Abstract
Objective:
We estimated premature mortality and identified causes of death and associated factors in people with active convulsive epilepsy (ACE) in rural Kenya.
Methods:
In this prospective population-based study, people with ACE were identified in a cross-sectional survey and followed up regularly for 3 years, during which information on deaths and associated factors was collected. We used a validated verbal autopsy tool to establish putative causes of death. Age-specific rate ratios and standardized mortality ratios were estimated. Poisson regression was used to identify mortality risk factors.
Results:
There were 61 deaths among 754 people with ACE, yielding a rate of 33.3/1,000 persons/year. Overall standardized mortality ratio was 6.5. Mortality was higher across all ACE age groups. Nonadherence to antiepileptic drugs (adjusted rate ratio [aRR] 3.37), cognitive impairment (aRR 4.55), and age (50+ years) (rate ratio 4.56) were risk factors for premature mortality. Most deaths (56%) were directly related to epilepsy, with prolonged seizures/possible status epilepticus (38%) most frequently associated with death; some of these may have been due to sudden unexpected death in epilepsy (SUDEP). Possible SUDEP was the likely cause in another 7%.
Conclusion:
Mortality in people with ACE was more than 6-fold greater than expected. This may be reduced by improving treatment adherence and prompt management of prolonged seizures and supporting those with cognitive impairment.
Epilepsy is one of the most common noncommunicable neurologic conditions in the world, estimated to affect approximately 70 million people, with up to 90% living in low- and middle-income countries (LMICs).1 It is associated with premature mortality, 1.3–9.3 times that of the background population in high-income countries (HICs) and much higher in institutionalized people and those with cerebral malformations.2,3 In LMICs there are few data, which are based on small cohorts with few deaths4–6 conducted in areas with high incidence of epilepsy5 that may not be representative.
Risk factors for mortality in epilepsy in developed countries3,7,8 include age at onset, duration of epilepsy (risk highest in the first 2 years after onset),3 high seizure frequency, convulsive seizures,3 and remote symptomatic etiology.9,10 In LMICs, however, risk factors for death have only been reported from China and included young age at onset of epilepsy, duration of epilepsy, and living close to a body of water.11
Causes of death (CODs) in epilepsy, including epilepsy-related CODs, have been studied in HICs.7,12 In LMICs, cause-specific mortality data are scant and have been reported in only 2 countries: China, where CODs included injuries and accidents, stroke, status epilepticus, and suicide13,14; and Ethiopia, where death was related to epilepsy.15,16
We used a population-based prospective cohort study of people with active convulsive epilepsy (ACE) in a resource-limited rural area of Kenya to determine the magnitude of and possible causes and risk factors associated with mortality.
METHODS
Study setting and study population.
The study setting and population characteristics have been described previously.17–19 Briefly, the study was within the Kilifi Health and Demographic Surveillance System (KHDSS) (http://www.kemri-wellcome.org/khdss/) in the Kilifi District of Kenya. In 2008 there were 233,800 people in the study area.20 Households were mapped using global positioning system, and maps were used to locate and follow up participants. Field personnel conduct re-enumeration and vital status updates of the population register 3 times per year.
About 80% of the population depends on subsistence farming and the rest are engaged in casual and formal employment or informal trading. Approximately 55% live in poverty.
Identification and follow-up of cohorts of participants with and without ACE.
We used the International League Against Epilepsy definitions of epilepsy21 but defined ACE as at least one convulsive seizure in the preceding 12 months,22,23 as this is the Kenyan criterion for offering antiepileptic treatment.24
We identified a cohort of people with ACE and the general population (people without ACE) in a baseline 3-stage population-wide prevalence screening between December 2007 and June 2008.19 In the first stage we asked household heads 2 questions to identify members with a history of convulsions, who were followed up with a more detailed epilepsy screening tool at a second stage. Those positive were then evaluated by an epilepsy-specialized clinician and the diagnoses were confirmed independently by a neurologic panel. Those diagnosed, if not on antiepileptic drugs (AEDs), were offered treatment. At this stage we collected clinical and demographic data including age, sex, level of schooling and employment (for adults), time (or age) of first seizure, and cognitive impairment (a clinician assessed the individual's response to questions [including orientation to person, place, and time] and ease of following instructions).
