Abstract
Phytochemicals are a rich source of anticancer drugs and chemopreventive agents. Several of these chemicals appear to exert at least some of their effects through interactions with topoisomerase II, an essential enzyme that regulates DNA supercoiling and removes knots and tangles from the genome. Topoisomerase II-active phytochemicals function by stabilizing covalent protein-cleaved DNA complexes that are intermediates in the catalytic cycle of the enzyme. As a result, these compounds convert topoisomerase II to a cellular toxin that fragments the genome. Because of their mode of action, they are referred to as topoisomerase II poisons as opposed to catalytic inhibitors. The first sections of this article discuss DNA topology, the catalytic cycle of topoisomerase II, and the two mechanisms (interfacial vs. covalent) by which different classes of topoisomerase II poisons alter enzyme activity. Subsequent sections discuss the effects of several phytochemicals on the type II enzyme, including demethyl-epipodophyllotoxins (semisynthetic anticancer drugs) as well as flavones, flavonols, isoflavones, catechins, isothiocyanates, and curcumin (dietary chemopreventive agents). Finally, the leukemogenic potential of topoisomerase II-targeted phytochemicals is described.
Keywords: Demethyl-epipodophyllotoxins, Bioflavonoids, Catechins, Isothiocyanates, Curcumin
Introduction
Topoisomerase II is the target for a broad spectrum of anticancer and chemopreventive agents. Many of these agents are dietary phytochemicals or are derived from these compounds. Topoisomerase II-targeted chemicals kill cells in a unique and insidious fashion: rather than depriving cells of the essential activities of the enzyme, they convert topoisomerase II into a toxic enzyme that fragments the genome. Hence, these compounds are referred to as “topoisomerase II poisons.”
In order to fully appreciate how phytochemicals are able to impact human health through their interactions with topoisomerase II, it is necessary to understand why cells need the type II enzyme, how it functions, and how topoisomerase II poisons alter the activity of the enzyme. Therefore, the early sections of this article will address these important issues. The later sections will discuss phytochemicals that are topoisomerase II poisons, including widely prescribed anticancer drugs as well as dietary compounds with chemopreventive (and potentially chemotherapeutic) properties.
DNA Topology
The human genome is encoded in a series of forty-six linear chromosomes that range in size from ~50 million to ~250 million DNA base pairs. Furthermore, the genetic information is stored in the form of a double helix in which the two DNA strands are plectonemically coiled around one another (Bates and Maxwell, 2005; Deweese et al., 2008; Liu et al., 2009). Although these structural features contribute to the physical integrity of the genome, they impose a number of topological constraints on the genetic material that affect all of its physiological functions (Bates and Maxwell, 2005; Deweese et al., 2008; Liu et al., 2009).
Topological properties of DNA, including under- and overwinding, knotting, and tangling, can only be changed when one or both strands of the double helix are broken (Bates and Maxwell, 2005; Deweese et al., 2008; Liu et al., 2009; Ketron and Osheroff, 2013). DNA underwinding (i.e., negative supercoiling) is important because the two strands of the double helix must be separated in order to replicate or express the genetic information. Because negative supercoiling destabilizes the double-stranded nature of DNA, it facilitates strand separation. Conversely, DNA overwinding (i.e., positive supercoiling) that occurs ahead of replication forks and transcription complexes inhibits strand separation and blocks these critical cellular processes (Bates and Maxwell, 2005; Deweese et al., 2008; Liu et al., 2009; Ketron and Osheroff, 2013).
Essential nucleic acid functions, such as DNA recombination and replication, generate knots and tangles within the double helix (Bates and Maxwell, 2005; Deweese et al., 2008; Liu et al., 2009; Ketron and Osheroff, 2013). DNA knots impair the ability to separate the two strands of the genetic material and intermolecular DNA tangles prevent segregation of chromosomes during mitosis. Consequently, these topological obstacles can be lethal to cells if they are not resolved. Enzymes that regulate the topological structure of DNA are called topoisomerases (Champoux, 2001; Corbett and Berger, 2004; Deweese et al., 2008; Vos et al., 2011; Ketron and Osheroff, 2013). Topoisomerases can be separated into two major classes, which are distinguished by the number of DNA strands they cleave and ligate. Type I topoisomerases act by cleaving one strand of the double helix. Thus, they are able to regulate levels of DNA supercoiling (Champoux, 1994; Leppard and Champoux, 2005; Pommier, 2009). In contrast, type II topoisomerases act by cleaving both strands of the double helix (Deweese and Osheroff, 2009; Nitiss, 2009a; Vos et al., 2011). As a result, they can regulate the superhelical density of DNA and also can resolve tangles and knots in duplex DNA. In order to maintain the integrity of the genome during the required DNA cleavage event, all topoisomerases form covalent bonds between active site tyrosyl residues and the DNA termini generated during the reaction (Champoux, 2001; Corbett and Berger, 2004; Deweese et al., 2008; Vos et al., 2011; Ketron and Osheroff, 2013). This covalent enzyme-cleaved DNA complex (known as the “cleavage complex”) is a hallmark of topoisomerases.
This article will focus on eukaryotic type II topoisomerases. A number of recent review articles that discuss type I topoisomerases (Baker et al., 2009; Pommier, 2009, 2013) or bacterial type II enzymes are available (Sissi and Palumbo, 2010; Collin et al., 2011).
Topoisomerase II
Eukaryotic type II topoisomerases are homodimeric enzymes. Humans express two isoforms of topoisomerase II, α and β (Austin and Marsh, 1998; Champoux, 2001; Corbett and Berger, 2004; Deweese et al., 2008; Deweese and Osheroff, 2009; Nitiss, 2009a; Vos et al., 2011). These two isoforms share extensive amino acid sequence identity (~70%), but are encoded by separate genes (located at chromosomal bands 17q21-22 and 3p24 in humans, respectively). Topoisomerase IIα and IIβ also can be distinguished by their protomer molecular masses (~170 kDa and ~180 kDa, respectively).
Topoisomerase IIα and IIβ have distinct patterns of expression and separate nuclear functions (Austin and Marsh, 1998; Champoux, 2001; Wang, 2002; Deweese et al., 2008; Deweese and Osheroff, 2009; Nitiss, 2009a; Vos et al., 2011). Topoisomerase IIα is essential for the survival of proliferating cells and protein levels rise dramatically during periods of cell growth. The enzyme is further regulated over the cell cycle, with protein concentrations peaking in G2/M. Topoisomerase IIα unlinks tangled daughter chromosomes following replication and resolves DNA knots that are formed during recombination. It also helps to alleviate the torsional stress that accumulates ahead of replication forks and transcription complexes. Furthermore, topoisomerase IIα is required for proper chromosome condensation, cohesion, and segregation and appears to play roles in centromere function and chromatin remodeling. Finally, the enzyme is important for the maintenance of proper chromosome organization and structure and is the major non-histone protein of the mitotic chromosome scaffold and the interphase nuclear matrix.
Topoisomerase IIβ is dispensable at the cellular level, and its presence cannot compensate for the loss of topoisomerase IIα in mammalian cells (Austin and Marsh, 1998; Champoux, 2001; Corbett and Berger, 2004; Deweese et al., 2008; Deweese and Osheroff, 2009; Nitiss, 2009a; Vos et al., 2011). However, the β isoform is required for proper neural development in mice (Yang et al., 2000). In contrast to topoisomerase IIα, the concentration of topoisomerase IIβ is independent of the cell cycle, and high levels of this isoform are found in most cell types regardless of proliferation status (Austin and Marsh, 1998; Isaacs et al., 1998; Linka et al., 2007). Furthermore, topoisomerase IIβ dissociates from chromosomes during mitosis. Ultimately, the physiological functions of the β isoform have yet to be fully defined. However, it has been suggested that topoisomerase IIβ plays an important role in the transcription of hormonally- or developmentally-regulated genes (Haince et al., 2006; Ju et al., 2006).
Much of what we understand regarding the mechanism of action of type II enzymes comes from experiments with topoisomerase II from eukaryotic species that express only a single form of the protein. Consequently, eukaryotic type II topoisomerases will be referred to collectively as topoisomerase II, unless the properties being discussed are specific to either the α or β isoform.
Topoisomerase II catalytic cycle
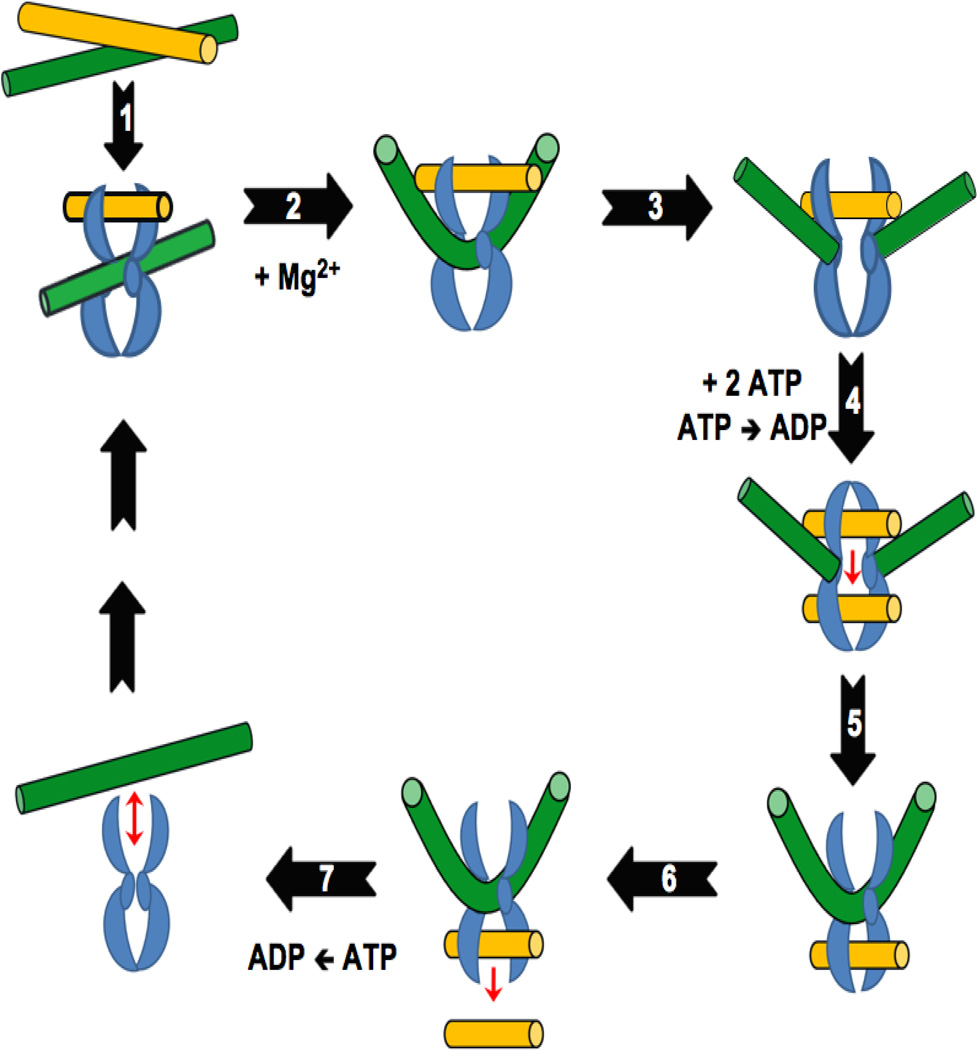
Topoisomerase II regulates the superhelical density of DNA and removes tangles and knots from the genetic material by the double-stranded DNA passage reaction depicted in Figure 1 (Champoux, 2001; Corbett and Berger, 2004; Deweese et al., 2009; Deweese and Osheroff, 2009; Liu et al., 2009; Deweese and Osheroff, 2010; Vos et al., 2011; Ketron and Osheroff, 2013). The enzyme requires divalent metal ions (Mg2+ appears to be the physiological ion) and ATP in order to carry out the complete catalytic cycle.
Figure 1.
Catalytic cycle of type II topoisomerases. Adapted from (Ketron and Osheroff, 2013). The homodimeric enzyme is shown in blue, the DNA double helix that is cleaved and acts as the DNA gate (G-segment) is shown in green, and the double helix that is transported through the DNA gate (T-segment) is shown in yellow. Details of the individual reaction steps are described in the text.
