Abstract
Signaling by the BCR-ABL fusion kinase drives Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) and chronic myelogenous leukemia (CML). Despite their clinical activity in many patients with CML, the BCR-ABL kinase inhibitors (BCR-ABL-KIs) imatinib, dasatinib, and nilotinib provide only transient leukemia reduction in patients in Ph+ ALL. While host-derived growth factors present in the leukemia microenvironment have been invoked to explain this drug resistance, their relative contribution remains uncertain. Using genetically-defined murine Ph+ ALL cells, we identified Interleukin 7 (IL-7) as the dominant host-factor that attenuates response to BCR-ABL-KIs. To identify potential combination drugs that could overcome this IL-7-dependent BCR-ABL-KI-resistant phenotype, we screened a small molecule library including FDA-approved drugs. Among the validated hits, the well-tolerated anti-malarial drug dihydroartemisinin (DHA) displayed potent activity in vitro and modest in vivo monotherapy activity against engineered murine BCR-ABL-KI–resistant Ph+ ALL. Strikingly, co-treatment with DHA and dasatinib in vivo strongly reduced primary leukemia burden and improved long-term survival in a murine model that faithfully captures the BCR-ABL-KI-resistant phenotype of human Ph+ ALL. This co-treatment protocol durably cured 90% of treated animals, suggesting that this cell-based screening approach efficiently identified drugs that could be rapidly moved to human clinical testing
Introduction
Targeted BCR-ABL inhibition with imatinib has improved outcome for CML1. However, the situation is starkly different for pediatric and adult Ph+ ALL 2. While continuous BCR-ABL-inhibition therapy -- using imatinib, nilotinib, or dasatinib -- potently kills Ph+ ALL cells in vitro, these drugs only induce transient remissions in patients with Ph+ ALL, ultimately allowing relapse and giving poor long-term outcomes. Although additional chemotherapy and hematopoietic stem cell transplantation (HSCT) improve the remission rate in Ph+ ALL patients, relapses remain common3, with an 18- to 24-month survival rate of 64% and an even worse relapse-free survival rate3. Differences in primary responses to BCR-ABL-KIs in patients with Ph+ ALL and CML remain poorly understood, and cannot be attributed solely to mutations in the ABL kinase domain (BCR-ABLMUTANTS)4.
We previously developed a murine leukemia model of Ph+ ALL, using primary, polyclonal Arf -/- p185BCR-ABL luciferase-expressing (Luc+) Leukemia-Initiating pre-B Cells (LICs), which captures key genetic features of human Ph+ ALL (i.e. BCR-ABL expression and CDKN2A deletion) and enables in vivo luminescent imaging to monitor response to therapy5. Such BCR-ABLWT LICs retain phenotypic and signaling properties essential for suspended leukemic blasts to interact with the surrounding host-environment6 not typically present in established leukemic cell lines and remain responsive to cytokines implicated in murine pre-B cell development both in vitro and in vivo6. These polyclonal BCR-ABLWT LICs can very efficiently initiate leukemias in syngeneic, immune-competent mice.6
In ‘recipient’; host mice engrafted with these LICs, residual BCR-ABLWT5 cells evade treatment with BCR-ABL inhibitors, paralleling what is seen in human Ph+ ALL patients4. BCR-ABL kinase domain (KD) mutations, including BCR-ABLT315I (resistant to imatinib, dasatinib and nilotinib), emerge only after long-term exposure to BCR-ABL-KIs5, 7. In contrast to the in vivo setting, BCR-ABLWT murine and human cells are quite sensitive to BCR-ABL-KIs in vitro8. This disparity between in vitro and in vivo sensitivity implicates the host microenvironment in attenuating the therapeutic response to BCR-ABL kinase inhibition. We previously reported that common gamma chain receptor host cytokines modulate primary imatinib resistance6, and other host-derived growth factors have been shown to attenuate BCR-ABL kinase inhibition9-11. Currently, no consensus exists on the relative importance of host cytokines or growth factors that attenuate the cellular responses to BCR-ABL-targeted agents.
We used a naive screening approach to identify IL-7 as the most potent among candidate host growth factors in inducing resistance to BCR-ABL-KIs in Ph+ ALL. Following on that study, we used a repurposing screen of existing drugs and clinical candidates to identify drugs that blocked proliferation of BCR-ABLWT LICs cultured with murine IL-7. This study identified both the majority of known anti-leukemic drugs and a few novel agents that could overcome cytokine-dependent cell survival signaling. Testing of combinations of these validated hits with dasatinib revealed that the antimalarial drug dihydroartemisinin (DHA) provides strong synergy with BCR-ABL-KI's against Ph+ALL cells responding to the tumor microenvironment. When used in vivo, DHA-dasatinib combination therapy eradicates dasatinib-refractory leukemia in vivo providing near complete long-term survival.
Materials and Methods
Development of LIC-based screening assay
Before use in any in vitro assay, LICs had to pass the following quality control parameters: test thawing (benchmark: 95% viability at 24 h); PCR genotyping to confirm Arf -/- status; mycoplasma testing (negative by Takara Bio Mycoplasma PCR assay, Clontech, Madison WI); sequence verification of BCR-ABL allele; and in vitro dasatinib potency confirmation.
