Abstract
Background
The matricellular protein connective tissue growth factor (CCN2) has been implicated in pathological fibrosis, but its physiologic role remains elusive. In vitro, transforming growth factor-β (TGF-β) induces CCN2 expression in mesenchymal cells. Because CCN2 can enhance pro-fibrotic responses elicited by TGF-β, it has been proposed that CCN2 functions as an essential downstream signaling mediator for TGF-β. To explore this notion, we characterized TGF-β-induced activation of fibroblasts from CCN2-null (CCN2−/−) mouse embryos.
Methods
The regulation of CCN2 expression was examined in vivo in a model of fibrosis induced by bleomycin. Cellular TGF-β signal transduction and regulation of collagen gene expression were examined in CCN2−/− MEFs by immunohistochemistry, Northern, Western and RT-PCR analysis, immunocytochemistry and transient transfection assays.
Results
Bleomycin-induced skin fibrosis in the mouse was associated with substantial CCN2 up-regulation in lesional fibroblasts. Whereas in vitro proliferation rate of CCN2−/− MEFs was markedly reduced compared to wild type MEFs, TGF-β-induced activation of the Smad pathways, including Smad2 phosphorylation, Smad2/3 and Smad4 nuclear accumulation and Smad-dependent transcriptional responses, were unaffected by loss of CCN2. The stimulation of COL1A2 and fibronectin mRNA expression and promoter activity, and of corresponding protein levels, showed comparable time and dose-response in wild type and CCN2−/− MEFs, whereas stimulation of alpha-smooth muscle actin and myofibroblast transdifferentiation showed subtle impairment in MEFs lacking CCN2.
Conclusion
Whereas endogenous CCN2 plays a role in regulation of proliferation and TGF-β-induced myofibroblast transdifferentation, it appears to be dispensable for Smad-dependent stimulation of collagen and extracellular matrix synthesis in murine embryonic fibroblasts.
Keywords: TGF-β, CTGF/CCN2, fibrosis, fibroblast, Type I collagen
INTRODUCTION
Systemic sclerosis (SSc) is characterized by excessive synthesis and accumulation of interstitial collagens in the skin, lungs and other tissues. The resulting fibrosis contributes to progressive functional impairment of affected organs, and accounts for the morbidity and mortality associated with SSc. Although the pathogenesis of fibrosis remains incompletely understood, substantial evidence indicates that activation of resident fibroblasts is the pivotal event that results in enhanced expression of collagen and other extracellular matrix (ECM) genes (1). Several cytokines and growth factors shown to induce fibroblast activation are implicated in both physiologic ECM accumulation (as seen during development and wound healing), and in pathological fibrosis (as seen in SSc and related conditions). In particular, transforming growth factor-β (TGF-β) elicits multiple responses in fibroblasts and mesenchymal cells, such as stimulation of collagen synthesis, myofibroblast transdifferentiation, and suppression of matrix-degrading metalloproteases, that in aggregate transform these cells into fibrogenic effectors. Indeed, TGF-β is considered to be the pivotal fibroblast-activating cytokine and master regulator of the fibrotic process in SSc (2). The complex mechanisms underlying the profibrotic responses elicited by TGF-β in fibroblasts are becoming better understood. Signaling through two distinct transmembrane cellular receptors, TGF-β induces Smad2/3 activation and formation of heteromeric Smad complexes, their accumulation within the nucleus, and direct binding to consensus DNA binding sites within the promoters of target genes (3). In addition to Smad-mediated signal transduction, the importance of alternate intracellular signaling pathways involving non-canonical Smad pathways as well as Smad-independent mechanisms, are increasingly recognized as playing important roles in TGF-β responses (4).
Connective tissue growth factor CCN2 is a 38-kD member of the CCN family of secreted cysteine-rich modular matricellular proteins with important roles in angiogenesis, chondrogenesis and wound healing (5). Reported effects of CCN2 on fibroblasts include stimulation of collagen production, chemotaxis, proliferation and integrin expression and matrix adhesion, and largely parallel the effects of TGF-β. However, CCN2 has also been shown to stimulate the synthesis of matrix-degrading metalloproteinases (6). Although the potential involvement of CCN2 in physiological and pathological matrix remodeling has been recognized (7), its precise biological function has remained controversial. Normally, mesencymal CCN2 expression can be detected during embryonic development and wound healing, whereas only relatively low levels are found in normal adult tissues. In contrast, pathological fibrosis is associated with strong CCN2 overexpression. In the absence of stimulation, normal fibroblasts have low levels of CCN2. TGF-β induces CCN2 expression in fibroblasts, but not in epithelial cells (8). This response is mediated via a unique TGF-β response element on the CCN2 gene promoter, and involves both Smad-dependent signaling pathways as well as PKC and Ras/MAPK-mediated Smad-independent pathways.
Because TGF-β and CCN2 are both involved in fibrosis, and since CCN2 is synthesized and secreted by mesenchymal cells upon their activation by TGF-β and many of the effects of CCN2 mirror those of TGF-β, CCN2 has been proposed as a downstream mediator for the profibrotic activities of TGF-β (9). Previous studies have provided evidence consistent with an indispensable role of endogenous CCN2 in TGF-β responses. For instance, Duncan et al. demonstrated that induction of collagen synthesis by TGF-β in normal rat kidney fibroblasts was effectively blocked with specific anti-CCN2 antibodies (10) Moreover, transfection of normal fibroblasts with antisense CCN2 resulted in abrogation of TGF-β responses, leading these investigators to conclude that endogenous CCN2 mediated the stimulation of collagen synthesis by TGF-β (10). However, more recent studies suggested that the functional relationship between TGF-β and CCN2 in the context of fibrosis may be significantly more complex, with CCN2 by itself having little direct effect, but functioning as a TGF-β costimulus required for optimal induction of profibrotic responses. For example, CCN2 was shown to enhance Smad signal transduction triggered by TGF-β (11). Another study showed that CCN2 interacted directly with TGF-β1 in the extracellular space, and enhanced ligand binding to the Type II TGF-β receptor, thereby potentiating TGF-β-induced cellular responses (12). In this way, CCN2 appeared to amplify TGF-β signaling by sensitizing target cells to respond to sub-threshold concentrations of TGF-β. These and similar studies notwithstanding, the precise nature of the relationship between TGF-β and CCN2 in the context of the fibrotic response remains incompletely understood.
In order to directly address the role of endogenous CCN2 in mediating the profibrotic effects of TGF-β, and in particular the stimulation of collagen synthesis, we examined TGF-β signal transduction and the regulation of gene expression in fibroblasts isolated from CCN2-null (CCN2−/−) mouse embryos (13). The results indicate that TGF-β-induced activation of the Smad signal transduction pathways was intact, and most profibrotic TGF-β responses occurred with comparable magnitude and time course in both wild type and CCN2−/− MEFs. These findings provide new insight into the mechanism of action of CCN2, and imply that while CCN2 may be involved in executing certain proliferative, adhesive and contractile functions of activated fibroblasts, it is not by itself directly responsible for mediating the important stimulatory effects of TGF-β on collagen gene expression.
MATERIALS AND METHODS
Culture of embryonic fibroblasts
Fibroblasts were isolated from CCN2−/− mouse embryos and wild type littermates (embryonic day 14.5) as described previously (14), and studied at low passage. Culture media were from Biowhittaker (Walkersville, MD); all other tissue culture reagents were from Gibco BRL (Grand Island, NY). Embryonic fibroblasts were cultured at 37°C in a 5% CO2 atmosphere in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS), 1% vitamin solution, 100 U/ml penicillin/streptomycin and 2 mM L-glutamine. When the fibroblasts reached confluence, fresh media with 0.1% FCS and indicated concentrations of TGF-β1 (Amgen, Thousand Oaks, CA) were added to the cultures. For proliferation assays, equal numbers of MEFs were seeded into 6-well plates in media containing 10% FCS. At the end of the indicated incubation periods, cells were harvested and counted. Viability of MEFs determined by Trypan blue dye exclusion was >90% in all experiments.
