SUMMARY
Mitochondrial morphology is maintained by the opposing activities of dynamin-based fission and fusion machines. In response to stress, this balance is dramatically shifted toward fission. This study reveals that the yeast transcriptional repressor cyclin C is both necessary and sufficient for stress-induced hyper-fission. In response to oxidative stress, cyclin C translocates from the nucleus to the cytoplasm where it is destroyed. Prior to its destruction, cyclin C both genetically and physically interacts with Mdv1p, an adaptor that links the GTPase Dnm1p to the mitochondrial receptor Fis1p. Cyclin C is required for stress-induced Mdv1p mitochondrial recruitment and the efficient formation of functional Dnm1p filaments. Finally, co-immunoprecipitation studies and fluorescence microscopy revealed an elevated association between Mdv1p and Dnm1p in stressed cells that is dependent on cyclin C. This study provides a mechanism by which stress-induced gene induction and mitochondrial fission are coordinated through translocation of cyclin C.
Keywords: Cdk8, oxidative stress, programmed cell death
Introduction
Mitochondria are dynamic organelles undergoing constant fusion and fission during normal cell division. The equilibrium between fission and fusion is controlled by the activity of conserved molecular machines driven by dynamin-like GTPases (see (Westermann, 2010) for review). In budding yeast, mitochondrial fission requires the GTPase Dnm1p that forms atypical helical filaments that first encircle, then constrict, mitochondria until scission is achieved (Mears et al., 2011). Recruitment of Dnm1p to the mitochondria requires the outer membrane protein Fis1p (Mozdy et al., 2000; Tieu et al., 2002) and one of two adaptor proteins, Mdv1p (Mozdy et al., 2000; Tieu and Nunnari, 2000) or Caf4p (Griffin et al., 2005). On the other side of the equation, the fusion of the inner and outer mitochondrial membranes requires the Mgm1p and Fzo1p GTPases, respectively (Meeusen et al., 2006; Rapaport et al., 1998). Several studies have demonstrated that the proper balance of fission and fusion is required for normal mitochondrial function (Ishihara et al., 2009; Wakabayashi et al., 2009).
The balance between fission and fusion is shifted dramatically toward fission in cells exposed to exogenous stress (Westermann, 2010). Mitochondrial hyper-fission is a conserved hallmark of the stress response (Igaki et al., 2000; Karbowski et al., 2002; Vieira et al., 2002) and is associated with the release of sequestered programmed cell death (PCD) inducing factors from this organelle (Breckenridge et al., 2003; Frank et al., 2001). Consistent with a connection between fission and PCD, yeast mutants lacking Dnm1p are more resistant to cytotoxic agents (Fannjiang et al., 2004).
The conserved protein kinase cyclin C-Cdk8p (Bourbon, 2008) associates with the RNA polymerase II holoenzyme mediator complex (Cooper et al., 1999; Liao et al., 1995). The cyclin C-Cdk8p kinase associates with two other proteins, Med12p and Med13p, to form a subcomplex termed the Cdk8 module (Bourbon, 2008) that plays both positive and negative roles in transcription depending on the locus (Chi et al., 2001; Hirst et al., 1999; van de Peppel et al., 2005; Vincent et al., 2001). Phenotypic studies in yeast have found that cyclin C-Cdk8p is required for several processes that respond to external cues including meiotic development (Cooper and Strich, 2002), pseudohyphal growth (Nelson et al., 2003) and PCD execution (Krasley et al., 2006). To relieve cyclin C-Cdk8 repression in yeast, cyclin C is destroyed (Cooper et al., 1997) following its translocation from the nucleus to the cytoplasm (Cooper et al., 2012). Here we provide evidence that, prior to its destruction, cytoplasmic cyclin C interacts with the fission machinery to promote stress-induced mitochondrial hyper-fission. These findings indicate that stress induced gene induction and mitochondrial fission are coordinated through cyclin C relocalization.
Results
Cyclin C-YFP localizes to the mitochondria following H2O2-induced oxidative stress in yeast
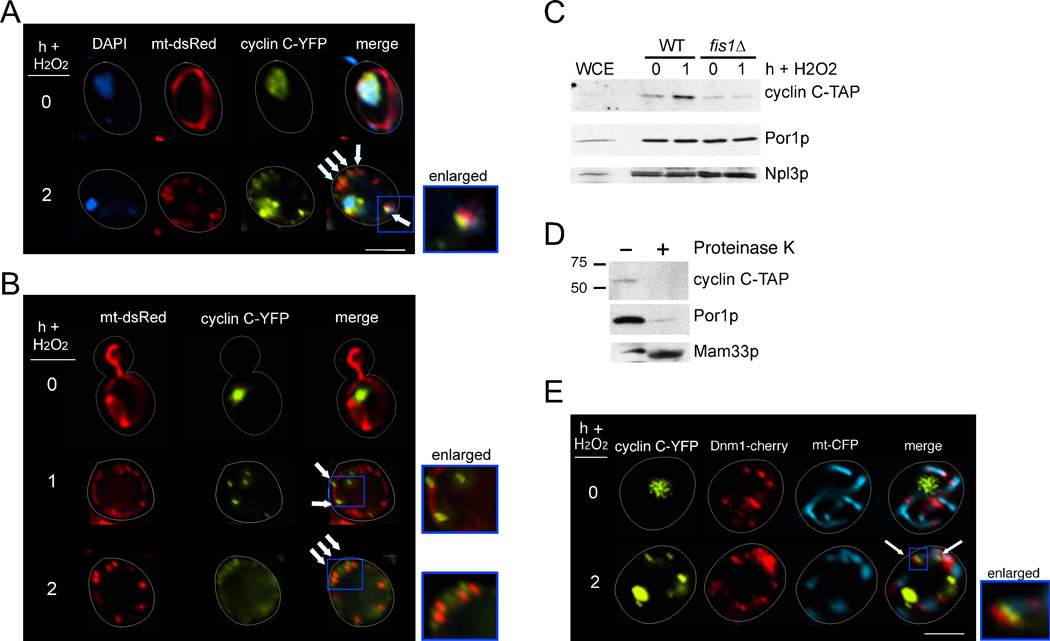
Our previous studies revealed that exposure to several stressors, including H2O2, induces cyclin C translocation from the nucleus to the cytoplasm where it forms punctate foci (Cooper et al., 2012). As mitochondria play a key role in mediating cell death, and cyclin C is required for normal PCD in yeast (Krasley et al., 2006), we first examined whether cyclin C foci associated with this organelle. Wild-type cells expressing cyclin C-YFP and the DsRed mitochondrial targeted protein (mt-DsRed) were treated with H2O2 (1 mM), fixed, then examined using fluorescence microscopy. As expected, cyclin C-YFP exhibited nuclear localization in the absence of stress while the mitochondria displayed a typical reticular structure (Figure 1A, upper panels). Following H2O2 exposure, cyclin C-YFP foci co-localized with the ends of mitochondrial fragments (arrows, Figure 1A, lower panels). We next imaged living cells to eliminate potential fixation artifacts. As before, cyclin C-YFP was not associated with the mitochondrial before stress (Figure 1B). However, one hour post H2O2 application, both fragmented and reticular mitochondria could be observed with cyclin C both free and associated with ends of the organelle (arrows). Two hours post stress revealed more substantial fragmentation with cyclin C-YFP more clearly at mitochondrial ends (arrows). By 3 h, cyclin-C-YFP could still detected at the mitochondria (Figure S1) although its visualization required increased exposure time due to its stress-induced degradation (Cooper et al., 2012).
Figure 1.
Stress-induced mitochondrial localization of cyclin C. (A) Fluorescence microscopy was conducted on mid-log phase cells expressing cyclin C-YFP and the DsRed mitochondrial targeting plasmid (mt-DsRed) before (0 h) and following (2 h) 1 mM H2O2 treatment. Arrows indicate sites of cyclin C-YFP and mitochondrial co-localization. (B) As in (A) except that living cells were visualized omitting DAPI staining. (C) Western blot analysis of cyclin C-TAP in whole cell extracts (WCE) or mitochondrial enriched fractions prepared from cultures with the indicated genotypes before and after H2O2 exposure (0.8 mM). The blot was stripped and reprobed for the presence of Npl3p (nuclear) and Por1p (mitochondrial) markers. (D) Western blot analysis of cyclin C-TAP in the 1 hr WT mitochondrial fraction described in (C) with (+) and without (−) Proteinase K treatment. Molecular weight markers (kDa) are indicated on the left of the panel. This blot was stripped and probed for components of the outer (Por1p) and inner (Mam33p) mitochondrial membranes. (E) WT cells expressing cyclin C-YFP, Dnm1-cherry and mt-CFP expression plasmids were grown to mid-log phase then treated with 1 mM H2O2 as indicated. Co-localization (arrows) of the mitochondria, cyclin C-YFP and Dnm1p-cherry was visualized by fluorescence microscopy. In all figures, the bars = 5µM unless otherwise stated. The enlarged regions are indicated by the blue boxes in all panels. See also Figure S1.