The maximum DMSO concentration tolerated by LICs in culture was determined to be 0.2% (by volume) and was maintained at ≤0.1 % (by volume) in all subsequent work. LICs were plated in 384-well micro-titer plates at a cellular density of 5 × 104 per mL (1250 LICs per well in 25 μL BCM10) and confirmed to give exponential growth during first 72 h (Supplementary Fig S1). To select assay reagents, 3 high throughput screening (HTS) relevant cellular assays were evaluated according to manufacturer's instructions (see Supplementary Fig S3). The luminescent CTG (CellTiter-Glo; Promega Madison, WI) assay, which monitors cellular ATP content, accurately detected low number of LICs producing results comparable to those obtained by flow cytometry, and provided ease of use in HTS (no prolonged incubation step required between reagent addition and assay readout). When carried out after 72 h, the CTG assay had a 10,000-fold dynamic range, low signal variance (Z'= 0.9 in semi-automated mode, and Z'=0.8 in fully automated mode), displayed minimal drift or edge effects, and had robust inter-plate and inter-day reproducibility.
Forward cytokine phenotypic screen
Lyophilized murine and human recombinant cytokines (R&D Systems, Minneapolis, MN) were diluted with 1 mg/mL BSA in sterile PBS to a final concentration 20 μg/mL and stored at 4°C. Anti-leukemia drugs, BCR-ABL-KIs, and non-BCR-ABL-specific-KIs (LC labs, Sigma), were solubilized in DMSO to obtain 10 mM stocks concentrations. Subsequent serial titrations were prepared using DMSO in 0.5 mL vials or 384-well drug master plates, and stored at −20°C.
To study the impact of murine cytokines on dasatinib action, LIC stocks containing different concentrations of candidate cytokines were prepared in multi-channel sterile reservoirs and plated into 384-well plates. Drug delivery to cell suspensions was accomplished with a V & P Scientific (San Diego, CA) pin tool (Supplementary Table 1). For murine-human comparisons, human SUP-B15 Ph+ ALL cells (DSMZ cell culture collection, Germany) in McCoy's media containing 20% fetal calf serum and penicillin/streptomycin (Invitrogen, Carlsbad, CA), were plated at 12,500 cells in 25 μL per well in black clear-bottom 384-well microtiter plates. Drugs were transferred by pin-transfer. After 72 h incubation at 37°C, 20% (by vol) MTS assay reagent (CellTiter 96 Aqueous One Solution, Promega, Madison, WI) was added, microtiter plates were incubated for 75 min at 37°C, and read colorimetrically at 490 nm.
High-throughput (HT) phenotypic drug screening
The IL-7 concentration of 0.85 ng/mL was confirmed (cytokine titration assays similar to Fig. 1a for dasatinib) to confer near-maximal and reproducible resistance against nilotinib and imatinib in BCR-ABLWT LICs (Fig. 1b and Supplementary Table 1). Primary screening of a collection of 5600 compounds (see Supplementary Information for details) highly enriched for approved drugs, development candidates, and compounds of known cellular activity from a variety of signaling mechanisms was carried out at a fixed dose (10 μM). Active compounds were retested to establish potency using a 10-step, 2-fold dilution scheme (see main text and Supplementary Table 1). In addition to BCR-ABLWT and BCR-ABLT315I LICs, secondary drug-screens were also performed against BCR-ABLF317L LICs, another clinically-known dasatinib-resistant ABL KD mutation, which gave identical results to that for BCR-ABLT315I (not shown). Multiple control assay plates were set-up during all screening runs to allow for inter-plate and inter-day comparisons at 0, 24, 48, and 72 hrs.
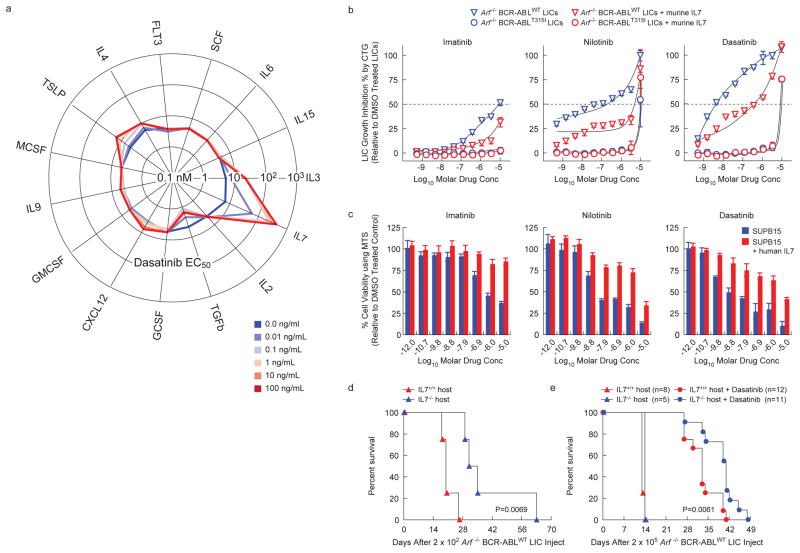
Figure 1. IL-7 induces resistance to BCR-ABL-KIs in Ph+ ALL without mutation of BCR-ABL.
