Abstract
Assessment of cortisol concentrations in hair is one of the latest innovations for measuring long-term cortisol exposure. We performed a systematic review of correlates of cortisol in human hair to inform the design, analysis and interpretation of future epidemiologic studies. Relevant publications were identified through electronic searches on PubMed, WorldCat, and Web of Science using keywords, “cortisol” “hair” “confounders” “chronic” “stress” and “correlates.” Thirty-nine studies were included in this review. Notwithstanding scarce data and some inconsistencies, investigators have found hair cortisol concentrations to be associated with stress-related psychiatric symptoms and disorders (e.g., PTSD), medical conditions indicating chronic activation of the hypothalamic-pituitary-adrenal axis (e.g., Cushing´s syndrome) and other life situations associated with elevated risk of chronic stress (e.g., shiftwork). Results from some studies suggest that physical activity, adiposity, and substance abuse may be correlates of hair cortisol concentrations. In contrast to measures of short-term cortisol release (saliva, blood, and urine), cigarette smoking and use of oral contraceptives appear to not be associated with hair cortisol concentrations. Studies of pregnant women indicate increased hair cortisol concentrations across successive trimesters. The study of hair cortisol presents a unique opportunity to assess chronic alterations in cortisol concentrations in epidemiologic studies.
Keywords: hair, cortisol, chronic stress, correlates, assessment, analysis, determinants, psychiatric disorders
INTRODUCTION
“It is not stress that kills us; it is our reaction to it.”
-Hans Selye (stress research pioneer)
Overview of the Stress Response
Despite its extensive study, psychosocial stress (hereafter called stress) remains challenging to describe. Investigators acknowledge stress as a process whereby a stimulus imposes demands or overwhelms an organism’s ability to cope, causing physiological and psychological responses[1–3]. The magnitude, duration and quality of these responses are contingent upon the individual´s interpretation of the stressor and how well the individual adapts or copes with the stressor[3].
Two neuroendocrine pathways are activated in stress response: one, the sympathetic-adrenal-medullary axis is activated rapidly–its activation has been described elsewhere[1]. The other, the hypothalamic-pituitary-adrenal (HPA) axis, is activated less swiftly: first, the hypothalamus discharges corticotrophin-releasing hormone, stimulating the dispensation of adrenocorticotropin-releasing hormone by the pituitary into systemic circulation, which then triggers the release of cortisol by the adrenal cortex[1]. Cortisol has emerged as an important objective biological measure of stress.
Cortisol and Health
Cortisol helps maintain homeostasis in the body by aiding metabolism and immune response[4–6]. The human body secretes approximately 10mg of cortisol daily; however, excess or insufficient amounts of cortisol are synthesized and released into systemic circulation under stressful conditions[1, 7–9]. Although acute alterations in cortisol concentrations may not immediately cause disease, long-term alterations of the HPA axis are associated with adverse health outcomes. For example, high concentrations of cortisol have been associated with Cushing’s disease[10], diabetes complications[11], adverse perinatal outcomes[12], and myocardial infarction[13]. Low cortisol concentrations have been associated with Addison’s disease[14], endometriosis[15], chronic pelvic pain[15, 16], and psychiatric disorders including post-traumatic stress disorder (PTSD)[17].
Measuring Chronic Cortisol Concentrations: Traditional Matrices of Cortisol
Saliva, blood, and urine, the predominant matrices from which cortisol is obtained, have been useful in establishing the diurnal profile of circulating cortisol and in elucidating the relationship between aberrations in diurnal cortisol patterns and various disorders. But these matrices have several limitations. First, they only inform about short-term cortisol concentrations, i.e. cortisol released within a few hours or one day[18]. Second, cortisol concentrations obtained using these specimens are easily influenced by a host of factors including study procedures[19], time of the day[20] and food consumption[21]. Third, the process of specimen collection can be invasive, particularly for blood, which in itself may induce stress and corresponding cortisol levels. Fourth, to deal with variability and the need to obtain approximate measurements of chronic cortisol concentrations, researchers need to take repeated measurements during 24 hours for several days[22]. This need for repeat sampling is expensive, burdensome for study participants and likely to increase incomplete sample collection and loss to follow-up. Given these limitations, investigators have searched for matrices that can ensure better, less invasive measurements of long-term cortisol concentrations. The most recent innovation is the isolation of cortisol from hair.
Why Hair? Why Now?
To the best of our knowledge, Raul et al were the first to examine cortisol concentrations in human hair[23]. Previously, three investigative teams[24–26] provided evidence of hair as a matrix for various glucocorticoids—for example, hair was used to detect exposure to corticosteroids, amphetamines and anabolic steroids in athletes[26]. Hair allows for retrospective assessment of long-term cortisol concentrations because it grows over weeks, months and years (e.g., 18 month hair cortisol concentrations were assessed in [27, 28]). Collecting hair is less invasive than obtaining blood and hair can be stored easily (Table 1). The proposed mechanisms by which cortisol is incorporated into hair is beyond the scope of this review. However, we refer interested readers to a highly relevant review on this topic[29].
Table 1.
Comparison of hair with other matrices of cortisol
| Characteristics | Hair | Other Matrices (saliva, blood and urine) |
|---|---|---|
|
Length of cortisol information |
Chronic | Acute |
| Storage | Can be stored at room temperature |
Require specific storage (e.g., refrigeration or freezing) |
|
Situational and intra-individual variability |
Cortisol in hair is not easily influenced by acute situational and individual factors—although evidence suggests that it might be influenced by hair hygiene and cosmetic related behavior (e.g., hair treatment and hair dyeing) |
Measurements are vulnerable to factors such as time of the day, cigarette smoking, and acute stress |
|
Repeated Measurements |
Less need for repeated measurements—although some individuals might be unwilling to give hair |
Need for repeated measurements— expensive, burdensome for study participants and likely to increase incomplete sample collection and loss to follow-up |
| Invasiveness | Small amount of hair is needed; not invasive |
Although saliva and urine are less invasive, obtainment of blood can be invasive and painful for participants. |
Correlations of Hair Cortisol Concentrations with Other Measures of Chronic Stress
In efforts to assess the validity of hair cortisol as a biological marker of chronic stress, investigators have examined the correlations of hair cortisol concentrations (HCC) with cortisol concentrations from repeated samplings of other matrices. Van Holland et al found that HCC was moderately correlated with mean salivary cortisol concentrations taken on three days (r=0.41, p=0.03, samples were taken at six time points on each day)[30]. Similarly, Vanaelst et al found that HCC was significantly correlated with area under the curve for salivary cortisol collected over two consecutive days (samples were taken at four time points on each day)[31]. Statistically significant correlation between 24-hour urinary cortisol concentrations and HCC has been reported[32], though no statistically significant correlations were found between HCC and cortisol in one-time samples of morning blood serum[32] and blood serum collected after an overnight fast[31]. Additionally, investigators have examined correlations of HCC with scores on the perceived stress scale (PSS), a widely used self-reported measure of chronic stress over a 4-week period. Overall, results have been mixed with some investigators reporting correlation coefficients of <0.10 in studies of young adults[33] and in a racially diverse adult sample[34]; and correlations of 0.2 in adrenal insufficiency patients[35], 0.24 in chronic pain patients and controls[36] and 0.47 in pregnant women[37]. On balance, available evidence suggests positive associations of HCC with PSS scores and repeated measures of cortisol from other matrices.
Correlates of Hair Cortisol
A previous review from 2012[29] found limited data on relevant correlates of HCC. Yet, as this is a rapidly developing field, we performed a systematic review of all relevant literature to shed light on correlates of HCC, broadly encompassing factors that may be important determinants, confounders, effect modifiers, interactions and mediators that could influence the relationship between HCC and covariates of interest in epidemiologic studies.
Firstly, it is inevitably of principal interest to understand how hair-related factors (e.g., natural hair color, frequency of hair wash) might affect cortisol concentrations in hair. Second, it is important to examine whether hair cortisol concentrations reflect an individual´s subjective experience of chronic psychological stress and related morbidities or disorders. Lastly, the relationships between cortisol concentrations in hair and important socio-demographic and lifestyle factors should be assessed. Our systematic review summarizes evidence from existing literature on the correlates and determinants of HCC. The findings will be used to inform the design, analysis and interpretation of clinical and population-based studies that may use HCC as measures of chronic hypo or hyper-activation of the HPA axis.
METHODS
Literature Search and Paper Identification
Pertinent publications on hair cortisol, published before September 2013, were identified from PubMed, WorldCat, and Web of Science using keywords, “cortisol” “hair” “confounders” “chronic” “stress” “correlates” and “determinants.” We also perused references of identified publications to find other relevant papers. We included papers published in English and in peer-reviewed journals.
Paper Selection, Data Extraction and Data Synthesis
Human studies were the primary consideration in this systematic review. For studies to be included, the investigators had to have specified the length and weight of hair analyzed, the cortisol extraction methods, participant recruitment procedures, and comprehensible quantitative figures on the primary relationships of interest. Lastly, hair must have been taken from the posterior vertex region. Hair from the posterior vertex region is the standard for hair cortisol analysis[38]. It has been shown to have higher cortisol content and lower inter-individual variability compared to hair sampled from other regions[39]. We grouped the findings according to the following themes: hair-specific characteristics, stress-related correlates, lifestyle and behavioral factors.
RESULTS
Characteristics of Studies Included
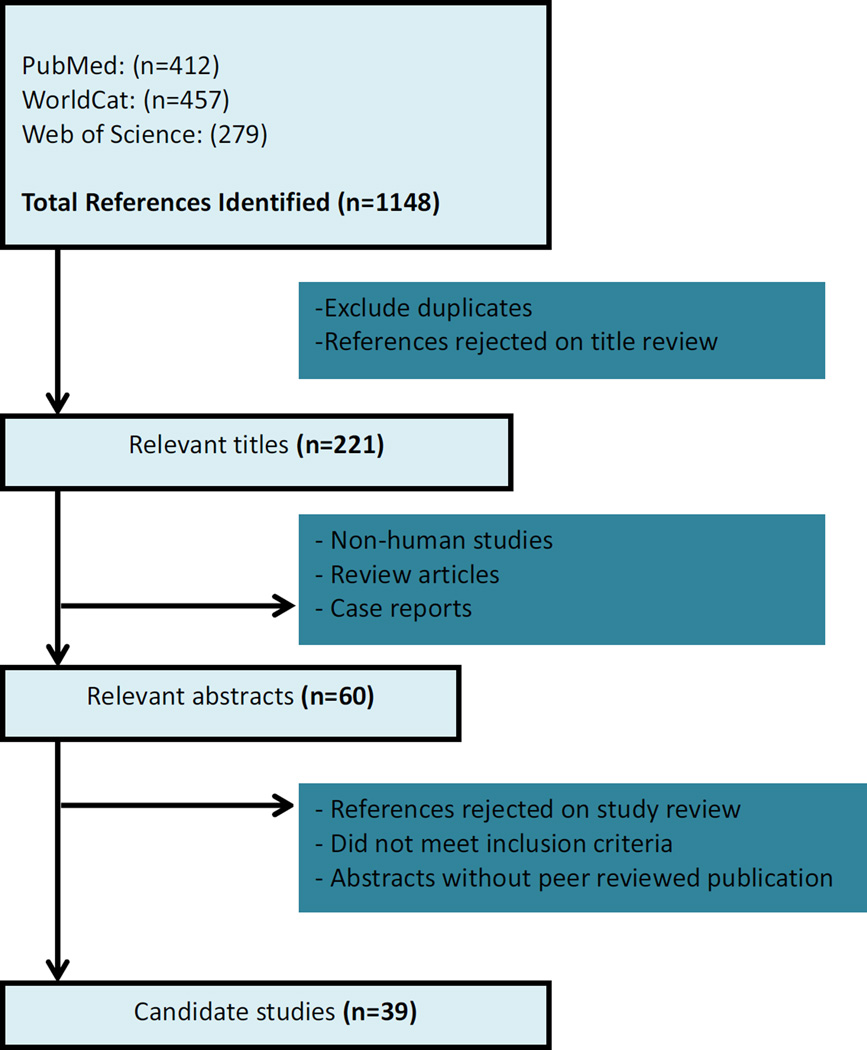
After elimination of duplicates, abstracts and papers that did not meet the inclusion criteria, 39 studies were chosen for inclusion in this review (Figure 1). Studies were conducted in multiple countries: Brazil, Canada, China, France, Germany, Israel, The Netherlands, Switzerland, Uganda, and the United States (Supplement Table). Approximately 33% of the studies were from Germany, published by pioneers who integrated hair cortisol measurement in their clinical and population-based studies[40–52]. In the sections that follow, we provide summary results of studies that assessed HCC in relation to hair-specific characteristics, stress-related correlates, lifestyle and behavioral factors.
Figure 1.
