Abstract
Phosphorus plays a critical role in diverse biological processes, and therefore, the regulation of phosphorus balance and homeostasis are critical to the well-being of the organism. Changes in environmental, dietary and serum concentrations of inorganic phosphorus are detected by sensors that elicit changes in cellular function and alter the efficiency by which phosphorus is conserved. Short-term, post-cibal responses which occur independently of hormones previously thought to be important in phosphorus homeostasis may play a larger role than previously appreciated in the regulation of phosphorus homeostasis. Several hormones and regulatory factors such as the vitamin D endocrine system, parathyroid hormone, and the phosphatonins (FGF-23, sFRP-4, MEPE) among others, may play a role only in the long-term regulation of phosphorus homeostasis. In this review we will discuss how organisms sense changes in phosphate concentrations and how changes in hormonal factors result in the conservation or excretion of phosphorus.
Phosphorus is required for diverse biological processes
Phosphorus plays a critical role in cellular biology (5). Many cellular processes require phosphorus in one form or another, and include nucleic acid synthesis and metabolism (37), energy metabolism (42, 46), cellular signaling (38), membrane integrity (53, 58), muscle function (10, 33), enzyme activity (74), lipid metabolism (25) and bone mineralization (30). Phosphorus is present in virtually every bodily fluid. In human plasma or serum, phosphorus exists in the form of inorganic phosphorus or phosphate (Pi), lipid phosphorus and phosphoric ester phosphorus. Total serum phosphorus concentrations range between 89–149 mg/L (2.87–4.81 mmol/L), inorganic phosphorus (phosphate, Pi) concentrations between 25.6–41.6 mg/L (0.83–1.34 mmol/L) (these change with age) (5), phosphoric ester phosphorus concentrations between 25–45 mg/dL (0.81–1.45 mmol/L) and lipid phosphorus concentration between 69–97 mg/L (2.23–3.13 mmol/L) (22). In mammals, bone a contains substantial amount of phosphorus (approximately 10 g per 100-g dry fat-free tissue); in comparison, muscle contains a the 0.2 g per 100-g fat free tissue, and brain 0.33-g per 100-g fresh tissue (22).
Given its widespread distribution and critical role in vital cellular processes it is not surprising that a deficiency of phosphorus results in clinical disease including muscle weakness, rhabdomyolysis, impaired leukocyte function, and abnormal bone mineralization resulting in rickets or osteomalacia (34, 35, 64).
Organs involved in phosphorus homeostasis
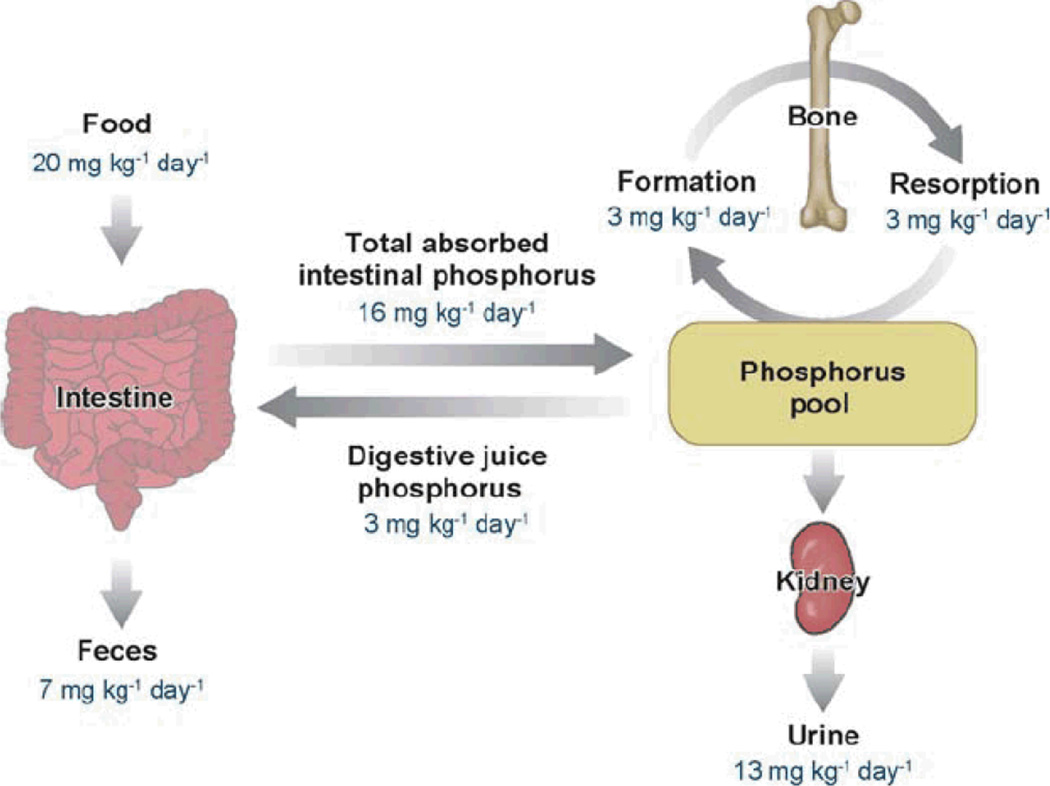
The intestine and kidney play important roles in the absorption of phosphorus (in the form of Pi) from the diet and in the excretion of phosphorus (in the form of Pi) in the urine, respectively (4, 5). The quantitative aspects of phosphorus homeostasis in humans are shown in Figure 1 (5). In states of neutral phosphorus balance, the amount of phosphorus absorbed in the intestine (about 1–1.5 g/24 hours) is equivalent to the amount excreted in the urine. Various hormones and factors involved in the regulation of phosphorus homeostasis alter the efficiency of Pi absorption in the intestine or the reabsorption of Pi in the proximal tubule of the kidney. Pi absorption in the intestine is increased by 1α,25(OH)2D3, although there is evidence for a 1α,25(OH)2D3-independent increase in Pi transport during Pi deprivation that occurs in the absence of the 1α,25(OH)2D3 receptor (17, 20, 21, 63, 72, 78, 81). In the kidney, Pi is reabsorbed along the proximal convoluted and proximal straight tubule and Pi reabsorption is influenced by numerous factors, most importantly PTH (see Table 1) (4). In addition, the movement of Pi from the extracellular fluid into soft tissue and bone is controlled by numerous factors as well. Serum Pi concentrations can be altered significantly without changes in the absorption of Pi in the intestine or changes in excretion in the kidney.
Figure 1.
Phosphorus homeostasis in normal humans (5).
TABLE I.