Extraction and analysis of RNA
For determination of mRNA levels, total RNA was isolated from confluent fibroblasts using TRIZOL Reagent (Gibco), and subjected to Northern analysis as previously described (15). Filters were sequentially hybridized with [32P]-labeled cDNA probes for human CCN2, COL1A2, fibronectin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 18S ribosomal RNA. Signal intensities were quantified by densitometry, and results were normalized with signal intensities of GAPDH mRNA or 18S ribosomal RNA in each sample. In some experiments, mRNA levels were determined by Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) analysis using the following PCR primers:GAPDH; forward, tgaccacagtccatgccatc,; reverse, gatccacgacggacacattg; COL6A1; forward, ctgtggtgcagacattcagg; reverse, agcactctcttcttggtggc; COL1A1; forward, gtattgctggacaacgtggt; reverse, aatgcctctgtcaccttgttc; Smad7; forward, agcacaccagctcggggttgat; reverse, aacgatctgcgctcgtccggcg
Western immunoblot analysis
At the end of the incubation, whole cell lysates were prepared from wild type and CCN2−/− MEFs and examined by immunoblot analysis as previously described (14). Equal aliquots (20 µg/lane) of proteins were subjected to SDS-PAGE in 4–20% gradient gels, and transferred onto Immobilon-P (PVDF) membranes (Millipore, Bedford, MA). Following blocking with 5% nonfat dry milk, membranes were incubated with antibodies against phospho-Smad2 (Cell Signaling Technology Inc., Beverly, MA), Type I collagen (Southern Biotech, Birmingham, AL), fibronectin (Chemicon International, Temecula, CA) Smad2/3, plasminogen activator inhibitor-1 (PAI-1) or actin (Santa Cruz Biotechnology, Santa Cruz, CA), followed by horseradish peroxidase-conjugated secondary antibodies. After washing, blots were developed with chemiluminescence reagents according to the manufacturer’s protocol (Pierce, Rockford, IL). Signal intensities were quantified by densitometry.
Immunocytochemistry
The expression and intracellular localization of endogenous Smads were studied by immunocytochemistry and fluorescence confocal microscopy, as described (10). Briefly, MEFs in media with 0.1% FCS were incubated with TGF-β1 (1 ng/ml). At the end of the indicated incubation periods, cultures were fixed with 100% methanol, and stained with primary antibodies against Smad2/3 or Smad4 (Santa Cruz), followed by horseradish peroxidase-conjugated secondary antibodies, and stained with fluorescein isothiocyanate (FITC). Nuclei were identified by 4,6-diamidino-2-phenylindone (DAPI) staining. Non-immunized IgG was used as negative control in every experiment. Following stringent washing of the slides, the pattern and subcellular distribution of fluorescence was evaluated by laser scanning confocal microscopy using a Zeiss LSM305 microscope, and data were analyzed using Release 3.2 Zeiss LSM Software. The data were then plotted as two-dimensional scatterplots to determine Pearson’s correlation coefficient, as described previously (15). A coefficient of −1.0 represents negative correlation between Smad and nucleus, 0 no correlation, and 1.0 complete correlation (ie. nuclear co-localization). The regulation of Type I collagen production and cytoskeletal organization in CCN2-deficient cells was evaluated by immunocytochemistry using antibodies to Type I collagen (Southern Biotech) or alpha-smooth muscle actin (Sigma, St. Louis, MO). Fibroblasts were categorized as alpha-smooth muscle actin-positive or negative by counting at least 50 cells/field in triplicate slides by an observer blinded to the identity of the sample. Fibroblasts were also stained with rhodamine-phalloidin to identify F-actin.
In vivo expression of CCN2
To characterize the pattern of CCN2 expression in vivo, lesional skin from mice injected with bleomycin s.c. for 21 d was analyzed by immunohistochemistry. Skin fibrosis was induced in 6–8 week old female C57BL/6 mice by daily injections of bleomycin (20 µg) as described (16). Skin tissue sections (4 µm) were deparaffinized in xylene and rehydrated with graded alcohol. Endogenous peroxidase activity was quenched with Peroxidase Blocking reagent (DAKO, Carpinteria CA). Antigen retrieval was performed by heating sections in target retrieval solution (DAKO, Carpinteria, CA) at 95°C in a water bath. Slides were washed in Tris-buffered saline with 0.05% Tween-20 (Sigma), incubated with blocking reagent (DAKO), and incubated with optimized dilutions of primary antibody to mouse CCN2/CTGF (Santa Cruz) overnight at 4°C, followed by biotinylated secondary antibody (Vector Laboratories, Burlingame, CA), and horseradish peroxidase-streptavidin (Vector). Color was developed with 3,3’-diaminobenzidine tetrahydrochloride substrate (Zymed), followed by counterstain with hematoxylin. As negative control, goat isotype IgG was used as primary antibody to ensure that effects of nonspecific binding were recognized. Fibroblasts in the dermis were identified based upon their characteristic spindle-shaped morphology. In each slide, a minimum of 30 cells in 6 microscopic fields at×400 magnification was scored as CCN2-positive or negative by two independent examiners blinded to the treatment. The ratio of positive cells/total cells examined was then calculated.
Transient Transfection Assays
Subconfluent cultures of wild type and CCN2−/− MEFs were transiently transfected using Superfect reagent (Qiagen, Valencia, CA), as described previously (15). The reporter plasmid [SBE]4-luc contains four tandem copies of an 8-bp palindromic consensus Smad binding element (SBE) that is specifically recognized by Smad3/4). The alpha-smooth muscle actin promoter-luc construct ASMA-luc (17) and 772COL1A2-CAT (containing the −772/+58 bp fragment of the human α2 (I) procollagen gene (COL1A2) were used for transfections. The amount of plasmid DNA used for each condition was equalized by adding pcDNA3 empty vector. A fixed amount (0.05 µg/well) of internal control reporter Renilla luciferase under the thymidine kinase promoter (pRL-TK from Promega, Madison, WI) was also cotransfected in every experiment to correct for minor variations in transfection efficiencies between the samples. Following transient transfection, MEFs were incubated with TGF-β1 (1 ng/ml) for a further 24 h. At the end of the incubation period, cultures were harvested and equal aliquots of cell lysates were assayed for their luciferase or chloramphenicol acetyltransferase (CAT) activities as described previously (15). All experiments were performed in triplicates and repeated at least three times.
Statistical analysis
For comparisons of the means in control and treated fibroblasts, statistical analysis using the Mann-Whitney test was employed. A p value < 0.05 was considered to be significant.
RESULTS
Expression of CCN2 in fibrotic dermis
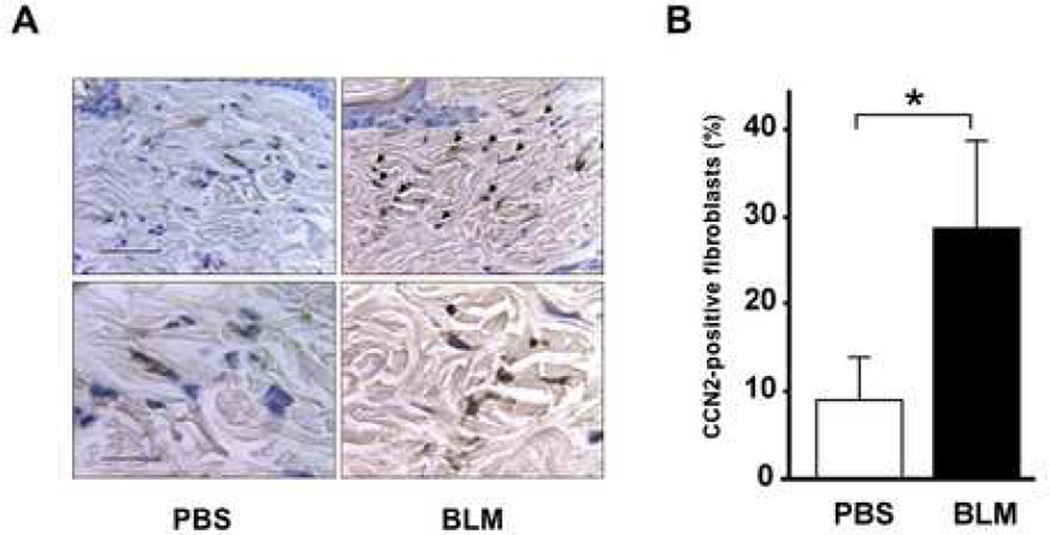
In order to characterize the expression and regulation of CCN2 in vivo, a murine model of fibrosis was used. Daily injections of bleomycin in 6 week-old BALB/c mice resulted in the development of skin fibrosis. By 21 days, there was dense sclerosis of the dermis and excessive accumulation of collagen in the relative absence of cellular infiltrates, with histological appearance similar to that seen in patients with SSc (data not shown). Dermal fibrosis in this mouse model is thought to be largely due to activation of resident fibroblasts by TGF-β (16). By immunohistochemisty, strong up-regulation of CCN2 expression was noted, whereas little CCN2 could be detected in the dermis from mice injected in parallel with PBS (Fig. 1), or from untreated mice (data not shown). CCN2 expression was found to be localized primarily to spindle-shaped fibroblast-like cells throughout the lesional dermis. Thus, these results indicate that CCN2 expression in dermal mesenchymal cells is normally quite low, but is markedly up-regulated during the fibrotic response, as observed in previous studies (7,8).
