Abstract
Preclinical studies show that estradiol enhances sensitization to cocaine in females by mechanisms not fully understood. These studies consistently show that ovariectomized (OVX) rats exhibit little or no sensitization to cocaine compared to OVX rats administered estradiol. In this study we varied the dose of cocaine (10, 15, and 30 mg/kg), the length of cocaine treatment (from 5 to 10 days) and the context of cocaine injections to determine if these factors play a role on estradiol's effects on cocaine sensitization. Because OVX rats are hormonally compromised, they are not representative of the natural state of the animal, and thus the physiological context of these studies remains unclear. To address this issue, we blocked ERs in gonadally intact females by icv administration of the antiestrogen ICI-182,780. Varying the dose or length of exposure to cocaine does not alter estradiol's effect on cocaine sensitization. In contrast, a highly context-dependent sensitization protocol results in robust sensitization even in OVX rats. Interestingly, using this protocol, sensitization in OVX rats diminished with time, suggesting that estradiol is necessary for the maintenance of cocaine sensitization. Blocking brain ERs with ICI completely abolishes the development and expression of cocaine sensization in gonadally intact female rats, even when tested in a highly context-dependent sensitization protocol. Given these findings, we propose that activation of brain ERs is required for the development and maintenance of sensitization and CPP.
Introduction
Drug abuse is a disease that affects more than 22.6 million Americans (Substance Abuse and Mental Health Services Administration, 2010). Of the illicit drugs of abuse, cocaine is one of the most widely used. Clinical studies show gender differences in addictive behaviors, in the subjective effects of drugs of abuse as well as in the response to treatment. For example, when women are exposed to cocaine, they show an earlier onset of cocaine use and become addicted at a faster rate than men (Griffin et al., 1989; Colell et al., 2013). Differences in social and environmental surroundings may contribute to some of these differences (van Etten et al., 1999; Du et al., 2013; Colell et al., 2013). Nonetheless, fundamental biological differences exist between the sexes that contribute to these differences in response to drugs (Luo et al., 2013; Rando, et al., 2013). The sex steroid milieu also plays a major role in modulating the effects of drugs of abuse. The consensus from previous studies is that progesterone attenuates the subjective effects of cocaine (Anker and Carroll, 2010, Evans and Foltin, 2006; Reed et al., 2011) whereas estradiol exacerbates drug-associated responses (Evans et al., 2002).
Studies using animal models show similar results. Our laboratory has found that in female rats, cocaine-induced behavioral sensitization is enhanced by estradiol (Febo et al., 2002; Puig et al., 2008; Segarra et al, 2010). Behavioral sensitization is defined as an increase in the response to a stimulus over time (Segal, 1971). This phenomenon has been observed in animals exposed repeatedly to drugs of abuse, such as psychostimulants (Robinson and Becker, 1986). Many factors can affect the process of sensitization, such as the type of drug, dose administered, length of treatment, age and sex of the animal, and the context in which the drug is administered (for review see Steketee and Kalivas, 2011).
Cocaine-induced sensitization of brain activity, as measured by fMRI, is also dependent on plasma estradiol (Febo et al., 2005). In these last studies we found that OVX-EB rats previously exposed to cocaine display a higher cocaine-induced BOLD signal than drug naive OVX-EB rats. These changes are not observed in OVX rats exposed to the same cocaine regimen (Febo et al., 2005). Although the main sources of plasma estradiol are the gonads, brain tissue also actively synthesizes estradiol de novo (Kretz et al., 2004, Fester et al., 2006, for reviews see Azcoitia et al., 2011, Fester et al, 2011). This neural estradiol is associated with the regulation of reproductive function, as well as with other non-reproductive functions such as neuronal survival, neurogenesis and modulation of synaptic function (Azcoitia et al., 2011).
Whereas some researchers fail to find an effect of estradiol on the behavioral response to cocaine in rodent (Collins et al, 2007) or primate animal models (Mello et al, 2007; Mello et al, 2008), others have found that estradiol facilitates acquisition (Jackson et al., 2006) and enhances cocaine self-administration (Lynch et al., 2001; Hu and Becker, 2008), cocaine-induced reinstatement (Larson et al., 2007), cocaine-induced rotational behavior (Hu and Becker, 2003) and cocaine sensitization (Peris et al, 1991, Sircar and Kim, 1999; Hu and Becker, 2003).
These differences in results may be attributed to differences in the way estradiol is administered (injection, pellet, Silastic implant), the dose administered, the type of estradiol administered (affecting half life and absorption rate), the length of treatment, if it is administered to a gonadally intact female, immediately or a week after ovariectomy, etc. All of these variables, in addition to differences in locally produced estrogens (neurosteroids) will affect the amount of estradiol present in the brain that is available to interact with its receptor, or act as a neuromodulator on other receptor systems or ion channels. Although there are differences in the acute response to cocaine, most studies agree that estradiol exacerbates the response to repeated cocaine administration. Therefore, we hypothesized that in females, estradiol triggers neuroadaptative changes that sensitize the brain to cocaine’s psychostimulant effects and enhances the rewarding properties of cocaine.
One mechanism by which estradiol may accomplish this is by increasing the incentive value of the stimulus. Our previous studies show that OVX rats display a higher locomotor response to an acute cocaine injection, compared to OVX-EB rats, that is maintained over time. One possibility is that OVX rats are more sensitive to the locomotor activating effects of cocaine, and, that if a lower dose is used, may display an increase in cocaine-induced hyperactivity throughout time. We rationalized that varying the dose, or increasing the length of exposure to cocaine in OVX rats, may enhance sensitization. Therefore to investigate if estradiol increases the incentive value of cocaine, we varied the dose and increased the length of exposure to cocaine (Experiments 2, 3A and 3B).
Another possibility is that estradiol may be enhancing associative learning processes of cocaine-related reward. Previous studies have shown that estradiol facilitates several aspects of learning and memory, (McEwen and Milner, 2007; Packard and Teather 1997; Barha and Galea, 2010; Holmes et al. 2002). We reasoned that increasing the number of exposures to the drug-associated context would enhance associative learning processes related to cocaine reward (Experiment 4).
In our past studies we used ovariectomized rats and provided estradiol to half of the animals. This animal model has the advantage of teasing out the effect of estradiol on cocaine-induced behaviors but adds the confound of estradiol deficiency in one group and non-physiological constantly elevated estradiol levels in the other group. In addition, it does not provide information to determine if estradiol’s actions are mediated by its interaction with the classical genomic estrogen receptor. To address these issues, we blocked ER in the brain of gonadally intact female rats with the anti-estrogen ICI-182,780. We surmised that if the effect is mediated by intracellular ERs, we should prevent estradiol’s enhancement of sensitization to cocaine (Wakeling et al., 1991; Wade et al., 1993) (Experiment 5).
To investigate if estradiol enhances rewarding properties of cocaine, we tested OVX and OVX-EB rats using the paradigm of conditioned place preference (CPP) (Experiment 6A). Finally, to determine if estrogen receptors play a major role in estradiol enhancement of conditioned place preference, we administered ICI-182,780 to gonadally intact female rats and assayed the rewarding effects of cocaine using conditioned place preference (Experiment 6B).
Materials and Methods
Subjects
Adult female Sprague-Dawley rats (180–220g) were purchased from Charles River Laboratories. Animals were housed in pairs in a temperature and humidity controlled room, under a 12-h light-dark cycle. Animals used for CPP experiments were kept in an inverted light-dark cycle with lights off at 7 AM. Animals used for sensitization experiments were kept in regular light-dark cycle with lights off at 7 PM. Water and Harlan Tek® rat chow were provided ad libitum. All animals were allowed to acclimate to the Animal Facilities for 5 days before any experimental manipulation. A separate group of animals was used for each experimental protocol. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Puerto Rico, Medical Sciences Campus and adhere to USDA, NIH and AAALAC guidelines.
Drugs and chemicals
The anesthetic used for surgery was a combination of ketamine (50 or 70 mg/kg, as detailed below) and xylazine (7 mg/kg) (Sigma-Aldrich, St. Louis, MO, USA) dissolved in a sterile 0.9% NaCl solution and injected intraperitoneally (i.p.). Cocaine-HCl (Sigma-Aldrich) was prepared in saline (0.9%) and injected i.p. at a dose of 10, 15 or 30 mg/kg. ICI-182, 780 (Sigma-Aldrich) was dissolved in 22.5% of (2-hydroxypropyl)-β-cyclodextrin and 0.45% saline solution and administered intracerebroventricularly (icv) via an Alzet mini osmotic pump. The pump was placed subcutaneously near the scapular region; the rate of delivery was 0.15 uL/hour. The concentration delivered of ICI-182,780 was 0.075ug/hour. Control animals received vehicle (22.5% of (2-hydroxypropyl)-β-cyclodextrin and 0.45% saline solution) using the same type of Alzet mini-pump, with the same delivery rate.