To confirm that cyclin C associates with the mitochondria, subcellular fractionation studies were performed from wild-type cultures expressing endogenously Tandem Affinity Purification (TAP) tagged cyclin C before and after (1 h) exposure to H2O2 (1 mM). A one hour timepoint was chosen as the time required to harvest and freeze the samples places the time between one and two hours. Western blot analysis revealed that cyclin C-TAP levels were elevated in the enriched mitochondrial fraction following H2O2 treatment (Figure 1C). These samples contained similar mitochondrial concentrations and nuclear contamination as determined by stripping the blot and probing for the presence of Por1p (Henriquez et al., 1990) and Npl3p (Bossie et al., 1992), respectively. However, cyclin C-TAP was not enriched in mitochondrial fractions prepared from a strain deleted for Fis1p, the receptor required to recruit the fission machinery suggesting that this association was physiological. Finally, treating the 1 h stressed mitochondrial-enriched sample with Proteinase K degraded both cyclin C-TAP and the outer membrane protein Por1p (Figure 1D). The protection of the inner matrix protein Mam33p (Seytter et al., 1998) from Proteinase K indicated that the mitochondria in these preparations were intact. Taken together, these results indicate that cyclin C translocates from the nucleus to the cytoplasm where it associates with the outer membrane of the mitochondria in a Fis1p dependent manner.
The punctate cyclin C-YFP foci pattern and its outer membrane localization has been observed for other mitochondrial proteins including Dnm1p (Bhar et al., 2006) and Num1p (Cerveny et al., 2007). To determine if cyclin C co-localized with Dnm1p in stressed cells, the localization of cyclin C-YFP and Dnm1p-Cherry was followed in a wild-type strain before and after H2O2 exposure. In the absence of stress, cyclin C-YFP was nuclear while Dnm1p-Cherry was found both associated and free of the mitochondria (Figure 1E). Following treatment with H2O2, the cyclin C-YFP signal was associated with Dnm1p-Cherry in 73% (±8%, n=3) of the cells. Interestingly, the cyclin C-YFP and Dnm1p-Cherry signals were adjoining rather than overlaying (see enlarged insert, Figure 1E). The finding that cyclin C associates with the fission machinery, combined with the requirement of Fis1p for binding the mitochondria, suggests a role regulating mitochondrial morphology.
Cdk8p and cyclin C are required for stress-induced mitochondrial fission
Yeast mitochondria form branched reticular networks under normal growing conditions that are converted to short tubules upon stress (Westermann, 2010; Youle and van der Bliek, 2012) (see Figure 2A). The stress-induced relocalization of cyclin C to the mitochondria suggested the possibility that it was involved in this fragmentation process. Therefore, mitochondrial morphology was followed in wild type and cnc1Δ strains harboring a mitochondrial targeted DsRed (mt-DsRed) expression plasmid before and after H2O2 treatment. Following peroxide application, ~90% of wild-type cells underwent fission (Figure 2B, quantitated in Figure 2C). However, only approximately 20% of the cnc1Δ mutant cells exhibited the fragmentation phenotype. Further studies revealed that cyclin C was required for fission in cells exposed to ethanol stress (Figure 2D, quantitated in Figure 2C) or 2 mM H2O2 (Figure 2E). These results indicate that cyclin C is required for extensive mitochondrial fission in response to multiple stress conditions.
Figure 2.
Cdk8p and cyclin C are required for stress-induced mitochondrial fission. (A) Representative images of reticular or fragmented mitochondria are shown. (B) Representative images of wild type and cnc1Δ Nomarski (Nom.) or mt-DsRed are shown following exposure to H2O2. (C) The percent of cells (mean ± s.e.m.) within the population displaying mitochondrial fission is given before and following H2O2 (1 mM) or ethanol (10% vol/vol) treatment for 2 h or 30 min, respectively. * p<0.05. (D) Confocal microscopic images of WT and cnc1Δ strains expressing mt-DsRed following exposure to ethanol (10% vol/vol) for 30 min. (E) Combined Nomarski and fluorescence images were obtained from WT and cnc1Δ cultures expressing mt-DsRed before and following exposure to H2O2 (2 mM) for 2 h. The percent of the population exhibiting fragmented mitochondria was calculated from three independent cultures (average ±s.e.m indicated). (F) A cdk8Δ strain (RSY1726) transformed with cyclin C-YFP and RFP-Nop1p expressing plasmids was visualized by fluorescence microscopy before and 2 h following H2O2 exposure (1 mM). DAPI staining indicates nuclear location. The arrows indicate the cyclin C-YFP foci observed in the stressed cdk8Δ cells. The frequency of cells containing a single focus associated with the nucleus is given on the right (mean ±s.e.m. n=3). The remainder of the culture exhibited either a diffuse nuclear signal or contained ≥2 nuclear associated foci. (G) The percentage of cells displaying fission (mean ± s.e.m.) in a cnc1Δ strain transformed with either the vector, wild type CNC1 or CNC1A110V 2 h following H2O2 treatment (1 mM). * = p<0.01 compared to the CNC1 expressing plasmid.
Cyclin C regulates mitochondrial morphology through a cytoplasmic activity
We previously demonstrated that Cdk8p remains in the nucleus or in the nucleolar compartment following H2O2 stress (Cooper et al., 2012). Interestingly, Cdk8p is also necessary for normal H2O2-induced mitochondrial fission (Figure 2C). These results are consistent with two models to explain cyclin C function. First, cyclin C mitochondrial relocalization may direct hyper-fission. Alternatively, cyclin C-Cdk8p may regulate the transcription of a gene (or genes) that in turn controls fission. To address these possibilities, we determined whether Cdk8p regulated cyclin C translocation. We reasoned that if cyclin C still interacted with the mitochondria in stressed cdk8Δ cultures, this result would point to a transcriptional role for these factors. Monitoring cyclin C-YFP subcellular localization in a stressed cdk8Δ culture revealed a single tight focus near the nucleus in 68% of the population (arrows, Figure 2F). This focus partially overlapped with a nucleolar marker (RFP-Nop1p) signal suggesting that cyclin C-YFP localization went from diffuse nuclear to the nucleolus but failed to efficiently enter the cytoplasm. These results indicate that Cdk8p is required for stress-induced cyclin C translocation to the cytoplasm possibly explaining its requirement for mitochondrial fission. A non-transcriptional role for cyclin C is also indicated when mitochondrial morphology was followed in a cnc1Δ mutant expressing a cyclin C derivative harboring a single amino acid substitution (A110V). This mutant remains nuclear following stress (Cooper et al., 2012) but still maintains normal transcriptional control (Cooper et al., 1999; Cooper et al., 2012). Compared to wild type, mitochondrial hyper-fission was significantly reduced in the strain expressing cyclin CA110V (Figure 2G). These results indicate that cytoplasmic translocation of cyclin C is required for stress-induced mitochondrial fragmentation.
Cyclin C is sufficient to induce mitochondrial fission
To test whether cyclin C is sufficient to induce mitochondrial fission, we sought to release cyclin C from the nucleus in the absence of stress. As noted above, cyclin C associates with three other proteins (Cdk8p, Med12p and Med13p) to form the Cdk8 module. We previously described a domain in the amino terminus of cyclin C (Holoenzyme Association Domain or HAD) that is required for transcriptional repression and binding to RNA polymerase II (Cooper et al., 1999). This domain was identified by a small internal deletion in which the residues KERQK were replaced with two alanines (HADΔ). We verified that the HAD is required for cyclin C interaction with the Cdk8 module using co-immunoprecipitation studies. A strain expressing endogenously myc-tagged Med13p was transformed with plasmids expressing GFP-cyclin C or GFP-cyclin CHADΔ. Protein extracts prepared from these unstressed log-phase cultures were immunoprecipitated with antibodies directed against GFP and the immunoprecipitates probed for the presence of Med13p-myc by Western blot analysis. This experiment revealed that HAD integrity was necessary for Med13p association (Figure 3A).
Figure 3.
Cytoplasmic cyclin C is sufficient to induce fission. (A) Extracts prepared from a wild-type strain expressing endogenously tagged MED13-myc allele, GFP-cyclin C or the HADΔ derivative as indicated were immunoprecipitated with GFP antibodies and the resulting immunoprecipitates probed for the presence of Med13-13myc. This blot was stripped and reprobed for GFP to ensure similar expression levels between the two GFP-cyclin C proteins. Extracts immunoprecipitated with myc or whole cell extracts (WCE) directly probed for myc controlled for the presence of Med13-13myc in the extracts. [ ] indicates no antibody control lanes. (B) Fluorescence microscopy monitoring the location of cyclin CHADΔ, the nucleus (DAPI) and mitochondria (mt-DsRed). Arrows indicate sites of mitochondria-cyclin CHADΔ interaction. Bar = 5 µM. (C) A cnc1Δ mutant expressing either wild type or cyclin CHADΔ and mt-DsRed were grown to mid-log phase then examined by fluorescence microscopy. The cells were scored based on the mitochondria exhibiting a fusion, fission or mixed morphology (see Materials and methods for scoring metric). The mean values obtained from three independent transformants are presented (±s.e.m.) along with the p value. (D) The experiment in (C) was repeated with a dnm1Δ cnc1Δ mutant strain. (E) A wild-type strain (RSY10) harboring either myc-cyclin C or myc-cyclin CHADΔ expression plasmids was subjected to an oxidative stress timecourse. Extracts prepared from these samples were probed for cyclin C and cyclin CHADΔ levels by Western blot analysis. Tub1p levels were used as a loading control. See also Figure S2.