Figure 1. Up-regulation of CCN2 expression in lesional dermis.
C57BL/6 mice (6–8 week old) received daily s.c. injections of bleomycin or PBS in parallel. At 21 days, lesional skin was harvested and tissue sections were examined by immunohistochemistry using antibodies to CCN2. A. Representative photomicrographs. Arrows indicate positively stained fibroblasts. Bars, 50 µm (upper panels), 20 µm (lower panels). B. The proportion of CCN2-positive fibroblastic cells. The results are expressed as means ± SD of percentage of CCN2-positive fibroblasts from six independent microscopic fields; p<0.05.
Reduced proliferative responses in CCN2-null MEFs
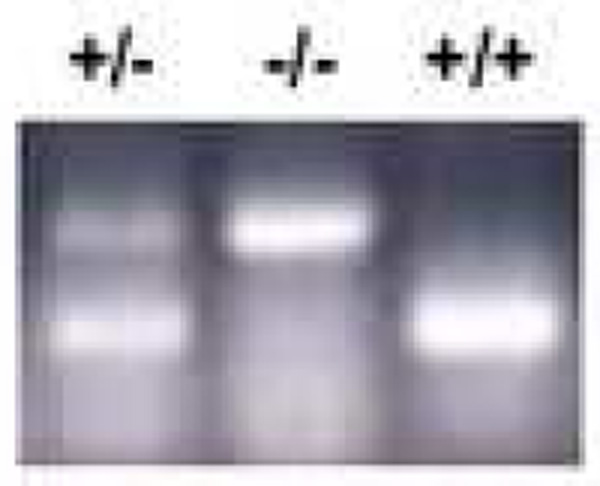
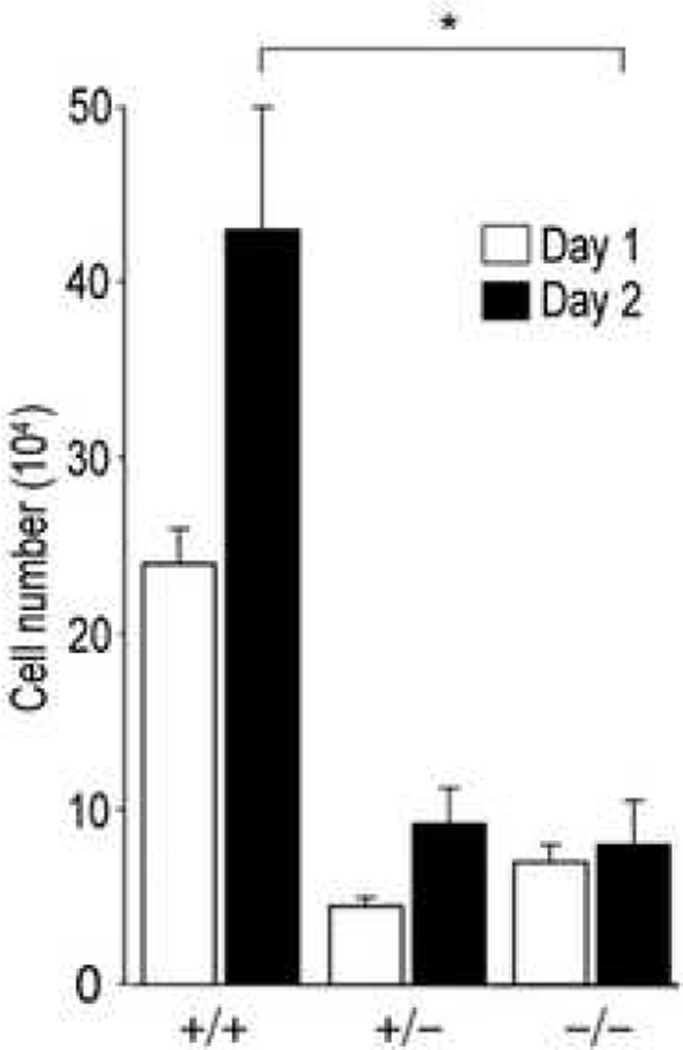
In order to characterize the functional role of endogenous CCN2 in fibroblast responses in vitro, the proliferation of CCN2−/− MEFs was first examined. Cultures of MEFs isolated from CCN2−/− and CCN2+/− mice and from wild type littermates were studied at low passage. Several individual MEF cell lines were examined in parallel, and yielded consistent results. Successful targeting of the CCN2 locus in these MEFs was confirmed by RT-PCR analysis (Fig. 2). Cells were seeded into 6-well plates and incubated for 1 or 2 days in media containing 10% FCS. At the end of the incubation, cell numbers and viability were determined. As shown in Fig. 3, CCN2−/− MEFs showed significantly reduced proliferation rates compared to CCN2+/− and CCN2+/+ MEFs, while cell viability at both time points was >95%.
Figure 2. PCR analysis of CCN2-null MEFs.
Genomic DNA was extracted from CCN2−/−, CCN2−/+ and CCN2−/− MEFs and used for PCR analysis with primers specific for CCN2.
Figure 3. Murine embryonic fibroblast proliferation.
Fibroblasts from CCN2−/−, CCN2−/+ and CCN2+/+ embryos were seeded in 6-well plates, and after 1 or 2 d incubation, cell numbers were determined. Results from a representative experiment (means ± SEM from triplicate determinations); p<0.05. Viability was >95%.