Assessment of Eligibility
Hair-Related Characteristics
Natural Hair Color, Frequency of Hair Washing, Hair Dyeing and Treatment
Natural hair color appears not to be associated with HCC in humans. In their early study, Raul et al reported no association between natural hair color and HCC[23]. Other investigators have confirmed these findings[27, 32, 41, 42, 53]. On balance, it appears reasonable to conclude that natural hair color is not a correlate or determinant of HCC.
Investigators have speculated that the frequency and temperature at which hair is washed may be inversely associated with HCC. However, most[27, 43, 46, 51, 53], though not all[39] studies on this topic indicated no association between HCC and hair washing. Li et al found that in a subsample of three participants, mean HCC decreased from 30.5±9.6 pg/mg to 6.5±5.9 pg/mg in hair immersed in shampoo solution for four hours[39]. Furthermore, in a subsample of eight participants, cortisol concentrations progressively declined after immersion of hair in hot water at 40°C, 65°C, and 80°C (Table 2)[39].
Table 2.
Correlates of Hair Cortisol Concentrations
| Study | Sample size for analysis | Summary of Findings |
|---|---|---|
| Hair-Related Factors | ||
| Natural Hair Color | ||
| Raul et al (2004) | 8 black, 3 blond, 24 brown, 7 grey | No differences in HCC of different hair colors (p=0.9773) AB Mean±SD: (black: 16.63±7.99; blond: 16.97±6.84; brown: 15.52±7.85; grey: 15.86±5.21) pg/mg |
| Sauvé et al (2007) | 4 blond, 4 brown, 4 black | No difference in HCC of different hair colorsCD |
| Kirschbaum et al (2009) | 142 womenE | No difference in HCC of different hair colors (p>0.20)CD |
| Manenschijn et al (2011) | 10 black, 75 brown, 94 blond, 6 red, 9 grey | No difference in HCC of different hair colors (p=0.413)D |
| Dettenborn et al (2012) | 33 light blond, 36 dark brown | No difference in HCC of different hair colors (p=0.524) Mean±SD: (light blond: 20.22±11.2; dark brown: 21.88±10.4) pg/mg |
| Dettenborn et al (2012) | 79 light-middle blond, 71 dark blond-light brown, 84 middle-dark brown |
No significant difference in HCC of different hair colors F(2,232)=2.538, p=0.081D |
| Groeneveld et al (2013) | 42 children (mean age, 50.1 months, SD=0.42 months, 45% boys)E |
HCC was not significantly related to hair colorCD |
| Hair Wash Frequency and Temperature | ||
| Kirschbaum et al (2009) | 142 womenE | No significant association between frequency of hair washes per week and HCC (p>0.20)CD |
| Manenschijn et al (2011) | 50 hair samples (≤2 times per week) 143 hair samples (≥3 times per week) |
No significant association between frequency of hair washes per week and HCC (p=0.673)D |
| Dettenborn et al (2012) | Frequency of hair washing: (0–1, 1.5–2, 2.5– 3, 3.5–4, 4.5–5, 5.5–6, 6.5–9 times per week) | No significant association between frequency of hair wash and HCC in first two segments: (first 3-cm segment: r=−0.061, p=0.335, n=249), (second 3-cm segment: r=−0.102, p=0.168, n=184). However, in third segment, HCC was inversely correlated with frequency of hair wash (r=−0.248, p=0.008, n=113) |
| Li et al (2012) | 3 participants | After immersion in shampoo solution for four hours, HCC decreased from 30.5±9.6 to 6.5±5.9 pg/mgC Mean±SD cortisol loss ratio was 75.5±27.4%, range: 45.1–98.4% |
| 8 participants | After 20 hour immersion, mean HCC decreased from (at 40°C: 13.8±5.8 to 4.4±2.9 pg/mg, p<0.001), (at 65°C: 8.8±4.1 to 1.4±0.4 pg/mg, p<0.01) and (at 80°C: 8.8±4.1 to 0.6±0.2 pg/mg, p<0.01) |
|
| Stalder et al (2012) | 155 individuals in study I (mean±SD of number of hair washes in the entire sample: 3.1±2.3)E 58 individuals in study II (mean±SD of number of hair washes in the entire sample: 3.7±1.6)E |
No significant association between frequency of hair washes per week and HCC (p>0.10 in study I, p>0.30 in study II)CD |
| Groeneveld et al (2013) | 42 children (mean age, 50.1 months, SD=0.42 months, 45% boys)E |
HCC was not significantly related to hair washing frequencyCD |
| Stalder et al (2013) | 1258 men and womenE | HCC was not correlated with number of hair washes per week (r =−0.046, p>0.05)C |
| Hair Dyeing and other Hair Treatments | ||
| Sauvé et al (2007) | 25 untreated hair, 14 dyed hair | Dyed hair had lower HCC (p=0.036)D |
| Dowlati et al (2010) | 5 dyed hair, 78 non-dyed hair | No difference in mean HCC of dyed and non-dyed hair (4.77±0.70 vs. 4.95±0.57, p=0.49) |
| Karlén et al (2011) | 95 students (40 students had colored/permed hair) |
There seemed to be a difference between mean HCC of colored/permed hair vs. non colored/permed hair (mean±SD 13.0±16.0pg/mg vs. 24.8±40.9 pg/mg, p=0.09), but it was not statistically significant |
| Manenschijn et al (2011) | 152 untreated hair, 43 treated hair (women) 90 used hair products, 104 did not use hair products |
Treated hair (dyed, bleached, permanent waved/straightened) had lower mean HCC than untreated hair (24.27 vs. 29.38 pg/mg, p=0.051) Use of hair product (hair spray, mousse, gel, wax) on the day of hair collection was not significantly associated with HCC: 24.83 pg/mg (product use) vs. 28.44 pg/mg (no product use), p=0.109 |
| Manenschijn et al (2012) | 96 healthy, non-obese controls, 14 patients with Cushing’s Syndrome, 6 patients suspected of cyclic Cushing’s syndromeE |
HCC was slightly lower in healthy women with treated hair, i.e. hair dyeing or bleaching (p=0.06)D No difference between HCC of treated and untreated hair in Cushing’s patientsCD |
| Stalder et al (2012) | Study I: 59 treated hair, 96 untreated hair Study II: 21 treated hair, 37 untreated hair |
Study I: No difference between treated semi-permanent color, coloration or permanent wave) and untreated hair (p>0.10)CD Study II: No difference between treated (as described above) and untreated hair (p>0.30)CD |
| Stalder et al (2013) | 1258 men and womenE (15.5% reported use of hair treatment) |
HCC was negatively associated with hair treatment after adjustment for sex (F1, 1195=4.22, p=0.04, ŋp2=0.0004) |
| Hair Segment | ||
| Xie et al (2011) | 8 female participants | Average linear HCC decline rate of 2.9±0.6 pg/mg (r=−0.948, p<0.05) per centimeter in the first five 1- cm hair segments |
| Manenschijn et al (2011) | 28 healthy women | HCC was not significantly different in six consecutive 3-cm segments (p=0.249)D |
| Steudte et al (2011) | 30 participants | HCC decreased 17.4% from the proximal 3-cm segment to the second 3-cm segment, then decreased 18.3% from the second 3-cm segment to the third |
| Dettenborn et al (2012) | 155 individuals | HCC decreased by 16.32% from first 3-cm hair segment to the second hair segment (p<0.001). HCC also decreased 12.29% from second 3-cm hair segment to third (p<0.001) |
| Skoluda et al (2012) | 273 individuals | HCC decreased from proximal to distal hair segments (F(1.55, 273.05)=131.9, p<0.001)D |
| Luo et al (2012) | 20 non-traumatized controlsE | HCC linearly declined from proximal to distal in the nontraumatized control group (F=41.07, p <0.0001) |
| Krumbholz et al (2013) | 1 woman (hair examined across pregnancy) | HCC decreased by >50% after 4 months of hair growth |
| Hair Texture and UV-Irradiation | ||
| Kirschbaum et al (2012) | 142 womenE | Hair curvature was not significantly related to HCC (p>0.20)D |
| Dettenborn et al (2012) | 360 individuals (70% of whom were aged 18–49 years old)E |
There was no influence of curls (p=0.468)D or waves (p=0.633)D on HCC when compared with straight hair in individuals aged 18–49 years old |
| Li et al (2012) | 12 hair samples exposed to 9-hour UV irradiation |
HCC decreased from 26.1±8.8pg/mg to 18.9±8.0pg/mg (p=0.047) The average loss ratio in HCC was 26.5±20.9% (r=0.616, p<0.05) |
| Stress-Related Factors | ||
| Demographic, Social and Occupational Factors | ||
| Dettenborn et al (2010) | 31 unemployed, 28 employed | Unemployed individuals had higher HCC for first 3-cm segment (p<0.05)D and second 3-cm segment (n=52, p<0.05)D |
| Manenschijn et al (2011) | 33 shift workers, 89 day workers | Workers who had a fast-forward rotating shift schedule had higher mean HCC compared with those who only worked during the day (mean(95% CI): 47.32(38.37–58.21) vs. 29.72(26.18–33.73) pg/mg, p<0.001). However, in individuals <40 years of age, shift workers had higher HCC (mean(95%CI): 48.53(36.56–64.29) vs. 26.42(22.91–30.55) pg/mg, p<0.001) while for those >40 years, there was no effect of shiftwork. There were no individuals who were exactly 40 years old |
| Chen et al (2013) | Cohort A (13 had elementary school education, 34 had high school education or below, 6 had college education or above) Cohort B (4 had elementary school education, 27 had high school education and below, 19 had college education or above) |
No significant difference in HCC among all three educational groups in cohort A (F=0.31, p=0.74) and in cohort B (F=1.98, p=0.15) |
| Groeneveld et al (2013) | 42 children (mean age, 50.1, SD=0.42 months); mean parental educational was 14.8 years (SD=1.9)E |
HCC was not significantly related to parents’ educational levelCD |
| Saleem et al (2013) | 56 cardiac rehabilitation patients with CAD (26 with normal HCC (median 111 ng/g) and 30 with elevated HCC (median 263 ng/g) |
Normal vs. high HCC group: total years of education (mean±SD: 16.5±3 vs 17±3.5 years, p=0.45), married (88.5% vs. 73.3% married, p=0.20) |
| Vaghri et al (2012) |
333 preschoolers |
Parental income was inversely correlated with HCC from 1–2cm hair segment (r=-0.18, p=0.001); in a subsample of 275 preschoolers, annual family income was not correlated with HCC (r=-0.07, p=0.235) |
| Age | ||
| Raul et al (2004) | 44 individuals | Linear regression predicting HCC from age (β=0.0296, standard error=0.0541, p=0.139)AB There was no relationship between HCC and age category (mean±SD: 0–15 years: 15.2±7.1; 16–30 years: 14.4±8.4; 31–50 years: 16.2±7.6; 51–90 years: 17.3±6.3 pg/mg, p=0.878) |
| Manenschijn et al (2011) | 195 individuals (18–63 years); Mean age: 36 years |
HCC was not correlated with participant age (p=0.388)D |
| Dettenborn et al (2012) | 360 individuals (1–91 years) | HCC was positively related with age (R2=0.010, p=0.030) For children 0–5 years, age in months was negatively correlated with HCC (r=−0.428, p=0.023). Children (1–9 years old, n=28) had significantly higher HCC compared with adults (n=34, 18–38 years old) (p<0.001)D |
| Kirschbaum et al (2012) | 103 mothers of newborns 2–4 days of age (mean±SD age: 29.7 ±5.5years), 19 mothers of toddlers 3–9 months (mean±SD age: 30.4± 2.8years) and 20 nonpregnant nulliparas (mean±SD age: 25.8±6.4years) |
HCC was not significantly associated with age across the entire group (p>0.20) CD |
| Manenschijn et al (2012) | 14 Cushing’s syndrome patients, 6 individuals with suspected cyclic Cushing’s Syndrome, 96 healthy controlsE |
HCC was not significantly related with age in either the patient or control groupCD |
| O’Brien et al (2012) | 135 adults (18–66 years old) | No correlation of age and HCC (r=0.07, p>0.05) HCC was not significantly correlated with tertiles of age (age ranges: young=18–21 years, n=44; middle=22–29 years, n=45; older=30–66 years, n=43) |
| Stalder et al (2012) | 155 individuals (18–46 years) Mean±SD age: 24.1±14.2 years |
HCC was not correlated with participant age (r=−0.02, p>0.05) CD |
| Stalder et al (2012) | 58 individuals (20–70 years) Mean±SD age: 30.5±12.1 years |
HCC was not correlated with participant age (r=−0.06, p>0.05) CD |
| Vaghri et al (2012) | 339 preschoolers (mean±SD age: 4.6±0.5 years) |
No statistically significant correlation between age and log-transformed, winsorized HCC variable (r=− 0.09, p=0.098) |
| Chen et al (2013) | Cohort A (23 participants 21–40 years; 29 participants >40 years); Cohort B (21 participants aged 21–40 years; 23 participants >40 years) |
No significant difference in HCC of those aged 21–40 years and those >40 years in cohort A (z=−0.179, p=0.86), and in cohort B (z=−0.51, p=0.61) |
| Gerber et al (2013) | 42 university male and female students | Age was not significantly associated with HCCCD |