Step 1: Topoisomerase II binds two segments of DNA. The first segment bound by the enzyme is the double helix that will be cleaved and is referred to as the “Gate-” or “G-segment.” The second segment is the double helix that will be transported through the transient DNA gate and is referred to as the “Transport-” or “T-segment.” DNA binding requires no cofactors. Step 2: In the presence of the active site Mg2+ ions, topoisomerase II samples the DNA for malleability (Lee et al., 2012). Sequences that can be cleaved are bent to an angle of ~150° (Dong and Berger, 2007; Schmidt et al., 2010; Hardin et al., 2011; Lee et al., 2012). Conversely, sequences that cannot be bent are not cleaved (Lee et al., 2012). Step 3: A double-stranded break is generated in the G-segment using a noncanonical two-metal-ion mechanism (Deweese and Osheroff, 2010; Schmidt et al., 2010). Cleavage is initiated by the nucleophillic attack of the two active site tyrosyl residues (one in each subunit of the homodimeric enzyme; Tyr805 and Tyr821 in human topoisomerase IIα and topoisomerase IIβ, respectively) on the DNA backbone, each of which makes a single-stranded DNA break. The resulting transesterification reaction results in the formation of a covalent phosphotyrosyl bond that links the protein to each of the newly generated 5’-DNA termini. It also generates a 3’-hydroxyl moiety on the opposite terminus of each cleaved strand. The scissile bonds in the two strands of the double helix are staggered and are located across the major groove from one another. Thus, topoisomerase II generates cleaved DNA molecules with 4-base 5’-single-stranded cohesive ends, each of which is covalently linked to a separate protomer subunit of the enzyme. Step 4: Two molecules of ATP are bound by the enzyme, which triggers the closing of the N-terminal protein gate, the opening of the DNA gate, and the translocation of the T-segment through the gate. Although hydrolysis of the cofactor is not a prerequisite for DNA translocation, it appears that this step proceeds more rapidly if it is preceded by hydrolysis of one of the bound ATP molecules. Step 5: Topoisomerase II ligates the cleaved DNA strands. Step 6: The T-segment is released through the C-terminal protein gate. Step 7: Upon hydrolysis of the second ATP molecule, topoisomerase II regains the ability to initiate a new round of catalysis.
The covalent enzyme-DNA linkage formed during DNA scission (Step 3) plays two important roles in the topoisomerase II reaction mechanism (Champoux, 2001; Corbett and Berger, 2004; Deweese et al., 2009; Deweese and Osheroff, 2009; Liu et al., 2009; Deweese and Osheroff, 2010; Vos et al., 2011; Ketron and Osheroff, 2013). First, it conserves the bond energy of the sugar-phosphate DNA backbone. Second, because it does not allow the cleaved DNA chain to dissociate from the enzyme, the protein-DNA linkage maintains the integrity of the genetic material during the cleavage event. The covalent topoisomerase II-cleaved DNA reaction intermediate is referred to as the “cleavage complex” and is critical for the pharmacological activities of phytochemicals on the enzyme, which are discussed later in this article. The DNA cleavage/ligation equilibrium of the enzyme greatly favors ligation (Champoux, 2001; Corbett and Berger, 2004; Deweese et al., 2009; Deweese and Osheroff, 2009; Liu et al., 2009; Deweese and Osheroff, 2010; Vos et al., 2011; Ketron and Osheroff, 2013). Thus, topoisomerase II-DNA cleavage complexes normally are short-lived and are readily reversible. As described below, compounds that increase the longevity of cleavage complexes can have serious cellular consequences.
Topoisomerase II as a cellular toxin
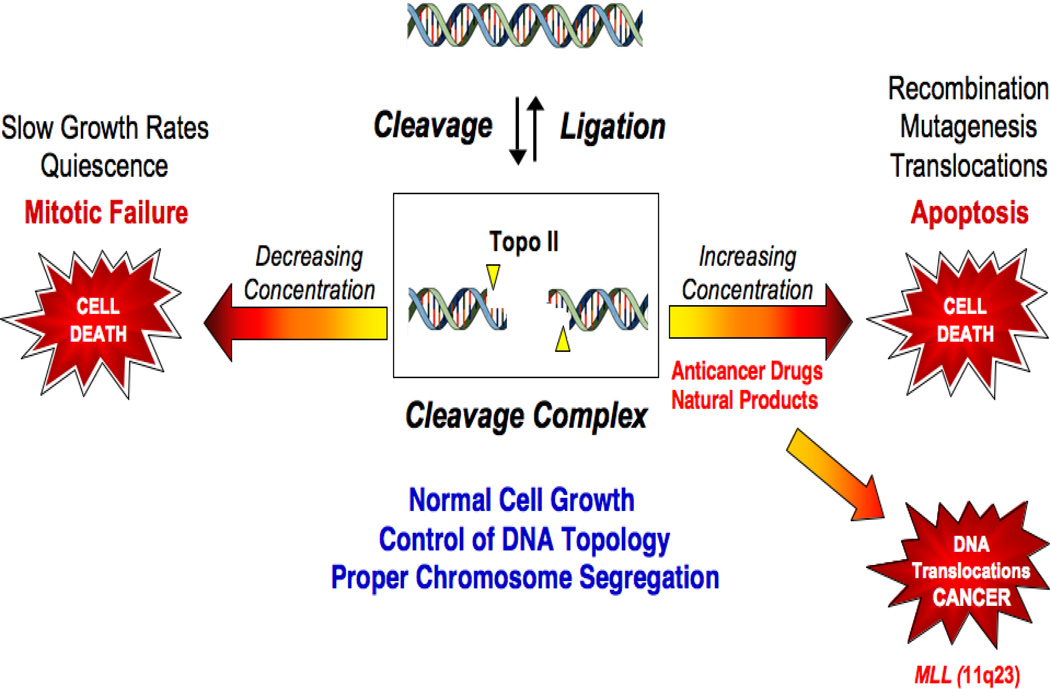
Because topoisomerases generate DNA strand breaks as obligate reaction intermediates, they are intrinsically dangerous proteins (Pommier and Marchand, 2005; McClendon and Osheroff, 2007; Deweese et al., 2008; Deweese and Osheroff, 2009; Nitiss, 2009b; Pommier, 2009; Pommier et al., 2010). Thus, while necessary for cell viability, these enzymes also have the capacity to fragment the genome (Figure 2). As a result of this dual “Dr. Jekyll/Mr. Hyde” persona, cells maintain levels of cleavage complexes in a critical balance. If topoisomerase IIα cleavage drops below threshold levels, daughter chromosomes remain entangled following replication (Wang, 1991; Champoux, 2001; Deweese et al., 2008; Nitiss, 2009a; Vos et al., 2011). As a result, chromosomes cannot segregate properly, and cells die as a result of catastrophic mitotic failure (Figure 2).
Figure 2.
Topoisomerase II-DNA cleavage complex equilibrium. Adapted from (Deweese and Osheroff, 2009). The formation of covalent DNA cleavage complexes is required for topoisomerases to perform their critical cellular functions. If the level of topoisomerase II-DNA cleavage complexes falls below threshold levels (left arrow), cells are unable to segregate their chromosomes and ultimately die of mitotic failure. If the level of cleavage complexes becomes too high (right arrow) the actions of DNA tracking systems can convert these transient complexes to permanent double-stranded breaks. The resulting DNA breaks, as well as the inhibition of essential DNA processes, initiate recombination/repair pathways and generate mutations, chromosome translocations, and other DNA aberrations. If the strand breaks overwhelm the cell, they can trigger apoptosis. This is the basis for the actions of several widely prescribed anticancer drugs that target topoisomerase II. However, if the increase in enzyme-mediated DNA strand breaks does not kill the cell, mutations or chromosomal aberrations may persist in surviving populations. In some cases, exposure to topoisomerase II-targeted agents has been associated with the formation of acute myeloid leukemias that involve the MLL (mixed lineage leukemia) gene at chromosome band 11q23 (lower right arrow).
Increased levels of topoisomerase IIα- or IIβ-DNA cleavage complexes also cause deleterious physiological effects, but for different reasons (Figure 2) (Pommier and Marchand, 2005; McClendon and Osheroff, 2007; Deweese et al., 2008; Deweese and Osheroff, 2009; Nitiss, 2009b; Pommier, 2009; Pommier et al., 2010). When replication forks, transcription complexes, or other DNA tracking proteins attempt to traverse covalently bound protein “roadblocks” in the genetic material, accumulated cleavage intermediates are converted to strand breaks that are no longer tethered by proteinaceous bridges. The ensuing damage induces recombination/repair pathways that can trigger mutations and other chromosomal aberrations. If the number of DNA breaks overwhelms the repair process, it can initiate cell death pathways (D'Arpa et al., 1990; Kaufmann, 1998; Fortune and Osheroff, 2000; McClendon and Osheroff, 2007; Bender and Osheroff, 2008). Conversely, if cells are not killed, DNA breaks can be converted to permanent chromosomal translocations that lead to specific forms of leukemia (Felix et al., 2006; Joannides and Grimwade, 2010).
Topoisomerase II poisons
Compounds that alter topoisomerase II activity can be separated into two categories. Chemicals that decrease the overall activity of the enzyme are known as catalytic inhibitors (Andoh and Ishida, 1998; Fortune and Osheroff, 1998; Bailly, 2012; Pommier, 2013). Conversely, chemicals that increase levels of topoisomerase II-DNA cleavage complexes are said to “poison” the enzyme and convert it to a cellular toxin that initiates the mutagenic and lethal consequences described in Figure 2 (Pommier and Marchand, 2005; McClendon and Osheroff, 2007; Deweese et al., 2008; Deweese and Osheroff, 2009; Nitiss, 2009b; Pommier, 2009; Pommier et al., 2010; Bailly, 2012; Chen et al., 2012; Pommier, 2013). Because of their actions, these latter compounds are referred to as “topoisomerase II poisons” to distinguish them from catalytic inhibitors that do not increase the concentration of cleavage complexes (Pommier and Marchand, 2005; McClendon and Osheroff, 2007; Deweese et al., 2008; Deweese and Osheroff, 2009; Nitiss, 2009b; Pommier, 2009; Pommier et al., 2010; Bailly, 2012; Chen et al., 2012; Pommier, 2013). Although some topoisomerase II poisons also inhibit overall activity, the “gain of function” induced by these compounds in the cell (i.e., increased levels of cleavage complexes) is a dominant phenotype. Thus, they kill cells by a fundamentally different mechanism than that of most protein-targeted drugs (which act by robbing the cell of an essential function). As discussed below, a number of phytochemicals that display anticancer or chemopreventive properties act as topoisomerase II poisons.
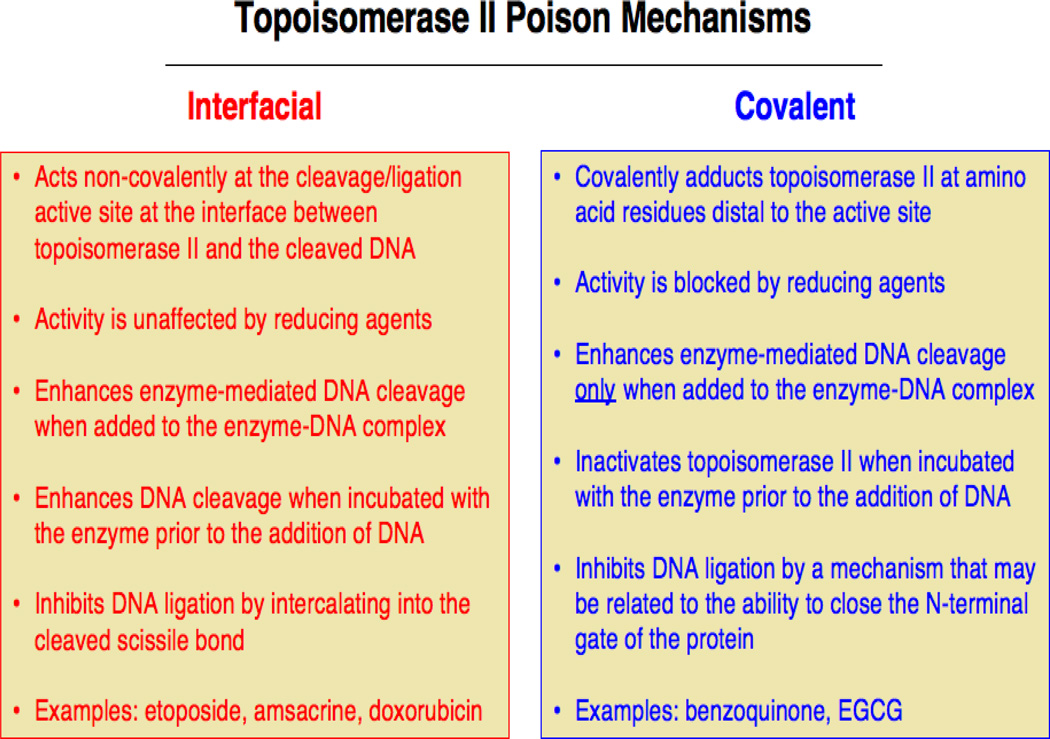
Chemicals that function as topoisomerase II poisons act by two distinct mechanisms (Figure 3). Compounds utilizing the first mechanism are referred to as interfacial topoisomerase II poisons (Pommier and Marchand, 2005; McClendon and Osheroff, 2007; Deweese et al., 2008; Deweese and Osheroff, 2009; Nitiss, 2009b; Pommier, 2009; Pommier et al., 2010; Pommier, 2013). These chemicals form non-covalent interactions with topoisomerase II at the protein-DNA interface in the vicinity of the active site tyrosine. They also interact with DNA within the ternary enzyme-DNA-poison complex and inhibit ligation by intercalating into the double helix at the cleaved scissile bond. Thus, they present a physical barrier to ligation and act as “molecular doorstops.” It is notable that the actions of interfacial topoisomerase II poisons are not affected by reducing agents, such as dithiothreitol, and that these compounds induce similar levels of enzyme-mediated DNA cleavage whether they are added to the binary topoisomerase II-DNA complex or are incubated with the enzyme prior to the addition of nucleic acid substrates.