We next determined whether the HAD was required for maintaining cyclin C in the nucleus in unstressed cultures. The CNC1HADΔ allele was fused to YFP and its localization monitored in unstressed cultures by fluorescence microscopy. These studies revealed that unlike cyclin C-YFP that demonstrated diffuse nuclear localization (see Figure 1B), > 90% of the cyclin CHADΔ-YFP expressing cells exhibited cytoplasmic foci (see Figure 3B for representative image). Closer examination revealed that cyclin CHADΔ-YFP co-localized to the mitochondria (arrows, Figure 3B). Importantly, the GFP-cyclin CHADΔ foci were found at mitochondrial regions that are either undergoing constriction or at sites of fission (see arrows, mt-DsRed panel). These results suggest that cyclin CHADΔ directs mitochondrial fission in the absence of stress. To test this hypothesis, mitochondrial morphology was monitored in log-phase unstressed cells expressing either cyclin C or cyclin CHADΔ. These experiments revealed that the mitochondria exhibited more fission in the cyclin CHADΔ expressing strains (Figure 3C). In addition, we observed an intermediate mitochondrial morphology in which the cells contained a mixed content of fragmented and fused mitochondria (see Figure S2A for additional example images). This “mixed” morphology was the largest category observed in cyclin CHADΔ expressing cells. Furthermore, this mitochondrial fragmentation required Dnm1p as >99% of a mid-log dnm1Δ culture expressing cyclin CHADΔ displayed the net phenotype indicative of loss of Dnm1p function (Figure 3D). These results indicate that cyclin CHADΔ-dependent fragmentation occurred through the normal fission machinery. Finally, since stress-induced cyclin C destruction occurs in the cytoplasm (Cooper et al., 2012), we next determined if precocious cytoplasmic cyclin C localization affected its destruction kinetics. A stress timecourse experiment was performed with wild-type cultures expressing myc-cyclin C or myc-cyclin CHADΔ. After normalizing to Tub1p levels, these experiments revealed that the half-life of cyclin C CHADΔ was less than half that of the wild type (14 min versus 32 min, respectively, Figure 3E). Taken together, these results indicate that cytoplasmic localization of cyclin C is sufficient to induce mitochondrial fission without an added stress signal. However, the fission extent was less than that observed for stressed cells (see Figure 2B). This result may suggest that the HAD domain is important for high efficiency fission to occur. Alternatively, an additional stress signal may be necessary to fully execute the fission program.
To address this issue in another way, we placed cyclin C on a high-copy plasmid that induced about 8-fold increase in protein levels (Figure S2B). Although most of cyclin C remained nuclear, this overexpression allowed release of the protein without stress (Figure S2C). Similar to cyclin CHADΔ, the presence of cyclin C in the cytoplasm induced mitochondrial fission to levels approaching those observed in stressed cells (Figure S2D). In addition, we found that precocious fission also renders cells more sensitive to Yca1p-independent ROS-induced programmed cell death (Figures S2E and S2F) suggesting a connection between these two processes. These findings indicate that cyclin C is sufficient to induce fission in the absence of stress and that the HAD plays a role in nuclear retention and perhaps regulating mitochondrial morphology.
An intact fission complex is required for normal cyclin C mitochondrial localization
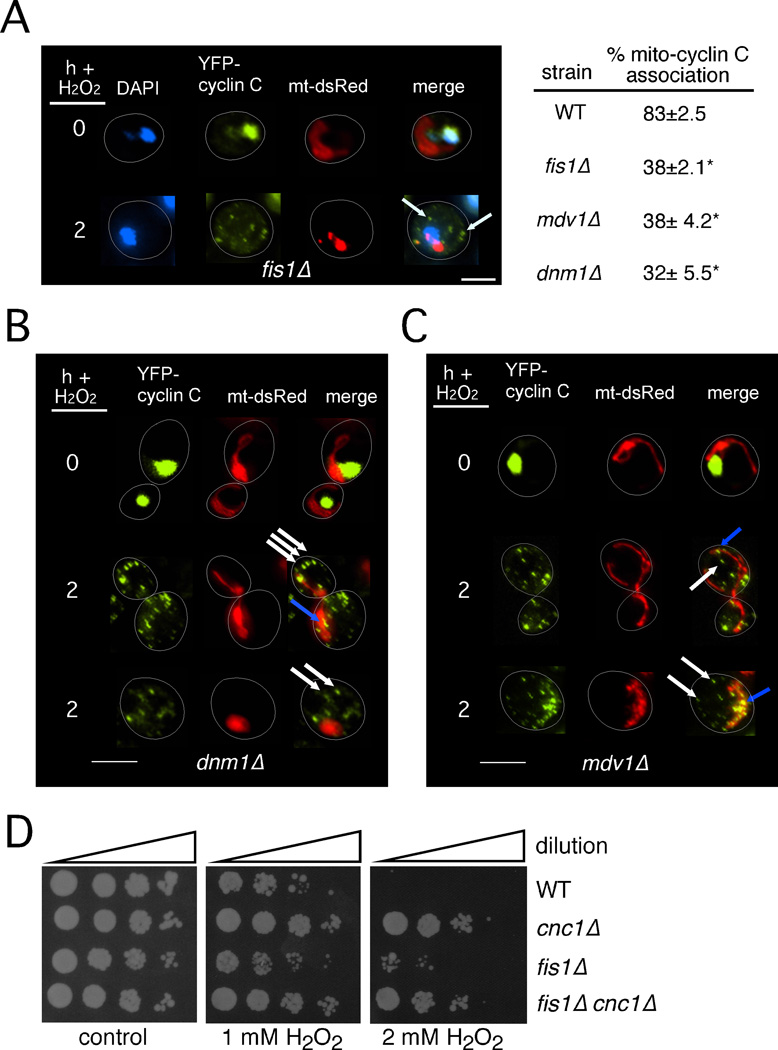
The results presented in Figure 1C indicated that the mitochondrial localization of cyclin C requires the outer membrane receptor Fis1p. To verify this conclusion, cyclin C-YFP localization was monitored in a fis1Δ mutant before and after H2O2 stress. Fluorescence microscopy revealed that cyclin C-YFP displayed normal nuclear localization in an unstressed fis1Δ mutant (Figure 4A). As expected, fis1Δ strains displayed the collapsed net mitochondrial morphology seen previously (Jakobs et al., 2003) that formed more condensed aggregates in stressed cells. Unlike wild type, the majority of the cyclin C-YFP cytoplasmic foci was independent of the mitochondria (arrows, Figure 4A) in the fis1Δ mutant. However, approximately a third of the population exhibited some association of cyclin C-YFP with the mitochondria. Evaluating the localization of cyclin C-YFP foci was difficult due to the large mitochondrial aggregates that formed in these stressed mutants perhaps leading to an overestimate of mitochondria-cyclin C-YFP co-localization. Similarly, Dnm1p and Mdv1p were required for stress-induced cyclin C mitochondrial localization (Figures 4B and 4C, see Figure 4A for quantitation). A wide field view of mitochondrial morphology in a stressed dnm1Δ strain is presented in Figure S3. These data indicate that a functional fission complex composed of Fis1p, Dnm1p and Mdv1p is required for the normal H2O2-induced mitochondrial association of cyclin C.
Figure 4.
Mitochondrial localization of cyclin C requires the fission complex. (A) Log phase fis1Δ cells harboring cyclin C-YFP and the mt-DsRed expression plasmids were treated with 1 mM H2O2 as indicated then examined by fluorescence microscopy. Arrows indicate cyclin C-YFP signals that do not associate with the mitochondria. Quantitation of the number of cyclin C-YFP foci associated with the mitochondria is given on the right (mean ±s.e.m. n=3). Asterisks indicate p<0.01 from wild type value. (B) A dnm1Δ strain harboring cyclin C-YFP and mt-DsRed constructs was grown and analyzed as described in (A). White and blue arrows indicate cyclin C-YFP foci either not associating or associating with the mitochondria, respectively. (C) The experiment described in (B) was repeated with an mdv1Δ mutant. (D) Wild type, cnc1Δ, fis1Δ and cnc1Δ fis1Δ mid-log phase cultures were treated with 1 or 2 mM H2O2 for 2 h then serially diluted (1:10) and plated onto rich growth medium. Plates were incubated three days prior to image collection. See also Figure S3.
