Abstract
Objectives
Recent investigations have associated airborne Particulate Matter (PM) with increased coagulation and thrombosis, but underlying biological mechanisms are still incompletely characterized. DNA methylation is an environmentally-sensitive mechanism of gene regulation that could potentially contribute to PM-induced hypercoagulability. We aimed to test whether altered methylation mediates environmental effects on coagulation.
Methods
We investigated 63 steel workers exposed to a wide range of PM levels, as a work-related condition with well-characterized prothrombotic exposure. We measured personal PM10 (PM≤10 μm in aerodynamic diameter), PM1 (≤1 μm), and air metal components. We determined leukocyte DNA methylation of NOS3 (nitric-oxide-synthase-3) and EDN1 (endothelin-1) through bisulfite-pyrosequencing and we measured Endogenous Thrombin Potential (ETP), as a global coagulation-activation test after standardized triggers.
Results
ETP increased in association with PM10 (β=20.0, 95%CI: 3.0, 37.0), PM1 (β=80.8 95%CI: 14.9, 146.7), and zinc (β=51.3, 95%CI: 0.01, 111.1) exposures. NOS3 methylation was negatively associated with PM10 (β=−0.2, 95%CI: −0.4, −0.03), PM1 (β=−0.8, 95%CI: −1.4, −0.1), zinc (β=−0.9, 95%CI: −1.4, −0.3) and iron (β=−0.7, 95%CI: −1.4, −0.01) exposures. Zinc exposure was negatively associated with EDN1 (β=−0.3, 95%CI: −0.8, −0.1) methylation. Lower NOS3 (β=−42.3; p<0.001) and EDN1 (β=−14.5; p=0.05) were associated with higher ETP. Statistical mediation analysis formally confirmed NOS3 and EDN1 hypomethylation as intermediate mechanisms for PM-related coagulation effects.
Conclusions
Our study showed for the first time, that gene hypomethylation contributes to environmentally-induced hypercoagulability.
Keywords: Air pollution, DNA methylation, coagulation
INTRODUCTION
DNA methylation is an epigenetic mark regulating the expression of human genes that is being increasingly proposed as a potential molecular mechanism underlying cardiovascular risks1. DNA methylation usually marks genes that are silenced, whereas hypomethylated genes are more accessible to transcription factors and therefore active or poised to be activated2. It represents an attractive potential disease mechanism because it can be modified by environmental risk factors2 and, might help to explain how the environment imposes aberrant gene expression reprogramming that can result in increased disease risk1. Several investigations have shown that environmental exposures are associated with changes in DNA methylation that can be detected in blood DNA samples2. Air pollution is a pervasive environmental threat that has been estimated to be responsible for ~800,000 deaths every year worldwide, mostly due to cardiovascular disease3. Ambient particulate matter (PM) has been associated with increased hospitalization and mortality due to cardiovascular disease in the general population3. The particulate component of PM has been associated with increased coagulation and risk of venous thrombosis.1,4 Potential causes for PM-related hypercoagulability include a direct effect of soluble PM components (such as metals) or ultrafine particles reaching the bloodstream, or a possible indirect effect following PM-induced pulmonary inflammation via release of inflammatory mediators, increased microvascular activation, and thrombosis5. DNA methylation has been shown to regulate biological processes underlying cardiovascular disease, such as inflammation and immune responses.1 In a previous study, we found an association between the expression of the inflammatory marker V-CAM and blood DNA hypomethylation6. PM or PM components, such as toxic metals, that enter into the bloodstream can react with DNA and induce hypomethylation.7 Human investigations have shown association of PM exposure with decreased DNA methylation, in peripheral blood of exposed individuals8 However, no study has yet linked changes in DNA methylation resulting from environmental exposures with biological endpoints participating in the pathways leading to altered coagulation. In the present work, we investigated blood DNA methylation of Nitric Oxide Synthase 3 (NOS3) and Endothelin-1 (EDN1) in a group of steel workers exposed to different levels of metal-rich air particles, as potential intermediate along the pathways contributing to the systemic pro-coagulant effects of PM. NOS3 and EDN1 have proinflammatory activity in human circulating leukocytes9,10NOS3 generates both nitric oxide (NO) and superoxide and it has been shown to determine oxidative stress through preferential generation of superoxide in the presence of pathological conditions, such as atherosclerosis or hyperlipidaemia11. Oxidative stress has been extensively implicated in PM-induced inflammatory responses,12 and may enhance blood coagulation and thrombosis.13 Whereas widely regarded as a vasoconstrictor, EDN1 has been shown to be a primary contributor in blood coagulation activation.14EDN1 may exert procoagulant effects by increasing vWF-Ag levels, and induce systemic activation of coagulation in animals.14 Transcription of both genes has been shown to be controlled by promoter DNA methylation.15,16
Foundry work is a specific condition of exposure, particularly well-suited for investigations of PM effects. In spite of state-of-art measures for exposure reduction, in modern foundry facilities some of the workers are still exposed to levels of airborne PM well above those found outdoors7. Because of the different proximity to emission sources, foundry workers are exposed to a wide range of PM levels, from low to very high7. As work routines have usually little variation over time, the differences in PM exposure levels tend to remain quite stable across participants, thus allowing investigating individuals with wide and stable contrasts of exposures without the need for an unexposed control population4. Airborne PM in foundry facilities is rich in metals4, which have been indicated as remarkably toxic components of ambient PM17. Metals contained in PM have been linked to cardiovascular diseases both in animal17 and human studies18. It has been shown that treatment with the water-soluble fraction of PM significantly accelerated the whole blood coagulation time in vitro19. Zinc was found to be the metal with the greatest procoagulant effect19. Zinc, a common constituent of ambient PM and a workplace toxin, can lead to functional changes in the lung and subsequent systemic inflammation and cardiovascular alterations in humans20. Animal rat models have demonstrated systemic inflammation and procoagulant effects following pulmonary zinc exposure21.
