Abstract
Clandestine laboratories constantly produce new synthetic cannabinoids to circumvent legislative efforts, complicating toxicological analysis. No extensive synthetic cannabinoid quantitative urinary methods are reported in the literature. We developed and validated a liquid chromatography tandem mass spectrometric (LC-MS/MS) method for simultaneously quantifying JWH-018, JWH-019, JWH-073, JWH-081, JWH-122, JWH-200, JWH-210, JWH-250, JWH-398, RCS-4, AM-2201, MAM-2201, UR-144, CP 47,497-C7, CP 47,497-C8 and their metabolites, and JWH-203, AM-694, RCS-8, XLR-11 and HU-210 parent compounds in urine. Non-chromatographically resolved alkyl hydroxy metabolite isomers were considered semi-quantitative. β-glucuronidase hydrolyzed urine was extracted with 1 ml Biotage SLE+ columns. Specimens were reconstituted in 150 µL mobile phase consisting of 50% A (0.01% formic acid in water) and 50% B (0.01% formic acid in 50:50 methanol:acetonitrile). 4 and 25 µL injections were performed to acquire data in positive and negative ionization modes, respectively. The LC-MS/MS instrument consisted of a Shimadzu UFLCxr system and an ABSciex 5500 Qtrap mass spectrometer with an electrospray source. Gradient chromatographic separation was achieved utilizing a Restek Ultra Biphenyl column with a 0.5 ml/min flow rate and an overall run time of 19.5 and 11.4 min for positive and negative mode methods, respectively. Quantification was by multiple reaction monitoring with CP 47,497 compounds and HU-210 ionized via negative polarity; all other analytes were acquired in positive mode. Lower and upper limits of linearity were 0.1–1.0 and 50–100 µg/l (r2 > 0.994). Validation parameters were evaluated at three concentrations spanning linear dynamic ranges. Inter-day analytical recovery (bias) and imprecision (N=20) were 88.3–112.2% and 4.3–13.5% coefficient of variation, respectively. Extraction efficiencies and matrix effect (N=10) were 44–110 and −73 to 52%, respectively. We present a novel LC-MS/MS method for simultaneously quantifying 20 synthetic cannabinoids and 21 metabolites, and semi-quantifying 12 alkyl hydroxy metabolites in urine.
Keywords: synthetic cannabinoids, urine, metabolites, analytical method, LCMSMS
1. INTRODUCTION
Synthetic cannabinoids bind CB1 and/or CB2 receptors and were originally developed for studying endocannabinoid pharmacology; however, now are abused drugs smoked or inhaled for psychoactive effects, but deceptively marketed as herbal incenses and air fresheners, Synthetic cannabinoid abuse resulted in increases in emergency room visits and occasional deaths [1–3]. Synthetic cannabinoid subclasses include napthoylindoles (JWH-015, JWH-018, JWH-019, JWH-073, JWH-081, JWH-122, JWH-200, JWH-210 and JWH-398), phenylacetylindoles (JWH-203, JWH-250, JWH-251, and RCS-8), benzoylindoles (RCS-4 and AM694), cyclohexylphenols (CP 47,497 C7 and C8 analogs) and dibenzopyrans (HU-210).
In July 2012 the United States Drug Enforcement Agency classified JWH-018, JWH-019, JWH-073, JWH-081, JWH-122, JWH-200, JWH-203, JWH-250, JWH-398, AM694, AM2201, RCS-4, RCS-8, HU-210, CP 47,497-C7, CP 47,497-C8 and their analogs as schedule I controlled substances [4,5]. Recently, UR-144, XLR11 and AKB48 were temporarily added to the Schedule I controlled substance list [6]. Most countries enacted similar legislation. Clandestine laboratories constantly synthesize new compounds in response to legislative efforts, complicating drug testing.
New synthetic cannabinoid structures may not cross-react in antibody-based techniques, leading laboratorians to consider mass spectrometric screening [7–10]. Mass spectrometry is flexible, allowing incorporation of new analytes as rapidly as reference standards become available. We recently published a liquid chromatography tandem mass spectrometric (LC-MS/MS) qualitative screening method employing spectral library searching simultaneously targeting 9 synthetic cannabinoids and 20 metabolites in urine [8]. Urinary quantitative methods were only published for single parent analytes and metabolites [11,12] or for metabolites of JWH-018 and JWH-073 [13–15]. The most comprehensive urine quantification method reported to-date targets 8 parent analyte families [16]. A comprehensive, up-to-date quantitative confirmatory synthetic cannabinoid method is required for confirming presumptive positive and negative screening results, comparing screening techniques and evaluating optimal cutoff concentrations. We present a fully-validated LC-MS/MS method targeting 53 analytes: JWH-018, JWH-019, JWH-073, JWH-081, JWH-122, JWH-200, JWH-210, JWH-250, JWH-398, RCS-4, AM2201, MAM2201, UR-144, CP 47,497-C7, CP 47,497-C8 and their metabolites, and JWH-203, AM694, RCS8, XLR11 and HU210 parent compounds in urine. Non-chromatographically resolved alkyl hydroxyl metabolite isomers were semi-quantitative.
2. METHODS
2.1. Reagents and supplies
All standards and deuterated internal standards were purchased from Cayman Chemical (Ann Arbor, MI), except 11-nor-9-carboxy-tetrahydrocannabinol-d9 was from Cerilliant (Round Rock, TX). Ammonium acetate, formic acid, acetonitrile and ethyl acetate were obtained from Sigma-Aldrich (St. Louis, MO), and methanol from Fisher Scientific (Fair Lawn, NJ). Water was purified by an ELGA Purelab Ultra Analytic purifier (Siemens Water Technologies, Lowell, MA). All solvents were HPLC grade or better. Abalone beta-glucuronidase powder containing 1,500,000 units/gram beta-glucuronidase and 150,000 units/g sulfatase was diluted with distilled water to contain 100,000 units/ml beta-glucuronidase and 10,000 units/ml sulfatase activity for enzymatic hydrolysis (Campbell Science, Rockton, Illinois). 1-ml Isolute SLE+ cartridges were utilized for preparing samples (Biotage, Inc, Charlotte, NC). A Cerex System 48 positive pressure manifold (SPEware Corp, Baldwin Park, CA) was employed for specimen extraction. Resprep C18 (3 ml/200 mg, Restek Inc, Bellefonte, PA) and Strata C8 solid phase extraction columns (6 ml/500 mg, Phenomenex, Torrance, CA) were evaluated during method development. Analytical chromatography was performed on an Ultra Biphenyl HPLC column (100 × 2.1 mm; 3 µm particle size) combined with a 10 × 2.1 mm guard column of identical phase purchased from Restek.
2.2. Instrumentation
An ABSciex API 5500 QTRAP® triple quadrupole/linear ion trap mass spectrometer with a TurboIonSpray source operated in electrospray (ESI) mode (ABSciex, Foster City, CA) was coupled with an LC-20ADxr high performance liquid chromatography (HPLC) system (Shimadzu Corp, Columbia, MD). Analyst version 1.6.1 and Multiquant 2.1 were employed for data acquisition and analysis, respectively.
2.3. Calibrators, quality control and internal standards
Blank urine was evaluated to ensure absence of detectable synthetic cannabinoids or metabolites prior to fortification with working stock solutions to prepare calibrators and quality control samples. Primary stock solution containing 53 synthetic cannabinoids and metabolites at 1000 µg/l was prepared in methanol (see analyte list in Table 1). Dilutions of the stock solution created calibrators at 0.1, 0.2, 0.5, 1.0, 5.0, 10, 25, 50 and 100 µg/l when fortifying 20 µL standard solution into 200 µL blank human urine.
Table 1.
Liquid chromatography tandem mass spectrometry parameters for synthetic cannabinoids and metabolites in human urine.
| Analyte | Peak # | Q1 mass (amu) |
Q3 masses (amu) |
DP (V) |
EP (V) |
CE (V) |
CXP (V) |
RT (min) |
|---|---|---|---|---|---|---|---|---|
| A. Positive Mode Method | ||||||||
| JWH-018 | 42 | 342.1 | 155.2, 127.2 | 46 | 10 | 33, 53 | 12, 12 | 12.30 |
| JWH-018 5-hydroxyindole | 33 | 358.2 | 155.1, 127.2 | 91 | 10 | 33, 65 | 16, 10 | 10.70 |
| JWH-018 6-hydroxyindole | 29 | 358.1 | 155.1, 127.2 | 76 | 10 | 31, 69 | 16, 10 | 10.20 |
| JWH-018 N-5-hydroxypentyl | 16 | 358.2 | 155.1, 127.2 | 56 | 10 | 29, 65 | 12, 14 | 8.48 |
| JWH-018 N-pentanoic acid | 15 | 372.2 | 155.0, 126.9 | 106 | 10 | 31, 71 | 14, 14 | 8.47 |
| JWH-019 | 45 | 356.0 | 154.9, 127.0 | 26 | 10 | 33, 65 | 16, 14 | 12.90 |
| JWH-019 5-hydroxyindole | 36 | 372.0 | 155.0, 127.0 | 11 | 10 | 33, 71 | 12, 12 | 11.40 |
| JWH-019 N-6-hydroxyhexyl | 23 | 372.0 | 154.9, 127.0 | 86 | 10 | 29, 73 | 14, 12 | 9.34 |
| JWH-073 | 39 | 328.0 | 155.2, 127.1 | 51 | 10 | 31, 63 | 12, 14 | 11.70 |
| JWH-073 5-hydroxyindole | 27 | 344.2 | 155.0, 127.0 | 51 | 10 | 33, 65 | 12, 18 | 9.88 |
| JWH-073 6-hydroxyindole | 24 | 344.2 | 155.1, 127.0 | 46 | 10 | 31, 67 | 10, 14 | 9.37 |
