresh spermatozoa were obtained concurrently with egg retrieval in 40 patients (78.4%). Within this subgroup, the average age of males and their female partners was 34 ± 1 and 40 ± 1, respectively [Table 1]. ICSI with fresh epididymal sperm resulted in a fertilization rate of 241/310 (77.7%), with five embryos not surviving to transfer (12.5%). Of the 35 patients with successful embryo transfer, 17 achieved a pregnancy (48.6%). Amongst the 17 pregnancies, twin gestations were confirmed in two (11.8%).
Table 1.
Results of 51 IVF cycles using PESA-obtained sperm shows similar outcomes using fresh or frozen spermatozoa
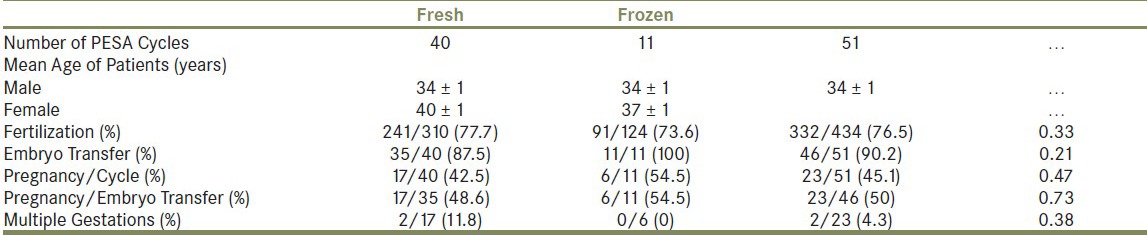
Frozen-thawed spermatozoa obtained by PESA were used along with fresh egg retrieval in 11 patients (21.6%). Within this subgroup, the average age of males and their female partners was 34 ± 1 and 37 ± 1, respectively [Table 1]. ICSI with frozen-thawed epididymal sperm resulted in a fertilization rate of 91/124 (73.6%). All embryos survived to transfer, and pregnancy was confirmed in six patients (54.5%). No multiple gestations were identified in the frozen-thawed subgroup [Table 1].
Overall, ICSI with fresh and frozen-thawed epididymal spermatozoa resulted in a cumulative fertilization rate of 76.5% [Table 1]. No significant differences in fertilization rates for fresh vs. frozen-thawed spermatozoa (P = 0.33) were identified.
The cumulative pregnancy rate per cycle of PESA was 45.1%. Amongst the 46 couples with successful embryo transfer, a total of 23 women (50%) achieved a pregnancy and two achieved twin gestations (4.3%). There was no significant difference in pregnancy rates between fresh and frozen-thawed groups (P = 0.47).
The majority of female patients were between 31 and 39 years of age (11 patients <30 years old, 16 patients between 31-34 years old, and 19 patients between 35-39 years old) with only five patients greater than 40 years old. Cumulative fertilization and pregnancy rates were highest for females aged 31-34 (81.5% and 50%, respectively) and lowest for those patients aged 40 or greater (70.8% and 40%, respectively). The amount of pregnancies obtained per embryo transfer were highest for those females <30 years old (five pregnancies out of nine embryo's transferred (55.6%)) and lowest for those females >40 years old (two pregnancies per five embryo's transferred (40%)). No complications occurred as a result of any of the PESA procedures.