Intact Smad-dependent TGF-β signal transduction in CCN2-null MEFs
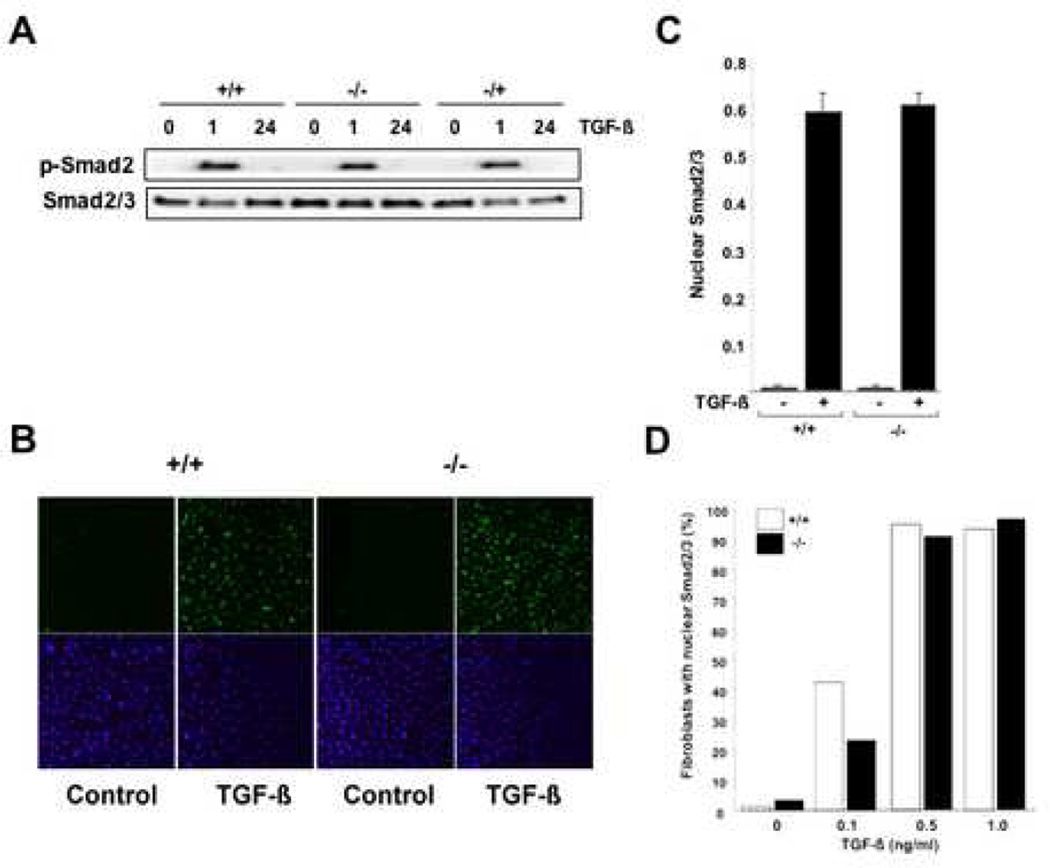
In normal fibroblasts, TGF-β induces rapid Smad2/3 phosphorylation, and enhances nuclear accumulation of the Smad heteromeric complex (14). Previous studies suggested that optimal Smad-dependent TGF-β responses were dependent on CCN2 (12). To investigate the role of CCN2 in ligand-induced Smad activation, endogenous Smad2/3 phosphorylation and nuclear accumulation were examined. Confluent MEFs were incubated with TGF-β1 (1 ng/ml) for 60 min or 24 h, and whole cell lysates were prepared and examined using antibodies specific for phosphorylated Smad2 and total Smad2/3. The results of Western analysis showed that in wild type MEFs TGF-β induced rapid and transient phospho-Smad2 accumulation in the absence of significant change in total Smad2/3 levels, as expected (Fig. 4A). Significantly, Smad2 phosphorylation was induced by TGF-β in CCN2−/− and CCN2+/+ MEFs with comparable time-course and magnitude. A critical step in the Smad-mediated TGF-β signal transduction pathways involves the nuclear accumulation of activated Smad2/3 together with Smad4. Therefore, the intracellular distribution of Smads regulated by TGF-β was next examined. The results of confocal microscopy showed that incubation of wild type MEFs with TGF-β for 60 min resulted in substantial nuclear accumulation of Smad2/3 (Fig. 4B) and Smad4 (data not shown), as expected. Quantification of Smad2/3 nuclear accumulation from multiple independent experiments indicated that TGF-β incubation resulted in a comparable ~6-fold increase in both wild type and CCN2−/− MEFs (Fig. 4C). Additional experiments showed that both the TGF-β dose response and the time course for Smad nuclear import were comparable in wild type and CCN2−/− MEFs (Fig. 4D; and data not shown).
Figure 4. Smad activation in CCN2−/− MEFs.
Confluent cultures of CCN2−/−, CCN2−/+ and CCN2−/− MEFs in parallel were incubated with TGF-β for indicated periods. A. Whole cell lysates were prepared and subjected to Western analysis. Representative autoradiograms are shown. B. Cultures were incubated with TGF-β for 60 min, fixed and examined by immunocytochemistry and confocal microscopy using antibodies to Smad2/3. Nuclei were identified by DAPI (blue). Representative images are shown. C. The proportion of MEFs showing predominantly nuclear Smad2/3 was determined as described in Materials and Methods. Results are expressed as means ± SEM from 5 independent determinations from three different microscopic fields. D. Comparable dose-response of TGF-β-induced Smad2/3 nuclear accumulation in CCN2−/− and CCN2−/− MEFs. Fibroblasts were categorized as showing predominantly nuclear Smad2/3 by a blinded observer.
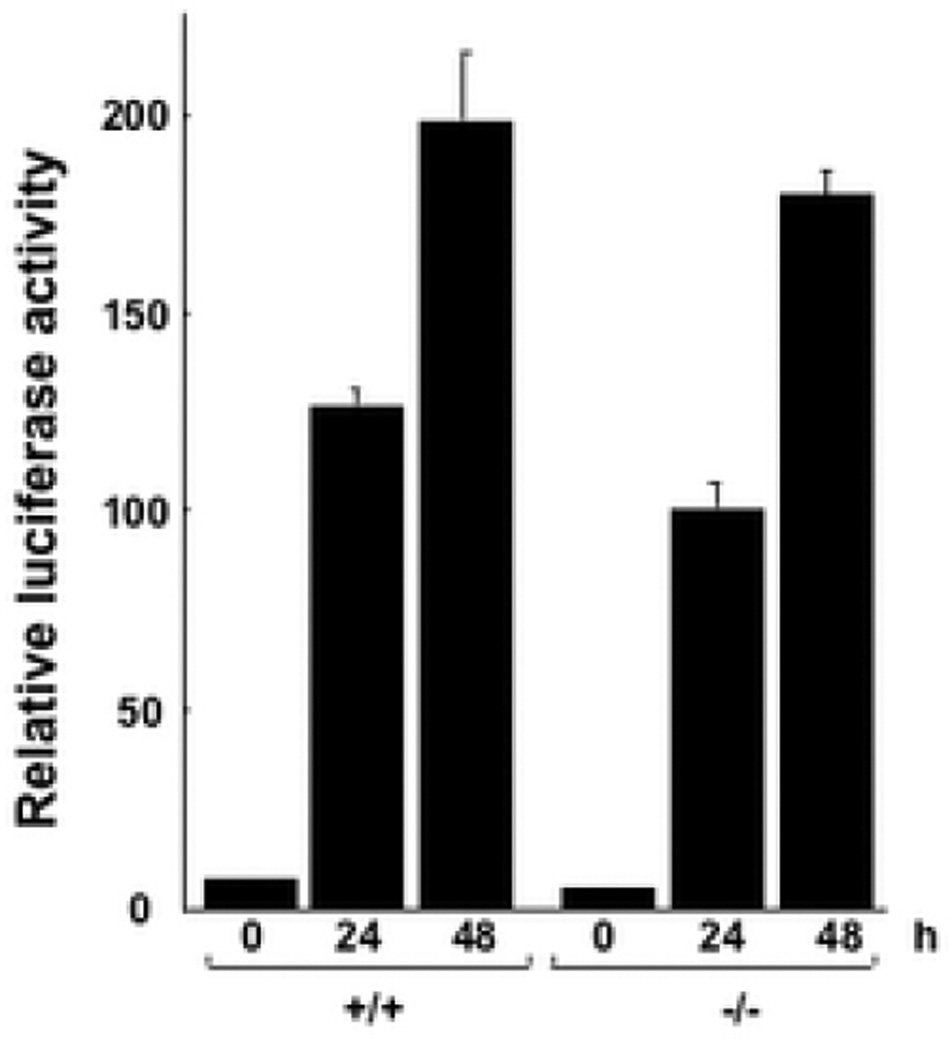
Smad-dependent TGF-β transcriptional responses involve direct binding of activated Smad3 to consensus cis-acting DNA elements on the target gene promoter, accompanied by recruitment of tissue-specific coactivators. To further investigate the role of endogenous CCN2 in Smad-driven transcription, transient transfection experiments were performed using a minimal Smad3-dependent reporter construct. Sub-confluent cultures of MEFs from CCN2−/− embryos and wild type littermates in parallel were transfected with p[SBE]4-luc, a Smad3-responsive minimal reporter construct. Following incubation with TGF-β1 for 24 or 48 h, cells were harvested and assayed for their luciferase activities. The results of transient transfection assays showed that TGF-β induced a time-dependent increase in luciferase activity of comparable magnitude in both wild type and CCN2−/− MEFs (Fig. 5). Taken together, these results clearly indicate that intracellular Smad-dependent signal transduction was unaffected by deletion of endogenous CCN2 in these MEFs.
