Abstract
Objective:
To assess pregabalin monotherapy for partial-onset seizures using a historical-controlled conversion-to-monotherapy design.
Methods:
Adults with inadequately controlled partial-onset seizures while receiving 1 or 2 antiepileptic drugs during an 8-week prospective baseline were randomized to double-blind monotherapy with pregabalin 600 or 150 mg/d (4:1) for 20 weeks (8-week conversion and 12-week monotherapy period). The primary endpoint was the seizure-related exit rate for pregabalin 600 mg/d, based on discontinuations due to predefined criteria. Efficacy was declared if the upper limit of the 95% confidence interval for the exit rate was below a historical-control threshold of 74%, with stepwise evaluation using a threshold of 68%.
Results:
The trial was stopped early for positive efficacy after an interim analysis in 125 patients. The full study population included 161 patients, with 148 evaluable for efficacy. The mean time since epilepsy diagnosis was 14 years. Overall, 54.3% (600 mg/d) and 46.9% (150 mg/d) of patients completed 20 weeks of double-blind treatment. Seizure-related exit rate in the 600 mg/d group (27.5%; 95% confidence interval, 17.8%–37.2%) was significantly below the 74% and 68% thresholds (p < 0.001 for both). Eight patients on 600 mg/d and 2 on 150 mg/d were seizure-free throughout pregabalin monotherapy. Pregabalin's overall safety profile was consistent with prior trials.
Conclusions:
Pregabalin monotherapy was safe and efficacious for patients with inadequately controlled partial-onset seizures.
Classification of evidence:
This study provides Class III evidence that patients with inadequately controlled partial-onset seizures switched to pregabalin monotherapy have fewer seizure-related exit events compared with historical controls switched to pseudo-placebo monotherapy.
Pregabalin (Lyrica; Pfizer Inc., New York, NY) is an antiepileptic drug (AED) approved in many countries for adjunctive treatment of partial-onset seizures in adults. The efficacy and safety of pregabalin for this use was established in prior trials (at doses from 150 to 600 mg/d).1–3 Pregabalin has also shown similar tolerability but inferior efficacy to lamotrigine as monotherapy for newly diagnosed partial seizures.4 The current trial investigated pregabalin as monotherapy for partial-onset seizures using a historical-control group comparison within the conversion-to-monotherapy paradigm.
In the United States, the Food and Drug Administration (FDA) has required separate trials for the approval of AEDs for monotherapy (usually after they have demonstrated efficacy as adjunctive treatment), requiring demonstration of superiority to a comparator. Previous studies have been performed in patients inadequately controlled on prior AEDs utilizing a conversion-to-monotherapy design; however, this approach is associated with several logistical and ethical challenges.5–7 Among these are the pragmatic and ethical issues relating to use of a fully active drug, placebo, or “pseudo-placebo” (usually a suboptimal dose of an active drug) as comparator. To address these issues, utilization of a historical-control group has been proposed.8 This approach uses results from previous conversion-to-monotherapy trials to create a “virtual” placebo arm against which the study drug's effect is measured. Recently the historical-control design was endorsed by an advisory panel of the FDA.8
The current study is the second reported use of the historical-control design in epilepsy monotherapy research; the first was a study of lamotrigine extended-release (XR) tablets (Lamictal XR; GlaxoSmithKline, Research Triangle Park, NC).9
METHODS
Study design.
This was a 20-week, double-blind, randomized (4:1), historical-controlled study conducted at 54 centers (44 United States, 3 Czech Republic, 1 Hong Kong, 6 Ukraine) in adults with partial-onset seizures inadequately controlled on 1 or 2 AEDs. The trial comprised an 8-week baseline (prior AED regimen maintained); 20-week, double-blind treatment phase (8 weeks' conversion-to-monotherapy [2-week pregabalin initiation, 6-week AED taper] and 12 weeks' pregabalin monotherapy); and 1-week open-label initiation (for patients entering a 6-month extension)/taper phase (figure 1).
Figure 1. Monotherapy study design and end-of-study options.