Surgical procedures
Ovariectomy
Following the acclimation period, a group of animals was ovariectomized using ketamine (50mg/kg) and xylazine (7mg/kg) (Sigma-Aldrich, St. Louis, MO, USA) as anesthetics. To avoid a second surgery, a 5-mm Silastic tubing implant (inner diameter 1.47 mm, outer diameter 1.96 mm, Dow Corning) was prepared (Legan et al., 1975) and placed subcutaneously in the midscapular region during surgery. Half of the animals received empty implants (OVX group) and the others received implants packed with 4mg of crystalline 17-β-estradiol benzoate (OVX-EB group). The amount of estradiol attained by these implants is in the high range of physiological concentrations of estradiol during proestrous, approximately 140 pg/ml (Febo et al, 2002; Legan et al., 1975). Estradiol replacement was started the day the ovaries were removed to avoid hormonal and neurochemical changes associated with ovariectomy, After a 7 day recovery period, animals were submitted to behavioral testing.
Administration of ICI-182,780
ICI-182, 780 is a steroidal pure anti-estrogen (Howell et al, 2000) that impairs dimerization of ER, increases ER turnover, disrupts nuclear localization, and downregulates the ER (Parker, MG, 1993; Dauvois et al., 1993, Howell et al, 2000). ICI 182,780 effectively blocks estradiol’s mitogenic effect on the uterus, and attenuates estradiol’s effect on growth and sexual receptivity (Wade et al., 1993). Therefore to test the hypothesis that centrally located ER promote cocaine-induced behavioral sensitization we administered ICI-182,780 to gonadally intact cycling female rats and assayed the stimulant effects of cocaine using the behavioral sensitization and conditioned place preference paradigms.
Gonadally intact cycling females were anesthetized with a ketamine (70mg/kg) and xylazine (7mg/kg) solution. A cannula was inserted into the right lateral ventricle (coordinates: AP=−0.72mm, ML= +1.6mm, DV=−4.0mm from Bregma using the Paxinos and Watson Brain Atlas (2007). The cannula was attached to an osmotic mini-pump (ALZET model 2006 and ALZET Brain Infusion Kits) for constant administration of ICI-182,780 or vehicle (0.15 ul/hour). The osmotic mini-pump and brain infusion tubing was inserted subcutaneously near the scapular region. Half of the animals received osmotic pumps filled with vehicle (VEH group) and the others received osmotic pumps with ICI-182, 780 (ICI group). After 7 days of recovery, all animals were submitted to behavioral testing. The stage of the estrous cycle of intact rats was determined by vaginal smears obtained after behavioral testing and recorded. At the end of the experiment, animals were euthanized and the accuracy of the lateral ventricle cannula was verified histologically. In all cases, cannulae were appropriately placed into the lateral ventricle.
Behavioral Apparatus
Horizontal and stereotyped activity was measured using 10 automated animal activity cage systems (Versamax™ system) purchased from AccuScan™ Instruments (Columbus, Ohio, USA). The activity cages were made from clear acrylic (42 cm × 42 cm × 30 cm), with 16 equally spaced (2.5 cm) infrared beams across the length and width of the cage at a height of 2 cm from the cage floor (horizontal beams). An additional set of 16 infrared beams was located at a height of 10 cm from the cage floor (vertical beams). All beams were connected to a Data AnalyserR that sends information to a personal computer that displays beam data through a WindowsR-based program (VersadatR). The Versamax™ system differentiates between stereotyped and horizontal locomotor activity based on repeated interruption of the same beam or sequential breaking of different beams, respectively.
Behavioral Tests
Cocaine-induced locomotor activity
The locomotor response to cocaine was measured in an isolated room with low illumination. To diminish the effects of novelty, animals were habituated to the activity cage for one hour on the seventh day after surgery (Day 0). The day that locomotor activity (LMA) was recorded, rats were placed in the activity cage for 30 minutes prior to any injection. This served to reduce novelty-induced activity and to determine basal LMA of the animals. Rats were then injected with saline or cocaine and LMA recorded for 60 additional min. Each session of behavioral testing consisted of at least one animal of each group being tested to minimize intergroup variation that may result from differences in time of testing and/or injections. Animals that show an increase in locomotor response after repeated cocaine administration (i.e., day 1 vs day 5) are considered sensitized.
Conditioned Place Preference
Behavioral tests were conducted in the room used for our sensitization experiments. The cages used for the sensitization experiments were fitted with an acrylic box that consisted of two chambers. Chamber 1: Smooth floor, clear ceiling and vertical black lined walls. Chamber 2: Textured floor, black ceiling and horizontal white lined walls. Each rat was tested in the same activity cage every day. Experiments were conducted during the dark phase of their daily light cycle. On days 1–2 (pre-conditioning days), the rats were allowed 30 min to roam freely through both chambers. The amount of time spent in each chamber was calculated to determine which side it preferred. If the rat shows a very high preference for one side, (>80%) it was not used in the study to avoid using subjects that may show aversion towards one compartment. For 12 days (days 3–14) experimental subjects were injected daily, alternating between saline and cocaine (15 mg/kg) injections. The cocaine injection was administered in the non-preferred side of the chamber, the saline injection in the preferred side. Saline animals received saline injections in both chambers. After the injection, rats were confined for 30 min to the chamber where they received the injection. The last 2 days, days 15–16 (post-conditioning days), the animals did not receive an injection. They were placed in the activity chamber and allowed to roam freely between the two chambers for 30 minutes. The time spent in each chamber was recorded and compared with that of the first days (pre-conditioning days). The rats conditioned to cocaine if there was a significant increase in the time spent in the chamber where they received cocaine.
Experimental subjects
Experiments 1, 2, 3A, 3B, 4, and 6A were conducted with ovariectomized rats without (OVX) or with (OVX-EB) estradiol treatment. Experiments 5 and 6B were conducted with gonadally intact female rats that received the anti-estrogen ICI 182, 780, or its vehicle, via an osmotic mini-pump connected to a cannula that was inserted into the lateral ventricle.
Statistical Analysis
To determine if rats showed sensitization, OVX and OVX-EB data were analyzed separately using a Repeated Measures ANOVA with days (1, 5 and 13) and minutes (35–90) as the repeated factors. The Bonferroni's Multiple Comparison Test was used for post-hoc analysis and the effect size was calculated using eta squared (η2).
To compare sensitization between OVX and OVX-EB, we calculated the percent change between day 1 and days 5 or 13 using the sum of the time points 15–45 min after cocaine injections. These time points were selected since they represent the time points where the locomotor activating effects of cocaine are more robust. The percent change was calculated as follows: (1) We added the total amount of activity during the time interval 15–45 of D1; (2) The average for D1 was calculated; (3) The total amount of activity during the time interval 15–45 of D5 (or D13) for each individual was calculated; (4) Percent change was calculated as follows: For Day 5=(D5 – mean D1/mean D1) × 100; for Day 13=(D13 – mean D1/mean D1) × 100. The percent change of OVX and OVX-EB rats were compared using an unpaired Student's t-Test and Cohen's d was used for pairwise comparisons.
The CPP data were analyzed using a MANOVA with pre- and post-conditioning as a repeated measures and estradiol and cocaine as the independent variables. We did not find significant within-group differences in the groups of gonadally intact females, thus the data were pooled, irrespective of the stage of the estrous cycle.
All graphics and statistical analysis of sensitization experiments were performed with Graph Pad Prism (v6). CPP experiments (MANOVA analysis) were analyzed with JMP 8. The effect size for pairwise comparisons was determined with Cohen's d using an online calculator (http://www.cognitiveflexibility.org/effectsize).
RESULTS
Experiment 1: Five daily cocaine (15 mg/kg) injections
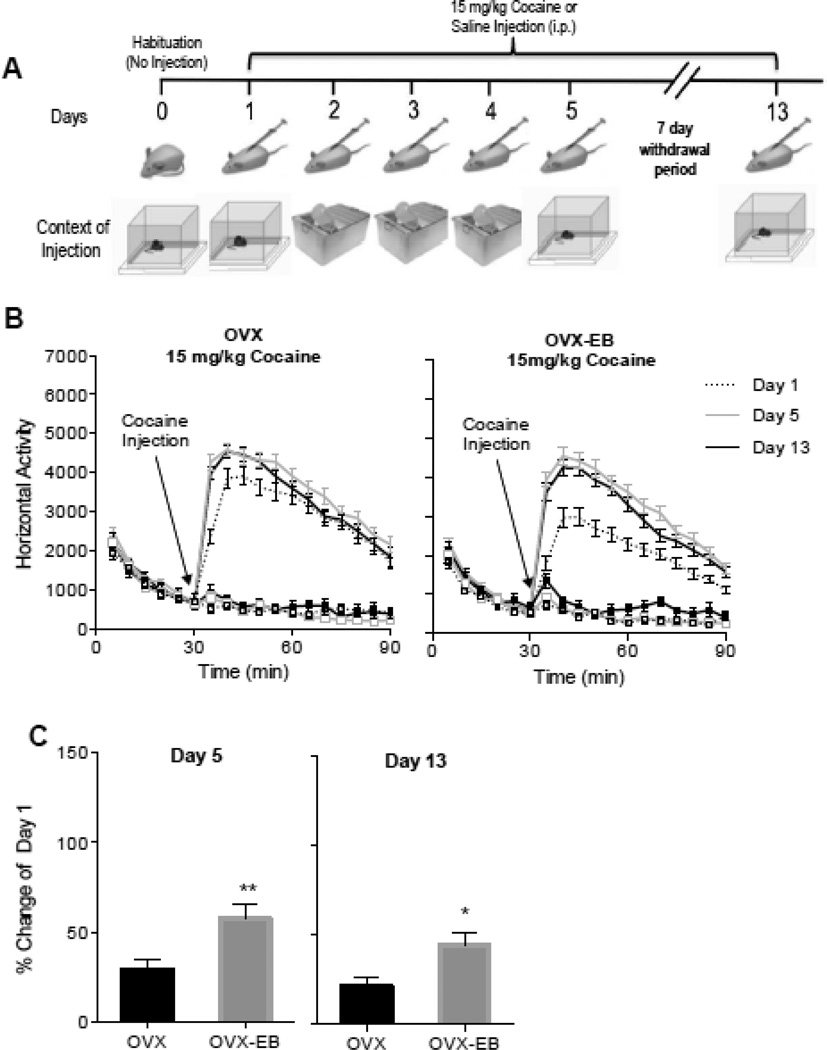
OVX and OVX-EB rats were injected with saline (0.9%, i.p.) or with cocaine (15 mg/kg) and locomotor activity was recorded for 60 minutes. On the days that locomotor activity was recorded (i.e. days 1, 5 and 13) rats were injected in the activity cage; on days 2, 3 and 4 rats were injected in the home cage (Fig 1A).