We followed up the identified ACE cohort every 3 months in the study clinic for dose adjustments, assessment of adverse events, monitoring adherence, and to replenish supplies of AEDs. Those who did not attend the clinic appointments were followed up in the community within 2 weeks by a fieldworker. During each review we administered a questionnaire to capture demographic information and potential risk factors for mortality.
If a member of the ACE cohort was not on treatment during one of the appointments, we classified him or her as nonadherent to AEDs as, by definition, he or she had active epilepsy. This was determined by asking to see the medication during all visits in the community or if they missed a clinic appointment and were not taking AEDs at the subsequent attendance.
We followed up the general population for vital status and migration during the routine re-enumeration within the KHDSS. Participants were followed up until death or the end of the study or were censored at out-migration (and during the last clinic contact or withdrawal of consent/refusal for people with ACE).
Postmortem examinations, which are the gold standard for the determination of COD, are not conducted in this rural area.20,25 To determine putative COD, we investigated the immediate CODs using the World Health Organization verbal autopsy tool (appendix e-1 on the Neurology® Web site at www.neurology.org) administered by a fieldworker trained in the sensitivity required to interview bereaved relatives. We were unable to examine death certificates as these, in keeping with local tradition, are destroyed with all personal effects of the deceased after the funeral (Francis Yaa, oral communication, October 12, 2012).
We conducted the verbal autopsy interviews within 1–4 months of death to allow for the culturally acceptable mourning period in this community. As much as possible we interviewed either the guardian or the carer of the deceased prior to death. The CODs were assigned independently by 3 clinicians trained in the use of the ICD-10. We considered the assigned COD as final if there was agreement between all coders. A fourth coder (C.R.N.) independently assigned COD in instances where initially an agreement could not be reached. Disagreements between the 4 coders were resolved by consensus, or the cause was designated as unclassified.
Statistical analysis.
We estimated mortality rates as the number of deaths divided by the total person-years of observation. To compare mortality between people with ACE and the general population, mortality in those with ACE was stratified in age bands and standardized mortality ratios (SMRs) were estimated using the age-specific rates of the general population. We performed a sensitivity analysis on the rate ratio to determine whether it was sensitive to losses to follow-up; the mortality rates in both the “lost” cases and the “lost” general population varied between 0.5 and 3 times the observed rates. Excess death rates were estimated as the absolute age-specific rate differences between the ACE and general population groups.
We used Poisson regression to examine the influence of 10 putative risk factors on mortality in the ACE cohort. We determined these at baseline (sex, age at onset, schooling, distance to health facilities, duration of epilepsy, income, and cognitive impairment) and/or during each of the 3-month reviews (seizure frequency, adherence to AEDs, and current age). The last 3 variables were modeled as time-dependent covariates.
In the multivariable analysis we explored the relationship between mortality and 1) AED adherence, 2) cognitive impairment, 3) schooling, and 4) age at onset, as these were significant in the univariable analysis.
The relationships of putative COD to ACE were categorized independently by 2 clinicians as 1) directly or indirectly related to epilepsy, 2) related to the underlying cause of epilepsy, 3) not related to epilepsy, or 4) unknown. Epilepsy-related CODs were defined as 1) death during a prolonged seizure–possible status epilepticus; 2) sudden death in epilepsy (possible sudden unexpected death in epilepsy [SUDEP])–sudden unwitnessed death or death without any preceding illness; or 3) death following a seizure-related accident, e.g., drowning, falling from heights. We also estimated cause-specific mortality proportions and compared the epilepsy-specific mortality proportions to the attributable fraction (1− (1/rate ratio)) to determine the performance of the verbal autopsy in capturing epilepsy-related mortality.
All analyses were carried out in STATA v. 11 (StataCorp, College Station, TX).
Standard protocol approvals, registrations, and patient consents.
We obtained written informed consent from all participants. When the person with ACE was a child, an adult who could not respond, or had died, a guardian/carer was interviewed. Approval for the study was obtained from the Kenya Medical Research Institute/National Ethical Review Committee.