Factors Influencing Phosphate Absorption or Reabsorption in The Intestine And Kidney
| Intestine | |
|---|---|
| Factors that increase Pi absorption | |
| |
| Factors that reduce Pi absorption | |
| |
| Kidney | |
| Factors that increase Pi reabsorption | |
|
|
| Factors that decrease Pi reabsorption | |
|
|
The regulation of phosphorus homeostasis
In the regulation of a metabolic process, it is important to consider: 1) how environmental signals to which the metabolic process responds are sensed; 2) the nature of the responses that follow both with respect to changes in mediators, and the effects of mediators in different organs; 3) temporal differences between the responses. As we will demonstrate, in the case of Pi, short-term responses that occur shortly (minutes to hours) after the feeding of a high Pi meal may play a larger role than previously appreciated. Furthermore, changes in the concentrations of hormones previously thought to be important in this regard the regulation of Pi homeostasis may only be of relevance during long-term changes (over a period of days) in dietary Pi intake.
Phosphate sensing and cellular responses to changes in phosphate concentrations in the environment
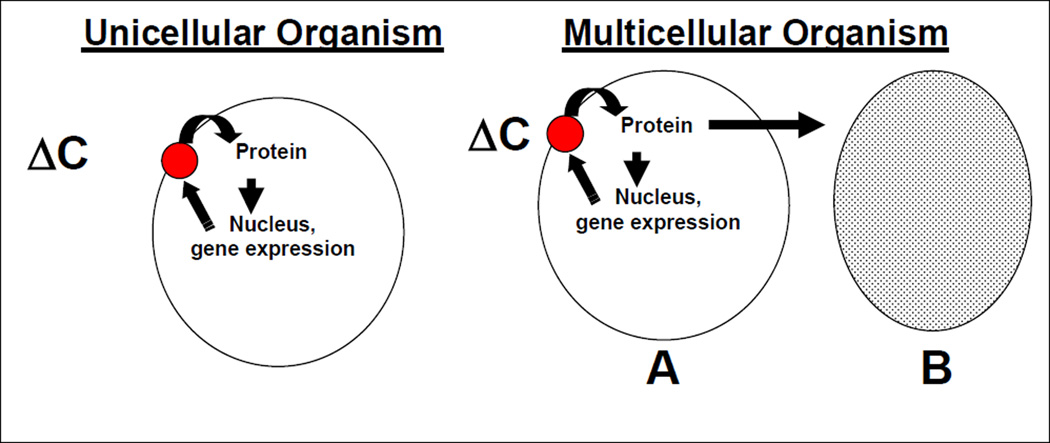
An important question regarding the regulation of phosphorus homeostasis is how the organism or individual senses changes in Pi concentrations in the environment and adjusts metabolic processes to accommodate such changes. Thus, in states of phosphorus deficiency, following a change in the concentrations of Pi, the acquisition and retention of Pi by the organism should be accelerated, whereas, in states of phosphorus excess, Pi acquisition and retention should be reduced. Individual cells or uni-cellular organisms sense changes in extracellular (or in some cases intracellular) Pi concentrations (ΔC) via specific “phosphate sensors” (41, 51, 76). The sensors, in turn, alter intracellular protein metabolism, generally by altering the phosphorylation state of intracellular proteins, and subsequent nuclear transcription events (Figure 2). Proteins synthesized in response to changes in gene transcription increase the efficiency with which phosphorus is retained by the cell, and may be components of the cellular Pi sensor.
Figure 2.
Mechanisms by which cells and organisms respond to alterations in the extracellular phosphorus concentrations. See text for details.
a. Phosphate-sensing in cells
Responses to changes in extra cellular phosphate in Escherichia coli and Saccharomyces cerevisiae are illustrative. For details the reader is referred to two recent reviews, (41, 51). Briefly, in E. coli a set of proteins in the peri-plasmic membrane (PstS, PstC, PstA and PstB in association with a protein PhoU) sense low concentrations of Pi in the external environment and enhance the uptake of Pi into the cell (41). Phosphorylation of histidine residues on a protein, PhoR occurs; phosphorylated PhoR, in turn, phosphorylates PhoB on aspartase residues. Phosphorylated PhoB acts as a transcription factor by binding to DNA sequences known as “PHO boxes” to increase the transcription of genes in the Pho regulon. When Pi concentrations on no longer limiting, PhoR his de-phosphorylated and no longer phosphorylates PhoB; PhoB in its on-phosphorylated state is incapable of binding PHO boxes and activating transcription. Many of the genes in the Pho regulon help the organism adapt to changes in phosphorus concentrations. In Saccharomyces cerevisiae, when Pi in the environment is limited, the cyclin-dependent kinase (CDK) inhibitor Pho81 inactivates the Pho80-Pho85 complex (51). As a result the transcription factor, Pho4 is un-phosphorylated and active, leading to the induction of PHO genes, one of which encodes a protein, Pho84, that functions has a high affinity Pi transporter and scavenges phosphate from the medium. When Pi is no longer limiting, Pho84 is degraded and the transcription factor Pho4 is phosphorylated and exported from the nucleus to the cytoplasm, thereby turning off the expression of the PHO genes.
In multicellular organisms, the same sorts of processes that occur in unicellular organisms are likely to occur in cells that are present in the intestine and kidney, although the specific sensors and effector proteins are likely to be different. However, in multi-cellular organisms it is likely that various signaling molecules are also elaborated by the sensing cell that subsequently alter of the efficiency of Pi absorption in other organs in a hormonal or autocrine/paracrine fashion. Markowitz et al have shown that renal epithelial cells maintained in culture are capable of responding directly to changes in environmental Pi concentrations by altering the efficiency of Pi uptake (48). The authors grew opossum kidney cells in high or low Pi media and were able to show changes in the efficiency of sodium-dependent Pi absorption within one hour of changing medium Pi concentrations. Given the manner in which the cells were grown, it is unlikely that changes in a hormonal factor were involved in changing the efficiency of sodium-dependent Pi uptake. Several investigators have suggested that intestinal cells may also respond directly to changes in Pi concentrations by altering the efficiency of Pi transport (45, 57, 63). Others have demonstrated that non-epithelial cells such as osteoblasts and marrow stromal cells are capable of responding to changes in medium Pi concentrations by altering BMP-4 expression, Runx2/Cbfa1 localization and alkaline phosphatase secretion (26–28). These results would suggest the presence of a phosphate sensor in mammalian cells. However, the exact biochemical nature of this sensor is not known.