Fig. 1. Locomotor activity of ovariectomized rats with and without estradiol after 5 daily injections of 15 mg/kg of cocaine.
A. Sensitization protocol. Context of injection is represented by activity chambers (square boxes) and standard home cages. B. Timecourse of cocaine-induced locomotor activity of OVX and OVX-EB rats injected for 5 days with cocaine (15 mg/kg). Rats showed an increase in cocaine-induced locomotor activity after repeated cocaine injections, this increase was more robust in rats that received estradiol. Plot lines with squares represent saline groups. C. Percent change from day 1 in cocaine-induced locomotor activity of day 5 and day 13. Asterisks represent a significant difference from OVX rats; * p<0.05; **p<0.01.
Consistent with our previous studies, we observed that OVX rats showed a higher locomotor response to an acute cocaine injection than OVX-EB rats (Fig 1B). Data were analyzed with a Two Way Repeated Measures ANOVA with Estradiol as the independent variable and Minutes as the repeated measure. Day 1-OVX-Coc (n=47) vs Day 1-OVX-EB-Coc (n=59), N=106: Estradiol Effect: F (1,104) = 15.79, p<0.0001; η2 = 0.08.
In addition, OVX and OVX-EB rats injected for 5 days with 15 mg/kg of cocaine hydrochloride show cocaine-induced sensitization (Fig 1B). Data were analyzed with a Two Way Repeated Measures ANOVA with Days and Minutes as the repeated measures. OVX-Coc: Days Effect: F(2,92)=6.38, p=0.0026, η2 = 0.03; Days × Minutes Effect: F(22,1012)=5.24, p<0.0001, η2 = 0.02; Post hoc: Day 1 vs Day 5 p=0.0013, Day 1 vs Day 13 p=0.0602; OVX-EB-Coc: Days Effect: F(2,116)=14.75, p<0.0001, η2 = 0.04; Days × Minutes: F(22,1276)=6.40, p<0.0001, η2 = 0.05; Post-hoc: Day 1 vs Day 5 p=0.0020, Day 1 vs Day 13 p<0001.
We also found that although cocaine-induced locomotor activity on days 5 and days 13 is similar between OVX and OVX-EB rats, the percent change in activity from day 1 is higher in the OVX-EB group (Fig 1C), indicating a more robust sensitization in OVX-EB rats. Data were analyzed with a Student’s t-Test, with Estradiol as the independent variable and percent change from Day 1 as the dependent variable. Day 5: t=3.82, df=68, p=0.0003, Cohen’s d=0.60; Day 13: t=4.71, df=68, p<0.0001, Cohen’s d=0.51.
Experiment 2: Increased number of cocaine injections: Ten daily cocaine (15 mg/kg) injections
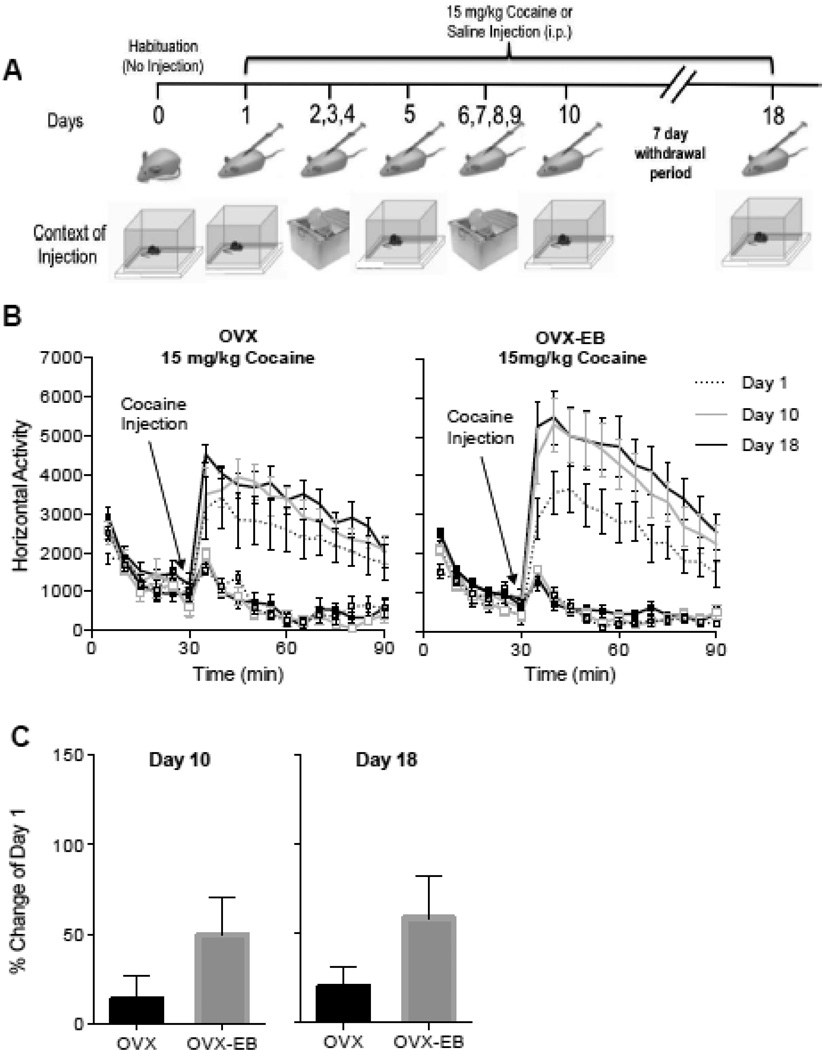
To rule out the possibility that increasing the time of exposure to cocaine would increase sensitization in OVX rats to levels observed in OVX-EB rats, we augmented the number of cocaine injections from 5 to 10 daily injections of cocaine and measured cocaine-induced locomotor activity on days 1 and 10 and after a drug-free week, day 18 (Fig 2A).
Fig. 2. Locomotor activity of ovariectomized rats with and without estradiol after 10 daily injections of 15 mg/kg of cocaine.
A. Sensitization protocol. Context of injection is represented by activity chambers (square boxes) and standard home cages. B. Timecourse of cocaine-induced locomotor activity of OVX and OVX-EB rats injected for 10 days with cocaine (15 mg/kg). OVX rats did not show an increase in cocaine-induced locomotor activity after 10 daily injections of cocaine i.p. whereas OVX-EB rats continued to show sensitization. Plot lines with squares represent saline groups. C. Percent change from day 1 of cocaine-induced locomotor activity on day 10 and day 18.
Our data illustrate that OVX-EB rats continue to display cocaine sensitization while OVX rats did not (Fig 2B). Cocaine treated animals (OVX-Coc and OVX-EB-Coc) were analyzed separately with a Two Way Repeated Measures ANOVA with Days and Minutes as the repeated measures. Results for OVX rats: Days Effect: F(2,10)=1.72, p=0.2275, η2 = 0.08; Days × Minutes Effect: F(22,110) =0.95, p=0.5311, η2 = 0.02; Post hoc: Day 1 vs Day 10 p=0.5311, Day 1 vs Day 18 p=0.1937. Results for OVX-EB rats: Days Effect: F(2,16)=5.32, p=0.0169, η2 = 0.12; Days X Minutes Effect: F(22,176)=1.43, p=0.1043, η2 = 0.01; Post-hoc Day 1 vs Day 10 p=0.055, Day 1 vs Day 18 p=0.0137.