RESULTS
At study start in December 2007, 232,164 participants entered the cohorts: 754 individuals with ACE and 231,410 people in the general population (without ACE) (table 1). The median (interquartile range) follow-up for people with ACE was 32.6 (27.7–41.1) months and for the general population was 32.6 (28.5–32.6) months. Within the ACE group we recorded a total of 7,331 contacts with a median of 11 follow-up contacts per person. We recorded 61 deaths in people with ACE and 3,291 in the general population at study end in November 2011. Outcomes by ACE status are shown in table 1. Among people with ACE, loss to follow-up did not vary by age group (p = 0.906), sex (p = 0.126), or school attendance (p = 0.061) of the participant.
Table 1.
Follow-up of study participants with active convulsive epilepsy (ACE) and the general population in Kilifi, Kenya
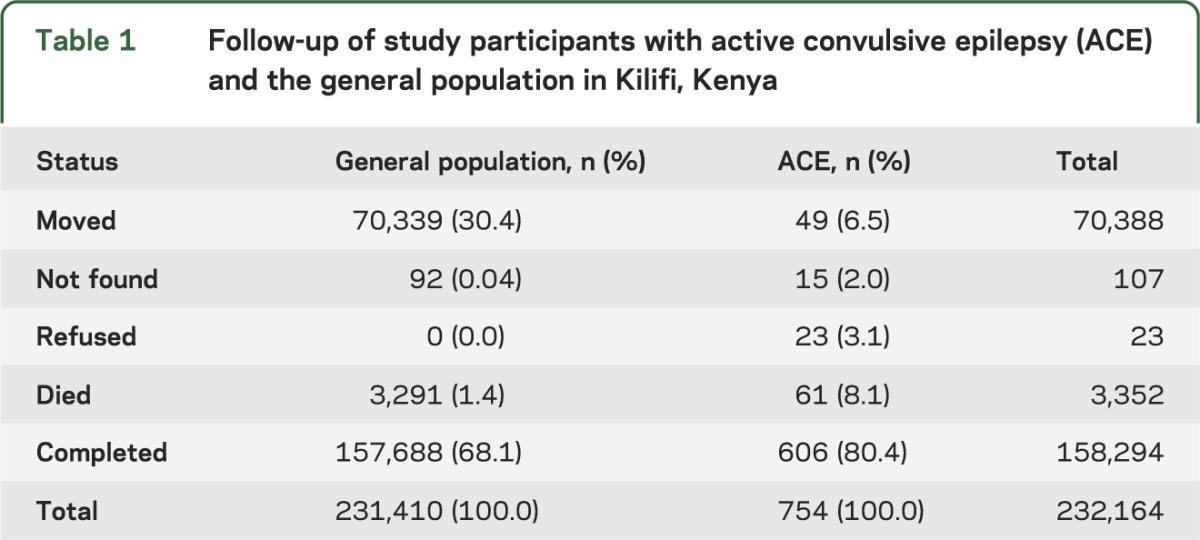
The total person-years of observation (pyo) was 1,833 for the ACE cohort and 540,909 for the general population, with crude mortality rates of 33.3/1,000 pyo (95% confidence interval [CI]: 25.9–42.8) and 6.1/1,000 pyo (95% CI: 5.9–6.3), respectively. The crude rate ratio was 5.5 (95% CI: 4.2–7.0) and the SMR was 6.5 (95% CI: 5.0–8.3). In the sensitivity analyses, overall mortality rate ratios varied between 3.2 and 7.9 (table e-1). Mortality was higher than in the general population in all age groups: age-specific rate ratios ranged from 4.4 (95% CI: 1.1–17.4) in the 0 to 5 years age group to 22.5 (95% CI: 12.5–40.6) in the 6 to 12 years age group. The excess death rate per 1,000 pyo increased with age, ranging from 14.4 in 0- to 5-year-olds to 55.3 in those aged ≥50 years (table e-2).
In the univariable analysis, current age, age at onset of seizures, AED nonadherence, and cognitive impairment were associated with mortality. Having attended school was protective against mortality (table 2). After adjusting for potential confounding, those not taking AEDs (rate ratio 3.37 [95% CI: 1.84–6.16]) and those with cognitive impairment (rate ratio 4.55 [2.49–8.33]) were at higher risk (table 3). Epilepsy-related deaths were also associated with not taking AEDs (adjusted rate ratio [aRR] 3.41 [1.57–7.42]) and cognitive impairment (aRR 4.34 [2.51–8.76]) (table e-3).
Table 2.
Univariable analysis of factors associated with mortality in people with active convulsive epilepsy in Kilifi, Kenya

Table 3.