| Manenschijn et al (2013) | 283 elderly adults (65–85 years) | HCC was not correlated with age (r=−0.02; p=0.76) |
| Saleem et al (2013) | 56 cardiac rehabilitation patients with CAD (26 in normal HCC (median 111 ng/g) and 30 in the elevated HCC (median 263ng/g) |
Age was not significantly associated with HCC |
| Stalder et al (2013) | 1258 men and womenE(age: 16–64 years) | HCC was positively correlated with age (r=0.11, p<0.0001)C |
| Sex | ||
| Raul et al (2004) | 16 males, 26 females | No differences in HCC of males and females (mean±SD: 15.6± 8.8 vs. 16.1±6.3 pg/mg, p=0.859) |
| Karlén et al (2011) | 95 students (24 males, 71 females) | No difference in HCC of males and females (mean±SD: 17.8±13.4 pg/mg vs 20.6±37.7 (p=0.73) |
| Manenschijn et al (2011) | 90 men, 105 women | No significant effect of gender (p=0.353)D |
| Manenschijn et al (2012) | Bipolar patients: 34 men, 62 women Healthy controls: 90 men, 105 women |
No sex differences in HCC of bipolar patients (p=0.12)D and healthy controls (p=0.87)D |
| Steudte et al (2011) | PTSD patients: 4 men, 6 women Controls: 11 men, 6 women |
No significant effects of gender on HCC (F(1,23)=1.01, p=0.33, ŋp2=0.04)D |
| Dettenborn et al (2012) | 252 adults (18–49 years) 52 children (1–9 years) 25 adolescents (10–17 years) 31 elderly (50– 91 years) |
Males had higher HCC in first 3-cm segment compared with females for adults 18–49 years (F(1,251)=9.573, p=0.002, ŋp2=0.037).D Males had higher in first 3-cm segment HCC compared with females for children 1–9 years (F(1,51)=5.304, p=0.025, ŋp2=0.078).D No sex differences were seen in HCC of adolescents (F(1,24)=0.837, p=0.370)D or elderly (F(1,30)=0.064, p=0.803)D |
| Manenschijn et al (2012) | 14 Cushing’s syndrome patients, 6 individuals with suspected Cushing’s Syndrome, 96 healthy controls E |
HCC was not significantly related with sex in either the patient or control groupCD |
| O’Brien et al (2012) | 135 adults (60% female) | Men had slightly higher HCC compared with women t(129) = 2.91, p<0.01 |
| Skoluda et al (2012) | Athletes: 304 individuals (58.9% female), Controls: 70 individuals (82.9% female) |
No significant differences in HCC between the sexes in athletes (p=0.06)D nor in controls (p=0.62)D Across the entire sample, males had higher HCC (F(1,372)=8.13, p<0.01)D |
| Stalder et al (2012) | Study I: N=155 (73.5% females) Study II: N=58 (67.2% females) |
Study I: No significant difference in HCC of males and females (F(1.154)=0.006, p>0.05)CD Study II: Non significant linear trend for higher HCC in males compared with females (F(1,57)=3.58, p=0.06, ŋp2=0.06)D |
| Vaghri et al (2012) | 167 preschool boys, 172 preschool girls | No statistically significant correlation between age and log-transformed, winsorized HCC variable (biserial correlation=−0.01, p=0.790) |
| Chen et al (2013) | Cohort B (41 men, 9 women) | No significant gender difference in HCC (z=−1.25, p=0.22) |
| Gerber et al (2013) | 42 university students (22 women, 20 men) |
Sex was not significantly associated with participants’ HCCCD |
| Groeneveld et al (2013) | 42 children (mean age, 50.1 months, SD=0.42 months, 45% boys) |
HCC was not significantly related to genderCD |
| Hinkelmann et al (2013) | 43 major depressive disorder patients, 41 age- and sex-matched controlsE |
HCC was not significantly associated with sex (p>0.10)CD |
| Manenschijn et al (2013) | 283 elderly adults (66.1% female) | HCC were significantly higher in men than in women (median, 26.3 pg/mg hair [interquartile range (IQR), 20.6 – 35.5 pg/mg hair] vs 21.0 pg/mg hair [IQR, 16.0 – 27.0 pg/mg hair]; p<0.001) |
| Saleem et al (2013) | 56 cardiac rehabilitation patients with coronary artery disease (26 in normal HCC group (median 111.4 ng/g) and 30 in the elevated HCC group (median 262.8 ng/g) |
Gender was not significantly different between normal HCC group (92.3% male) and high HCC group (80% male) (p=0.19) |
| Stalder et al (2013) | 1258 men and womenE (about 84.8% were men) |
HCC was not significantly with sex (F1, 1216=1.83, p>0.05)CD |
| Race and Ethnicity | ||
| O’Brien et al (2012) | 135 adults categorized into two groups: minorities (African-Cuban, Afro-Cuban, Asian, Brazilian, Indian, Latino-Hispanic and Pacific Islander (n=67)); and non-minorities (European or White American (n=68)) |
HCC was not correlated with race (r=−0.09, p>0.05). However, there was significant interaction of race and SES on HCC (F(2, 122)=3.26, p<0.05, ŋp2=0.16) |
| Psychiatric Symptoms and Disorders | ||
| Dowlati et al (2010) | 34 depressed patients, 87 non-depressed patients (all attending a cardiac rehabilitation center) |
Mean HCC was not different between depressed and non-depressed patients (log mean±SD: 4.96±0.58 vs. 5.04±0.59, p=0.53) |
| Karlén et al (2011) | 95 students (20 with serious life events) | Mean HCC were higher students who had experienced serious life events within the last 3 months vs. those who had not (mean±SD: 33.0±57.0 vs 16.3±22.5 pg/mg, p=0.045) |
| Steudte et al (2011) | 10 traumatized individuals with PTSD, 16 traumatized controls without PTSD | Traumatized PTSD patients had significantly higher HCC compared with traumatized non-PTSD controls (F(1,25)=5.35, p=0.03, ŋp2=0.18)D |
| Steudte et al (2011) | 15 generalized anxiety disorder patients (GAD), 15 controls |
HCC were significantly lower in GAD patients compared with controls for first 3-cm segment (F(1,27)=11.80, p=0.002, ηp2=0.304)D and second 3-cm segment (F(1,24)=8.894, p=0.006, ηp2=0.270)D. A non-significant trend for lower HCC in GAD patients was observed for the third segment (F(1,17)=4.138, p=0.058, ηp2=0.196)D |
| Dettenborn et al (2012) | 23 depressed patients, 64 healthy controls | Depressed patients had elevated HCC in the first 3-cm (mean±SD: 26.7±20.8 vs. 18.7±11.5 pg/mg, p<0.05) and second 3-cm hair segments (mean±SD: 21.9±23.7 vs. 13.4±9.6 pg/mg, p<0.05) compared with controls |
| Luo et al (2012) | 32 individuals with PTSD, 32 traumatized non-PTSD controls, 20 non-traumatized non-PTSD controls |
For the hair segment corresponding to 2 months before and 1 month after the earthquake, HCC were elevated in traumatized individuals (PTSD and non-PTSD) compared with the non-traumatized non- PTSD controls (F=3.88, p=0.0499 and F=6.27, p=0.013, respectively)D. For the hair segment corresponding to 2 to 4 months after the earthquake, traumatized non-PTSD controls had significantly higher HCC compared with traumatized PTSD individuals (F=6.17, p=0.0137)D and non-traumatized non- PTSD controls (F=11.74, p=0.0007)D. For the period corresponding to 5 to 7 months after the earthquake, traumatized non-PTSD controls had higher HCC compared with traumatized individuals with PTSD (F=4.11, p=0.0438)D |
| Manenschijn et al (2012) | 100 bipolar disorder (BD) patients, 195 healthy controls |
No difference between mean HCC of BD patients and that of controls (mean(95%CI): 31.84(28.38– 35.81) vs. 28.18(25.94–30.62) pg/mg, p=0.233). However, in a subsample, individuals with older age of onset (≥30 years) had higher HCC compared with those with early onset (<30 years old) (β=0.335, p=0.004) |
| Gerber et al (2013) | 42 undergraduate students (22 women, 20 men) |
HCC were negatively associated with depressive symptoms (as assessed using the Beck Depression Inventory (BDI)), β=−0.24, p=0.009. |
| Grassi-Oliveira et al (2012) | 23 treatment seeking crack cocaine- dependent women |
HCC was significantly related with negative life events exposure 90 days before hospital admission (r =0.56; p=0.007) and 30 days (r =0.42; p=0.048) prior to admission at the hospital, but not with 60 days prior to admission (r=0 .23; p =0.293) |
| Groeneveld et al (2013) | 42 children (mean age, 50.1 months, SD=0.42 months, 45% boys); 21 children scored high on a fearfulness questionnaire |
HCC significantly increased after the start of school, but only in children who scored high for fearfulness F(1, 19)=4.67, p=0.04, ŋp2=0.20. |
| Hinkelmann et al (2013) | 43 major depressive disorder patients, 41 age- and sex-matched controlsE |
HCC was not associated with current depression, nor with atypical depressionCD |
| Saleem et al (2013) | 56 patients who completed a one-year cardiac rehabilitation program(26 of whom had normal HCC at baseline, 30 had normal HCC a baseline)E |
The prevalence of depression did not differ between the normal HCC group (26.9%) and the high HCC group (30%) (p=0.80) |
| Steudte et al (2013) | 25 PTSD patients, 25 traumatized controls, 28 non-traumatized controls |
HCC was 59% lower in PTSD patients and 51% lower in traumatized controls (TC), compared to non- traumatized controls (NTC) (mean±SD HCC: PTSD 6.44±6.05 pg/mg; TC 7.78±7.01 pg/mg; NTC 15.75±14.77 pg/mg; p<0.05). No differences in HCC between PTSD and TC groups (p=0.535). HCC was not correlated with Beck Depression Index (BDI-II) scores (r=−0.127, p=0.256). HCC was inversely correlated with number of different lifetime traumatic events, frequency of traumatic experiences, time since traumatization, and severity of intrusion symptoms. |
| Other Medical Conditions | ||
| Cardiovascular Disease, Cardio-Metabolic Syndrome, and Chronic Pain | ||
| Van Uum et al (2008) | 15 chronic pain patients receiving opioid treatments, 39 non-obese controls |
Chronic pain patients had higher HCC compared with controls (mean(range): 83.1(33.0–205) pg/mg vs. 46.1(27.2–200) pg/mg, p<0.01) |
| Pereg et al (2010) | 56 acute myocardial infarction patients (AMI), 56 non-AMI hospital controls |
AMI patients had significantly elevated median HCC compared with non-AMI hospital controls (median (range): 295.3(105.4–809.3) vs. 224.9(76.58–949.9) ng/g, p<0.01) |
| Karlén et al (2011) | 95 students (28 reported taking pharmaceuticals) |
No difference in HCC according to use of pharmaceuticals (mean±SD: 22.9±44.9 pg/mg vs 18.6±27.2 pg/mg, p=0.57) |
| Manenschijn et al (2011) | 46 healthy participants | No correlation between HCC and systolic (p=0.109)D or diastolic blood pressure (p=0.365)D |
| O’Brien et al (2012) | 135 adults (18–66 years, 65% female, 48% minority) |
HCC was positively correlated with systolic blood pressure (r=0.25, p<0.01); however no significant correlation was observed between HCC and diastolic blood pressure (r=0.08, p>0.05) |
| Manenschijn et al (2013) | 283 elderly adults (age range: 65–85 years) | HCC was significantly associated with CVD. The odds ratio (OR) was 1.9 (p=0.09) for the second, 2.0 (p=0.08) for the third and 2.7 (p=0.01) for the fourth quartile. Highest levels of HCC quartile assocated with type 2 diabetes mellitus (OR 3.2, p=0.04). |
| Saleem et al (2013) | 56 patients who completed a one-year cardiac rehabilitation program(26 of whom had normal HCC at baseline, 30 had normal HCC a baseline)E |
High baseline HCC (i.e. HCC ≥153.2 ng/g) predicted less improvement in verbal memory performance after one year (F(1, 50)=5.50, p=0.02,.Number of weeks since acute coronary event, percutaneous coronary intervention, and myocardial infarction did not differ between normal and high HCC groups, however history of coronary artery bypass surgery differed. Between the normal and high HCC groups: prevalence of hypertension (53.8% vs 63.3%, p=0.47), diabetes (7.7% vs 16.7%, p=0.31), maximum heart rate (118±18 vs 121±22 bpm, p=0.55), SBP (176±24 vs 177±27 mmHg, p=0.90), DBP (76±9 vs 80±11 mm Hg, p=0.18), and maximal oxygen intake (20±5 vs 20±5, p=0.98) seemed to differ, though the p-values were not significant. |
| Stalder et al (2013) | 1258 men and womenE (about 24% had metabolic syndrome (MetS)) |
HCC was positively associated with metabolic syndrome (MetS): [mean(±SD), no MetS: 7.70 (±8.12) pg/mg; MetS: 10.78 (±11.37) pg/mg, p<0.0001]. Furthermore, in the adjusted partial correlations, HCC was not significantly associated with mean arterial pressure (r=0.047, p>0.05), high-density lipoprotein cholesterol (r=−0.037, p>0.05), triglycerides (r=−0.015, p>0.05), and glucose (r=0.005, p>0.05). However, HCC was associated with low-density lipoprotein cholesterol (r=−0.080, p<0.01), and glycated hemoglobin (r=0.116, p<0.001) |
| Adrenocorticoidal Conditions | ||