Endogenous Thrombin Potential (ETP) is a global functional assay that describes overall coagulability and represents the time course of formation and decay of thrombin, a key enzyme in clot formation in response to triggering by low levels of tissue factor and/or calcium chloride22. ETP decreases in patient with anticoagulant treatment23, increases in patients with thrombophilia24, and has been correlated with higher risk of venous thromboembolism25. A recent study, has also demonstrated that ETP, measured in the presence of thrombomodulin, increased in association to PM exposure.26
The aim of our study was to investigate the effects of PM and its metal components on blood DNA methylation of NOS3 and EDN1 and to evaluate the associations of NOS3 and EDN1 methylation with ETP, taken as a global coagulation test, in order to characterize possible links among PM and metal exposure, DNA methylation, and prothrombotic states. We used mediation analysis to formally characterize for the role of DNA methylation as an intermediate process linking PM and metal exposures to increased coagulation function.
MATERIAL AND METHODS
Study Participants
We recruited 63 male healthy workers (27–55 years; mean=44 years,), free of cardiovascular disease, employed in a steel production plant in Brescia, Northern Italy. Participants were recruited in different work areas to ensure a wide range of exposure levels and had all been working in the current job position for at least one year. Twenty-five participants (40%) were current smokers, who reported a mean number of 13.0 (SD=7.2) cigarettes smoked every day. The average body mass index (BMI) of the study participants was 26.5 Kg/m2 (SD=2.7). A self-administered questionnaire was used to collect detailed information on lifestyle, drugs intake and recent medical conditions. Factory's clinical files were used to abstract information on occupational and past medical history. To enhance statistical power and avoid potential masking of effects due to day-to-day variability, we evaluated both DNA methylation and ETP on blood samples collected from the study participants on two different days of the same workweek: respectively in the morning of the first day of the workweek (sample 1) and at the same hour on the fourth day of work (sample 2). Individual written informed consent and approval from the local Institutional Review Board were obtained before the study.
Exposure assessment
Particle mass (PM10 and PM1) and individual particle metal components (aluminum, manganese, nickel, zinc, arsenic, lead, iron and chromium) were measured as previously described26.
DNA methylation analysis
Seven ml of blood were drawn and DNA extraction, bisulfite treatment, and PCR-pyrosequencing were performed as previously described27. We developed the assays for NOS3 and EDN1 methylation by locating their promoters using Genomatix Software (Genomatix Software Inc, Ann Arbor, MI) and amplified the sequences as shown in table S1. (Primer concentrations and sequences, and PCR cycling conditions are shown in Tables S2–S3). We developed pyrosequencing assays by selecting amplicons in promoter CpG-rich areas. We designed the assays to cover the greatest number of CpG sites possible within the promoter region, taking into account the limitations of the method, (PCR amplicon <= 350 bp, primers that avoided CpGs, target sequence <=40bp). Compared to other common methods of DNA methylation analysis, pyrosequencing-based assays have the advantage to produce individual measures of methylation at more than one CpG dinucleotide, thus reflecting more accurately DNA methylation in the region. In each assay we measured %5mC at different CpG dinucleotides within a CpG island located in the gene promoter (Table S1). Each sample was tested twice for each assay to increase reliability. In statistical analyses, we used the average of the two analytical replicates for each sample. Even the single replicates, however, showed limited measurement error, as indicated by the high correlation coefficients between the two measures obtained on the same sample. The correlation coefficients between the two replicates were for EDN1, 0.99 (95% CI: 0.99–1.00) in sample 1 and 0.99 (95% CI: 0.98–0.99) in sample 2, and for NOS3, 0.90 (95% CI: 0.85–0.95) in sample 1 and 0.84 (95% CI: 0.77–0.92) in sample 2. To increase the reproducibility all pyrosequencing assays were run in duplicate.
Endogenous thrombin potential (ETP)
Blood for ETP testing was drawn into vacuum tubes (Becton Dickinson, Meylan, France) containing 0.109 M trisodium citrate at a ratio of 9:1 (blood/anticoagulant) and centrifuged for 15 minutes at 2,880g at room (controlled) temperature and plasma was aliquoted in plastic tubes, quickly frozen in liquid nitrogen and stored at −80°C until testing.
As previously described in detail, ETP – representing the plasma balance between the action of pro-coagulants and anti-coagulants – was measured through a thrombin generation assay, and in the presence of thrombomodulin to mimic much more closely the conditions operating in vivo 26
Statistical analysis
Effect of the exposures on DNA methylation
For each subject, we measured DNA methylation in two blood samples (sample 1 and 2) collected on two days of the same workweek. In each blood sample, the pyrosequencing-based DNA methylation analysis produced three values for NOS3 (methylation at three CpG dinucleotide positions) and four for EDN1 (methylation at four CpG dinucleotide positions). Thus, we assumed DNA methylation data has a nonhierarchical structure where participants are cross-classified by blood samples and CpG dinucleotide sites, with each subject potentially belonging to any combination of levels of different factors.
We use linear mixed-effect model, to take into account each CpG dinucleotide position measured in the two samples7,27.
This modeling approach allows for obtaining a robust estimate of the average global effect on DNA methylation in the gene, while fully representing the individual CpG resolution of the data. To identify the associations of the exposures with DNA methylation, we evaluated the level of each exposure in relation to the measures of DNA methylation regardless of whether they were measured on samples 1 or sample 2, i.e., we assumed that exposure produced similar effects at the two time points. We estimated the effects of PM10, PM1, or PM metal components on DNA methylation using the following model:
| (1) |
where β0 is the overall intercept; β1 is the regression coefficient representing the exposure effect; β2 is the regression coefficient for the difference between Samples 1 and 2; β3 is the regression coefficient for the difference between CpG dinucleotides position; i=1,2,…,63 represents the subject; j=1,2…n (where n=3 for NOS3 and n=4 for EDN1) represents the CpG dinucleotides position; k=1,2 represents the sample, ξijk is the random effect for the interaction between position and sample for each subject and εijk is the residual error term. Multivariate models included as potential confounders: age, BMI, current smoking status (yes/no), NSAIDs and percent monocytes in the differential blood count. The percent monocytes variable was fitted to account for possible variation in the proportion of leukocytes subtypes associated with the exposures. Models adjusted for granulocyte or lymphocyte proportions, instead of monocytes, produced similar results (data not shown). An unstructured covariance structure was used to model the within-subject errors. The Kenword–Roger approximation was used to estimate denominator degrees of freedom. Significance tests were based on least squares (LS) means and Type III sums of squares.