Adjusted rate ratios for mortality in people with active convulsive epilepsy in Kilifi, Kenya

More than half (56%) of the putative CODs in the population with ACE were directly or indirectly related to epilepsy. Most deaths (38%) occurred during a prolonged seizure (possible status epilepticus) and about 7% (n = 4) were possible SUDEP. About 40% of the CODs were either unrelated to epilepsy or unknown (table 4). The frequency of epilepsy-related COD was higher (52% of total deaths) in those aged 19–49 years than in those younger than 19 years (35%) or the elderly (43%). Cardiovascular-related CODs were significant in the ≥50-year-olds (table 5).
Table 4.
Causes of death in people with active convulsive epilepsy in Kilifi, Kenya

Table 5.
Age-specific proportional mortality in people with active convulsive epilepsy in Kilifi, Kenya

The overall attributable fraction for ACE was 82% and was highest (>90%) in the 6 to 28 years age groups (table e-2).
DISCUSSION
Mortality in people with ACE in this population was 6 times higher than that of the general population, and this was higher than that reported in other studies from LMICs.13,14 The high mortality rate in our study (compared to other studies that reported rates from 0.024 to 16.4/1,000 pyo26–29) may be partly due to the fact that we assessed only convulsive epilepsy, which is usually associated with higher mortality than nonconvulsive epilepsies.3,7 The inclusion of only those with a seizure within the preceding year formed a group with more severe epilepsy than is usually included in epidemiologic studies of active epilepsy (seizures within the last 5 years) and could also have contributed to the high mortality.
The highest age-specific mortality rate ratio was in the 6–28 years age group, which also had the lowest mortality rate in the general population. In Ugandan and Chinese studies relative mortality was also highest in this age group.5,13 Mortality in this age group could result from remote symptomatic epilepsy,9,10 from etiologies occurring earlier in life (e.g., infections of the brain, cerebral palsy), or from the higher risk of head injuries. The epilepsy-related mortality in these ages could also be due to difficulties in adherence to AEDs, SUDEP, or recent onset of seizures.3,10 The lower rate ratios in the other age groups may reflect the relatively higher mortality rates seen in the corresponding age groups in the general population. Previous studies in Kilifi suggest that mortality in these age groups is related to perinatal, nutritional, and infectious causes in the very young30,31 and HIV-related and cardiovascular causes in the older age groups.25 In our study, prolonged seizures (possible status epilepticus) were the most important cause of death in people with epilepsy. In China, status epilepticus and drowning were significant.11 These studies highlight the importance of seizure control in preventing mortality.
Excess mortality increased with age and it is likely that recurrent seizures in the old aggravate the severity of coexisting conditions (e.g., cardiovascular disease). The attributable fraction (82%) was higher than the epilepsy-specific mortality proportion determined through verbal autopsy (55.7%), suggesting that some of the epilepsy-related deaths may have been misclassified. Alternatively, the attributable fraction estimate could have been confounded by factors such as poverty or infectious diseases.
In the univariable analysis, those who were nonadherent to AEDs and/or who had cognitive impairment were at higher risk of mortality. In the adjusted analysis, those not on AEDs had a 3-fold increased risk compared to those on at least one AED. This association is important in view of the large treatment gap (62%) reported in Kilifi32 and the fact that epilepsy-related CODs, particularly prolonged seizures, were the most important in this population. Our findings are similar to those of a British study33 but dissimilar to those of a Ugandan study,5 which did not find higher mortality among people who were not adherent. Possible reasons for the difference include the fact that the Ugandan study was relatively small (61 patients) and used self-reported adherence, which has low sensitivity.32 Adherence in that study was assessed at only 2 time points over a 6-year period in a lifetime epilepsy cohort, in which some cases could have entered remission. Nonadherence leads to poorly controlled seizures, which may increase epilepsy severity (with respect to duration of seizures) and thus impart higher risk of epilepsy-related mortality. Poor seizure control may also increase seizure risk in hazardous environments, causing death by accidents (e.g., drowning and falling from heights). Age at onset and duration of ACE, which were associated with mortality in China,11 were not significant in our study possibly because the Chinese study investigated associations with specific causes of death (e.g., status epilepticus or drowning). Cognitive impairment is likely to increase the risk of mortality through injury resulting from seizures in risky environments, particularly if individuals are not supervised. Cognitive impairment is also likely to be a marker of more severe epileptic encephalopathies (e.g., Lennox-Gastaut syndrome in children), which confer a higher risk of premature mortality than more benign syndromes.34
This high risk of epilepsy-related mortality highlights the need to prevent seizures and reduce risk factors for mortality. Risk factors for epilepsy such as adverse perinatal events and parasitic infections should be targeted.19 Education of patients and carers on the importance of treatment and adherence to medication and monitoring and supporting people with cognitive impairment (e.g., assigning a household member as a designated helper) could reduce the risks associated with epilepsy-related causes of death. Additionally, AEDs that are effective against more severe epilepsy syndromes associated with cognitive impairment should be availed at the primary care level, although this is likely to have considerable resource implications. Factors associated with nonadherence in this population include long distances to AED distribution points and traditional animistic beliefs about epilepsy.32 Increasing access to AEDs by ensuring adequate supply at the primary care level and creation of awareness about treatment may well lead to a significant reduction in premature mortality.
