Abstract
Aim:
To evaluate the possible protective effect of pomegranate extract (PE) on rats following renal ischemia–reperfusion (I/R) injury.
Materials and Methods:
Twenty-four Wistar rats were divided into three groups. Sham group underwent laparotomy then waited for 45 minutes without ischemia. I/R group were subjected to left renal ischemia for 45 minutes followed by 60 minutes of reperfusion. I/R + PE group were subjected to the same renal I/R as the I/R group were also given 225 mg/kg PE peroral 30 minutes prior to the ischemia. Malondialdehyde (MDA), total antioxidant capacity (TAC), total oxidant status (TOS), and oxidative stress index (OSI) were determined on the blood samples and kidney tissues. Histopathological analyses were conducted on the kidney tissues.
Results:
Serum TAC levels were significantly decreased in I/R group when compared with S group (P = 0.001). Serum MDA levels were increased in I/R group; however, it was not statistically significant. In rat kidney tissues, TOS levels and OSI index were significantly increased after I/R injury, while TAC levels were decreased. In I/R + PE group, PE reversed the negative effects of I/R injury. PE pretreatment was effective in decreasing tubular necrosis score.
Conclusion:
PE pretreatment ameliorated the oxidative damage and histopathological changes occurring following renal I/R injury.
Keywords: Antioxidant, ischemia-reperfusion, kidney, pomegranate extract, rat
INTRODUCTION
Renal ischemia-reperfusion usually occurs due to trauma, sepsis, renal transplantation, and some of vascular surgery and this situation may be a reason of renal failure. It is an important factor for the pathogenesis of organ transplantation and rejection.[1] Although various mechanisms were reported about the pathogenesis of I/R injury, the data about the treatment are very limited. The ischemia of kidney starts a series of incidents including cellular dysfunction and necrosis.[2] However, reperfusion can paradoxically create more tissue injury by initiating the complex cellular events which results in renal injury. The combination of apoptosis and necrosis results with the death of renal cells. It seems to be that the effect of I/R injury on renal cells are multifactorial such as interdependent, involving hypoxia, free radical damage, and inflammatory responses. High levels of oxygen-free radicals play a critical role in the injury resulting with cell damage, initiation of apoptosis and necrosis.[3] In recent data, various number of studies reported that consumption of vegetables, fruits, and phytochemicals reduce the side effects of reactive oxygen species due to I/R injury.[4,5] The results of these studies showed that exogen administration of compounds in fruits have cytoprotective and antioxidant activity. Antioxidants such as ellagic acid (EA)[6] and pomegranate flowers[7] have shown protective effects which is caused by cytotoxic agents.
In ancient cultures, the pomegranate extract (PE) was used as drugs for medicinal effect. It has been used for treating inflammatory diseases, cholesterol-lowering properties, and to prevent atherogenesis and urolithiasis.[5,8,9] Pomegranate fruit is very rich of polyphenol antioxidants, that includes tannins and anthocyanins. These antioxidants reduce macrophage lipid peroxidation, cellular cholesterol accumulation and this situation results with the development of atherosclerosis.[10,11] The soluble polyphenol content in PE varies from 0.2 to 1.0%, depending on the variety, and primarily consists of anthocyanins, catechins, tannins, gallic acid, and EA.[10] These antioxidants are more potent than many other antioxidants compared with Vitamins C and E, coenzyme Q-10, and α-lipoic acid.[12] Also, it is shown that pomegranate contains higher levels of antioxidants compared to red wine and green tea.[13]
To the best of our knowledge, the effects of PE on renal I/R injury has not yet been studied. The aim of the present study was therefore to determine the possible protective effects of PE against I/R injury in the kidney of male rats by determining biochemical parameters and by histological examination.
MATERIALS AND METHODS
Animals and experimental design
This study was approved by Dicle University Animal Ethical Committee and was carried out in accordance with the “Animal Welfare Act and the Guide for the Care and Use of Laboratory animals prepared by the Dicle University, Animal Ethical Committee.” This experimental study was performed with 24 mature, male, 3-month Wistar albino rats weighing 200 to 250 g. All animals were housed under standard conditions at an ambient temperature of 25°C ± 2°C and 12/12 hours of light–dark cycle in animal cages and treated in compliance with the National Institutes of Health guidelines. All experimental procedures in compliance with the animal use regulations of Dicle University Experimental Research Center, Diyarbakır, Turkey. The PE was obtained from Sigma Chemicals (St. Louis, MO, USA).