| JWH-073 N-4-hydroxybutyl | 10 | 344.1 | 155.0, 127.0 | 36 | 10 | 29, 51 | 12, 18 | 7.68 |
| JWH-073 N-butanoic acid | 13 | 358.1 | 155.0, 127.0 | 61 | 10 | 31, 61 | 14, 12 | 7.80 |
| JWH-081 | 43 | 372.1 | 185.1, 157.2 | 181 | 10 | 33, 51 | 16, 10 | 12.70 |
| JWH-081 N-5-hydroxypentyl | 18 | 388.2 | 185.1, 113.9 | 56 | 10 | 29, 99 | 16, 14 | 9.09 |
| JWH-122 | 46 | 356.1 | 169.0, 115.0 | 51 | 10 | 33, 91 | 16, 12 | 12.90 |
| JWH-122 N-5-hydroxypentyl | 22 | 372.1 | 169.0, 115.0 | 51 | 10 | 29, 85 | 12, 14 | 9.29 |
| JWH-200 | 5 | 385.0 | 155.1, 126.8 | 126 | 10 | 29, 69 | 10, 14 | 5.41 |
| JWH-200 5-hydroxyindole | 1 | 401.1 | 155.0, 76.9 | 31 | 10 | 29, 125 | 14, 8 | 2.99 |
| JWH-200 6-hydroxyindole | 2 | 401.1 | 155.0, 127.0 | 120 | 10 | 29, 71 | 12, 16 | 3.48 |
| JWH-203 | 38 | 340.9 | 124.9, 89.1 | 6 | 10 | 35, 103 | 14, 8 | 11.50 |
| JWH-210 | 48 | 370.1 | 183.1, 214.1 | 51 | 10 | 33, 33 | 18, 18 | 13.50 |
| JWH-210 5-hydroxyindole | 41 | 386.2 | 183.0, 155.1 | 51 | 10 | 35, 49 | 14, 14 | 12.20 |
| JWH-210 N-5-hydroxypentyl | 30 | 386.2 | 183.1, 155.1 | 30 | 10 | 31, 47 | 16, 12 | 10.20 |
| JWH-210 N-5-carboxypentyl | 28 | 400.1 | 183.0, 155.0 | 40 | 10 | 33, 49 | 14, 18 | 10.10 |
| JWH-250 | 35 | 336.1 | 121.2, 91.1 | 50 | 10 | 27, 61 | 12, 14 | 11.10 |
| JWH-250 5-hydroxyindole | 20 | 352.1 | 121.0, 91.0 | 66 | 10 | 27, 65 | 14, 12 | 9.19 |
| JWH-250 N-5-hydroxypentyl | 8 | 352.2 | 121.0, 186.2 | 50 | 10 | 27, 21 | 12, 14 | 7.02 |
| JWH-250 N-5-carboxypentyl | 9 | 366.1 | 121.1, 200.1 | 56 | 10 | 27, 23 | 14, 16 | 7.04 |
| JWH-398 | 47 | 377.0 | 188.8, 126.0 | 81 | 10 | 33, 97 | 22, 16 | 13.20 |
| JWH-398 N-5-hydroxypentyl | 26 | 393.1 | 188.8, 160.9 | 51 | 10 | 29, 59 | 18, 14 | 9.85 |
| JWH-398 N-pentanoic acid | 25 | 406.9 | 189.1, 161.1 | 31 | 10 | 31, 65 | 18, 14 | 9.82 |
| AM2201 | 37 | 360.1 | 155.1, 127.2 | 106 | 10 | 33, 57 | 10, 10 | 11.40 |
| AM2201 6-hydroxyindole | 19 | 376.2 | 127.1, 77.0 | 66 | 10 | 67, 111 | 10, 12 | 9.09 |
| AM2201 N-4-hydroxypentyl | 14 | 376.1 | 155.0, 126.9 | 46 | 10 | 33, 69 | 14, 18 | 8.22 |
| MAM2201 | 40 | 374.1 | 169.0, 115.0 | 30 | 10 | 35, 91 | 14, 14 | 12.00 |
| MAM2201 N-4-hydroxypentyl | 17 | 390.1 | 169.0, 141.1 | 166 | 10 | 35, 59 | 18, 12 | 9.02 |
| MAM2201 N-pentanoic acid | 21 | 386.1 | 169.1, 141.1 | 61 | 10 | 33, 49 | 20, 16 | 9.26 |
| AM694 | 31 | 435.9 | 230.8, 202.9 | 196 | 10 | 35, 61 | 18, 20 | 10.40 |
| RCS-4 | 34 | 322.1 | 135.1, 77.2 | 56 | 10 | 31, 73 | 12, 10 | 10.70 |
| RCS-4 N-5-hydroxypentyl | 6 | 338.2 | 135.0, 77.0 | 141 | 10 | 27, 73 | 12, 12 | 6.47 |
| RCS-4 N-5-carboxypentyl | 7 | 352.1 | 135.0, 107.0 | 51 | 10 | 31, 59 | 14, 12 | 6.49 |
| RCS-4 M9 metabolite | 3 | 324.1 | 120.9, 92.9 | 21 | 10 | 27, 63 | 14, 14 | 4.09 |
| RCS-4 M10 metabolite | 4 | 324.1 | 120.9, 93.0 | 16 | 10 | 31, 63 | 14, 14 | 4.31 |
| RCS8 | 44 | 376.0 | 120.9, 90.9 | 146 | 10 | 31, 65 | 10, 12 | 12.80 |
| UR-144 N-5-hydroxypentyl | 11 | 328.0 | 124.9, 97.0 | 141 | 10 | 25, 37 | 18, 14 | 7.78 |
| UR-144 N-pentanoic acid | 12 | 342.0 | 125.0, 244.1 | 61 | 10 | 27, 31 | 10, 18 | 7.78 |
| XLR11 | 32 | 330.1 | 125.1, 232.0 | 156 | 10 | 31, 33 | 6, 24 | 10.50 |
| JWH-018-d9 | 351.1 | 155.0, 127.0 | 36 | 10 | 33, 53 | 14, 16 | 12.30 | |
| JWH-018 5-hydroxyindole-d9 | 367.1 | 155.0, 127.0 | 56 | 10 | 35, 73 | 18, 12 | 10.60 | |
| JWH-018 6-hydroxyindole-d9 | 367.1 | 155.0, 127.0 | 46 | 10 | 33, 73 | 14, 14 | 10.20 | |
| JWH-018 N-5-hydroxypentyl-d5 | 363.1 | 155.0, 127.0 | 56 | 10 | 29, 65 | 14, 14 | 8.43 | |
| JWH-073-d7 | 335.1 | 155.0, 127.0 | 76 | 10 | 33, 65 | 14, 16 | 11.60 | |
| JWH-073 5-hydroxyindole-d7 | 351.1 | 155.0, 127.0 | 51 | 10 | 33, 51 | 12, 16 | 9.81 | |
| JWH-073 6-hydroxyindole-d7 | 351.1 | 155.0, 127.0 | 36 | 10 | 33, 61 | 12, 10 | 9.31 | |
| JWH-073 N-4-hydroxybutyl-d5 | 349.1 | 155.0, 127.0 | 46 | 10 | 29, 57 | 14, 14 | 7.62 | |
| JWH-073 N-butanoic acid-d5 | 363.1 | 155.0, 127.0 | 61 | 10 | 31, 61 | 12, 16 | 7.74 | |
| JWH-081-d9 | 381.2 | 185.0, 157.0 | 51 | 10 | 35, 53 | 18, 12 | 12.70 | |
| JWH-122-d9 | 365.2 | 169.0, 114.9 | 86 | 10 | 35, 95 | 16, 14 | 12.90 | |
| JWH-122 N-5-hydroxypentyl-d5 | 377.1 | 169.0, 114.9 | 56 | 10 | 29, 97 | 16, 14 | 9.23 | |
| JWH-200-d5 | 390.1 | 155.1, 127.0 | 41 | 10 | 29, 65 | 14, 18 | 5.35 | |
| JWH-210-d9 | 379.2 | 183.0, 223.1 | 36 | 10 | 33, 33 | 18, 18 | 13.40 | |
| JWH-250-d5 | 341.1 | 121.0, 91.0 | 46 | 10 | 27, 63 | 14, 12 | 11.00 | |
| JWH-398-d9 | 385.9 | 189.0, 125.9 | 36 | 10 | 37, 93 | 20, 18 | 13.10 | |
| AM2201-d5 | 365.1 | 155.0, 127.0 | 31 | 10 | 35, 73 | 14, 12 | 11.30 | |
| AM2201 N-4-hydroxypentyl-d5 | 381.1 | 155.1, 127.1 | 50 | 10 | 29, 77 | 16, 16 | 8.15 | |
| RCS-4-d9 | 331.1 | 135.0, 77.0 | 61 | 10 | 33, 75 | 12, 10 | 10.60 | |
| UR-144-d5 | 317.0 | 125.0, 218.6 | 61 | 10 | 31, 33 | 12, 18 | 11.40 | |
| XLR11-d5 | 335.1 | 125.1, 237.1 | 161 | 10 | 31, 33 | 12, 20 | 10.40 | |
| B. Negative Mode Method | ||||||||
| CP 47,497-C7 | 51 | 317.1 | 245.1, 159.1 | −90 | −10 | −42, −64 | −29, −15 | 6.26 |
| CP 47,497-C7-hydroxy dimethylheptyl metabolite | 49 | 333.2 | 261.2, 158.9 | −15 | −10 | −50, −76 | −15, −15 | 4.49 |
| CP 47,497-C8 | 52 | 331.1 | 259.1, 159.0 | −30 | −10 | −46, −68 | −23, −15 | 6.54 |
| CP 47,497-C8-hydroxy dimethyloctyl metabolite | 50 | 347.1 | 159.1, 185.0 | −180 | −10 | −72, −66 | −17, −19 | 4.91 |
| HU210 | 53 | 385.6 | 301.3, 281.1 | −36 | −12 | −48, −58 | −27, −26 | 6.93 |
| CP 47,497-C7-d11 | 328.2 | 256.2, 159.0 | −160 | −10 | −44, −68 | −23, −15 | 6.22 | |
| CP 47,497-C8-d7 | 338.2 | 266.1, 159.0 | −150 | −10 | −46, −64 | −33, −17 | 6.52 | |
| 11-nor-9-carboxy-tetrahydrocannabinol-d9 | 352.1 | 254.2, 194.1 | −50 | −10 | −40, −44 | −21, −17 | 6.14 |
Q1= quadrupole 1, Q3= quadrupole 3, DP= declustering potential, EP= entrance potential, CE= collision energy, CXP= collision cell exit potential, RT= retention time.
Bold masses depict quantification transitions.
Quality control (QC) samples were prepared with different vials of reference standard solutions than calibrators. Three mixed QC working solutions containing the same analytes as present in calibrators (see Table 1), ranging from 0.3–30 µg/l, were prepared in methanol (see Table 2 for analyte QC concentrations).
Table 2.
Analytical recovery and imprecision data for synthetic cannabinoids and metabolites in human urine by liquid chromatography tandem mass spectrometry.
| Analyte | Target Low µg/l |
Intra-day, N=4 |
Inter-day, N=20 |
||||
|---|---|---|---|---|---|---|---|
| Accuracy (%CV) | Accuracy (%CV) | ||||||
| Low | Mid | High | Low | Mid | High | ||
| JWH-018 | 0.6 | 109.2 (8.1) | 102.6 (7.9) | 101.6 (9.8) | 102.3 (7.6) | 103.4 (7.6) | 101.2 (8.1) |
| JWH-018 5-hydroxyindole | 0.3 | 109.2 (6.3) | 108.1 (8.7) | 106.6 (5.0) | 107.2 (7.9) | 108.1 (6.9) | 103.8 (7.5) |
| JWH-018 6-hydroxyindole | 0.3 | 104.2 (4.8) | 107.5 (6.8) | 106.0 (7.4) | 106.2 (7.3) | 110.4 (6.5) | 102.0 (10.0) |
| JWH-018 N-hydroxypentyl | 0.3 | 97.5 (12.9) | 98.9 (7.7) | 96.1 (7.0) | 96.0 (9.4) | 102.4 (7.2) | 92.8 (7.6) |
| JWH-018 N-pentanoic acid | 0.3 | 110.8 (9.3) | 112.4 (3.1) | 109.8 (4.7) | 111.0 (6.4) | 112.0 (4.3) | 109.6 (5.2) |
| JWH-019 | 0.3 | 104.2 (11.5) | 100.2 (7.8) | 100.2 (10.7) | 101.3 (10.8) | 103.3 (7.9) | 99.6 (9.2) |
| JWH-019 5-hydroxyindole | 0.3 | 100.8 (11.2) | 99.8 (5.9) | 96.7 (8.6) | 100.0 (11.7) | 103.6 (10.0) | 96.3 (7.2) |
| JWH-019 N-hydroxyhexyl | 0.3 | 111.7 (5.2) | 111.8 (3.2) | 107.3 (5.2) | 107.5 (7.9) | 112.0 (4.4) | 105.6 (7.4) |
| JWH-073 | 0.3 | 108.3 (8.1) | 100.7 (8.5) | 100.2 (6.1) | 98.2 (10.1) | 101.4 (8.6) | 97.3 (7.1) |
| JWH-073 5-hydroxyindole | 0.3 | 105.0 (9.9) | 106.0 (8.8) | 105.3 (5.3) | 103.5 (8.1) | 107.6 (7.4) | 100.7 (7.7) |
| JWH-073 6-hydroxyindole | 0.3 | 102.5 (9.3) | 99.3 (9.8) | 99.7 (7.1) | 100.7 (8.8) | 103.0 (7.6) | 96.0 (8.2) |
| JWH-073 N-hydroxybutyl | 0.3 | 105.8 (4.0) | 105.4 (6.7) | 101.6 (5.7) | 102.7 (8.6) | 106.5 (7.8) | 97.6 (5.9) |
| JWH-073 N-butanoic acid | 0.3 | 102.5 (9.7) | 103.8 (8.7) | 101.4 (7.0) | 105.5 (7.8) | 105.4 (6.7) | 99.0 (5.3) |
| JWH-081 | 0.3 | 105.8 (6.5) | 102.0 (7.8) | 99.8 (9.4) | 98.8 (9.4) | 104.1 (7.2) | 96.4 (7.5) |
| JWH-081 N-hydroxypentyl | 0.3 | 112.5 (7.8) | 107.8 (6.4) | 104.7 (2.2) | 108.2 (8.1) | 111.1 (5.6) | 104.6 (7.0) |
| JWH-122 | 0.3 | 102.5 (6.7) | 95.5 (7.2) | 90.0 (8.1) | 101.3 (9.8) | 105.4 (8.7) | 98.5 (8.7) |
| JWH-122 N-hydroxypentyl | 0.3 | 101.7 (11.2) | 100.9 (9.2) | 99.3 (4.0) | 99.7 (8.3) | 103.7 (7.7) | 97.4 (7.3) |
| JWH-200 | 0.3 | 103.3 (9.5) | 102.0 (9.4) | 98.4 (6.6) | 99.3 (9.1) | 103.1 (6.8) | 97.5 (6.7) |