We had large attrition of people without ACE, mainly out-migrants. Due to cost and logistical implications, we did not determine their survivorship status, although their demographic characteristics were not significantly different from those in the study. Mortality rate ratios, however, were more than 3 even after sensitivity analysis.
We used a prevalence cohort, which might underestimate short-term mortality due to the early mortality of people with severe ACE. A prospectively identified population-based cohort of incident cases may provide more accurate ratios but would be more expensive.
We were unable to estimate cause-specific SMRs due to lack of cause-specific mortality data in the general population. We did not use a validated tool to screen for cognitive impairment, and it is likely we included only those with the most severe form, hence the strong association with premature mortality.
As is common in many resource-poor settings, postmortem examination, the gold standard for identifying COD, was unavailable. The use of death certificates in epilepsy, however, is fraught with problems, since epilepsy is only attributed as a COD when seizure-related clinical events are observed immediately preceding death.35 To overcome this, we carried out verbal autopsies and CODs were attributed by moderation based on the verbal autopsies. CODs should therefore be considered putative rather than definitive. We were, for instance, unable to establish definite status epilepticus as a COD in people who died during prolonged seizures since this was a population-based study in which terminal seizure events were not timed and were dependent on recall by witnesses. There is therefore a likelihood of overlap between possible SUDEP and prolonged seizures.
It is likely that certain epilepsy syndromes and seizure types are associated with mortality in people with epilepsy. However, we were unable to determine specific seizure types (other than convulsive), epilepsy syndrome, or etiology in this cohort due to limited seizure type information during follow-up.
Our findings highlight the urgency of prioritizing epilepsy as a major public health problem. Increased resources need to be allocated to improve access to and utilization of diagnostic, treatment, and preventive services. The health system needs to increase and sustain capacity to provide appropriate health services to people with epilepsy at the primary care level (e.g., in terms of training and retaining nurses or clinical officers to diagnose and manage epilepsy as well as provide AEDs). Preventive interventions must be given priority, with the identification of preventable causes in communities as well as risk factors of mortality in epilepsy through deployment of measures such as health education and promotion.
Supplementary Material
ACKNOWLEDGMENT
The authors are grateful to Karren Visser and Gail Bell for critically reviewing this manuscript. This work is published with the permission of the Director of KEMRI.
GLOSSARY
- ACE
active convulsive epilepsy
- AED
antiepileptic drug
- aRR
adjusted rate ratio
- CI
confidence interval
- COD
cause of death
- HIC
high-income country
- ICD-10
International Classification of Diseases, 10th revision
- KHDSS
Kilifi Health and Demographic Surveillance System
- LMICs
low- and middle-income countries
- pyo
person-years of observation
- SMR
standardized mortality ratio
- SUDEP
sudden unexpected death in epilepsy
Footnotes
Editorial, page 552
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
The study was conceived by A.K.N., B.N., J.W.S., and C.R.N.; designed by A.K.N. and C.R.N.; and set up by A.K.N., E.C., R.O., C.R.N., and E.B. A.K.N., E.C., and R.O. coordinated and monitored data collection. R.O. created the database and managed data. Data analysis was done by A.K.N. with input from C.B., G.F., I.K., J.W.S., and C.R.N. A.K.N. wrote the first draft, and all authors contributed to the subsequent drafts and approved the final manuscript. Statistical analyses were conducted by A.K.N. with input from C.B., I.K., and G.F.