Figure 3.
Distinguishing characteristics of interfacial vs. covalent topoisomerase II poisons. Details are provided in the text.
Unlike interfacial poisons, compounds that use the second mechanism contain protein reactive groups. Because many of the original compounds that were examined underwent redox cycling as a prerequisite for activity, compounds that utilize this second mechanism originally were referred to as redox-dependent topoisomerase II poisons (Wang et al., 2001; Lindsey et al., 2004; Bender et al., 2006; Deweese and Osheroff, 2009; Lin et al., 2011). However, recent studies indicate that some of these compounds can be activated in the absence of redox cycling (Wang et al., 2001; Lindsey et al., 2004; Bender et al., 2006; Deweese and Osheroff, 2009; Lin et al., 2011; Ketron et al., 2013). Therefore, because the common feature of these poisons is the covalent adduction of the enzyme (see below), we suggest that it is more correct to call them covalent topoisomerase II poisons.
Covalent topoisomerase II poisons all incorporate sulfhydryl-reactive groups such as quinones, isothiocyanates, or maleimides (Wang et al., 2001; Lindsey et al., 2004; Bender et al., 2006; Deweese and Osheroff, 2009; Lin et al., 2011; Ketron et al., 2013). In contrast to interfacial topoisomerase II poisons, covalent poisons modify the enzyme at amino acid residues outside of the active site (Wang et al., 2001; Lindsey et al., 2004; Bender et al., 2006; Deweese and Osheroff, 2009; Lin et al., 2011), and their ability to poison topoisomerase II can be abrogated by reducing agents. In addition, compounds within this second group enhance DNA cleavage when added to the protein-DNA complex, but display the distinguishing feature of inhibiting topoisomerase II activity when incubated with the enzyme prior to the addition of DNA.
There is evidence that some covalent topoisomerase II poisons function (at least in part) by crosslinking or closing the N-terminal protein gate of topoisomerase II (Bender et al., 2006). Such an action could provide a mechanistic basis for stabilizing pre-existing cleavage complexes, while excluding DNA binding to unoccupied enzymes. However, the precise details by which covalent topoisomerase II poisons increase levels of DNA cleavage complexes have yet to be determined.
A number of topoisomerase II poisons described to date are derived from natural sources. Among these, phytochemicals have proven to be a rich source of topoisomerase II-active compounds. As discussed below, some phytochemical-based topoisomerase II poisons are widely utilized anticancer drugs. Others are normal components of the human diet and display chemopreventive (and potentially chemotherapeutic) properties.
Demethyl-epipodophyllotoxins
Etoposide and the related compound teniposide (Figure 4) are two of the most successful anticancer drugs in the world. They are used to treat a variety of cancers, including small cell lung cancer, germ-line malignancies, sarcomas, leukemias, and lymphomas (Hande, 1998a, b; Baldwin and Osheroff, 2005; Deweese and Osheroff, 2009; Bailly, 2012; Chen et al., 2012).
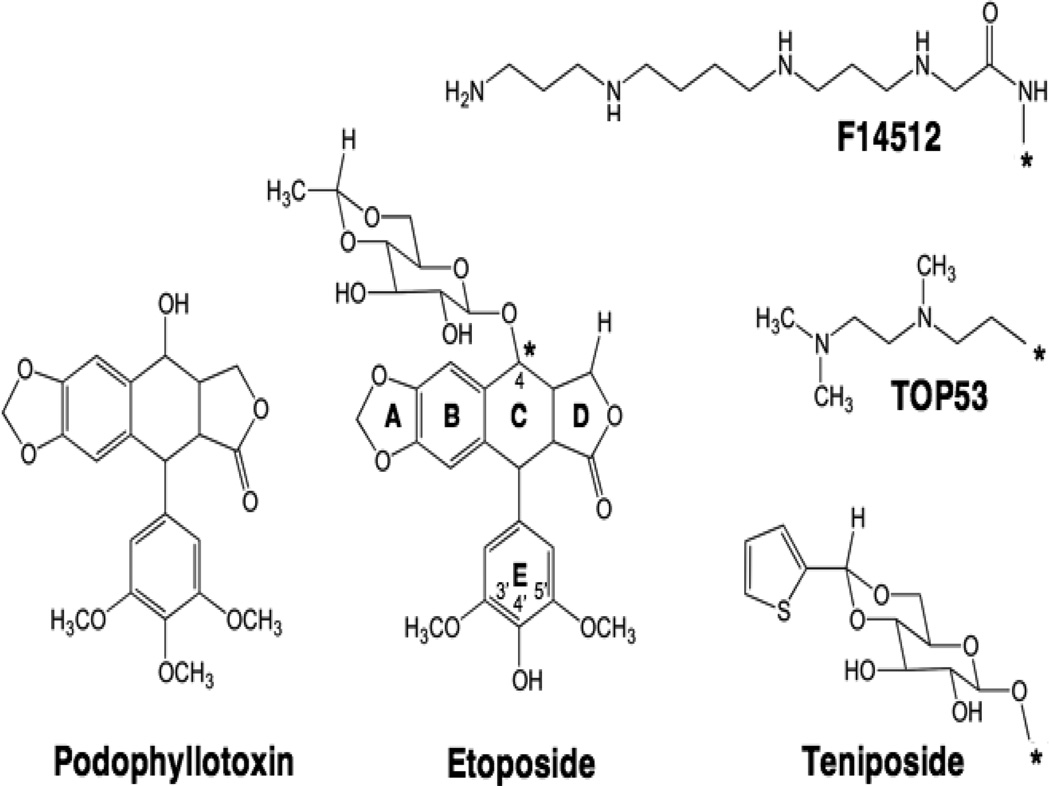
Figure 4.
Structures of podophyllotoxin and demethylated epimers. Podophyllotoxin and etoposide are shown. C-4 (*) substitutions of the glycosidic moiety on etoposide are shown for teniposide, TOP-53 and F14512.
Etoposide is derived from podophyllotoxin (Figure 4) (Stahelin and von Wartburg, 1991; Baldwin and Osheroff, 2005). This natural product is produced by Podophyllum peltatum, more commonly known as the mayapple or American mandrake plant. Podophyllotoxin has been used as a folk remedy for over a thousand years and is an antimitotic drug that acts by preventing microtubule formation (Hande, 1998b, a; Baldwin and Osheroff, 2005). The clinical use of this compound as an antineoplastic agent was prevented by high toxicity. In the 1950’s, investigators at Sandoz Pharmaceuticals initiated a discovery program in the hope of overcoming the prohibitive toxicity of the parent compound (Stahelin and von Wartburg, 1991; Hande, 1998b; Baldwin and Osheroff, 2005). As a result, a series of semi-synthetic podophyllotoxin derivatives was generated. Ultimately two analogs, etoposide and teniposide, displayed increased antineoplastic activity and decreased toxicity. Further analysis revealed, however, that these drugs no longer interacted with microtubules (Loike and Horwitz, 1976). It was determined several years later that they act as topoisomerase II poisons (Chen et al., 1984; Ross et al., 1984; Yang et al., 1985). Etoposide was approved for clinical use in the mid-1980s and for several years was the most widely prescribed anticancer drug in the world (Hande, 1998b, a; Baldwin and Osheroff, 2005).
Of all the known topoisomerase II poisons, etoposide is by far the best characterized (Baldwin and Osheroff, 2005). Studies with this drug have provided important conceptual frameworks that paved the way for later work on other anticancer agents. Etoposide was one of the first chemotherapeutic drugs demonstrated to kill cells by targeting topoisomerase II and was the first shown to inhibit the DNA ligation activity of the type II enzyme (Chen et al., 1984; Ross et al., 1984; Yang et al., 1985; Osheroff, 1989). It is an interfacial topoisomerase II poison that enters the binary enzyme-DNA complex primarily through interactions with the protein (Burden et al., 1996; Kingma et al., 1999).
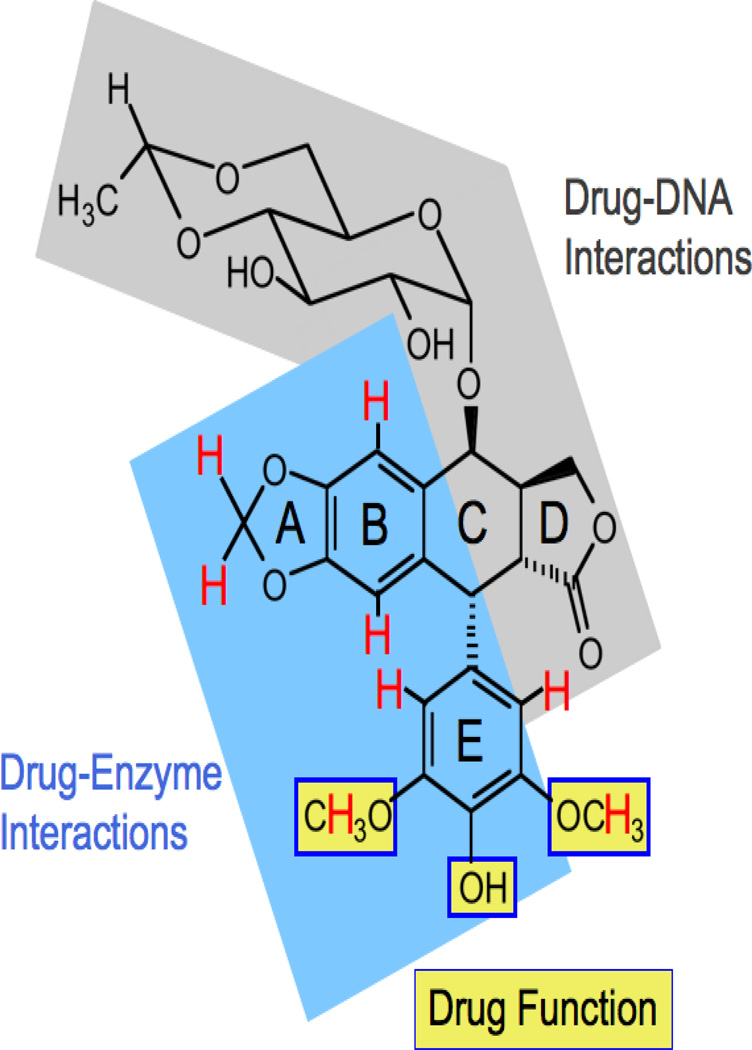
Etoposide remains the only topoisomerase II poison for which we have structural data that describes its interactions with the enzyme. Studies that coupled saturation transfer difference NMR spectroscopy of the binary human topoisomerase IIα-etoposide complex with drug activity/binding strongly suggest that the binding of etoposide by topoisomerase IIα is driven by interactions with the A– and B–rings, and potentially by stacking interactions with the pendent E–ring (Figure 5) (Wilstermann et al., 2007; Bender et al., 2008; Pitts et al., 2011). In addition, the 3’-, 4’-, and 5’-substituents on the E-ring are important for drug function and interact with the enzyme, but do not appear to contribute significantly to binding (Wilstermann et al., 2007; Bender et al., 2008). Finally, portions of the D-ring and (to a lesser extent) the C-4 carbohydrate moiety mediate interactions between etoposide and DNA (Pitts et al., 2011). These findings largely have been substantiated by a recent structure of the ternary human topoisomerase IIβ-DNA-etoposide complex (Wu et al., 2011).