b. Phosphate sensing in animals
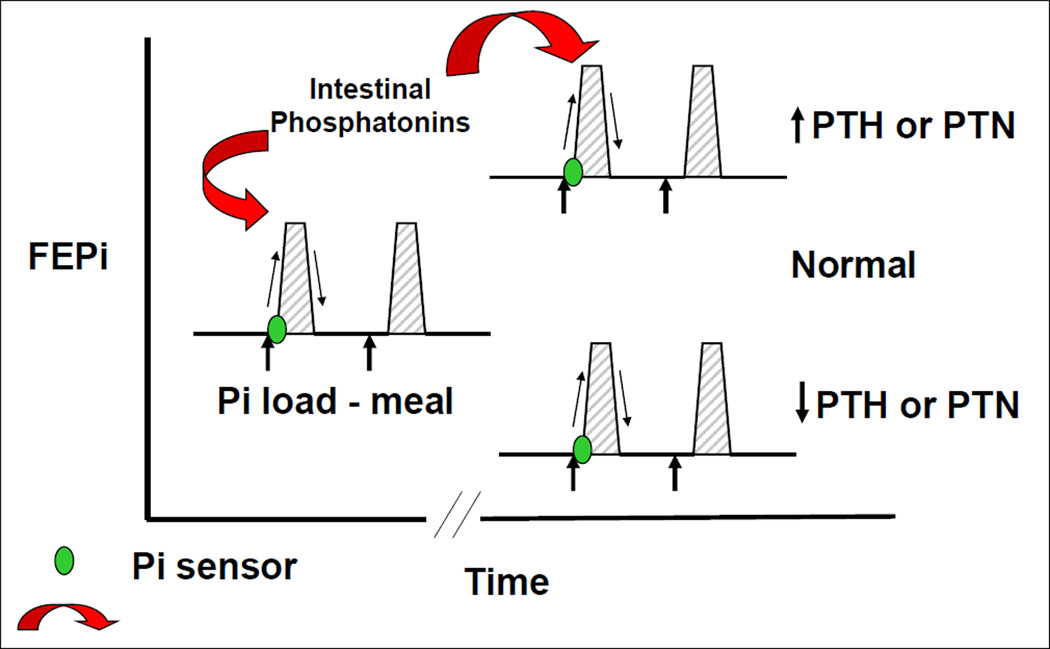
Martin and colleagues demonstrated the presence of phosphate signaling in the gastrointestinal tract and the parathyroid gland in rodents (50). Uremic animals were fed a high phosphate diet for 4 weeks, and were then administered a low phosphate diet on the day of the experiment. There was a rapid reduction in serum concentrations of parathyroid hormone within 2 hours without changes in serum calcium. Serum phosphate concentrations decreased. When uremic rats fed a high phosphate diet were gavaged with a high phosphate diet on the day of the experiment, PTH levels increased with only modest changes in serum phosphate during the first 30 minutes of the experiment. Phosphonoformic acid, a phosphorus uptake inhibitor, also rapidly increased PTH concentrations with no significant changes in serum phosphorus. The administration of intravenous phosphate was associated with a rapid increase in PTH with no changes in serum calcium and modest increases in serum phosphorus. These data would suggest the presence of a phosphate sensor in the intestine and in the parathyroid gland as well. The nature of the sensor is not known. Sensors involved in calcium and PTH homeostasis such as the calcium sensing receptor (12) are present in the intestine where they may play a role in sensing concentration of luminal amino acids (16). Whether the Ca sensing receptor or a similar g-protein coupled receptor is involved in sensing phosphate remains unknown. Our laboratory performed experiments in which phosphate was administered into the duodenum of intact or parathyroidectomized rats (6). Changes in the fractional excretion of phosphate were examined 5, 10, 20 and 30 minutes after the infusion of phosphate into the duodenum. In the intact rats, we observed a rapid increase in the fractional excretion of phosphate in the kidney without changes in serum phosphate concentrations. Rats infused sodium chloride into the duodenum showed no changes in the fractional excretion of phosphate. In thyro-parathyroidectomized rats, there was a similar increase in the fractional excretion of phosphate following the administration of intra-duodenal phosphate. The latter experiments clearly show that increases in the renal fractional excretion of phosphate following intra-duodenal phosphate infusion are independent of PTH. We also measured serum concentrations of the phosphaturic peptides, PTH, FGF-23, and sFRP-4 in both intact and parathyroidectomized rats following the infusion of intra-duodenal phosphate or intra-duodenal sodium chloride. There were no significant differences between the serum concentrations of these three phosphaturic peptides throughout the experiment. Our data show that there is a sensor for phosphate in the intestine that causes an increase in the fractional excretion of phosphate in the kidney within a short period of time following the exposure of the intestinal mucosa to increased phosphate concentrations. Furthermore, the response that occurs in the kidney is not mediated by any of the known phosphaturic factors that regulate the excretion of phosphorus in the kidney. An important caveat is that concentrations of MEPE and FGF-7 were not measured and it is not known whether concentrations of these phosphaturic substances increased following the instillation of Pi into the intestine. It should also be kept in mind that the amount of Pi instilled in to the intestine was large compared to normal dietary Pi intake. What then is the signal emanating in the intestine that tells the kidney to increase the fractional excretion of phosphate? Renal nerves are not involved in this process since we demonstrated that renal denervation did not alter the phosphaturic response following the intra-duodenal infusion of phosphate. We next prepared homogenates of the duodenum and infused filtered proteins derived from the intestine into rats. We demonstrated the presence of a factor in the duodenal mucosa that was capable of inducing phosphaturia in the intact rats. Taken together the data shows that the intestine senses an increase in luminal phosphate concentrations and releases a substance into the circulation that inhibits renal phosphate reabsorption.
Experiments performed by Segawa and colleagues have shown that the intestine is capable of responding to changes in dietary phosphate independent of vitamin D (63). In vitamin D receptor knockout mice these investigators were able to demonstrate the up-regulation of the sodium-phosphate cotransporter IIB by the low dietary phosphate. This would suggest that intestinal cells have the ability to detect changes in luminal phosphate concentrations and increase the uptake of phosphate via up regulation of the synthesis of the sodium-phosphate cotransporter.