DISCUSSION
Currently, there exists a wide variety of treatment options available for men with OA.[6] Microsurgical reconstruction remains a commonly used procedure, as it is a reliable means of restoring fertility in men with OA.[6] The success rates of microsurgical reconstruction vary. A review of the literature has identified overall patency rates and live delivery rates for vasectomy reversal to be approximately 86% and 58%, respectively.[12] Among these studies, live birth rates ranged from as low as 26% to as high as 84% with outcomes inversely dependent on the duration of obstruction.[13,14,15] A large series by Belker and colleagues demonstrated that for intervals of nine to 14 years and 15 years or more, pregnancy rates were 44% and 30%, respectively.[15] Subsequent studies reported cumulative pregnancy rates of 37% and 43% after 10 and 15 years of obstruction.[16,17]
Percutaneous sperm retrieval, either from the epididymis or testicle (i.e., testicular sperm aspiration or TESA), in conjunction with IVF/ICSI, is an accepted alternative to microsurgical reconstruction for cases of OA.[18] While improvements in technology over the past few years has seen a rise in PESA success rates, these outcomes have yet to be thoroughly documented. Case reports, such as that of a successful pregnancy derived from PESA in an 81-year-old male with OA, serve as modern benchmarks.[19] A recent 2013 study by Esteves and colleagues[18] provides insight by examining PESA and TESA outcomes in the current technology era. The authors examined success and stratified outcomes via the etiology of obstruction.[18] Sperm retrieval rates using both PESA and TESA were 97.3% when both techniques were employed; however, these numbers dropped significantly when only PESA was used. For example, in patients with congenital bilateral absence of the vas deferens (CBAVD), PESA had success rates of 96.8% while success rates were lower in cases of OA post-vasectomy (69.5%) and post-infection (76.4%).[18] These values prompted Esteves and colleagues to suggest that PESA was sufficient in CBAVD while TESA would likely be required in all other cases of OA.[18] These findings and suggestions are in contradiction to the findings of the current study where we report excellent success rates using PESA. Interestingly, the average age of the male population in the Esteves study was 47 years old post-vasectomy and 43 years old post-infection.[18] This is a substantial difference from that of our population, which was 34 years old. Moreover, other intangibles like surgeon and embryology lab techniques and experience may play a role in the differences observed.
An earlier 2002 study by Pasqualotto et al.[10] noted a pregnancy per cycle rate of 34.6% with PESA-derived sperm. Overall, the authors identified a fertilization rate of 58.7% for PESA, which is improved compared to Esteves[18] but still not as good as the results reported herein. Moreover, the mean age of patients was 31 years old - similar to that observed in this report. Similarly, work by Levine et al.[20] examined 94 cases of PESA, of which viable sperm were identified in 97%.[20] This rate of sperm retrieval is close to what was obtained in this report. Moreover, fertilization and clinical pregnancy rates of 58% and 39%, respectively,[20] mirror those found in Pasqualotto et al.[10] but still lag behind the current report. Levine and colleges[20] concluded that PESA, performed in a similar way to how it was described in the current report, was an effective, safe, and reproducible method of obtaining sperm for IVF/ICSI.
Further evidence for PESA effectiveness comes from a 2008 study by Naru et al.,[11] in which pregnancy rates among 517 couples undergoing sperm retrieval and ICSI were examined. Of the 69 PESA cycles for men with OA, 43.5% achieved a pregnancy. Another study by He et al.[21] investigated ICSI outcomes in a subset of 92 patients with OA and reported fertilization and pregnancy rates of 64% and 40%, respectively, amongst 51 cycles of PESA and ICSI. As such, these aforementioned studies support the use of PESA in conjunction with IVF/ICSI. Indeed, the use of PESA in conjunction with ICSI/IVF has offered comparable fertility outcomes to surgical correction, especially in cases of prolonged post-vasectomy obstruction.[6,22] In the case of our report, it is possible that the use of intangibles such as newer sperm buffers, embryological techniques, and equipment led to the improved outcomes, but that is merely speculation.
Our successful outcomes using PESA-derived spermatozoa allow more flexibility to the couple desiring fertility. Indeed, the use of frozen spermatozoa allows patients to visit the urologist and have PESA-derived spermatozoa obtained at their convenience. This flexibility may potentially decrease stress and lead to improved outcomes. While dogma suggests that fresh spermatozoa are best, multiple prior studies have reported no significant differences in clinical outcomes when using either fresh or frozen-thawed epididymal spermatozoa.[23,24] A meta-analysis of the literature published in 2004 found no significant differences in fertilization rates, implantation rates, or ongoing pregnancy rates with the use of fresh or frozen-thawed epididymal spermatozoa for ISCI.[25] These outcomes are consistent with the results observed in the current report where no significant differences in fertilization or pregnancy rates between fresh and frozen-thawed epididymal spermatozoa were identified. Therefore, while the overall amount of spermatozoa obtained from PESA and cryopreserved may be smaller, the outcomes are unaffected. This was confirmed by Esteves et al. where the authors noted that in 29% of patients with PESA-derived sperm after vasectomy, enough excess sperm was obtained for cryopreservation.[18]
Couples using PESA-derived frozen spermatozoa should be comforted in that the outcomes are not compromised and no effects on congenital malformation rates compared to IVF using ejaculated sperm were identified.[26] This allows the couple to plan an in-office PESA and cryopreserve the sample in anticipation of IVF. By decreasing the stress of this procedure, on top of the need to harvest eggs in the female and multiple clinical appointments, the experience can be less bothersome and more convenient to the couple involved.