We next investigated whether a connection existed between the roles of cyclin C in promoting PCD and mitochondrial fission. Previous studies analyzing the stress sensitivity of fis1Δ stains were complicated by the findings that the strain also carried a whi2 gene mutation (Cheng et al., 2008; Fannjiang et al., 2004). Therefore, we first confirmed the presence of the wild-type WHI2 allele by DNA sequence analysis. Following exposure to different H2O2 concentrations, the fis1Δ mutant was resistant to H2O2 compared to wild type but the phenotype was not as strong as that observed in the cnc1Δ mutant (Figure 4D). In addition, the cnc1Δ fis1Δ double mutant displayed H2O2 sensitivity similar to the cnc1Δ single mutant. These results suggest that cyclin C-dependent regulation of PCD may not be solely due to its ability to induce mitochondrial fission (see discussion).
Productive Dnm1p filament formation requires cyclin C
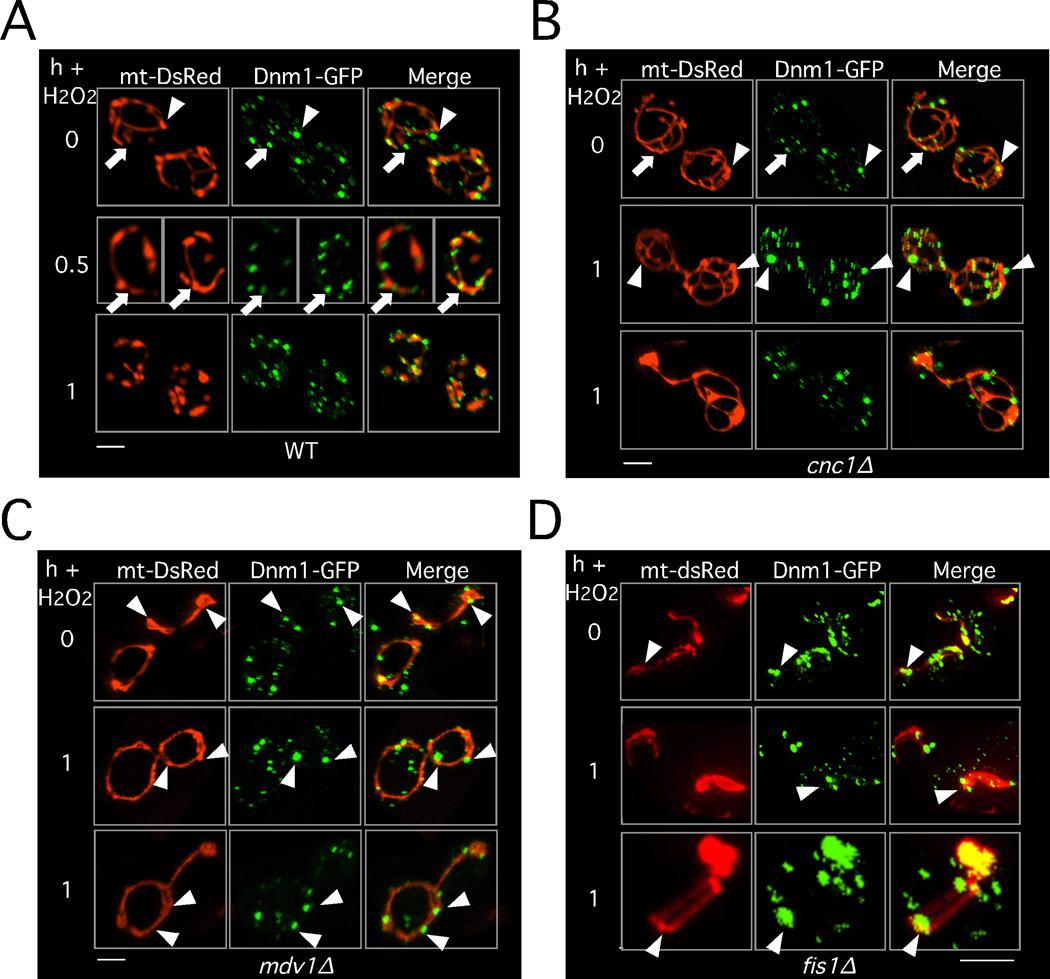
Under normal growing conditions, Dnm1p dynamically associates with the mitochondrial surface forming puncta that mark potential sites for mitochondrial division (Cerveny et al., 2007; Legesse-Miller et al., 2003; Mozdy et al., 2000; Otsuga et al., 1998; Sesaki and Jensen, 1999). Under unstressed conditions, Dnm1p foci have been characterized as either non-productive aggregates associating with the mitochondrial edge (arrowhead, Figure 5A) or productive spirals centered on the mitochondrial axis (arrow, Figure 5A) associated with mitochondrial constriction (Schauss et al., 2006). Shortly after H2O2 exposure, the number of productive Dnm1p foci increased and by one hour, the majority of the foci appeared productive. To ask whether cyclin C regulates Dnm1p-mitochondrial association and/or filament formation, this experiment was repeated in cnc1Δ cells. A similar profile of Dnm1p foci was observed in the unstressed cnc1Δ mutant and the wild-type control (Figure 5B). After a one hour H2O2 exposure, the Dnm1p-GFP foci were generally larger than those observed in the wild type (Figure S4A) but appeared associated with the mitochondria (Figure 5B) indicating that cyclin C is not required for recruiting Dnm1p to the mitochondria. Larger than normal Dnm1p-GFP foci were also observed in ethanol stress cells (Figure S4B). However, the vast majority of the Dnm1p foci were non-productive as determined by the lack of mitochondrial constriction and their association with the mitochondrial edge (arrowheads, Figure 5B). Similar Dnm1p structures were reported in unstressed mdv1Δ (Karren et al., 2005; Naylor et al., 2006) or fis1Δ (Mozdy et al., 2000; Suzuki et al., 2005) mutants. Moreover, Dnm1p focus formation in stressed mdv1Δ or fis1Δ cells (see Figures 5C and 5D) phenocopied the results observed in the cnc1Δ strain (compare one hour timepoints). As expected by the Dnm1p-GFP morphology, stressed mdv1Δ strains do not undergo fission (Figure S4C). Taken together, these results indicate that, similar to Mdv1p and Fis1p, cyclin C is required for the increased formation of productive Dnm1p spirals on the mitochondria in stressed cells.
Figure 5.
Cyclin C is required for functional Dnm1p filament formation. Dnm1p-GFP subcellular localization was visualized by confocal microscopy before and following H2O2 stress (1 mM) for the times indicated in (A) WT, (B) cnc1Δ or (C) mdv1Δ cells harboring Dnm1p-GFP and mt-DsRed plasmids. Arrows indicate functional Dnm1p-GFP foci as determined by their centered location with respect to the mitochondrial axis and the constriction of the mitochondrial diameter. Arrowheads indicate non-functional aggregates as indicated by their association with the edge of the mitochondria and the lack of mitochondrial constriction. See also Figure S4.
Cyclin C interacts with Mdv1p and is required for its normal stress-induced mitochondrial localization
The similar aberrant Dnm1p localization phenotypes in the cnc1Δ and mdv1Δ mutant strains suggested a functional connection between these two factors. Mdv1p serves as an adaptor between the Fis1p receptor and Dnm1p (Karren et al., 2005; Naylor et al., 2006; Tieu et al., 2002). Under normal growing conditions, several Mdv1p localization profiles are observed with the most predominant type containing puncta both associated (white arrows, Figure 6A) and independent (blue arrows) of the mitochondria. In response to stress, this pattern shifts to primarily mitochondrial associated at sites of fission (white arrows, right panel Figure 6A). To test whether cyclin C regulated Mdv1p localization, we utilized an Mdv1p-GFP profile scoring system established in a previous study (Koirala et al., 2010). In unstressed wild-type cells, over half of the population exhibited Mdv1p-GFP foci associated with the mitochondria (Figure 6B) with two additional categories (puncta with smooth cytoplasmic and smooth cytoplasmic) also being observed. A similar Mdv1p-GFP localization profile was observed in unstressed cnc1Δ mutants. In the wild-type cultures exposed to H2O2, the Mdv1p-GFP signal primarily reorganizes to mitochondrial puncta at presumptive scission sites. However, this pattern was largely absent in the cnc1Δ strain. Although mitochondrial puncta were observed, much of the GFP signal remained diffuse in the cytoplasm. These results indicate that cyclin C is required for normal Mdv1p mitochondrial loading and/or its stable association with this organelle.
Figure 6.