| JWH-200 5-hydroxyindole | 1.5 | 109.2 (6.6) | 107.2 (7.7) | 108.7 (5.0) | 104.5 (6.4) | 104.1 (7.5) | 103.2 (7.6) |
| JWH-200 6-hydroxyindole | 0.3 | 110.0 (6.5) | 107.3 (9.3) | 108.7 (3.4) | 104.3 (8.5) | 106.0 (7.9) | 104.8 (6.1) |
| JWH-203 | 1.5 | 94.0 (7.7) | 87.9 (3.8) | 88.8 (5.4) | 98.0 (9.9) | 96.8 (10.7) | 96.2 (10.0) |
| JWH-210 | 0.3 | 110.0 (2.5) | 102.9 (10.0) | 101.2 (7.5) | 105.0 (8.0) | 106.3 (8.6) | 101.5 (7.5) |
| JWH-210 5-hydroxyindole | 0.6 | 100.0 (10.4) | 94.3 (6.9) | 94.5 (9.2) | 98.7 (10.7) | 106.8 (7.8) | 104.6 (8.4) |
| JWH-210 N-hydroxypentyl | 0.3 | 96.7 (13.5) | 95.5 (7.1) | 93.9 (10.0) | 97.8 (9.3) | 101.8 (9.2) | 91.3 (7.6) |
| JWH-210 N-5-carboxypentyl | 0.3 | 104.2 (13.7) | 103.2 (3.3) | 96.2 (8.0) | 99.7 (10.3) | 103.8 (6.8) | 97.6 (7.2) |
| JWH-250 | 0.6 | 107.5 (5.1) | 101.6 (7.9) | 101.3 (5.6) | 104.9 (7.3) | 103.9 (8.3) | 98.7 (6.9) |
| JWH-250 5-hydroxyindole | 0.3 | 110.0 (6.5) | 103.5 (7.1) | 104.7 (8.0) | 105.7 (7.3) | 107.3 (7.0) | 103.6 (8.3) |
| JWH-250 N-hydroxypentyl | 0.6 | 108.8 (5.8) | 109.3 (7.6) | 104.5 (9.5) | 106.6 (6.1) | 107.9 (6.6) | 99.1 (9.4) |
| JWH-250 N-5-carboxypentyl | 0.6 | 115.4 (1.4) | 111.5 (8.7) | 111.8 (10.9) | 111.3 (5.4) | 111.4 (6.7) | 105.2 (9.8) |
| JWH-398 | 1.5 | 98.3 (10.9) | 102.6 (7.9) | 101.4 (2.4) | 100.1 (10.0) | 105.9 (8.2) | 101.7 (11.2) |
| JWH-398 N-hydroxypentyl | 1.5 | 112.5 (4.5) | 105.3 (6.2) | 101.1 (5.1) | 105.2 (8.5) | 106.1 (5.4) | 102.0 (6.1) |
| JWH-398 N-pentanoic acid | 1.5 | 99.7 (9.1) | 101.0 (9.7) | 99.4 (5.9) | 95.2 (10.2) | 93.6 (7.6) | 92.5 (7.2) |
| AM2201 | 0.3 | 105.0 (8.4) | 105.0 (9.6) | 104.1 (5.6) | 105.3 (7.1) | 107.9 (6.0) | 102.6 (7.3) |
| AM2201 6-hydroxyindole | 1.5 | 109.2 (7.8) | 107.1 (8.4) | 106.8 (6.6) | 109.1 (6.7) | 107.2 (6.9) | 104.4 (8.2) |
| AM2201 N-hydroxypentyl | 0.3 | 96.7 (12.95) | 98.1 (10.0) | 96.6 (6.5) | 94.2 (10.0) | 99.7 (9.2) | 93.3 (6.3) |
| MAM2201 | 0.3 | 96.7 (9.8) | 97.7 (5.1) | 88.9 (3.3) | 92.0 (7.7) | 94.0 (6.3) | 88.3 (5.3) |
| MAM2201 N-hydroxypentyl | 0.3 | 110.0 (10.5) | 110.0 (5.6) | 107.3 (3.4) | 104.7 (9.4) | 110.4 (5.5) | 105.3 (5.6) |
| MAM2201 N-pentanoic acid | 0.6 | 104.6 (9.4) | 105.9 (6.5) | 101.9 (5.6) | 103.1 (8.1) | 105.6 (6.6) | 101.2 (6.7) |
| AM694 | 0.3 | 97.5 (12.3) | 103.5 (7.0) | 102.2 (8.0) | 100.8 (11.2) | 107.3 (6.4) | 103.9 (7.5) |
| RCS-4 | 0.3 | 106.7 (8.5) | 105.1 (11.8) | 101.2 (6.2) | 104.7 (8.5) | 108.4 (7.6) | 101.6 (8.4) |
| RCS-4 N-hydroxypentyl | 0.6 | 105.8 (6.9) | 102.8 (7.7) | 99.8 (8.7) | 102.2 (8.3) | 105.6 (8.1) | 96.7 (7.4) |
| RCS-4 N-5-carboxypentyl | 0.6 | 118.3 (1.1) | 111.8 (6.8) | 110.9 (10.2) | 112.2 (5.7) | 111.7 (6.1) | 106.7 (8.2) |
| RCS-4 M9 metabolite | 0.6 | 112.5 (4.4) | 110.0 (8.4) | 107.8 (7.6) | 105.9 (7.5) | 108.9 (6.6) | 104.1 (6.1) |
| RCS-4 M10 metabolite | 0.6 | 112.1 (6.5) | 109.7 (8.7) | 107.3 (7.2) | 105.9 (7.9) | 109.4 (7.0) | 103.3 (6.6) |
| RCS8 | 0.3 | 110.0 (10.2) | 103.6 (11.7) | 98.9 (7.3) | 97.5 (12.1) | 99.0 (10.7) | 92.7 (8.6) |
| UR-144 N-hydroxypentyl | 0.3 | 95.0 (14.2) | 91.6 (8.5) | 88.0 (6.9) | 90.0 (9.3) | 97.7 (10.3) | 88.5 (5.5) |
| UR-144 N-pentanoic acid | 0.6 | 100.8 (10.3) | 96.7 (8.6) | 94.2 (4.2) | 103.6 (8.0) | 102.9 (8.4) | 97.0 (5.2) |
| XLR11 | 0.6 | 101.7 (5.8) | 100.6 (6.0) | 100.6 (6.5) | 101.7 (7.1) | 101.7 (9.2) | 97.2 (9.7) |
| CP 47,497-C7 | 1.5 | 113.7 (4.2) | 103.0 (6.4) | 95.6 (5.9) | 106.2 (9.4) | 106.4 (7.1) | 97.4 (7.3) |
| CP 47,497-C7-hydroxy metabolite | 1.5 | 105.8 (12.1) | 112.6 (7.0) | 111.0 (7.4) | 103.7 (10.2) | 108.8 (9.4) | 105.2 (10.4) |
| CP 47,497-C8 | 1.5 | 112.7 (5.6) | 105.3 (8.0) | 105.1 (7.8) | 106.2 (9.1) | 105.4 (7.1) | 103.1 (6.8) |
| CP 47,497-C8-hydroxy metabolite | 1.5 | 101.0 (16.8) | 93.6 (9.1) | 95.7 (5.2) | 99.0 (13.5) | 99.9 (12.2) | 98.1 (11.0) |
| HU210 | 1.5 | 95.5 (9.7) | 95.9 (12.7) | 86.5 (6.0) | 97.3 (10.8) | 101.0 (11.7) | 99.2 (11.8) |
| % target (range) | 94.0 – 118.3 | 87.9 – 112.6 | 86.5 – 111.8 | 90.0 – 112.2 | 93.6 – 112.1 | 88.3 – 109.6 | |
| %CV (range) | 1.1 – 16.8 | 3.1 – 12.7 | 2.2 – 10.9 | 5.4 – 13.5 | 4.3 – 12.2 | 5.2 – 11.8 | |
Mid and high quality control concentrations were 7.5 and 30 µg/l, respectively.
Deuterated internal standard (N=24, see Table 1) stock solutions were diluted in methanol producing a mixed internal standard solution of 10 µg/l. 20µL of 10 µg/l mixed internal standard solution was added to blank urine, yielding 1 µg/l fortified urine internal standard concentrations.
All primary and working solutions were stored at −20°C in amber glass vials.
2.4. Specimen preparation approaches evaluated during method development
Preliminary synthetic cannabinoids recovery studies were conducted in triplicate during method development to evaluate potential sample preparation approaches with Resprep C18 columns, Strata C8 columns and SLE. Three sets of specimens were prepared: urine fortified prior to extraction, urine fortified after extraction and neat.
For Resprep C18 and Strata C8 sample preparations, 0.5 ml blank urine containing 10 µg/l JWH-018, RCS8, JWH-250 N-5-hydroxypentyl and JWH-250 N-5-carboxypentyl was diluted with 2.5 ml 400mM ammonium acetate buffer, pH 4.0 prior to addition of 50 µL glucuronidase solution (100,000 units glucuronidase activity/ml). Screwtop glass tubes were capped and incubated at 55°C for 2 h, then centrifuged at 1600g, 4°C for 5 min prior to application on conditioned columns. Resprep C18 and Strata C8 columns were conditioned with acetonitrile and 400 mM ammonium acetate buffer, pH 4.0 and washed with 3 ml water and 400 mM ammonium acetate buffer, pH 4.0:acetonitrile (80:20, v/v). Columns were dried via 40 psi positive pressure for 5 min prior to elution with 3 ml 2% glacial acetic acid in acetonitrile followed by 3 ml hexane:ethyl acetate (90:10, v/v). Combined eluents were dried completely under nitrogen at 40°C prior to reconstitution with 150 µL mobile phase A:B 50:50 (v/v).
For SLE preparation, 200 µL blank urine containing 10 µg/l JWH-018, RCS8, JWH-250 N-5-hydroxypentyl and JWH-250 N-5-carboxypentyl was diluted with 0.3 ml 400 mM ammonium acetate buffer, pH 4.0 prior to addition of 40 µL glucuronidase solution (100,000 units glucuronidase activity/ml). Polypropylene microcentrifuge tubes were capped and incubated at 55°C for 2 h. Samples were centrifuged at 15,000g, 4°C for 5 min after addition of 0.5 ml acetonitrile. Samples were transferred onto SLE columns and gently driven onto column phase with fine pressure control by slowly increasing pressure up to 1 l/min (achieving 21 ml/min through each column). After equilibration at ambient pressure for 5 min, analytes were eluted with 6 ml ethyl acetate into 16×100 mm conical polypropylene tubes. Positive pressure was gradually applied up to 5 l/min (100 ml/min through each column) with fine pressure control until elution was complete. All sample extracts were completely dried at 45°C under nitrogen in a Zymark TurboVap. Samples were reconstituted in 150 µL mobile phase A:B 50:50 (v/v), vortexed 15 s prior to centrifugation at 4°C, 4000g for 5 min and transferred to autosampler vials containing 200 µL glass inserts.
2.5. LC-MS/MS
Chromatographic separation was performed on an Ultra Biphenyl column equipped with a guard column containing identical packing material. Two LC-MS/MS methods were required, a 19.5 min positive ionization mode method with 4 µL injection volume and a 11.4 min negative ionization mode method with 25 µL injection volume. For positive and negative mode methods, the column oven and auto-sampler were maintained at 40 and 4°C, respectively. Gradient elution was performed for both methods with (A) 0.01% formic acid in water and (B) 0.01% formic acid in acetonitrile:methanol (50:50, v/v) at a flow rate of 0.5 ml/min. The initial gradient conditions for the positive mode method were 40% B, held for 30 s, then increased to 90% B over 14.5 min, increased to 98% B at 15.1 min held until 17.6 min, returned to 40% B at 17.7 min and held until 19.5 min. Flow rate was ramped from 0.5 ml/min to 1.0 ml/min at 15.2 min and returned to 0.5 ml/min at 18.0 min. HPLC eluent was diverted to waste for the first 1.5 min and after 16.5 min of analysis. Initial gradient conditions for the negative mode method were 40% B, held for 30 s, then increased to 90% B over 6.5 min, increased to 98% B at 7.1 min held until 9.5 min, returned to 40% B at 9.6 min and held until 11.4 min. Flow rate was ramped from 0.5 ml/min to 1.0 ml/min at 7.3 min and returned to 0.5 ml/min at 9.9 min. The divert valve was directed to waste for the first 3.0 min and after 7.1 min.