Effect of exposures on ETP
The effect of PM mass and PM metal component on ETP was estimated with the following model:
| (2) |
where β0 is the overall intercept; β1 is the regression coefficient representing the exposure effect; i=1,2,…,63 represents the subject; j=1,2 represents the sample; ξij is the random effect for sample and each subject and εij is the residual error term. In these models, we included as independent variables the same covariates as in model (1).
Effect of DNA methylation differences on ETP
We evaluated the association of NOS3 and EDN1 DNA methylation with ETP, using linear mixed-effect models:
| (3) |
where β0 is the overall intercept; β1 is the regression coefficient representing the DNA methylation effect; i=1,2,…,63 represents the subject; j=1,2 represents the sample; ξij is the random effect for the sample and subject, and εij is the residual error term. Likelihood ratio tests were used to test for the significance of β1. The same covariates used in model (1) were included as independent variables.
General procedures used in mixed-regression models
For each of the models described above, we checked regression assumptions by performing diagnostic tests, including the Shapiro-Wilk test to verify normality of residuals and the White test to verify homogeneity of variance of the residuals. Cook's distance was use to estimate the influence of particular data points to avoid that large or high residuals could distort the accuracy of the linear regression models. For all models, we conducted an exploratory set of analyses modelling each individual CpG position separately. The results from these set of models were less robust than those from the global modelling approach described above, as indicated by larger confidence intervals for the analyses on individual CpG site. In general, the associations with individual CpG positions were similar in direction and size within each amplicon. Only the results for the global tests reflecting the average association with methylation in the amplicon analysed are reported throughout the paper. The β regression coefficients were scaled to represent the increase in the dependent variable estimated for an increment equal to the difference between the 75th and 25th percentile in the independent variable. A p-value <0.05 was considered statistically significant.
Mediation analysis
Finally, we performed mediation analysis28 to explore the role of DNA methylation as a mediator of the association between exposures and ETP. This approach decomposes the total observed effect of exposure on ETP into a direct effect of exposure and an indirect effect of exposure that acts via the mediator of interest (i.e., DNA methylation). Mediation analysis requires a significant relation of the outcome to the exposure, a significant relation of the outcome to the mediator and a significant relation of the mediator to the exposure; as potential mediators, we therefore analyzed only the methylation markers that satisfied all these assumptions. We assessed the indirect effect (IE) of DNA methylation with the classic casual step approach29; we fitted the linear mixed model of ETP on DNA methylation and exposures and the model of DNA methylation on exposures, using the estimates of these model to assess the mediation effect of the exposures through DNA methylation (indirect effect)28. All statistical analyses were performed in SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Personal exposure levels
Estimated personal levels of exposure to PM and metals are shown in Table 1. For instance, PM10 ranged between 73.7 and 1220.2 (mean=233.4; SD=214.6), PM1 between 1.7 and 30.5, (mean=8.5; SD=6.2), and zinc between 0.3 and 129.1, (mean=18.9; SD=26.4).
Table 1.
Estimated personal exposure levels to PM and metalsa (n=63)
| Pollutants (μg/m3) | Mean (SD) | Min | 25th percentile | Median | 75th percentile | 90th percentile | Max | |
|---|---|---|---|---|---|---|---|---|
| PM10 | 233.4 | (214.6) | 73.7 | 152.2 | 179.4 | 222.9 | 383.5 | 1220.2 |
| PM1 | 8.5 | (6.2) | 1.7 | 3.5 | 9.0 | 11.4 | 13.3 | 30.5 |
| Aluminium | 8.5 | (18.1) | 0.4 | 1.5 | 2.0 | 7.4 | 13.9 | 84.1 |
| Manganese | 11.3 | (30.4) | 0.1 | 1.2 | 4.6 | 10.8 | 17.0 | 174.8 |
| Nickel | 0.3 | (0.2) | 0.0 | 0.2 | 0.3 | 0.5 | 0.5 | 0.7 |
| Zinc | 18.9 | (26.4) | 0.3 | 1.5 | 8.5 | 32.3 | 36.4 | 129.1 |
| Arsenic | 0.1 | (0.1) | 0.0 | 0.0 | 0.1 | 0.2 | 0.3 | 0.3 |
| Lead | 7.5 | (17.5) | 0.1 | 0.6 | 2.9 | 9.5 | 10.8 | 99.9 |
| Iron | 32.0 | (22.1) | 1.0 | 18.0 | 25.6 | 48.7 | 59.8 | 88.4 |
| Chromium | 0.1 | (0.1) | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.5 |
Metals were measured in PM10.
Effects of PM and metal component on methylation
In unadjusted models, PM10 and PM1 levels were significantly associated with decreased NOS3 methylation (Table 2). Zinc levels were significantly and negatively associated with both NOS3 and EDN1 methylation. In covariate-adjusted models, PM10 and PM1 levels were significantly associated with decreased NOS3 methylation (β=−0.2 95%CI: −0.4; −0.03, for PM10; and β=−0.8, 95%CI: −1.4; −0.1, for PM1), zinc levels were significantly and negatively associated with both NOS3 and EDN1 methylation (β=−0.9, 95%CI: −1.4; −0.3, and β=−0.3, 95%CI: −0.8; −0.1, respectively) and iron showed a borderline-significant negative association with NOS3 methylation (β=−0.7, 95%CI: −1.4; −0.01)
Table 2.