Stress-induced Mdv1p mitochondrial localization requires cyclin C. (A) A wild-type strain transformed with Mdv1-GFP and mt-DsRed expressing plasmids were analyzed by confocal microscopy before and following H2O2 treatment (1 mM for 2h). White arrows indicate sites of active fission, blue arrows identify Mdv1p foci not associated with the mitochondria. (B) Mdv1p subcellular localization patterns in WT and cnc1Δ strains before and following H2O2 stress (1 mM for 2 h) were determined by confocal microscopy. The categories for Mdv1-GFP localization patterns (Schauss et al., 2006) are given on the left. Values are mean ±s.e.m. **=p<0.01. (C) Wild-type strain expressing cyclin C-YFP, Mdv1p-DsRed and mt-CFP was subjected to H2O2 stress (1 mM for 2 h). Subcellular localization of the proteins and mitochondria was monitored by fluorescence microscopy. The percentage of cyclin C-YFP foci associating with Mdv1p-DsRed is indicated (n=3). Arrows indicate regions of co-localization between cyclin C-YFP and Mdv1p in the enlarged images. (D) and (E) A two-hybrid reporter strain (gal1-HIS3, PJ69-4A) transformed with Gal4p DNA binding domain (DBD) and activator fusion protein combinations as indicated was patched onto medium selecting for reporter gene activation (-His). The previously reported Mdv1-DBD and Fis1-AD interaction (Griffin et al., 2005) was used as a positive control. Vec = vector control. (F) Extracts prepared from unstressed and stressed wild-type strain expressing endogenous cyclin C-TAP and Mdv1p-HA were incubated with αTAP or αHA antibodies then probed for the presence of cyclin C-TAP (top panel). Control immunoprecipitation of Mdv1p-HA is shown in the bottom panel. The control extracts not expressing endogenous Mdv1p-HA (−) are indicated. (G) A wild-type strain expressing mt-CFP, cyclin CHADΔ-YFP and Mdv1p-dsRed was grown to mid-log phase then examined by fluorescence microscopy. The arrows indicate areas of co-localization between the two proteins and the mitochondria. See also Figure S5.
We next determined whether cyclin C and Mdv1p interacted using three separate approaches. First, co-localization of Mdv1p-DsRed and cyclin C-YFP in stressed cells was monitored by fluorescence microscopy. These results indicated a strong co-localization of the two proteins with 81% of the cyclin C-YFP foci co-localizing with Mdv1-Cherry (Figure 6C). Next, we examined whether the two proteins could interact in a two-hybrid assay. Previous studies found that Fis1p interacted with Mdv1p and Caf4p using this approach (Griffin et al., 2005; Tieu et al., 2002). Interestingly, cyclin C also interacted with Mdv1p-DBD in this system (Figure 6D). However, cyclin C did not interact with Fis1p in two-hybrid studies (Figure S5) suggesting that cyclin C did not associate with all fission machinery components. Our studies with cyclin CHADΔ suggested that the HAD may have a role in mediating fission. Therefore, we examined whether cyclin CHADΔ could interact with Mdv1p in the two-hybrid system. These studies found that the HAD was not required for Mdv1p bindings (Figure 6E). Therefore, if the HAD mediates stress-induced fission, it does so independent of Mdv1p binding.
Finally, co-immunoprecipitation assays were conducted with and the endogenously expressed Mdv1p-HA and the cyclin C-TAP fusion protein. Extracts were prepared from wild-type cultures expressing these proteins before and after treatment with H2O2 (1 mM). These studies revealed that cyclin C-TAP co-immunoprecipitated with Mdv1p-HA in stressed but not unstressed cells (Figure 6F). This interaction appears transient as the cyclin C-TAP signal was most robust in the one hour timepoint but reduced by two hours after H2O2 addition. This result may reflect the unstable nature of the fission complex or the overall loss of cyclin C-TAP levels due to its stress-induced destruction (left panel, Figure 6F and see Figure S5B). Finally, we asked whether cyclin C-Mdv1p interaction required a stress signal. To address this question, we took advantage of our finding that cyclin CHADΔ was released from the nucleus without stress. Therefore, the association of cyclin CHADΔ-YFP and Mdv1p-DsRed was monitored in unstressed cells. These results indicate that, similar to cyclin C, the HADΔ derivative associates with Mdv1p (arrows, Figure 6G). Taken together, these experiments indicate that cyclin C interacts with Mdv1p and that this interaction is driven by co-localization of these proteins in the same subcellular compartment.
Cyclin C is required for normal Mdv1p-Dnm1p association
Our results indicate that cyclin C is required for stress-induced hyper-fission. Therefore, e next sought a mechanism to explain this observation. Extensive mitochondrial fragmentation can be accomplished by inhibiting fusion, enhancing fission, or both. However, we do not detect down regulation of the fusion protein Fzo1p (Figure S6A) or induction of Mdv1p or Dnm1p fission genes in cnc1Δ mutants (Figures S6B). These findings are consistent with our earlier conclusion that cyclin C regulates fission through its cytoplasmic, not nuclear, activities. Alternatively, based on our imaging analysis, increased mitochondrial fragmentation in stressed cells could be due to enhanced interaction of fission machinery components in a cyclin C-dependent manner. However, co-immunoprecipitation experiments found no differences in either Mdv1p-Fis1p association (Figure S6C). Interestingly, we observed a modest upregulation of Mdv1p and Fis1p in the cnc1Δ mutants (Figure S6C, middle and bottom panels) that was due to increased transcription (Figure S6D). However, this increase in gene expression did not overcome the need for cyclin C for stress-induced fission.
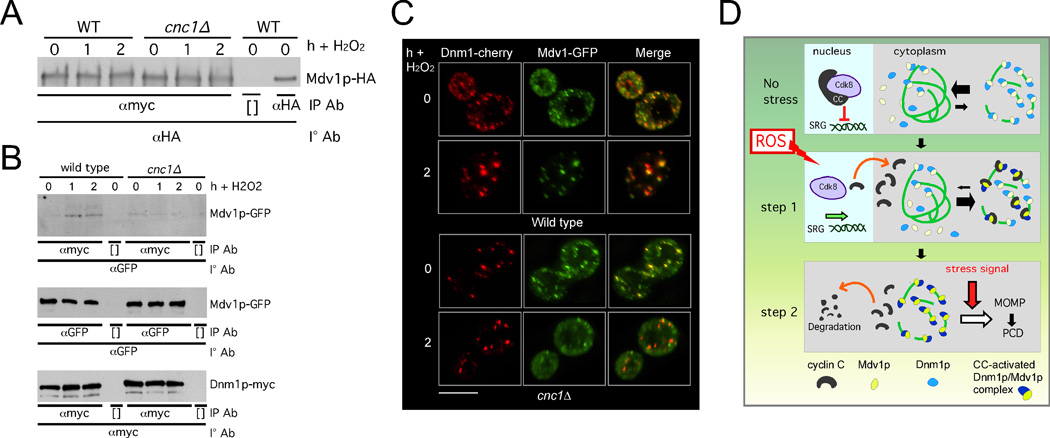
In our experiments examining Mdv1p-mitochondrial association in a cnc1Δ mutant, a predominant phenotype we observed was smooth cytoplasmic and smooth mitochondrial (see Figure 6B). A similar result was obtained with Mdv1p mutants defective for their homodimerization (Koirala et al., 2010). Therefore, we measured the ability of Mdv1p to homodimerize in stressed cnc1Δ mutants. Co-immunoprecipitation experiments were conducted with a wild type or cnc1Δ mutant expressing Mdv1p-3HA and Mdv1p-13myc before and following H2O2 stress. In the wild-type extracts, no increase in Mdv1p dimerization was observed in stressed cells (Figure 7A). Similarly, Mdv1p-Mdv1p dimerization was also unchanged in the mutant extracts. These results indicate that Mdv1p dimerization efficiency does not change in stressed cells and that this process is not regulated by cyclin C.
Figure 7.
Cyclin C is required for stress-elevated Dnm1p-Mdv1p interaction. (A) WT and cnc1Δ strains expressing MET25-Mdv1p-HA and MET25-Mdv1-myc were grown under non-inducing conditions for the MET25 promoter. Extracts prepared from samples taken before and following H2O2 addition were immunoprecipitated with αmyc then the immunoprecipitates probed for the presence of Mdv1p-HA. (B) Extracts prepared from stressed and unstressed wild type and cnc1Δ strains expressing Mdv1p-GFP and Dnm1p-myc were subjected to co-immunoprecipitation experiments as indicated (top panel). Bottom two panels control for Dnm1p-myc and Mdv1p-GFP expression levels in these extracts. [ ] indicate no antibody controls. (C) Co-localization of Mdv1p-GFP and Dnm1-cherry was examined in wild type and cnc1Δ strains before and following H2O2 treatment (1 mM) as indicated. Bar = 5µM. (D) A two-step model for cyclin C regulation of mitochondrial morphology and PCD. In unstressed cells, cyclin C (CC) and Cdk8 repress stress responsive genes. The mitochondria exhibit fused morphology in the majority of cells with Dnm1p and Mdv1p being located both in the cytoplasm and at the mitochondria. Step 1. Stress-induced translocated cyclin C associates with Mdv1p promoting Mdv1p-Dnm1p complex formation and extensive mitochondrial fragmentation. Step 2. Cyclin C disassociates from the fission complex and is destroyed by ubiquitin-mediated degradation. An additional stress signal, in combination with hyper-fission, is needed to complete the PCD pathway. See also Figure S6.