Mass spectrometric data were collected in scheduled multiple reaction monitoring (MRM) mode with a target scan time of 0.5 s. Positive and negative mode method MRM detection windows were 50 and 40 s, respectively. CP 47,497-C7, CP 47,497-C7 C7-hydroxydimethylheptyl metabolite, CP 47,497-C8, CP 47,497-C8 C8-hydroxydimethyloctyl metabolite, HU210, CP 47,497-C7-d11, CP 47,497-C8-d7 and 11-nor-9-carboxy-tetrahydrocannabinol-d9 were acquired in negative ionization mode; all other analytes and internal standards were acquired in positive ionization mode. MS/MS parameters (Table 1) were optimized via direct infusion of individual analytes at 10 or 50 µg/l in initial mobile phase for positive and negative mode, respectively. Optimized source parameters for positive and negative modes were: gas−1 60, gas−2 50, curtain gas 45, source temperature 500°C; ion spray voltage was 5500 and −4500 for positive and negative modes, respectively. Nitrogen collision gas was set at medium for all experiments. Quadrupoles one and three were set to unit resolution. Quantifier and qualifier ion transitions were monitored for each analyte and internal standard.
2.6. Hydrolysis optimization
Hydrolysis conditions were optimized with blank urine fortified to contain 2500 µg/l JWH-018 N-5-hydroxypentyl-glucuronide. Amount of enzyme, pH, temperature and duration of incubation were evaluated for optimally hydrolyzing glucuronides.
2.7. Data analysis
Peak area ratios of analytes to corresponding internal standards were calculated for each concentration to construct daily calibration curves via linear least-squares regression with a 1/x2 weighting factor. See Table 3 for calibration linear ranges.
Table 3.
Synthetic cannabinoids and metabolites in human urine by liquid chromatography tandem mass spectrometry: limits of detection (LOD), linear ranges and calibration results (N=6).
| Analyte | Internal Standard |
LOD (µg/l) |
Linear range (µg/l) |
Y-intercept Mean (SD) |
Slope Mean (SD) |
R2 (range) |
|---|---|---|---|---|---|---|
| JWH-018 | JWH-018-d9 | 0.2 | 0.2–50 | 0.78 (0.03) | 0.02 (0.01) | 0.995–0.999 |
| JWH-018 5-hydroxyindole | JWH-018 5-hydroxyindole-d9 | 0.1 | 0.1–50 | 1.08 (0.06) | 0.02 (0.01) | 0.994–0.998 |
| JWH-018 6-hydroxyindole | JWH-018 6-hydroxyindole-d9 | 0.1 | 0.1–50 | 0.96 (0.06) | 0.02 (0.01) | 0.994–0.998 |
| JWH-018 N-hydroxypentyl | JWH-018 N-hydroxypentyl-d5 | 0.1 | 0.1–50 | 1.19 (0.04) | 0.04 (0.01) | 0.996–0.997 |
| JWH-018 N-pentanoic acid | JWH-018 N-hydroxypentyl-d5 | 0.1 | 0.1–50 | 0.71 (0.04) | 0.01 (0.01) | 0.996–0.999 |
| JWH-019 | JWH-018-d9 | 0.1 | 0.1–50 | 0.63 (0.06) | 0.01 (0.01) | 0.995–0.999 |
| JWH-019 5-hydroxyindole | JWH-018 5-hydroxyindole-d9 | 0.1 | 0.1–50 | 0.95 (0.11) | 0.02 (0.01) | 0.995–0.997 |
| JWH-019 N-hydroxyhexyl | JWH-122 N-hydroxypentyl-d5 | 0.1 | 0.1–50 | 1.04 (0.05) | 0.03 (0.01) | 0.995–0.998 |
| JWH-073 | JWH-073-d7 | 0.1 | 0.1–50 | 0.48 (0.02) | 0.01 (0.01) | 0.995–0.998 |
| JWH-073 5-hydroxyindole | JWH-073 5-hydroxyindole-d7 | 0.1 | 0.1–50 | 0.94 (0.06) | 0.02 (0.01) | 0.995–0.998 |
| JWH-073 6-hydroxyindole | JWH-073 6-hydroxyindole-d7 | 0.1 | 0.1–50 | 0.94 (0.06) | 0.02 (0.01) | 0.995–0.998 |
| JWH-073 N-hydroxybutyl | JWH-073 N-hydroxybutyl-d5 | 0.1 | 0.1–50 | 1.34 (0.11) | 0.02 (0.01) | 0.995–0.998 |
| JWH-073 N-butanoic acid | JWH-073 N-butanoic acid-d5 | 0.1 | 0.1–50 | 1.43 (0.08) | 0.02 (0.02) | 0.996–0.998 |
| JWH-081 | JWH-081-d9 | 0.1 | 0.1–50 | 0.78 (0.04) | 0.05 (0.01) | 0.994–0.998 |
| JWH-081 N-hydroxypentyl | JWH-122 N-hydroxypentyl-d5 | 0.1 | 0.1–50 | 1.06 (0.07) | 0.02 (0.01) | 0.995–0.997 |
| JWH-122 | JWH-122-d9 | 0.1 | 0.1–50 | 1.05 (0.08) | 0.02 (0.01) | 0.995–0.997 |
| JWH-122 N-hydroxypentyl | JWH-122 N-hydroxypentyl-d5 | 0.1 | 0.1–50 | 0.99 (0.05) | 0.02 (0.01) | 0.995–0.997 |
| JWH-200 | JWH-200-d5 | 0.1 | 0.1–50 | 8.01 (0.38) | 0.12 (0.05) | 0.996–0.997 |
| JWH-200 5-hydroxyindole | JWH-073 6-hydroxyindole-d7 | 1.0 | 1–100 | 0.77 (0.30) | −0.01 (0.03) | 0.994–0.999 |
| JWH-200 6-hydroxyindole | JWH-073 6-hydroxyindole-d7 | 0.1 | 0.1–50 | 0.81 (0.30) | 0.01 (0.01) | 0.996–0.998 |
| JWH-203 | UR-144-d5 | 0.5 | 0.5–50 | 0.37 (0.04) | 0.08 (0.05) | 0.994–0.998 |
| JWH-210 | JWH-210-d9 | 0.1 | 0.1–50 | 1.30 (0.05) | 0.02 (0.02) | 0.995–0.999 |
| JWH-210 5-hydroxyindole | JWH-018-d9 | 0.2 | 0.2–50 | 1.24 (0.45) | 0.09 (0.06) | 0.995–0.999 |
| JWH-210 N-hydroxypentyl | JWH-018 N-hydroxypentyl-d5 | 0.1 | 0.1–50 | 1.38 (0.06) | 0.03 (0.01) | 0.994–0.997 |
| JWH-210 N-5-carboxypentyl | JWH-122 N-hydroxypentyl-d5 | 0.1 | 0.1–50 | 0.91 (0.09) | 0.01 (0.01) | 0.996–0.998 |
| JWH-250 | JWH-250-d5 | 0.2 | 0.2–50 | 0.61 (0.04) | 0.02 (0.02) | 0.995–0.998 |
| JWH-250 5-hydroxyindole | JWH-073 6-hydroxyindole-d7 | 0.1 | 0.1–50 | 0.77 (0.02) | 0.01 (0.004) | 0.995–0.998 |
| JWH-250 N-hydroxypentyl | JWH-073 N-hydroxybutyl-d5 | 0.2 | 0.2–50 | 1.41 (0.09) | 0.08 (0.02) | 0.995–0.997 |
| JWH-250 N-5-carboxypentyl | JWH-073 N-butanoic acid-d5 | 0.2 | 0.2–50 | 1.34 (0.09) | 0.03 (0.03) | 0.995–0.999 |
| JWH-398 | JWH-398-d9 | 0.5 | 0.5–50 | 0.93 (0.07) | 0.06 (0.07) | 0.996–0.999 |
| JWH-398 N-hydroxypentyl | JWH-122 N-hydroxypentyl-d5 | 0.5 | 0.5–50 | 0.08 (0.005) | 0.01 (0.004) | 0.995–0.999 |
| JWH-398 N-pentanoic acid | JWH-122 N-hydroxypentyl-d5 | 1.0 | 1–100 | 0.05 (0.006) | 0.01 (0.007) | 0.996–0.999 |
| AM2201 | AM2201-d5 | 0.1 | 0.1–50 | 7.60 (0.26) | 0.10 (0.03) | 0.994–0.999 |
| AM2201 6-hydroxyindole | JWH-073 6-hydroxyindole-d7 | 1.0 | 1–100 | 0.56 (0.04) | −0.02 (0.04) | 0.996–0.999 |
| AM2201 N-hydroxypentyl | AM2201 N-hydroxypentyl-d5 | 0.1 | 0.1–50 | 1.33 (0.08) | 0.03 (0.01) | 0.995–0.998 |
| MAM2201 | AM2201-d5 | 0.1 | 0.1–50 | 10.14 (2.22) | 0.22 (0.11) | 0.995–0.996 |
| MAM2201 N-hydroxypentyl | JWH-122 N-hydroxypentyl-d5 | 0.1 | 0.1–50 | 0.68 (0.03) | 0.01 (0.004) | 0.995–0.999 |
| MAM2201 N-pentanoic acid | JWH-122 N-hydroxypentyl-d5 | 0.2 | 0.2–50 | 0.82 (0.07) | 0.02 (0.01) | 0.995–0.999 |
| AM694 | XLR11-d5 | 0.1 | 0.1–50 | 1.99 (0.52) | 0.05 (0.03) | 0.996–0.998 |
| RCS-4 | RCS-4-d9 | 0.1 | 0.1–50 | 0.73 (0.04) | 0.01 (0.004) | 0.995–0.997 |
| RCS-4 N-hydroxypentyl | JWH-073 N-hydroxybutyl-d5 | 0.2 | 0.2–50 | 1.27 (0.09) | 0.04 (0.01) | 0.996–0.998 |
| RCS-4 N-5-carboxypentyl | JWH-073 N-butanoic acid-d5 | 0.2 | 0.2–50 | 1.39 (0.06) | 0.03 (0.02) | 0.995–0.997 |
| RCS-4 M9 metabolite | JWH-073 N-hydroxybutyl-d5 | 0.2 | 0.2–50 | 0.77 (0.13) | 0.03 (0.02) | 0.995–0.998 |
| RCS-4 M10 metabolite | JWH-073 N-hydroxybutyl-d5 | 0.2 | 0.2–50 | 0.77 (0.13) | 0.03 (0.02) | 0.995–0.998 |
| RCS8 | RCS-4-d9 | 0.1 | 0.1–50 | 0.46 (0.05) | 0.01 (0.01) | 0.995–0.998 |
| UR-144 N-hydroxypentyl | JWH-073 N-butanoic acid-d5 | 0.2 | 0.2–50 | 1.91 (0.17) | 0.06 (0.03) | 0.994–0.997 |
| UR-144 N-pentanoic acid | JWH-073 N-butanoic acid-d5 | 0.2 | 0.2–50 | 1.30 (0.07) | 0.05 (0.02) | 0.995–0.998 |
| XLR11 | XLR11-d5 | 0.2 | 0.2–50 | 1.32 (0.03) | 0.03 (0.01) | 0.995–0.999 |
| CP 47,497-C7 | CP 47,497-C7-d11 | 0.5 | 0.5–50 | 1.10 (0.06) | 0.11 (0.07) | 0.995–0.998 |
| CP 47,497-C7-hydroxy metabolite | 11-nor-9-tetrahydrocannabinol-d9 | 0.5 | 0.5–50 | 1.50 (0.46) | 0.21 (0.16) | 0.995–0.999 |
| CP 47,497-C8 | CP 47,497-C8-d7 | 0.5 | 0.5–50 | 0.75 (0.07) | 0.02 (0.02) | 0.995–0.999 |
| CP 47,497-C8-hydroxy metabolite | 11-nor-9-tetrahydrocannabinol-d9 | 0.5 | 0.5–50 | 2.21 (0.61) | 0.10 (0.16) | 0.994–0.999 |
| HU210 | CP 47,497-C8-d7 | 0.5 | 0.5–50 | 0.09 (0.02) | 0.03 (0.01) | 0.996–0.998 |
Limit of quantification was the lower limit of linearity.