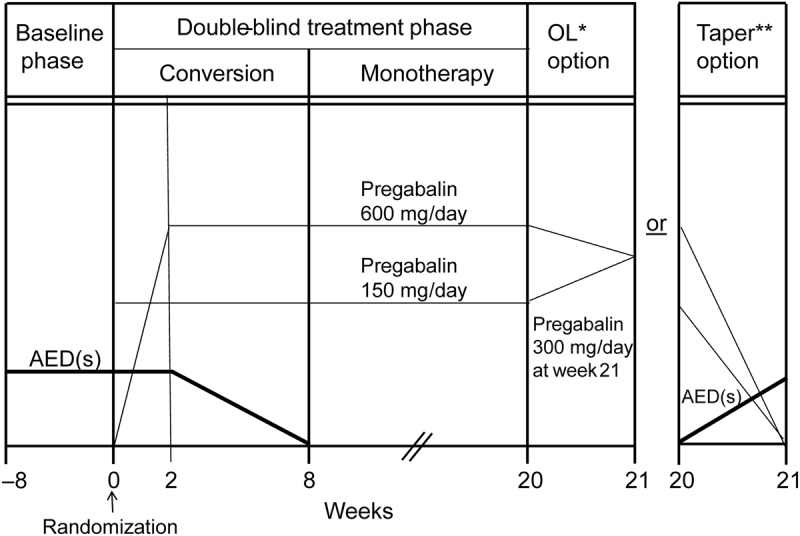
*OL: open-label continuation of pregabalin; **Taper: gradual decrease of pregabalin while initiating new antiepileptic drugs (AEDs).
Standard protocol approvals, registrations, and patient consents.
The protocol adhered to the International Conference on Harmonization Good Clinical Practice Guidelines and received local ethics board approval. Patients provided written informed consent before participation. ClinicalTrials.gov identifier: NCT00524030.
Patients.
Patients were aged 18 years or older with a diagnosis of epilepsy with partial-onset seizures (simple or complex partial, with/without secondary generalization) by the 1981 clinical criteria defined by the International League Against Epilepsy.10 Patients had to be on stable treatment with 1 or 2 AEDs, with ≥4 partial seizures in the 8 weeks before screening, and ≥4 partial seizures during the 8-week baseline with no 4-week seizure-free period. Exclusion criteria included seizures caused by an underlying condition, primary generalized seizures, status epilepticus within the past year, or seizures occurring only in cluster patterns. Patients were also excluded for the following: any clinically relevant medical condition that was anticipated to interfere with the interpretation of the trial results, significant ECG findings, history of alcohol/substance abuse (unless in remission for ≥12 months), creatinine clearance ≤60 mL/min, previous pregabalin use, current treatment with benzodiazepines or barbiturates for indications other than epilepsy, or concomitant medication that could alter the response to study medication or affect seizure frequency. Patients were required to have a CT or MRI scan within the past 2 years demonstrating no progressive structural abnormality.
Study medication, randomization, and blinding.
Patients were randomized to blinded treatment with pregabalin 600 or 150 mg/d (capsules identical in size/color) in a 4:1 ratio. The 150 mg/d dose was not powered for efficacy and was included for blinding. Randomization was according to a computer-generated pseudorandom code using the method of random permuted blocks. Randomization codes were provided by Pfizer's Clinical Statistics department, and study medication was dispensed accordingly through an interactive voice recognition system.
Patients assigned to pregabalin 150 mg/d (75 mg twice a day) received this dose throughout the double-blind phase. Patients assigned to pregabalin 600 mg/d began treatment with 150 mg/d (75 mg twice a day) during week 1 then 300 mg/d (150 mg twice a day) week 2, followed by 600 mg/d (300 mg twice a day) thereafter. Patients entering the open-label extension11 had their dose adjusted to a 300 mg/d starting dose by week 21. Otherwise, pregabalin was tapered over 1 week and discontinued while alternative AEDs were introduced (figure 1).
Efficacy endpoints.
The primary endpoint was the proportion of the pregabalin 600 mg/d group meeting ≥1 of the predefined seizure-related exit criteria: 1) doubling of the 28-day seizure rate during the double-blind phase vs baseline; 2) doubling of the 2-day seizure rate during the double-blind phase vs baseline; 3) secondarily generalized tonic-clonic seizure if none had been experienced within 2 years of study entry; 4) status epilepticus; or 5) clinically significant, unacceptable increase in the frequency or intensity of seizures (as assessed by the investigator).
Secondary efficacy endpoints included the following: exit rate for the pregabalin 150 mg/d group; time to seizure-related exit; proportion completing 20 weeks' double-blind treatment; proportion meeting individual seizure-related exit criteria; mean time on pregabalin monotherapy; and proportion seizure-free during the last 28 days on monotherapy, the entire monotherapy phase, or the double-blind phase.