(a) Radar plot representing the effect of 15 hematopoietic cytokines on dasatinib potency (EC50) against Arf-/- BCR-ABLWT LICs. CTG assay measured LIC populations after 72-h dasatinib exposures in presence of increasing concentrations of indicated cytokines. Each spoke represents an individual cytokine; each line graph represents dasatinib proliferation inhibition EC50 (nM) at indicated titration of the parent cytokine (see Excel Supplement). (b) Arf-/- BCR-ABLWT (triangles) and Arf-/- BCR-ABLT315I (circles) LICs were treated with drug or DMSO for 72-h in the absence (blue) or presence (red) of 1 ng/mL mIL-7, in triplicates. For each drug concentration, LIC growth measured by CTG assay was normalized to DMSO-treated LICs and fit using nonlinear regression (+/- sd). (c) Viability of human Ph+ ALL SUPB15 cells after 72-h drug-treatment in absence or presence of 25 ng/mL hIL-7, was measured by MTS assay. Values were normalized to DMSO-treated cells. (d, e) Kaplan-Meier survival curves showing overall survival in IL-7+/+ and IL-7-/- Bl6 host-mice receiving day 0 tail vein injection of (d) 2×102 Arf-/-BCR-ABLWT LICs (vehicle treated), with median survival of 20 days in IL-7+/+(n=4) versus 33 days in IL-7-/- mice (n=4), P=0.0069, or (e) 2×105 Arf-/- BCR-ABLWT LICs. Data are an average of 2 independent experiments in which vehicle or dasatinib therapy was initiated on day 7 or 9. Survivals of vehicle-treated IL-7+/+and IL-7-/- mice were comparable. Dasatinib treatment caused a median survival of 33 days in IL-7+/+ (n=12) versus 41 days in IL-7-/- (n=11) mice, P=0.0061.
Supplementary Data
Supplementary data includes: 1) Supplemental Information (PDF), which contains materials and methods for generation of LICs, cytokine screening, FACS assessment of viability and cell cycle percentages, detailed description of chemical library screened, data processing, quality control and hit scoring criteria for HT drug screens, cluster analysis using therapeutic drug classes, Supplementary figures S1-S11, table 1 and references; and an Excel Supplement containing complete data-sets for cytokine and HT drug screens.
Results
Determining which leukemia-microenvironment cytokines induce drug-resistance against BCR-ABL-KIs
In the absence of drug treatment, both Arf -/- p185WT and Arf -/- p185T315I pre-B cells (referred to as BCR-ABLWT leukemia initiating cells (LICs)12 and BCR-ABLT315I LICs, respectively) have identical growth properties in vitro (Supplementary Fig. S1), and demonstrate similar, potent leukemia initiation capacity and aggressive progression in vivo (Supplementary Fig. S2). They retain both phenotypic and signaling characteristics of leukemic blasts suggesting that they are a ready model for the effects of cytokines upon blasts. Fifteen leukemia-microenvironment cytokines were evaluated for their ability to protect murine BCR-ABLWT LICs from dasatinib in vitro.
IL-7 provided the strongest protection against dasatinib (Fig. 1a). In addition, IL3, thymic stromal lymphopoietin (TSLP, IL-7-like cytokine), and IL4 conferred some resistance, but with greatly reduced potency. In contrast, TGF-β enhanced dasatinib activity. Additionally, IL-7 significantly attenuated the potency of all FDA-approved BCR-ABL-KIs against murine BCR-ABLWT LICs (Fig. 1b) and the human Ph+ ALL cell line SUP-B15 (Fig. 1c). However, IL-7 did not affect the potency of tested non-BCR-ABL–targeted drugs (Supplementary Fig. S4). Thus, IL-7 drives a specific cellular response that provides resistance to all clinically-available BCR-ABL-KIs. The specificity was further confirmed by three in vitro findings: 1) the presence of IL-7 during prolonged dasatinib treatment significantly increased viability of Arf-/- BCR-ABLWT LICs (Supplementary Fig. S5); 2) transient BCR-ABL inhibition dramatically increased LIC responsiveness to IL-7 (Supplementary Fig. S6c-d)13; and 3) removing IL-7 completely reversed the protection effect and restored dasatinib's potency (data not shown).
Physiological levels of IL-7 confer an aggressive, dasatinib-resistant phenotype to Ph+ ALL in vivo
To confirm the physiological relevance of this finding, we studied the effect of IL-7 on leukemia progression and dasatinib responsiveness in vivo. Implantation of IL-7+/+, but not IL-7-/-, host mice with relatively small numbers of BCR-ABLWT LICs (200, low burden model) led to decreased survival (Fig. 1d), suggesting that murine Ph+ ALL progression accelerates in response to IL-7. Injecting higher numbers of LICs (200,000) overcame this trend (200,000, high burden model, Fig. 1e), suggesting the phenomena is most important during expansion of the initial seeding leukemia cell population. Treatment of animals in the high burden model revealed a significantly shortened survival benefit from dasatinib monotherapy of BCR-ABLWT-driven leukemias in IL-7-deficient hosts (Fig. 1e). From a therapeutic perspective, these findings show that in vivo responses to dasatinib therapy in Ph+ ALL are modulated by IL-7, independent of BCR-ABL KD mutations. These studies also suggest that using the BCR-ABLWT LIC's in the presence of IL-7 as a screening system could identify physiologically relevant pharmacological approaches to reversing this cytokine-dependent drug resistance.