| Thomson et al (2010) | 6 female Cushing’s syndrome patients, 32 healthy controls (21 females, 11 males) |
Cushing’s patients had significantly higher HCC compared with controls: (median(range): 679(279–2500) vs. 116(26–204) ng/g, p<0.001) |
| Gow et al (2011) | 93 adrenal insufficiency (AI) patients on hydrocortisone replacement therapy, 62 household partners |
AI patients had higher median HCC than controls (median(range): 230.7(22.7–1377) vs. 184.7(57.7– 1479) ng/g, p=0.08), although the result was not significance. Hydrocortisone dose was also correlated with HCC (r=0.03, p=0.004) |
| Manenschijn et al (2011) | 195 healthy controls, 9 hypercortisolemics | Hypercortisolemics had significantly elevated HCC compared with healthy controls (p<0.0001)CD |
| Manenschijn et al (2012) | 14 patients with Cushing’s syndrome (CS), 6 patients with cyclic Cushing’s syndrome, 96 non-obese healthy controls |
Patients with CS (excluding cyclic CS patients) had significantly elevated mean HCC compared with healthy individuals (mean(95%CI): 399.7(171.8–930.0) vs. 27.3(24.6–30.4) pg/mg, p<0.0001) |
| Adiposity | ||
| Manenschijn et al (2011) | 39 shift workers, 89 day workers | HCC positively correlated with participant BMI (b=0.262, p<0.05). HCC increased across BMI groups as follows: (mean(95%CI): (BMI<25kg/m2: 31.26(26.79–36.48)); (BMI 25–30kg/m2: 36.06(30.48–42.76)); (BMI >30 kg/m2: 60.95(43.95–84.72) pg/mg, p=0.002) |
| Manenschijn et al (2011) | 195 healthy people, 9 hypercortisolemic, 1 hypocortisolemic |
HCC was positively correlated with waist circumference (r=0.392, p<0.05) and WHR (r=0.425, p<0.05). HCC was not significantly correlated with BMI (p=0.646)D or hip circumference (p=0.096)D |
| Manenschijn et al (2012) | Bipolar patients: 100 individuals; BMI median (IQR): 25.3(23.5–28.0 kg/m2) Healthy controls: 195 people: BMI median (IQR): 23.7(21.7–26.5 kg/m2) |
No significant relationship between HCC and BMI in bipolar (r=0.163, p=0.11) and control individuals (r=0.042, p=0.59) |
| O’Brien et al (2012) | 135 adults | HCC was not significantly correlated with waist-to-hip ratio (r=−0.03, p>0.05) |
| Stalder et al (2012) | 155 adults; BMI: (mean±SD: 22.2±3.4; range:16.5–35.8) kg/m2 |
HCC positively correlated with participant weight (r=0.29, p≤0.001) and BMI (r=0.33, p≤0.0001) |
| Stalder et al (2012) | 58 university students; BMI: (mean±SD:24.0±4.9; range:16.9–42.1) kg/m2 |
HCC positively correlated with participant weight (r=0.36, p<0.05) and BMI (r=0.42, p≤0.001) |
| Manenschijn et al (2013) | 283 elderly adults (65–85 years old, 66.1% female) |
No significant correlation between HCC and BMI (r=0.06, p=0.36), waist circumference (r=0.11, p=0.08) |
| Saleem et al (2013) | 56 patients who completed a one-year cardiac rehabilitation program(26 of whom had normal HCC at baseline, 30 had normal HCC at baseline)E |
Between the normal and high HCC groups, mean±SD of BMI did not differ (27.1±5.0 kg/m2 vs 27.4±3.5 kg/m2, p=0.75), neither did waist circumference (96.2±10.1 cm vs. 98.8±8.8, p=0.29) |
| Pregnancy | ||
| D’Anna-Hernandez et al (2011) | 21 non-smoking pregnant women (<17 weeks gestational age) |
HCC increased across successive trimesters and then decreased during the first 2 to 3 months postpartum. HCC in third trimester was higher than in the first (t=4.1, p=0.001)D as well as post-partum (t=2.9, p=0.004)D |
| Kirschbaum et al (2012) | 103 mothers with newborn 2–4 days of age, 19 mothers of toddlers 3 to 9 months, 20 non-pregnant nulliparous women (n=20). |
HCC of mothers of newborns 2–4 days of age (their hair corresponded with the third trimester) was significantly elevated in the first 3-cm segment compared with that of control women (t(1,120)=4.77, p<0.0001) D; however there was no significant difference in HCC of the two groups of women for segments 2 and 3 |
| Krumbholz et al (2013) | 1 woman (HCC over a pregnancy) | The highest HCC (13.4 pg/mg) was observed for the hair segment that reflected the last month of pregnancy and first month postpartum |
| Early Life Adversity | ||
| Yamada et al (2007) | 60 NICU infants > 25 weeks gestational age, NICU term infants, healthy term infants |
HCC for NICU infants was elevated compared with HCC for healthy term infants (mean±SD: 2.06±2.05 vs. 0.11±0.42 nmol/g, p=0.004). Total number of days on the ventilator was associated with HCC (increased 0.2 nmol/g, p=0.03, on average, for each additional day). There was no difference between HCC of term infants in NICU and HCC of preterm infants in NICU |
| Grassi-Oliveira et al (2012) | 23 treatment seeking crack cocaine- dependent women |
No correlation between HCC and severity of early life stress CD |
| Hinkelmann et al (2013) | 43 major depressive disorder patients, 41 age- and sex-matched controlsE |
HCC was lower in subjects who experienced childhood maltreatment compared to controls (F1,71=4.11, p=0.05)C |
| Steudte et al (2013) | 25 PTSD patients, 25 traumatized controls, 28 non-traumatized controlsE |
No significant correlation was observed between HCC and childhood trauma scores (r=-0.144, p=0.197) |
| Lifestyle and Behavioral Factors | ||
| Alcohol Use | ||
| Stalder et al (2010) | 23 alcoholics in acute withdrawal, 25 abstinent alcoholics, 20 controls |
HCC (mean±SD) was highest in acute withdrawal alcoholics (51.99±43.30 pg/mg), compared with abstinent alcoholics (13.98±10.63 pg/mg, p<0.001) and controls (16.35±12.59 pg/mg, p<0.001). No |
| difference between HCC of abstinent alcoholics and controls (p=0.91) | ||
| Manenschijn et al (2013) | 283 elderly adults (17.4% who had never drank, 52.8% light drinkers, 26.2% moderate drinkers, 3.6% (very) excessive drinkers) |
HCC was positively associated with alcohol consumption. HCC were 21.2 pg/mg hair (IQR, 15.2–29.4) in non-drinkers, 21.7 pg/mg hair (IQR, 16.9–28.9) in light drinkers, 24.5 pg/mg hair (IQR, 17.1–36.3) in moderate drinkers, and 30.4 pg/mg hair (IQR, 25.0–45.0) in excessive drinkers (p=0.05) |
| Stalder et al (2013) | 1258 men and womenE (43% reported regular consumption of ≥3 alcoholic drinks/week) |
No association between HCC and self-reported alcohol consumption (F1, 1208 = 0.11, p>0.05)D; HCC was positively associated with serum γ-glutamyltransferase (γGT), (r=0.11, p<0.0001) |
| Cigarette Smoking | ||
| Dettenborn et al (2012) | 18–49 year oldsE | No influence of smoking status on HCC (p=0.836)D |
| Stalder et al (2012) | Study I: 155 adults (17.6% smokers), Study II: 58 students at the Technical University of Dresden (27.6% smokers) |
Study I: No association between smoking status and HCC (p>0.10)D Study II: No association between smoking status and HCC (p>0.30)D |
| Skoluda et al (2012) | 304 amateur endurance athletes (7.1% habitual smokers); 70 controls (20% habitual smokers) |
Smoking was not associated with HCC (F(1, 364)=0.02, p=0.88)D |
| Hinkelmann et al (2013) | 43 major depressive disorder patients; 41 age- and sex-matched controlsE |
HCC was not significantly associated with smoking status (p>0.10)CD |
| Manenschijn et al (2013) | 283 elderly adults (38.2% never smoked; 52.7% former smokers, 8.8% current smokers) |
No difference in HCC based on smoking status. HCC were 21.2 pg/mg hair (IQR, 16.6–26.9) in those who never smoked, 22.1 pg/mg hair (IQR, 17.4–33.6) in former smokers, and 26.3 pg/mg hair (IQR, 18.7- 32.3) in current smokers (p=0.22) |
| Stalder et al (2013) | 1258 men and womenE (33.5% smokers) | HCC was not associated with smoking status (p>0.05)CD |
| Oral Contraceptives and Medication Intake | ||
| Dettenborn et al (2012) | (16.7% of 360 individuals in the sample used oral contraceptives)E 252 individuals aged 18–49 years oldE 29 elderly individuals (medication intake analysis)E |
No influence of oral contraceptive use on HCC in 18–49 year old women (p=0.110)D No influence of overall medication intake on HCC in 18–49 year olds (p=0.610)D No influence of medication intake in elderly group (F(1,29)=0.245, p=0.625)D |
| Stalder et al (2012) | Study I: 72 oral contraceptive users, 42 not taking oral contraceptives Study II: 16 oral contraceptive users, 23 not taking oral contraceptives |
Study I: No association between oral contraceptive use and HCC (p>0.10)D Study II: No association between oral contraceptive use and HCC (p>0.30)D |
| Groeneveld et al (2013) | 42 children (mean age, 50.1 months, SD=0.42 months, 45% boys)E |
HCC of children was not related to use of corticosteroids or other medicationsCD |
| Hinkelmann et al (2013) | 43 major depressive disorder patients, 41 age- and sex-matched controlsE |
HCC was not associated with antidepressant treatment (p>0.1)D |
| Saleem et al (2013) | 56 patients who completed a one-year cardiac rehabilitation program(26 of whom had normal HCC at baseline, 30 had normal |
Use of concomitant medications seemed to differ with specific drugs between normal and high HCC groups, however no statistically significant difference was observed; beta-blocker (80.8% vs 70%, p=0.35); calcium channel blocker (26.9% vs 10%, p=0.10); diuretics (23.1% vs 20%, p=0.78); |
| HCC at baseline)E | antihypertensives (50% vs 63.3%, p=0.32); antidiabetics (3.8% vs 16.7%, p=0.12); antidepressants (3.8% vs 10%, p=0.37) ; anxiolytics (11.5% vs 3.3%, p=0.23) |
|
| Steudte et al (2013) | 25 PTSD patients (36% used regular medication (RM)), 25 traumatized controls (20% used RM), 28 non-traumatized controls (29% used RM)E |
No relationship between HCC and medication intake (p>0.31)D |
| Steudte et al (2013) | 25 PTSD patients (96% female, 7 women used oral contraceptives (OC)), 25 traumatized controls (92% female, 4 used OC), 28 non-traumatized controls (89 female, 9 used OC)E |
No relationship between HCC and oral contraceptive use (p>0.31)D |
| Physical Activity | ||
| Skoluda et al (2012) | 304 amateur endurance athletes, 70 controls |
On average, athletes had higher HCC compared with controls (mean±SD: 18.18±9.6 vs. 12.43±6.2 pg/mg; and gender did not alter the effect of endurance sports on HCC, p=0.048). Additionally, they observed a significant correlation between HCC and training kilometers run per week (r=0.32, p<0.001), training hours per week (r=0.22, p<0.001) and number of competitions per year (r=0.29, p<0.001), but not with number of training years (r=0.008, p=0.89). |
| Stalder et al (2013) | 1258 men and womenE (physical activity score range: 3–15) |
No association of HCC with light or moderate physical activity; positive association with vigorous physical activity (p>0.05)CD |
| Gerber et al (2013) | 42 university students (vigorous physical activity: 0–213 weekly minutes; moderate physical activity: 164– 775 weekly minutes) |
HCC positively correlated with vigorous physical activity (β=0.33, p=0.05, ?R2=0.106). HCC was not significantly correlated with moderate physical activity (r=−0.08, p>0.05) |
| Diet | ||
| Stalder et al (2013) | 1258 men and womenE (self-reported daily fruit and vegetable consumption) |
No association between HCC and fruit and vegetable consumption (p>0.05)CD |
We used SAS 9.2 to calculate these values
We excluded two outlier values
Authors did not report exact p-values
Authors did not report mean HCC, median HCC, correlation or odds ratio
Sample size used in analysis might be different from total sample size (and/or) authors did not specify the proportion of subjects in comparison groups
As with frequency of hair washing, findings on the relationship between hair treatment and HCC have been inconsistent. Some investigators[27, 32, 51, 54] but not all[33, 46, 55] have documented substantial influences of hair dye and other hair treatments on HCC. Manenschijn et al found that treated hair had lower HCC compared with untreated hair while use of hair product (e.g., spray, mousse, gel and wax) on the day of hair sample collection was not significantly associated with mean HCC[27]. In aggregate, available data suggest modest influence of hair dyes and other treatments on HCC. Hence, investigators may consider collecting information on these behaviors in future large-scale epidemiologic studies that rely on HCC as a biomarker of chronic stress.