We also found a trend in the percent change towards higher cocaine-induced locomotor activity in the OVX-EB group (FIG. 2C). Data were analyzed with a Student’s t-Test, with Estradiol as the independent variable and percent change from Day 1 as the dependent variable. Day 10: t=1.42, df=12.29, p=0.1817, Cohen’s d=0.74; Day 18: t=1.46, df=10.75, p<0.1720, Cohen’s d=0.78. Number of animals: OVX-Coc n=6, OVX-Sal n=8, OVX-EB-Coc n=9, OVX-EB-Sal n= 12; N=35.
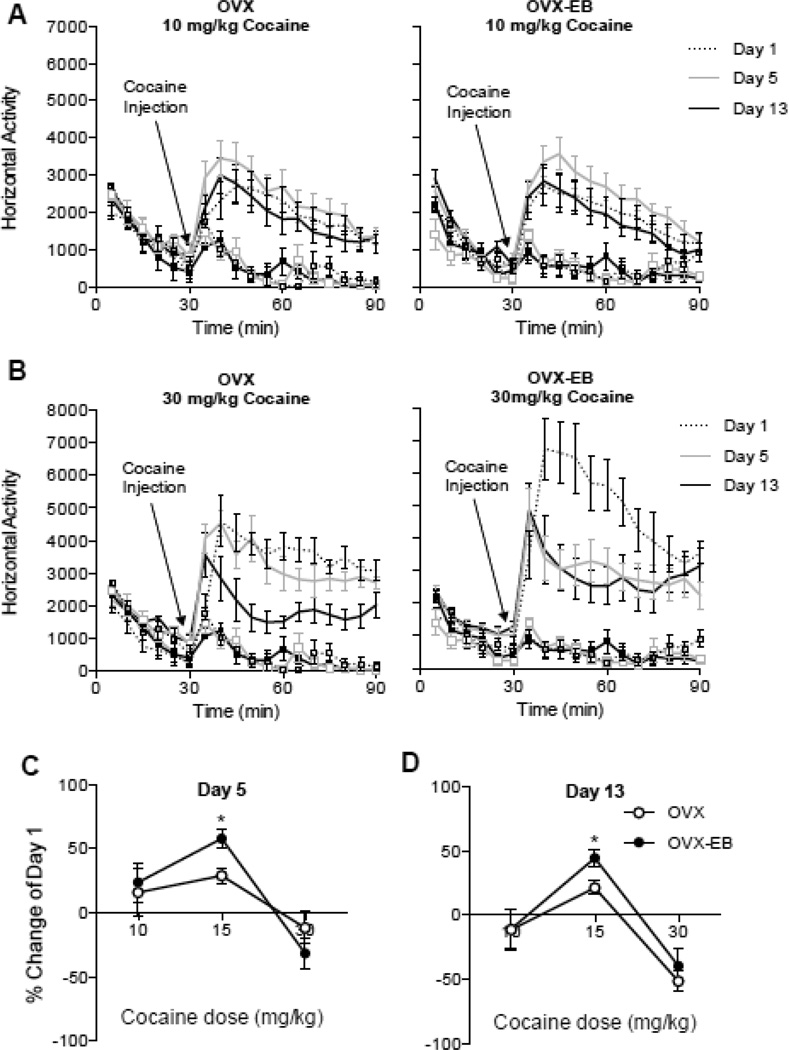
Experiments 3A and 3B: Varying cocaine dose: Five daily cocaine (10 or 30 mg/kg) injections
To investigate if varying the dose of cocaine could enhance sensitization in OVX rats, we injected a group of rats with 10 mg/kg (Experiment 3) and another group of rats with 30 mg/kg, (Experiment 4) of cocaine hydrochloride daily for 5 days. Cocaine-induced locomotor activity was recorded on days 1 and 5 and after a drug-free week, day 13.
Our data indicate that 10 nor 30 mg/kg of cocaine induced long lasting sensitization in OVX or OVX-EB rats (Fig 3A and Fig 3B). With the dose of 10 mg/kg we did observe an overall trend towards increased locomotor activity in OVX and OVX-EB rats on day 5 (Fig 3A). Cocaine treated animals (OVX-Coc and OVX-EB-Coc) were analyzed separately with a Two Way Repeated Measures ANOVA with Days and Minutes as the repeated measures. Results for OVX rats: Days Effect; F(2,18)=3.466, p=0.0533, η2 = 0.03; Days × Minutes Effect: F(22,198) =1.843, p=0.0152; η2 = 0.02; Post hoc Day 1 vs Day 5 p=0.1163, Day 1 vs Day 13 p>0.9999; Results for OVX-EB rats: Days Effect: F(2,16)=2.936, p=0.0820, η2 = 0.05; Days × Minutes Effect: F(22,176)=1.036, p=0.4235; η2 = 0.01; Post-hoc Day 1 vs Day 5 p=0.1761, Day 1 vs Day 13 p>0.9999. Interestingly, a detailed Bonferroni Post-hoc analysis revealed differences in the initial time points after cocaine injection on days 1 and 5, being higher at day 5. Results for OVX: MIN 35: p=0.001; MIN 40: p=0.001; MIN 45 p=0.0115. Results for OVX-EB: MIN 35: p=0.0435; MIN 40: p=0.0098; MIN 45 p=0.0001.
Fig. 3. Locomotor activity of ovariectomized rats with and without estradiol after 5 daily injections of 10 or 30 mg/kg of cocaine.
A. Sensitization protocol. Context of injection is represented by activity chambers (square boxes) and standard home cages. B. Timecourse of cocaine-induced locomotor activity of OVX and OVX-EB rats injected for 5 days with cocaine (10 mg/kg) or C. 30 mg/kg. A cocaine dose of 10 or 30 mg/kg failed to induce robust sensitization in OVX or OVX-EB rats (3A and 3B). Animals injected with 30 mg/kg showed a decrease in cocaine-induced locomotor activity over time, particularly at day 13, suggestive of tolerance to the higher dose of cocaine (3B). C. and D. Percent change from day 1 of cocaine-induced locomotor activity on day 5 and day 13. Plot lines with squares represent saline groups. Asterisks represent a significant difference from OVX rats, * p<0.05.
With the dose of 30 mg/kg we observed a decrease in cocaine-induced locomotor activity throughout time (FIG 3B). Cocaine treated animals were analyzed separately with a Repeated Measures ANOVA using DAYS and MINUTES as the repeated measures. Results for OVX rats: Days Effect: F(2,12)=13.24, p=0.0009, η2 = 0.22; Days × Minutes Effect: F(22,132) =3.467, p=0.0001; η2 = 0.11; Post hoc Day1 vs Day 5 p=0.8745, Day 1 vs Day 13 p=0.0009. Results for OVX-EB rats: Days Effect: F(2,12)=4.227, p=0.0408, η2 = 0.18; Days X Minutes Effect: F(22,132)=4.006, p<0.0001; η2 = 0.11; Post-hoc Day 1 vs Day 5 p=0.0581, Day 1 vs Day 13 p>0.0503.
Our data indicate that cocaine doses of 10 and 30 mg/kg do not induce a robust sensitization in OVX and OVX-EB rats, in contrast to animals injected with 15 mg/kg that do show sensitization at days 5 (Fig 3C) and 13 (Fig 3D). Data were analyzed with a Two Way ANOVA with Dose and Estradiol as the independent variables. Dose Effect: Day 5: F (2,133) =11.38, p<0.0001; η2 = 0.13; Estradiol Effect: (F (1,133) =0.2131, p<0.6451), η2 = 0.001. Day 13: F (2,133) =22.44, p<0.0001; η2 = 0.24; Estradiol Effect: (F (1,133) =1.134, p<0.2889), η2 = 0.01.