High-throughput screens against primary (IL-7-induced) and acquired (BCR-ABL-mutation) BCR-ABL-KI drug-resistant phenotypes
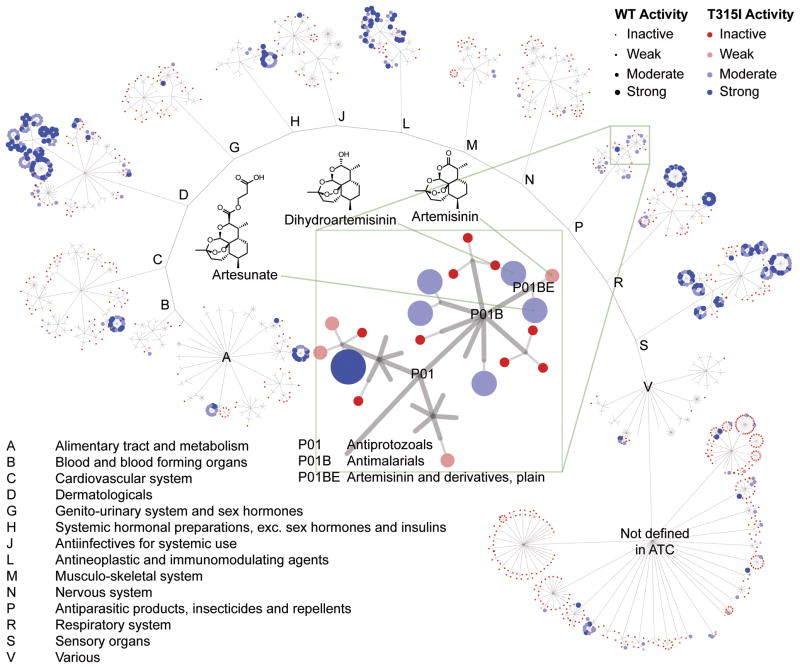
We hypothesized that pharmacologic agents could be identified that would overcome IL-7 induced BCR-ABL-KI-resistance. A collection of 5600 compounds highly enriched for approved drugs, development candidates, and compounds of known cellular activity from a variety of signaling mechanisms, including all active ingredients from approved oncology and anti-infective drugs, was screened against BCR-ABLWT and BCR-ABLT315I LICs in the presence of IL-7 (0.85 ng/mL) at a fixed drug concentration (10 μM). The quality of the screen and minimum significant activity (> 10% growth inhibition) was confirmed by receiver operating characteristic analysis and other statistical metrics (Supplementary Fig. S7-S10). 627 compounds showed statistically significant activity (≥10% growth inhibition). These hits were augmented with available structural analogs and drugs of the same pharmacologic classes to yield 706 active compounds. Concentration–response relationships were established for these compounds against BCR-ABLWT and drug-resistant BCR-ABLT315I LICs in the presence of IL-7, giving 229 validated hits with reasonable potency, representing a final hit rate of 4%. While a high hit rate, this is congruent with our historical experience screening this collection in growth inhibitory assays against cancer cell lines and primary cultures. All validated hits were equipotent in inhibiting the growth of BCR-ABLWT and BCR-ABLT315I LICs, suggesting they are not acting through BCR-ABL. This set included 15 conventional anti-leukemic drugs (see Excel Supplement), some of which enhance BCR-ABL-KI-therapy in vivo3, 5, thus validating the detection of appropriate phenotypic responses. Furthermore, cluster analysis indicated that this set effectively sampled the space of all existing therapeutic classes (Fig. 2). See Excel Supplement for a detailed annotation of validated hits.
Figure 2. Screen to identify compounds preventing growth of wild type and BCR-ABLMUTANT Ph+ ALL cells in the presence of IL-7.
Several hundred validated hits were identified. The hits are shown as a graph with clustering hits using ATC therapeutic classification (http://www.whocc.no/atc/structure_and_principles/). A single compound can be represented more than once because of multiple therapeutic indications, as is the case for the corticosteroids (highly active clusters in A, C, D, H, R, and S). This demonstrates that the active compounds come from drugs used to treat many indications. See Supplemental materials and methods and Supplementary Figure S11 for details on clustering methodology and analysis.
In vitro and in vivo anti-Ph+ ALL activity of dihydroartemisinin
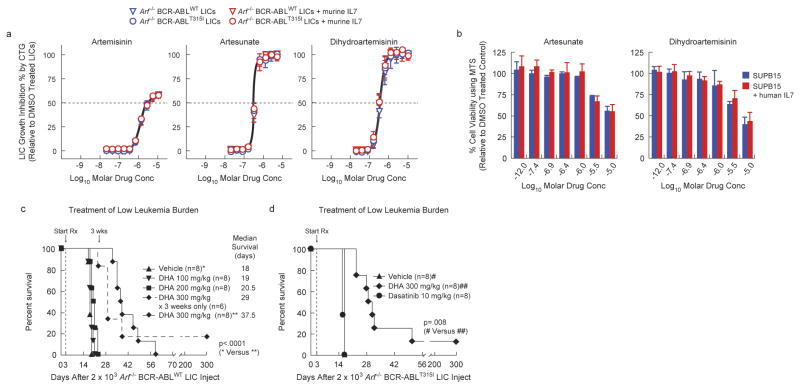
Although our validated hits included compounds with varying mechanisms of action and compound in varying stages of development, we focused on approved drugs with favorable human pharmacokinetic and toxicity profiles that have previously been used with pediatric populations but not for leukemia, which quickly narrowed focus to the artemisinin class of antimalarials (ARTs), which includes artemisinin, artesunate, and dihydroartemisinin (DHA)14. As a class, the ARTs were equipotent against BCR-ABLWT and BCR-ABLT315I LICs (Fig. 3a), but the anti-LIC potency of artemisinin (ca. 1 μM EC50) was weaker than that of artesunate or DHA (ca. 200 nM EC50). DHA and artesunate also killed the human Ph+ ALL cell line SUP-B15 with similar potency in the presence of IL-7 (Fig. 3a, 3b). Studies have shown that DHA kills other genotypes of leukemic cells while selectively sparing normal human lymphocytes15, 16. Therefore, DHA was chosen for further analysis because of its potency, cellular selectivity, oral formulation, favorable pharmacokinetics, known bioavailability in the hematopoietic system, and tolerability in all human age groups.