Thus, information reviewed so far would support: 1) the capacity of unicellular organisms to respond to changes in environmental phosphate; 2) the capacity of intestinal cells and renal cells to respond directly to changes in phosphate concentrations in the medium and the capacity of the intestinal cells to respond to changes in dietary phosphate content; 3) the capacity of the intestine and kidney to alter phosphate handling independent of 1, 25-dihydroxyvitamin D, PTH and the phosphatonins.
c. PTH, the vitamin D endocrine system, and the phosphatonins in the regulation of phosphate homeostasis
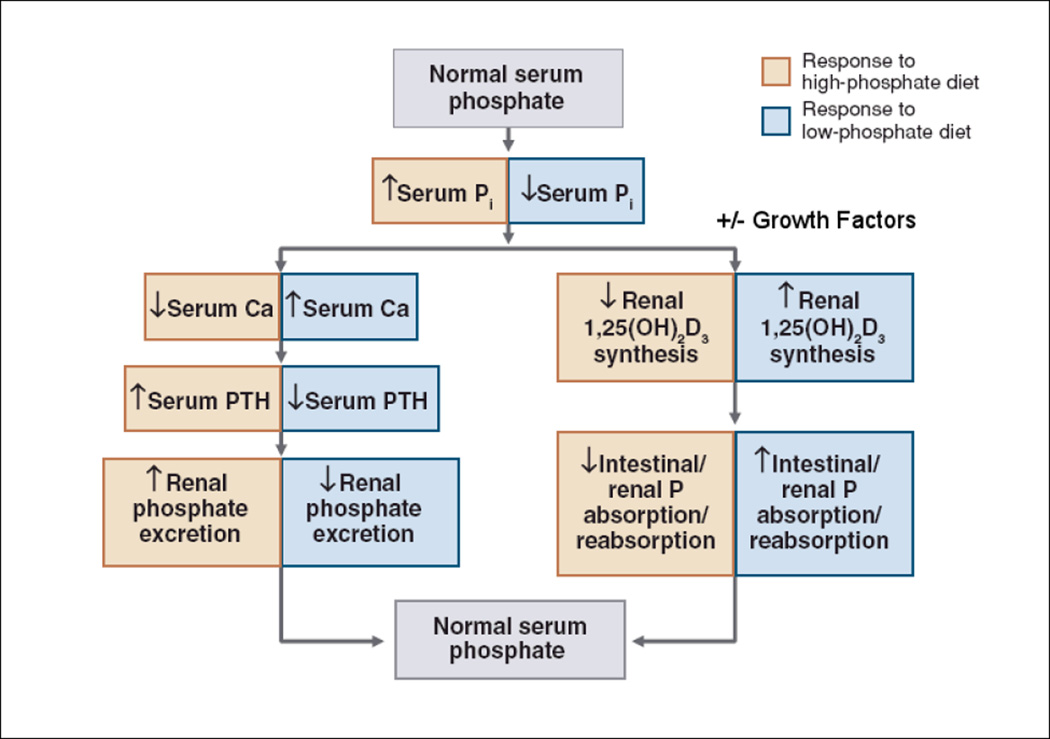
What is the role of the vitamin D endocrine system, PTH, and the phosphatonins in the regulation of phosphate transport? The role of these factors in regulating phosphate concentrations is shown in Figure 4. The phosphatonins, FGF-23 and sFRP-4 (3, 7, 43, 56, 62, 65–68), decrease, and IGF-1 increases (18) the activity of the 25-hydroxyvitamin D 1α-hydroxylase (“growth factors” in Figure 4). They may, if one accepts the debatable proposition that their levels are modulated by serum phosphate concentrations (see discussion below), also directly modulate renal phosphate reabsorption (13, 44, 52, 54, 71).
Figure 4.
Adaptations to changes in dietary phosphate (5).
Animals fed a low phosphate diet have decreased serum phosphate concentrations that are associated with a reciprocal increase in circulating plasma calcium concentrations. The increase in plasma calcium concentrations inhibits PTH release, which in turn, reduces the renal excretion of phosphate. Inorganic phosphate may also directly influence PTH release (50). Additionally, a low phosphate diet and reductions in serum Pi are associated with increased 1α,25(OH)2D3 synthesis as a result of stimulation of 25-hydroxyvitamin D 1α-hydroxylase activity (72, 77, 78). It is important to note that the increases in 25-hydroxyvitamin D 1α-hydroxylase activity that occur in animals fed a low phosphate diet, are independent of PTH, as they occur in parathyroidectomized animals. Conversely, when animals are fed a high phosphate diet, serum calcium concentrations decrease and PTH release is increased. Given the importance of phosphate ions in cellular function, a variety of other mechanisms must exist in order to maintain phosphate balance. For example, dietary phosphate deprivation markedly decreases Pi excretion yet this adaptation to decreases in Pi intake is similar in the presence and absence of PTH (73).
The "phosphatonins" induce renal phosphate wasting in patients with tumor-induced osteomalacia (TIO) (14, 23). Such patients typically exhibit low serum phosphate concentrations, normal or slightly low serum calcium concentrations, normal PTH concentrations, low or inappropriately normal serum 1α,25(OH)2D concentrations, renal phosphate wasting and a defect in bone mineralization. Conditioned media from tumors associated with this syndrome produce substances that inhibit sodium-dependent Pi transport in cultured opossum kidney cells. Of note, this phenotype is also observed in patients with autosomal dominant hypophosphatemic rickets (ADHR), X-linked hypophosphatemic rickets (XLH), and autosomal recessive hypophosphatemic rickets (ARHR) and the serum of such patients also contains phosphatonin-like activity (9, 39, 62, 64). Subsequent work by several laboratories has shown that factors such as fibroblast growth factor (FGF-23), secreted frizzled related protein (sFRP-4), fibroblast growth factor (FGF-7) and MEPE are present in these tumors and may contribute to the phosphaturia associated with this syndrome (3, 5, 9, 11, 19, 59, 60, 67). Identification of these phosphatonin molecules has led to the recognition that these proteins are also involved in numerous pathophysiological conditions associated with phosphate wasting and may be important regulators of phosphate homeostasis (Table 2) (64). While there is no question as to the importance of these factors in the pathophysiology of phosphate in the various disorders described, it remains somewhat uncertain as to whether the phosphatonins play a role in normal phosphate homeostasis.
TABLE 2.
The pathophysiology of disorders of phosphate homeostasis associated with altered phosphatonin production/circulating concentrations (64).