2.8. Method validation
Specificity, sensitivity, linearity, imprecision, analytical recovery, extraction efficiency, matrix effect, stability, dilution integrity and carry-over were evaluated during method validation.
2.9. Specificity
Analyte peak identification criteria were relative retention time within ± 0.1min of the lowest calibrator and qualifier/quantifier transition peak area ratios ± 20% of mean calibrator transition ratios. We employed ± 0.1 min as a peak identification retention time requirement based upon our observations of calibrator retention time drift during method development. Retention times did not drift by more than ± 0.05 min, but we employed a wider ± 0.1 min retention time window requirement because larger retention time variation is expected with authentic specimen analysis over time. Potential endogenous interferences were assessed by analyzing ten blank urine specimens from different individuals. In addition, 83 potential interferences from commonly used drugs were evaluated by fortifying drugs into low QC samples. Final interferent concentrations were 500 µg/l (see Supplementary Table 1 for interferent list). Synthetic cannabinoids also were individually fortified into low QC samples at 200 µg/l (20 µg/l for parent analytes and hydroxyindole minor metabolites). No interference was noted if all analytes in the low QC sample quantified within ± 20% of target concentrations with acceptable qualifier/quantifier transition ratios. At least 25 scans were acquired across each peak for accurate peak area determination.
2.10. Sensitivity and linearity
Limit of detection (LOD) was evaluated over three runs with duplicates from 3 different urine sources and defined as the lowest concentration producing a peak eluting within ± 0.1 min of analyte retention time for the lowest calibrator with signal-to-noise ≥3:1, Gaussian peak shape and qualifier/quantifier transition peak area ratios ± 20% of mean calibrator transition ratios for all replicates. Limit of quantification (LOQ) also was evaluated in the same manner, and defined as the lowest concentration that met LOD criteria with signal-to-noise ≥10:1 and measured concentration within ± 20% of target. Performance at the LOQ was confirmed in each batch of specimens and was each analytes’ lowest limit of linearity.
Preliminary experiments with six sets of calibrators determined the most appropriate calibration model comparing goodness-of-fit via normalized residuals inspection for unweighted linear least squares, linear least squares employing 1/x and 1/x2 weighting. Calibration curves were fit by linear least squares regression with at least 6 concentrations across the linear dynamic range for each analyte. Calibrators were required to quantify within ± 20% and during method validation correlation coefficients (R2) were required to exceed 0.99.
2.11. Analytical recovery and imprecision
Intra- and inter-day analytical recovery (bias) and imprecision were determined from four replicates at three different QC concentrations across the linear dynamic range of the assay. Analytical recovery was determined by comparing the mean result for all analyses to the nominal concentration value (i.e. mean % of expected concentration). Inter-day imprecision and analytical recovery were evaluated on five different runs with four replicates in each run, analyzed on five separate days (n=20). Imprecision was expressed as % coefficient of variation (% CV) of calculated concentrations. One-way analysis of variance (ANOVA) was conducted on low, medium and high QCs to evaluate inter- and intra-day differences in analyte concentrations.
2.12. Extraction efficiency and matrix effect
Extraction efficiency and matrix effect were evaluated via three sets of samples as described by Matuszewski et al. (n=10 for each set) [17]. In the first set, urine samples were fortified with analytes and internal standards prior to SLE. In set 2, urine samples were fortified with analytes and internal standards after SLE, and the third set contained analytes and internal standards in mobile phase. Extraction efficiency, expressed as a percentage, was calculated by dividing analyte mean peak areas of set 1 by set 2. Absolute matrix effect was calculated by dividing the mean peak area of the analyte in set 2 by the mean analyte area in set 3. The value was converted to a percentage and subtracted from 100 to represent the amount of signal suppressed by the presence of matrix.
2.13. Analyte stability
Analyte stability also was evaluated with blank human urine fortified with analytes of interest at low and high QC concentrations (n=3). Analyte short-term temperature stability was evaluated for fortified human urine stored in the dark in polypropylene microcentrifuge tubes for 16 h at room temperature, 72 h at 4°C, 72 h on the autosampler (4°C), and after three freeze-thaw cycles at −20°C. On the day of analysis, internal standard was added to each specimen and analyzed as described. Analyte autosampler stability was assessed by re-injecting QC specimens after 72 h, and comparing calculated concentrations to values obtained against the original calibration curve.
2.14. Dilution integrity
Dilution integrity was evaluated by diluting a fortified urine sample (n=3) containing all analytes at 400 µg/l 1:20 (v/v). Internal standards were added and samples extracted as described. Dilution integrity was maintained if specimens quantified within ± 20% of 20 µg/l.
2.15. Carry-over
Carry-over was investigated in triplicate by injecting extracted blank urine samples containing internal standards immediately after samples containing target analytes at 400 µg/l. Blank urine specimens could not meet LOD criteria to document absence of carryover.
2.16. Authentic specimens
Anonymous, randomly collected authentic urine specimens were analyzed to assess method utility.
3. RESULTS
3.1. Chromatography
Baseline chromatographic resolution between all analytes was not possible; all isobaric compounds were baseline resolved except for isomeric hydroxypentyl compounds and JWH-019, JWH-122, JWH-019 hydroxyhexyl and JWH-122 N-hydroxypentyl. Although, JWH-019, JWH-122 and JWH-019 hydroxyhexyl, JWH-122 hydroxypentyl isobaric pairs were not chromatographically baseline resolved, specific product ions differentiated the isobars. We added JWH-018 N-5-hydroxypentyl, JWH-019 N-6-hydroxyhexyl, JWH-073 N-4-hydroxybutyl, JWH-081 N-5-hydroxypentyl, JWH-122 N-5-hydroxypentyl, JWH-210 N-5-hydroxypentyl, JWH-250 N-5-hydroxypentyl, JWH-398 N-5-hydroxypentyl, AM2201 N-4-hydroxypentyl, MAM2201 N-4-hydroxypentyl, RCS-4 N-5-hydroxypentyl and UR-144 N-5-hydroxypentyl alkyl hydroxy metabolite standards, but since isomeric baseline separation was not possible, we can only identify these peaks as alkyl hydroxy metabolites without assigning hydroxy position on the pentyl chain. We employed ± 0.1 min as a peak identification retention time requirement based upon our observations of calibrator retention time drift during method development. Retention times did not drift by more than ± 0.05 min, but we employed a wider ± 0.1 min retention time window requirement because larger retention time variation is expected with authentic specimen analysis over time.
3.2. Evaluation of potential sample preparation approaches
For simplicity sake, we randomly selected two parent compounds and two metabolites for screening sample preparation approaches before method validation for all analytes. Resprep C18 and Strata C8 columns provided efficient recoveries for synthetic cannabinoid metabolites (83.1–98.7% recovery), but less than 27.3% synthetic cannabinoid parent analyte recovery from urine (Supplementary Table 2). SLE achieved 61.3–103.3% extraction efficiencies for all our urinary synthetic cannabinoid analytes of interest (Supplementary Table 2). Matrix effect was −14.0–6.0 for all sample preparations.
3.3. Hydrolysis optimization
We previously evaluated synthetic cannabinoid hydrolysis containing JWH-018, JWH-073, JWH-122, JWH-210, JWH-250, AM2201 and RCS-4 metabolites [8], but we wanted to verify optimal hydrolysis conditions for the larger urine specimen volume required in this method. We did not have fresh authentic specimens during hydrolysis optimization, therefore, we confirmed equivalent JWH-018 N-5-hydroxypentyl-glucuronide hydrolysis efficiency as observed during hydrolysis optimization for our previous qualitative method. We found that addition of 300 µL 400 mM ammonium acetate, pH 4.0, 40 µL 100,000 units beta-glucuronidase/ml and hydrolysis at 55°C for 2 h achieved optimal JWH-018 N-5-hydroxypentyl-glucuronide hydrolysis (>95.8% conversion to un-conjugated JWH-018 N-hydroxypentyl metabolite).
3.4. Specificity
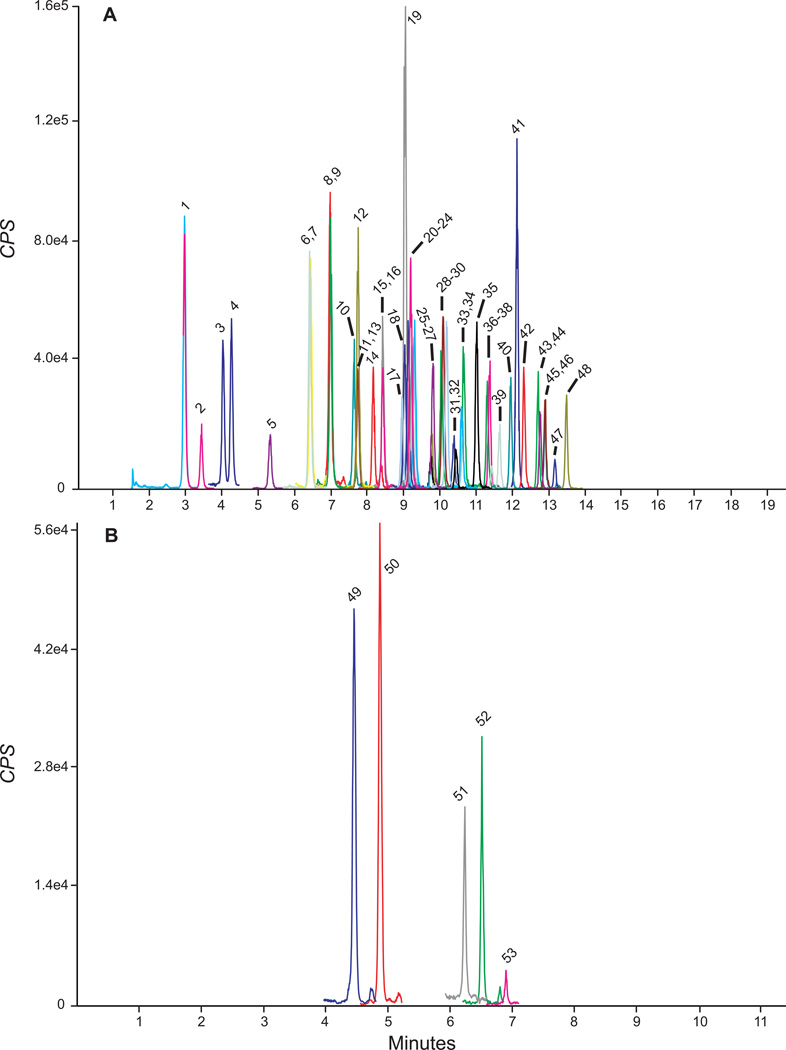
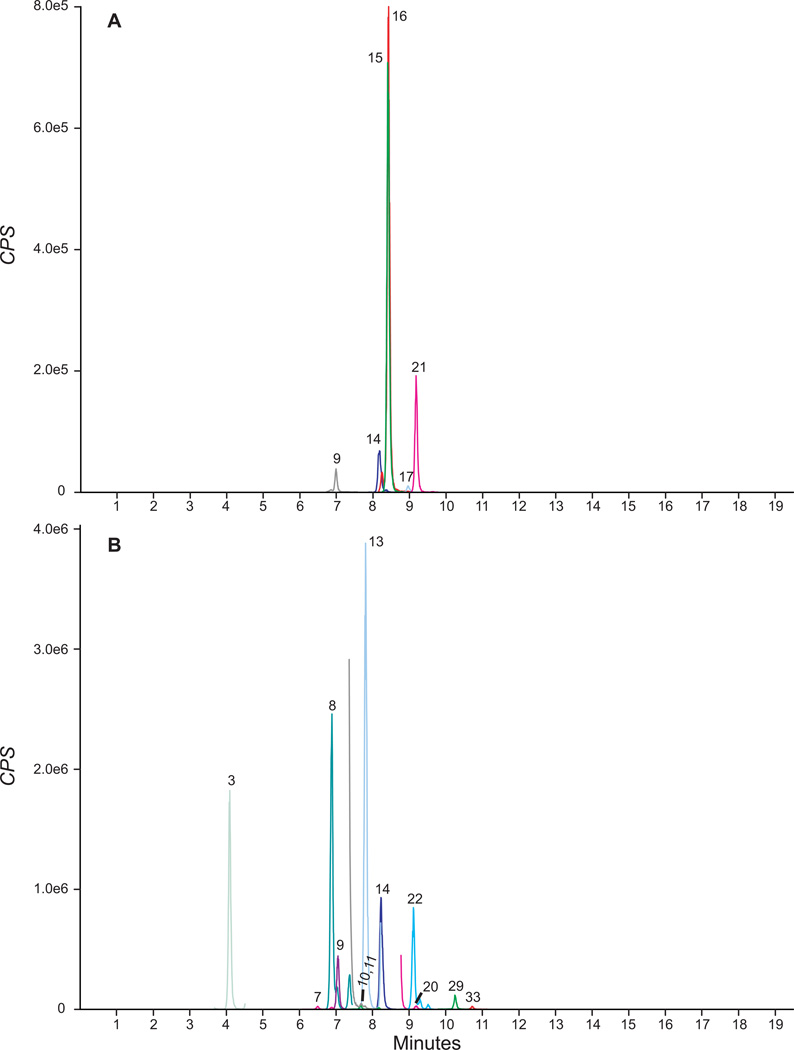
Urine samples from ten synthetic cannabinoid-abstinent individuals contained no peaks fulfilling LOD criteria. None of the 83 potential exogenous interferences fortified at 500 µg/l into low QC samples produced transition ratio or quantification criteria failure. MRM ion chromatograms from a blank urine specimen, a blank urine specimen fortified at low QC concentrations and anonymous, random urine specimens are depicted in Figures 1 and 2. No synthetic cannabinoid parent analytes or hydroxyindole metabolites fortified into low QCs at 20 µg/l interfered with any other analytes. JWH-250 N-5-carboxypentyl interfered with JWH-210 5-hydroxyindole when fortified into low QCs at >100 µg/l; no other hydoxypentyl or pentanoic metabolites interfered with other analyte low QC quantification when fortified at 200 µg/l.
