Abstract
Background:
Invasive urothelial bladder carcinomas have a poor prognosis even with cystectomy and chemotherapy. A high number of these patients have Her2 overexpression. The goal of this study is to assess the Her2 status in muscle invasive urothelial bladder carcinoma, to evaluation heterogeneity and discordance with metastases.
Patients and Methods:
We retrospectively analyzed 21 specimens of transurethral resection or cystectomy in patients with invasive urothelial bladder carcinoma. We selected one representative section from primary tumors and metastases for immunohistochemistry analysis. Staining was evaluated according to the same criteria of breast cancer. A chromogenic in situ hybridization (CISH) was performed in case of 2+ score or in heterogeneous samples.
Results:
Median age of our patients was 62 years. Intratumoral heterogeneity was observed in 2 cases (less than 1%). One case showed a Her2 3+ score (high grade, pT2 stage) and 3 cases showed a 2+ score (all low grades, stage T2, T4, M1, respectively). Two metastatic lymph nodes scored 1+ for the first (primary 1+) and 2+ for the second (primary 1+). Two cases showed CISH gene amplification. The first one scored 2+ and had area of 3+ score. The second one scored 1+ and had area with 2+ score.
Four patients died from disease, one of them had Her2 3+ score.
Conclusion:
Her2 overexpression can be observed in muscle invasive urothelial bladder carcinoma in an important number of patients. Evaluation criteria must be standardized, especially with heterogeneous cases. Metastases tests can also readdress the expression of Her2, which gives the patient a supplementary therapeutic tool.
Keywords: Her2, immunohistochemistry, prognosis, targeted therapy, urothelial invasive bladder carcinoma
INTRODUCTION
Urinary bladder cancer is the fourth most common malignancy in men and the ninth most common in women. Eighty percent of urothelial bladder carcinoma are superficial at the time of diagnosis and have a high survival rate.[1] The invasive form is however associated with a lower survival rate decreasing from 80% to 50%. Metastases occur most of the time in lymph nodes but also in lung, liver, and skeleton. In fact, 30% of patients are invasive or metastatic at the time of diagnosis.
Approximately 10 to 15% of superficial tumors progress to invasive stage. This risk is dependent on stage and grade, which are subjects of inter-and intra-observer variation.[1] More accurate prognostic factors have been developed such as polysomy 17 and Her2 overexpression.
The human epidermal growth factor receptor (Her2) encodes for a tyrosine kinase receptor of the epidermal growth factor. Its activation increases the mitotic activity and metastatic potential of the cell leading to oncogenic transformation. Several studies on transitional bladder carcinoma examined the overexpression of Her2 protein, with a range reported between 17% and 76% of invasive carcinoma.[2] This overexpression seems to be correlated with earlier time recurrence, higher grade, and worse prognosis. Her2 can be assessed with IHC staining or with Fluorescent in situ hybridization (FISH). Variant results of correlation between those techniques have been reported. Her2 overexpression can be observed in the primary tumor and in the metastatic lesions but correlation is still a controversy.
A reliable evaluation is needed to introduce targeted therapy in the management of invasive urothelial bladder carcinoma.
The goal of this study is to evaluate the status and pathological heterogeneity of Her2 overexpression in urothelial muscle invasive bladder carcinoma. We also studied Her2 expression in primary and metastatic samples.
PATIENTS AND METHODS
Patients
We selected 31 patients with muscle invasive urothelial carcinoma (pT2 and more) from the department of pathology, Salah Azaiez institute in 18 years period from 1993 to 2011. Patients who did not have complete follow-up or representative sections were excluded. Data of 21 patients were collected from surgical records. A total of 21 specimens from primary tumors were included and two additional metastatic lymph nodes were added.
Tumors were staged and graded according to the World Health Organization (WHO) 2004.
All patients had clinical follow up (age, sex, stage, grade, treatment, survival). Samples for histological examination were obtained after endoscopic resection (10 cases) and/or cystectomy (18 cases).
All samples have been reviewed and pathologically staged by two pathologists with a double blind examination. One representative block was selected to immunohistochemistry (IHC) analysis. In cases with metastatic lymph nodes, one block was chosen to IHC analysis. Heterogeneity was defined by at least one Her2 negative field in a Her2-positive tumor.
Immunohistochemistry
In each case, sections cut containing representative area were stained immunohistochemically. We used a Her2 antibody type Leica clone NCL-N-CD11.
Only membrane staining was scored according to the same standard criteria used in breast cancer. Her2 positivity was assessed using the following scoring system:
0 : No membrane staining or less than 10% of cells.
1+: Partial membrane staining in more than 10% of cells.