| Clinical Disorder | Clinical phenotype | Pathophysiology |
|---|---|---|
| Hypophosphatemic Disorders | ||
| Tumor-induced osteomalacia (TIO) | Hypophosphatemia, hyperphosphaturia, reduced 1α, 25(OH)2D concentrations or inappropriately normal 1α, 25(OH)2D concentrations for the level of serum phosphate, osteomalacia or mineralization defect | Excess of production of phosphatonins -- FGF-23, sFRP- 4, MEPE, FGF-7. |
| X-linked hypophosphatemic rickets (XLH) | As in TIO | Mutations in the endopeptidase PHEX that result in increased concentrations of FGF-23, sFRP-4 and MEPE. |
| Autosomal dominant hypophosphatemic rickets (ADHR) | As in TIO | Mutations in the FGF-23 gene that results in the formation of a mutant form of FGF-23 that is resistant to proteolysis. |
| Autosomal recessive hypophosphatemia (AR HP) | As in TIO | Mutations in the gene for DMP-1; associated with elevated concentrations of FGF-23. |
| Hyperphosphatemic Disorders | ||
| Tumoral calcinosis | Hyperphosphatemia, hypophosphaturia, elevated or normal 1α, 25(OH)2D concentrations, ectopic calcification | Mutations in the genes for GALNT3, FGF-23, and Klotho. Some patients with GALNT3 and FGF-23 mutations have diminished concentrations of intact FGF-23. The one patient with a Klotho mutation had very high FGF-23 concentrations. |
| Renal failure | Hyperphosphatemia, hypophosphaturia, reduced 1α, 25(OH)2D concentrations. | Elevated FGF-23 and FGF-7 concentrations |
The most extensively studied phosphatonin is FGF-23, a 251 amino acid secreted protein (1, 5, 8, 9). Recombinant FGF-23 administered intraperitoneally to mice or rats, induces phosphaturia and inhibits 25-hydroxyvitamin D 1α-hydroxylase activity (1, 5, 8, 9). The minimal sequence needed for phosphaturic activity resides between amino acids 176 and 210 (8). Transgenic animals over expressing FGF-23 are hypophosphatemic, phosphaturic and show the presence of rickets and reduced serum 1α,25(OH)2D concentrations or 25-hydroxyvitamin D 1α-hydroxylase activity (2, 43, 68). Conversely, mice in which the FGF-23 gene has been ablated demonstrate hyperphosphatemia, reduced phosphate excretion, markedly elevated serum 1α,25(OH)2D concentrations and renal 25-hydroxyvitamin D 1α-hydroxylase mRNA expression, vascular calcification and early mortality (65, 69). The ablation of the vitamin D receptor in FGF-23 null mice has been reported to rescue this phenotype supporting an important role for vitamin D in the pathogenesis of the abnormal phenotype seen in FGF-23 null mice (70). Feeding FGF-23 null mice a low phosphate diet results in decreased serum Pi and a reversal of vascular calcification. When the FGF-23 null mice were fed a vitamin D deficient diet, serum Pi and vascular calcification were improved minimally (75). FGF-23 binds and signals through FGF receptors 1c, 3c, and FGFR4 (40) although this is not been established in mice in vivo (47). A co-receptor, klotho, is necessary for FGF-23 to exhibit bioactivity (40, 80). The role of klotho in FGF-23 signaling is supported by the observation that klotho knock out mice have a phenotype identical to that of FGF-23 knock out mice (79).
From a physiological perspective, it would be appropriate for FGF-23 concentrations to be regulated by the intake of dietary phosphorus and by serum phosphate concentrations. In humans, in the short-term (over a period of hours) the feeding of meals containing increasing amounts of phosphate does not increase serum FGF-23 concentrations even though the previously administered meal induces a robust and dose-dependent phosphaturia (52). Nishida et al have shown that the administration of high phosphate diets to humans increases the fractional excretion of phosphate within an hour of the ingestion of a high phosphate meal (52). In this study, there were modest increases in PTH and no biologically relevant changes in FGF-23 after administration of a high phosphate diet (52). Ito et al have also failed to demonstrate an effect of infused Pi on FGF-23 concentrations (31). Likewise, in a study by Larsson et al human subjects fed normal, high or low phosphate diets for a period of 72 hours showed no differences in serum FGF-23 concentrations (44). Other studies conducted over a period of days, however, have shown changes in serum FGF-23 concentrations following alterations in the content of phosphate in the diet. For example, Ferrari et al administered a high or a low phosphate diet to humans over several days. Concomitant changes in dietary calcium designed to minimize changes in PTH were also made. Modest decreases or increases of serum FGF-23 within normal range were observed following the intake of a low or high phosphate diet, respectively (24). Others have shown similar changes when dietary phosphate is altered over a period of several weeks (13). In mice, Perwad et al have shown that a high phosphate diet increased and a low phosphate diet decreased, serum FGF-23 levels in these animals within 5 days of a changing dietary phosphate intake (55). The changes in serum FGF-23 correlated with changes in serum phosphate concentrations. Studies from our laboratory performed in rats fed a low, normal or high phosphate diet demonstrate that serum FGF-23 levels significantly decrease in animals fed a low phosphate diet, and increase in animals fed a high phosphate diet within 24 hours of altering dietary phosphate intake but do not correlate with serum phosphate in the animals fed a high phosphate diet (71). These data suggest that early and rapid changes in renal phosphate excretion occur following ingestion of a high of phosphate meal and are independent of FGF-23.
FGF-23 synthesis is regulated by 1α,25(OH)2D. Increasing doses of 1α,25(OH)2D increase FGF-23 concentrations in the serum within 24 hours but statistically significant changes are observed 4 hours after 1α,25(OH)2D treatment (36, 61). In the physiologic sense, it is possible that FGF-23 is a negative feedback regulator of the 25-hydroxyvitamin D 1α-hydroxylase enzyme.
The Wnt antagonist, secreted frizzled related protein-4 (sFRP-4) is highly expressed in tumors associated with renal phosphate wasting and osteomalacia (19). Recombinant sFRP-4 is phosphaturic in rats and prevents the up-regulation of the 25-hydroxyvitamin D 1α-hydroxylase enzyme seen in the presence of hypophosphatemia (3). sFRP-4 decreases Na+-Pi co-transporter abundance in the brush border membrane of the proximal tubule, and reduces the surface expression of the Na+-Pi IIa co-transporter in proximal tubules of the kidney, as well as, on the surface opossum kidney cells (7). sFRP-4 expression is increased in bone samples and serum from X-linked hypophosphatemic mice, in mice with a global knockout of the phex gene but not in mice in which the phex gene has been knocked out in bone alone (82). sFRP-4 protein concentrations are increased in the kidneys of rats fed a high phosphate diet for two weeks but not in animals fed a low phosphate diet suggesting a possible role for sFRP-4 during increases in phosphate intake (71). This suggests that sFRP-4 concentrations are altered in the kidney of animals fed high phosphate diet and could play a role in the long-term adaptations to high phosphate intake.