Association of PM and metals with blood NOS3 and EDN1 methylation.
| NOS3 | EDN1 | |||
|---|---|---|---|---|
|
|
||||
| Exposures | β c | (95% CI) | β c | (95% CI) |
| Unadjusted regression | ||||
| PM10 | −0.2 | (−0.34 −0.03) | −0.02 | (−0.1; 0.1) |
| PM1 | −0.7 | (−1.3; −0.1) | 0.1 | (−0.3; 0.60) |
| Aluminium | −0.08 | (−0.2; 0.08) | −0.05 | (−0.2; 0.07) |
| Manganese | −0.03 | (−0.2; 0.1) | −0.03 | (−0.1; 0.08) |
| Nickel | −0.1 | (−0.8; 0.5) | −0.2 | (−0.7; 0.3) |
| Zinc | −0.8 | (−1.4; −0.3) | −0.3 | (−0.7; −0.1) |
| Arsenic | 0.1 | (−0.6; 0.9) | −0.3 | (−0.9; 0.2) |
| Lead | −0.1 | (−0.4; 0.1) | −0.07 | (−0.2; 0.1) |
| Iron | −0.6 | (−1.3; 0.07) | −0.2 | (−0.7; 0.3) |
| Chromium | −0.1 | (−0.4; 0.1) | −0.03 | (−0.2; 0.2) |
| Multivariable regression b | ||||
| PM10 | −0.2 | (−0.4; −0.03) | 0.02 | (−0.1; 0.1) |
| PM1 | −0.8 | (−1.4; −0.1) | 0.3 | (−0.2; 0.7) |
| Aluminium | −0.08 | (−0.2; 0.1) | −0.03 | (−0.2; 0.09) |
| Manganese | −0.05 | (−0.2; 0.1) | 0.02 | (−0.1; 0.1) |
| Nickel | −0.2 | (−0.8; 0.5) | −0.1 | (−0.6; 0.3) |
| Zinc | −0.9 | (−1.4; −0.3) | −0.3 | (−0.8; −0.1) |
| Arsenic | 0.02 | (−0.7; 0.8) | −0.3 | (−0.8; 0.3) |
| Lead | −0.2 | (−0.4; 0.1) | 0.00 | (−0.2; 0.2) |
| Iron | −0.7 | (−1.4; −0.01) | −0.2 | (−0.7; 0.3) |
| Chromium | −0.1 | (−0.4; 0.1) | 0.03 | (−0.2; 0.2) |
To estimate long-term effects of PM mass and PM metal component, the level of estimated individual exposure to PM mass and PM metal component, taken as a measure of usual exposure to particles, was examined in relation to all the measures of DNA methylation performed in the study, regardless of whether they were measured on samples taken on the first day of work (i.e., after 2 days off) or after 3 consecutive days of exposure to PM mass and PM metal component in the plant. In the models, the two samples collected at different times are exchangeable, thus assuming that PM mass and PM metal component effects operating over an extended time frame produced similar modifications at the two time points.
Multivariable mixed models adjusted for age, BMI, current smoking status (yes/no), NSAID, %Monocytes.
β for an increment equal to the difference between the 75th and 25th percentile of PM mass and PM metal component.
Effects of PM and metal components on ETP
We found that both PM10 and PM1 were positively and significantly associated with ETP. Multivariable regression models adjusting for age, BMI, current smoking status (yes/no), NSAIDs and percent monocytes, confirmed the positive associations of PM10 (β=20.0 95%CI 3.0; 37.0) and PM1 (β=80.8 95%CI 14.9; 146.7) with ETP and a positive significant association of zinc (β=51.3 95%CI 0.01; 111.1) with ETP (Table 3).
Table 3.
Association of PM and metals with Endogenous ETP
| Exposures | β a | (95% CI) |
|---|---|---|
| Unadjusted regression | ||
| PM10 | 17.9 | (0.9; 34.8) |
| PM1 | 85.0 | (20.0; 149.9) |
| Aluminium | 9.7 | (−7.4; 26.9) |
| Manganese | 1.9 | (−14.5; 18.4) |
| Nickel | 14.4 | (−54.9; 83.8) |
| Zinc | 43.8 | (−17.0; 104.5) |
| Arsenic | −30.8 | (−112.2; 50.7) |
| Lead | 6.6 | (−20.0; 33.3) |
| Iron | 43.9 | (−28.9; 116.7) |
| Chromium | 18.5 | (−8.1; 45.0) |
| Multivariable regression b | ||
| PM10 | 20.0 | (3.0; 37.0) |
| PMT | 80.8 | (14.9; 146.7) |
| Aluminium | 7.5 | (−9.6; 24.5) |
| Manganese | 7.4 | (−9.6; 24.5) |
| Nickel | 19.2 | (−47.5; 85.9) |
| Zinc | 51.3 | (0.01; 111.1) |
| Arsenic | −1.6 | (−82.6; 79.4) |
| Lead | 15.3 | (−11.9; 42.6) |
| Iron | 53.6 | (−16.5; 123.7) |
| Chromium | 22.9 | (−4.0; 49.8) |
Regression coefficient (β) for an increment equal to the difference between the 75th and 25th percentile of PM or metals.
Multivariable mixed-effect models adjusted for age, BMI, current smoking status (yes/no), use of NSAID, and %monocytes.