Experiment 4: Maintain context of cocaine injections (15 mg/kg) constant
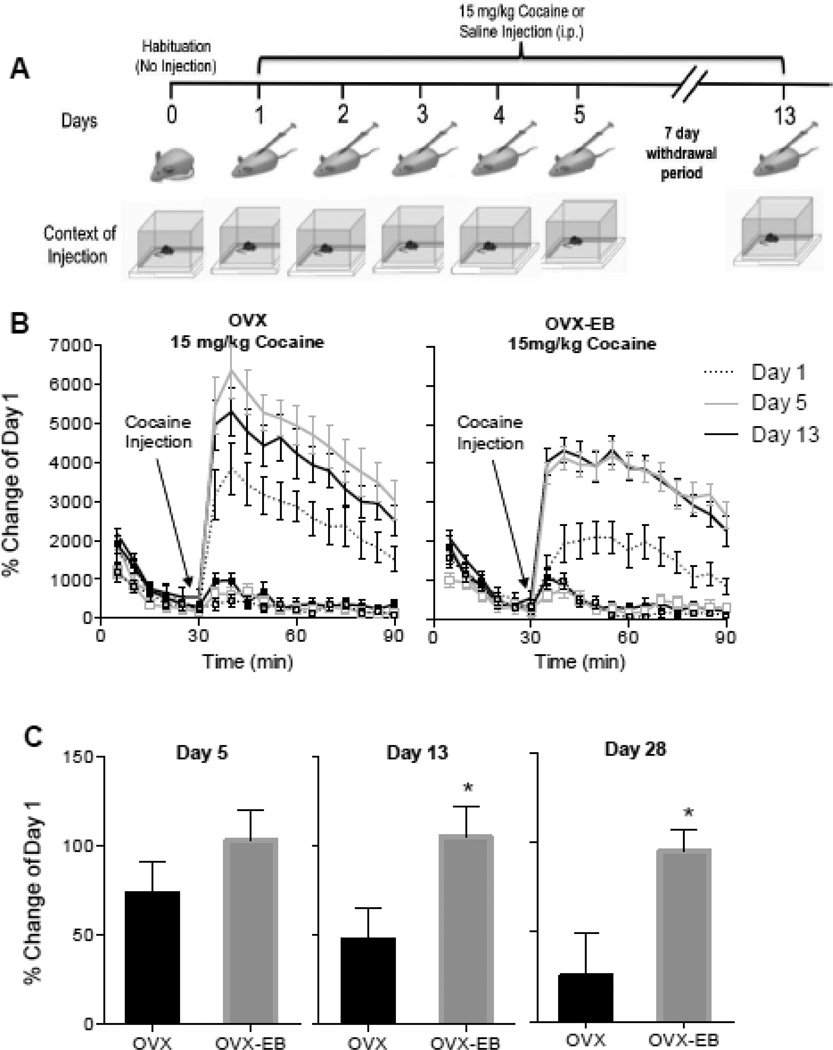
In Fig 1, and in all our previous studies, rats were injected with cocaine in the activity chambers (days 1, 5 and 13) and in their home cages (days 2, 3 and 4). To determine if estradiol facilitates processes of associative learning involved in cocaine-induced behavioral sensitization, cocaine was injected in the activity chamber every day (days 1–5, 13 and 28). Rats were allowed to spend an hour in the chamber after injection and during this time their locomotor activity was measured (Fig 4A).
Fig. 4. Locomotor activity of OVX and OVX-EB rats after 5 daily injections of cocaine (15 mg/kg) in the same activity chamber.
A. Sensitization protocol. Context of injection is represented by activity chambers (square boxes) and standard home cages. B. Timecourse of cocaine-induced locomotor activity of OVX and OVX-EB rats injected in the same activity chamber for 5 days with cocaine (15 mg/kg). Administration of cocaine (15 mg/kg) in the same activity chamber every day induced robust cocaine sensitization in OVX, as well as OVX-EB rats. Plot lines with squares represent saline groups. C. Percent change from day 1 of cocaine-induced locomotor activity on day 5, day 13 and day 28. In OVX-EB, but not OVX rats, this sensitization persisted when animals were given a first (day 13) and second (day 28) challenge of cocaine. Asterisks represent a significant difference from OVX rats, * p<0.05.
We found that under these conditions, OVX and OVX-EB rats rats showed an increase in cocaine-induced locomotor activity over time, indicative of a robust sensitization. Data were analyzed with a Two Way Repeated Measures ANOVA with Days and Minutes as the repeated measures. Results for OVX rats: Days Effect: F(2,24)=10.77, p=0.0005, η2 = 0.18; Days × Minutes Effect: F(12,144)=0.7853, p=0.6649; η2 = 0.03; Post hoc Day 1 vs Day 5 p=0.0002, Day 1 vs Day 13 p=0.0140. Results for 17 OVX-EB rats: Days Effect: F(2,24)=15.84, p<0.0001, η2 = 0.33; Days × Minutes Effect: F(12,144)=0.6538, p=0.7928, η2 = 0.03; Post-hoc Day 1 vs Day 5: p=0.0001, Day 1 vs Day 13: p=0001.
Although both group of rats showed a robust sensitization on day 5, if rats are left 7 or 15 days without receiving cocaine and are then challenged with cocaine on days 13 or 28, OVX-EB rats maintained the sensitized response to cocaine, whereas OVX rats showed a gradual decrease in the locomotor response to cocaine, indicating that estradiol enhances the expression of sensitization (Fig 4C). The percent change was compared using Student’s unpaired t-Test. OVX vs OVX-EB Day 5: t=1.228, df=23.95, p=0.2313, Cohen's d= 0.94; Day 13: t=2.393, df=23.93, p<0.0249; Day 28: t=2.624; df=5.940; p=0.0397, Cohen's d=1.8.
Experiment 5: Effect of the anti-estrogen ICI 182,780 on cocaine sensitization
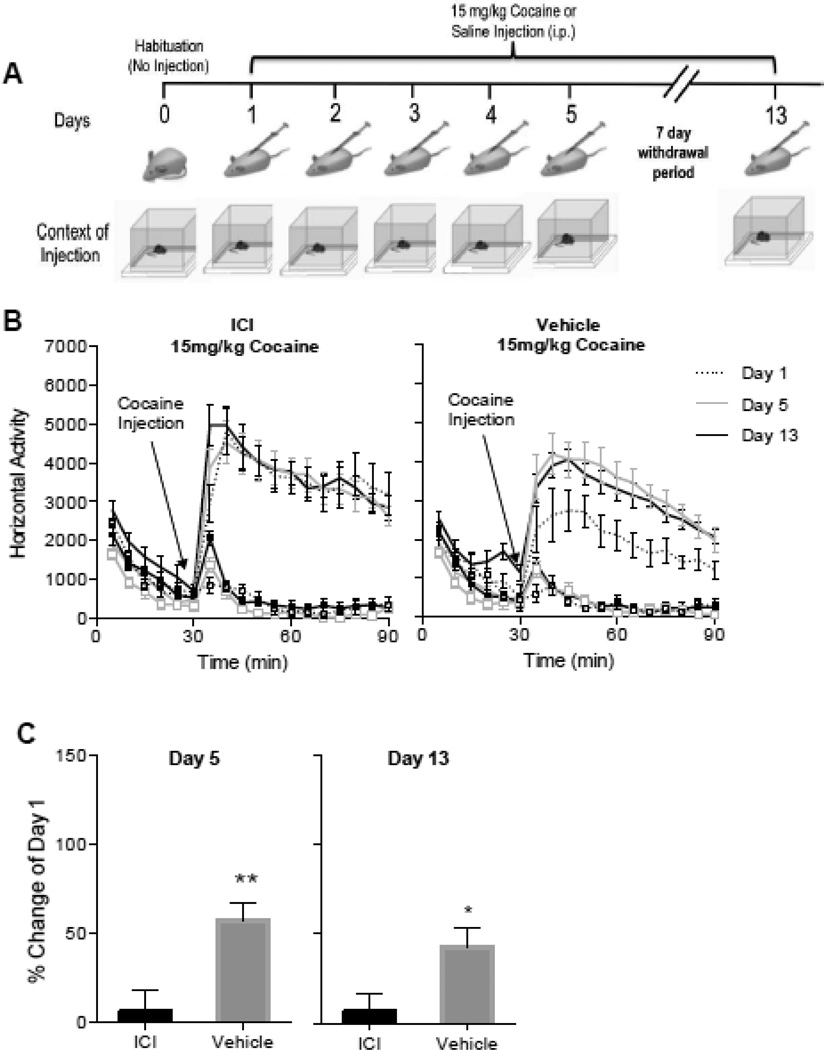
To investigate the role of ER in cocaine-induced behavioral sensitization, gonadally intact female rats were infused icv with ICI-182,780 or with vehicle. After a week, rats were injected with cocaine (15 mg/kg) for 5 consecutive days and on day 13 (Fig 5A).
Fig. 5. Cocaine-induced locomotor activity of intact female rats treated with the antiestrogen ICI 182,780 or with vehicle.
A. Sensitization protocol. Context of injection is represented by activity chambers (square boxes) and standard home cages. B.Timecourse of cocaine-induced locomotor activity of ICI and VEH rats injected for 5 days with cocaine (15 mg/kg). Gonadally intact females that received vehicle showed an increase in cocaine-induced locomotor activity with time. In contrast, rats that received the anti-estrogen ICI 182,780 failed to show sensitization. Plot lines with squares represent saline groups. C. Percent change from day 1 of cocaine-induced locomotor activity on day 5 and day 13. Sensitization of vehicle rats was maintained after one week of drug withdrawal when challenged with the same dose of cocaine. Asterisks represent a significant difference from ICI rats; * p<0.05; **p<0.01.
Rats treated with vehicle showed an increase in cocaine-induced locomotor activity over time. In contrast, ICI-182,780 completely blocked the development and expression of sensitization to cocaine despite the use of a context dependent protocol (Fig 5B), Data were analyzed with a Two Way Repeated Measures ANOVA with Days and Minutes as the repeated measures. Results for ICI treated females: Days Effect: F(2,24)=0.1968, p=0.8227, η2 = 0.002; Days × Minutes Effect: F(22,264) =1.764, p=0.0206; η2 = 0.03; Post hoc Day 1 vs Day 5 p>0.9999, Day 1 vs Day 13 p>0.9999; Results for VEHICLE treated females: Days Effect: F(2,20)=10.78, p=0.0007, η2 = 0.23; Days × Minutes Effect: F(22,220)=0.7469, p=0.7873, η2 = 0.01; Post-hoc Day 1 vs Day 5 p=0.0007, Day 1 vs Day 13 p>0.0034.