Figure 1.
Extracted ion chromatograms showing quantification MRMs in blank urine fortified at low quality control concentrations (0.3 – 1.5 µg/l). Panels A and B are positive and negative mode injections, respectively. See Table 1 for peak numbering information and Table 3 for quality control concentrations.
Figure 2.
Extracted ion chromatograms showing quantification MRMs in authentic urine specimens containing A) 11.3, 18.3, 0.9, 1.8, 0.3 and 3.9 µg/l JWH-018 N-hydroxypentyl, JWH-018 N-pentanoic acid, JWH-250 N-5-carboxypentyl, AM2201 N-hydroxypentyl, MAM2201 N-hydroxypentyl and MAM2201 N-pentanoic acid, respectively, B) 0.2, 0.7, 0.2, 45.5, 6.7, 0.2, 1.4, 5.0, 10.2, 0.3, 36.4 and 0.2 µg/l JWH-018 5-hydroxyindole, JWH-018 6-hydroxyindole, JWH-073 N-hydroxybutyl, JWH-073 N-butanoic acid, JWH-122 N-hydroxypentyl, JWH-250 5-hydroxyindole, JWH-250 N-hydroxypentyl, JWH-250 N-5-carboxypentyl, AM2201 N-hydroxypentyl, RCS-4 N-5-carboxypentyl, RCS-4 M9 metabolite and UR-144 N-hydroxypentyl, respectively. Specimen B also contained 138.4 and 211.6 µg/l JWH-018 N-hydroxypentyl and JWH-018 N-pentanoic acid (determined after diluted re-analysis, data not shown). See Table 1 for peak numbering.
3.5. Sensitivity and linearity
Initial experiments were conducted with six sets of calibration curves fit via unweighted linear least squares and linear least squares with 1/x and 1/x2 weighting factor to identify the most appropriate calibration model. Inspection of residuals indicated linear least squares with 1/x2 weighting factor produced the best fit for the calibration data. All correlation coefficients exceeded 0.994 (Table 3).
Table 3 details LOD, LOQ, linearity and mean calibration results. LOD were between 0.05 and 1.0 µg/l; LOQ were between 0.1 and 1.0 µg/l. Assays were linear to 50 µg/l for all analytes except 100 µg/l for JWH-200 5-hydroxyindole, JWH-398 N-pentanoic acid and AM2201 6-hydroxyindole.
3.6. Analytical recovery and imprecision
Analytical recovery and imprecision were evaluated at three concentrations across the linear dynamic range. Analytical recovery in urine ranged from 83.3–118.3% of expected concentrations for intra-day and inter-day analytical recoveries (Table 2). Intra-day and inter-day imprecision were 0.8–9.1 and 4.3–13.5% CV, respectively (Table 2). There were few significant effects of day on most analyte QC concentrations (F4,15 = 0.03–3.02, p>0.05); however, in 25 cases day did have an influence (F4,15 = 3.12–9.98, p<0.05) for JWH-250 carboxypentyl, AM694 and RCS-4 carboxypentyl low QCs; JWH- 019 5-hydroxyindole, JWH-203, JWH-210 5-hydroxyindole, JWH-398, RCS8, UR-144 N-hydroxypentyl, UR-144 N-pentanoic acid, XLR11 and CP 47,497-C8 mid QCs and JWH-018 6-hydroxyindole, JWH-019 N-hydroxyhexyl, JWH-203, JWH-210 5-hydroxyindole, JWH-250 N-hydroxypentyl, JWH-250 N-5-carboxypentyl, JWH-398, AM2201, RCS-4, RCS-4 N-5-carboxypentyl, RCS8, CP 47,497-C8 and HU210 high QCs.
3.7. Extraction efficiency and matrix effect
Extraction efficiencies and matrix effects for synthetic cannabinoids in urine are presented in Table 4. Mean extraction efficiencies were 43.7–109.3% (n=10). Mean matrix effects (% suppressed signal) were −73.1–51.7% (n=10, Table 4).
Table 4.
Mean extraction efficiencies and matrix effects for synthetic cannabinoids and metabolites extracted from urine by supported-liquid extraction.
| Analyte | Low QC (µg/l) |
Extraction efficiency (%, N = 10) |
Matrix effect (% of signal suppressed, N = 10) |
||
|---|---|---|---|---|---|
| Low | High | Low | High | ||
| JWH-018 | 0.6 | 65.1% | 59.6% | −8.1% | −11.6% |
| JWH-018 5-hydroxyindole | 0.3 | 89.8% | 92.5% | 22.6% | 7.3% |
| JWH-018 6-hydroxyindole | 0.3 | 89.7% | 93.9% | 20.6% | 6.5% |
| JWH-018 N-hydroxypentyl | 0.3 | 94.4% | 96.1% | 30.8% | 12.3% |
| JWH-018 N-pentanoic acid | 0.3 | 93.5% | 97.5% | 35.1% | 16.4% |
| JWH-019 | 0.3 | 66.9% | 58.9% | −15.4% | −19.1% |
| JWH-019 5-hydroxyindole | 0.3 | 94.3% | 90.5% | 16.5% | 6.7% |
| JWH-019 N-hydroxyhexyl | 0.3 | 88.1% | 91.0% | 28.4% | 12.5% |
| JWH-073 | 0.3 | 62.5% | 60.0% | 16.1% | 6.7% |
| JWH-073 5-hydroxyindole | 0.3 | 91.8% | 93.9% | 23.6% | 7.8% |
| JWH-073 6-hydroxyindole | 0.3 | 92.2% | 93.1% | 25.3% | 10.3% |
| JWH-073 N-hydroxybutyl | 0.3 | 92.5% | 95.4% | 31.4% | 14.9% |
| JWH-073 N-butanoic acid | 0.3 | 96.1% | 99.4% | 33.4% | 16.6% |
| JWH-081 | 0.3 | 75.9% | 68.7% | −10.4% | −15.1% |
| JWH-081 N-hydroxypentyl | 0.3 | 90.9% | 94.1% | 31.2% | 13.3% |
| JWH-122 | 0.3 | 70.7% | 61.3% | −17.8% | −20.0% |
| JWH-122 N-hydroxypentyl | 0.3 | 89.1% | 93.6% | 42.7% | 11.0% |
| JWH-200 | 0.3 | 96.0% | 94.6% | 24.1% | 10.3% |
| JWH-200 5-hydroxyindole | 1.5 | 92.9% | 97.7% | 27.3% | 10.0% |
| JWH-200 6-hydroxyindole | 0.3 | 94.3% | 95.1% | 25.0% | 9.8% |
| JWH-203 | 1.5 | 67.4% | 52.9% | 11.8% | 11.0% |
| JWH-210 | 0.3 | 72.5% | 58.1% | −24.8% | −23.1% |
| JWH-210 5-hydroxyindole | 0.6 | 92.9% | 91.7% | 21.9% | 3.1% |
| JWH-210 N-hydroxypentyl | 0.3 | 92.4% | 93.9% | 36.4% | 14.9% |
| JWH-210 N-5-carboxypentyl | 0.3 | 99.2% | 98.8% | 44.4% | 23.4% |
| JWH-250 | 0.6 | 63.4% | 62.9% | 14.9% | 5.3% |
| JWH-250 5-hydroxyindole | 0.3 | 92.3% | 95.1% | 29.0% | 12.9% |
| JWH-250 N-hydroxypentyl | 0.6 | 91.5% | 99.7% | 51.7% | 23.3% |
| JWH-250 N-5-carboxypentyl | 0.6 | 85.3% | 94.7% | 41.8% | 22.1% |
| JWH-398 | 1.5 | 70.8% | 55.1% | −27.2% | −27.9% |
| JWH-398 N-hydroxypentyl | 1.5 | 84.4% | 89.5% | 26.5% | 7.7% |
| JWH-398 N-pentanoic acid | 1.5 | 96.6% | 96.5% | 35.5% | 13.8% |
| AM2201 | 0.3 | 76.1% | 72.4% | 8.9% | 0.8% |
| AM2201 6-hydroxyindole | 1.5 | 89.3% | 94.3% | 29.9% | 14.5% |
| AM2201 N-hydroxypentyl | 0.3 | 93.8% | 97.6% | 26.4% | 12.5% |
| MAM2201 | 0.3 | 77.3% | 73.8% | 45.5% | 26.6% |
| MAM2201 N-hydroxypentyl | 0.3 | 90.2% | 95.8% | 34.1% | 14.5% |
| MAM2201 N-pentanoic acid | 0.6 | 94.3% | 98.0% | 31.8% | 16.7% |
| AM694 | 0.3 | 77.2% | 78.6% | 25.9% | 8.2% |
| RCS-4 | 0.3 | 62.9% | 61.4% | 9.7% | 0.7% |
| RCS-4 N-hydroxypentyl | 0.6 | 95.8% | 97.4% | 37.5% | 21.4% |
| RCS-4 N-5-carboxypentyl | 0.6 | 88.4% | 94.3% | 40.0% | 18.4% |
| RCS-4 M9 metabolite | 0.6 | 93.8% | 99.1% | 46.6% | 24.8% |
| RCS-4 M10 metabolite | 0.6 | 94.3% | 98.9% | 45.2% | 23.6% |
| RCS8 | 0.3 | 74.6% | 63.7% | −12.0% | −14.9% |
| UR-144 N-hydroxypentyl | 0.3 | 95.0% | 92.6% | 30.2% | 17.1% |
| UR-144 N-pentanoic acid | 0.6 | 94.9% | 96.0% | 31.3% | 14.3% |
| XLR11 | 0.6 | 57.3% | 55.4% | 5.0% | −0.8% |
| CP 47,497-C7 a | 1.5 | 86.7% | 82.3% | −34.4% | −41.1% |
| CP 47,497-C7-hydroxy metabolite a | 1.5 | 91.0% | 89.7% | −54.1% | −59.2% |
| CP 47,497-C8 a | 1.5 | 92.9% | 80.9% | −41.4% | −43.0% |
| CP 47,497-C8-hydroxy metabolite a | 1.5 | 92.5% | 85.0% | −44.8% | −50.6% |
| HU210 a | 1.5 | 92.6% | 88.7% | −68.6% | −73.1% |
| JWH-018-d9 | 69.8% | 64.9% | −6.6% | −13.7% | |
| JWH-018 5-hydroxyindole-d9 | 94.3% | 97.9% | 17.8% | 5.9% | |
| JWH-018 6-hydroxyindole-d9 | 95.0% | 103.9% | 26.9% | 9.5% | |
| JWH-018 N-hydroxypentyl-d5 | 100.2% | 107.0% | 29.0% | 16.1% | |
| JWH-073-d7 | 64.1% | 65.2% | 23.1% | 9.4% | |
| JWH-073 5-hydroxyindole-d7 | 94.6% | 102.8% | 24.0% | 8.5% | |
| JWH-073 6-hydroxyindole-d7 | 96.9% | 102.1% | 21.9% | 11.0% | |
| JWH-073 N-hydroxybutyl-d5 | 97.1% | 107.6% | 29.7% | 13.4% | |
| JWH-073 N-butanoic acid-d5 | 99.0% | 104.7% | 34.8% | 18.0% | |
| JWH-081-d9 | 78.9% | 74.2% | −9.3% | −13.8% | |
| JWH-122-d9 | 72.3% | 66.6% | −15.9% | −19.2% | |
| JWH-122 N-hydroxypentyl-d5 | 94.8% | 102.9% | 32.8% | 15.2% | |
| JWH-200-d5 | 99.8% | 105.3% | 19.5% | 7.4% | |
| JWH-210-d9 | 78.1% | 63.3% | −24.0% | −23.0% | |
| JWH-250-d5 | 69.7% | 71.9% | 22.4% | 11.2% | |
| JWH-398-d9 | 72.6% | 61.7% | −22.6% | −22.3% | |
| AM2201-d5 | 76.2% | 77.3% | 10.6% | −4.1% | |
| AM2201 N-hydroxypentyl-d5 | 96.6% | 109.3% | 30.8% | 12.6% | |
| RCS-4-d9 | 64.2% | 66.1% | 11.1% | −0.1% | |
| UR-144-d5 | 53.9% | 44.8% | −15.3% | −12.0% | |
| XLR11-d5 | 60.4% | 59.6% | 5.4% | −1.2% | |
| CP 47,497-C7-d11 a | 92.7% | 92.1% | −42.4% | −47.5% | |
| CP 47,497-C8-d7 a | 90.5% | 89.7% | −41.2% | −44.5% | |
| 11-nor-9-carboxy-tetrahydrocannabinol-d9 a | 82.3% | 84.4% | −52.7% | −60.2% | |
Data acquired in negative ionization mode
High quality control concentrations were 30 µg/l; deuterated internal standard concentrations were 1 µg/l.