The rats were randomly assigned to three experimental groups: Group-1: Sham group (n = 8): Sham-operated animals underwent exposure of the left renal pedicles but did not receive any I/R, Group-2: I/R group (n = 8): Animals were subjected to 45 min of left renal ischemia followed by 60 min of reperfusion; Group-3: I/R + PE group (n = 8): Animals were given PE (225 mg/kg dose, PE [Punicagranatum] [40% EA = 100 mg] 250 mg) perorally 30 minutes prior to the ischemia and then subjected to 45 min of left renal ischemia followed by 60 min of reperfusion.
Surgical procedure
The animals were anesthetized with an intramuscular injection of ketamine and xylazine (90 mg/kg and 10 mg/kg, respectively). The abdominal region was sterilized with povidone iodine solution, the abdomen was entered through a midline minimal incision, and left kidney was isolated. In the S group, the abdomen was closed without any further procedure. In the I/R and I/R + PE groups, left renal artery was occluded using non traumatic microvascular clamps for 45 min, and occlusion of blood flow was confirmed by visual inspection of the kidneys. The animals received 50 ml/kg of warm saline instilled into the abdominal cavity during the entire procedure. After declamping, we confirmed that renal blood flow had been restored prior to closing the incision. At the end of 60 min of reperfusion, 5 cc blood was taken from cardiac cavity and then all rats were sacrificed. The abdomen was reentered and left nephrectomy was carried out; the half of left kidney was used for further enzymatic analysis, whereas the other half of kidney was stored in 10% formalin for histopathological examination.
Measurement of the malondialdehyde
Malondialdehyde (MDA) levels were estimated by the double heating method of Draper and Hadley.[14] The principle of this method is spectrophotometric measurement of the color generated by the reaction of thiobarbituric acid (TBA) with MDA. For this purpose, 2.5 ml of trichloroacetic acid solution (%10) was added to 0.5 ml serum in each centrifuge tube, and the tubes were placed in a boiling water bath for 15 min. After cooling in tap water, the tubes were centrifuged at 1 000 g for 10 min and 2 ml of the supernatant was added to 1 ml of TBA solution (6.7 g/l) in a test tube, and the tube was placed in a boiling water bath for 15 min. The solution was then cooled in tap water and its absorbance was measured using a spectrophotometer (Shimadzu UV-1208, Japan) at 532 nm. The concentration of MDA was calculated by the absorbance coefficient of the MDA–TBA complex (absorbance coefficient of 1.56 × 105 cm-1 M-1) and is expressed as μmol/l.
Measurement of the total antioxidant capacity
Total antioxidant capacity (TAC) of supernatant fractions was determined using a novel automated measurement method developed by Erel.[15] In this method, hydroxyl radical, which is the most potent biological radical, is produced. In the assay, ferrous ion solution, which is present in Reagent 1, is mixed with hydrogen peroxide, which is present in Reagent 2. The sequential produced radicals such as brown-colored dianisidinyl radical cation, produced by the hydroxyl radical, are also potent radicals. Using this method, antioxidant effect of the sample against the potent-free radical reactions, which is initiated by the produced hydroxyl radical, is measured. The results are expressed as nmol Trolox Equiv./mg protein.
Measurement of total oxidant status
Total oxidant status (TOS) of supernatant fractions was determined using a novel automated measurement method, developed by Erel.[16] Oxidants present in the sample oxidize the ferrous ion-o-dianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules, which are abundantly present in the reaction medium. The ferric ion makes a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay is calibrated with hydrogen peroxide and the results are expressed in terms of nmol H2O2 Equiv./mg protein.
Determination of oxidative stress index
The percent ratio of TOS level to TAC level was accepted as the oxidative stress index (OSI). The OSI value was calculated according to the following formula: OSI (Arbitrary Unit) = TOS (nmol H2O2 Equivalent/mg protein)/TAC (nmol Trolox Equivalent/mg protein).[17]
Histopathological assessment
Kidneys were fixed in a 10% formalin solution and embedded in paraffin for histopathological assessment. Embedded tissues were cut into 5 μm-thick sections with a microtome and stained with hematoxylin and eosin (H and E). An experienced pathologist, blinded to the treatment conditions and the groups, examined the histological preparations with a light microscope (Nikon ECLIPSE 80i, Japan). Renal injury was graded as follows: Grade 0, no diagnostic change; grade 1, demonstrated tubular cell swelling, brush border loss, nuclear condensation with up to 1/3 of tubular profile showing nuclear loss; grade 2 is as grade 1, but greater than 1/3 and less than 2/3 of tubular profile showing nuclear loss; grade 3, greater than 2/3 of tubular profile showing nuclear loss. A minimum of 10 fields for each kidney slide were examined.