STUDY FUNDING
Supported by grants from The Wellcome Trust (No. 083744) to C.R.N. and strategic training award (No. 084538) to KEMRI-Wellcome Trust Research Programme.
DISCLOSURE
A. Ngugi, C. Bottomley, G. Fegan, E. Chengo, R. Odhiambo, E. Bauni, B. Neville, and I. Kleinschmidt report no disclosures. J. Sander served on scientific advisory boards for GlaxoSmithKline, Eisai, and UCB; has received funding for travel from UCB; serves on the editorial board of Lancet Neurology; serves on the speakers' bureaus of UCB and GlaxoSmithKline; received research support from the NIH, CBRC, European Union Seventh Framework Programme, Wellcome Trust, Nationaal Epilepsie Fonds Nederland, UCB (institutional), Eisai (institutional), GlaxoSmithKline (institutional), and The Brain Research Trust; and his current post is endowed by the UK Epilepsy Society and supported by the Dr. Marvin Weil Epilepsy Research Fund. C. Newton reports no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia 2010;51:883–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shackleton DP, Westendorp RGJ, Kasteleijn-Nolst Trenité DG, De Craen AJM, Vandenbroucke JP. Survival of patients with epilepsy: an estimate of the mortality risk. Epilepsia 2002;43:445–450 [DOI] [PubMed] [Google Scholar]
- 3.Forsgren L, Hauser WA, Olafsson E, Sander JW, Sillanpää M, Tomson T. Mortality of epilepsy in developed countries: a review. Epilepsia 2005;46(suppl 1):18–27 [DOI] [PubMed] [Google Scholar]
- 4.Coleman R, Loppy L, Walraven G. The treatment gap and primary health care for people with epilepsy in rural Gambia. Bull World Health Organ 2002;80:378–383 [PMC free article] [PubMed] [Google Scholar]
- 5.Kaiser C, Asaba G, Kasoro S, Rubaale T, Kabagambe G, Mbabazi M. Mortality from epilepsy in an onchocerciasis-endemic area in West Uganda. Trans R Soc Trop Med Hyg 2007;101:48–55 [DOI] [PubMed] [Google Scholar]
- 6.Snow RW, Williams RE, Rogers JE, Mung'ala VO, Peshu N. The prevalence of epilepsy among a rural Kenyan population. Its association with premature mortality. Trop Geogr Med 1994;46:175–179 [PubMed] [Google Scholar]
- 7.Lhatoo SD, Sander JW. Cause-specific mortality in epilepsy. Epilepsia 2005;46(suppl 1):36–39 [DOI] [PubMed] [Google Scholar]
- 8.Neligan A, Bell GS, Johnson AL, Goodridge DM, Shorvon SD, Sander JW. The long-term risk of premature mortality in people with epilepsy. Brain 2011;134(Pt 2):388–395 [DOI] [PubMed] [Google Scholar]
- 9.Beghi E, Leone M, Solari A. Mortality in patients with a first unprovoked seizure. Epilepsia 2005;46(suppl 1):40–42 [DOI] [PubMed] [Google Scholar]
- 10.Cockerell OC, Johnson AL, Sander JW, Hart YM, Goodridge DM, Shorvon SD. Mortality from epilepsy: results from a prospective population-based study. Lancet 1994;344:918–921 [DOI] [PubMed] [Google Scholar]
- 11.Ding D, Wang W, Wu J, et al. Premature mortality risk in people with convulsive epilepsy: long follow-up of a cohort in rural China. Epilepsia 2013;54:512–517 [DOI] [PubMed] [Google Scholar]
- 12.Hitiris N, Mohanraj R, Norrie J, Brodie MJ. Mortality in epilepsy. Epilepsy Behav 2007;10:363–376 [DOI] [PubMed] [Google Scholar]
- 13.Ding D, Wang W, Wu J, et al. Premature mortality in people with epilepsy in rural China: a prospective study. Lancet Neurol 2006;5:823–827 [DOI] [PubMed] [Google Scholar]
- 14.Mu J, Liu L, Zhang Q, et al. Causes of death among people with convulsive epilepsy in rural West China: a prospective study. Neurology 2011;77:132–137 [DOI] [PubMed] [Google Scholar]
- 15.Tekle-Haimanot R, Abebe M, Gebre-Mariam A. Community-based study of neurological disorders in rural Central Ethiopia. Neuroepidemiology 1990;9:263–277 [DOI] [PubMed] [Google Scholar]