We next asked if the interaction between Mdv1p and Dnm1p was regulated by cyclin C. Co-immunoprecipitation studies from extracts prepared from wild-type cells revealed a low but detectable co-immunoprecipitation between Mdv1p and Dnm1p that increased in response to stress (Figure 7B, top panel). A similar low-level interaction between Mdv1p and Dnm1p was observed in unstressed cnc1Δ extracts. Unlike wild type, an elevated interaction between Mdv1p and Dnm1p was not seen in cnc1Δ stressed extracts. These results were not due to changes in Mdv1p-GFP or Dnm1p-myc levels in the mutant strain (middle and bottom panels, see Figure S6E). These findings indicate that cyclin C is required for enhanced interaction between Dnm1p and Mdv1p in stressed cells. To examine this question in another way, Dnm1p-Cherry and Mdv1p-GFP localization was examined by confocal microscopy before and after oxidative stress in wild type and cnc1Δ mutants. Prior to H2O2 application, the percentages of Mdv1p foci overlapping with Dnm1p were similar in both wild type and mutant strains (25%±8.5 vs. 22%±3.5, respectively, Figure 7C). Following stress, Dnm1p and Mdv1p exhibited nearly complete co-localization in uniformed sized complexes in the wild-type control. However, Mdv1p-Dnm1p interaction efficiency did not change in the stressed cnc1Δ mutant (29%±3) and was significantly reduced compared to wild type (65%±8, p =0.007). These results indicate that cyclin C does not appreciably affect Mdv1p and Dnm1p function in unstressed cells. However, in stressed cells, cyclin C is required for both Mdv1p recruitment to the mitochondria (Figure 6B) and its enhanced association with Dnm1p. These results suggest a model that cyclin C enhances Mdv1p-Dnm1p interaction, which in turn drives formation of productive Dnm1p spirals responsible for the elevated mitochondrial fission associated with stress.
Discussion
Mitochondrial morphology is controlled by the opposing activities of the fusion and fission machinery. In many organisms tested, cellular damage results in a dramatic shift in mitochondrial morphology from highly interconnected tubules to extensive fragmentation. Failure to undergo elevated fission reduces the ability of the cell to survive exogenous stress. Although the same machinery is required for normal and stress-induced fission, how the cell shifts the balance toward fission has remained enigmatic. This report provides evidence that the nuclear transcription factor cyclin C is both necessary and sufficient to induce extensive mitochondrial fragmentation. Cyclin C and its kinase Cdk8p negatively regulate a subset of stress response genes. In response to stress, this repression is relieved by cyclin C relocalization to the cytoplasm where it is destroyed. Prior to its destruction, cyclin C interacts with Mdv1p and is required for stress-enhanced Mdv1p-Dnm1p association. These results reveal a mechanism that coordinates stress gene induction with mitochondrial fission through cyclin C function.
How does cyclin C enhance mitochondrial fission in stressed cells? One possibility is that the cyclin C-Cdk8p kinase regulates the transcription of genes that control the fission/fusion balance. Alternatively, cyclin C plays a cytoplasmic role, independent of Cdk8p, to mediate mitochondrial fission. Several results presented in this paper argue against a transcriptional role for cyclin C in controlling fission. First, we presented several pieces of data indicating that loss of cyclin C does not alter mitochondrial morphology in unstressed cells. Previous studies have demonstrated that reducing or overexpressing components of the fission or fusion machinery changes mitochondrial fission under normal growth conditions (Bleazard et al., 1999; Hermann et al., 1998; Otsuga et al., 1998; Sesaki and Jensen, 1999). In addition, the HADΔ mutation causes loss of transcriptional repressor ability (Cooper and Strich, 1999). However, unlike the strains harboring the cnc1Δ allele, cyclin CHADΔ enhances, rather than prevents, fission. Finally, the presence of the A110V mutation still permits cyclin C transcriptional regulation but prevents its translocation to the cytoplasm (Cooper et al., 2012). In this study, we demonstrate that cyclin CA110V can no longer mediate stress-induced fission. These results all point to a cytoplasmic, not transcriptional, role for cyclin C in controlling mitochondrial fission.
In unstressed wild-type cells, the majority of Dnm1p is assembled into inactive aggregates located on the sides of mitochondria (Legesse-Miller et al., 2003; Schauss et al., 2006). However, in response to stress, Dnm1p foci are predominately in the “activated” state as defined by their centered location on the mitochondrial axis and the appearance of a membrane constriction at this site (see Figure 6A). At this point, it is not clear whether these “activated” foci are converted from inactive aggregates or are generated de novo although the latter seems more likely. In either case, the appearance of “active” Dnm1p foci is not observed in stressed cnc1Δ mutants indicating that the cyclin is required for this critical step in the fission process. A similar phenotype was observed in mdv1Δ mutants (this study and (Lackner et al., 2009; Naylor et al., 2006) suggesting a functional interaction between these two proteins. This possibility is supported by our findings using three independent methods that cyclin C and Mdv1p physically interact. These results suggest that cyclin C stimulates, and/or stabilizes, an elevated interaction between Mdv1p and Dnm1p that in turn promotes stress-induced mitochondrial fragmentation.
How does cyclin C increase fission efficiency in stressed cells? Based on several observations, we propose that cyclin C is most likely playing a regulatory rather than structural role in this process. First, the number of cyclin C molecules in the cell is greatly reduced compared to Dnm1p or Mdv1p. For the latter two proteins, their estimated abundance is approximately 9600 and 3700 molecules per cell based on Western blot analysis probing for identical epitope tags (Ghaemmaghami et al., 2003). However, the same study did not detect cyclin C (or Cdk8p) in their assays suggesting a far smaller concentration as is typical for many transcription factors. In addition, our co-immunoprecipitation experiments found that the cyclin C-Mdv1p interaction was detected predominately early in the stress timecourse. However, this interaction was transient being somewhat reduced by 2 h post H2O2 addition. This result was not anticipated as the interaction of cyclin C-YFP and Mdv1p-Cherry is clearly observed at this latter timepoint. Given the stringent conditions employed in our co-immunoprecipitation experiments compared to imaging live cells, these observations may reflect differences in complex stability early and late in the oxidative stress response. Interestingly, the Mdv1p-Dnm1p interaction observed in stressed cells remained elevated through the two h timepoint well within the timeframe in which mitochondrial fission is actively ongoing. Taken together, these results suggest a model that cyclin C is required early in the process to establish productive Fis1p-Mdv1p-Dnm1p complexes capable of executing a scission reaction. Once established, loss of cyclin C from this complex, and its subsequent destruction, may represent a mechanism by which the cell attenuates this accelerated mitochondrial fission process.
Programmed cell death (PCD) in yeast displays many of the signature events observed in metazoans including extensive mitochondrial fission, loss of mitochondrial integrity and release of pro-apoptotic factors (reviewed in (Carmona-Gutierrez et al., 2010; Frohlich et al., 2007). These findings indicate that basic mechanics of PCD have been conserved from yeast to humans. Although some regulators have not been found in yeast (e.g., p53), caspases (reviewed in (Wilkinson and Ramsdale, 2011) and a Bcl-2 family member (Buttner et al., 2011) have been identified suggesting that portions of regulatory pathway governing PCD have remained intact throughout evolution. In our previous studies, we found that cyclin C is a negative regulator of stress response genes (Cooper et al., 1997; Cooper et al., 2012) and positively regulates programmed cell death (PCD) in yeast (Krasley et al., 2006). Combined with our current studies, these results suggest a dual role for cyclin C relocalization in controlling the ROS response in yeast (Figure 7D). In the first step, relocalization of the yeast cyclin C to the cytoplasm relieves its repressor function thus allowing normal induction of stress response genes (Cooper et al., 2012). In the second step, cyclin C triggers mitochondrial fragmentation. However, extensive fission alone is not sufficient to induce cell death. Therefore, an additional stress signal appears required to evoke the final stages of the PCD pathway. Using cyclin C as a messenger to the mitochondria would allow the cell to coordinate its stress response between the nucleus and other organelles. This dual role may explain why cnc1Δ mutants are more resistant to H2O2 than either dnm1Δ, mdv1Δ or fis1Δ strains. Derepression of stress response genes such as catalase and Hsp70 prior to H2O2 exposure may partially protect the cell by detoxifying the reactive oxygen and allowing damaged proteins to refold correctly (see (Morano et al., 2012) for review). Another, not mutually exclusive, possibility is that, unlike fis1Δ mutants, loss of cyclin C activity does not alter mitochondrial morphology in unstressed cells. Therefore, more reticular mitochondria, unlike the aggregates observed in fis1Δ mutants, may be better equipped to deal with ROS-induced cell damage. Taken together, these findings indicate that cyclin C plays multiple roles in the stress response to thus allowing cells to coordinate actions in the nucleus and mitochondria.
Materials and Methods
Strains, and plasmids
The strains used in this study are derived from a W303a-related strain RSY10 (Strich et al., 1989) and listed in the Supplemental Materials and methods section. In accordance with the mediator nomenclature unification effort (Bourbon et al., 2004), the yeast cyclin C-Cdk8p kinase will use CNC1 (a.k.a.SSN8/SRB11/UME3) and CDK8 (a.k.a. SSN3/SRB10/UME5) gene designations, respectively. Please see Supplemental Materials and methods section for details about plasmids used in this study.