Assessment methods.
Seizure frequency was based on entries by patients or their guardian/caregiver in paper daily diaries. Changes in seizure frequency were automatically calculated upon entry of seizure data into a spreadsheet, facilitating assessment of seizure frequency-related exit criteria. Safety assessments included monitoring of adverse events (AEs), clinically significant symptoms/signs, laboratory tests, physical and neurologic examination findings and progression/worsening of underlying disease, and review of concomitant medications.
Statistical analyses.
A sample size of 200 patients in the 600 mg/d group was estimated to provide ≥90% power (α = 0.05, 2-sided) to detect a significant difference in seizure-related exit rate vs a historical-control threshold of 74% and based on an assumed all-cause exit rate of 53%.8
The exit rate was calculated as (1 − the Kaplan-Meier product limit estimate for survival function) × 100%. Treatment was considered efficacious if the upper bound of the 2-sided 95% confidence interval (CI) for the 600 mg/d group exit rate was below the 74% historical-control threshold. If the primary endpoint comparison was significant, testing proceeded using a threshold of 68% (included for regulatory purposes). Patients were evaluable for efficacy if they received ≥1 dose of double-blind treatment, and had a baseline and ≥1 efficacy assessment. Patients discontinuing for reasons other than meeting exit criteria were considered censored at last visit provided blinded review by an Independent Data Monitoring Committee (IDMC) considered the observation as randomly censored and not a seizure-related exit. Safety analyses were conducted for patients receiving ≥1 dose of study drug.
Interim analysis.
A planned interim analysis was conducted by the IDMC after 50% of the planned 250 patients completed the study. The IDMC recommended that the study be stopped early for efficacy based on predefined stopping rules. The decision was based on the primary efficacy analysis (seizure-related exit rate in the 600 mg/d group) for the cohort available at the interim analysis (134 patients; 125 evaluable for efficacy); the full study population included an additional 27 patients who completed the trial after the interim analysis cutoff.
Classification of evidence.
The primary research question was to determine the efficacy of pregabalin monotherapy for partial-onset seizures as compared with a historical control. This study provides Class III evidence that pregabalin 600 mg/d is efficacious as monotherapy for patients with partial-onset seizures. The seizure-related exit rate was significantly lower with pregabalin 600 mg/d (31.9%; 95% CI, 20.7%–43.1%) compared with a historical-control threshold of 74% (p < 0.001) at the primary efficacy (interim) analysis (n = 102). In the full study population (n = 120), the seizure-related exit rate was 27.5% (95% CI, 17.8%–37.2%; p < 0.001 vs the 74% threshold).
RESULTS
Patients.
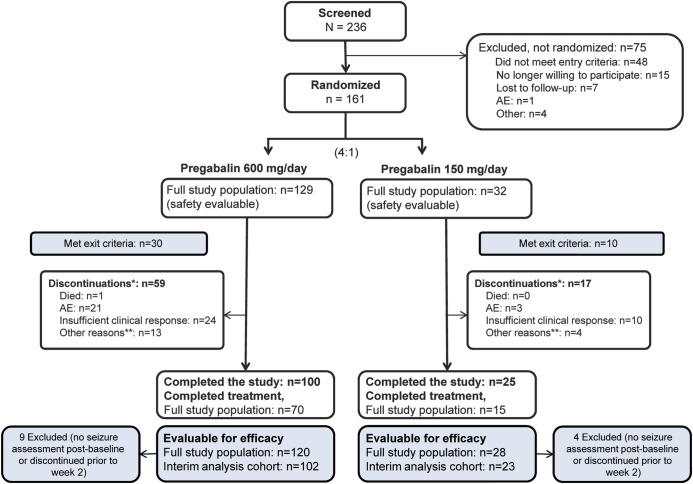
In total, 161 patients were randomized and received ≥1 dose of study medication (figure 2). The first patient was enrolled in September 2007 and the last completed the study in June 2011. Seventy of 129 patients (54.3%) in the 600 mg/d group and 15 of 32 (46.9%) in the 150 mg/d group completed 20 weeks of double-blind treatment.
Figure 2. Patient disposition and study populations.
*Includes patients meeting seizure-related exit criteria. For patients meeting exit criteria, the primary reason for discontinuation was recorded as “insufficient clinical response” unless the patient also experienced an adverse event (AE) requiring discontinuation at the same time, in which case the primary reason for discontinuation was reported as the AE. **Other reasons for discontinuation included loss to follow-up, no longer willing to participate, protocol violation, pregnancy, or other.