Statistical analysis
All data were expressed as mean and standard deviation (SD). Differences between groups were evaluated by Kruskal-Wallis variance analysis followed by a Mann-Whitney U-test with Bonferroni correction for binary comparisons. The Pearson Chi-Square test was used to compare kidney pathology grades. P < 0.05 were considered statistically significant. All data were processed using the SPSS 15.0 for Windows (SPSS INC., Chicago, IL, USA) statistical package.
RESULTS
The biochemical results are summarized in Table 1. Serum MDA levels were increased with I/R damage, but was not statistically significant when compared with S group. On the other hand, rats treated with PE prior to I/R showed statistically significant reduction on serum MDA levels (P = 0.016). Serum TAC levels were lower in I/R group than S group (P = 0.001). Also, PE pretreatment supported TAC levels significantly (P = 0.001).
Table 1.
Oxidant and antioxidant parameters in rat groups
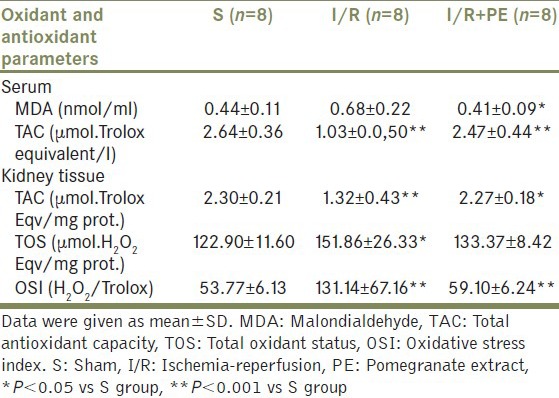
I/R procedure significantly increased TOS levels (P = 0.016) and OSI index (P = 0.001) showing increased oxidative stress and decreased TAC levels (P = 0.002) in rat kidney tissues when compared with S group. After PE treatment, OSI index (P = 0.003) was significantly decreased and TAC levels (P = 0.003) were increased; however, there were no difference about TOS levels.
Kidney histopathological analysis
The renal tissues in S group exhibited normal structure with no pathological changes [Figure 1a]. Histopathological examination of the tissues in I/R group exhibited Grade 3 tubular cell damage in five rats [Figure 1b], Grade 2 damage in two rats, and Grade 1 damage in one rat. PE-treated rats’ kidney tissues showed no severe tubular cell damage; however, mild damage was seen in four rats and moderate tubular cell damage was observed in four rats [Figure 1c] and also histopathological scores were significantly decreased than I/R group. The histopathological changes were graded and summarized in Figure 2.
Figure 1.
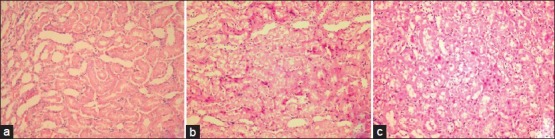
(a) Sham group. Normal histomorphological features of the renal tubules. Mild tubular cell swelling is seen in a few tubular structures (H and E stain, ×200). (b) Ischemia reperfusion group. Note tubular cell swelling, brush border loss, and nuclear condensation with nuclear losses (H and E stain, ×200). (c) Ischemia reperfusion+Pomegranate extract group. Note tubular cell swelling, slight brush border loss, and a few instances of nuclear condensation with some nuclear losses (H and E stain, ×200)
Figure 2.