Figure 3. The artemisinin family of antimalarial drugs circumvents IL-7 induced resistance to dasatinib in Ph+ ALL cells in vitro and Ph+ ALL in IL-7+/+ host mice.
(a) Arf -/- BCR-ABLWT (triangles) and Arf -/- BCR-ABLT315I (circles) LICs were treated with artemisinin, artesunate, dihydroartemisinin, or DMSO for 72 h in the absence (blue) or presence (red) of 1 ng/mL murine IL-7, in triplicates. For each drug concentration, LIC growth (measured by the CTG assay) was normalized to DMSO-treated LICs and fit using nonlinear regression (+/- sd). (b) Human Ph+ ALL SUPB15 cells were treated with artesunate, dihydroartemisinin, or DMSO (no drug) for 72 h in the absence (blue) or presence (red) of 25 ng/mL human IL-7. Viability was measured by the MTS assay. Values were normalized to DMSO-treated cells. (c, d) Kaplan-Meier survival curves showing the overall survival in response to therapy (survival benefit) in IL-7+/+ Bl6 host mice that received day 0 tail vein injection of (c) 2×103 Arf-/- BCR-ABLWT Luc+ LICs; on day 3, once daily for 5 days of each week, vehicle, 100 mg/kg DHA, 200 mg/kg DHA, 300 mg/kg DHA, or 300 mg/kg DHA (for 3 weeks only) therapy was initiated in mice bearing low burdens of BCR-ABLWT-driven leukemia, or (d) 2×103 Arf-/- BCR-ABLT315I Luc+ LICs; on day 3, once daily for 5 days of each week, vehicle, 300 mg/kg DHA, or 10 mg/kg dasatinib was given to mice bearing low burdens of BCR-ABLT315I-driven leukemia.
Due to our interest in modeling the approved drug DHA, which is given orally for malaria, followup studies in vivo utilized the oral route rather than the parenteral route more commonly used in oncology studies. DHA monotherapy conferred a significant dose-dependent survival benefit to IL-7+/+ host mice bearing low-burden BCR-ABLWT leukemia (Fig. 3c) although this required escalation to high doses (300 mg/kg) for maximal effect. During these studies no significant changes in body weight were observed. This dose falls within the tolerated and bioavailable doses used in prior animal modeling for malaria.17
By conventional allometry this dose corresponds to roughly 45 mg/kg in a human, which is 4-fold higher than the current efficacious dose used for malaria in humans (up to 12 mg/kg). The limited murine pharmacokinetic data for DHA18 indicates that it is more rapidly cleared in mice and has lower bioavailability than in humans suggesting that lower relative doses would be efficacious in humans. Additionally, existing human and murine pharmacokinetic data indicate that plasma concentrations that should be achieved in this model would be well above concentrations efficacious in vitro against LIC's, even at doses more closely aligned with those used for malaria.19 BCR-ABL mutations, which are often selected with dasatinib treatment in this model5, 7, were absent in leukemic samples collected from terminally moribund DHA-treated mice. DHA monotherapy also gave a similar response in mice with low-burden BCR-ABLT315I leukemia, which is completely refractory to dasatinib (Fig. 3d). Thus, unlike BCRABL-KIs, DHA acts against Ph+ ALL via a BCR-ABL kinase–independent mechanism.
DHA synergistically enhances response to dasatinib
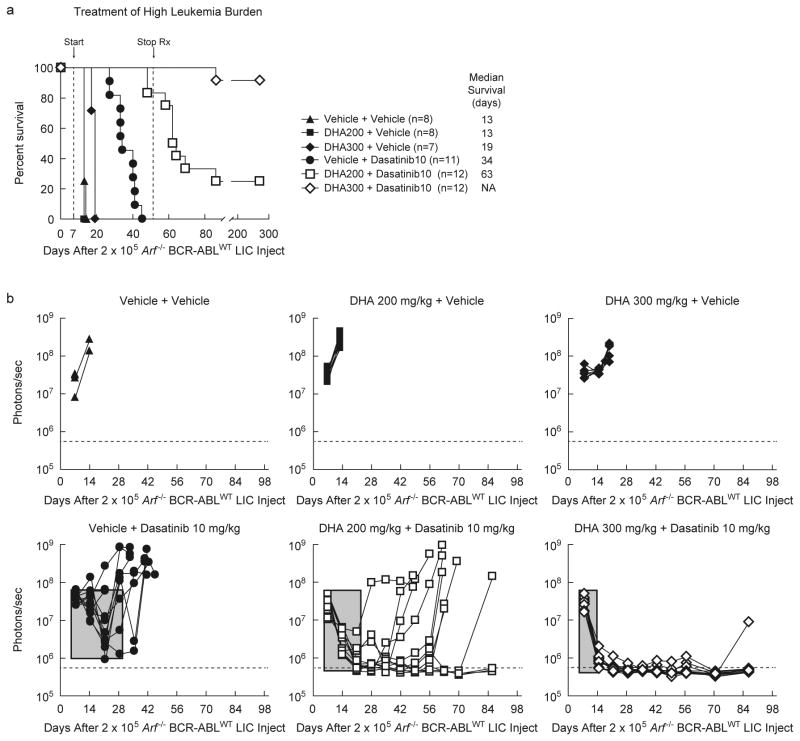
Because BCR-ABLWT leukemias in IL-7-deficient mice were more sensitive to dasatinib (Fig. 1e), and DHA displayed anti-Ph+ ALL activity in IL-7+/+ host mice (Fig. 3c, 3d), the potential for synergy between the two drugs was evaluated. Cohorts of IL-7+/+ immune-competent mice bearing high burdens of Arf-/- BCR-ABLWT -driven leukemia were treated with dasatinib alone (10 mg/kg), DHA alone (200 or 300 mg/kg) or binary combinations (Fig. 4a). Despite continuous monotherapy, dasatinib induced only weak initial responses, and all animals later relapsed (Fig. 4b) with complete mortality within 6 weeks of initiating therapy (Fig. 4a). DHA alone was completely ineffective (200 mg/kg) or poorly effective (300 mg/kg) in the high-burden leukemia model (Fig. 4a). However, the combination of DHA and dasatinib provided a strong and rapid initial response, and led to significantly increased overall survival (Fig. 4a & Fig. 4b), in comparison to the treatment with either of the two drugs alone. Of the 12 mice given 200 mg/kg DHA and 10 mg/kg dasatinib, 3 survived long-term. All mice given 300 mg/kg DHA and 10 mg/kg dasatinib (n=12) survived the treatment period. As discussed above, the plasma concentrations expected to be reached with such doses are likely providing exposure well above that efficacious against LIC's and within those that could be reached in humans with good tolerability.17, 19
Figure 4. Dihydroartemisinin works synergistically with dasatinib to cure Ph+ ALL.