Association of NOS3 and EDN1 methylation with ETP
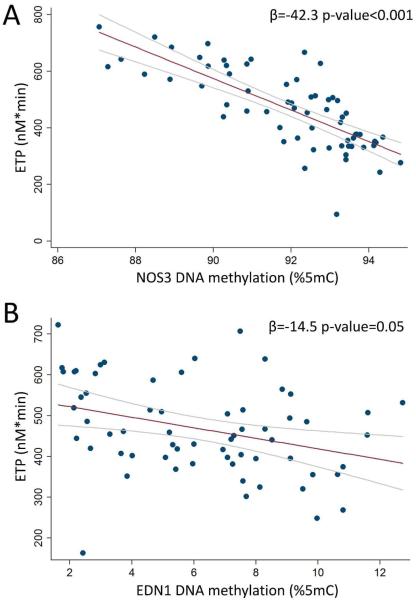
We found negative significant associations between NOS3 methylation and ETP (β=−42.3p<0.001; Figure 1, panel A), and between EDN1 and ETP (β=−14.5, p=0.05, Figure 1, panel B). Neither NOS3 nor EDN-1 methylation were significantly associated with plasma CRP (p>=0.17), an inflammatory marker previously associated with PM exposure in this same study population26.
Figure 1. Associations of NOS3 (A) and EDN1 (B) blood DNA methylation with Endogenous ETP.
The scatterplot shows the mean methylation values [between measures at sample1 and sample 2] of the CpG sites measured at each gene sequences.
Regression coefficients (β) and corresponding p-values adjusted for age, BMI, current smoking status (yes/no), NSAID, and % monocytes are reported. The regression coefficients express the effect on ETP estimated for one unit increase in NOS3 and EDN1.
Mediation analysis
We used mediation analysis to formally test whether the associations of PM1, PM10, and zinc with ETP were mediated by NOS3 and EDN1 hypomethylation. In mediation analysis, NOS3 hypomethylation was estimated to mediate 29% of the positive association between PM10 exposure and higher ETP (Figure 2A, Indirect Effect: 26.23); 26% of the association between PM1 exposure and higher ETP (Figure 2B, Indirect Effect: 35.83); 45% of the association between zinc exposure and higher ETP (Figure 2C, Indirect Effect: 34.18). EDN1 hypomethylation showed to mediate 34% of the positive association between zinc exposure and higher ETP (Figure 2D, Indirect Effect: 23.20).
Figure 2. Mediation of the effect of the exposures on ETP through NOS3 and EDN1 hypomethylation.
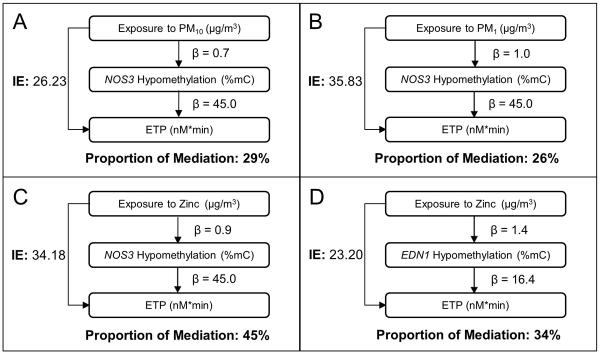
Results from mediation analysis on (A) PM10, NOS3, and ETP; (B) PM1, NOS3, and ETP; (C) zinc, NOS3, and ETP; (D) zinc, EDN1, and ETP. The figure shows, for the two potential mediators (NOS3 or EDN1 methylation), the βs obtained from the mixed-effect mediation models, the estimates of indirect effect (IEs), and proportion of mediation.
β1 is an estimate of the decrease in DNA methylation caused by the exposures; β2 is an estimate of the increase in ETP for each unit decrease in DNA methylation
DISCUSSION
In the present study based on a population of healthy individuals with well-characterized measures of exposure to a wide range of metal-rich PM levels, we demonstrated intermediate roles for decreased NOS3 and EDN1 methylation in the path linking inhalation of PM and its metal components to coagulation activation. Our results showed remarkable consistency in the observed relations among exposures, DNA methylation, and ETP. PM mass and zinc exposure were found to exhibit effects on both ETP and DNA methylation. In turn, we found a negative correlation of NOS3 and EDN1 methylation with ETP, suggesting that the negative effects of the exposures on DNA methylation may result in increased coagulation activity, as reflected in ETP. Mediation analysis confirmed that moderate-to-large proportions of PM effects were mediated by decreased DNA methylation.
A key finding of our study is that DNA methylation of NOS3 and EDN1 promoters was negatively associated with PM and zinc. Zinc have been proposed to play a central role in PM-induced adverse health effects20,21. Our results suggest that zinc exposure induces hypomethylation, which is expected to facilitate NOS3 and EDN1 expression. Our study showed associations with relatively small differences in DNA methylation. This finding is consistent with the effect sizes shown in previous investigations on the effects of PM on blood DNA methylation7,27. At the level of one haploid genome, DNA methylation is binary, rather than quantitative, as each allele is either methylated or unmethylated. The methylation proportion commonly measured by DNA methylation analytic methods, including pyrosequencing, is an overall measure of all the haploid genomes from the cells constituting to the test samples. This percent measure reflects the proportion of haploid genomes in the sample of cells from which the test DNA was purified that was found to be methylated at the specific methylation site(s) investigated. Blood leukocytes include several cell type subsets with specific interconnected functions. Standard immune regulation models posit that small proportions of circulating leukocytes in specific subsets can initiate and maintain inflammatory responses. As small differences in DNA methylation in blood may be localized in a cell subtype with specific immune or inflammatory function and, therefore, play critical roles in mounting inflammatory responses. Further research is needed to identify the effects of PM on specific isolated leucocyte subtypes. Animal models have shown that smoke exposure – a source of direct exposure to PM at high concentrations – induces NOS3 transcription30, and in-vitro experiments have shown that diesel exhaust particulate extracts cause NOS3 activation and NO production31. NOS3 expression has been extensively studied in endothelial cells and linked to antithrombotic activity via reduction of platelet migration, aggregation, and adhesion to the endothelia. However NOS3 is expressed at high levels also in circulating blood leukocytes, where it contributes to producing NO and sustaining pro-inflammatory responses and subsequent activation of the coagulation cascade10. NOS3-related NO production can also participate to generate reactive oxygen species, which can also affect blood coagulation13. NOS3 deficient mice exhibit reduced coagulation function, as reflected in longer time to occlusion in a model of carotid injury and thrombosis32. NO generation by NOS isoforms in circulating leukocytes has been suggested to participate in the PM-induced activation of inflammatory pathways that has consistently been demonstrated in human observational studies, as well as in animal and in vitro experiments33.