Figure 5.
Summary of etoposide substituents that interact with human topoisomerase IIα. Adapted from (Pitts et al., 2011). Protons that interact with the enzyme (as determined by saturation transfer difference NMR spectroscopy) are shown in red. The blue region on etoposide, including portions of the A–, B– and E–rings, has been proposed to interact with topoisomerase II in the binary drug-enzyme complex. E–ring substituents highlighted with yellow boxes are important for drug function and interact with the enzyme, but did not appear to contribute significantly to binding. It has been proposed that interactions between etoposide and DNA in the ternary complex (shaded in gray) are driven primarily by the D-ring, with additional contributions from the C-4 sugar.
The two critical modifications of podophyllotoxin that convert it from a tubulin-targeted drug to a topoisomerase II poison are the substitution of a hydroxyl moiety for the methoxy group at C-4’ and the inclusion of the carbohydrate group at C-4 (Figure 4). The C-4’ position appears to sit in a constrained pocket in topoisomerase II (Bender et al., 2008; Wu et al., 2011). Consequently, the presence of the C-4’ methoxy prevents the parent compound from binding to the type II enzyme (Bender et al., 2008). The inclusion of a bulky group at C-4 (although not necessary for interactions with topoisomerase II) appears to prevent the binding of etoposide to tubulin (Loike and Horwitz, 1976).
Because the C-4 carbohydrate of etoposide does not interact with topoisomerase II (Wilstermann et al., 2007; Bender et al., 2008), it is possible to substitute other functional groups at this position (Bailly, 2012) (Figure 4). For example, TOP-53 is an etoposide derivative that contains a C-4 aminoalkyl side chain (Kitamura et al., 1997). In contrast to the carbohydrate moiety of etoposide, every proton associated with the side chain of TOP-53 contacts topoisomerase IIα in the binary enzyme-drug complex (Wilstermann et al., 2007). The inclusion of the aminoalkyl group increases the binding affinity of TOP-53 for topoisomerase IIα and significantly enhances the potency and efficacy of the drug against the type II enzyme (Byl et al., 2001; Wilstermann et al., 2007). Alternatively, F14512 is a novel etoposide derivative that contains a spermine group in place of the C-4 glycosidic moiety (Barret et al., 2008). The presence of the spermine enhances the selectivity of the drug for cancers that overexpress an active polyamine transport system (Annereau et al., 2008; Barret et al., 2008; Kruczynski et al., 2009). In addition, because the spermine converts the drug into a DNA binder (Barret et al., 2008), F14512 is a more potent and efficacious topoisomerase II poison than etoposide (Gentry et al., 2011). F14512 forms highly stable ternary topoisomerase II-DNA-drug complexes (Gentry et al., 2011) and currently is in clinical trials.
Bioflavonoids
Bioflavonoids are a diverse group of polyphenolic compounds that are constituents of many fruits, vegetables, legumes, and plant leaves (Kurzer and Xu, 1997; Scalbert and Williamson, 2000; Galati and O'Brien, 2004; Yao et al., 2004; Kandaswami et al., 2005; Siddiqui et al., 2006). They are an integral component of the human diet and represent the most abundant natural source of antioxidants (Kurzer and Xu, 1997; Scalbert and Williamson, 2000; Dragsted, 2003; Galati and O'Brien, 2004; Yao et al., 2004; Sang et al., 2005a; Siddiqui et al., 2006).
It is believed that the dietary intake of bioflavonoids provides a number of health benefits to adults (Adlercreutz et al., 1993; Kurzer and Xu, 1997; Lamartiniere, 2000; Scalbert and Williamson, 2000; Galati and O'Brien, 2004; Yao et al., 2004; Kandaswami et al., 2005; Siddiqui et al., 2006). Epidemiological studies suggest that these compounds help protect against cancer, cardiovascular disease, osteoporosis, age-related diseases, and inflammation. The mechanistic basis for the physiological actions of bioflavonoids is not fully described, as they have a variety of effects on human cells. Beyond their antioxidant properties, many of these polyphenols are potent inhibitors of tyrosine kinases (Akiyama et al., 1987; Hagiwara et al., 1988; Geahlen et al., 1989; Cushman et al., 1991; Yang et al., 2001; Hollosy and Keri, 2004; Kandaswami et al., 2005), display anti-proliferative, pro-apoptotic, and genotoxic effects, and decrease the expression or function of several proteins that are involved in cell-cycle progression (Ren et al., 2003; Williams et al., 2004; Kandaswami et al., 2005; Fresco et al., 2006; Sarkar et al., 2006; Siddiqui et al., 2006).
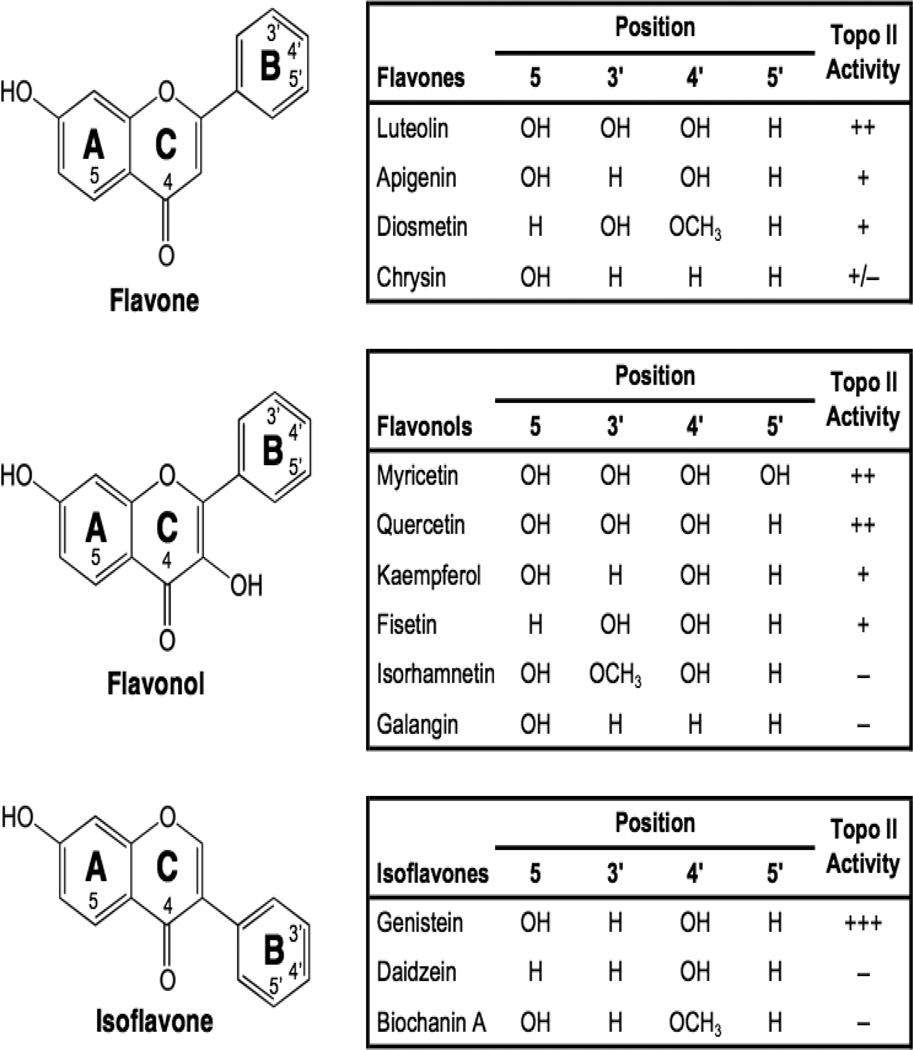
A variety of flavones, isoflavones, and flavonols have been examined for their abilities to enhance DNA cleavage mediated by human topoisomerase IIα and IIβ, and several were found to be potent topoisomerase II poisons in vitro and in cultured human cells (Figure 6) (Austin et al., 1992; Constantinou et al., 1995; Bandele and Osheroff, 2007; Lopez-Lazaro et al., 2010). Among the bioflavonoids, genistein appears to have the highest activity against the human type II enzymes (Austin et al., 1992; Bandele and Osheroff, 2007). Many of the chemopreventive, cytotoxic, and genotoxic properties of flavones, isoflavones, and flavonols are consistent with their activities as topoisomerase II poisons. To this point, the sensitivity of cells to genistein has been correlated to the activity of the type II enzyme (Markovits et al., 1995; Lopez-Lazaro et al., 2007a).
Figure 6.
Structures of selected bioflavonoids. Adapted from (Bandele and Osheroff, 2007). Flavones, flavonols, and isoflavones are shown, and the ability of each to enhance topoisomerase II-mediated DNA cleavage is indicated as >8-fold (+++), 6- to 8-fold (++), 3- to 6-fold (+), 2- to 3-fold (+/−), or <2-fold (−) over baseline.
With the exception discussed below, flavones, isoflavones, and flavonols are interfacial topoisomerase II poisons and increase levels of cleavage complexes primarily by inhibiting enzyme-mediated DNA ligation (Austin et al., 1992; Bandele and Osheroff, 2007). Structure-activity studies indicate that (like etoposide) the presence of a C-4’ hydroxyl on the pendant ring (in this case, the B-ring) does not mediate binding of bioflavonoids to topoisomerase II, but is important for the activity against the type II enzyme (Bandele and Osheroff, 2007). Once again, the C-4’ hydroxyl appears to interact with a constrained pocket in topoisomerase II, as the inclusion a methoxy group at this position impairs the ability of compounds to bind the enzyme (Bandele and Osheroff, 2007). The C-5 hydroxyl also appears to be important for bioflavonoid function and binding (Austin et al., 1992; Constantinou et al., 1995; Bandele and Osheroff, 2007). It has been proposed that this substituent forms a pseudo ring with the C-4 keto group that helps to maintain the planarity of the A- and C-rings (Kozerski et al., 2003).
In general, flavones, isoflavones, and flavonols appear to be more efficacious against topoisomerase IIβ than the α isoform (Bandele and Osheroff, 2007). Furthermore, cells that are depleted of topoisomerase IIβ are resistant to genistein (Lopez-Lazaro et al., 2007a). Therefore, it is believed that many of the cellular effects of flavones, isoflavones, and flavonols as topoisomerase II poisons are mediated primarily by the β isoform (Lopez-Lazaro et al., 2007a).