3.8. Analyte stability, dilution integrity and carryover
Analytes at low, mid and high QC concentrations in urine extracts were stable for 72 h at 4°C in the autosampler, n=4 (data not shown). All synthetic cannabinoid metabolites at low and high QC concentrations (n=3) were stable for 16 h at room temperature; all parent analytes were unstable except JWH-200, CP 47,497-C7, CP 47,497-C8 and HU210 (Table 5). All analytes were stable for 72 h at 4°C (Table 5). All synthetic cannabinoid metabolites at low and high QC concentrations (n=3) were stable after three freeze/thaw cycles; all parent analytes were unstable except JWH-200, JWH-398, AM694, CP 47,497-C7, CP 47,497-C8 and HU210 (Table 5).
Table 5.
Synthetic cannabinoids and metabolites stabilities in human urine.
| Analyte | Low QC µg/l |
16 h room temperature (% target, n=3) |
72 h 4°C (% target, n=3) |
3 freeze and thaw cycles (% target, n=3) |
|||
|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | ||
| JWH-018 | 0.6 | 65.0% | 73.4% | 86.7% | 93.9% | 76.7% | 79.0% |
| JWH-018 5-hydroxyindole | 0.3 | 93.3% | 84.9% | 90.0% | 89.4% | 96.7% | 85.0% |
| JWH-018 6-hydroxyindole | 0.3 | 84.4% | 83.5% | 92.2% | 85.5% | 90.0% | 82.9% |
| JWH-018 N-hydroxypentyl | 0.3 | 91.1% | 86.3% | 86.7% | 85.4% | 92.2% | 84.3% |
| JWH-018 N-pentanoic acid | 0.3 | 115.6% | 110.5% | 108.9% | 109.0% | 114.4% | 106.2% |
| JWH-019 | 0.3 | 56.7% | 71.4% | 81.1% | 95.6% | 73.3% | 76.4% |
| JWH-019 5-hydroxyindole | 0.3 | 88.9% | 84.7% | 92.2% | 90.3% | 84.4% | 84.1% |
| JWH-019 N-hydroxyhexyl | 0.3 | 93.3% | 90.7% | 95.6% | 98.7% | 100.0% | 88.7% |
| JWH-073 | 0.3 | 72.2% | 70.2% | 82.2% | 85.0% | 70.0% | 74.3% |
| JWH-073 5-hydroxyindole | 0.3 | 90.0% | 89.7% | 95.6% | 97.1% | 98.9% | 87.7% |
| JWH-073 6-hydroxyindole | 0.3 | 87.8% | 82.2% | 92.2% | 83.8% | 87.8% | 84.1% |
| JWH-073 N-hydroxybutyl | 0.3 | 101.1% | 91.0% | 100.0% | 91.1% | 101.1% | 86.2% |
| JWH-073 N-butanoic acid | 0.3 | 105.6% | 102.8% | 104.4% | 101.6% | 111.1% | 93.3% |
| JWH-081 | 0.3 | 67.8% | 70.7% | 86.7% | 94.7% | 74.4% | 77.2% |
| JWH-081 N-hydroxypentyl | 0.3 | 97.8% | 93.2% | 101.1% | 101.2% | 107.8% | 91.7% |
| JWH-122 | 0.3 | 62.2% | 70.6% | 88.9% | 99.8% | 72.2% | 75.2% |
| JWH-122 N-hydroxypentyl | 0.3 | 92.2% | 86.4% | 87.8% | 90.9% | 91.1% | 85.1% |
| JWH-200 | 0.3 | 90.0% | 93.1% | 90.0% | 94.8% | 90.0% | 87.7% |
| JWH-200 5-hydroxyindole | 1.5 | 107.3% | 105.1% | 106.7% | 104.4% | 109.1% | 106.5% |
| JWH-200 6-hydroxyindole | 0.3 | 103.3% | 105.7% | 102.2% | 105.5% | 105.6% | 105.5% |
| JWH-203 | 1.5 | 76.4% | 65.8% | 84.2% | 92.3% | 82.0% | 77.8% |
| JWH-210 | 0.3 | 57.8% | 69.6% | 87.8% | 97.2% | 74.4% | 82.6% |
| JWH-210 5-hydroxyindole | 0.6 | 85.6% | 85.0% | 91.1% | 99.0% | 101.7% | 84.3% |
| JWH-210 N-hydroxypentyl | 0.3 | 92.2% | 83.3% | 90.0% | 85.9% | 90.0% | 81.8% |
| JWH-210 N-5-carboxypentyl | 0.3 | 94.4% | 93.8% | 98.9% | 98.2% | 105.6% | 92.9% |
| JWH-250 | 0.6 | 78.3% | 77.3% | 88.9% | 90.3% | 76.7% | 80.8% |
| JWH-250 5-hydroxyindole | 0.3 | 97.8% | 90.5% | 98.9% | 90.9% | 101.1% | 91.2% |
| JWH-250 N-hydroxypentyl | 0.6 | 108.3% | 86.3% | 100.6% | 88.5% | 106.7% | 82.3% |
| JWH-250 N-5-carboxypentyl | 0.6 | 107.2% | 100.7% | 107.2% | 101.1% | 111.7% | 95.1% |
| JWH-398 | 1.5 | 57.3% | 70.5% | 82.2% | 98.6% | 80.9% | 85.2% |
| JWH-398 N-hydroxypentyl | 1.5 | 88.4% | 85.6% | 90.7% | 94.1% | 89.3% | 85.7% |
| JWH-398 N-pentanoic acid | 1.5 | 95.8% | 85.4% | 92.9% | 89.3% | 99.3% | 85.2% |
| AM2201 | 0.3 | 85.6% | 76.4% | 92.2% | 92.8% | 82.2% | 78.5% |
| AM2201 6-hydroxyindole | 1.5 | 99.8% | 93.4% | 106.9% | 95.4% | 104.0% | 92.9% |
| AM2201 N-hydroxypentyl | 0.3 | 87.8% | 92.8% | 90.0% | 93.6% | 92.2% | 86.7% |
| MAM2201 | 0.3 | 70.0% | 72.1% | 84.4% | 84.5% | 70.0% | 77.0% |
| MAM2201 N-hydroxypentyl | 0.3 | 95.6% | 96.3% | 94.4% | 102.6% | 96.7% | 93.1% |
| MAM2201 N-pentanoic acid | 0.6 | 102.2% | 97.7% | 101.1% | 103.9% | 104.4% | 95.2% |
| AM694 | 0.3 | 78.9% | 81.6% | 87.8% | 107.4% | 82.2% | 84.6% |
| RCS-4 | 0.3 | 80.0% | 72.5% | 96.7% | 86.0% | 70.0% | 72.9% |
| RCS-4 N-hydroxypentyl | 0.6 | 101.1% | 92.4% | 99.4% | 96.5% | 99.4% | 88.4% |
| RCS-4 N-5-carboxypentyl | 0.6 | 107.2% | 103.9% | 108.3% | 105.7% | 111.7% | 97.0% |
| RCS-4 M9 metabolite | 0.6 | 105.6% | 101.9% | 100.6% | 105.1% | 103.3% | 97.5% |
| RCS-4 M10 metabolite | 0.6 | 102.8% | 101.6% | 99.4% | 103.0% | 102.2% | 97.0% |
| RCS8 | 0.3 | 56.7% | 67.4% | 85.6% | 88.9% | 65.6% | 79.4% |
| UR-144 N-hydroxypentyl | 0.3 | 86.7% | 89.8% | 86.7% | 88.9% | 83.3% | 84.9% |
| UR-144 N-pentanoic acid | 0.6 | 106.1% | 101.9% | 103.3% | 102.0% | 109.4% | 93.6% |
| XLR11 | 0.6 | 76.1% | 79.2% | 85.0% | 92.4% | 77.8% | 80.1% |
| CP 47,497-C7 | 1.5 | 84.4% | 84.7% | 82.9% | 98.9% | 80.9% | 84.3% |
| CP 47,497-C7-hydroxy metabolite | 1.5 | 101.3% | 111.7% | 108.2% | 113.5% | 95.6% | 98.9% |
| CP 47,497-C8 | 1.5 | 85.6% | 87.9% | 89.3% | 96.2% | 83.3% | 89.3% |
| CP 47,497-C8-hydroxy metabolite | 1.5 | 103.1% | 107.2% | 103.6% | 113.1% | 97.1% | 97.2% |
| HU210 | 1.5 | 88.4% | 83.7% | 93.6% | 95.7% | 91.6% | 85.9% |
Bold type indicates >20% analyte loss
Dilution integrity was acceptable (within ± 20% of expected diluted concentration) for all analytes after diluting a sample containing analytes at 400 µg/l 1:20 with blank urine.
There was no evidence of carryover for synthetic cannabinoids. Negative specimens injected after samples containing analytes at 400 µg/l did not have analyte peaks satisfying assay LOD criteria (n=3).
3.9. Demonstration of method applicability
The method was applied to measurement of synthetic cannabinoids and metabolites in anonymous, randomly collected urine specimens (Figure 2).
4. DISCUSSION
Despite initial DEA scheduling of synthetic cannabinoids in March 2011, abuse is an ongoing problem, with the 2012 Monitoring the Future survey reporting that 11.3% of 12th graders ingested synthetic cannabinoids in the past year [18]. Another recent survey of undergraduate students at a southeastern university found 14% students reported lifetime synthetic cannabinoid use [19]. A validated and sensitive LC-MS/MS method for simultaneously quantifying synthetic cannabinoids and metabolites in urine is necessary for confirming intake by clinical and forensic laboratories. Few quantitative urinary synthetic cannabinoids methods exist; most published LC-MS/MS quantitative methods target JWH-018 and JWH-073 metabolites after β-glucuronidase hydrolysis [11–16]. We present a fully validated and sensitive quantitative LC-MS/MS method for simultaneously measuring the most comprehensive synthetic cannabinoid panel to-date in urine. This quantitative synthetic cannabinoid method is useful for evaluating cutoff concentrations to optimally document synthetic cannabinoid intake, for determining windows of synthetic cannabinoid detection and identifying optimal synthetic cannabinoid analytes for documenting recent intake.