Figure 5. SMAD-dependent transcriptional activity in CCN2−/− MEFs.
Subconfluent cultures of wild type and CCN2−/− MEFs were transiently transfected with p[SBE]4-luc and following 48 h incubation with TGF-β luciferase activities were determined. The results, normalized by renilla luciferase activity to correct for small variations in transfection efficiency between samples, represent the means ± SEM from triplicate determinations. Representative results from three experiments.
Regulation of fibrotic gene expression by TGF-β in CCN2-null MEFs
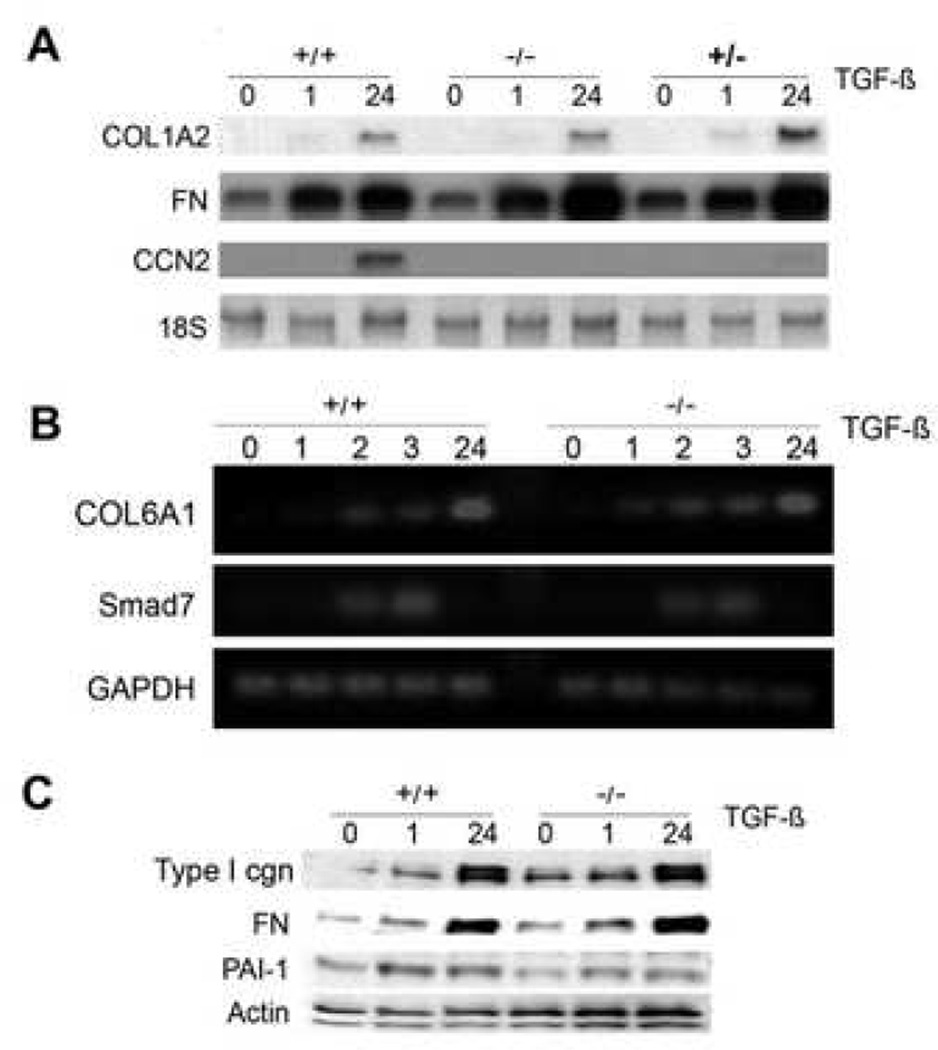
Previous studies implicated CCN2 in the pathogenesis of fibrosis in a variety of conditions. In particular, it has been suggested that endogenous CCN2, by functioning downstream of TGF-β, may be responsible for at least some of the profibrotic responses elicited by TGF-β in fibroblasts (9,10). To investigate the role of endogenous CCN2 in mediating the stimulatory effects of TGF-β, the regulation of collagen and ECM gene expression by TGF-β was examined in CCN2−/− MEFs in vitro. For this purpose, total RNA was isolated from confluent cultures of wild type and CCN2−/− MEFs incubated with TGF-β, and subjected to Northern and RT-PCR analysis. The results demonstrated that whereas CCN2 mRNA was undetectable in wild type MEFs in the absence of TGF-β stimulation, strong CCN2 expression was induced by TGF-β at 24 h, consistent with the notion that in normal fibroblasts CCN2 levels are low in the absence of activation (Fig. 6A). As expected, CCN2 mRNA was undetectable in CCN2−/− MEFs in the absence or presence of TGF-β. Incubation of the cultures with TGF-β resulted in time-dependent up-regulation of COL1A2 and fibronectin mRNA transcripts. Significantly, the stimulatory response elicited by TGF-β was comparable in wild type, CCN2−/+ and CCN2−/− MEFs. In complementary experiments, total RNA was subjected to RT-PCR analysis. The results showed comparable TGF-β stimulation of COL1A1 and COL6A1 mRNA expression in CCN2−/− MEFs and wild type MEFs (Fig. 6B and data not shown).
Figure 6. Regulation of ECM gene expression by TGF-β in CCN2−/− MEFs.
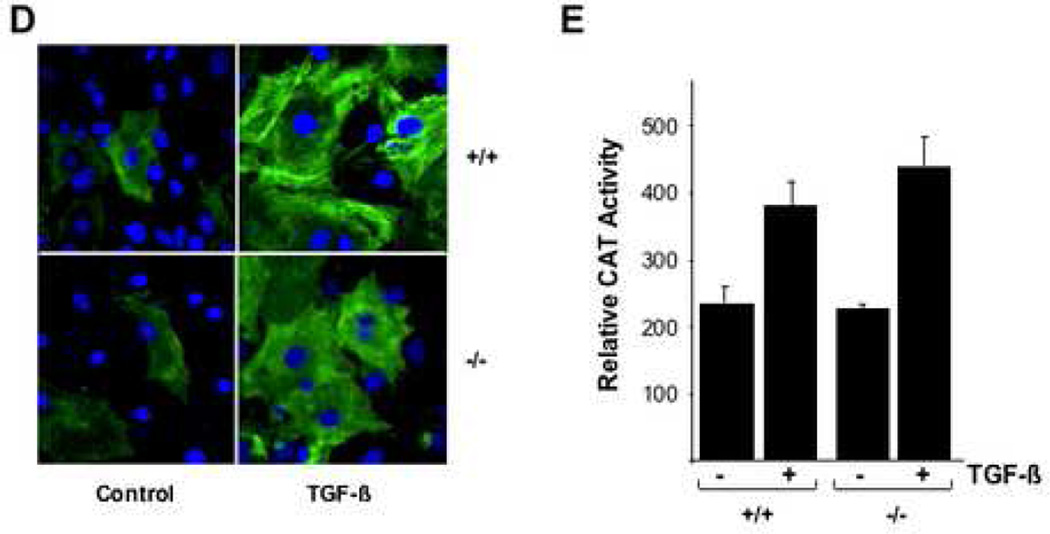
Subconfluent cultures of wild type, CCN2+/− and CCN2−/− MEFs were incubated with TGF-β for the indicated periods, and mRNA levels were examined by A. Northern analysis; or B. RT-PCR analysis. Representative results shown. C. Whole cell lysates were subjected to Western analysis. D. Confocal microscopy was performed in confluent cultures incubated with TGF-β for 48 h and stained with antibodies to Type I collagen (green color). E. Cultures were transiently transfected with 772COL1A2-CAT, and following 48 h incubation with TGF-β, cell lysates were assayed for their CAT activities. The results, normalized by renilla luciferase activity to correct for small variations in transfection efficiency between samples, represent the means ± SEM from triplicate determinations.