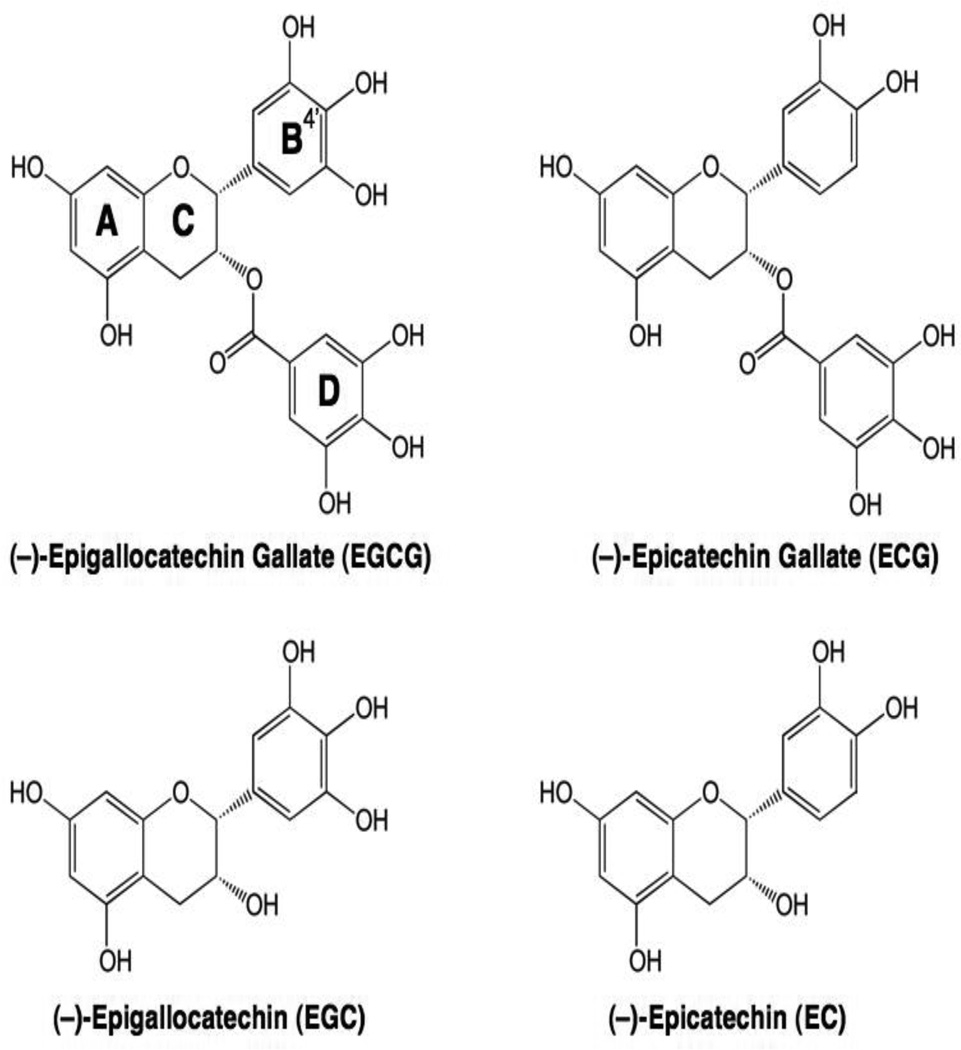
Catechins represent another major and important class of bioflavonoids (Galati and O'Brien, 2004; Yao et al., 2004; Kanwar et al., 2012). Green tea, which is one of the most commonly consumed beverages in the world, is a rich source of catechins and has been suggested to reduce the incidence of breast, prostate, colorectal, and lung cancer in humans (Sang et al., 2005a; Isbrucker et al., 2006a; Isbrucker et al., 2006b; Yang et al., 2007). The most abundant catechins in green tea are (−)-epigallocatechin gallate (EGCG) and the related compounds (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG), and (−)-epicatechin (EC) (Figure 7) (Sang et al., 2005a; Isbrucker et al., 2006a; Isbrucker et al., 2006b; Yang et al., 2007). Although EGCG and EGC are potent topoisomerase II poisons, neither ECG nor EC (which contain only two hydroxyl groups on their B-rings) display any substantial activity against the human type II enzymes (Austin et al., 1992; Bandele et al., 2008). Thus, the ability of green tea catechins to poison topoisomerase II reflects the presence of three hydroxyl groups on the B-ring, with the D-ring having little relevance.
Figure 7.
Structures of EGCG and related catechins.
Surprisingly (and in major contrast to the flavones, isoflavones, and flavonols), EGCG and EGC appear to be covalent (rather than interfacial) topoisomerase II poisons (Bandele et al., 2008; Bandele and Osheroff, 2008). The mechanistic differences between bioflavonoid classes appear to be related to structural elements in the B- and C-rings (Figure 7) (Bandele et al., 2008). Although the C-4’ hydroxyl of the B-ring is critical for bioflavonoids to act as interfacial topoisomerase II poisons (Austin et al., 1992; Constantinou et al., 1995; Bandele and Osheroff, 2007), the inclusion of two additional B-ring hydroxyl groups increases redox activity (Valcic et al., 1999; Valcic et al., 2000) and is required for compounds to act as covalent topoisomerase II poisons (Bandele et al., 2008; Bandele and Osheroff, 2008). In addition, the C-ring in flavones, isoflavones, and flavonols is aromatic, planar, and includes the C-4 keto group that allows the formation of the proposed pseudo ring with the C-5 hydroxyl (Kozerski et al., 2003). All of these elements are required for binding to human type II topoisomerases (Bandele and Osheroff, 2007; Bandele et al., 2008). Because EGCG and EGC contain the catechin C-ring, they are unable to act as interfacial topoisomerase II poisons and function exclusively as covalent poisons. Furthermore, because ECG and EC lack the critical third hydroxyl group on their B-rings that would allow them to function as covalent poisons, they show virtually no activity against topoisomerase II. As predicted from the above, if three hydroxyl groups are included on the B-ring of a flavonol such as myricetin (see Figure 6), the compound acts as a dual function topoisomerase II poison and displays both interfacial and covalent characteristics (Bandele et al., 2008).
During the brewing process, a portion of EGCG (and related green tea catechins) undergoes epimerization. As a result, the stereochemistry of the bond linking the B- and C-rings inverts, converting EGCG to (−)-gallocatechin gallate (GCG) (Sang et al., 2005b; Ishino et al., 2010). Despite this stereochemical alteration, GCG poisons topoisomerase II with an activity that is similar to that of EGCG (Timmel et al., 2013).
Isothiocyanates
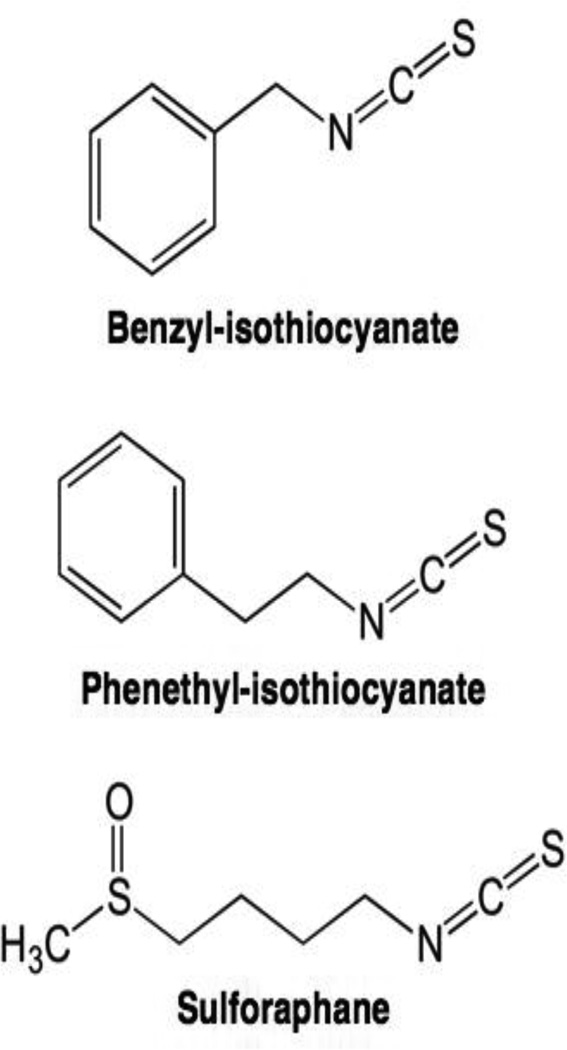
Dietary glucosinolates are found in cruciferous vegetables, including broccoli, cabbage, cauliflower, and kale (Stan et al., 2008). They are converted to bioactive isothiocyanates such as benzyl-isothiocyanate, phenethyl-isothiocyanate, and sulforaphane (Figure 8), upon hydrolysis by myrasinase (Herr and Buchler, 2010). Many of these compounds inhibit cell proliferation, display chemopreventive properties, and inhibit tumor growth in xenograft models (Chung et al., 2000; Singh et al., 2004; Warin et al., 2009).
Figure 8.
Structures of selected isothiocyanate-based topoisomerase II poisons.
Isothiocyanates are topoisomerase II poisons in vitro, and silencing topoisomerase IIα in cultured mouse embryonic fibroblasts decreases DNA damage induced by these compounds (Lin et al., 2011). As found for reactive quinone-based topoisomerase II poisons (Bender et al., 2007), isothiocyanates act as covalent poisons and modify several cysteine residues in human topoisomerase IIα (Lin et al., 2011). Consistent with a mechanism that requires cysteine modification, the ability of isothiocyanates to induce topoisomerase II-mediated DNA cleavage is abolished when compounds are co-incubated with excess glutathione (Lin et al., 2011).
Curcumin
Curcumin (Figure 9) is the principal flavor and color component of turmeric, a common spice used in curries and a variety of other Asian cuisines (Goel et al., 2008; Gupta et al., 2012). Beyond its culinary uses, curcumin is believed to positively impact human health and commonly is used in traditional Chinese herbal medicine and Ayurvedic medicine (Goel et al., 2008; Gupta et al., 2012). The compound has chemopreventive properties against a variety of human malignancies and currently is in clinical trials as an anticancer agent (Satoskar et al., 1986; Sharma et al., 2001; Mahady et al., 2002; Dhillon et al., 2008; Hatcher et al., 2008; Patel et al., 2010).
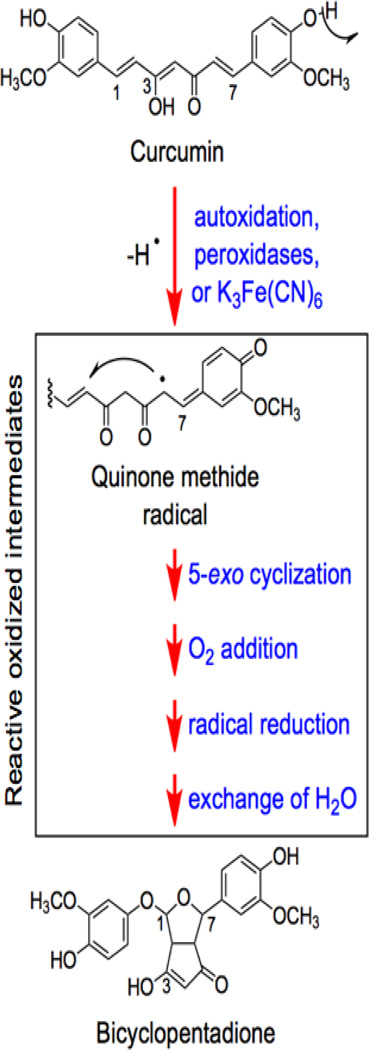
Figure 9.
Oxidative transformation of curcumin. Adapted from (Griesser et al., 2011)
Curcumin has poor oral bioavailability and is unstable under physiological conditions (Tonnesen and Karlsen, 1985; Wang et al., 1997; Pfeiffer et al., 2003; Anand et al., 2007; Griesser et al., 2011). Thus, it has been suggested that some of its biological effects are mediated by metabolites (Ireson et al., 2001; Anand et al., 2008; Shen and Ji, 2009, 2012). Curcumin can undergo spontaneous autoxidation in aqueous solutions at physiological pH (Figure 9) (Griesser et al., 2011), and this reaction gives rise to novel quinone-containing products that have potential for biological activity. Treatment of human cells with curcumin induces DNA cleavage complexes formed by topoisomerase IIα and IIβ (Lopez-Lazaro et al., 2007b). Cleavage complex formation is prevented by the addition of an antioxidant, suggesting the importance of oxidative pathways in curcumin activity against the type II enzymes (Lopez-Lazaro et al., 2007b).
A recent study demonstrated that reactive oxidized metabolites of curcumin, but neither the parent compound nor the stable bicyclopentadione end product (Figure 9), are covalent topoisomerase II poisons (Ketron et al., 2013). These metabolites display similar abilities to induce DNA cleavage mediated by human topoisomerase IIα and IIβ. Although breakdown products of curcumin, including vanillin, ferulic acid, and feruloylmethane, are bioreactive, none display activity against the human type II enzymes (Ketron et al., 2013).
Basis for the selectivity of topoisomerase II poisons
Topoisomerase II poisons are in wide use as anticancer chemotherapeutics (Pommier and Marchand, 2005; McClendon and Osheroff, 2007; Deweese et al., 2008; Deweese and Osheroff, 2009; Nitiss, 2009b; Pommier, 2009; Pommier et al., 2010; Bailly, 2012; Chen et al., 2012; Pommier, 2013). Considering that all cells in the human body contain type II topoisomerases, an obvious question arises: why do topoisomerase II poisons preferentially kill cancer cells?
First, due to the mechanism of action of these agents (i.e. the generation of DNA strand breaks), the higher the cellular level of topoisomerase II, the more lethal they become. Most cancer cells proliferate rapidly and contain higher than normal levels of type II enzymes (Nitiss, 2009b). Therefore, treatment with topoisomerase II poisons generates more DNA strand breaks and induces greater toxicity.
Second, the transient DNA breaks generated by type II topoisomerases are converted to permanent strand breaks upon collision with DNA tracking machinery, such as replication and transcription complexes. Since cancer cells are distinguished by high rates of metabolism and frequent replication, cleavage complexes stabilized by topoisomerase II poisons in these cells are more likely to be converted to permanent (and potentially lethal) DNA strand breaks.