(a) Kaplan-Meier survival curves showing the survival benefit in response to therapy in IL-7+/+ Bl6 host mice that received day 0 tail vein injection of 2×105 Arf -/- BCR-ABLWT Luc+ LICs. On day 7, six mouse groups bearing high burdens of BCR-ABLWT-driven leukemia were started on daily treatment for 5 days of each week, using two-dose regimens - 2 vehicles or 1 vehicle and 1 agent or 2 agents (see label), with 2 doses of each regimen given separately, 8 h apart, by oral gavage. On day 52, all therapy was terminated. (b) For 6 mouse groups in (a), separate graphs represent quantitative assessment of serial whole-animal luminescent signals (p/s/cm2/sr) acquired weekly. Solid lines represent imaging intensity of signal (y-axis) as a measure of leukemia burden from individual mice in the group, which is traced in real time on the x-axis. On day 7, before initiating therapy, all 6 groups had comparable and high burden of leukemia. The horizontal dotted line represents the empirically determined lower limit of sensitivity for luminescent signal as an experimental cut-off for detectable levels of persistent leukemia. Rectangular gray box quantifies experimental primary therapeutic response to indicated regimens; box-width depicts approximate time (days) to induce maximal initial response and box-depth represents maximal decrease achieved in leukemia burden signal. Increasing depth and decreasing width indicate stronger and quicker primary responses, respectively.
4 weeks after completion of combination therapy, 1 mouse succumbed to an isolated CNS relapse, which may be related to the poor CNS availability of dasatinib20. Remarkably, the 14 surviving mice taken off dasatinib-combined therapy (3/12 at 200 mg/kg and 11/12 at 300 mg/kg DHA) showed no leukemia for one year, the longest period monitored. In this leukemia model, all animals left untreated succumb to full blown leukemia within 4 weeks of injecting a very low numbers of LICs (Supplementary Fig. S2), strongly suggesting that the drug combination cured the disease. All dasatinib–DHA regimens were well tolerated in leukemic and non-leukemic mice, with no noticeable changes in weight gain or clinical signs, in congruence with prior rodent work utilizing similar dosages of DHA17.
Discussion
We identified IL-7 as the dominant (most potent) cytokine that confers resistance to all FDA-approved BCR-ABL-inhibitors. IL-7 is essential for murine T cell and B cell development21, has recently been implicated in human post-fetal B cell development 21, 22, and is a major contributor to the regulation and maintenance of mature T lymphoid cells23. IL-7-rich niches such as bone marrow harbor early persistent drug-refractory leukemias in dasatinib-treated mice, at which point the vast majority of leukemic cells do not harbor BCR-ABL KD mutations5 Ultimately, BCR-ABL mutations emerge under continuous drug exposures5, 24, causing secondary drug resistance. Our findings suggest that IL-7 maintains leukemic cell viability, probably through induction of phosphorylation of STAT5, despite continuous BCR-ABL inhibition in vivo, thereby facilitating the subsequent acquisition of drug resistance conferring kinase domain mutations. There is a well appreciated relationship between phosphorylation of STAT5 and resistance to imatinib in cells.25-27 Additionally, human Ph+ ALL patients with increased levels of pSTAT5, whose phosphorylation is triggered by IL-7, respond poorly to imatinib therapy.27
The demonstration of IL-7-induced drug resistance provided a rationale for ‘complementing’ pharmacological BCR-ABL-inhibition with a second drug that induces IL-7-independent leukemic cell death. We assayed 5600 compounds enriched with approved drugs to identify such agents. Our studies confirmed the activities of the majority of known anti-leukemia drugs and also identified several new classes of the compounds with activity against both IL-7-cultured wild-type and BCR-ABL mutant LICs. We characterized the anti-leukemia activity of one such drug: the orally-bioavailable, well-tolerated, antimalarial agent dihydroartemisinin (DHA) that selectively kills leukemic cells while sparing normal lymphocytes15, 16. Mechanistically, DHA acts independently of BCR-ABL-KIs (Fig. 3). In combination with dasatinib, DHA enhanced the depth of remission induction (Fig. 4b) and achieved near-complete long-term survival in a robust murine model of human Ph+ ALL. It is noteworthy that the combination of DHA and dasatinib therapy is significantly more efficacious than the clinically-used triple combination between dasatinib, dexamethasone, and asparaginase when assessed in the same model5.