2+: Weak, circumferential membrane staining in more than 10% of cells, or intense membrane staining in less than 30% of cases.
3+: Intense membrane staining in more than 30% of cells.
Protein overexpression was considered present if IHC score was 3+. Specimens with 2+ score were selected of chromogenic in situ hybridization (CISH) analysis.
Metastatic tumors and lymph nodes were stained and scored with the same criteria.
Chromogenic in situ hybridization
Only cases scored 2 + or/and had an intratumoral heterogeneity were analyzed by CISH to evaluate Her2 gene copy number. All samples were carried out on a single block according to the instruction from the test kits.
RESULTS
Patients
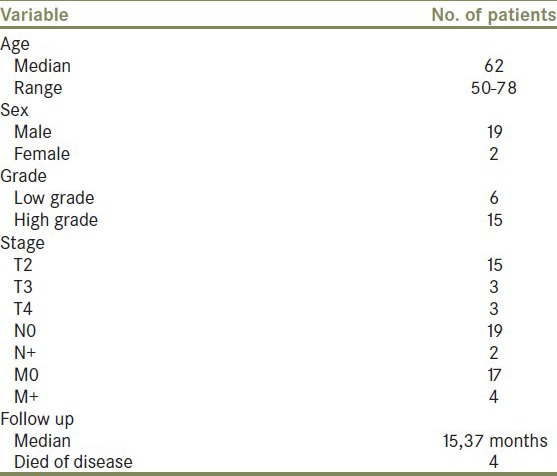
The average age of our patients was 62 years (range, 50-78 years). There were 19 males and 2 females with sex ratio M/F of 8.
There were 15 cases (75%) with stage T2, 3 cases (15%) with stage T3, and 3 cases with stage T4. Two cases of lymph node involvement and four cases of metastasis were observed (lung, liver).
Four deaths related directly to disease progression were observed; one case was associated with Her2 positive (3+) and had metastatic disease.
The time of follow up was of 3 months in Her3+ patient, 25 months in Her2+ patients, and 11 months in Her2-negative patients.
The relationship between the distribution of age, sex, and tumor stage and grade is summarized in Table 1.
Table 1.
Relationship between the distribution of age, sex, and tumor stage and grade
Microscopic aspects
We observed two cases with glandular differentiation, two cases with micro papillary features, one case with sarcomatoide differentiation, and four cases with squamous features. Most of our patients were high grade (17 cases, 70%).
Immunohistochemistry
One sample scored 3+, three scored 2+, and 17 scored 0/1+ [Figure 1].
Figure 1.
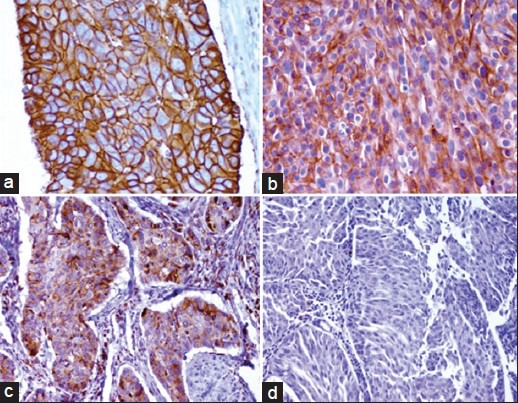
(a) Her2 (3+) score, (b) Her2 (2+) score, (c) Her2 (1+) score, (d) Her2 (0) score
Heterogeneous staining was observed in two cases; in one case, we found 10% of Her2 3+ area in a Her2+ tumor and in the other case more than 10% of Her2+ area in a Her1+ tumor.
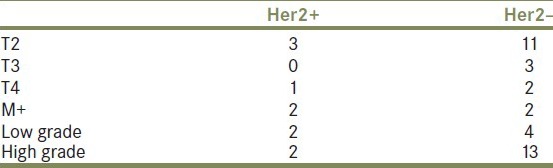
Her2-positive staining was more frequent in higher stage (2/4 cases were stage pT4 or M+) or higher grade (2 cases with high grade 3). However, among the four cases of death, only one patient had Her2-positive staining.
In Her2-negative tumors, 63% (12/19) were high grade and 57% (11/16) were stage T2, two cases with lymph node involvement and one case with metastases (lung not histologically examined).
IHC analysis of two added metastatic lymph nodes showed Her2 1+ staining in 1+ primary tumor and 2+ staining in 1+ primary tumor.
The distribution of Her2-positive and negative cases among stages and grades is summarized in Table 2.
Table 2.