Hair Segment
HCC has been shown to decrease as one moves distally from the scalp[42, 44, 49, 56–58]. This attenuation in cortisol concentration has been attributed to exposure to water, sunlight and other elements. Of note, Steudte et al reported that HCC decreased 17.4% from the proximal 3-cm segment to the second 3-cm segment, then decreased 18.3% from the second 3-cm segment to the third[49]. Manenschijn et al, however, found no difference in HCC among six consecutive 3-cm segments of hair from healthy women (Table 2)[27].
Other Factors
Other hair-related factors that have been studied include hair texture and ultraviolet (UV) irradiation. While HCC appears to not be influenced by curls, hair curvature, or waves[42, 43], investigators have reported that 9-hour UV irradiation, compared with no exposure, is associated with statistically significant reductions in HCC (Table 2)[39]. More studies are needed to further examine the effect of hair texture and UV irradiation on HCC.
Taken together, while the available data suggest that natural hair color and texture are not associated with HCC, data are still scarce or inconclusive on the relationship between the various hair treatments and HCC. Thus, investigators may consider collecting information on these behaviors along with precisely defining hair segment for collection and analysis in future large-scale epidemiologic studies that rely on HCC as a biomarker of chronic stress.
Stress-Related Correlates
Demographic, Social and Occupational Stressors
Socio-economic factors such as income, educational level, occupation, and neighborhood characteristics are important determinants of health and are closely related with psychosocial stress[59]. Results of studies exploring the association between these factors and cortisol concentrations in saliva, urine and blood serum have been mixed[60]. To date, only a few studies have examined the relationship between HCC and socioeconomic factors. In a sample of 333 children from 23 neighborhoods in Vancouver, Canada, Vaghri et al observed that maternal and paternal education were both inversely correlated with HCC (r=−0.18, p=0.001). However, in a subsample of 275 preschoolers, annual family income was not significantly correlated with HCC (r=−0.07, p=0.235) (Table 2)[61]. Chen et al observed no relationship between HCC and educational level in a study of adults in Nanjing, China[62].
With regard to employment-related factors, shiftwork is of increasing importance in health studies, particularly because chronic shiftwork alters the circadian rhythm of cortisol production leading to HPA dysfunction[63]. In a small but important early study, investigators observed elevated mean HCC in participants who had a fast-forward rotating shift schedule compared with those who only worked during the day (47.32 vs. 29.72 pg/mg, p<0.001)[64]. However, there appeared to be an effect of age on the relationship between HCC and shiftwork (Table 2)[64]. Unemployment has also been examined in relation to HCC, however with mixed results. Dettenborn et al found higher HCC in unemployed individuals compared to those employed[41].
Age
Results of studies on the relationship between age and cortisol in other matrices have been quite mixed with investigators reporting both positive[66, 67] and negative associations[68]. With regard to the hair cortisol literature, some[23, 27, 34, 43, 46, 53, 54, 61, 62, 65, 69, 70], but not all[42, 51] investigators have reported no relationship between age and HCC. However, inferences from most studies documenting no associations are hindered by limited variability in the age of study participants. A study of 360 individuals aged 1 to 91 years suggests a complex non-linear relationship between age and HCC[42]. Across the age spectrum, the authors found that HCC were elevated in children <10 years old and in adults aged 50–91 years[42]. Furthermore, HCC was inversely related with age (in months) of children ≤5 years (r=−0.428, p=0.023).
In a smaller study with a comparable age range (2–90 years old), Raul et al found no association of age with HCC[23]. Available data suggest a complex relationship of HCC with age. Studies of individuals across broader age spectrums are needed to more thoroughly explore the relationship between age and HCC.
Sex
Findings on the relationship between sex and cortisol concentrations in other matrices have been inconsistent with some investigators finding higher concentrations in men[71] and others finding higher cortisol concentrations in women[66]. HCC studies on this topic have also been mixed with some investigators finding associations between HCC and sex[34, 42, 44, 46, 69, 72] and others finding none[23, 27, 33, 51–54, 61, 62, 65, 70, 73]. Dettenborn et al found higher HCC among males than among females in two age groups: adults aged 18–49 years and children <10 years of age. However, no differences were found in adults >49 years nor in individuals aged 10–17 years (Table 2)[42].
Race and Ethnicity
Race and ethnicity are important indicators of exposure to social stressors[74]. Equally vital, they are determinants of hair texture and hair growth rate[75]. Yet race and ethnicity have not been adequately studied in HCC studies. The average rate of hair growth used in most hair cortisol studies and other hair analysis is 1-cm/month, however, hair growth is known to vary between 0.7cm and 3.6cm per month[76]. Loussouarn et al observed lower hair density and hair growth rate in Africans compared with Caucasians[75]. These findings are consistent with racial differences in hair growth characteristics. In a study by O’Brien et al, participants were classified into two groups: minorities (African-Cuban, Afro-Cuban, Asian, Brazilian, Indian, Latino-Hispanic and Pacific Islander) and non-minorities (European or white American), and investigators observed no relationship between racial category and HCC[34]. However, the authors found a significant interaction of race and socio-economic status (SES) on HCC with minorities having elevated HCC at low and high SES[34]. Additional studies are needed to ascertain variations in hair growth rate among diverse populations. Considering inconsistencies in findings concerning HCC in relation to gender and given the importance of race and ethnicity as important indicators of social stressors, hair texture, and hair growth rate, investigators should account for sex, race and ethnicity in the design, analysis, and interpretation of hair cortisol studies.
Psychiatric Symptoms and Disorders
Despite some inconsistencies, findings from studies examining cortisol concentrations in saliva, blood, and urine generally point to a relationship between HPA dysregulation, psychiatric disorders, and aberrations in cortisol concentrations or diurnal cycles[17, 56, 77, 78]. A meta-analysis of 47 studies that examined salivary, blood plasma/serum, and urinary cortisol showed that compared with no-trauma controls, PTSD patients had suppressed morning cortisol concentrations and afternoon/evening cortisol concentrations[17]. Studying the association of HCC with stressful events and psychological symptoms or disorders is particularly important for establishing the validity of hair cortisol as a biomarker of chronic stress.
Using hair samples that corresponded to 1 to 2 months after the 2008 Wenchuan earthquake, Luo et al determined that HCC were elevated in PTSD and traumatized controls (TC) compared with non-traumatized controls (NTC) (Table 2). Furthermore, for an additional 2 to 4 months after the earthquake, TC had the most elevated average HCC compared with the other two groups (Table 2)[56]. Steudte et al also observed higher HCC in individuals with PTSD compared to TC (60% of PTSD patients and 22.2% of TC experienced traumatic events in the past year)[48]. However, in a separate study, Steudte et al observed lower HCC in PTSD patients and TC compared to NTC (75% of participants had experienced their most traumatic event >5 years ago) and no difference in HCC of PTSD patients and TC[50]. Furthermore, they found no associations between salivary cortisol levels and traumatization or PTSD[50]. On balance, available data indicate that trauma exposure and PTSD are associated with aberrant HCC, and that while cortisol levels may rise shortly after trauma, they may decrease over time. Like trauma, serious adverse life events, frequently precursors to impaired mental health, have also been associated with HCC[33, 79].
Depression has also been studied in relation to HCC, however the results have been mixed. One study found that depressed patients had elevated HCC compared with healthy controls[40]. In another study of cardiac rehabilitation patients, investigators observed no significant difference in HCC between depressed and nondepressed subjects[55]. The authors suggested that the absence of an association may be due to already elevated stress and cortisol levels in the cardiac rehabilitation population[55]. Investigations of the relationship between HCC and scores on the Beck Depression Index (BDI) have found both negative associations[80] and no associations[50, 80]. Saleem et al found no difference in prevalence of depression between normal HCC and high HCC subjects [65].
Other psychiatric conditions that have been studied in hair cortisol research include bipolar disorder and generalized anxiety disorder (GAD). Manenschijn et al found no overall difference between mean HCC of bipolar disorder patients, most of whom were receiving pharmacological treatment, compared with healthy controls[73]. However in a subsample, it was observed that individuals with older age of onset (≥30 years) had higher HCC compared with those with early onset (<30 years old) (Table 2). The authors suggested that stressful life events and HPA axis dysregulation may play a role in later development of bipolar disorder while early onset may be related to genetics or fluctuations in sex hormones[73]. Finally, Steudte et al examined cortisol concentrations for GAD patients and controls. While they found no difference in area under the diurnal curve for 24-hour salivary cortisol concentrations between the two groups, GAD patients had lower HCC compared with controls (Table 2)[49]. The investigators suggested that the experience of participating in a study might have contributed to acute fluctuations in salivary cortisol concentrations such that no differences in salivary cortisol concentrations were detectable. They also reasoned that HCC might be a more accurate measure of long-term HPA axis activity as it is not easily susceptible to acute influences[49].
Finally, in a sample of young school children, HCC significantly increased after school entry, but only in children who scored high on the fearfulness subscale of the Child Behavior Questionnaire[53]. Collectively, findings suggest that trauma exposure, psychiatric disorders, serious averse life events and symptoms of negative moods and emotions may be associated with HCC. More studies are needed to firmly establish HCC as a clear biomarker of chronic stress, and to elucidate the temporal relationship of HCC deviations with psychiatric disorders while jointly considering the mediating effects of stressors and stress response.
Other Medical Conditions
Adrenocorticoidal Conditions
Cushing’s syndrome, Addison’s disease and other conditions affecting the hypothalamus, pituitary or adrenal cortex are associated with aberrations in cortisol concentrations or diurnal patterns in saliva, blood serum/plasma and urine[81]. Studies examining HCC and adrenocorticoidal conditions have been consistent with these findings. In a study of adrenal insufficiency (AI) patients receiving hydrocortisone replacement therapy and household partner controls, AI patients had higher HCC than controls. HCC was also positively correlated with glucocorticoid dose[35]. The authors speculated that patients with adrenal insufficiency may be over-treated and could be at risk for adverse effects of elevated cortisol concentrations.
Investigators examining the relationship between Cushing’s syndrome and HCC have found significantly higher HCC in Cushing’s patients compared with healthy controls (Table 2)[27, 28, 54]. In their study of non-obese healthy controls and patients with cyclic Cushing’s syndrome, Manenschijn et al observed 86% sensitivity and 98% specificity for Cushing’s syndrome, using the upper-limit reference range for non-overweight healthy controls (75.9 pg/mg hair)[54]. These findings on Cushing’s syndrome lend support to the capability of hair as a valid matrix of chronic cortisol exposure.
Chronic Pain, Cardiovascular Disease and Metabolic Syndrome
Results support an association between aberrations in cortisol concentrations from other matrices and cardiovascular disease[82–84], cardio-metabolic syndromes[85] and chronic pelvic pain[15, 16]. Positive significant associations have been observed of high HCC with severe chronic pain[36], one year verbal memory and history of coronary artery bypass graft surgery in cardiac rehabilitation patients[65], cardiovascular disease and events[13, 69].
The results on cardio-metabolic parameters have been mixed. HCC has not been associated with diastolic blood pressure (BP)[27, 34, 65], and while O’Brien et al found a significant positive correlation between HCC and systolic BP (r=0.25, p<0.01)[34], two other studies observed no significant association[27, 65]. Stalder et al observed a positive correlation in unadjusted analysis of HCC and mean arterial pressure, but after adjustment, the correlation did not exist (r=0.047, p>0.05). They also observed mixed results between HCC and cardio-metabolic parameters (Table 2)[51]. In one study, mean values of various cardio-metabolic fitness parameters did not differ between normal and high HCC groups [65]. To date, HCC has not been associated with cancer, osteoporosis and chronic nonspecific lung diseases[69].