Elevated levels of EDN1 have been shown in blood of children34 and seniors35 exposed to air pollution, as well as in cigarette smokers36. Previous investigations have demonstrated that EDN1 is highly expressed in blood leukocytes, and that leukocyte-derived EDN1 can start and sustain inflammatory reactions, and activate coagulation14.
Consistent with those previous findings, our study found negative associations between ETP levels and DNA methylation of both EDN1 and NOS3 promoters.
In a recent work on the same population, we observed an association of PM exposure with increased ETP26. Here we confirm the association of PM with ETP, and also report a positive association between zinc exposure and ETP. ETP represents the total amount of thrombin generated in plasma that results from the balance of the pro- and anticoagulant drivers. Increased ETP has been proposed as a global index of hypercoagulability and has been associated with increased risk of recurrent venous thromboembolism in prospective studies25. Taken together, our results provide further evidence for PM procoagulant effects and indicate DNA methylation of NOS3 and EDN1 as epigenetic alterations underlying PM-related hypercoagulability. Our mediation analysis confirmed this finding by formally testing that decreased blood DNA methylation in both genes is a mediator of the effects of the exposures on coagulation.
Blood leukocytes are a mixed cell population and data from a previous combined analysis of DNA methylation studies has suggested that changes in the proportions of leukocyte cell types may affect DNA methylation37. In our study, we ensured that associations with DNA methylation of NOS3 and EDN1 were independent of differences in the proportions of major cell types by adjusting all results by percent monocytes in multivariable models, as well as by conducting sensitivity analyses further adjusting for lymphocyte and monocyte proportions. However, because we used unfractionated leukocytes, we cannot determine which of the leukocyte subtypes was sensitive to the effects of the exposure on DNA methylation. DNA methylation of genes activated during acute-phase responses may decrease rapidly even in the absence of any cell division,38 so, it is plausible that PM effects on NOS3 and EDN methylation observed in our study may represent actual changes in DNA methylation within cells. On the other hand, it is also equally plausible that cells with specific methylation patterns may proliferate in response to PM and determine an overall change in DNA methylation profiles as measured on unfractionated blood DNA.
We also note that the effects observed in blood leukocytes cannot be extended to other relevant cell types, such endothelia and other vascular tissues. Future studies investigating isolated leukocyte subtypes and additional tissue types are warranted.
Our statistical approach modeled the overall DNA methylation over multiple CpG sites measured in each of the two genes we evaluated. This approach is consistent with the notion that adjacent CpG sites tend to have correlated methylation values and may act in concert to control gene expression39. Exploratory analysis of individual CpG sites did not show major departures at any of the sites from the findings on the overall gene methylation.
The study participants showed a wide range of exposure levels, owing to the differences in PM levels in the different job positions across the work facility. The differences in the estimated individual levels of exposure in our study group were large (range=73–1220 μg/m3) and provided sufficient contrast for identifying exposure-related changes in DNA methylation. A major advantage of our approach is that we could obtain a large contrast in exposures without including an external reference population of individuals working outside the factory. In studies of occupational groups, inclusion of referents working in a different workplace has been demonstrated to have high potential for bias due to differences in personal characteristics determining hiring into the different types of jobs, such as socioeconomic factors or physical constitution40.
The observed associations showed remarkable internal coherence, as well as consistency with previous experimental and observational data. Also, the wide ranges of estimated personal levels of exposure and relatively controlled environment in the foundry provided a setting that was extremely well-suited for evaluating exposure-related effects, while reducing bias and chance findings4. However, although foundry workers in our study were in a modern facility with state-of-art systems for exposure reduction, we cannot exclude that exposures other than PM and metals, such as heat, carbon monoxide, and non-ionizing radiations might have contributed to the observed effects7. Some common SNPs in coagulation pathway genes have been significantly associated with ETP41. Whereas the investigation of gene-epigene interactions would further the understanding of the biological processer related to PM-induced ETP activation, the small sample size precluded to pursue this objective in the present study.
We analysed DNA methylation, a primary epigenetic mechanism regulating the expression of human genes, but we did not measure mRNA expression of the same genes. DNA methylation alterations, once established, are relatively persistent and therefore particularly amenable to capture environmental effects on gene reprogramming. Conversely, mRNA expression is highly variable and shows dramatic fluctuations even over a short time span (e.g., hours). In facts low DNA methylation is found not just in genes that are active at the exact time of their assessment, but also in genes that are poised to be activated in the presence of other molecular signals. Consequently, mRNA expression in human in-vivo studies has often been found to be uncorrelated with DNA methylation42. For our study, we selected two candidate genes (NOS3 and EDN1) that have important functions in mediating PM effects and have been shown to be regulated by DNA methylation, thus maximizing the functional significance of our observations, as also confirmed by the associations of their methylation levels with ETP. The extent to which differences in methylation in other genes are involved in mediating the procoagulant effects of PM and metal exposures remains to be determined.