Cell growth and survival assays
Cells were grown in either rich, non-selective medium (YPDA) or synthetic minimal medium (SC) allowing plasmid selection as previously described (Cooper et al., 1997). Galactose inducible gene expression (gal1-mt-CFP and MDV1-dsRed) was achieved by adding galactose (1% final concentration) to cultures grown in SC with raffinose as the carbon source. All MET25 inducible plasmids (MDV1-MYC, MDV1-HA, FIS1-MYC) were grown under non-inducing conditions as described (Koirala et al., 2010). Viability studies were conducted with mid-log phase (6 × 106 cells/ml) treated with 1 or 2 mM H2O2 for 2 h then serially diluted (1:10) and plated on minimal complete medium with or without plasmid selection as indicated in the text. TUNEL assays were conducted essentially as previously described (Krasley et al., 2006; Madeo et al., 1997). At least 400 cells were counted per timepoint from three independent cultures. DHE oxidation assays were performed as described (Buttner et al., 2007) and DHE positive cells were quantitated by direct cell count using fluorescence microscopy. All statistical analysis was performed using the student's T test with p <0.05 considered significant. All analyses were conducted with at least three independent cultures with 200 or more cells counted per timepoint.
Subcellular fractionation
Subcellular fractionation of yeast mitochondria were accomplished essentially as described previously (Diekert et al., 2001) with the following modifications. The enriched mitochondrial fraction was purified from a mid-log phase culture (4 L per timepoint) before and one hour after treatment with H2O2 (1 mM). Due to the low abundance of cyclin C-TAP, approximately one third of the enriched mitochondrial preparation was loaded per sample. Whole cell extract samples represent 1/100 of mitochondrial preparation. Proteinase protection assays of mitochondrial bound cyclin C were conducted by adding 100 µg/ml of recombinant Proteinase K (Roche) for 15 min on ice. The control sample was incubated under the same conditions without added protease.
Immunofluorescence Microscopy
Localization studies of chimeric fusion proteins were performed on fixed or living cells as indicated in the text. Cells were fixed in 3.7% para-formaldehyde and stained with 4’, 6-diamidino-2-phenylindole (DAPI). For all experiments, the cells were grown to mid-log (5 × 106 cells/ml), treated with 1 mM H2O2 for the timepoints indicated, then analyzed by fluorescence microscopy as described in the Supplemental Materials and methods. The images (0.2 µM slices at 0.2 µM spacing) were analyzed as described above. In all panels, the bar = 5 µM unless otherwise stated.
Fluorescence microscopy scoring methods
To measure co-localization signals (Figures 1E, 4A, 4B, 4C, 6C, 7B, 7C), total foci exhibiting co-localization (with the mitochondria or another protein signal) were divided by the total foci observed. At least 30 cells were counted from three independent samples. Mitochondrial fission was scored positive if no reticular mitochondria were observed that transversed half the cell diameter. Fusion was scored when cells exhibited one or more reticular mitochondria the diameter of the cell. Fission and fusion was scored for 200 cells from three independent isolates. The intermediate or mixed mitochondria phenotype (Figure 3C) described cells containing both ≥3 mitochondrial fragments in addition to an elongated mitochondrion equal to the diameter of the cell. The cyclin C-YFP-nucleolus association (Figure 2D) was scored positive when one condensed YFP signal was observed within or adjacent to the nucleolar signal. 50 cells were counted from three independent isolates. Statistical analysis was performed using the Student’s T-test with p<0.05 used to indicate significant differences.
Molecular biology methods
Western blot and co-immunoprecipitation analyses from yeast extracts were performed essentially as described (Cooper et al., 1997) with the modifications indicated in the Supplemental Materials and methods section. Western blot signals were detected using secondary antibodies conjugated to alkaline phosphatase (Sigma) and the CDP-Star chemiluminescence kit (Tropix). Quantitation of Western blot signals was accomplished using the chemiluminescence imager (Kodak Inc.).
Supplementary Material
Highlights.
Cyclin C exhibits stress-induced nuclear to mitochondrial relocalization.
Cyclin C is required for stress-enhanced Mdv1p-Dnm1p association.
Cyclin C is required for stress-induced mitochondrial fragmentation.
Acknowledgements
We thank N. L. van Berkum, D. Chan, E. Craig, S. Fields, E. Golemis, M. Escobar-Henriques, M. Henry, E. Hettema, M. Lisby, R. Jensen, J. Nunnari and J. Shaw for strains and plasmids. We thank The Yeast Resource Center (NIH grant #P41 RR11823 awarded to T. N. Davis) for the two-hybrid plasmids. We thank M. Scarnati for help with the dnmΔ strain construction and cyclin C over-expression viability assays. We thank M. Henry for the Mam33p antibody. We thank B. Cain (Nikon Inc.) for technical support with image analysis. We thank members of the Cooper and Strich laboratories for critical reading of this manuscript. This work was supported by grants to R.S. from the National Institutes of Health (CARO1099003, GMRO1086788) and the WW Smith Charitable Trust (#CO604) to K.F.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bhar D, Karren MA, Babst M, Shaw JM. Dimeric Dnm1-G385D interacts with Mdv1 on mitochondria and can be stimulated to assemble into fission complexes containing Mdv1 and Fis1. J Biol Chem. 2006;281:17312–17320. doi: 10.1074/jbc.M513530200. [DOI] [PubMed] [Google Scholar]
- Bleazard W, McCaffery JM, King EJ, Bale S, Mozdy A, Tieu Q, Nunnari J, Shaw JM. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat Cell Biol. 1999;1:298–304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossie MA, DeHoratius C, Barcelo G, Silver P. A mutant nuclear protein with similarity to RNA binding proteins interferes with nuclear import in yeast. Mol Biol Cell. 1992;3:875–893. doi: 10.1091/mbc.3.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbon HM. Comparative genomics supports a deep evolutionary origin for the large, four-module transcriptional mediator complex. Nuc Acid Res. 2008;36:3993–4008. doi: 10.1093/nar/gkn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbon HM, Aguilera A, Ansari AZ, Asturias FJ, Berk AJ, Bjorklund S, Blackwell TK, Borggrefe T, Carey M, Carlson M, et al. A Unified Nomenclature for Protein Subunits of Mediator Complexes Linking Transcriptional Regulators to RNA Polymerase II. Mol Cell. 2004;14:553–557. doi: 10.1016/j.molcel.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner S, Eisenberg T, Carmona-Gutierrez D, Ruli D, Knauer H, Ruckenstuhl C, Sigrist C, Wissing S, Kollroser M, Frohlich KU, et al. Endonuclease G regulates budding yeast life and death. Mol Cell. 2007;25:233–246. doi: 10.1016/j.molcel.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Buttner S, Ruli D, Vogtle FN, Galluzzi L, Moitzi B, Eisenberg T, Kepp O, Habernig L, Carmona-Gutierrez D, Rockenfeller P, et al. A yeast BH3-only protein mediates the mitochondrial pathway of apoptosis. Embo J. 2011;30:2779–2792. doi: 10.1038/emboj.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Gutierrez D, Eisenberg T, Buttner S, Meisinger C, Kroemer G, Madeo F. Apoptosis in yeast: triggers, pathways, subroutines. Cell Death Differ. 2010;17:763–773. doi: 10.1038/cdd.2009.219. [DOI] [PubMed] [Google Scholar]
- Cerveny KL, Studer SL, Jensen RE, Sesaki H. Yeast mitochondrial division and distribution require the cortical num1 protein. Dev Cell. 2007;12:363–375. doi: 10.1016/j.devcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Cheng WC, Teng X, Park HK, Tucker CM, Dunham MJ, Hardwick JM. Fis1 deficiency selects for compensatory mutations responsible for cell death and growth control defects. Cell Death Differ. 2008;15:1838–1846. doi: 10.1038/cdd.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Huddleston MJ, Zhang X, Young RA, Annan RS, Carr SA, Deshaies RJ. Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Genes Dev. 2001;15:1078–1092. doi: 10.1101/gad.867501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KF, Mallory MJ, Smith JB, Strich R. Stress and developmental regulation of the yeast C-type cyclin Ume3p (Srb11p/Ssn8p) EMBO J. 1997;16:4665–4675. doi: 10.1093/emboj/16.15.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KF, Mallory MJ, Strich R. Oxidative stress-induced destruction of the yeast C-type cyclin Ume3p requires phosphatidylinositol-specific phospholipase C and the 26S proteasome. Mol Cell Biol. 1999;19:3338–3348. doi: 10.1128/mcb.19.5.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KF, Scarnati MS, Krasley E, Mallory MJ, Jin C, Law MJ, Strich R. Oxidative-stress-induced nuclear to cytoplasmic relocalization is required for Not4-dependent cyclin C destruction. J Cell Sci. 2012;125:1015–1026. doi: 10.1242/jcs.096479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KF, Strich R. Functional analysis of the Ume3p/ Srb11p-RNA polymerase II holoenzyme interaction. Gene Expr. 1999;8:43–57. [PMC free article] [PubMed] [Google Scholar]