Distribution of HER2-positive and negative cases among stages and grades
Chromogenic in situ hybridization
Four samples were analyzed. Only two cases showed gene amplification. The first one scored 2+ and had area of 3+ score [Figure 2a]. The second one scored 1+ and had area with 2+ score [Figure 2b]. The two other samples scored 2+.
Figure 2.
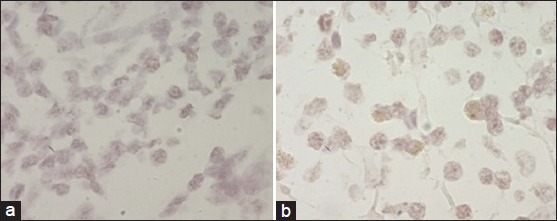
(a) CISH amplification in a sample scored 2+ with 3+ score area. (b) CISH amplification in a sample scored 1+ with 2+ area
DISCUSSION
Since the important prognostic and therapeutic impact that Her2 status had in breast carcinoma, more interest has been given to its expression in other cancers. In 1990, Zhau et al. reported first an increased amplification and an overexpression of the Her2 in bladder carcinoma.[1] Since then, several studies tried to evaluate its status and prognostic role.
Incidence of Her2 overexpression in muscle-invasive urothelial bladder carcinoma is variable in the literature. It occurs in a median of 45%, and ranges from 4 to 32% in invasive stages and from 2 to 50% in high-grade forms.[1,2]
The largest series (1005 cases) reported by Laé et al. suggested an incidence reaching 9.2%, which was lower than that reported in the literature (23-80%).[3,4]
Some authors consider both 2+ and 3+ score as a positive Her2 status and other consider only 3+ score. In our study, Her2 2+/3+ staining was observed in 19% (4 cases: Only one cases 3+ score) of tumor samples. This rate is similar to the rates described previously.
Heterogeneous IHC staining, found in only 5% of invasive breast carcinoma, was less frequent (1%) in our study. M Laé et al. found 35% of intratumoral heterogeneity, which made analysis more difficult.[3,4,5] However, the study of Edwards et al. found a uniform IHC staining of Her2 in all 39 cases tested.[4] These discordant results are explained by the diversity of kits, antibodies, and interpretations, and highlight the necessity to standardize kits and assessment criteria. That is why many authors recommend to carry out a FISH in these cases, in order to avoid ambiguity.
Gene amplification in invasive urothelial bladder carcinoma reported in the literature ranges from 0% to 30% in urothelial muscle-invasive bladder carcinoma.[6,7] Laé et al. reported a 5.1% of gene amplification in their series.
Several studies compared IHC to FISH results.[3,4,5,6,7,8,9,10,11,12] Sauter et al. reported in 141 cases an overexpression in 43%, but only 7% of gene amplification (all of these cases showed Her2 overexpression); similar results have been reported by Zhau et al. This discordance seems to be more frequent than in breast cancer where it has been reported in 36% of cases.[8] It can be explained by the several mechanisms leading to protein expression (translocation, mutation), the bias in sampling, cross-reactivity of antibodies, or by interobserver variation. Thus, they suggest that the decision algorithm used in breast cancer can be used in bladder carcinoma; selection of patients with invasive urothelial carcinoma might rely on strong IHC staining and when it is difficult to assess, a FISH or CISH analysis become necessary. In our series, we performed a CISH analysis, in Her2 2+ specimen and in heterogeneous cases, in order to confirm the diagnosis. Two cases had CISH amplification.
Her2 status in primary tumor and in distant metastases has been also compared in several studies.
100% concordance for Her2/neu amplification and 88% concordance in overexpression have been reported.[3] Differences between primary and metastatic tumor can be explained by the heterogeneity of overexpression as reported by Jimenez et al. Other theories have been advanced, such as growth-stimulating signals from Her2 are different in primary and in metastatic lesions.[9]
In our series, Her2 overexpression in metastatic lymph node lesions have been assessed in two cases and showed a concordant result in a case (1+ score) and discordant result in the other one; a 2+ score in metastases but 1+ score in primary tumor.
The prognostic impact of Her2 overexpression is still a controversy.
Several studies (Jamez, Undewood, and Kringer) found that Her2 overexpression is predictive of bladder cancer-related death in patient with invasive tumors.[10] B. Kolla et al. observed a significantly high disease-free survival in Her2-negative patients compared to Her2-positive patients; this difference was more profound in patients with locally advanced disease (T2b, T4, N+). But high controversial results have been published; Rafeael et al. reported no significant difference in survival between Her2-positive and negative patients.[9]
Recently, an anti-Her2 antibody (Trastuzumab) was proposed as therapeutic tool in invasive urothelial carcinoma of the bladder. Response rates range from 3% to 63%.[13]
All these results suggest that IHC assessment of Her2 overexpression might represent an additional tool to evaluate individual prognosis of bladder patients.