Third, cancer cells display the hallmark characteristics of genomic instability and impaired DNA damage response pathways (Friedberg et al., 2006). Thus, they are more susceptible than normal cells to the effects of DNA damaging agents, such as topoisomerase II poisons.
As discussed above, many dietary topoisomerase II poisons appear to have chemopreventive properties. It is likely that the above criteria apply (at least in part) to these compounds as well. Ingested at low levels, topoisomerase II-targeted phytochemicals may preferentially affect cells that are progressing toward malignancy. To this point, compounds such as genistein, curcumin and green tea catechins have been investigated in animal and human trials as anticancer agents (Sarkar and Li, 2004; Von Low et al., 2007; Dhillon et al., 2008; Hatcher et al., 2008; Patel et al., 2010; Kanwar et al., 2012).
Topoisomerase II-associated leukemias
Despite the importance of topoisomerase II as a target for anticancer drugs and chemopreventive agents, evidence suggests that DNA strand breaks generated by the enzyme can trigger chromosomal translocations associated with specific types of leukemia (Felix et al., 2006; McClendon and Osheroff, 2007; Deweese and Osheroff, 2009; Joannides and Grimwade, 2010; Joannides et al., 2011; Cowell and Austin, 2012). To this point, 2–3% of patients who receive regimens that include etoposide subsequently develop acute myeloid leukemias (AMLs) (Baldwin and Osheroff, 2005; Felix et al., 2006; McClendon and Osheroff, 2007; Deweese and Osheroff, 2009; Joannides and Grimwade, 2010; Joannides et al., 2011; Cowell and Austin, 2012). Most of these leukemias are characterized by translocations with breakpoints in the MLL (mixed lineage leukemia) gene at chromosomal band 11q23. The MLL protein is a histone methyltransferase that regulates (among other substrates) the Hox genes, which control proliferation in hematopoietic cells. Several breakpoints in MLL have been identified and are located in close proximity to topoisomerase II-DNA cleavage sites that are induced by etoposide (Felix et al., 1995; Lovett et al., 2001a; Lovett et al., 2001b; Whitmarsh et al., 2003; Robinson et al., 2008).
In addition to treatment-related leukemias, ~80% of infants with AML or acute lymphoblastic leukemia (ALL) display translocations that involve the MLL gene (Strick et al., 2000; Felix et al., 2006; McClendon and Osheroff, 2007; Deweese and Osheroff, 2009). The chromosomal translocations associated with these cancers have been observed in utero, indicating that infant leukemias are initiated during gestation. Epidemiological studies indicate that the risk of developing these infant leukemias increases >3-fold by the maternal consumption (during pregnancy) of foods that are rich in bioflavonoids and other naturally occurring topoisomerase II poisons (Ross et al., 1994; Greaves, 1997; Spector et al., 2005). Consistent with this finding, treatment of cultured human cells with dietary bioflavonoids induces cleavage within the MLL gene (Strick et al., 2000). Furthermore, compounds that display the highest activity in in vitro topoisomerase II-DNA cleavage assays show the greatest propensity to generate breaks in the MLL gene in cultured cells (Strick et al., 2000). Thus, the same topoisomerase II-active phytochemicals that help to maintain health in human adults appear to have harmful effects on developing embryos.
Conclusions
Topoisomerase II poisons play critical roles in curing and preventing cancer. However, in some cases, they are linked to the development of specific forms of the disease. Many topoisomerase II poisons are derived from natural sources, and some of the most important compounds that affect the type II enzyme originate in plants. Many of these compounds are routinely consumed as part of the human diet. Clearly, phytochemicals are a rich source of structurally and mechanistically diverse topoisomerase II poisons. It is likely that plants will continue to yield novel anticancer agents that function through the type II enzyme, and drug discovery efforts in this area are strongly encouraged.
Acknowledgements
Work in the senior author’s laboratory is supported by National Institutes of Health research grant GM33944. A.C.K. was supported by training grant T32 GM065086 from the National Institutes of Health. We are grateful to Katie J. Aldred, R. Hunter Lindsey, and MaryJean Campbell for critical reading of the manuscript.
Contributor Information
Adam C. Ketron, Department of Biochemistry and the Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine, Nashville, Tennessee 37232 USA
Neil Osheroff, Email: neil.osheroff@vanderbilt.edu, Departments of Biochemistry and Medicine (Hematology/Oncology) and the Vanderbilt Institute of Chemical Biology, Vanderbilt University School of Medicine, Nashville, Tennessee 37232 USA.
REFERENCES
- Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Andoh T, Ishida R. Catalytic inhibitors of DNA topoisomerase II. Biochimica et Biophysica Acta. 1998;1400:155–171. doi: 10.1016/s0167-4781(98)00133-x. [DOI] [PubMed] [Google Scholar]
- Annereau JP, Brel V, Dumontet C, Guminski Y, Imbert T, Broussas M, Vispe S, Breand S, Guilbaud N, Barret JM, Bailly C. A fluorescent biomarker of the polyamine transport system to select patients with AML for F14512 treatment. Leuk Res. 2008;34:1383–1389. doi: 10.1016/j.leukres.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Austin CA, Marsh KL. Eukaryotic DNA topoisomerase IIβ. Bioessays. 1998;20:215–226. doi: 10.1002/(SICI)1521-1878(199803)20:3<215::AID-BIES5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Austin CA, Patel S, Ono K, Nakane H, Fisher LM. Site-specific DNA cleavage by mammalian DNA topoisomerase II induced by novel flavone and catechin derivatives. Biochem J. 1992;282:883–889. doi: 10.1042/bj2820883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly C. Contemporary challenges in the design of topoisomerase II inhibitors for cancer chemotherapy. Chem Rev. 2012;112:3611–3640. doi: 10.1021/cr200325f. [DOI] [PubMed] [Google Scholar]
- Baker NM, Rajan R, Mondragon A. Structural studies of type I topoisomerases. Nucleic Acids Res. 2009;37:693–701. doi: 10.1093/nar/gkn1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin EL, Osheroff N. Etoposide, topoisomerase II and cancer. Curr Med Chem Anti-Cancer Agents. 2005;5:363–372. doi: 10.2174/1568011054222364. [DOI] [PubMed] [Google Scholar]
- Bandele OJ, Clawson SJ, Osheroff N. Dietary polyphenols as topoisomerase II poisons: B ring and C ring substituents determine the mechanism of enzyme-mediated DNA cleavage enhancement. Chem Res Toxicol. 2008;21:1253–1260. doi: 10.1021/tx8000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandele OJ, Osheroff N. Bioflavonoids as poisons of human topoisomerase IIα and IIβ. Biochemistry. 2007;46:6097–6108. doi: 10.1021/bi7000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandele OJ, Osheroff N. (−)-Epigallocatechin gallate, a major constituent of green tea, poisons human type II topoisomerases. Chem Res Toxicol. 2008;21:936–943. doi: 10.1021/tx700434v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barret JM, Kruczynski A, Vispe S, Annereau JP, Brel V, Guminski Y, Delcros JG, Lansiau A, Guilbaud N, Imbert T, Bailly C. F14512, a potent antitumor agent targeting topoisomerase II vectored into cancer cells via the polyamine transport system. Cancer Res. 2008;68:9845–9853. doi: 10.1158/0008-5472.CAN-08-2748. [DOI] [PubMed] [Google Scholar]
- Bates AD, Maxwell A. DNA Topology. New York: Oxford University Press; 2005. [Google Scholar]
- Bender RP, Ham AJ, Osheroff N. Quinone-induced enhancement of DNA cleavage by human topoisomerase IIα: adduction of cysteine residues 392 and 405. Biochemistry. 2007;46:2856–2864. doi: 10.1021/bi062017l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender RP, Jablonksy MJ, Shadid M, Romaine I, Dunlap N, Anklin C, Graves DE, Osheroff N. Substituents on etoposide that interact with human topoisomerase IIα in the binary enzyme-drug complex: contributions to etoposide binding and activity. Biochemistry. 2008;47:4501–4509. doi: 10.1021/bi702019z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender RP, Lehmler HJ, Robertson LW, Ludewig G, Osheroff N. Polychlorinated biphenyl quinone metabolites poison human topoisomerase IIα: altering enzyme function by blocking the N-terminal protein gate. Biochemistry. 2006;45:10140–10152. doi: 10.1021/bi0524666. [DOI] [PubMed] [Google Scholar]
- Bender RP, Osheroff N. DNA topoisomerases as targets for the chemotherapeutic treatment of cancer. In: Dai W, editor. Checkpoint Responses in Cancer Therapy. Totowa, New Jersey: Humana Press; 2008. pp. 57–91. [Google Scholar]
- Burden DA, Kingma PS, Froelich-Ammon SJ, Bjornsti M-A, Patchan MW, Thompson RB, Osheroff N. Topoisomerase II-etoposide interactions direct the formation of drug-induced enzyme-DNA cleavage complexes. J Biol Chem. 1996;271:29238–29244. doi: 10.1074/jbc.271.46.29238. [DOI] [PubMed] [Google Scholar]
- Byl JA, Cline SD, Utsugi T, Kobunai T, Yamada Y, Osheroff N. DNA topoisomerase II as the target for the anticancer drug TOP-53: mechanistic basis for drug action. Biochemistry. 2001;40:712–718. doi: 10.1021/bi0021838. [DOI] [PubMed] [Google Scholar]
- Champoux JJ. Mechanism of catalysis by eukaryotic DNA topoisomerase I. Advances in Pharmacology. 1994;29:71–82. doi: 10.1016/s1054-3589(08)60540-2. [DOI] [PubMed] [Google Scholar]
- Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- Chen GL, Yang L, Rowe TC, Halligan BD, Tewey KM, Liu LF. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984;259:13560–13566. [PubMed] [Google Scholar]
- Chen W, Qiu J, Shen YM. Topoisomerase IIα, rather than IIβ, is a promising target in development of anti-cancer drugs. Drug Discov Ther. 2012;6:230–237. [PubMed] [Google Scholar]
- Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- Collin F, Karkare S, Maxwell A. Exploiting bacterial DNA gyrase as a drug target: current state and perspectives. Appl Microbiol Biotechnol. 2011;92:479–497. doi: 10.1007/s00253-011-3557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou A, Mehta R, Runyan C, Rao K, Vaughan A, Moon R. Flavonoids as DNA topoisomerase antagonists and poisons: structure-activity relationships. J Nat Prod. 1995;58:217–225. doi: 10.1021/np50116a009. [DOI] [PubMed] [Google Scholar]
- Corbett KD, Berger JM. Structure, molecular mechanisms, and evolutionary relationships in DNA topoisomerases. Annu Rev Biophys Biomol Struct. 2004;33:95–118. doi: 10.1146/annurev.biophys.33.110502.140357. [DOI] [PubMed] [Google Scholar]
- Cowell IG, Austin CA. Mechanism of generation of therapy related leukemia in response to anti-topoisomerase II agents. Int J Environ Res Public Health. 2012;9:2075–2091. doi: 10.3390/ijerph9062075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman M, Nagarathnam D, Burg DL, Geahlen RL. Synthesis and protein-tyrosine kinase inhibitory activities of flavonoid analogues. J Med Chem. 1991;34:798–806. doi: 10.1021/jm00106a047. [DOI] [PubMed] [Google Scholar]
- D'Arpa P, Beardmore C, Liu LF. Involvement of nucleic acid synthesis in cell killing mechanisms of topoisomerase poisons. Cancer Res. 1990;50:6919–6924. [PubMed] [Google Scholar]