On balance, HCC appears to be associated with chronic disorders, particularly disorders that are strongly associated with stress (e.g., chronic pain and cardiovascular disease). There is a need for prospective studies to further elucidate the relationship of HCC with incident disease and disease progression. For example, epidemiologic studies that investigate the relationship between HCC and adrenocorticoidal conditions should take into account the duration for which individuals have had the condition, the duration of treatment, and the type of treatment that individuals receive as all these factors may influence the relationship between HCC and adrenocorticoidal conditions.
Pregnancy
Evidence of increased cortisol production during pregnancy is well-established[43, 86]. Findings on the relationship between HCC and pregnancy have been consistent with a positive association. D’Anna-Hernandez et al studied HCC among pregnant women and found that HCC was significantly higher in the third trimester compared with the first, and then decreased during the first 2 to 3 months postpartum (Table 2)[87]. In their study of mothers of newborns 2–4 days of age, mothers of toddlers aged 3–9 months, and control women who were nulliparous and non-pregnant, Kirschbaum et al found that mean HCC among mothers of newborns 2–4 days of age (their hair samples corresponded with the third trimester) was two-fold higher than that of non-pregnant nulliparous women (Table 2)[43]. Krumbholz et al also found significantly elevated HCC in hair segment reflecting the last month of pregnancy and first month of delivery[58]. Given well-documented evidence of a relationship between pregnancy and cortisol increase, investigators should exclude pregnant women from hair cortisol studies unless of course when pregnancy is the focus of the research.
Early Life Adversity
In their study of term infants and preterm infants who were >25 weeks gestational age at birth, Yamada et al found that neonatal intensive care unit (NICU) infants had significantly higher HCC compared with healthy term (≥37 weeks) infants (mean±SD: 2.06±2.05 vs. 0.11±0.42 nmol/g, p=0.004). Total number of days on the ventilator was associated with HCC and there was no significant difference in HCC for NICU term infants and NICU preterm infants[88]. In a study of depressed patients and healthy subjects, Hinkelmann et al observed lower mean HCC in subjects who experienced childhood maltreatment[52]. However, Grassi-Oliveira et al did not observe a correlation between severity of early life stress and HCC in a sample of women seeking treatment for substance abuse[79].
Although studies on early life adversity and HCC in humans have been few, compelling results have been observed in studies of non-human primates. Two studies that examined monkeys showed that those separated from their mothers in early life had aberrant HCC when placed in new social environments compared with those that were reared by their mothers[89, 90]. Additionally, Laudenslager et al observed that monkeys whose social groups were relocated during the perinatal period had elevated HCC compared with monkeys raised in a constant environment[91]. On balance, results from studies of humans and non-human primates suggest that early life adversity may influence cortisol concentrations, and have long-term negative effects on development. Thus, investigators should consider early life adversity in studies of HCC where necessary. There is a need for more studies to elucidate the relationship between HCC and perinatal and other early life experiences.
Adiposity
Results of studies examining relationships between adiposity and cortisol in blood, urine, and saliva have been varied. Investigators have reported no association[92], inverse[93–95] and positive associations[96, 97] of cortisol concentrations with a full range of measures meant to reflect body fatness or adiposity. In Table 2, we provide a summary of the relatively sparse literature devoted to assessing HCC in relation to different measures of adiposity. Of note, some[27, 46, 51, 64], but not all[34, 73] available studies have found positive relationships between HCC and at least one adiposity measure including body mass index (BMI), waist circumference (WC) and waist-to-hip-ratio (WHR).
Positive associations between HCC and BMI have been reported in various populations including fast-forward rotating shift workers[64], university students and adults[51]. However, other studies have found no relationship between HCC and BMI[27, 65, 69, 73] or WC[65]. Still, others have shown HCC to be positively correlated with WC[27, 51, 69] and WHR[27, 51] but not with hip circumference[27]. Despite some inconsistencies, available data suggest that HCC may be associated with adiposity in some, but not all populations studied to date. Studies with objective measures of adiposity and central obesity are needed to further clarify suggested associations.
Lifestyle and Behavioral Factors
Alcohol Use
Studies of other matrices suggest that aberrations in cortisol concentrations are associated with alcoholism[98] and alcohol relapse[99]. Two studies have found positive associations between HCC and high alcohol intake[45, 69]. A third study reported no associated between HCC and regular alcohol consumption (≥3 days/week), but observed positive associations between HCC and serum γ-glutamyltransferase (γGT)[51]. Overall, these findings suggest a positive relationship between high alcohol intake and HCC. More studies are needed to explore the relationship between HCC and alcohol use or abuse. Investigators should ascertain alcohol consumption among study participants and examine alcohol’s effect on HCC where necessary.
Cigarette Smoking
Evidence suggests that nicotine alters the HPA axis and acutely increases cortisol concentrations in saliva, urine and blood plasma[100–104]. However, the association may only be acute[101]. Thus far, HCC has not been associated with cigarette smoking[42, 44, 46, 51, 69]. More studies are needed to provide further evidence about the relationship between cigarette-smoking status and HCC, especially since smokers report higher levels of stress than non-smokers[105].
Oral Contraceptives and other Medications
The results of studies examining the effect of oral contraceptive use on cortisol concentrations in other matrices have been inconsistent. A summary of the relationship between oral contraceptive use and cortisol concentrations in blood plasma and saliva is given by Dettenborn et al[42]. So far, investigators have reported no significant positive or negative relationships between HCC and oral contraceptive use[42, 46, 50]. See Table 2 for report of findings on the association between HCC and other medications.
Physical Activity
Investigators have previously reported on the influence of moderate to high intensity physical activity to acutely raise concentrations of circulating cortisol in blood[106]. Positive associations have been observed for HCC and participation in endurance sports[44] and vigorous physical activity. However, no associations have been observed for moderate physical activity[70]. Further, Stalder et al observed no association between HCC and physical activity level as assessed by summing up 5-point frequency ratings in the areas of mild, moderate and vigorous physical activity over the preceding 6 months[51]. On balance, evidence suggests an association between HCC and vigorous physical activity.
DISCUSSION
The study of correlates of hair cortisol concentrations is still at an early stage. Yet, available data obtained through this systematic review are suggestive of the validity of this biomarker. Importantly, our findings suggest that hair cortisol may be associated with early life adversity, stress-related psychiatric conditions, and medical conditions indicating chronic activation of the HPA axis or high stress levels. Further research is needed to underpin the validity of the biomarker as well as to obtain deeper understanding of its potentially different associations to reactive psychiatric conditions versus psychiatric disorders with stronger hereditary etiology.
The literature suggests that some factors (e.g., natural hair color, cigarette smoking, oral contraceptive use, and medication use) may not have important influences on HCC. Other factors, such as sex, adiposity, hair treatments, and substance abuse, may be potential determinants of HCC and thus investigators may want to include these measures in future studies. For a number of other factors (e.g., age, physical activity and hair texture) available evidence are insufficient, particularly since most existing hair cortisol studies assess the effect of socio-demographic and hair-specific behaviors secondary to some other primary outcomes. As such, studies were not usually conducted in ways that maximized opportunities for comprehensive assessments of these secondary covariates[42].
Over the last decade, hair as a measure of cortisol has emerged as a promising biomarker of chronic stress and alterations of the HPA axis. Hair cortisol concentrations have shown favorable intra-individual variability in some studies. In one study, Stalder et al observed a relatively strong correlation between HCC after an average 375.3 day interval (r=0.78, p<0.0001, adjusted for perceived stress scores and number of competitions per year)[47]. Three repeated measurements at two month intervals showed correlation coefficients ranging from 0.53–0.79 (p<0.001)[47]. These findings support the reliable intra-individual stability of HCC.
Many gaps in understanding the fidelity and determinants of hair cortisol remain. One major limitation of current hair cortisol research is that most studies have been cross-sectional. Little is known about how HCC change across the human life-course; about the degree with which HCC changes across preclinical and clinical manifestations of disease; how HCC change with pharmacological and non-pharmacological treatments of conditions including mood and anxiety disorders; and how time since last episode and severity of illness influence HCC.
Other under-studied areas include the influences of diet, hair loss, and seasonal variations on HCC and the extent to which different methods of laboratory analysis affect HCC observed and reported in studies[75, 76, 107]. To date, there have been few studies of large representative populations and few studies have included ethnically, racially and economically diverse populations. Studies conducted in increasingly diverse populations will enrich the literature and provide investigators with much needed information that can be used to design, analyze and interpret HCC in clinical and population based studies.
Our assessment of correlates of HCC suggests that hair cortisol may indeed constitute an important biomarker of HPA-axis dysregulation and chronic psychosocial stress. Investigators who elect to integrate this emerging biological marker into their studies should be strategic about accounting for covariates that may confound or modify associations of primary interests. Results of hair cortisol studies should be interpreted with consideration of appropriate factors including study size and segments of hair studied. The assessment of cortisol in hair presents unique strengths—and challenges that if overcome may revolutionize the study of the potential health effects of chronic stress in large-scale epidemiologic studies.
Supplementary Material
Limitations of Cortisol Concentrations Obtained from Saliva, Blood and Urine.
Only inform about short-term cortisol concentrations (24 to 36 hours)
Easily influenced by individual and environmental characteristics
Can be invasive, especially for blood
Require repeated measurements during 24 hours over several days to obtain average chronic concentrations of cortisol
Gaps in Current Hair Cortisol Research.
Lack of prospective studies to elucidate the association of HCC with major disease risk as well as how HCC change over preclinical and clinical manifestations of disease, and with pharmacological and non-pharmacological treatments
Other under-studied areas include the effects of diet, hair loss, and seasonal variations on HCC, and the extent to which the different methods of laboratory analysis affect HCC observed and reported in studies
Little racial/ethnic variation in current studies
ACKNOWLEDGMENTS
This research was supported by grants from the National Institutes of Health (NIMHD: T37-MD001449; and NCRR/NCATS: 8UL1TR000170).