Smad7 is an endogenous antagonist of Smad-dependent TGF-β signaling that is itself induced by TGF-β. By targeting the ALK5 TGF-β receptor for ubiquitination and proteasomal degradation, Smad7 down-regulates ALK5-mediated Smad2/3 phosphorylation and activation, thus participating in a negative feedback loop that serves to limit the TGF-β response. It was recently reported that in mesangial cells, recombinant CCN2 suppressed the basal expression of Smad7, suggesting that interference with Smad7-mediated negative feedback regulation of TGF-β signaling could potentially account for the ability of CCN2 to amplify profibrotic responses (11). In order to evaluate if endogenous CCN2 may modulate the basal expression of Smad7 or its induction by TGF-β, Smad7 mRNA expression was examined in CCN2−/− MEFs. The results of RT-PCR analysis indicated that TGF-β induced a rapid and transient increase in Smad7 mRNA levels in wild type MEFs, as expected; the magnitude and kinetics of Smad7 stimulation were comparable in CCN2−/− MEFs (Fig. 6B and data not shown).
In order to investigate the role of endogenous CCN2 in the regulation of ECM protein expression, the stimulation by TGF-β of Type I collagen, fibronectin and PAI-1 synthesis was examined in CCN2−/− MEFs. The results of Western analysis showed that TGF-β-induced stimulation of all three ECM proteins occurred with comparable time course in wild type and CCN2−/− MEFs (Fig. 6C). Additional studies indicated that the induction of these ECM proteins followed similar TGF-β dose response in wild type and CCN2−/− MEFs (data not shown). Qualitatively comparable stimulation of Type I collagen accumulation by TGF-β in wild type and CCN2−/− MEFs was confirmed by immunocytochemistry (Fig. 6D). To examine the regulation of COL1A2 promoter activity by TGF-β, wild type and CCN2−/− MEFs at confluence were transiently transfected with 772COL1A2-CAT, and CAT activities were determined following 48 h incubation with TGF-β. The results of transfection assays showed that TGF-β induced a comparable ~2-fold increase in CAT activity in both wild type and CCN2−/− MEFs (Fig. 6E).
Myofibroblasts differentiation in CCN2−/− MEFs
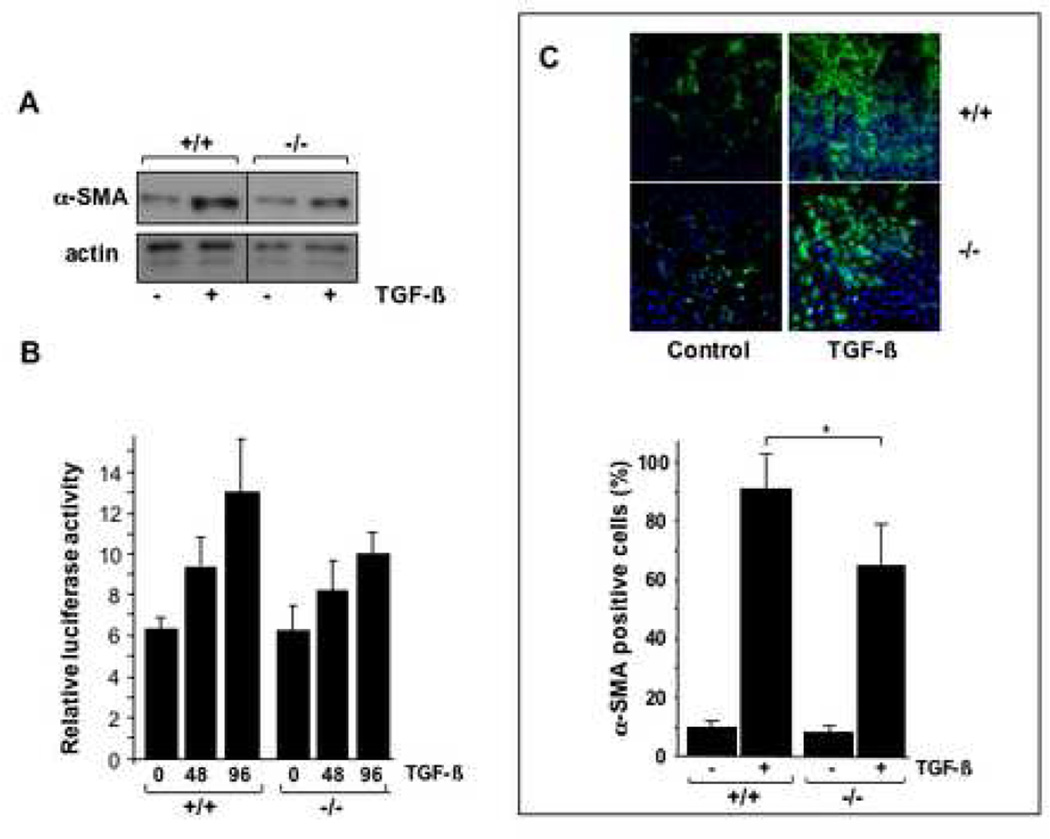
A pivotal feature of TGF-β profibrotic response in mesenchymal cells is their transdifferentiation into alpha smooth muscle actin-expressing myofibroblasts. These contractile cells play an important role in the development and persistence of pathological scar, and therefore are major potential targets in the pathogenesis of fibrosis. To investigate the role of endogenous CCN2 in myofibroblast transdifferentiation, the regulation of alpha smooth muscle actin was examined in wild type and CCN2−/− MEFs in parallel. Subconfluent cultures of MEFs were incubated with TGF-β for 48 h, and whole cell lysates were subjected to Western analysis. Densitometric scanning of immunoblots from three independent experiments showed that TGF-β induced a 4.2-fold increase in cellular levels of alpha smooth muscle actinin wild type MEFs, and a somewhat diminished (2.3-fold) increase in CCN2−/− MEFs, suggesting partially impaired induction of myofibroblast transdifferentiation in the absence of CCN2 (Fig. 7A). To investigate the mechanistic basis accounting this apparent deficit, the transcriptional regulation of the alpha smooth muscle actin gene was examined. Wild type and CCN2−/− MEFs were transiently transfected with ASMA-luciferase plasmids, and following incubation with TGF-β1 for 48 or 96 h, cell lysates were assayed for their luciferase activities. The results indicated that TGF-β incubation resulted in an up to 2-fold increase in alpha smooth muscle actin promoter activity in wild type MEFs and a 60% increase (p<0.005) in CCN2−/− MEFs (Fig. 7B).
Figure 7. Induction of myofibroblasts differentiation in CCN2−/− MEFs.