Distinct from its anti-malarial mechanism of action, induction of cell death by ARTs in transformed cells is associated with accelerating the degradation of c-MYC 28. c-MYC expression is downstream of activated STAT529, which is maintained by IL-7 signaling, and STAT5 activation30 and c-MYC expression31, 32 are necessary to sustain BCR-ABL transformed cells. This phenomena has recently been implicated in other stroma-induced drug resistance10 as well as used as an explanation for resistance to imatinib10, 33. However, we do not see changes in pSTAT5 levels in response to treatment with artemisinins (data not shown). Thus, DHA may synergize with BCR-ABL-KI therapy by accelerating the degradation of c-MYC, thereby countering the protective effects of IL-7, as has been reported by others. Alternatively, as has also been reported DHA may affect BCR-ABL levels directly.34, 35
We have shown that phenotypic screening using primary leukemia cells selected to maintain responsiveness to host cytokines allows identification of therapeutic agents or developmental compounds that hold promise for the treatment of Ph+ leukemias. This system provides an unbiased platform for the discovery of such new therapies and to evaluate the potential utility of such agents by demonstrating proof-of-concept in vivo using a murine model that closely recapitulates human disease. Finally, our results strongly support further study of BCR-ABL-KI/DHA combination therapy for the treatment of patients with high-risk Ph+ ALL.
Supplementary Material
Acknowledgments
We acknowledge Charles J. Sherr, Gerard Zambetti and Joe Opfermann for critically reading the manuscript. We thank Chandra Savage and Branden Williams for assistance with mouse therapeutic studies, Christopher R. Calabrese and Monique Payton for assistance with animal imaging, Alexander J. Kovalic and Per Holmfeldt for assistance with in vitro growth factor screening experiments, Narendra P. Singh for suggestions on drug-preparation for animal use, Armand Guiguemde and Jimmy Cui at the High Throughput Screening Center at St. Jude for assistance with high-throughput drug screening, Heather Mulder for performing BCR-ABL KD mutation analyses, Richard A. Ashmun and Jim Houston for performing flow cytometric cell-sorting and analyses, and Owen Witte (UCLA) for the MSCV-BCR-ABL-IRES-GFP retroviral vector. This work was supported in part by an American Association for Cancer Research (AACR) Centennial Career Development Award for Childhood Cancer Research (R.T.W.), NIH 1R21NS066460-01 (R.T.W. and R.K.G), Gabrielle's Angel Foundation Medical Research Award (R.T.W.), NIH/NCI Comprehensive Cancer Center Core Grant CA-21765 and by the American and Lebanese Syrian Associated Charities (ALSAC) of St Jude Children's Research Hospital.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Reference List
- 1.Bjorkholm M, Ohm L, Eloranta S, et al. Success story of targeted therapy in chronic myeloid leukemia: a population-based study of patients diagnosed in Sweden from 1973 to 2008. J Clin Oncol. 2011;29:2514–2520. doi: 10.1200/JCO.2011.34.7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. The New England journal of medicine. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 3.Ravandi F, O'Brien S, Thomas D, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116:2070–2077. doi: 10.1182/blood-2009-12-261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicolini FE, Mauro MJ, Martinelli G, et al. Epidemiologic study on survival of chronic myeloid leukemia and Ph(+) acute lymphoblastic leukemia patients with BCR-ABL T315I mutation. Blood. 2009;114:5271–5278. doi: 10.1182/blood-2009-04-219410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulos N, Mulder HL, Calabrese CR, et al. Chemotherapeutic agents circumvent emergence of dasatinib-resistant BCR-ABL kinase mutations in a precise mouse model of Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;117:3585–3595. doi: 10.1182/blood-2010-08-301267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams RT, den Besten W, Sherr CJ. Cytokine-dependent imatinib resistance in mouse BCR-ABL+, Arf-null lymphoblastic leukemia. Genes & development. 2007;21:2283–2287. doi: 10.1101/gad.1588607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams RT, Sherr CJ. The INK4-ARF (CDKN2A/B) locus in hematopoiesis and BCR-ABL-induced leukemias. Cold Spring Harbor symposia on quantitative biology. 2008;73:461–467. doi: 10.1101/sqb.2008.73.039. [DOI] [PubMed] [Google Scholar]
- 8.Snead JL, O'Hare T, Adrian LT, et al. Acute dasatinib exposure commits Bcr-Abl-dependent cells to apoptosis. Blood. 2009;114:3459–3463. doi: 10.1182/blood-2007-10-113969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HP, Frankel AE, Hogge DE. A diphtheria toxin interleukin-3 fusion protein synergizes with tyrosine kinase inhibitors in killing leukemic progenitors from BCR/ABL positive acute leukemia. Leukemia research. 2010;34:1035–1042. doi: 10.1016/j.leukres.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 10.McMillin DW, Delmore J, Weisberg E, et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nature medicine. 2010;16:483–489. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiwase DK, White DL, Powell JA, et al. Blocking cytokine signaling along with intense Bcr-Abl kinase inhibition induces apoptosis in primary CML progenitors. Leukemia. 2010;24:771–778. doi: 10.1038/leu.2009.299. [DOI] [PubMed] [Google Scholar]