LIST OF ABBREVIATIONS
- AI
Adrenal Insufficiency
- AMI
Acute Myocardial Infarction
- BDI
Beck Depression Index
- BMI
Body Mass index
- BP
Blood Pressure
- CS
Cushing’s Syndrome
- γGT
γ-Glutamyltransferase
- HCC
Hair Cortisol Concentrations
- HPA
Hypothalamic-Pituitary-Adrenal
- NICU
Neonatal Intensive Care Unit
- NTC
Non-Traumatized Controls
- PTSD
Post-Traumatic Stress Disorder
- SD
Standard Deviation
- TC
Traumatized Controls
- WC
Waist Circumference
- WHR
Waist-to-Hip Ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None Declared
REFERENCES
- 1.King S, Hegadoren K. Stress hormones: how do they measure up? Biological Reseach for Nursing. 2002;4(2):92–103. doi: 10.1177/1099800402238334. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S, Janicki-Deverts D, Miller G. Psychological stress and disease. JAMA: The Journal of the American Medical Association. 2007;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 3.Lazarus R, Folkman S. Stress, appraisal, and coping. New York: Springer Publishing Company; 1984. [Google Scholar]
- 4.O'Connor T, O'Halloran D, Shanahan F. The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. Quarterly Journal of Medicine. 2000;93(6):323–333. doi: 10.1093/qjmed/93.6.323. [DOI] [PubMed] [Google Scholar]
- 5.McEwen B, Seeman T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896(1):30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 6.Smith L, Cidlowski J. Glucocorticoid-induced apoptosis of healthy and malignant lymphocytes. Progress in brain research. 2010;182:1–30. doi: 10.1016/S0079-6123(10)82001-1. Epub 2010/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Earle T, Wolfgang L, Weinberg J. Differential effects of harassment on cardiovascular and salivary cortisol stress reactivity and recovery in women and men. Journal of Psychosomatic Research. 1999;46(2):125–141. doi: 10.1016/s0022-3999(98)00075-0. [DOI] [PubMed] [Google Scholar]
- 8.Lindholm H, Ahlberg J, Sinisalo JCH, Hirvonen A, Partinen M, et al. Morning cortisol levels and perceived stress in irregular shift workers compared with regular daytime workers. Sleep Disorders. 2012:2012. doi: 10.1155/2012/789274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarkovic M, Stefanova E, Ciric J, Penezic Z, Kostic V, Sumarac-Dumanovic M, et al. Prolonged psychological stress suppresses cortisol secretion. Clinical endocrinology. 2003;59(6):811–816. doi: 10.1046/j.1365-2265.2003.01925.x. Epub 2004/02/21. [DOI] [PubMed] [Google Scholar]
- 10.Ceccato F, Barbot M, Zilio M, Ferasin S, Occhi G, Daniele A, et al. Performance of salivary cortisol in the diagnosis of Cushing's syndrome, adrenal incidentaloma, and adrenal insufficiency. European journal of endocrinology / European Federation of Endocrine Societies. 2013;169(1):31–36. doi: 10.1530/EJE-13-0159. Epub 2013/04/24. [DOI] [PubMed] [Google Scholar]
- 11.Chiodini I, Adda G, Scillitani A, Coletti F, Morelli V, Di Lembo S, et al. Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care. 2007;30(1):83–88. doi: 10.2337/dc06-1267. [DOI] [PubMed] [Google Scholar]
- 12.Giurgescu C. Are maternal cortisol Levels related to preterm birth? Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2009;38(4):377–390. doi: 10.1111/j.1552-6909.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- 13.Pereg D, Gow R, Mosseri M, Lishner M, Rieder M, Van Uum S, et al. Hair cortisol and the risk for acute myocardial infarction in adult men. Stress. 2011;14(1):73–81. doi: 10.3109/10253890.2010.511352. [DOI] [PubMed] [Google Scholar]
- 14.Ten S, New M, Maclaren N. Addison’s disease 2001. Journal of Clinical Endocrinology and Metabolism. 2001;86(7):2909–2922. doi: 10.1210/jcem.86.7.7636. [DOI] [PubMed] [Google Scholar]
- 15.Petrelluzzi K, Garcia M, Petta C, Grassi-Kassisse D, Spadari-Bratfisch R. Salivary cortisol concentrations, stress and quality of life in women with endometriosis and chronic pelvic pain. Stress. 2008;11(5):390–397. doi: 10.1080/10253890701840610. [DOI] [PubMed] [Google Scholar]
- 16.Heim C, Ehlert U, Hanker J, Hellhammer D. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosomatic medicine. 1998;60(3):309–318. doi: 10.1097/00006842-199805000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Morris M, Compas B, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clinical Psychology Review. 2012;32(4):301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kidambi S, Raff H, Findling J. Limitations of nocturnal salivary cortisol and urine free cortisol in the diagnosis of mild Cushing's syndrome. European journal of endocrinology / European Federation of Endocrine Societies. 2007;157(6):725–731. doi: 10.1530/EJE-07-0424. Epub 2007/12/07. [DOI] [PubMed] [Google Scholar]
- 19.Wolfram M, Bellingrath S, Feuerhahn N, Kudielka B. Cortisol responses to naturalistic and laboratory stress in student teachers: comparison with a non-stress control day. Stress and Health : Journal of the International Society for the Investigation of Stress. 2013;29(2):143–149. doi: 10.1002/smi.2439. [DOI] [PubMed] [Google Scholar]
- 20.Adam E, Hawkley L, Kudielka B, Cacioppo J. Day-to-day dynamics of experience--cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(45):17058–17063. doi: 10.1073/pnas.0605053103. Epub 2006/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson E, Checkley S, Papadopoulos A, Poon L, Daley S, Wardle J. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosomatic medicine. 1999;61(2):214–224. doi: 10.1097/00006842-199903000-00014. Epub 1999/04/16. [DOI] [PubMed] [Google Scholar]
- 22.Kobelt A, Hemsworth P, Barnett J, Butler K. Sources of sampling variation in saliva cortisol in dogs. Research in veterinary science. 2003;75(2):157–161. doi: 10.1016/s0034-5288(03)00080-8. Epub 2003/08/02. [DOI] [PubMed] [Google Scholar]
- 23.Raul J, Cirimele V, Ludes B, Kintz P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin Biochem. 2004;37(12):1105–1111. doi: 10.1016/j.clinbiochem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Bevalot F, Gaillard Y, Lhermitte M, Pepin G. Analysis of corticosteroids in hair by liquid chromatography-electrospray ionization mass spectrometry. Journal of Chromatography B: Biomedical Sciences and Applications. 2000;740(2):227–236. doi: 10.1016/s0378-4347(00)00085-2. [DOI] [PubMed] [Google Scholar]
- 25.Cirimele V, Kintz P, Doray S, Ludes B. Determination of chronic abuse of the anaesthetic agents midazolam and propofol as demonstrated by hair analysis. International Journal of Legal Medicine. 2002;116(1):54–57. doi: 10.1007/s004140100240. [DOI] [PubMed] [Google Scholar]
- 26.Gaillard Y, Vayssette F, Pépin G. Compared interest between hair analysis and urinalysis in doping controls. Results for amphetamines, corticosteroids and anabolic steroids in racing cyclists. Forensic Science International. 2000;107(1–3):361–379. doi: 10.1016/s0379-0738(99)00179-6. [DOI] [PubMed] [Google Scholar]
- 27.Manenschijn L, Koper J, Lamberts S, van Rossum E. Evaluation of a method to measure long term cortisol levels. Steroids. 2011;76(10–11):1032–1036. doi: 10.1016/j.steroids.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Thomson S, Koren G, Fraser L, Rieder M, Friedman T, Van Uum S. Hair analysis provides a historical record of cortisol levels in Cushing's syndrome. Experimental and Clinical Endocrinology and Diabetes. 2010;118(2):133–138. doi: 10.1055/s-0029-1220771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stalder T, Kirschbaum C. Analysis of cortisol in hair - State of the art and future directions. Brain, Behavior and Immunity. 2012;26(7):1019–1029. doi: 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Van Holland BJ, Frings-Dresen MH, Sluiter JK. Measuring short-term and long-term physiological stress effects by cortisol reactivity in saliva and hair. International Archives of Occupational and Environmental Health. 2012;85(8):849–852. doi: 10.1007/s00420-011-0727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanaelst B, Huybrechts I, Bammann K, Michels N, de Vriendt T, Vyncke K, et al. Intercorrelations between serum, salivary, and hair cortisol and child-reported estimates of stress in elementary school girls. Psychophysiology. 2012;49(8):1072–1081. doi: 10.1111/j.1469-8986.2012.01396.x. [DOI] [PubMed] [Google Scholar]
- 32.Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum S. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clinical and Investigative Medicine. 2007;30(5):E183–E191. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- 33.Karlén J, Ludvigsson J, Frostell A, Theodorsson E, Faresjö T. Cortisol in hair measured in young adults - a biomarker of major life stressors? BioMed Central Clinical Pathology. 2011;11(1):1–6. doi: 10.1186/1472-6890-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Brien K, Tronick E, Moore C. Relationship between Hair Cortisol and Perceived Chronic Stress in a Diverse Sample. Stress and Health [Internet] 2012;(5) doi: 10.1002/smi.2475. [DOI] [PubMed] [Google Scholar]
- 35.Gow R, Koren G, Rieder M, Van Uum S. Hair cortisol content in patients with adrenal insufficiency on hydrocortisone replacement therapy. Clinical endocrinology. 2011;74(6):687–693. doi: 10.1111/j.1365-2265.2011.04001.x. [DOI] [PubMed] [Google Scholar]
- 36.Van Uum S, Fraser L, Paul T, Sauve B, Koren G, Morley-Forster P. Elevated content of cortisol in hair of patients with severe chronic pain: A novel biomarker for stress. Stress. 2008;11(6):483–488. doi: 10.1080/10253890801887388. [DOI] [PubMed] [Google Scholar]
- 37.Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. The relationship between stress and hair cortisol in healthy pregnant women. Clinical and Investigative Medicine. 2007;30(2):E103–E107. doi: 10.25011/cim.v30i2.986. [DOI] [PubMed] [Google Scholar]
- 38.Society of hair testing. Forensic Science International. 1997;84(1–3):3–6. doi: 10.1016/s0379-0738(96)02057-9. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Xie Q, Gao W, Xu Y, Wang S, Deng H, et al. Time course of cortisol loss in hair segments under immersion in hot water. Clin Chim Acta: International Journal of Clinical Chemistry and Diagnostic Laboratory Medicine. 2012;413(3–4):434–440. doi: 10.1016/j.cca.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 40.Dettenborn L, Muhtz C, Skoluda N, Stalder T, Steudte S, Hinkelmann K, et al. Introducing a novel method to assess cumulative steroid concentrations: Increased hair cortisol concentrations over 6 months in medicated patients with depression. Stress. 2012;15(3):348–353. doi: 10.3109/10253890.2011.619239. [DOI] [PubMed] [Google Scholar]
- 41.Dettenborn L, Tietze A, Bruckner F, Kirschbaum C. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology. 2010;35(9):1404–1409. doi: 10.1016/j.psyneuen.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Dettenborn L, Tietze A, Kirschbaum C, Stalder T. The assessment of cortisol in human hair - associations with sociodemographic variables and potential confounders. Stress. 2012;15(6):578–588. doi: 10.3109/10253890.2012.654479. [DOI] [PubMed] [Google Scholar]
- 43.Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34(1):32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 44.Skoluda N, Dettenborn L, Stalder T, Kirschbaum C. Elevated hair cortisol concentrations in endurance athletes. Psychoneuroendocrinology. 2012;37(5):611–617. doi: 10.1016/j.psyneuen.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Stalder T, Kirschbaum C, Heinze K, Steudte S, Foley P, Tietze A, et al. Use of hair cortisol analysis to detect hypercortisolism during active drinking phases in alcohol-dependent individuals. Biological Psychology. 2010;85(3):357–360. doi: 10.1016/j.biopsycho.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Stalder T, Steudte S, Alexander N, Miller R, Gao W, Dettenborn L, et al. Cortisol in hair, body mass index and stress-related measures. Biological Psychology. 2012;90(3):218–223. doi: 10.1016/j.biopsycho.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Stalder T, Steudte S, Miller R, Skoluda N, Dettenborn L, Kirschbaum C. Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology. 2012;37(5):602–610. doi: 10.1016/j.psyneuen.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Steudte S, Kolassa I, Stalder T, Pfeiffer A, Kirschbaum C, Elbert T. Increased cortisol concentrations in hair of severely traumatized Ugandan individuals with PTSD. Psychoneuroendocrinology. 2011;36(8):1193–1200. doi: 10.1016/j.psyneuen.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Steudte S, Stalder T, Dettenborn L, Klumbies E, Foley P, Beesdo-Baum K, et al. Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Research. 2011;186(2–3):310–314. doi: 10.1016/j.psychres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Steudte S, Kirschbaum C, Gao W, Alexander N, Schonfeld S, Hoyer J, et al. Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.03.011. Epub 2013/04/30. [DOI] [PubMed] [Google Scholar]
- 51.Stalder T, Kirschbaum C, Alexander N, Bornstein S, Gao W, Miller R, et al. Cortisol in hair and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98(6):2573–2580. doi: 10.1210/jc.2013-1056. [DOI] [PubMed] [Google Scholar]
- 52.Hinkelmann K, Muhtz C, Dettenborn L, Agorastos A, Wingenfeld K, Spitzer C, et al. Association between childhood trauma and low hair cortisol in depressed patients and healthy control subjects. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 53.Groeneveld M, Vermeer H, Linting M, Noppe G, van Rossum EM. Children's hair cortisol as a biomarker of stress at school entry. Stress. 2013:1–5. doi: 10.3109/10253890.2013.817553. vI. [DOI] [PubMed] [Google Scholar]