CONCLUSION
HER2 overexpression, expressed in a median of 45% of invasive urothelial bladder carcinoma, became an interesting tool to assess the patient prognosis and to select those who can be candidates for targeted therapy. Evaluation criteria must be standardized, especially with heterogeneous cases. Metastases tests can also readdress the expression of Her2, which gives the patient a supplementary therapeutic tool. Trastuzumab (targeted therapy for Her2) can improve the overcome of patients with Her2 overexpression. More investigation and clinical trials are needed in order to standardize diagnostic criteria and tools and the optimal use of Her2 expression in targeted therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None
REFERENCES
- 1.Stein JP, Lieskovsky G, Cote R. Radical cystectomy in the treatment of bladder cancer: Long term results in 1045 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 2.Lonn U, Lonn S, Friberg S, Nilsson B, Silfverswärd C, Stenkvist B. Prognostin value of amplification of c-erb-B2 in bladder carcinoma. Clin Cancer Res. 1995;1:1189–94. [PubMed] [Google Scholar]
- 3.Laé M, Couturier J, Oudard S, Radvanyi F, Beuzeboc P, Vieliiefond A. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: Results in 1005 patients. Ann Oncol. 2010;21:815–9. doi: 10.1093/annonc/mdp488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Underwood M, Bartlett J, Reeves J, Gardiner S, Scott R, Cooke T. C-erbB-2: Gene amplification: A marquerin recurrent bladder tumors? Cancer Res. 1995;55:2422–30. [PubMed] [Google Scholar]
- 5.Surenda B, Amlesh S, Mano K, Narmada P, Ashok K, Prem M. Prognostic significance of Her2/neu overexpression in patients with muscle invasiveurinary bladder cancer treated with radical cystectomy. Int Urol Nephrol. 2008;40:321–7. doi: 10.1007/s11255-007-9283-x. [DOI] [PubMed] [Google Scholar]
- 6.Latif Z, Watters AD, Dunn I, Grigor KM, Underwood MA, Bartlett JM. HER2/neu overexpression in the development of muscle invasive transitional cell carcinoma of the bladder. Br J Cancer. 2003;89:1305–9. doi: 10.1038/sj.bjc.6601245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCann A, Dervan PA, Johnson PA, Gullick WJ, Carney DN. C-erbB-2 oncoprotein expression in primary humantumors. Cancer. 1990;65:88–92. doi: 10.1002/1097-0142(19900101)65:1<88::aid-cncr2820650119>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.Sauter G, Moch H, Moore D, Caroll P, Kerschmann R, Chew K, et al. Heterogeneity of erbB-2 bene amplification in bladder cancer. Cancer Res. 1993;53:2199–203. [PubMed] [Google Scholar]
- 9.Jimenez RE, Hussain M, Bianco F, Jr, Vaishampayan U, Tabazcka P, Sakr WA, et al. Her-2/neu overexpression in muscle urothelial carcinoma of the bladder: Prognostic significance and comparative analysis in primary and metastatic tumors. Clin Cancer Res. 2001;7:2440–7. [PubMed] [Google Scholar]
- 10.Coogan CL, Estrada CR, Kapur S, Bloom KJ. HER-2/neu protein overexpression and gene amplification in humain transitional cell carcinoma of the bladder. Urology. 2004;63:786–90. doi: 10.1016/j.urology.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Gardmark T, Wester K, De la torre M, Carlsonn J, Malmstron PU. Analysis of HER2 expression in primary urinary bladder carcinoma and corresponding metastases. BJUInt. 2005;95:982–6. doi: 10.1111/j.1464-410X.2005.05452.x. [DOI] [PubMed] [Google Scholar]
- 12.De Pinieux G, Colin D, Vincent-Salomon A, Couturier J, Amsallem D, Beuzeboc P, et al. Confrontation of immunohistochemistry and fluorescent in situ hybridization for the assessment of HER-2/neu (c-erbb-2) status in urothelial carcinoma. Virchows Arch. 2004;444:415–9. doi: 10.1007/s00428-004-0986-4. [DOI] [PubMed] [Google Scholar]
- 13.Hussain MH, MacVicar GR, Petrylak DP. Trastuzumab, paclitaxel, carboplatin, and gencitabine in advanced human epidermal growth factor receptor-2/neu6positif urothelial carcinoma/results of a multicenter phase II National cancer Institute tria. J Clin Oncol. 2007;25:2218–24. doi: 10.1200/JCO.2006.08.0994. [DOI] [PubMed] [Google Scholar]