- 12.Williams RT, Roussel MF, Sherr CJ. Arf gene loss enhances oncogenicity and limits imatinib response in mouse models of Bcr-Abl-induced acute lymphoblastic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6688–6693. doi: 10.1073/pnas.0602030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jalkanen SE, Vakkila J, Kreutzman A, et al. Poor cytokine-induced phosphorylation in chronic myeloid leukemia patients at diagnosis is effectively reversed by tyrosine kinase inhibitor therapy. Experimental hematology. 2011;39:102–113 e101. doi: 10.1016/j.exphem.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 14.White NJ. Qinghaosu (artemisinin): the price of success. Science (New York, NY. 2008;320:330–334. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 15.Lai H, Singh NP. Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer letters. 1995;91:41–46. doi: 10.1016/0304-3835(94)03716-v. [DOI] [PubMed] [Google Scholar]
- 16.Singh NP, Lai HC. Synergistic cytotoxicity of artemisinin and sodium butyrate on human cancer cells. Anticancer research. 2005;25:4325–4331. [PubMed] [Google Scholar]
- 17.Nontprasert A, Pukrittayakamee S, Prakongpan S, et al. Assessment of the neurotoxicity of oral dihydroartemisinin in mice. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2002;96:99–101. doi: 10.1016/s0035-9203(02)90256-7. [DOI] [PubMed] [Google Scholar]
- 18.Batty KT, Gibbons PL, Davis TM, et al. Pharmacokinetics of dihydroartemisinin in a murine malaria model. The American journal of tropical medicine and hygiene. 2008;78:641–642. [PubMed] [Google Scholar]
- 19.Newton PN, van Vugt M, Teja-Isavadharm P, et al. Comparison of oral artesunate and dihydroartemisinin antimalarial bioavailabilities in acute falciparum malaria. Antimicrobial agents and chemotherapy. 2002;46:1125–1127. doi: 10.1128/AAC.46.4.1125-1127.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagas JS, van Waterschoot RA, van Tilburg VA, et al. Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin Cancer Res. 2009;15:2344–2351. doi: 10.1158/1078-0432.CCR-08-2253. [DOI] [PubMed] [Google Scholar]
- 21.von Freeden-Jeffry U, Vieira P, Lucian LA, et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. The Journal of experimental medicine. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parrish YK, Baez I, Milford TA, et al. IL-7 Dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J Immunol. 2009;182:4255–4266. doi: 10.4049/jimmunol.0800489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schluns KS, Kieper WC, Jameson SC, et al. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nature immunology. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 24.Mullighan CG, Phillips LA, Su X, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science (New York, NY. 2008;322:1377–1380. doi: 10.1126/science.1164266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casetti L, Martin-Lanneree S, Najjar I, et al. Differential contributions of STAT5A and STAT5B to stress protection and tyrosine kinase inhibitor resistance of chronic myeloid leukemia stem/progenitor cells. Cancer research. 2013;73:2052–2058. doi: 10.1158/0008-5472.CAN-12-3955. [DOI] [PubMed] [Google Scholar]
- 26.Quentmeier H, Eberth S, Romani J, et al. BCR-ABL1-independent PI3Kinase activation causing imatinib-resistance. Journal of hematology & oncology. 2011;4:6. doi: 10.1186/1756-8722-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warsch W, Kollmann K, Eckelhart E, et al. High STAT5 levels mediate imatinib resistance and indicate disease progression in chronic myeloid leukemia. Blood. 2011;117:3409–3420. doi: 10.1182/blood-2009-10-248211. [DOI] [PubMed] [Google Scholar]
- 28.Lu JJ, Meng LH, Shankavaram UT, et al. Dihydroartemisinin accelerates c-MYC oncoprotein degradation and induces apoptosis in c-MYC-overexpressing tumor cells. Biochemical pharmacology. 2010;80:22–30. doi: 10.1016/j.bcp.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Lord JD, McIntosh BC, Greenberg PD, et al. The IL-2 receptor promotes lymphocyte proliferation and induction of the c-myc, bcl-2, and bcl-x genes through the trans-activation domain of Stat5. J Immunol. 2000;164:2533–2541. doi: 10.4049/jimmunol.164.5.2533. [DOI] [PubMed] [Google Scholar]
- 30.Hoelbl A, Schuster C, Kovacic B, et al. Stat5 is indispensable for the maintenance of bcr/abl-positive leukaemia. EMBO molecular medicine. 2010;2:98–110. doi: 10.1002/emmm.201000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawyers CL, Callahan W, Witte ON. Dominant negative MYC blocks transformation by ABL oncogenes. Cell. 1992;70:901–910. doi: 10.1016/0092-8674(92)90241-4. [DOI] [PubMed] [Google Scholar]
- 32.Sawyers CL. The role of myc in transformation by BCR-ABL. Leukemia & lymphoma. 1993;11(Suppl 1):45–46. doi: 10.3109/10428199309047862. [DOI] [PubMed] [Google Scholar]
- 33.Albajar M, Gomez-Casares MT, Llorca J, et al. MYC in Chronic Myeloid Leukemia: Induction of Aberrant DNA Synthesis and Association with Poor Response to Imatinib. Mol Cancer Res. 2011;9:564–576. doi: 10.1158/1541-7786.MCR-10-0356. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Shen P, Zhang G, et al. Dihydroartemisinin inhibits the Bcr/Abl oncogene at the mRNA level in chronic myeloid leukemia sensitive or resistant to imatinib. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2013;67:157–163. doi: 10.1016/j.biopha.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Zhang G, Wu X, et al. Growth inhibitory effect of dihydroartemisinin on Bcr/Abl+ chronic myeloid leukemia K562 cells involve AKT, ERK and NF-kappaB modulation. Journal of cancer research and clinical oncology. 2012;138:2095–2102. doi: 10.1007/s00432-012-1292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.