- 54.Manenschijn L, Koper J, De Jong F, Feelders R, Van Rossum E, Van Den Akker E, et al. A novel tool in the diagnosis and follow-up of (cyclic) Cushing's syndrome: Measurement of long-term cortisol in scalp hair. Journal of Clinical Endocrinology and Metabolism. 2012;97(10):E1836–E1843. doi: 10.1210/jc.2012-1852. [DOI] [PubMed] [Google Scholar]
- 55.Dowlati Y, Herrmann N, Swardfager W, Thomson S, Oh PI, Van Uum S, et al. Relationship between hair cortisol concentrations and depressive symptoms in patients with coronary artery disease. Journal of Neuropsychiatric Disease and Treatment. 2010;6:393–400. [PMC free article] [PubMed] [Google Scholar]
- 56.Luo H, Hu X, Liu X, Ma X, Guo W, Qiu C, et al. Hair cortisol level as a biomarker for altered hypothalamic-pituitary-adrenal activity in female adolescents with posttraumatic stress disorder after the 2008 Wenchuan earthquake. Biological Psychiatry. 2012;72(1):65–69. doi: 10.1016/j.biopsych.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 57.Xie Q, Gao W, Li J, Qiao T, Jin J, Deng H, et al. Correlation of cortisol in 1-cm hair segment with salivary cortisol in human: hair cortisol as an endogenous biomarker. Clinical Chemistry and Laboratory Medicine. 2011;49(12):2013–2019. doi: 10.1515/CCLM.2011.706. [DOI] [PubMed] [Google Scholar]
- 58.Krumbholz A, Anielski P, Reisch N, Schelling G, Thieme D. Diagnostic value of concentration profiles of glucocorticosteroids and endocannabinoids in hair. Therapeutic Drug Monitoring. 2013 doi: 10.1097/FTD.0b013e3182953e43. Epub 2013/08/15. [DOI] [PubMed] [Google Scholar]
- 59.Cutler D, Lleras-Muney A, Vogl T. Socioeconomic status and health: dimensions and mechanisms. Working Paper Series No 14333. 2008;(14333):1–50. [Google Scholar]
- 60.Dowd J, Simanek A, Aiello A. Socio-economic status, cortisol and allostatic load: a review of the literature. International Journal of Epidemiology. 2009;38(5):1297–1309. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaghri Z, Guhn M, Weinberg J, Grunau R, Yu W, Hertzman C. Hair cortisol reflects socio-economic factors and hair zinc in preschoolers. Psychoneuroendocrinology. 2013;38(3):331–340. doi: 10.1016/j.psyneuen.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Z, Li J, Zhang J, Xing X, Gao W, Lu Z, et al. Simultaneous determination of hair cortisol, cortisone and DHEAS with liquid chromatography-electrospray ionization-tandem mass spectrometry in negative mode. Journal of Chromatography B. 2013;929:187–194. doi: 10.1016/j.jchromb.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 63.Wong I, Ostry A, Demers P, Davies H. Job strain and shift work influences on biomarkers and subclinical heart disease indicators: a pilot study. Journal of Occupational and Environmental Hygiene. 2012;9(8):467–477. doi: 10.1080/15459624.2012.693831. [DOI] [PubMed] [Google Scholar]
- 64.Manenschijn L, De Jong F, Koper J, Van Rossum E, Van Kruysbergen R. Shift work at young age is associated with elevated long-term cortisol levels and body mass index. Journal of Clinical endocrinology and metabolism. 2011;96(11):E1862–E1865. doi: 10.1210/jc.2011-1551. [DOI] [PubMed] [Google Scholar]
- 65.Saleem M, Herrmann N, Swardfager W, Oh P, Shammi P, Koren G, et al. Higher cortisol predicts less improvement in verbal memory performance after cardiac rehabilitation in patients with coronary artery disease. Cardiovascular Psychiatry and Neurology. 2013:2013. doi: 10.1155/2013/340342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Larsson C, Gullberg B, Rastam L, Lindblad U. Salivary cortisol differs with age and sex and shows inverse associations with WHR in Swedish women: a cross-sectional study. BioMed Central: Endocrine Disorders. 2009;9(16):1–11. doi: 10.1186/1472-6823-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao Z, Lu F, Xie Y, Fu YR, Bogdan A, Touitou Y. Cortisol secretion in the elderly Influence of age, sex and cardiovascular disease in a Chinese population. Steroids. 2003;68(6):551–555. doi: 10.1016/s0039-128x(03)00083-7. [DOI] [PubMed] [Google Scholar]
- 68.Goldberg S, Smith GS, Barnes A, Ma Y, Kramer E, Robeson K, et al. Serotonin modulation of cerebral glucose metabolism in normal aging. Neurobiology of Aging. 2004;25(2):167–174. doi: 10.1016/s0197-4580(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 69.Manenschijn L, Schaap L, van Schoor N, van der Pas S, Peeters G, Lips P, et al. High long-term cortisol levels, measured in scalp hair, are associated with a history of cardiovascular disease. J Clin Endocrinol Metab. 2013;98(5):2078–2083. doi: 10.1210/jc.2012-3663. [DOI] [PubMed] [Google Scholar]
- 70.Gerber M, Jonsdottir I, Kalak N, Elliot C, Pühse U, Holsboer-Trachsler E, et al. Objectively assessed physical activity is associated with increased hair cortisol content in young adults. Stress. 2013:1–7. doi: 10.3109/10253890.2013.823599. [DOI] [PubMed] [Google Scholar]
- 71.Lamb E, Noonan K, Burrin J. Urine-free cortisol excretion: evidence of sex-dependence. Annals of Clinical Biochemistry. 1994;31:455–458. doi: 10.1177/000456329403100505. [DOI] [PubMed] [Google Scholar]
- 72.Manenschijn L, Schaap L, van Schoor N, van der Pas S, Peeters G, Lips P, et al. High long-term cortisol levels, measured in scalp hair, are associated with a history of cardiovascular disease. Journal of Clinical Endocrinology and Metabolism. 2013;98(5):2078–2083. doi: 10.1210/jc.2012-3663. [DOI] [PubMed] [Google Scholar]
- 73.Manenschijn L, Spijker A, Koper J, Jetten A, Giltay E, Haffmans J, et al. Long-term cortisol in bipolar disorder: Associations with age of onset and psychiatric co-morbidity. Psychoneuroendocrinology. 2012;37(12):1960–1968. doi: 10.1016/j.psyneuen.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 74.Williams D, Jackson P. Social sources of racial disparities in health. Health Affairs. 2005;24(2):325–334. doi: 10.1377/hlthaff.24.2.325. [DOI] [PubMed] [Google Scholar]
- 75.Loussouarn G. African hair growth parameters. British Journal of Dermatology. 2001;145(2):294–297. doi: 10.1046/j.1365-2133.2001.04350.x. [DOI] [PubMed] [Google Scholar]
- 76.Wennig R. Potential problems with the interpretation of hair analysis results. Forensic Science International. 2000;107(1–3):5–12. doi: 10.1016/s0379-0738(99)00146-2. [DOI] [PubMed] [Google Scholar]
- 77.Kiraly S, Ancill R, Dimitrova G. The relationship of endogenous cortisol to psychiatric disorder: a review. Canadian Journal of Psychiatry. 1997;42(4):415–420. doi: 10.1177/070674379704200409. [DOI] [PubMed] [Google Scholar]
- 78.Burke H, Davis M, Otte C, Mohr D. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 79.Grassi-Oliveira R, Pezzi J, Daruy-Filho L, Viola T, Francke I, Leite C, et al. Hair cortisol and stressful life events retrospective assessment in crack cocaine users. The American Journal of Drug and Alcohol Abuse. 2012;38(6):535–538. doi: 10.3109/00952990.2012.694538. [DOI] [PubMed] [Google Scholar]
- 80.Gerber M, Kalak N, Elliot C, Holsboer-Trachsler E, Pühse U, Brand S. Both hair cortisol levels and perceived stress predict increased symptoms of depression: an exploratory study in young adults. Neuropsychobiology. 2013;68(2):100–109. doi: 10.1159/000351735. [DOI] [PubMed] [Google Scholar]
- 81.De Leo M, Cozzolino A, Colao A, Pivonello R. Subclinical Cushing's syndrome. Best Practice and Research Clinical Endocrinology and Metabolism. 2012;26(4):497–505. doi: 10.1016/j.beem.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 82.Whitworth J, Mangos G, Kelly J. Cushing, cortisol, and cardiovascular disease. Hypertension. 2000;36(5):912–916. doi: 10.1161/01.hyp.36.5.912. [DOI] [PubMed] [Google Scholar]
- 83.Tauchmanovà L, Rossi R, Biondi B, Pulcrano M, Nuzzo V, Palmieri E, et al. Patients with subclinical Cushing's syndrome due to adrenal adenoma have increased cardiovascular risk. Journal of Clinical endocrinology and metabolism. 2002;87(11):4872–4878. doi: 10.1210/jc.2001-011766. [DOI] [PubMed] [Google Scholar]
- 84.Dekker M, Koper J, Van Aken M, Pols H, De Jong F, Lamberts S, et al. Salivary cortisol is related to atherosclerosis of carotid arteries. Journal of Clinical endocrinology and metabolism. 2008;93(10):3741–3747. doi: 10.1210/jc.2008-0496. [DOI] [PubMed] [Google Scholar]
- 85.Litchfield W, Hunt S, Jeunemaitre X, Fisher N, Hopkins P, Williams R, et al. Increased urinary free cortisol: a potential intermediate phenotype of essential hypertension. Hypertension. 1998;31(2):569–574. doi: 10.1161/01.hyp.31.2.569. [DOI] [PubMed] [Google Scholar]
- 86.Scott E, McGarrigle H, Lachelin G. The increase in plasma and saliva cortisol levels in pregnancy is not due to the increase in corticosteroid-binding globulin levels. The Journal of Clinical Endocrinology and Metabolism. 1990;71(3):639–644. doi: 10.1210/jcem-71-3-639. [DOI] [PubMed] [Google Scholar]
- 87.D'Anna-Hernandez K, Ross R, Natvig C, Laudenslager M. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiology and behavior. 2011;104(2):348–353. doi: 10.1016/j.physbeh.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, et al. Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology. 2007;92(1):42–49. doi: 10.1159/000100085. [DOI] [PubMed] [Google Scholar]
- 89.Feng X, Wang L, Yang S, Qin D, Wang J, Li C, et al. Maternal separation produces lasting changes in cortisol and behavior in rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(34):14312–14317. doi: 10.1073/pnas.1010943108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dettmer A, Novak M, Suomi S, Meyer J. Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: Hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology. 2012;37(2):191–199. doi: 10.1016/j.psyneuen.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laudenslager M, Natvig C, Corcoran C, Blevins M, Pierre P, Bennett A. The influences of perinatal challenge persist into the adolescent period in socially housed bonnet macaques (Macaca radiata) Developmental Psychobiology. 2012;55(3):316–322. doi: 10.1002/dev.21030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park S, Blumenthal J, Georgiades A, Lee S. Association of cortisol and the metabolic syndrome in Korean men and women. Journal of Korean Medical Science. 2011;26(7):914–918. doi: 10.3346/jkms.2011.26.7.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosmond R, Holm G, Bjorntorp P. Food-induced cortisol secretion in relation to anthropometric, metabolic and haemodynamic variables in men. International Journal of Obesity. 2000;24(4):416–422. doi: 10.1038/sj.ijo.0801173. [DOI] [PubMed] [Google Scholar]
- 94.Power C, Li L, Hertzman C. Associations of early growth and adult adiposity with patterns of salivary cortisol in adulthood. Journal of Clinical Endocrinology and Metabolism. 2006;91(11):4264–4270. doi: 10.1210/jc.2006-0625. [DOI] [PubMed] [Google Scholar]
- 95.Jones A, McMillan M, Jones R, Kowalik G, Steeden J, Deanfield J, et al. Adiposity is associated with blunted cardiovascular, neuroendocrine and cognitive responses to acute mental stress. PLoS One. 2012;7(6):e39143. doi: 10.1371/journal.pone.0039143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marin P, Darin N, Amemiya T, Andersson B, Jern S, Bjorntorp P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41(8):882–886. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- 97.Peeke P, Chrousos G. Hypercortisolism and Obesity. Annals of the New York Academy of Sciences. 1995;771(1):665–676. doi: 10.1111/j.1749-6632.1995.tb44719.x. [DOI] [PubMed] [Google Scholar]
- 98.Besemer F, Pereira A, Smit J. Alcohol-induced Cushing syndrome: Hypercortisolism caused by alcohol abuse. Netherlands Journal of Medicine. 2011;69(7):318–323. [PubMed] [Google Scholar]
- 99.Sinha R. New Findings on Biological Factors Predicting Addiction Relapse Vulnerability. Current Psychiatry Reports. 2011;13(5):398–405. doi: 10.1007/s11920-011-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mendelson J, Goletiani N, Sholar M, Siegel A, Mello N. Effects of smoking successive low- and high-nicotine cigarettes on hypothalamic-pituitary-adrenal axis hormones and mood in men. Neuropsychopharmacology. 2008;33(4):749–760. doi: 10.1038/sj.npp.1301455. [DOI] [PubMed] [Google Scholar]
- 101.Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. Journal of Clinical Endocrinology and Metabolism. 2007;92(3):819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- 102.Steptoe A, Ussher M. Smoking, cortisol and nicotine. International Journal of Psychophysiology. 2006;59(3):228–235. doi: 10.1016/j.ijpsycho.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 103.Kellar K, Davila-Garcia M, Xiao Y. Pharmacology of neuronal nicotinic acetylcholine recceptors: effects of acute and chronic nicotine. Nicotine and Tobacco Research. 1999;1(Suppl. 2):S117–S120. doi: 10.1080/14622299050011921. discussion S39-S40. [DOI] [PubMed] [Google Scholar]
- 104.Wilkins J, Carlson H, Van Vunakis H, Hill M, Gritz E, Jarvik M. Nicotine from cigarette smoking increases circulating levels of cortisol, growth hormone, and prolactin in male chronic smokers. Psychopharmacology. 1982;78(4):305–308. doi: 10.1007/BF00433730. [DOI] [PubMed] [Google Scholar]
- 105.Maxson P, Edwards S, Ingram A, Miranda M. Psychosocial differences between smokers and non-smokers during pregnancy. Addictive Behaviors. 2012;37(2):153–159. doi: 10.1016/j.addbeh.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 106.Hill E, Zack E, Battaglini C, Viru M, Viru A, Hackney A. Exercise and circulating cortisol levels: the intensity threshold effect. Journal of Endocrinological Investigation. 2008;31(7):587–591. doi: 10.1007/BF03345606. [DOI] [PubMed] [Google Scholar]
- 107.Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37(5):589–601. doi: 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.