- Deweese JE, Burch AM, Burgin AB, Osheroff N. Use of divalent metal ions in the DNA cleavage reaction of human type II topoisomerases. Biochemistry. 2009;48:1862–1869. doi: 10.1021/bi8023256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deweese JE, Osheroff MA, Osheroff N. DNA topology and topoisomerases: teaching a "knotty" subject. Biochem Mol Biol Educ. 2008;37:2–10. doi: 10.1002/bmb.20244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deweese JE, Osheroff N. The DNA cleavage reaction of topoisomerase II: wolf in sheep's clothing. Nucleic Acids Res. 2009;37:738–748. doi: 10.1093/nar/gkn937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deweese JE, Osheroff N. The use of divalent metal ions by type II topoisomerases. Metallomics. 2010;2:450–459. doi: 10.1039/c003759a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- Dong KC, Berger JM. Structural basis for gate-DNA recognition and bending by type IIA topoisomerases. Nature. 2007;450:1201–1205. doi: 10.1038/nature06396. [DOI] [PubMed] [Google Scholar]
- Dragsted LO. Antioxidant actions of polyphenols in humans. Int J Vitam Nutr Res. 2003;73:112–119. doi: 10.1024/0300-9831.73.2.112. [DOI] [PubMed] [Google Scholar]
- Felix CA, Kolaris CP, Osheroff N. Topoisomerase II and the etiology of chromosomal translocations. DNA Repair (Amst) 2006;5:1093–1108. doi: 10.1016/j.dnarep.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Felix CA, Lange BJ, Hosler MR, Fertala J, Bjornsti M-A. Chromosome band 11q23 translocation breakpoints are DNA topoisomerase II cleavage sites. Cancer Research. 1995;55:4287–4292. [PubMed] [Google Scholar]
- Fortune JM, Osheroff N. Merbarone inhibits the catalytic activity of human topoisomerase IIα by blocking DNA cleavage. J Biol Chem. 1998;273:17643–17650. doi: 10.1074/jbc.273.28.17643. [DOI] [PubMed] [Google Scholar]
- Fortune JM, Osheroff N. Topoisomerase II as a target for anticancer drugs: when enzymes stop being nice. Prog Nucleic Acid Res Mol Biol. 2000;64:221–253. doi: 10.1016/s0079-6603(00)64006-0. [DOI] [PubMed] [Google Scholar]
- Fresco P, Borges F, Diniz C, Marques MP. New insights on the anticancer properties of dietary polyphenols. Med Res Rev. 2006;26:747–766. doi: 10.1002/med.20060. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. 2nd ed. Washington, DC: ASM Press; 2006. [Google Scholar]
- Galati G, O'Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004;37:287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Geahlen RL, Koonchanok NM, McLaughlin JL, Pratt DE. Inhibition of protein-tyrosine kinase activity by flavanoids and related compounds. J Nat Prod. 1989;52:982–986. doi: 10.1021/np50065a011. [DOI] [PubMed] [Google Scholar]
- Gentry AC, Pitts SL, Jablonsky MJ, Bailly C, Graves DE, Osheroff N. Interactions between the etoposide derivative F14512 and human type II topoisomerases: implications for the C4 spermine moiety in promoting enzyme-mediated DNA cleavage. Biochemistry. 2011;50:3240–3249. doi: 10.1021/bi200094z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "curecumin": from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Greaves MF. Aetiology of acute leukaemia. Lancet. 1997;349:344–349. doi: 10.1016/s0140-6736(96)09412-3. [DOI] [PubMed] [Google Scholar]
- Griesser M, Pistis V, Suzuki T, Tejera N, Pratt DA, Schneider C. Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventive agent curcumin. J Biol Chem. 2011;286:1114–1124. doi: 10.1074/jbc.M110.178806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Sung B, Kim JH, Prasad S, Li S, Aggarwal BB. Multitargeting by turmeric, the golden spice: from kitchen to clinic. Mol Nutr Food Res. 2012 doi: 10.1002/mnfr.201100741. Epub. Aug. 13 2012. [DOI] [PubMed] [Google Scholar]
- Hagiwara M, Inoue S, Tanaka T, Nunoki K, Ito M, Hidaka H. Differential effects of flavonoids as inhibitors of tyrosine protein kinases and serine/threonine protein kinases. Biochem Pharmacol. 1988;37:2987–2992. doi: 10.1016/0006-2952(88)90286-9. [DOI] [PubMed] [Google Scholar]
- Haince JF, Rouleau M, Poirier GG. Transcription. Gene expression needs a break to unwind before carrying on. Science. 2006;312:1752–1753. doi: 10.1126/science.1129808. [DOI] [PubMed] [Google Scholar]
- Hande KR. Clinical applications of anticancer drugs targeted to topoisomerase II. Biochim Biophys Acta. 1998a;1400:173–184. doi: 10.1016/s0167-4781(98)00134-1. [DOI] [PubMed] [Google Scholar]
- Hande KR. Etoposide: four decades of development of a topoisomerase II inhibitor. Eur J Cancer. 1998b;34:1514–1521. doi: 10.1016/s0959-8049(98)00228-7. [DOI] [PubMed] [Google Scholar]
- Hardin AH, Sarkar SK, Seol Y, Liou GF, Osheroff N, Neuman KC. Direct measurement of DNA bending by type IIA topoisomerases: implications for non-equilibrium topology simplification. Nucleic Acids Res. 2011;39:5729–5743. doi: 10.1093/nar/gkr109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr I, Buchler MW. Dietary constituents of broccoli and other cruciferous vegetables: implications for prevention and therapy of cancer. Cancer Treat Rev. 2010;36:377–383. doi: 10.1016/j.ctrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Hollosy F, Keri G. Plant-derived protein tyrosine kinase inhibitors as anticancer agents. Curr Med Chem Anticancer Agents. 2004;4:173–197. doi: 10.2174/1568011043482124. [DOI] [PubMed] [Google Scholar]
- Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, Steward WP, Gescher A. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61:1058–1064. [PubMed] [Google Scholar]
- Isaacs RJ, Davies SL, Sandri MI, Redwood C, Wells NJ, Hickson ID. Physiological regulation of eukaryotic topoisomerase II. Biochim Biophys Acta. 1998;1400:121–137. doi: 10.1016/s0167-4781(98)00131-6. [DOI] [PubMed] [Google Scholar]
- Isbrucker RA, Bausch J, Edwards JA, Wolz E. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 1: genotoxicity. Food Chem Toxicol. 2006a;44:626–635. doi: 10.1016/j.fct.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Isbrucker RA, Edwards JA, Wolz E, Davidovich A, Bausch J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: dermal, acute and short-term toxicity studies. Food Chem Toxicol. 2006b;44:636–650. doi: 10.1016/j.fct.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Ishino N, Yanase E, Nakatsuka S. Epimerization of tea catechins under weakly acidic and alkaline conditions. Biosci Biotechnol Biochem. 2010;74:875–877. doi: 10.1271/bbb.90884. [DOI] [PubMed] [Google Scholar]
- Joannides M, Grimwade D. Molecular biology of therapy-related leukaemias. Clin Transl Oncol. 2010;12:8–14. doi: 10.1007/s12094-010-0460-5. [DOI] [PubMed] [Google Scholar]
- Joannides M, Mays AN, Mistry AR, Hasan SK, Reiter A, Wiemels JL, Felix CA, Coco FL, Osheroff N, Solomon E, Grimwade D. Molecular pathogenesis of secondary acute promyelocytic leukemia. Mediterr J Hematol Infect Dis. 2011;3:e2011045. doi: 10.4084/MJHID.2011.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju BG, Lunyak VV, Perissi V, Garcia-Bassets I, Rose DW, Glass CK, Rosenfeld MG. A topoisomerase IIβ-mediated dsDNA break required for regulated transcription. Science. 2006;312:1798–1802. doi: 10.1126/science.1127196. [DOI] [PubMed] [Google Scholar]
- Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, Lee MT. The antitumor activities of flavonoids. In Vivo. 2005;19:895–909. [PubMed] [Google Scholar]
- Kanwar J, Taskeen M, Mohammad I, Huo C, Chan TH, Dou QP. Recent advances on tea polyphenols. Front Biosci (Elite Ed) 2012;4:111–131. doi: 10.2741/363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann SH. Cell death induced by topoisomerase-targeted drugs: more questions than answers. Biochim Biophys Acta. 1998;1400:195–211. doi: 10.1016/s0167-4781(98)00136-5. [DOI] [PubMed] [Google Scholar]
- Ketron AC, Gordon ON, Schneider C, Osheroff N. Oxidative metabolites of curcumin poison human type II topoisomerases. Biochemistry. 2013;52:221–227. doi: 10.1021/bi3014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketron AC, Osheroff N. DNA Topology and Topoisomerases. Encyc of Molec Life Sci. 2013 (In press) [Google Scholar]
- Kingma PS, Burden DA, Osheroff N. Binding of etoposide to topoisomerase II in the absence of DNA: decreased affinity as a mechanism of drug resistance. Biochemistry. 1999;38:3457–3461. doi: 10.1021/bi982855i. [DOI] [PubMed] [Google Scholar]
- Kitamura R, Bandoh T, Tsuda M, Satoh T. Determination of a new podophyllotoxin derivative, TOP-53, and its metabolite in rat plasma and urine by high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl. 1997;7:283–288. doi: 10.1016/s0378-4347(96)00388-x. [DOI] [PubMed] [Google Scholar]
- Kozerski L, Kamienski B, Kawecki R, Urbanczyk-Lipkowska Z, Bocian W, Bednarek E, Sitkowski J, Zakrzewska K, Nielsen KT, Hansen PE. Solution and solid state 13C NMR and X-ray studies of genistein complexes with amines. Potential biological function of the C-7, C-5, and C4'-OH groups. Org Biomol Chem. 2003;1:3578–3585. doi: 10.1039/b305991j. [DOI] [PubMed] [Google Scholar]
- Kruczynski A, Vandenberghe I, Pillon A, Pesnel S, Goetsch L, Barret JM, Guminski Y, Le Pape A, Imbert T, Bailly C, Guilbaud N. Preclinical activity of F14512, designed to target tumors expressing an active polyamine transport system. Invest New Drugs. 2009;29:9–21. doi: 10.1007/s10637-009-9328-3. [DOI] [PubMed] [Google Scholar]
- Kurzer MS, Xu X. Dietary phytoestrogens. Annu Rev Nutr. 1997;17:353–381. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- Lamartiniere CA. Protection against breast cancer with genistein: a component of soy. Am J Clin Nutr. 2000;71:1705S–1707S. doi: 10.1093/ajcn/71.6.1705S. [DOI] [PubMed] [Google Scholar]
- Lee S, Jung SR, Heo K, Byl JA, Deweese JE, Osheroff N, Hohng S. DNA cleavage and opening reactions of human topoisomerase IIα are regulated via Mg2+-mediated dynamic bending of gate-DNA. Proc Natl Acad Sci U S A. 2012;109:2925–2930. doi: 10.1073/pnas.1115704109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppard JB, Champoux JJ. Human DNA topoisomerase I: relaxation, roles, and damage control. Chromosoma. 2005;114:75–85. doi: 10.1007/s00412-005-0345-5. [DOI] [PubMed] [Google Scholar]
- Lin RK, Zhou N, Lyu YL, Tsai YC, Lu CH, Kerrigan J, Chen YT, Guan Z, Hsieh TS, Liu LF. Dietary isothiocyanate-induced apoptosis via thiol modification of DNA topoisomerase IIα. J Biol Chem. 2011;286:33591–33600. doi: 10.1074/jbc.M111.258137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey RH, Jr, Bromberg KD, Felix CA, Osheroff N. 1,4-Benzoquinone is a topoisomerase II poison. Biochemistry. 2004;43:7563–7574. doi: 10.1021/bi049756r. [DOI] [PubMed] [Google Scholar]
- Linka RM, Porter AC, Volkov A, Mielke C, Boege F, Christensen MO. C-terminal regions of topoisomerase IIα and IIβ determine isoform-specific functioning of the enzymes in vivo. Nucleic Acids Res. 2007;35:3810–3822. doi: 10.1093/nar/gkm102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Deibler RW, Chan HS, Zechiedrich L. The why and how of DNA unlinking. Nucleic Acids Res. 2009;37:661–671. doi: 10.1093/nar/gkp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loike JD, Horwitz SB. Effects of podophyllotoxin and VP-16-213 on microtubule assembly in vitro and nucleoside transport in HeLa cells. Biochemistry. 1976;15:5435–5443. doi: 10.1021/bi00670a003. [DOI] [PubMed] [Google Scholar]
- Lopez-Lazaro M, Willmore E, Austin CA. Cells lacking DNA topoisomerase IIβ are resistant to genistein. J Nat Prod. 2007a;70:763–767. doi: 10.1021/np060609z. [DOI] [PubMed] [Google Scholar]
- Lopez-Lazaro M, Willmore E, Austin CA. The dietary flavonoids myricetin and fisetin act as dual inhibitors of DNA topoisomerases I and II in cells. Mutat Res. 2010;696:41–47. doi: 10.1016/j.mrgentox.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Lopez-Lazaro M, Willmore E, Jobson A, Gilroy KL, Curtis H, Padget K, Austin CA. Curcumin induces high levels of topoisomerase I- and II-DNA complexes in K562 leukemia cells. J Nat Prod. 2007b;70:1884–1888. doi: 10.1021/np070332i. [DOI] [PubMed] [Google Scholar]