A comparison of the percent change in cocaine-induced locomotor activity over time reveals that gonadally intact rats, similar to OVX-EB rats, develop sensitization to cocaine, whereas ICI treated rats do not. The percent change between ICI and VEH (Day 5 vs Day 1 and Day 13 vs Day 1) were compared using Student’s t-Test. Day 5: t=0.3.351, df=21.94, p=0.0029; Cohen's d=1.4; Day 13: t=2.448, df=21.68, p<0.0230, Cohen's d=1.00. These data indicate that ERs play an essential role in cocaine-induced behavioral sensitization in the female rat. They also attest to our model of estradiol replacement in our experiments of cocaine-induced behavioral sensitization since gonadally intact females behave similarly to OVX-EB rats whereas those treated with ICI-182,780 behave similar to OVX rats (Compare Figures 1B with 5B).
Experiment 6A: Effect of estradiol on cocaine-induced CPP
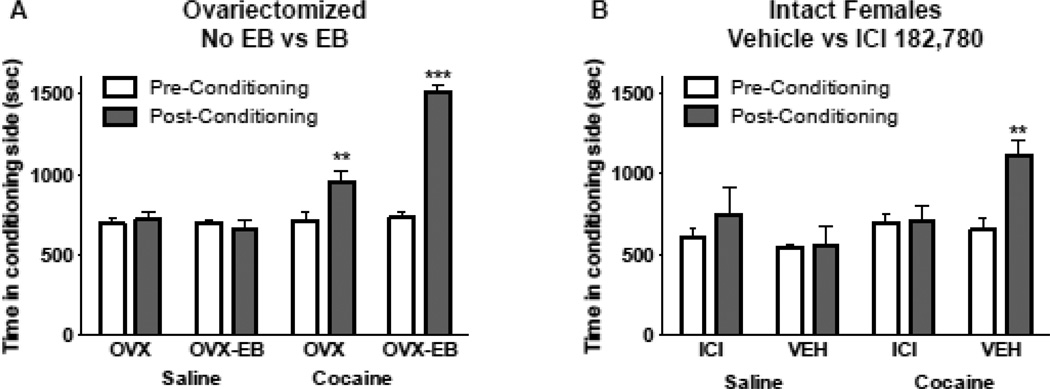
To extend our previous studies (Segarra et al. 2010), we conducted an extended CPP (12 days of alternating saline and cocaine (15 mg/kg) injections) and found that cocaine induced CPP more robustly in OVX-EB than in OVX rats (Fig 6A). Data were analyzed with a MANOVA with Pre and Post conditioning as a Repeated Measures and estradiol and cocaine as the independent factors (N=24, n=6 rats per group). Cocaine Effect: F(1,20) = 56.1016, p<0.0001; Estradiol effect: F(1,20) = 11.4917, p=0.0029; Estradiol × Cociane Effect: F (1,20) = 34.9033, p <0.0001.
Fig. 6. Conditioned place preference to cocaine of OVX and OVX-EB rats (A) and of intact female rats treated with the anti-estrogen ICI 182,780 or with vehicle (B).
Time spent in the activity chamber before (Pre-Conditioning) and after (Post-Conditioning) 12 alternating injections of saline and cocaine (15 mg/kg). A. OVX and OVX-EB rats showed an increase in the time spent in the chamber where they were injected with cocaine (pre-conditioning vs post-conditioning), with OVX-EB rats showing a greater change. Rats injected with saline did not show changes in the time spent in the chamber where they were injected with saline. B. Gonadally intact females also showed CPP to cocaine, however, ICI 182,780 treatment blocked conditioning. These data show that gonadally intact female rats show conditioned place preference (CPP) to cocaine (15 mg/kg), that estradiol enhances this conditioning and that estrogen receptors are necessary for the development and/or expression of conditioning to cocaine.
Experiment 6B: Effect of the anti-estrogen ICI-180,780 on cocaine-induced CPP
To investigate the role of ER in cocaine-induced CPP, gonadally intact female rats were infused icv with ICI-182,780 or with vehicle. After a week, rats were tested for CPP to cocaine. Rats treated with ICI 182,780 did not show an increase in time spent in the chamber where they were injected with cocaine whereas those treated with vehicle did (Fig 6B). These data indicate that ERs play an essential role in cocaine-induced CPP in the female rat. They also attest to our model of estradiol replacement in our experiments of cocaine-induced CPP. Data were analyzed with a MANOVA with Pre and Post conditioning as a Repeated Measures and ICI 182,780 and cocaine as the independent factors (n=20, 5 rats per group). Cocaine Effect: F(1,16) = 7.7094, p=0.0135; ICI 182,780 Effect: F(1,16) = 1.4308, p=0.2491; Cocaine × ICI 182,780 Effect: F (1,16) = 4.7452, p <0.0447.
Discussion
Our current study shows that estradiol, as well as a highly context-dependent sensitization protocol, enhance sensitization and CPP to cocaine in female rats. In the absence of gonadal estradiol, increasing the length of cocaine treatment or altering the dose of cocaine, did not enhance cocaine sensitization. However, when OVX rats were tested in a highly context-dependent sensitization protocol, they became sensitized to cocaine to a similar degree as OVX-EB rats tested with a protocol that is not highly context-dependent (compare Fig 1B with Fig 4B). Nevertheless, context-dependent sensitization of OVX rats was short lived, and decreased gradually with time (Fig 4C) even though their estradiol-treated counterparts continued to display a sensitized response to cocaine when challenged a week (day 13) and 2 weeks (day 28) after cocaine withdrawal, highlighting the importance of estradiol in cocaine sensitization (Fig 4C). Our experiments also addressed the issue of constant estradiol exposure versus fluctuating estradiol levels, as seen in intact female rats. Similar to OVX-EB rats, gonadally intact cycling females showed sensitization and CPP to cocaine whereas those that received the anti-estrogen ICI 182,780 did not (Fig 5B and 6B), indicating that ERs are required.
In the current study we report that OVX rats displayed a small but significant sensitization (Fig 1) in contrast to our previous studies in which OVX rats did not display sensitization (Febo et al., 2002, Puig-Ramos et al, 2008). We must mention that for Fig 1 we pooled data from 5 experiments (OVX-cocaine n=47, OVX-EB-cocaine n=59). However, when our sample size is relatively small, the large variability between subjects may mask differences that exist between treatments. This is illustrated in experiment 2; OVX rats (n=10) injected for 10 days with 15 mg/kg of cocaine, did not display sensitization (Fig 2), Nonetheless, consistent in our studies and that of others, is that females treated with estradiol always display greater sensitization.
Interestingly, rats injected with ICI did not sensitize to cocaine, even though we used a highly context-dependent protocol that induced sensitization in OVX rats. These data suggest that in the absence of ovaries (OVX rats) estrogens produced by peripheral conversion of adrenal steroids, or neurosteroids produced locally in the brain, are sufficient to induce neuroadaptative changes that result in sensitization and CPP to cocaine, albeit to a lesser degree than in animals that have their ovaries or that receive estradiol via a Silastic implant.
An interesting finding in this and in our previous studies, is that OVX rats display a higher locomotor response to their first cocaine injection, than OVX-EB rats (compare Fig 1B, left and right panels). One possibility is that estradiol produces this effect via its actions on mesocorticolimbic dopamine signaling. Dopaminergic neurons in the substantia nigra and ventral tegmental area are rich in ER (Kritzer and Creutz, 2008). Ovariectomy decreases dopamine transporters (Le Saux and Di Paolo, 2006; Sánchez et al, 2012) and induces changes in D2/D3 receptor function (Febo et al., 2003), effects that are prevented or reversed by treatment with estradiol. One might argue that since OVX rats show a higher initial response to cocaine, further increases are unlikely due to a ceiling effect. However, when OVX rats are injected in a highly context dependent paradigm, sensitization is as robust as in OVX-EB rats, although not as persistent. The same phenomenon is observed between gonadally intact rats treated with ICI versus VEH (compare Fig 6B, left and right panels).
A review of the literature shows that the dose of cocaine most commonly used to induce sensitization is 15 mg/kg, particularly in female rats (Sircar and Kim, 1999; Chin et al, 2002; Febo et al, 2002; Sell et al., 2002; Segarra et al, 2010). High cocaine doses (for example of 40 mg/kg) decrease cocaine-induced locomotor activity over time, a phenomenon reminiscent of tolerance in self-administration studies (King et al. 1992). Lower doses of intraperitoneal cocaine, such as 5 mg/kg, fail to induce sensitization (Cailhol and Mormedes, 1999; Hu and Becker, 2003). However, doses that approximate 15 mg/kg (10 mg/kg; 20 mg/kg) exert different effects on locomotor activity that vary depending on the experimental conditions. Consistent with these studies, we found that, animals injected with 10 mg/kg of cocaine did not show a robust sensitization to cocaine, although we do observe a trend to increased locomotor activity on day 5 that disappears by day 13. However other studies report sensitization at this dose (Peris et al. (1991); Hu and Becker (2003)). There are several methodological differences among these studies that may account for these differences. In the Peris et al. study, rats received 3 daily injections of saline prior to the cocaine injections and used categorical ranking scales to measure sensitization. On the other hand, the Hu and Becker study used rotational behavior to measure sensitization, which involves an ipsilateral injection of 6-hydroxydopamine in the substantia nigra. It is possible that the experimental conditions of these last two studies render the animals more “sensitive” to the locomotor activating effects of lower doses of cocaine. Thus, due to methodological differences, it is very difficult to compare results between studies.