Our results linked for the first time a test for global coagulation function such as thrombin generation assessed by ETP and DNA hypomethylation of inflammatory genes with procoagulant functions in blood leukocytes, and – in turn – DNA hypomethylation of the same genes and PM and PM metal components exposures. Among the metal components evaluated, we found associations with zinc levels, consistent with previous studies showing that zinc exposure activates blood coagulation19. Our results, as also supported by mediation analysis, indicate that reduced methylation of NOS3 and EDN1 is a potential intermediate mechanism underlying the effects of PM and metals on blood coagulation. To the best of our knowledge, this is the first time that DNA methylation has been formally considered as a mediator for the relationship between PM and blood coagulation.The proportion of mediation of NOS3 methylation in the relationship between PM1 or PM10 and ETP represented a modest, but not negligible, effect. The proportions of effect of zinc on ETP mediated by NOS3 or ET1 were moderately larger. Due to the small sample size, our estimates of proportions mediated may lack in precision. Further studies with a larger sample size are warranted to confirm the findings of the present investigation.
Supplementary Material
WHAT IS KNOWN
Particulate matter exposure is associated with increased coagulation and thrombosis, but the biological mechanism has not yet been clarified.
DNA methylation represents a potential mechanism because it can be modified by environmental risk factors.
Foundry workers are exposed to PM components and show increased cardiovascular risk
WHAT THIS PAPER ADDS
In a group of foundry workers PM and zinc exposure levels were negatively associated with DNA methylation in leukocyte and in turn DNA hypo-methylation resulted associated to increased ETP.
Our study showed that gene hypomethylation contributes to environmentally-induced hypercoagulability.
Acknowledgments
FUNDINGS: This work was supported by research grants from the National Institute of Environmental Health Sciences (ES000002, ES020010, ES019773, ES020268) and CARIPLO Foundation (2007-5469) and Dote Ricerca: Fondo Sociale Europeo, Regione Lombardia
ABBREVIATIONS
- EDN1
endothelin-1
- ETP
Endogenous Thrombin Potential
- NOS3
nitric-oxide-synthase-3
- PM
particulate matter
- PM1
PM≤1 μm in aerodynamic diameter
- PM10
PM≤10 μm in aerodynamic diameter
Footnotes
CONTRIBUTORSHIP: Letizia Tarantini: performed laboratory analysis and wrote the paper
Matteo Bonzini: designed research and interpreted data
Armando Tripodi: designed research and interpreted data
Laura Angelici: performed statistical analysis
Francesco Nordio: performed statistical analysis
Laura Cantone: contributed to laboratory analysis
Pietro Apostoli: designed research
Pier Alberto Bertazzi: designed research and provided the funding
Andrea Baccarelli: designed research, interpreted data and wrote the paper
COMPETING INTEREST: The Authors declare no competing interests
REFERENCES
- 1.Baccarelli A, Rienstra M, Benjamin EJ. Cardiovascular epigenetics: basic concepts and results from animal and human studies. Circ Cardiovasc Genet. 2010;3:567–73. doi: 10.1161/CIRCGENETICS.110.958744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollati V, Baccarelli A. Environmental epigenetics. Heredity. 2011;105:105–12. doi: 10.1038/hdy.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 4.Bollati V, Marinelli B, Apostoli P, et al. Exposure to metal-rich particulate matter modifies the expression of candidate microRNAs in peripheral blood leukocytes. Environ Health Perspect. 2010;118:763–8. doi: 10.1289/ehp.0901300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilmour PS, Schladweiler MC, Nyska A, et al. Systemic imbalance of essential metals and cardiac gene expression in rats following acute pulmonary zinc exposure. J Toxicol Environ Health A. 2006;69:2011–32. doi: 10.1080/15287390600746173. [DOI] [PubMed] [Google Scholar]
- 6.Baccarelli A, Tarantini L, Wright RO, et al. Repetitive element DNA methylation and circulating endothelial and inflammation markers in the VA normative aging study. Epigenetics. 2010;5 doi: 10.4161/epi.5.3.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baccarelli A, Wright RO, Bollati V, et al. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179:572–8. doi: 10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr Opin Pediatr. 2009;21:243–51. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browatzki M, Pfeiffer CA, Schmidt J, et al. Endothelin-1 induces CD40 but not IL-6 in human monocytes via the proinflammatory transcription factor NF-kappaB. Eur J Med Res. 2005;10:197–201. [PubMed] [Google Scholar]
- 10.Muhl H, Pfeilschifter J. Endothelial nitric oxide synthase: a determinant of TNFalpha production by human monocytes/macrophages. Biochem Biophys Res Commun. 2003;310:677–80. doi: 10.1016/j.bbrc.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 11.Wever RM, Luscher TF, Cosentino F, et al. Atherosclerosis and the two faces of endothelial nitric oxide synthase. Circulation. 1998;97:108–12. doi: 10.1161/01.cir.97.1.108. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson K, Tran L, Jimenez LA, et al. Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol. 2005;2:10. doi: 10.1186/1743-8977-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorlach A. Redox regulation of the coagulation cascade. Antioxid Redox Signal. 2005;7:1398–404. doi: 10.1089/ars.2005.7.1398. [DOI] [PubMed] [Google Scholar]
- 14.Halim A, Kanayama N, el Maradny E, et al. Endothelin-1 increased immunoreactive von Willebrand factor in endothelial cells and induced micro thrombosis in rats. Thromb Res. 1994;76:71–8. doi: 10.1016/0049-3848(94)90208-9. [DOI] [PubMed] [Google Scholar]
- 15.Chan Y, Fish JE, D'Abreo C, et al. The cell-specific expression of endothelial nitric-oxide synthase: a role for DNA methylation. J Biol Chem. 2004;279:35087–100. doi: 10.1074/jbc.M405063200. [DOI] [PubMed] [Google Scholar]