- Lovett BD, Lo Nigro L, Rappaport EF, Blair IA, Osheroff N, Zheng N, Megonigal MD, Williams WR, Nowell PC, Felix CA. Near-precise interchromosomal recombination and functional DNA topoisomerase II cleavage sites at MLL and AF-4 genomic breakpoints in treatment-related acute lymphoblastic leukemia with t(4;11) translocation. Proc Natl Acad Sci U S A. 2001a;98:9802–9807. doi: 10.1073/pnas.171309898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett BD, Strumberg D, Blair IA, Pang S, Burden DA, Megonigal MD, Rappaport EF, Rebbeck TR, Osheroff N, Pommier YG, Felix CA. Etoposide metabolites enhance DNA topoisomerase II cleavage near leukemia-associated MLL translocation breakpoints. Biochemistry. 2001b;40:1159–1170. doi: 10.1021/bi002361x. [DOI] [PubMed] [Google Scholar]
- Mahady GB, Pendland SL, Yun G, Lu ZZ. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002;22:4179–4181. [PubMed] [Google Scholar]
- Markovits J, Junqua S, Goldwasser F, Venuat AM, Luccioni C, Beaumatin J, Saucier JM, Bernheim A, Jacquemin-Sablon A. Genistein resistance in human leukaemic CCRF-CEM cells: selection of a diploid cell line with reduced DNA topoisomerase IIβ isoform. Biochem Pharmacol. 1995;50:177–186. doi: 10.1016/0006-2952(95)00131-i. [DOI] [PubMed] [Google Scholar]
- McClendon AK, Osheroff N. DNA topoisomerase II, genotoxicity, and cancer. Mutat Res. 2007;623:83–97. doi: 10.1016/j.mrfmmm.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss JL. DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer. 2009a;9:327–337. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009b;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheroff N. Effect of antineoplastic agents on the DNA cleavage/religation reaction of eukaryotic topoisomerase II: inhibition of DNA religation by etoposide. Biochemistry. 1989;28:6157–6160. doi: 10.1021/bi00441a005. [DOI] [PubMed] [Google Scholar]
- Patel VB, Misra S, Patel BB, Majumdar AP. Colorectal cancer: chemopreventive role of curcumin and resveratrol. Nutr Cancer. 2010;62:958–967. doi: 10.1080/01635581.2010.510259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer E, Heoehle SI, Solyom AM, Metzler M. Studies on the stability of turmeric constituents. J Food Engin. 2003;56:257–259. [Google Scholar]
- Pitts SL, Jablonksy MJ, Duca M, Dauzonne D, Monneret C, Arimondo PB, Anklin C, Graves DE, Osheroff N. Contributions of the D-ring to the activity of etoposide against human topoisomerase IIα: potential interactions with DNA in the ternary enzyme-drug-DNA complex. Biochemistry. 2011;50:5058–5066. doi: 10.1021/bi200531q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y. DNA topoisomerase I inhibitors: chemistry, biology, and interfacial inhibition. Chem Rev. 2009;109:2894–2902. doi: 10.1021/cr900097c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y. Drugging topoisomerases: lessons and challenges. ACS Chem Biol. 2013;8:82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y, Marchand C. Interfacial inhibitors of protein-nucleic acid interactions. Curr Med Chem Anti-Cancer Agents. 2005;5:421–429. doi: 10.2174/1568011054222337. [DOI] [PubMed] [Google Scholar]
- Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23:519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- Robinson BW, Cheung NK, Kolaris CP, Jhanwar SC, Choi JK, Osheroff N, Felix CA. Prospective tracing of MLL-FRYL clone with low MEIS1 expression from emergence during neuroblastoma treatment to diagnosis of myelodysplastic syndrome. Blood. 2008;111:3802–3812. doi: 10.1182/blood-2007-07-096065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JA, Potter JD, Robison LL. Infant leukemia, topoisomerase II inhibitors, and the MLL gene. J Natl Cancer Inst. 1994;86:1678–1680. doi: 10.1093/jnci/86.22.1678. [DOI] [PubMed] [Google Scholar]
- Ross W, Rowe T, Glisson B, Yalowich J, Liu L. Role of topoisomerase II in mediating epipodophyllotoxin-induced DNA cleavage. Cancer Res. 1984;44:5857–5860. [PubMed] [Google Scholar]
- Sang S, Hou Z, Lambert JD, Yang CS. Redox properties of tea polyphenols and related biological activities. Antioxid Redox Signal. 2005a;7:1704–1714. doi: 10.1089/ars.2005.7.1704. [DOI] [PubMed] [Google Scholar]
- Sang S, Lee MJ, Hou Z, Ho CT, Yang CS. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J Agric Food Chem. 2005b;53:9478–9484. doi: 10.1021/jf0519055. [DOI] [PubMed] [Google Scholar]
- Sarkar FH, Adsule S, Padhye S, Kulkarni S, Li Y. The role of genistein and synthetic derivatives of isoflavone in cancer prevention and therapy. Mini Rev Med Chem. 2006;6:401–407. doi: 10.2174/138955706776361439. [DOI] [PubMed] [Google Scholar]
- Sarkar FH, Li Y. The role of isoflavones in cancer chemoprevention. Front Biosci. 2004;9:2714–2724. doi: 10.2741/1430. [DOI] [PubMed] [Google Scholar]
- Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol. 1986;24:651–654. [PubMed] [Google Scholar]
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- Schmidt BH, Burgin AB, Deweese JE, Osheroff N, Berger JM. A novel and unified two-metal mechanism for DNA cleavage by type II and IA topoisomerases. Nature. 2010;465:641–644. doi: 10.1038/nature08974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- Shen L, Ji HF. Contribution of degradation products to the anticancer activity of curcumin. Clin Cancer Res. 2009;15:7108. doi: 10.1158/1078-0432.CCR-09-1749. [DOI] [PubMed] [Google Scholar]
- Shen L, Ji HF. The pharmacology of curcumin: is it the degradation products? Trends Mol Med. 2012;18:138–144. doi: 10.1016/j.molmed.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Siddiqui IA, Adhami VM, Saleem M, Mukhtar H. Beneficial effects of tea and its polyphenols against prostate cancer. Mol Nutr Food Res. 2006;50:130–143. doi: 10.1002/mnfr.200500113. [DOI] [PubMed] [Google Scholar]
- Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- Sissi C, Palumbo M. In front of and behind the replication fork: bacterial type IIA topoisomerases. Cell Mol Life Sci. 2010;67:2001–2024. doi: 10.1007/s00018-010-0299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector LG, Xie Y, Robison LL, Heerema NA, Hilden JM, Lange B, Felix CA, Davies SM, Slavin J, Potter JD, Blair CK, Reaman GH, Ross JA. Maternal diet and infant leukemia: the DNA topoisomerase II inhibitor hypothesis: a report from the children's oncology group. Cancer Epidemiol Biomarkers Prev. 2005;14:651–655. doi: 10.1158/1055-9965.EPI-04-0602. [DOI] [PubMed] [Google Scholar]
- Stahelin HF, von Wartburg A. The chemical and biological route from podophyllotoxin glucoside to etoposide: ninth Cain memorial Award lecture. Cancer Res. 1991;51:5–15. [PubMed] [Google Scholar]
- Stan SD, Kar S, Stoner GD, Singh SV. Bioactive food components and cancer risk reduction. J Cell Biochem. 2008;104:339–356. doi: 10.1002/jcb.21623. [DOI] [PubMed] [Google Scholar]
- Strick R, Strissel PL, Borgers S, Smith SL, Rowley JD. Dietary bioflavonoids induce cleavage in the MLL gene and may contribute to infant leukemia. Proc Natl Acad Sci U S A. 2000;97:4790–4795. doi: 10.1073/pnas.070061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmel MA, Byl JA, Osheroff N. Epimerization of green tea catechins during brewing does not affect the ability to poison human type II topoisomerases. Chem Res Toxicol. 2013 doi: 10.1021/tx4000667. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen HH, Karlsen J. Studies on curcumin and curcuminoids VI. Kinetics of curcumin degradation in aqueous solution. Z Lebensm Unters Forsch. 1985;180:402–404. doi: 10.1007/BF01027775. [DOI] [PubMed] [Google Scholar]
- Valcic S, Burr JA, Timmermann BN, Liebler DC. Antioxidant chemistry of green tea catechins. New oxidation products of (−)-epigallocatechin gallate and (−)-epigallocatechin from their reactions with peroxyl radicals. Chem Res Toxicol. 2000;13:801–810. doi: 10.1021/tx000080k. [DOI] [PubMed] [Google Scholar]
- Valcic S, Muders A, Jacobsen NE, Liebler DC, Timmermann BN. Antioxidant chemistry of green tea catechins. Identification of products of the reaction of (−)-epigallocatechin gallate with peroxyl radicals. Chem Res Toxicol. 1999;12:382–386. doi: 10.1021/tx990003t. [DOI] [PubMed] [Google Scholar]
- Von Low EC, Perabo FG, Siener R, Muller SC. Review. Facts and fiction of phytotherapy for prostate cancer: a critical assessment of preclinical and clinical data. In Vivo. 2007;21:189–204. [PubMed] [Google Scholar]
- Vos SM, Tretter EM, Schmidt BH, Berger JM. All tangled up: how cells direct, manage and exploit topoisomerase function. Nat Rev Mol Cell Biol. 2011;12:827–841. doi: 10.1038/nrm3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Mao Y, Chen AY, Zhou N, LaVoie EJ, Liu LF. Stimulation of topoisomerase II-mediated DNA damage via a mechanism involving protein thiolation. Biochemistry. 2001;40:3316–3323. doi: 10.1021/bi002786j. [DOI] [PubMed] [Google Scholar]
- Wang JC. DNA topoisomerases: why so many? J Biol Chem. 1991;266:6659–6662. [PubMed] [Google Scholar]
- Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol. 2002;3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15:1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- Warin R, Chambers WH, Potter DM, Singh SV. Prevention of mammary carcinogenesis in MMTV-neu mice by cruciferous vegetable constituent benzyl isothiocyanate. Cancer Res. 2009;69:9473–9480. doi: 10.1158/0008-5472.CAN-09-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh RJ, Saginario C, Zhuo Y, Hilgenfeld E, Rappaport EF, Megonigal MD, Carroll M, Liu M, Osheroff N, Cheung NK, Slater DJ, Ried T, Knutsen T, Blair IA, Felix CA. Reciprocal DNA topoisomerase II cleavage events at 5'-TATTA-3' sequences in MLL and AF-9 create homologous single-stranded overhangs that anneal to form der(11) and der(9) genomic breakpoint junctions in treatment-related AML without further processing. Oncogene. 2003;22:8448–8459. doi: 10.1038/sj.onc.1207052. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Wilstermann AM, Bender RP, Godfrey M, Choi S, Anklin C, Berkowitz DB, Osheroff N, Graves DE. Topoisomerase II-drug interaction domains: identification of substituents on etoposide that interact with the enzyme. Biochemistry. 2007;46:8217–8225. doi: 10.1021/bi700272u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Li TK, Farh L, Lin LY, Lin TS, Yu YJ, Yen TJ, Chiang CW, Chan NL. Structural basis of type II topoisomerase inhibition by the anticancer drug etoposide. Science. 2011;333:459–462. doi: 10.1126/science.1204117. [DOI] [PubMed] [Google Scholar]
- Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EB, Guo YJ, Zhang K, Chen YZ, Mack P. Inhibition of epidermal growth factor receptor tyrosine kinase by chalcone derivatives. Biochim Biophys Acta. 2001;1550:144–152. doi: 10.1016/s0167-4838(01)00276-x. [DOI] [PubMed] [Google Scholar]
- Yang L, Rowe TC, Liu LF. Identification of DNA topoisomerase II as an intracellular target of antitumor epipodophyllotoxins in Simian Virus 40-infected monkey cells. Cancer Res. 1985;45:5872–5876. [PubMed] [Google Scholar]
- Yang X, Li W, Prescott ED, Burden SJ, Wang JC. DNA topoisomerase IIβ and neural development. Science. 2000;287:131–134. doi: 10.1126/science.287.5450.131. [DOI] [PubMed] [Google Scholar]
- Yao LH, Jiang YM, Shi J, Tomas-Barberan FA, Datta N, Singanusong R, Chen SS. Flavonoids in food and their health benefits. Plant Foods Hum Nutr. 2004;59:113–122. doi: 10.1007/s11130-004-0049-7. [DOI] [PubMed] [Google Scholar]