Our data show that: (1) pairing drug administration with a context or (2) administration of estradiol enhances the locomotor response to cocaine (15 mg/kg) of ovariectomized rats. Moreover, the context-dependent sensitization displayed by OVX rats dissipates faster than in their estradiol-treated counterparts, suggesting that estradiol may be facilitating associative learning processes linked with drugs of abuse and/or that delays extinction of these memories. Previous studies show that animals that receive a drug repeatedly in the same context, show a more robust sensitization than those that receive the drug unpaired with the testing environment (Anagnostaras and Robinson, 1996; Vezina and Leyton, 2009). These animals show increased dendritic spine density and branching in the NAc, adaptations not found in animals sensitized in a context-independent paradigm (Singer et al., 2009).
Interestingly, estradiol increases dendritic spine density (Segarra and McEwen, 1991; Woolley and McEwen, 1992) and synaptic proteins (Choi et al, 2003). Moreover it regulates ion channels (Carrer et al.2003), facilitates synaptic transmission (Teyler et al, 1980, Wong and Moss. 1992) and long term potentiation (Foy et al, 1999; Grassi et al., 2011) events associated with enhanced plasticity. These data imply that estradiol and ER may promote cocaine-induced plasticity of brain substrates that facilitate and strengthen the associations between the testing environment and the psychostimulant properties of cocaine.
Estradiol can also enhance sensitization to cocaine by activating the anaplastic lymphoma kinase (Alk), an estrogen-responsive gene that promotes development of cocaine-induced sensitization and CPP (Singh et al., 2005; Lasek et al, 2011). In the striatum, the estrogen receptor alpha (ERα) directly regulates transcription of Alk (Singh et al, 2005). Lasek et al (2011) show that in the presence of estradiol, the ERα binds to a promoter region of the Alk gene and enhances cocaine sensitization and reward. According to this model, we would expect increased levels of Alk in the NAc of OVX-EB rats that may enhance synaptogenesis and neuronal plasticity as well as cocaine-induced sensitization and CPP.
Recently, cognitive enhancers have been the subject of much debate. Cognitive enhancers facilitate processes of learning and memory, and may do so by boosting attention and memory consolidation (Tuboly and Vécsei, 2013). Drugs, such as amphetamine and methylphenidate, exercise, sleep, certain foods and hormones enhance cognitive performance (Chen et al., 2011), (Chepkova et al, 1995; Ramanathan et al, 2012) (for review see Tuboly and Vécsei, 2013). Estradiol shares several intracellular pathways with cognitive processes, such as mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3 kinase /protein kinase B (P13K/Akt) (D’Astous et al., 2006; Chan and Ye, 2012). It is possible that in the context of drug abuse, estradiol, acts as a cognitive enhancer, facilitating processes of learning and memory associated with the drug. Since a common finding in addicted individuals is a decreased ability to inhibit maladaptive behaviors (Connolly et al., 2012; Goldstein and Volkow, 2011), estradiol, by inducing excessive response to the drug and drug-related cues, may act exacerbate addictive behavior. Further experiments are required to determine the mechanisms by which estradiol facilitates drug-induced plasticity. These studies will no doubt assist in the development of pharmacological strategies to treat addiction, particularly in women.
Table 1.
Summary of the methodology used in each experimental protocol
| Experiment | Dose (mg/kg) |
# cocaine injections |
# injections same context |
Hormone Groups |
Behaviors Measured |
|---|---|---|---|---|---|
| Exp 1 | 15 | 5 | 2 | OVX vs. OVX-EB | Sensitization |
| Exp 2 | 15 | 10 | 2 | OVX vs. OVX-EB | Sensitization |
| Exp 3A | 10 | 5 | 2 | OVX vs. OVX-EB | Sensitization |
| Exp 3B | 30 | 5 | 2 | OVX vs. OVX-EB | Sensitization |
| Exp 4 | 15 | 5 | 5 | OVX vs. OVX-EB | Sensitization |
| Exp 5 | 15 | 5 | 5 | ICI vs. VEH | Sensitization |
| Exp 6A | 15 | 6 | 5 | OVX vs. OVX-EB | CPP |
| Exp 6B | 15 | 6 | 5 | ICI vs. VEH | CPP |
HIGHLIGHTS.
Blocking estrogen receptors disrupts sensitization and CPP to cocaine in females
Estradiol facilitates associations between cocaine and drug context.
Estradiol strengthens the acquisition and maintenance of cocaine sensitizations
Acknowledgements
The authors wish to acknowledge funding by grants U54NS39405 from NIDDK, S06-GH08224 and R25Gm061838 from NIGMS. The authors also like to acknowledge Amanda González, Josel Diaz, Paola Arce and Janice Vivaldi in proofreading the manuscript and assistance with the references.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav. Neurosci. 1996;110(6):1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. The role of progestins in the behavioral effects of cocaine and other drugs of abuse: human and animal research. Neurosci. Biobehav. Rev. 2010;35(2):315–333. doi: 10.1016/j.neubiorev.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcoitia I, Yague JG, García-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–147. doi: 10.1016/j.neuroscience.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Barha CK, Galea LAM. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim. Biophys. Acta. 2010;1800:1056–1067. doi: 10.1016/j.bbagen.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Cailhol S, Mormedes P. Strain and sex differences in the locomotor response and behavioral sensitization to cocaine in hyperactive rats. Brain Res. 1999;842(1):200–205. doi: 10.1016/s0006-8993(99)01742-4. [DOI] [PubMed] [Google Scholar]
- Carrer HF, Araque A, Buño W. Estradiol regulates the slow Ca2+-activated K+ current in hippocampal pyramidal neurons. J Neurosci. 2003;23(15):6338–6344. doi: 10.1523/JNEUROSCI.23-15-06338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CB, Ye K. Phosphoinositide 3-kinase enhancer (PIKE) in the brain: is it simply a phosphoinositide 3-kinase/Akt enhancer? Neuroscience. 2012;23(2):153–161. doi: 10.1515/revneuro-2011-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, Blitzer RD, Alberini CM. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469(7331):491–497. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepkova AN, French P, De Wied D, Ontskul AH, Ramakers GM, Skrebitski VG, Gispen WH, Urban IJ. Long-lasting enhancement of synaptic excitability of CA1/subiculum neurons of the rat ventral hippocampus by vasopressin and vasopressin (4–8) Brain Res. 1995;701(1–2):255–266. doi: 10.1016/0006-8993(95)01006-7. Brain, Res. [DOI] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HB, Burrell S, Lu D, Jenab S, Perrotti LI, Quiñones-Jenab V. Endogenous gonadal hormones modulate behavioral and neurochemical responses to acute and chronic cocaine administration. Brain Res. 2002;945(1):123–130. doi: 10.1016/s0006-8993(02)02807-x. 26. [DOI] [PubMed] [Google Scholar]
- Choi JM, Romeo RD, Brake WG, Bethea CL, Rosenwaks Z, McEwen BS. Estradiol increases pre- and post-synaptic proteins in the CA1 region of the hippocampus in female rhesus macaques (Macaca mulatta) Endocrinology. 2003;144(11):4734–4738. doi: 10.1210/en.2003-0216. [DOI] [PubMed] [Google Scholar]
- Colell E, Sánchez-Niubó A, Domingo-Salvany A. Sex differences in the cumulative incidence of substance use by birth cohort. Int. J. Drug Policy. 2013;24(4):319–325. doi: 10.1016/j.drugpo.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Collins SL, Evans SM, Foltin RW, Haney M. Intranasal cocaine in humans: effects of sex and menstrual cycle. Pharmacol. Biochem. Behav. 2007;86(1):117–124. doi: 10.1016/j.pbb.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Foxe JJ, Nierenberg J, Shpaner M, Garavan H. The neurobiology of cognitive control in successful cocaine abstinence. Drug Alcohol Depend. 2012;121(1–2):45–53. doi: 10.1016/j.drugalcdep.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Astous M, Mendez P, Morissette M, Garcia-Segura LM, Di Paolo T. Implication of the phosphatidylinositol-3 kinase/protein kinase B signaling pathway in the neuroprotective effect of estradiol in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice. Mol. Pharmacol. 2006;69(4):1492–1498. doi: 10.1124/mol.105.018671. [DOI] [PubMed] [Google Scholar]
- Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993;106:1377–1388. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- Du J, Huang D, Zhao M, Hser YI. Drug-abusing offenders with co-morbid mental disorders: gender differences in problem severity, treatment participation, and recidivism. Biomed. Environ. Sci. 2013;26(1):32–39. doi: 10.3967/0895-3988.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31(3):659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159(4):397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xuj, Xie X, Brinton RD, Thompson RF, Berger TW. 17 β estradiol enhances NMDA receptor-mediated EPSP’s and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Febo M, Ferris CF, Segarra AC. Estrogen influences cocaine-induced blood oxygen level-dependent signal changes in female rats. J Neurosci. 2005;25(5):1132–1136. doi: 10.1523/JNEUROSCI.3801-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, González-Rodríguez LA, Capó-Ramos DE, González-Segarra NY, Segarra AC. Estrogen-dependent alterations in D2/D3-induced G protein activation in cocaine-sensitized female rats. J Neurochem. 2003;86(2):405–412. doi: 10.1046/j.1471-4159.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- Febo M, Jiménez-Rivera CA, Segarra AC. Estrogen and opioids interact to modulate the locomotor response to cocaine in the female rat. Brain Res. 2002;943(1):151–161. doi: 10.1016/s0006-8993(02)02748-8. [DOI] [PubMed] [Google Scholar]