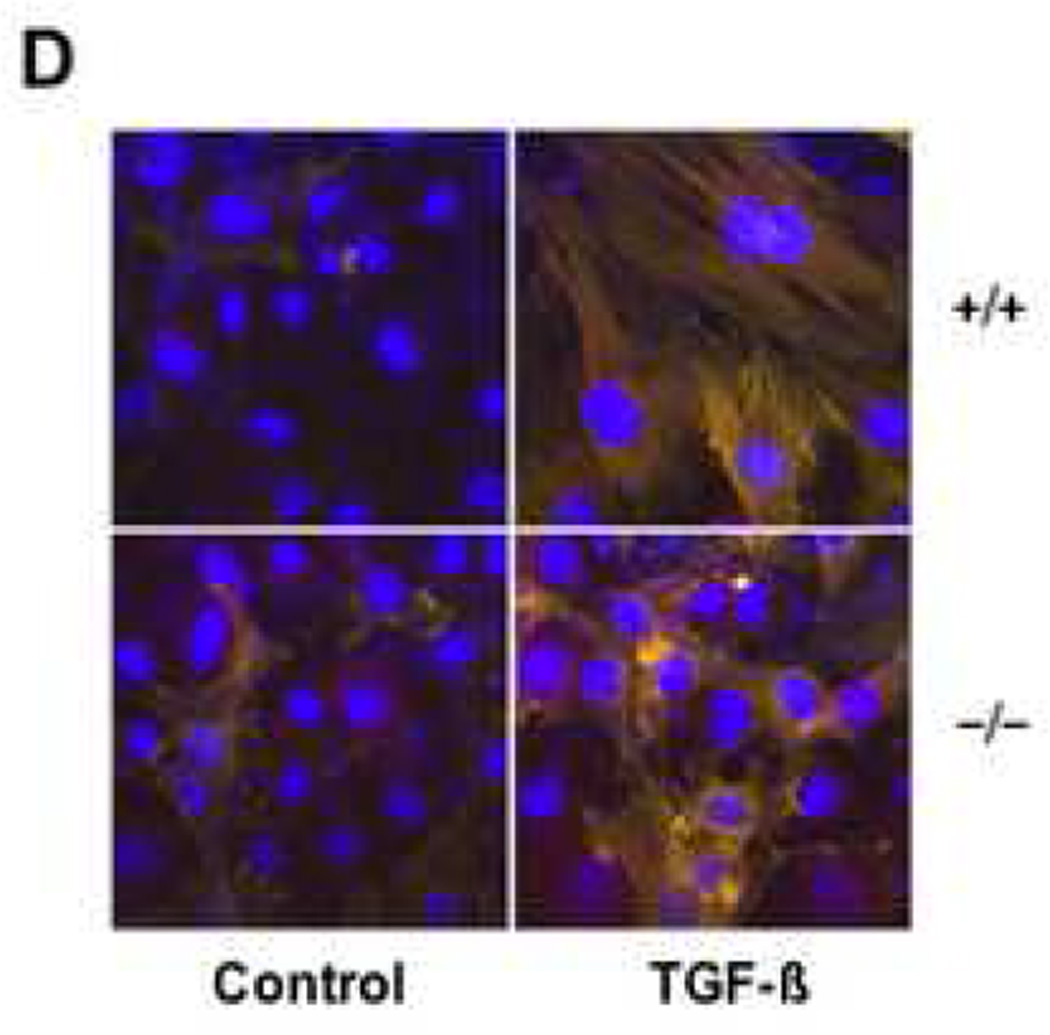
A. Subconfluent cultures of wild type and CCN2−/− MEFs were incubated in parallel with TGF-β for 48 h, and whole cell lysates were texamined by Western analysis. Representative results are shown. B. Wild type and CCN2−/− MEFs were transiently transfected with ASMA-luc, followed by incubation with TGF-β for 48 or 96 h. Cultures were harvested and luciferase activities determined. Results represent the means ± SEM from three determinations. C. Wild type and CCN2−/− MEFs were allowed to adhere to plastic for 12 h, and then incubated with TGF-β for 48 h. Cells were then fixed and subjected to indirect immunofluorescence with anti-alpha smooth muscle actin antibodies, as described in Materials and Methods. Nuclei were detected by DAPI staining (blue). Compared to wild type MEFs, CCN2−/− MEFs display reduced filopodia, alpha smooth muscle actin localization at the cell periphery and stress fiber formation. Lower panel, alpha smooth muscle actin expression was quantified. D. Wild type and CCN2−/− MEFs were incubated in parallel with TGF-β for 72 h, and stained with phalloidin to detect F-actin. Compared to wild type MEFs, CCN2−/− MEFs showed disorganized actin network.
To further explore the effect of endogenous CCN in myofibroblast transdifferentiation, the regulation of cytoskeletal stress fiber formation by TGF-β was examined by immunocytochemistry. For these studies, MEFs (5,000 cells/well) were seeded in 8-well chamber slides, and stimulated with TGF-β (1 ng/ml) for 48 or 96 h. At the end of the incubation, MEFs were stained for alpha smooth muscle actin and visualized by confocal microscopy. The results indicated that TGF-β induced a substantial increase in cellular levels of alpha smooth muscle actin protein, and enhanced the formation of characteristic alpha smooth muscle actin stress fibers in wild type MEFs, with >95% of the cells displaying prominent stress fibers after 96 h of TGF-β incubation. The basal level of alpha smooth muscle actin expression was consistently noted to be elevated in unstimulated MEFs compared to normal human dermal fibroblasts (data not shown). CCN2−/− MEFs also showed induction of alpha smooth muscle actin stress fibers when incubated with TGF-β, but the frequency of alpha smooth muscle actin-positive MEFs was 20–30% lower than among wild type MEFs (Fig. 7C). Quantitative analysis confirmed this observation (Fig. 7C, lower panel). Furthermore, as shown in Fig. 7D, the formation of the F-actin filament network appeared to be impaired and disorganized in CCN2−/− MEFs.
Discussion
Mesenchymal cells are responsible for the synthesis and accumulation of collagen and other ECM components during physiologic matrix remodeling such as organ development and wound healing. In these processes, mesenchymal cell activation induced by cytokines and growth factors including TGF-β, PDGF and CCN2 is spatially and temporally controlled. In contrast, pathological fibrosis is characterized by excessive matrix accumulation due to sustained and deregulated fibroblast activation that underlies the pathogenesis of these disorders. Understanding fibroblast activation, and the intracellular signal transduction pathways involved is crucial for the development of therapeutic approaches to fibrosis. The pleiotropic cytokine TGF-β stimulates collagen synthesis, ECM accumulation and myofibroblast transdifferentiation via the canonical Smad signaling cascade (18–20). In addition, alternate non-canonical Smad pathway signal transduction, as well as Smadindependent mechanisms, also appear to play important roles in mediating TGF-β responses, although their involvement in the fibrotic process still remains to be firmly established (4).
CCN2 has been shown to induce mesenchymal cell activation, including stimulation of collagen and ECM synthesis, proliferation, migration and adhesion, and has been proposed to play an indispensable role in mediating some of the profibrotic activities of TGF-β (9). For instance, induction of collagen synthesis by TGF-β in normal rat kidney fibroblasts was effectively blocked with specific anti-CCN2 antibodies or by antisense oligonucleotide (10). However, it is becoming clear that the effects of CCN2 on fibroblasts and role in ECM remodeling are complex. For example, CCN2 was shown to stimulate collagen production in cultured fibroblasts only when insulin was added to the culture media (21). Furthermore, in contrast to lesional SSc dermal fibroblasts, healthy control dermal fibroblasts showed only a modest and inconsistent stimulatory response. Similarly, mesangial fibroblasts failed to respond to CCN2 by itself, whereas Smad activation by TGF-β, and Smad-mediated cellular responses, were markedly enhanced in these cells (11). In the presence of exogenous CCN2, TGF-β-induced Smad2/3 phosphorylation and nuclear translocation, and induction of PAI-1 and Type III collagen, were enhanced. The additive effects of CCN2 with TGF-β in these cells were attributed to suppression of the inhibitory Smad7 by CCN2 (11). Chen et al. had recently shown that when normal dermal fibroblasts were allowed to adhere to CCN2 in vitro, substantial increases in the mRNA levels and the secretion of MMP1 (collagenase-1) and MMP3 (stromelysin-1) were observed (6). Up-regulation of MMP expression induced by adhesion of these fibroblasts to CCN2 appeared to be mediated via the α6β1 integrin and HSP. In this scenario, CCN2-induced fibroblast stimulation would conceivably result in enhanced collagen turnover and ECM degradation. Based upon other studies, it appears that CCN2 can stimulate not only collagen synthesis and ECM accumulation, but also the production of matrix-degrading MMPs. Together, these findings therefore indicate that the role of CCN2 in ECM regulation, and its functional relationship with the preeminent profibrotic signal, TGF-β, are complex and perhaps cell type-specific.
In order to better understand the functional relationship between TGF-β and CCN2 in the context of fibrotic procesess, MEFs from CCN2−/− mice have been used. Previous studies showed that CCN2−/− MEFs had intact ability to bind to CCN2, but showed reduced FAK early phosphorylation, with substantially impaired spreading, reduced filopodia, and failure of alpha-smooth muscle actin to organize along the cell periphery or into stress fibers (22). However, at later time points, no difference between wild type and CCN2−/− MEF phenotype was observable, suggesting that endogenous CCN2 potentiated, but was not required for, the MEF adherene to and spreading on fibronectin (22). Indeed, it was recently shown that TGF-β was able to induce a majority of its normal responses in CCN2−/− MEFs in vitro (23). In that report, TGF-β-induced stimulation of Type I collagen and alpha-smooth muscle actin protein and mRNA expression were reduced compared to wild type MEFs. Surprisingly, and in seeming contrast to our present findings, even wild type MEFs showed only a modest stimulation of Type I collagen gene expression (23).