- Cooper KF, Strich R. Saccharomyces cerevisiae C-type cyclin Ume3p/Srb11p is required for efficient induction and execution of meiotic development. Euk cell. 2002;1:66–74. doi: 10.1128/EC.01.1.66-74.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekert K, de Kroon AI, Kispal G, Lill R. Isolation and subfractionation of mitochondria from the yeast Saccharomyces cerevisiae. Methods Cell Biol. 2001;65:37–51. doi: 10.1016/s0091-679x(01)65003-9. [DOI] [PubMed] [Google Scholar]
- Fannjiang Y, Cheng WC, Lee SJ, Qi B, Pevsner J, McCaffery JM, Hill RB, Basanez G, Hardwick JM. Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev. 2004;18:2785–2797. doi: 10.1101/gad.1247904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Frohlich KU, Fussi H, Ruckenstuhl C. Yeast apoptosis--from genes to pathways. Semin Cancer Biol. 2007;17:112–121. doi: 10.1016/j.semcancer.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O'Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- Griffin EE, Graumann J, Chan DC. The WD40 protein Caf4p is a component of the mitochondrial fission machinery and recruits Dnm1p to mitochondria. J Cell Biol. 2005;170:237–248. doi: 10.1083/jcb.200503148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez R, Blobel G, Aris JP. Isolation and sequencing of NOP1. A yeast gene encoding a nucleolar protein homologous to a human autoimmune antigen. J Biol Chem. 1990;265:2209–2215. [PubMed] [Google Scholar]
- Hermann GJ, Thatcher JW, Mills JP, Hales KG, Fuller MT, Nunnari J, Shaw JM. Mitochondrial fusion in yeast requires the transmembrane GTPase Fzo1p. J Cell Biol. 1998;143:359–373. doi: 10.1083/jcb.143.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst M, Kobor MS, Kuriakose N, Greenblatt J, Sadowski I. GAL4 is regulated by the RNA polymerase II holoenzyme-associated cyclin-dependent protein kinase SRB10/CDK8. Mol Cell. 1999;3:673–678. doi: 10.1016/s1097-2765(00)80360-3. [DOI] [PubMed] [Google Scholar]
- Igaki T, Kanuka H, Inohara N, Sawamoto K, Nunez G, Okano H, Miura M. Drob-1, a Drosophila member of the Bcl-2/CED-9 family that promotes cell death. Proc Natl Acad Sci USA. 2000;97:662–667. doi: 10.1073/pnas.97.2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- Jakobs S, Martini N, Schauss AC, Egner A, Westermann B, Hell SW. Spatial and temporal dynamics of budding yeast mitochondria lacking the division component Fis1p. J Cell Sci. 2003;116:2005–2014. doi: 10.1242/jcs.00423. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karren MA, Coonrod EM, Anderson TK, Shaw JM. The role of Fis1p-Mdv1p interactions in mitochondrial fission complex assembly. J Cell Biol. 2005;171:291–301. doi: 10.1083/jcb.200506158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koirala S, Bui HT, Schubert HL, Eckert DM, Hill CP, Kay MS, Shaw JM. Molecular architecture of a dynamin adaptor: implications for assembly of mitochondrial fission complexes. T J Cell Biol. 2010;191:1127–1139. doi: 10.1083/jcb.201005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasley E, Cooper KF, Mallory MJ, Dunbrack R, Strich R. Regulation of the oxidative stress response through Slt2p-dependent destruction of cyclin C in Saccharomyces cerevisiae. Genetics. 2006;172:1477–1486. doi: 10.1534/genetics.105.052266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner LL, Horner JS, Nunnari J. Mechanistic analysis of a dynamin effector. Science. 2009;325:874–877. doi: 10.1126/science.1176921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legesse-Miller A, Massol RH, Kirchhausen T. Constriction and Dnm1p recruitment are distinct processes in mitochondrial fission. Mol Biol Cell. 2003;14:1953–1963. doi: 10.1091/mbc.E02-10-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S-M, Zhang J, Jeffery DA, Koleske AJ, Thompson CM, Chao DM, Viljoen M, van Vuuren HJJ, Young RA. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature. 1995;374:193–196. doi: 10.1038/374193a0. [DOI] [PubMed] [Google Scholar]
- Madeo F, Frohlich E, Frohlich KU. A yeast mutant showing diagnostic markers of early and late apoptosis. J Cell Biol. 1997;139:729–734. doi: 10.1083/jcb.139.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears JA, Lackner LL, Fang S, Ingerman E, Nunnari J, Hinshaw JE. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nat Struct Mol Biol. 2011;18:20–26. doi: 10.1038/nsmb.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen S, DeVay R, Block J, Cassidy-Stone A, Wayson S, McCaffery JM, Nunnari J. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006;127:383–395. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Morano KA, Grant CM, Moye-Rowley WS. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics. 2012;190:1157–1195. doi: 10.1534/genetics.111.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozdy AD, McCaffery JM, Shaw JM. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol. 2000;151:367–380. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor K, Ingerman E, Okreglak V, Marino M, Hinshaw JE, Nunnari J. Mdv1 interacts with assembled dnm1 to promote mitochondrial division. J Biol Chem. 2006;281:2177–2183. doi: 10.1074/jbc.M507943200. [DOI] [PubMed] [Google Scholar]
- Nelson C, Goto S, Lund K, Hung W, Sadowski I. Srb10/Cdk8 regulates yeast filamentous growth by phosphorylating the transcription factor Ste12. Nature. 2003;421:187–190. doi: 10.1038/nature01243. [DOI] [PubMed] [Google Scholar]
- Otsuga D, Keegan BR, Brisch E, Thatcher JW, Hermann GJ, Bleazard W, Shaw JM. The dynamin-related GTPase, Dnm1p, controls mitochondrial morphology in yeast. J Cell Biol. 1998;143:333–349. doi: 10.1083/jcb.143.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport D, Brunner M, Neupert W, Westermann B. Fzo1p is a mitochondrial outer membrane protein essential for the biogenesis of functional mitochondria in Saccharomyces cerevisiae. J Biol Chem. 1998;273:20150–20155. doi: 10.1074/jbc.273.32.20150. [DOI] [PubMed] [Google Scholar]
- Schauss AC, Bewersdorf J, Jakobs S. Fis1p and Caf4p, but not Mdv1p, determine the polar localization of Dnm1p clusters on the mitochondrial surface. J Cell Sci. 2006;119:3098–3106. doi: 10.1242/jcs.03026. [DOI] [PubMed] [Google Scholar]
- Sesaki H, Jensen RE. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J Cell Biol. 1999;147:699–706. doi: 10.1083/jcb.147.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seytter T, Lottspeich F, Neupert W, Schwarz E. Mam33p, an oligomeric, acidic protein in the mitochondrial matrix of Saccharomyces cerevisiae is related to the human complement receptor gC1q-R. Yeast. 1998;14:303–310. doi: 10.1002/(SICI)1097-0061(19980315)14:4<303::AID-YEA217>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Strich R, Slater MR, Esposito RE. Identification of negative regulatory genes that govern the expression of early meiotic genes in yeast. Proc Natl Acad Sci USA. 1989;86:10018–10022. doi: 10.1073/pnas.86.24.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Neutzner A, Tjandra N, Youle RJ. Novel structure of the N terminus in yeast Fis1 correlates with a specialized function in mitochondrial fission. J Biol Chem. 2005;280:21444–21452. doi: 10.1074/jbc.M414092200. [DOI] [PubMed] [Google Scholar]
- Tieu Q, Nunnari J. Mdv1p is a WD repeat protein that interacts with the dynamin-related GTPase, Dnm1p, to trigger mitochondrial division. J Cell Biol. 2000;151:353–366. doi: 10.1083/jcb.151.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu Q, Okreglak V, Naylor K, Nunnari J. The WD repeat protein, Mdv1p, functions as a molecular adaptor by interacting with Dnm1p and Fis1p during mitochondrial fission. J Cell Biol. 2002;158:445–452. doi: 10.1083/jcb.200205031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Peppel J, Kettelarij N, van Bakel H, Kockelkorn TT, van Leenen D, Holstege FC. Mediator expression profiling epistasis reveals a signal transduction pathway with antagonistic submodules and highly specific downstream targets. Mol Cell. 2005;19:511–522. doi: 10.1016/j.molcel.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Vieira HL, Boya P, Cohen I, El Hamel C, Haouzi D, Druillenec S, Belzacq AS, Brenner C, Roques B, Kroemer G. Cell permeable BH3-peptides overcome the cytoprotective effect of Bcl-2 and Bcl-X(L) Oncogene. 2002;21:1963–1977. doi: 10.1038/sj.onc.1205270. [DOI] [PubMed] [Google Scholar]
- Vincent O, Kuchin S, Hong SP, Townley R, Vyas VK, Carlson M. Interaction of the Srb10 Kinase with Sip4, a Transcriptional Activator of Gluconeogenic Genes in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:5790–5796. doi: 10.1128/MCB.21.17.5790-5796.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Ramsdale M. Proteases and caspase-like activity in the yeast Saccharomyces cerevisiae. Biochem Soc tTans. 2011;39:1502–1508. doi: 10.1042/BST0391502. [DOI] [PubMed] [Google Scholar]
- Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.