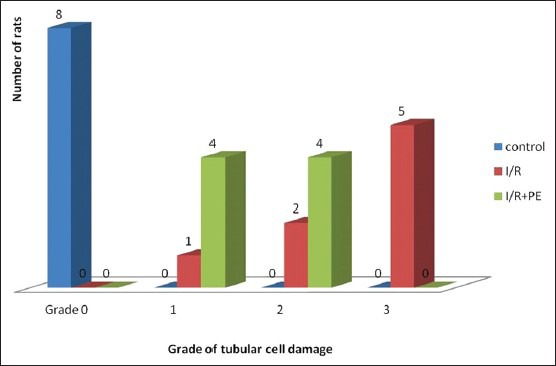
Histopathological evaluation of renal tissue for each group. Scores were significantly lower in IR+PE group than in the I/R group. (Pearson Chi-Square test, P = 0,024). I/R: Ischemia/reperfusion, PE: Pomegranate extract
DISCUSSION
This study showed that I/R-induced oxidative stress decreased with the pretreatment of PE according to the biochemical results of both serum and renal tissue and histopathological examination. The histopathological scores of I/R group was significantly higher than S group (P = 0.001) and lower in I/R + PE group (P = 0.024).
TOS levels and OSI index in kidney tissue were significantly increased after I/R injury showing I/R injury-induced oxidative stress in line with previous studies which has reported decreased TAC levels or low activity of antioxidant enzymes including superoxide dismutase (SOD) and catalase (CAT).[18,19] Reactive oxygen species (ROS) play a major role in the pathogenesis of I/R-induced renal injury. ROS reacts with lipids, proteins, and nucleic acids leading to lipid peroxidation in biological membranes.[20] The protective enzymes SOD, CAT and the antioxidant molecule glutathione reacts against the devastating actions of ROS and these molecules compose the TAC.[21] TAC levels were measured in this study lower in I/R group. High oxidative stress rates may reduce the endogenous antioxidant protective mechanisms. The result of an increase in oxidants and/or a decrease in antioxidant capacity can be defined as oxidative stress. Therefore, measurement of oxidants along with antioxidant components may reflect the status of oxidative stress. In addition, the ratio of the TOS levels to TAC levels is accepted OSI, which is an indicator of oxidative stress. OSI has been suggested to be more valuable parameter than TAC or TOS level alone to reflect the oxidative status.[22] OSI levels were significantly increased in I/R group like increased TOS levels demonstrating the oxidative stress.
Development of therapies to prevent the progression of the oxidative damage induced by I/R injury is important and for this purpose, several natural antioxidant agents such as melatonin, vitamin E, tempol, garlic oil, and nigella sativa have been used.[18,19,20,21] Pomegranate is also one of these agents[5,23] and in several studies, pomegranate has been used for its antioxidant property.[6,9,24] Therefore, the effects of PE on oxidative stress caused by renal ischemia in rats were studied in this study and PE supported antioxidant activity in both kidney tissue and serum. Seeram et al. have reported that the polyphenols including punicalagin, the major fruit ellagitannin, and EA are responsible for the antioxidant and anti-atherosclerotic activities of pomegranate juice. Also, they reported that the level of antioxidant activity was pomegranate juice > pomegranate tannin > punicalagin > EA.[24] In this study, PE reduced the oxidative stress markers in serum and renal tissue and ameliorated the histopathological changes of I/R-induced renal injury.
CONCLUSION
Our data suggest that PE may be an effective chemoprotective agent against renal I/R injury. It may provide protection by reducing the concentration of oxidant products by scavenging free radicals and supporting the antioxidant system. However, further research is needed to understand the possible mechanisms by which PE is able to prevent renal I/R injury.