Matrix extracellular phosphoglycoprotein (MEPE) is abundantly over-expressed found in tumors associated with renal phosphate wasting and osteomalacia (59). Recombinant MEPE is phosphaturic and reduces serum phosphate concentrations when administered to mice in vivo (60). The protein inhibits sodium-dependent phosphate uptake in opossum kidney cells. The protein has also been demonstrated to reduce intestinal Pi absorption directly (49). MEPE also inhibits bone mineralization in vitro, and MEPE null mice have increased bone mineralization (29). Thus, it is possible that MEPE is important in the pathogenesis of hypophosphatemia in renal phosphate wasting observed in patients with TIO. However, MEPE infusion does not recapitulate the defect in vitamin D metabolism seen in patients with TIO (60). Infusion of MEPE reduces serum phosphate concentrations, and serum 1α,25(OH)2D concentrations increase following MEPE as would be expected in the face of hypophosphatemia. Thus, in patients with TIO, it is likely that MEPE contributes to the hypophosphatemia, but other products such as FGF-23 and sFRP-4 inhibit 1α,25(OH)2D concentrations by inhibiting the activity of the 25-hydroxyvitamin D 1α-hydroxylase. MEPE may play a role in the pathogenesis of X-linked hypophosphatemic rickets, in which there is phosphate wasting, and evidence for a mineralization defect that is independent of low phosphate concentrations in the extracellular fluid (82). MEPE expression is increased in mice with the Hyp mutation, and mice with a global knockout of the phex gene but not in mice with a bone specific knockout of the phex gene. It is not known whether MEPE is regulated by phosphate concentrations although Jain et al have demonstrated that it is correlated with serum Pi concentration in normal humans (32).
Another growth factor, FGF-7, also known as keratinocyte growth factor, is overexpressed in tumors associated with phosphate wasting and osteomalacia (15). FGF-7 inhibits sodium-dependent phosphate transport in OK cells, and we have demonstrated that FGF-7 inhibits renal phosphate reabsorption in vivo. FGF-7 is present in normal plasma and is significantly increased in patients with renal failure (personal observations). Whether or not FGF-7 is regulated by phosphate concentrations is unknown.
Conclusions
The regulation of phosphate transport in vivo should be thought of in terms of rapid, short-term adaptive processes that occur within a timeframe of a few hours after the administration of a meal containing large amounts of phosphate. These processes involve phosphate sensing in the intestine and the elaboration of novel factors that alter the efficiency of phosphate transport in the kidney and possibly directly in the intestine itself. The more long-term adaptations to changes in phosphate transport are likely to be mediated by the vitamin D endocrine system and PTH. The role of the phosphatonins in normal human physiology remains to be established, although there is no question as to their importance in the pathophysiology of diseases associated with alterations in serum phosphate homeostasis.
Figure 3.
Intestinal phosphate sensing increases the fractional excretion of phosphorus in the kidney following increases in intestinal luminal phosphate concentrations by the release of an intestinal mediator ("intestinal phosphatonin").
Acknowledgments
Supported by NIH grants DK 076829 and DK 65830 to Dr. Kumar.
REFERENCES
- 1.Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 2.Bai X, Miao D, Li J, Goltzman D, Karaplis AC. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology. 2004;145:5269–5279. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- 3.Berndt T, Craig TA, Bowe AE, Vassiliadis J, Reczek D, Finnegan R, Jan De Beur SM, Schiavi SC, Kumar R. Secreted frizzled-related protein 4 is a potent tumor-derived phosphaturic agent. J Clin Invest. 2003;112:785–794. doi: 10.1172/JCI18563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berndt T, Knox FG. Renal regulation of phosphate excretion. New York: Raven Press, LTD; 1992. chapter 71. [Google Scholar]
- 5.Berndt T, Kumar R. Phosphatonins and the regulation of phosphate homeostasis. Annu Rev Physiol. 2007;69:341–359. doi: 10.1146/annurev.physiol.69.040705.141729. [DOI] [PubMed] [Google Scholar]
- 6.Berndt T, Thomas LF, Craig TA, Sommer S, Li X, Bergstralh EJ, Kumar R. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci U S A. 2007;104:11085–11090. doi: 10.1073/pnas.0704446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berndt TJ, Bielesz B, Craig TA, Tebben PJ, Bacic D, Wagner CA, O'Brien S, Schiavi S, Biber J, Murer H, Kumar R. Secreted frizzled-related protein-4 reduces sodium-phosphate co-transporter abundance and activity in proximal tubule cells. Pflugers Arch. 2006;451:579–587. doi: 10.1007/s00424-005-1495-2. [DOI] [PubMed] [Google Scholar]
- 8.Berndt TJ, Craig TA, McCormick DJ, Lanske B, Sitara D, Razzaque MS, Pragnell M, Bowe AE, O'Brien SP, Schiavi SC, Kumar R. Biological activity of FGF-23 fragments. Pflugers Arch. 2007;454:615–623. doi: 10.1007/s00424-007-0231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berndt TJ, Schiavi S, Kumar R. "Phosphatonins" and the regulation of phosphorus homeostasis. Am J Physiol Renal Physiol. 2005;289:F1170–F1182. doi: 10.1152/ajprenal.00072.2005. [DOI] [PubMed] [Google Scholar]
- 10.Bessman SP, Carpenter CL. The creatine-creatine phosphate energy shuttle. Annu Rev Biochem. 1985;54:831–862. doi: 10.1146/annurev.bi.54.070185.004151. [DOI] [PubMed] [Google Scholar]
- 11.Bowe AE, Finnegan R, Jan de Beur SM, Cho J, Levine MA, Kumar R, Schiavi SC. FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem Biophys Res Commun. 2001;284:977–981. doi: 10.1006/bbrc.2001.5084. [DOI] [PubMed] [Google Scholar]
- 12.Brown EM. Clinical lessons from the calcium-sensing receptor. Nat Clin Pract Endocrinol Metab. 2007;3:122–133. doi: 10.1038/ncpendmet0388. [DOI] [PubMed] [Google Scholar]
- 13.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 14.Cai Q, Hodgson SF, Kao PC, Lennon VA, Klee GG, Zinsmiester AR, Kumar R. Brief report: inhibition of renal phosphate transport by a tumor product in a patient with oncogenic osteomalacia. N Engl J Med. 1994;330:1645–1649. doi: 10.1056/NEJM199406093302304. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter TO, Ellis BK, Insogna KL, Philbrick WM, Sterpka J, Shimkets R. Fibroblast growth factor 7: an inhibitor of phosphate transport derived from oncogenic osteomalacia-causing tumors. J Clin Endocrinol Metab. 2005;90:1012–1020. doi: 10.1210/jc.2004-0357. [DOI] [PubMed] [Google Scholar]