- 16.Vallender TW, Lahn BT. Localized methylation in the key regulator gene endothelin-1 is associated with cell type-specific transcriptional silencing. FEBS Lett. 2006;580:4560–6. doi: 10.1016/j.febslet.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Corey LM, Baker C, Luchtel DL. Heart-rate variability in the apolipoprotein E knockout transgenic mouse following exposure to Seattle particulate matter. J Toxicol Environ Health A. 2006;69:953–65. doi: 10.1080/15287390500362105. [DOI] [PubMed] [Google Scholar]
- 18.Chen LC, Lippmann M. Effects of metals within ambient air particulate matter (PM) on human health. Inhal Toxicol. 2009;21:1–31. doi: 10.1080/08958370802105405. [DOI] [PubMed] [Google Scholar]
- 19.Sangani RG, Soukup JM, Ghio AJ. Metals in air pollution particles decrease whole-blood coagulation time. Inhal Toxicol. 2010;22:621–6. doi: 10.3109/08958371003599037. [DOI] [PubMed] [Google Scholar]
- 20.Fine JM, Gordon T, Chen LC, et al. Metal fume fever: characterization of clinical and plasma IL-6 responses in controlled human exposures to zinc oxide fume at and below the threshold limit value. J Occup Environ Med. 1997;39:722–6. doi: 10.1097/00043764-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Gilmour PS, Nyska A, Schladweiler MC, et al. Cardiovascular and blood coagulative effects of pulmonary zinc exposure. Toxicol Appl Pharmacol. 2006;211:41–52. doi: 10.1016/j.taap.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Siegemund A, Petros S, Siegemund T, et al. The endogenous thrombin potential and high levels of coagulation factor VIII, factor IX and factor XI. Blood Coagul Fibrinolysis. 2004;15:241–4. doi: 10.1097/00001721-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Kakkar VV, Hoppenstead DA, Fareed J, et al. Randomized trial of different regimens of heparins and in vivo thrombin generation in acute deep vein thrombosis. Blood. 2002;99:1965–70. doi: 10.1182/blood.v99.6.1965. [DOI] [PubMed] [Google Scholar]
- 24.Wielders S, Mukherjee M, Michiels J, et al. The routine determination of the endogenous thrombin potential, first results in different forms of hyper- and hypocoagulability. Thromb Haemost. 1997;77:629–36. [PubMed] [Google Scholar]
- 25.Tripodi A, Legnani C, Chantarangkul V, et al. High thrombin generation measured in the presence of thrombomodulin is associated with an increased risk of recurrent venous thromboembolism. J Thromb Haemost. 2008;6:1327–33. doi: 10.1111/j.1538-7836.2008.03018.x. [DOI] [PubMed] [Google Scholar]
- 26.Bonzini M, Tripodi A, Artoni A, et al. Effects of inhalable particulate matter on blood coagulation. J Thromb Haemost. 2010;8:662–8. doi: 10.1111/j.1538-7836.2009.03694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarantini L, Bonzini M, Apostoli P, et al. Effects of particulate matter on genomic DNA methylation content and iNOS promoter methylation. Environ Health Perspect. 2009;117:217–22. doi: 10.1289/ehp.11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vander Weele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology. 2009;20:18–26. doi: 10.1097/EDE.0b013e31818f69ce. [DOI] [PubMed] [Google Scholar]
- 29.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 30.Wright JL, Dai J, Zay K, et al. Effects of cigarette smoke on nitric oxide synthase expression in the rat lung. Lab Invest. 1999;79:975–83. [PubMed] [Google Scholar]
- 31.Sumanasekera WK, Ivanova MM, Johnston BJ, et al. Rapid effects of diesel exhaust particulate extracts on intracellular signaling in human endothelial cells. Toxicol Lett. 2007;174:61–73. doi: 10.1016/j.toxlet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Iafrati MD, Vitseva O, Tanriverdi K, et al. Compensatory mechanisms influence hemostasis in setting of eNOS deficiency. Am J Physiol Heart Circ Physiol. 2005;288:H1627–32. doi: 10.1152/ajpheart.00819.2004. [DOI] [PubMed] [Google Scholar]
- 33.Pope CA, Muhlestein JB, May HT, et al. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–8. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- 34.Calderon-Garciduenas L, Vincent R, Mora-Tiscareno A, et al. Elevated plasma endothelin-1 and pulmonary arterial pressure in children exposed to air pollution. Environ Health Perspect. 2007;115:1248–53. doi: 10.1289/ehp.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L, Ruddy T, Dalipaj M, et al. Effects of indoor, outdoor, and personal exposure to particulate air pollution on cardiovascular physiology and systemic mediators in seniors. J Occup Environ Med. 2009;51:1088–98. doi: 10.1097/JOM.0b013e3181b35144. [DOI] [PubMed] [Google Scholar]
- 36.Haak T, Jungmann E, Raab C, et al. Elevated endothelin-1 levels after cigarette smoking. Metabolism. 1994;43:267–9. doi: 10.1016/0026-0495(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 37.Zhu ZZ, Hou L, Bollati V, et al. Predictors of global methylation levels in blood DNA of healthy subjects: a combined analysis. Int J Epidemiol. 2010 doi: 10.1093/ije/dyq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruniquel D, Schwartz RH. Selective, stable demethylation of the interleukin-2 gene enhances transcription by an active process. Nat Immunol. 2003;4:235–40. doi: 10.1038/ni887. [DOI] [PubMed] [Google Scholar]
- 39.Hodges E, Smith AD, Kendall J, et al. High definition profiling of mammalian DNA methylation by array capture and single molecule bisulfite sequencing. Genome Res. 2009;19:1593–605. doi: 10.1101/gr.095190.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearce N, Checkoway H, Kriebel D. Bias in occupational epidemiology studies. Occup Environ Med. 2007;64:562–8. doi: 10.1136/oem.2006.026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segers O, van Oerle R, ten Cate H, et al. Thrombin generation as an intermediate phenotype for venous thrombosis. Thromb Haemost. 2010;103:114–22. doi: 10.1160/TH09-06-0356. [DOI] [PubMed] [Google Scholar]
- 42.Dioni L, Hoxha M, Nordio F, et al. Effects of short-term exposure to inhalable particulate matter on telomere length, telomerase expression, and telomerase methylation in steel workers. Environ Health Perspect. 2010;119:622–7. doi: 10.1289/ehp.1002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.