CONCLUSIONS
This study highlights that fact that PESA, coupled with ICSI, is an effective means of achieving pregnancy in men with OA. The procedure is fast, cost-effective, reliable, requires only local anesthesia, and yields excellent results. Frozen spermatozoa yielded similar rates of fertilization and pregnancy outcomes compared to freshly-obtained samples. Thus, the use of PESA-obtained spermatozoa can be routinely attempted in men with OA.
ACKNOWLEDGMENTS
Portions of this study were previously published in abstract form at the American Society for Reproductive Medicine (ASRM) annual meeting in 2011.
Footnotes
Source of Support: Nil
Conflict of Interest: None
REFERENCES
- 1.Mosher WD, Pratt WF. Fecundity and infertility in the United States: Incidence and trends. Fertil Steril. 1991;56:192–3. [PubMed] [Google Scholar]
- 2.Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol. 1989;142:62–5. doi: 10.1016/s0022-5347(17)38662-7. [DOI] [PubMed] [Google Scholar]
- 3.Fedder J, Cruger D, Oestergaard B, Petersen GB. Etiology of azoospermia in 100 consecutive non vasectomized men. Fertil Steril. 2004;82:1463–5. doi: 10.1016/j.fertnstert.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 4.Tuttelmann F, Werny F, Cooper TG, Kliesch S, Simoni M, Nieschlag E. Clinical experience with azoospermia: Aetiology and chances for spermatozoa detection upon biopsy. Int J Androl. 2011;34:291–8. doi: 10.1111/j.1365-2605.2010.01087.x. [DOI] [PubMed] [Google Scholar]
- 5.Schlegel PN. Causes of azoospermia and their management. Reprod Fertil Dev. 2004;16:561–72. doi: 10.10371/RD03087. [DOI] [PubMed] [Google Scholar]
- 6.Shridharani A, Sandlow JI. Vasectomy reversal versus IVF with sperm retrieval: which is better? Curr Opin Urol. 2010;20:503–9. doi: 10.1097/MOU.0b013e32833f1b35. [DOI] [PubMed] [Google Scholar]
- 7.Van Peperstraten A, Proctor ML, Johnson NP, Philipson G. Techniques for surgical retrieval of sperm prior to intra-cytoplasmic sperm injection (ICSI) for azoospermia. Cochrane Database Syst Rev. 2008:CD002807. doi: 10.1002/14651858.CD002807.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrivastav P, Nadkarni P, Wensvoort S, Craft I. Percutaneous epididymal sperm aspiration for obstructive azoospermia. Hum Reprod. 1994;9:2058–61. doi: 10.1093/oxfordjournals.humrep.a138393. [DOI] [PubMed] [Google Scholar]
- 9.Craft I, Tsirigotis M, Bennett V, Taranissi M, Khalifa Y, Hogewind G, et al. Percutaneous epididymal sperm aspiration and intracytoplasmic sperm injection in the management of infertility due to obstructive azoospermia. Fertil Steril. 1995;63:1038–42. doi: 10.1016/s0015-0282(16)57544-x. [DOI] [PubMed] [Google Scholar]
- 10.Pasqualotto FF, Rossi-Ferragut LM, Rocha CC, Iaconelli A, Jr, Borges E., Jr Outcome of in vitro fertilization and intracytoplasmic injection of epididymal and testicular sperm obtained from patients with obstructive and nonobstructive azoospermia. J Urol. 2002;167:1753–6. [PubMed] [Google Scholar]
- 11.Naru T, Sulaiman MN, Kidwai A, Ather MH, Waqar S, Virk S, et al. Intracytoplasmic sperm injection outcome using ejaculated sperm and retrieved sperm in azoospermic men. Urol J. 2008;5:106–10. [PubMed] [Google Scholar]
- 12.Lee R, Li PS, Schlegel PN, Goldstein M. Reassessing reconstruction in the management of obstructive azoospermia: Reconstruction or sperm acquisition? Urol Clin North Am. 2008;35:289–301. doi: 10.1016/j.ucl.2008.01.005. x. [DOI] [PubMed] [Google Scholar]