- 16.Chattopadhyay N, Cheng I, Rogers K, Riccardi D, Hall A, Diaz R, Hebert SC, Soybel DI, Brown EM. Identification and localization of extracellular Ca(2+)-sensing receptor in rat intestine. Am J Physiol. 1998;274:G122–G130. doi: 10.1152/ajpgi.1998.274.1.G122. [DOI] [PubMed] [Google Scholar]
- 17.Chen TC, Castillo L, Korycka-Dahl M, DeLuca HF. Role of vitamin D metabolites in phosphate transport of rat intestine. J Nutr. 1974;104:1056–1060. doi: 10.1093/jn/104.8.1056. [DOI] [PubMed] [Google Scholar]
- 18.Condamine L, Menaa C, Vrtovsnik F, Friedlander G, Garabedian M. Local action of phosphate depletion and insulin-like growth factor 1 on in vitro production of 1,25-dihydroxyvitamin D by cultured mammalian kidney cells. J Clin Invest. 1994;94:1673–1679. doi: 10.1172/JCI117512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Beur SM, Finnegan RB, Vassiliadis J, Cook B, Barberio D, Estes S, Manavalan P, Petroziello J, Madden SL, Cho JY, Kumar R, Levine MA, Schiavi SC. Tumors associated with oncogenic osteomalacia express genes important in bone and mineral metabolism. J Bone Miner Res. 2002;17:1102–1110. doi: 10.1359/jbmr.2002.17.6.1102. [DOI] [PubMed] [Google Scholar]
- 20.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 21.DeLuca HF. Vitamin D: revisited 1980. Clin Endocrinol Metab. 1980;9:1–26. [PubMed] [Google Scholar]
- 22.Diem K, Lentner C. Giegy Scientific Tables. New York: Geigy Pharmacueticals; 1970. [Google Scholar]
- 23.Econs MJ, Drezner MK. Tumor-induced osteomalacia--unveiling a new hormone. N Engl J Med. 1994;330:1679–1681. doi: 10.1056/NEJM199406093302310. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari SL, Bonjour JP, Rizzoli R. FGF-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2004 doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 25.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 26.Fujita T, Izumo N, Fukuyama R, Meguro T, Nakamuta H, Kohno T, Koida M. Phosphate provides an extracellular signal that drives nuclear export of Runx2/Cbfa1 in bone cells. Biochem Biophys Res Commun. 2001;280:348–352. doi: 10.1006/bbrc.2000.4108. [DOI] [PubMed] [Google Scholar]
- 27.Goseki-Sone M, Yamada A, Asahi K, Hirota A, Ezawa I, Iimura T. Phosphate depletion enhances tissue-nonspecific alkaline phosphatase gene expression in a cultured mouse marrow stromal cell line ST2. Biochem Biophys Res Commun. 1999;265:24–28. doi: 10.1006/bbrc.1999.1624. [DOI] [PubMed] [Google Scholar]
- 28.Goseki-Sone M, Yamada A, Hamatani R, Mizoi L, Iimura T, Ezawa I. Phosphate depletion enhances bone morphogenetic protein-4 gene expression in a cultured mouse marrow stromal cell line ST2. Biochem Biophys Res Commun. 2002;299:395–399. doi: 10.1016/s0006-291x(02)02646-3. [DOI] [PubMed] [Google Scholar]
- 29.Gowen LC, Petersen DN, Mansolf AL, Qi H, Stock JL, Tkalcevic GT, Simmons HA, Crawford DT, Chidsey-Frink KL, Ke HZ, McNeish JD, Brown TA. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem. 2003;278:1998–2007. doi: 10.1074/jbc.M203250200. [DOI] [PubMed] [Google Scholar]
- 30.Hansen NM, Felix R, Bisaz S, Fleisch H. Aggregation of hydroxyapatite crystals. Biochim Biophys Acta. 1976;451:549–559. doi: 10.1016/0304-4165(76)90150-1. [DOI] [PubMed] [Google Scholar]
- 31.Ito N, Fukumoto S, Takeuchi Y, Takeda S, Suzuki H, Yamashita T, Fujita T. Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. Journal of bone and mineral metabolism. 2007;25:419–422. doi: 10.1007/s00774-007-0779-3. [DOI] [PubMed] [Google Scholar]
- 32.Jain A, Fedarko NS, Collins MT, Gelman R, Ankrom MA, Tayback M, Fisher LW. Serum levels of matrix extracellular phosphoglycoprotein (MEPE) in normal humans correlate with serum phosphorus, parathyroid hormone and bone mineral density. J Clin Endocrinol Metab. 2004;89:4158–4161. doi: 10.1210/jc.2003-032031. [DOI] [PubMed] [Google Scholar]
- 33.Jencks WP. From chemistry to biochemistry to catalysis to movement. Annu Rev Biochem. 1997;66:1–18. doi: 10.1146/annurev.biochem.66.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Knochel JP. The pathophysiology and clinical characteristics of severe hypophosphatemia. Arch Intern Med. 1977;137:203–220. [PubMed] [Google Scholar]
- 35.Knochel JP, Barcenas C, Cotton JR, Fuller TJ, Haller R, Carter NW. Hypophosphatemia and rhabdomyolysis. Trans Assoc Am Physicians. 1978;91:156–168. [PubMed] [Google Scholar]
- 36.Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK. 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1036–G1042. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 37.Kornberg A. The enzymatic replication of DNA. CRC critical reviews in biochemistry. 1979;7:23–43. doi: 10.3109/10409237909102568. [DOI] [PubMed] [Google Scholar]
- 38.Krebs EG, Beavo JA. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- 39.Kumar R. The phosphatonins and the regulation of phosphate homeostasis. Annales d'endocrinologie. 2006;67:142–146. doi: 10.1016/s0003-4266(06)72570-7. [DOI] [PubMed] [Google Scholar]
- 40.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamarche MG, Wanner BL, Crepin S, Harel J. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS microbiology reviews. 2008;32:461–473. doi: 10.1111/j.1574-6976.2008.00101.x. [DOI] [PubMed] [Google Scholar]
- 42.Lardy HA, Ferguson SM. Oxidative phosphorylation in mitochondria. Annu Rev Biochem. 1969;38:991–1034. doi: 10.1146/annurev.bi.38.070169.005015. [DOI] [PubMed] [Google Scholar]
- 43.Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 44.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee DB, Walling MW, Brautbar N. Intestinal phosphate absorption: influence of vitamin D and non-vitamin D factors. Am J Physiol. 1986;250:G369–G373. doi: 10.1152/ajpgi.1986.250.3.G369. [DOI] [PubMed] [Google Scholar]
- 46.Lehninger AL, Wadkins CL. Oxidative phosphorylation. Annu Rev Biochem. 1962;31:47–78. doi: 10.1146/annurev.bi.31.070162.000403. [DOI] [PubMed] [Google Scholar]