Our goal during validation of the current method was to assess whether more β-glucuronidase enzyme was required to achieve similarly optimal hydrolysis as this method requires a larger 200 µL urine volume compared to 100 µL urine for the previous validated synthetic cannabinoid screening method [8]. We evaluated JWH-018 N-5-hydroxypentyl-glucuronide, the only synthetic cannabinoid glucuronide metabolite reference standard commercially available at the time of method development, for evaluating the effect of increasing urine volume on hydrolysis efficiency, as we did not have fresh authentic specimens. Using the current method’s hydrolysis conditions achieved similar JWH-018 N-5-hydroxypentyl-glucuronide hydrolysis as observed during development of the previous qualitative synthetic cannabinoid assay inferring that the current conditions would also achieve optimal hydrolysis of JWH-073, JWH-122, JWH-210, JWH-250, AM2201 and RCS-4 metabolites. It is unknown whether these hydrolysis conditions are optimal for other synthetic cannabinoid glucuronide conjugates.
Previously, de Jager et al. presented a synthetic cannabinoid urinary method targeting JWH-018 N-5-hydroxypentyl, JWH-018 N-pentanoic acid, JWH-019 5-hydroxyindole, JWH-073 N-4-hydroxybutyl, JWH-073 N-butanoic acid, JWH-122 N-5-hydroxypentyl, JWH-200 5-hydroxyindole, JWH-250 5-hydroxyindole, JWH-250 N-5-carboxypentyl, JWH-398 N-hydroxypentyl and RCS-4 N-5-hydroxypentyl metabolites [16]. 500 µL urine was hydrolyzed with β-glucuronidase prior to liquid-liquid extraction and analysis on an ABSciex 5500 QTRAP LCMSMS achieving linear ranges of 0.1–10 µg/l. Our current LCMSMS method achieves similar LLOQ with increased linearity to 50 or 100 µg/l with only a 200 µL urine sample. Other methods targeting only JWH-018 and JWH-073 metabolites achieved LLOQ of 0.1–1.8 µg/l, similar or lower LLOQ than our current method but only 2–14 analytes [11–15]. Two of the previous methods employed 1 and 2 ml sample volumes, 5–10 fold larger than our method [11,12], with Chimalakonda et al. and Yanes et al. only requiring 40 and 100 µL urine, respectively [13,15].
Our method was unable to achieve baseline chromatographic resolution for alkyl hydroxy metabolite isomers (i.e. JWH-018 N-2, N-3, N-4, and N-5-hydroxypentyl compounds have similar retention times) providing semi-quantitative total alkyl hydroxy metabolite concentrations for any synthetic cannabinoid. This is a potential limitation for distinguishing the synthetic cannabinoid ingested, as AM2201 metabolically forms JWH-018 N-5-hydroxypentyl with minimal JWH-018 N-4-hydroxypentyl, and JWH-018 forms less JWH-018 N-5-hydroxypentyl than JWH-018 N-4-hydroxypentyl. Our quantitative method includes the AM2201 specific metabolites AM2201 N-4-hydroxypentyl and AM2201 6-hydroxyindole, but these compounds are less abundant than JWH-018 N-5-hydroxypentyl after AM2201 intake. We added JWH-018 N-5-hydroxypentyl, JWH-019 N-6-hydroxyhexyl, JWH-073 N-4-hydroxybutyl, JWH-081 N-5-hydroxypentyl, JWH-122 N-5-hydroxypentyl, JWH-210 N-5-hydroxypentyl, JWH-250 N-5-hydroxypentyl, JWH-398 N-5-hydroxypentyl, AM2201 N-4-hydroxypentyl, MAM2201 N-4-hydroxypentyl, RCS-4 N-5-hydroxypentyl and UR-144 N-5-hydroxypentyl alkyl hydroxy metabolite standards, but since isomeric separation was not possible, we can only identify these peaks as alkyl hydroxy metabolites without assigning hydroxy position on the side chain.
We opted to include parent analytes, even though metabolites predominate in urine [20], to evaluate if parent analytes assist determining recent intake. This complicated sample preparation during method development, observing low recoveries of nonpolar parent analytes (<27%) with traditional C8 and C18 reverse phase solid phase extraction columns; SLE+ achieved >44% parent analyte recoveries.
Poor availability of analytical reference standards impedes method development for synthetic cannabinoids and other emerging drugs of abuse. Deuterated analogs compensate for variable analyte recovery or matrix effect enabling accurate quantification. Commercially available deuterated internal standards were utilized when available; deuterated analytes with similar functional groups and nearest retention time were utilized when matched deuterated internal standards were unavailable. There was one exception for this internal standard selection scheme; we selected deuterated JWH-018 parent analyte as an internal standard for JWH-210 5-hydroxyindole after observing poor QC performance with JWH-018 5-hydroxyindole-d9 as internal standard. JWH-018-d9 co-elutes with JWH-210 5-hydroxyindole better accounting for matrix effect.
ANOVA revealed 25 of 159 cases where statistically significant between-batch differences in measured QC concentration occurred. The explanation for these differences is unknown, possibly due to variable matrix effect, extraction efficiencies, or day-to-day variability in fortifying calibrators and QCs. Matched deuterated internal standards were not available for 16 of 25 cases that may explain between-day variability for these analytes. Although statistically significant, these effects are not likely clinically relevant since all QC concentrations were within 80–120% of target and maximum differences between days was 22.8%.
We are uncertain why >100 µg/l JWH-250 N-5-carboxypentyl concentrations interfered with JWH-210 5-hydroxyindole quantification at 0.6 µg/l. JWH-210 5-hydroxyindole ion ratio criteria were within 80–120% of target, but the low QC quantified at 193% of target. Interference appears unlikely because retention times differ by 5.2 min and parent masses by 20 amu for these two analytes. It also seems unlikely that JWH-250 N-5-carboxypentyl would be converted to JWH-210 5-hydroxyindole during sample preparation or analysis. JWH-250 N-5-carboxypentyl standard may have a trace JWH-210 5-hydroxyindole impurity. False positive results could occur when both JWH-250 N-5-carboxypentyl and JWH-210 5-hydroxyindole co-occur in specimens, presence of JWH-210 N-hydroxypentyl and/or JWH-210 N-5-carboxypentyl is required for documenting JWH-210 intake.
All analytes were stable under all evaluated conditions except most parent analytes showed >20% loss after 16 h at room temperature or after three freeze/thaw cycles in fortified urine. Similarly, Dresen et al. showed >15% JWH-073, JWH-081 and methandamide decreases in fortified serum after 72 h [21] and we previously showed THC instability in urine after 16h at room temperature [22]. Instability appears to be related to analyte polarity with more polar JWH-200, CP 47,497-C7, CP 47,497-C8 and HU210 being stable while other parent analytes were unstable. Parent analyte instability may partially explain not detecting parent synthetic cannabinoids in urine [20].
We present a fully-validated quantitative LC-MS/MS method for the most comprehensive synthetic cannabinoid urine method to-date targeting 53 analytes: JWH-018, JWH-019, JWH-073, JWH-081, JWH-122, JWH-200, JWH-210, JWH-250, JWH-398, RCS-4, AM2201, MAM2201, UR-144, CP 47,497-C7, CP 47,497-C8 and their metabolites, and JWH-203, AM694, RCS8, XLR11 and HU210 parent compounds. 200 µL urine was hydrolyzed with β-glucuronidase before SLE extraction. Two injections were required to acquire data for 53 analytes; 4 µL positive mode injection with a 19.5 min runtime for 48 analytes and 25 µL negative mode injection with a 11.4 min runtime for CP 47,497-C7, CP 47,497-C7 hydroxy metabolite, CP 47,497-C8, CP 47,497-C8 hydroxy metabolite and HU210. This method should accommodate new synthetic cannabinoids as certified reference standards become available. Inclusion of new analytes requires method re-validation for the new analytes; specificity, sensitivity, linearity, imprecision, analytical recovery, extraction efficiency, matrix effect, stability, dilution integrity and carry-over would need to be evaluated for new analytes. However, use of broad spectrum SLE+ sample preparation and scheduled MRM methodologies should eliminate re-optimization of sample preparation and most instrument settings.
Supplementary Material
Highlights.
Most comprehensive urine assay for 20 synthetic cannabinoids and 33 metabolites.
Enables cutoff concentration evaluation to optimally detect synthetic cannabinoids
Useful for determining best analytes to document synthetic cannabinoid intake
The method enables rapid addition of new synthetic cannabinoids.
ACKNOWLEDGMENT
The authors would like to recognize Hua-Fen Liu, Xiaohong Chen and Sumandeep Rana’s advice during method development. This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Substance Abuse and Mental Health Services Administration. Drug abuse warning network, 2011: national estimates of drug-related emergency department visits. Rockville, MD: 2013. [PubMed] [Google Scholar]
- 2.Wikstrom M, Thelander G, Dahlgren M, Kronstrand R. J Anal Toxicol. 2013;37:43. doi: 10.1093/jat/bks086. [DOI] [PubMed] [Google Scholar]
- 3.Young AC, Schwarz E, Medina G, Obafemi A, Feng SY, Kane C, Kleinschmidt K. Am J Emerg Med. 2012;30 doi: 10.1016/j.ajem.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Drug Enforcement Agency, United States Department of Justice. Fed Regist. 2011;76:11075. [Google Scholar]
- 5.Drug Enforcement Agency. United States Department of Justice. Fed Regist. 2013;78:664. [Google Scholar]
- 6.Drug Enforcement Agency. United States Department of Justice. Fed Regist. 2013;78:28735. [Google Scholar]
- 7.Guale F, Shahreza S, Walterscheid JP, Chen HH, Arndt C, Kelly AT, Mozayani A. J Anal Toxicol. 2013;37:17. doi: 10.1093/jat/bks084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wohlfarth A, Scheidweiler KB, Chen XH, Liu HF, Huestis MA. Anal Chem. 2013;85:3730. doi: 10.1021/ac3037365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu AH, Gerona R, Armenian P, French D, Petrie M, Lynch KL. Clin Toxicol (Phila) 2012;50:733. doi: 10.3109/15563650.2012.713108. [DOI] [PubMed] [Google Scholar]
- 10.Wissenbach DK, Meyer MR, Remane D, Philipp AA, Weber AA, Maurer HH. Anal Bioanal Chem. 2011;400:3481. doi: 10.1007/s00216-011-5032-1. [DOI] [PubMed] [Google Scholar]
- 11.Beuck S, Moller I, Thomas A, Klose A, Schlorer N, Schanzer W, Thevis M. Anal Bioanal Chem. 2011;401:493. doi: 10.1007/s00216-011-4931-5. [DOI] [PubMed] [Google Scholar]
- 12.ElSohly MA, Gul W, Elsohly KM, Murphy TP, Madgula VL, Khan SI. J Anal Toxicol. 2011;35:487. doi: 10.1093/anatox/35.7.487. [DOI] [PubMed] [Google Scholar]
- 13.Chimalakonda KC, Moran CL, Kennedy PD, Endres GW, Uzieblo A, Dobrowolski PJ, Fifer EK, Lapoint J, Nelson LS, Hoffman RS, James LP, Radominska-Pandya A, Moran JH. Anal Chem. 2011;83:6381. doi: 10.1021/ac201377m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moran CL, Le VH, Chimalakonda KC, Smedley AL, Lackey FD, Owen SN, Kennedy PD, Endres GW, Ciske FL, Kramer JB, Kornilov AM, Bratton LD, Dobrowolski PJ, Wessinger WD, Fantegrossi WE, Prather PL, James LP, Radominska-Pandya A, Moran JH. Anal Chem. 2011;83:4228. doi: 10.1021/ac2005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanes EG, Lovett DP. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;909:42. doi: 10.1016/j.jchromb.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 16.de Jager AD, Warner JV, Henman M, Ferguson W, Hall A. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;897:22. doi: 10.1016/j.jchromb.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Anal Chem. 2003;75:3019. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 18.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on drug use: 2012 overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan, Ann Arbor; 2013. [Google Scholar]
- 19.Stogner JM, Miller BL. J Subst Use. 2013 in press. [Google Scholar]
- 20.Meyer MR, Peters FT. Ther Drug Monit. 2012;34:615. doi: 10.1097/FTD.0b013e31826d0915. [DOI] [PubMed] [Google Scholar]
- 21.Dresen S, Kneisel S, Weinmann W, Zimmermann R, Auwarter V. J Mass Spec. 2011;46:163. doi: 10.1002/jms.1877. [DOI] [PubMed] [Google Scholar]
- 22.Scheidweiler KB, Desrosiers NA, Huestis MA. Clin Chim Acta. 2012;413:1839. doi: 10.1016/j.cca.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.