- Fester L, Prange-Kiel J, Jarry H, Rune GM. Estrogen synthesis in the hippocampus. Cell Tissue Res. 2011;345:285–294. doi: 10.1007/s00441-011-1221-7. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi S, Tozzi A, Costa C, Tantucci M, Colcell E, Scarduzio M, Calabresi P, Pettorossi VE. Neural 17 β estradiol facilitates long-term potentiation in the hippocampal CA1 region. Neuroscience. 2011;192:67–73. doi: 10.1016/j.neuroscience.2011.06.078. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch. Gen. Psychiatry. 1989;46(2):122–126. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LAM. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the Radial Arm Maze. Behav. Neurosci. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Howell A, Kent Osborne C, Morris C, Wakeling AE. ICI 182,780 (Faslodex ™) Development of a novel,“Pure” Antiestrogen. Cancer. 2000;89(4):817–825. doi: 10.1002/1097-0142(20000815)89:4<817::aid-cncr14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Becker JB. Acquisition of cocaine self-administration in ovariectomized female rats: effect of estradiol dose or chronic estradiol administration. Drug Alcohol Depend. 2008;94:56–62. doi: 10.1016/j.drugalcdep.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31(1):129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- King GR, Joyner C, Lee T, Kuhn C, Ellinwood EH. Intermittent and continuous cocaine administration: residual behavioral states during withdrawal. Pharmacol. Biochem. Behav. 1992;43(1):243–248. doi: 10.1016/0091-3057(92)90664-2. [DOI] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:495–499. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Creutz LM. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci. 2008;28(38):9525–9535. doi: 10.1523/JNEUROSCI.2637-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp. Clin. Psychopharmacol. 2007;15(5):461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Lasek AW, Gesch J, Giorgettoi F, Kharazia V, Heberlein U. Alk is a transcriptional target of LMO4 and ERα that promotes cocaine sensitization and reward. J Neurosci. 2011;31(40):14134–14141. doi: 10.1523/JNEUROSCI.3415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975;96(1):50–56. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Di Paolo T. Influence of oestrogenic compounds on monoamine transporters in rat striatum. J Neuroendocrinol. 2006;18(1):25–32. doi: 10.1111/j.1365-2826.2005.01380.x. [DOI] [PubMed] [Google Scholar]
- Luo X, Zhang S, Hu S, Bednarski SR, Erdman E, Farr OM, Hong KI, Sinha R, Mazure CM, Li CS. Error processing and gender-shared and –specific neural predictors of relapse in cocaine dependence. Brain. 2013;136(4):1231–1244. doi: 10.1093/brain/awt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol. Biochem. Behav. 2001;68(4):641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Milner TA. Hippocampal formation: Shedding light on the influence of sex and stress on the brain. Brain Res. Rev. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Mendelson JH. Sex and menstrual cycle effects on progressive ratio measures of cocaine self-administration in cynomolgus monkeys. Neuropsychopharmacology. 2007;32(9):1956–1966. doi: 10.1038/sj.npp.1301314. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS, Knudson IM, Kelly M, Mendelson JH. Effects of estradiol on cocaine self-administration and cocaine discrimination by female rhesus monkeys. Neuropsychopharmacology. 2008;33(4):783–795. doi: 10.1038/sj.npp.1301451. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neurobiol. Learn Mem. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Parker MG. Action of“pure” antiestrogens in inhibiting estrogen receptor action. Breast Cancer Res. Treat. 1993;26:131–137. doi: 10.1007/BF00689686. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2007 doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Peris J, Decambre N, Coleman-Hardee ML, Simpkins JW. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated striatal [3H]dopamine release. Brain Res. 1991;566(1–2):255–264. doi: 10.1016/0006-8993(91)91706-7. [DOI] [PubMed] [Google Scholar]
- Puig-Ramos A, Santiago GS, Segarra AC. A kappa opioid receptor agonist, decreases cocaine-induced behavioral sensitization in female rats. Behav. Neurosci. 2008;122(1):151–160. doi: 10.1037/0735-7044.122.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan G, Cilz NI, Kurada L, Hu B, Wang X, Lei S. Vasopressin facilitates GABAergic transmission in rat hippocampus via activation of V(1A) receptors. Neuropharmacology. 2012;63(7):1218–1226. doi: 10.1016/j.neuropharm.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando K, Tuit K, Hannestad J, Guarnaccia J, Sinha R. Sex differences in decreased limbic and cortical grey matter volume in cocaine dependence: a voxelbased morphometric study. Addict. Biol. 2013;18(1):147–160. doi: 10.1111/adb.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC, Evans SM, Bedi G, Rubin E, Foltin RW. The effects of oral micronized progesterone on smoked cocaine self-administration in women. Horm. Behav. 2011;59(2):227–235. doi: 10.1016/j.yhbeh.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behaviour produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396(2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Sánchez MG, Morissette M, Di Paolo T. Effect of a chronic treatment with 17β-estradiol on striatal dopamine neurotransmission and the Akt/GSK3 signaling pathway in the brain of ovariectomized monkeys. Psychoneuroendocrinology. 2012;37(2):280–291. doi: 10.1016/j.psyneuen.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Segal DS. Behavioral and neurochemical correlates of repeated damphetamine administration. Adv. Biochem. Psychopharmacol. 1971;13:247–262. [PubMed] [Google Scholar]
- Segarra AC, Agosto-Rivera JL, Febo M, Lugo-Escobar N, Menéndez-Delmestre R, Puig-Ramos A, Torres-Diaz YM. Estradiol: a key biological substrate mediating the response to cocaine in female rats. Horm. Behav. 2010;58(1):33–43. doi: 10.1016/j.yhbeh.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra AC, McEwen BS. Estrogen increases spine density in ventromedial hypothalamic neurons of peripubertal rats. Neuroendocrinology. 1991;54(4):365–372. doi: 10.1159/000125915. [DOI] [PubMed] [Google Scholar]
- Sell SL, Thomas ML, Cunningham KA. Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug Alcohol Depend. 2002;67(3):281–290. doi: 10.1016/s0376-8716(02)00085-6. [DOI] [PubMed] [Google Scholar]
- Singer BF, Tanabe LM, Gorny G, Jake-Matthews C, Li Y, Kolb B, Vezina P. Amphetamine-induced changes in dendritic morphology in rat forebrain correspond to associative drug conditioning rather than nonassociative drug sensitization. Biol. Psychiatry. 2009;65(10):835–840. doi: 10.1016/j.biopsych.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RR, Barnes CJ, Talukder AH, Fuqua SA, Kumar R. Negative regulation of estrogen receptor alpha transactivation functions by LIM domain only 4 protein. Cancer Res. 2005;65(22):10594–10601. doi: 10.1158/0008-5472.CAN-05-2268. [DOI] [PubMed] [Google Scholar]
- Sircar R, Kim D. Female gonadal hormones differentially modulate cocaineinduced behavioral sensitization in Fischer, Lewis, and Sprague-Dawley rats. J Pharmacol. Exp. Ther. 1999;289(1):54–65. [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol. Rev. 2011;63(2):348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler TJ, Vardaris RM, Lewis D, Rawitch AB. Gonadal steroids; effects on excitability of hippocampal pyramidal cells. Science. 1980;209:1017–1018. doi: 10.1126/science.7190730. [DOI] [PubMed] [Google Scholar]
- Tuboly G, Vécsei L. Somatostatin and cognitive function in neurodegenerative disorders. Mini Rev. Med. Chem. 2013;13(1):34–46. doi: 10.2174/138955713804484794. [DOI] [PubMed] [Google Scholar]
- Van Etten ML, Neumark YD, Anthony JC. Male-female differences in the earliest stages of drug involvement. Addiction. 1999;94(9):1413–1419. doi: 10.1046/j.1360-0443.1999.949141312.x. [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56(Suppl 1):160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade GN, Blaustein JD, Gray JM, Meredith JM. ICI 182,780: a pure antiestrogen that affects behaviors and energy balance in rats without acting in the brain. Am J Physiol. 1993;265:R1399–R1403. doi: 10.1152/ajpregu.1993.265.6.R1392. [DOI] [PubMed] [Google Scholar]
- Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci. 1992;12:3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12(7):2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]