- 16.Tekle-Haimanot R, Forsgren L, Ekstedt J. Incidence of epilepsy in rural central Ethiopia. Epilepsia 1997;38:541–546 [DOI] [PubMed] [Google Scholar]
- 17.Edwards T, Scott AG, Munyoki G, et al. Active convulsive epilepsy in a rural district of Kenya: a study of prevalence and possible risk factors. Lancet Neurol 2008;7:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ngugi AK, Bottomley C, Chengo E, et al. The validation of a three-stage screening methodology for detecting active convulsive epilepsy in population-based studies in health and demographic surveillance systems. Emerg Themes Epidemiol 2012;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngugi AK, Bottomley C, Kleinschmidt I, et al. Prevalence of active convulsive epilepsy in sub-Saharan Africa and associated risk factors: cross-sectional and case-control studies. Lancet Neurol 2013;12:253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott JA, Bauni E, Moisi JC, et al. Profile: The Kilifi Health and Demographic Surveillance System (KHDSS). Int J Epidemiol 2012;41:650–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia 1993;34:592–596 [DOI] [PubMed] [Google Scholar]
- 22.Meinardi H, Scott RA, Reis R, Sander JW. The treatment gap in epilepsy: the current situation and ways forward. Epilepsia 2001;42:136–149 [DOI] [PubMed] [Google Scholar]
- 23.Thurman DJ, Beghi E, Begley CE, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 2011;52(suppl 7):2–26 [DOI] [PubMed] [Google Scholar]
- 24.Ministry of Health, Kenya Central nervous system. In: Kimathi NA, Macheni JN, Muriithi A, editors. Clinical Guidelines, 2nd ed Nairobi: Ministry of Health, Govenrment of Kenya; 2002:55–60 [Google Scholar]
- 25.Bauni E, Ndila C, Mochamah G, et al. Validating physician-certified verbal autopsy and probabilistic modeling (InterVA) approaches to verbal autopsy interpretation using hospital causes of adult deaths. Popul Health Metr 2011;9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey AS, Nolan T, Carlin JB. Community-based study of mortality in children with epilepsy. Epilepsia 1993;34:597–603 [DOI] [PubMed] [Google Scholar]
- 27.Hauser E, Freilinger M, Seidl R, Groh C. Prognosis of childhood epilepsy in newly referred patients. J Child Neurol 1996;11:201–204 [DOI] [PubMed] [Google Scholar]
- 28.Sillanpaa M, Jalava M, Kaleva O, Shinnar S. Long-term prognosis of seizures with onset in childhood. N Engl J Med 1998;338:1715–1722 [DOI] [PubMed] [Google Scholar]
- 29.Zielinski JJ. Epilepsy and mortality rate and cause of death. Epilepsia 1974;15:191–201 [DOI] [PubMed] [Google Scholar]
- 30.Berkley JA, Ross A, Mwangi I, et al. Prognostic indicators of early and late death in children admitted to district hospital in Kenya: cohort study. BMJ 2003;326:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moisi JC, Gatakaa H, Berkley JA, et al. Excess child mortality after discharge from hospital in Kilifi, Kenya: a retrospective cohort analysis. Bull World Health Organ 2011;89:725–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mbuba CK, Ngugi AK, Fegan G, et al. Risk factors associated with the epilepsy treatment gap in Kilifi, Kenya: a cross-sectional study. Lancet Neurol 2012;11:688–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology 2005;64:1131–1133 [DOI] [PubMed] [Google Scholar]
- 34.Autry AR, Trevathan E, Van Naarden Braun K, Yeargin-Allsopp M. Increased risk of death among children with Lennox-Gastaut syndrome and infantile spasms. J Child Neurol 2010;25:441–447 [DOI] [PubMed] [Google Scholar]
- 35.Aspray TJ. The use of verbal autopsy in attributing cause of death from epilepsy. Epilepsia 2005;46(suppl 1):15–17 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