- 13.Thomas AJ., Jr Vasoepididymostomy. Urol Clin North Am. 1987;14:527–38. [PubMed] [Google Scholar]
- 14.Silber SJ, Grotjan H.E. Microscopic vasectomy reversal 30 years later: A summary of 4010 cases by the same surgeon. J Androl. 2004;25:845–859. doi: 10.1002/j.1939-4640.2004.tb03150.x. [DOI] [PubMed] [Google Scholar]
- 15.Belker AM, Thomas AJ, Jr, Fuchs EF, Konnak JW, Sharlip ID. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. J Urol. 1991;145:505–11. doi: 10.1016/s0022-5347(17)38381-7. [DOI] [PubMed] [Google Scholar]
- 16.Kolettis PN, Sabanegh ES, D’Amico AM, Box L, Sebesta M, Burns JR. Outcomes for vasectomy reversal performed after obstructive intervals of at least 10 years. Urology. 2002;60:885–8. doi: 10.1016/s0090-4295(02)01888-5. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs EF, Burt RA. Vasectomy reversal performed 15 years or more after vasectomy: Correlation of pregnancy outcome with partner age and with pregnancy results of in vitro fertilization with intracytoplasmic sperm injection. Fertil Steril. 2002;77:516–9. doi: 10.1016/s0015-0282(01)03219-8. [DOI] [PubMed] [Google Scholar]
- 18.Esteves SC, Lee W, Benjamin DJ, Seol B, Verza S, Jr, Agarwal A. Reproductive potential of men with obstructive azoospermia undergoing percutaneous sperm retrieval and intracytoplasmic sperm injection according to the cause of obstruction. J Urol. 2013;189:232–7. doi: 10.1016/j.juro.2012.08.084. [DOI] [PubMed] [Google Scholar]
- 19.Taitson PF, Melo CS, Mancebo AC, Melo UB, MC BS. Pregnancy after percutaneous epididymal sperm aspiration in an 81-year-old man with obstructive azoospermia. Andrologia. 2012;44:355–7. doi: 10.1111/j.1439-0272.2012.01287.x. [DOI] [PubMed] [Google Scholar]
- 20.Levine LA, Lisek EW. Successful sperm retrieval by percutaneous epididymal and testicular sperm aspiration. J Urol. 1998;159:437–40. doi: 10.1016/s0022-5347(01)63943-0. [DOI] [PubMed] [Google Scholar]
- 21.He X, Cao Y, Zhang Z, Zhao J, Wei Z, Zhou P, et al. Spermatogenesis affects the outcome of ICSI for azoospermic patients rather than sperm retrieval method. Syst Biol Reprod Med. 2010;56:457–64. doi: 10.3109/19396368.2010.513078. [DOI] [PubMed] [Google Scholar]
- 22.Esteves SC, Miyaoka R, Agarwal A. Sperm retrieval techniques for assisted reproduction. Int Braz J Urol. 2011;37:570–83. doi: 10.1590/s1677-55382011000500002. [DOI] [PubMed] [Google Scholar]
- 23.Tournaye H, Merdad T, Silber S, Joris H, Verheyen G, Devroey P, et al. No differences in outcome after intracytoplasmic sperm injection with fresh or with frozen-thawed epididymal spermatozoa. Hum Reprod. 1999;14:90–5. doi: 10.1093/humrep/14.1.90. [DOI] [PubMed] [Google Scholar]
- 24.Nagy Z, Liu J, Cecile J, Silber S, Devroey P, Van Steirteghem A. Using ejaculated, fresh, and frozen-thawed epididymal and testicular spermatozoa gives rise to comparable results after intracytoplasmic sperm injection. Fertil Steril. 1995;63:808–15. doi: 10.1016/s0015-0282(16)57486-x. [DOI] [PubMed] [Google Scholar]
- 25.Nicopoullos JD, Gilling-Smith C, Almeida PA, Norman-Taylor J, Grace I, Ramsay JW. Use of surgical sperm retrieval in azoospermic men: A meta-analysis. Fertil Steril. 2004;82:691–701. doi: 10.1016/j.fertnstert.2004.02.116. [DOI] [PubMed] [Google Scholar]
- 26.Fedder J, Loft A, Parner ET, Rasmussen S, Pinborg A. Neonatal outcome and congenital malformations in children born after ICSI with testicular or epididymal sperm: A controlled national cohort study. Hum Reprod. 2013;28:230–40. doi: 10.1093/humrep/des377. [DOI] [PubMed] [Google Scholar]