- 47.Liu S, Vierthaler L, Tang W, Zhou J, Quarles LD. FGFR3 and FGFR4 do not mediate renal effects of FGF23. J Am Soc Nephrol. 2008;19:2342–2350. doi: 10.1681/ASN.2007121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Markovich D, Verri T, Sorribas V, Forgo J, Biber J, Murer H. Regulation of opossum kidney (OK) cell Na/Pi cotransport by Pi deprivation involves mRNA stability. Pflugers Arch. 1995;430:459–463. doi: 10.1007/BF00373881. [DOI] [PubMed] [Google Scholar]
- 49.Marks J, Churchill LJ, Debnam ES, Unwin RJ. Matrix extracellular phosphoglycoprotein inhibits phosphate transport. J Am Soc Nephrol. 2008;19:2313–2320. doi: 10.1681/ASN.2008030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin DR, Ritter CS, Slatopolsky E, Brown AJ. Acute regulation of parathyroid hormone by dietary phosphate. Am J Physiol Endocrinol Metab. 2005;289:E729–E734. doi: 10.1152/ajpendo.00065.2005. [DOI] [PubMed] [Google Scholar]
- 51.Mouillon JM, Persson BL. New aspects on phosphate sensing and signalling in Saccharomyces cerevisiae. FEMS yeast research. 2006;6:171–176. doi: 10.1111/j.1567-1364.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 52.Nishida Y, Taketani Y, Yamanaka-Okumura H, Imamura F, Taniguchi A, Sato T, Shuto E, Nashiki K, Arai H, Yamamoto H, Takeda E. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70:2141–2147. doi: 10.1038/sj.ki.5002000. [DOI] [PubMed] [Google Scholar]
- 53.Op den Kamp JA. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- 54.Perwad F, Azam M, Zhang M, Yamashita T, Tennenhouse H, Portale A. Dietary phosphorus regulates serum FGF-23 concentrations and 1, 25(OH)2D3 metabolism in mice. Journal of Bone and Mineral Research. 2004;19:S251. [Google Scholar]
- 55.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 56.Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293:F1577–F1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 57.Rizzoli R, Fleisch H, Bonjour JP. Role of 1,25-dihydroxyvitamin D3 on intestinal phosphate absorption in rats with a normal vitamin D supply. J Clin Invest. 1977;60:639–647. doi: 10.1172/JCI108815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothfield L, Finkelstein A. Membrane biochemistry. Annu Rev Biochem. 1968;37:463–496. doi: 10.1146/annurev.bi.37.070168.002335. [DOI] [PubMed] [Google Scholar]
- 59.Rowe PS, de Zoysa PA, Dong R, Wang HR, White KE, Econs MJ, Oudet CL. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics. 2000;67:54–68. doi: 10.1006/geno.2000.6235. [DOI] [PubMed] [Google Scholar]
- 60.Rowe PS, Kumagai Y, Gutierrez G, Garrett IR, Blacher R, Rosen D, Cundy J, Navvab S, Chen D, Drezner MK, Quarles LD, Mundy GR. MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone. 2004;34:303–319. doi: 10.1016/j.bone.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280:2543–2549. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- 62.Schiavi SC, Kumar R. The phosphatonin pathway: new insights in phosphate homeostasis. Kidney Int. 2004;65:1–14. doi: 10.1111/j.1523-1755.2004.00355.x. [DOI] [PubMed] [Google Scholar]
- 63.Segawa H, Kaneko I, Yamanaka S, Ito M, Kuwahata M, Inoue Y, Kato S, Miyamoto K. Intestinal Na-P(i) cotransporter adaptation to dietary P(i) content in vitamin D receptor null mice. Am J Physiol Renal Physiol. 2004;287:F39–F47. doi: 10.1152/ajprenal.00375.2003. [DOI] [PubMed] [Google Scholar]
- 64.Shaikh A, Berndt T, Kumar R. Regulation of phosphate homeostasis by the phosphatonins and other novel mediators. Pediatr Nephrol. 2008 doi: 10.1007/s00467-008-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shimada T, Kakitani M, Hasegawa H, Yamzaki Y, Ohguma A, Takeuchi Y, ujita T, Fukumota S, Tomizuka K, Yamashita Y. Targeted ablation of FGF-23 causes hyperphosphatemia, increased 1,25-dihydroxyvitamin D Levels and severe growth retardation. J Bone Miner Res. 2002;17(Suppl 1):S168. [Google Scholar]
- 67.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004;314:409–414. doi: 10.1016/j.bbrc.2003.12.102. [DOI] [PubMed] [Google Scholar]
- 69.Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, H JA-P, Lanske B. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sitara D, Razzaque MS, St-Arnaud R, Huang W, Taguchi T, Erben RG, Lanske B. Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals. Am J Pathol. 2006;169:2161–2170. doi: 10.2353/ajpath.2006.060329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sommer S, Berndt T, Craig T, Kumar R. The phosphatonins and the regulation of phosphate transport and vitamin D metabolism. J Steroid Biochem Mol Biol. 2007;103:497–503. doi: 10.1016/j.jsbmb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 72.Steele TH, Engle JE, Tanaka Y, Lorenc RS, Dudgeon KL, DeLuca HF. Phosphatemic action of 1,25-dihydroxyvitamin D3. Am J Physiol. 1975;229:489–495. doi: 10.1152/ajplegacy.1975.229.2.489. [DOI] [PubMed] [Google Scholar]
- 73.Steele TH, Underwood JL. Renal response to phosphorus deprivation in the isolated rat kidney. Kidney Int. 1978;13:124–128. doi: 10.1038/ki.1978.18. [DOI] [PubMed] [Google Scholar]
- 74.Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 75.Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, Quarles LD. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol. 2007;18:2116–2124. doi: 10.1681/ASN.2006121385. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki S, Ferjani A, Suzuki I, Murata N. The SphS-SphR two component system is the exclusive sensor for the induction of gene expression in response to phosphate limitation in synechocystis. J Biol Chem. 2004;279:13234–13240. doi: 10.1074/jbc.M313358200. [DOI] [PubMed] [Google Scholar]
- 77.Tanaka Y, Deluca HF. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973;154:566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- 78.Tanaka Y, Deluca HF. Role of 1,25-dihydroxyvitamin D3 in maintaining serum phosphorus and curing rickets. Proc Natl Acad Sci U S A. 1974;71:1040–1044. doi: 10.1073/pnas.71.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Molecular endocrinology (Baltimore, Md. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 80.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 81.Williams KB, DeLuca HF. Characterization of intestinal phosphate absorption using a novel in vivo method. Am J Physiol Endocrinol Metab. 2007;292:E1917–E1921. doi: 10.1152/ajpendo.00654.2006. [DOI] [PubMed] [Google Scholar]
- 82.Yuan B, Takaiwa M, Clemens TL, Feng JQ, Kumar R, Rowe PS, Xie Y, Drezner MK. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J Clin Invest. 2008;118:722–734. doi: 10.1172/JCI32702. [DOI] [PMC free article] [PubMed] [Google Scholar]