Footnotes
Source of Support: Nil
Conflict of Interest: None
REFERENCES
- 1.Donnahoo KK, Meldrum DR, Shenkar R, Chung CS, Abraham E, Harken AH. Early renal ischemia, with or without reperfusion, activates NFkappaB and increases TNF-alpha bioactivity in the kidney. J Urol. 2000;163:1328–32. [PubMed] [Google Scholar]
- 2.Sivarajah A, Chatterjee PK, Patel NS, Todorovic Z, Hattori Y, Brown PA, et al. Agonists of peroxisome-proliferator activated receptor-gamma reduce renal ischemia/reperfusion injury. Am J Nephrol. 2003;23:267–76. doi: 10.1159/000072088. [DOI] [PubMed] [Google Scholar]
- 3.Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250:G749–53. doi: 10.1152/ajpgi.1986.250.6.G749. [DOI] [PubMed] [Google Scholar]
- 4.Yousef MI, Saad AA, El-Shennawy LK. Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem Toxicol. 2009;47:1176–83. doi: 10.1016/j.fct.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Tugcu V, Kemahli E, Ozbek E, Arinci YV, Uhri M, Erturkuner P, et al. Protective effect of a potent antioxidant, pomegranate juice, in the kidney of rats with nephrolithiasis induced by ethylene glycol. J Endourol. 2008;22:2723–31. doi: 10.1089/end.2008.0357. [DOI] [PubMed] [Google Scholar]
- 6.Yuce A, Atessahin A, Ceribasi AO. Amelioration of cyclosporine A-induced renal, hepatic and cardiac damages by ellagic acid in rats. Basic Clin Pharmacol Toxicol. 2008;103:186–91. doi: 10.1111/j.1742-7843.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- 7.Celik I, Temur A, Isik I. Hepatoprotective role and antioxidant capacity of pomegranate (Punicagranatum) flowers infusion against trichloroacetic acid-exposed in rats. Food Chem Toxicol. 2009;47:145–9. doi: 10.1016/j.fct.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Esmaillzadeh A, Tahbaz F, Gaieni I, Alavi-Majd H, Azadbakht L. Cholesterol-lowering effect of concentrated pomegranate juice consumption in type II diabetic patients with hyperlipidemia. Int J Vitam Nutr Res. 2006;76:147–51. doi: 10.1024/0300-9831.76.3.147. [DOI] [PubMed] [Google Scholar]
- 9.de Nigris F, Williams-Ignarro S, Sica V, Lerman LO, D’Armiento FP, Byrns RE, et al. Effects of a pomegranate fruit extract rich in punicalagin on oxidation-sensitive genes and eNOS activity at sites of perturbed shear stress and atherogenesis. Cardiovasc Res. 2007;73:414–23. doi: 10.1016/j.cardiores.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Aviram M, Dornfeld L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis. 2001;158:195–8. doi: 10.1016/s0021-9150(01)00412-9. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan M, Hayek T, Raz A, Coleman R, Dornfeld L, Vaya J, et al. Pomegranate juice supplementation to atherosclerotic mice reduces macrophage lipid peroxidation, cellular cholesterol accumulation and development of atherosclerosis. J Nutr. 2001;131:2082–9. doi: 10.1093/jn/131.8.2082. [DOI] [PubMed] [Google Scholar]
- 12.Aviram M, Dornfeld L, Kaplan M, Coleman R, Gaitini D, Nitecki S, et al. Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: Studies in atherosclerotic mice and in humans. Drugs Exp Clin Res. 2002;28:49–62. [PubMed] [Google Scholar]
- 13.Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl AcadSci USA. 2005;102:14813–8. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Draper HH, Csallany AS, Hadley M. Urinary aldehydes as indicators of lipid peroxidation in vivo. Free Radic Biol Med. 2000;29:1071–7. doi: 10.1016/s0891-5849(00)00367-1. [DOI] [PubMed] [Google Scholar]
- 15.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–9. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–11. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Bolukbas C, Bolukbas FF, Horoz M, Aslan M, Celik H, Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis. 2005;5:95. doi: 10.1186/1471-2334-5-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aktoz T, Aydogdu N, Alagol B, Yalcin O, Huseyinova G, Atakan IH. The protective effects of melatonin and vitamin E against renal ischemia-reperfusion injury in rats. Ren Fail. 2007;29:535–42. doi: 10.1080/08860220701391738. [DOI] [PubMed] [Google Scholar]
- 19.Savas M, Yeni E, Ciftci H, Yildiz F, Gulum M, Keser BS, et al. The antioxidant role of oral administration of garlic oil on renal ischemia-reperfusion injury. Ren Fail. 2010;32:362–7. doi: 10.3109/08860221003611711. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, et al. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000;58:658–73. doi: 10.1046/j.1523-1755.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 21.Bayrak O, Bavbek N, Karatas OF, Bayrak R, Catal F, Cimentepe E, et al. Nigella sativa protects against ischaemia/reperfusion injury in rat kidneys. Nephrol Dial Transplant. 2008;23:2206–12. doi: 10.1093/ndt/gfm953. [DOI] [PubMed] [Google Scholar]
- 22.Harma M, Erel O. Oxidative stress in women with preeclampsia. Am J Obstet Gynecol. 2005;192:656–657. doi: 10.1016/j.ajog.2004.07.094. author reply 657. [DOI] [PubMed] [Google Scholar]
- 23.Johanningsmeier SD, Harris GK. Pomegranate as a functional food and nutraceutical source. Annu Rev Food Sci Technol. 2011;2:181–201. doi: 10.1146/annurev-food-030810-153709. [DOI] [PubMed] [Google Scholar]
- 24.Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360–7. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]