The present study was therefore undertaken to specifically examine TGF-β regulation by collagen gene expression in embryonic cells lacking endogenous CCN2. If CCN2 is an obligate downstream signaling intermediate for TGF-β, as has been previously prosposed, we would predict that CCN2−/− MEFs would fail to appropriately up-regulate collagen gene expression in response to TGF-β. Alternately, if CCN2 suppressed endogenous Smad7 expression, as has been shown for exogenous CCN2 in mesangial cells, it could be anticipated that MEFs lacking CCN2−/− would have elevated basal levels or TGF-β induction of Smad7 expression, with consequent impairment in Smad signaling and attenuated TGF-β responses. However, our results revealed that ligand-induced Smad signal transduction was entirely unaffected by loss of CCN2, as shown by comparable Smad phosphorylation and nuclear accumulation, as well as induction of Smad3-dependent transcriptional responses in transiently transfected CCN2−/− MEFs. Furthermore, basal Type I collagen protein levels and mRNA expression levels were unaffected, and the stimulation of collagen gene expression and COL1A2 promoter activity by TGF-β remained unaltered. These results therefore indicate that endogenous CCN2 was not required for these profibrotic response induced by TGF-β in MEFs. In contrast, the results showed that while TGF-β elicited stimulation of alpha smooth muscle actin gene expression in both wild type and CCN2−/− MEFs, the loss of CCN2 was accompanied by attenuated TGF-β response. In the absence of endogenous CCN2, attenuated alpha smooth muscle actin protein expression and promoter activity were seen. Furthermore, the magnitude of myofibroblast transdifferentiation was reduced, associated with apparent disorganization of F-actin cytoskeleton. These findings indicate that in contrast to collagen gene expression, cytoskeletal rearrangement induced by TGF-β may be partially dependent on endogenous CCN2. Because the cytoskeleton is functionally involved in cell attachment and migration that are key components of the matrix remodeling process, the results suggest that CCN2 may have an important role in these physiologic processes. Furthermore, MEFs lacking CCN2 consistently showed reduced proliferative capacity.
The present results indicate that endogenous CCN2 is not absolutely required for optimal stimulation of embryonic fibroblast collagen gene expression by TGF-β, but does have a role in proliferation, myofibroblast transdifferentiation and cytoskeletal rearrangement. Taken together with recent findings, these observations suggest that endogenous CCN2 is functionally involved in mediating fibrogenic responses by facilitating cell binding to the ECM, cell proliferation and migration and matrix contraction in response to TGF-β stimulation. By enhancing fibroblast-ECM interactions and consequent matrix remodeling, CCN2 may play an important role in physiologic tissue repair and in the pathogenesis of fibrosis.
Acknowledgement
Supported by grants from the National Institutes of Health (AR-42309, AR-49025 and AR-052686).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004 Jan 6;140(1):37–50. [PubMed] [Google Scholar]
- 2.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007 Mar;117(3):557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massague J, Gomis RR. The logic of TGF-beta signaling. FEBS Lett. 2006 May 22;580(12):2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 4.Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005 Aug 15;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 5.Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004 Jan 3;363(9402):62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- 6.Chen CC, Chen N, Lau LF. The angiogenic factors Cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts. J Biol Chem. 2001 Mar 30;276(13):10443–10452. doi: 10.1074/jbc.M008087200. [DOI] [PubMed] [Google Scholar]
- 7.Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993 Jun;4(6):637–645. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996 Apr;7(4):469–480. [PubMed] [Google Scholar]
- 9.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997 Sep;8(3):171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 10.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB J. 1999 Oct;13(13):1774–1786. [PubMed] [Google Scholar]
- 11.Wahab NA, Weston BS, Mason RM. Modulation of the TGFbeta/Smad signaling pathway in mesangial cells by CTGF/CCN2. Exp Cell Res. 2005 Jul 15;307(2):305–314. doi: 10.1016/j.yexcr.2005.03.022. Epub 2005 Apr 22). [DOI] [PubMed] [Google Scholar]
- 12.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002 Aug;4(8):599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivkovic S, Yoon BS, Popoff S, Safadi FF, Libuda DE, Stephenson RS, Daluiski A, Lyons K. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori Y, Chen SJ, Varga J. Modulation of endogenous Smad expression in normal skin fibroblasts by transforming growth factor-β. Exp Cell Res. 2000;258:374–383. doi: 10.1006/excr.2000.4930. [DOI] [PubMed] [Google Scholar]
- 15.Chen SJ, Ning H, Ishida W, Sodin-Semrl S, Takagawa S, Mori Y, Varga J. The early-immediate gene EGR-1 is induced by transforming growth factor-beta and mediates stimulation of collagen gene expression. J Biol Chem. 2006;281:21183–21197. doi: 10.1074/jbc.M603270200. 2006. [DOI] [PubMed] [Google Scholar]
- 16.Hu B, Wu Z, Phan SH. Smad3 Mediates TGF-β- Induced α-Smooth Muscle Actin Expression. Am J Respir Cell Mol Biol. 2003 Sep;29:397–404. doi: 10.1165/rcmb.2003-0063OC. 2003. [DOI] [PubMed] [Google Scholar]
- 17.Takagawa S, Lakos G, Mori Y, Yamamoto T, Nishioka K, Varga J. Sustained activation of fibroblast transforming growth factor-beta/Smad signaling in a murine model of scleroderma. J. Invest. Dermatol. 2003;121:41–50. doi: 10.1046/j.1523-1747.2003.12308.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen SJ, Yuan W, Lo S, Trojanowska M, Varga J. Interaction of smad3 with a proximal smad-binding element of the human alpha2(I) procollagen gene promoter required for transcriptional activation by TGF-beta. J Cell Physiol. 2000 Jun;183(3):381–392. doi: 10.1002/(SICI)1097-4652(200006)183:3<381::AID-JCP11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 19.Poncelet AC, Schnaper HW. Sp1 and Smad proteins cooperate to mediate transforming growth factor-beta 1-induced alpha 2(I) collagen expression in human glomerular mesangial cells. J Biol Chem. 2001 Mar 9;276(10):6983–6992. doi: 10.1074/jbc.M006442200. Epub 2000 Dec 12. Erratum in: J Biol Chem 2001 Dec 14; 276(50):47746. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Ou J, Inagaki Y, Greenwel P, Ramirez F. Synergistic cooperation between Sp1 and Smad3/Smad4 mediates transforming growth factor beta1 stimulation of alpha 2(I)-collagen (COL1A2) transcription. J Biol Chem. 2000 Dec 15;275(50):39237–39245. doi: 10.1074/jbc.M003339200. [DOI] [PubMed] [Google Scholar]
- 21.Gore-Hyer E, Pannu J, Smith EA, Grotendorst G, Trojanowska M. Selective stimulation of collagen synthesis in the presence of costimulatory insulin signaling by connective tissue growth factor in scleroderma fibroblasts. Arthritis Rheum. 2003 Mar;48(3):798–806. doi: 10.1002/art.10953. [DOI] [PubMed] [Google Scholar]
- 22.Shi-wen X, Stanton LA, Kennedy L, Pala D, Chen Y, Howat SL, Renzoni EA, Carter DE, Bou-Gharios G, Stratton RJ, Pearson JD, Beier F, Lyons KM, Black CM, Abraham DJ, Leask A. CCN2 is necessary for adhesive responses to transforming growth factor-beta1 in embryonic fibroblasts. J Biol Chem. 2006 Apr 21;281(16):10715–10726. doi: 10.1074/jbc.M511343200. Epub 2006 Feb 16. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy L, Liu S, Shi-Wen X, Chen Y, Eastwood M, Carter DE, Lyons KM, Black CM, Abraham DJ, Leask A. CCN2 is necessary for the function of mouse embryonic fibroblasts. Exp Cell Res. 2007 Mar 10;313(5):952–964. doi: 10.1016/j.yexcr.2006.12.006. [DOI] [PubMed] [Google Scholar]