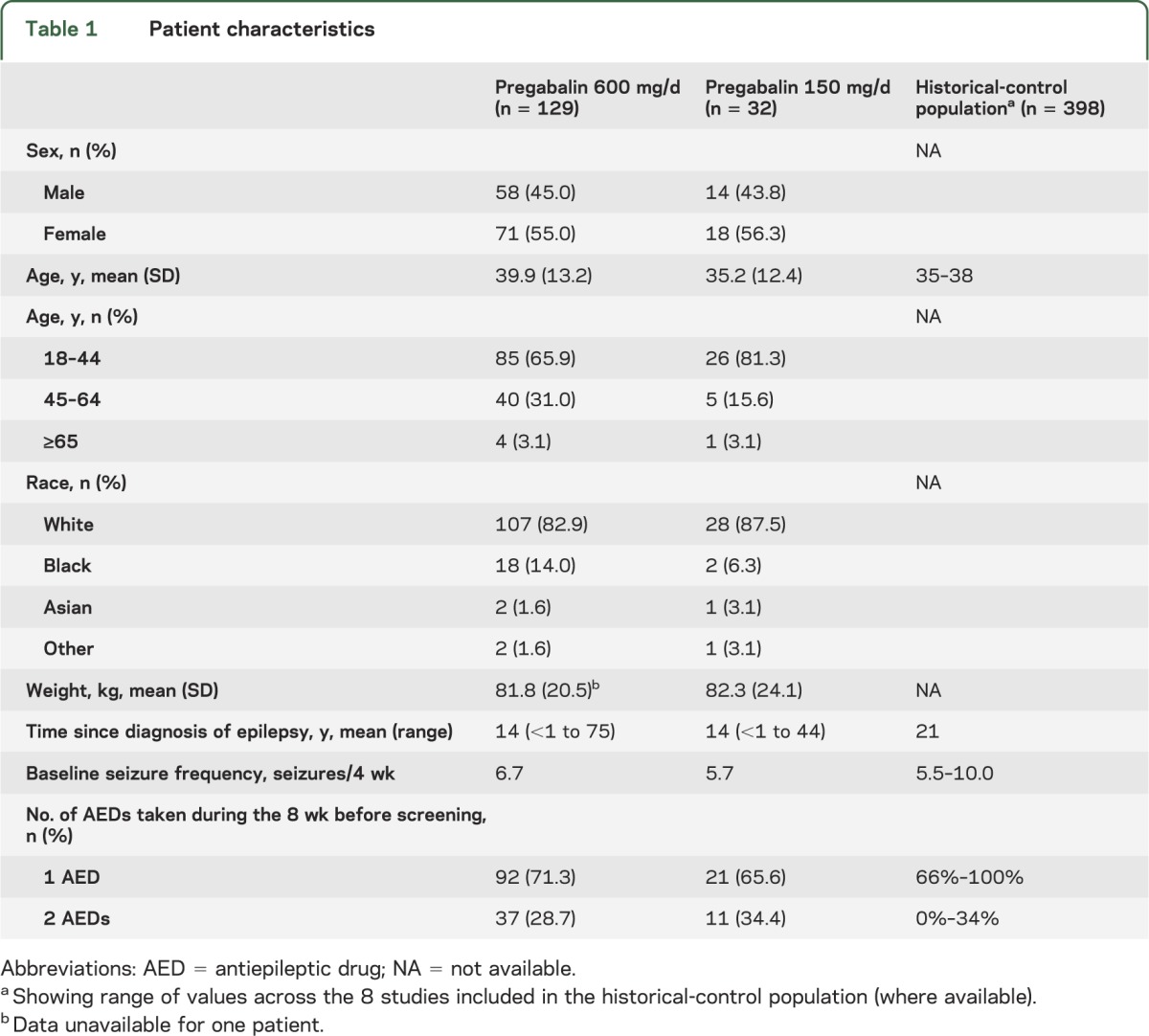
Overall, 89 patients (55.3%) were female and 135 white (83.9%) (table 1). The mean age was 40 years in the 600 mg/d group and 35 years in the 150 mg/d group. Mean time since epilepsy diagnosis was 14 years in both groups. All patients had received ≥1 AED before study enrollment; the most frequently reported were carbamazepine (47/161, 29.2%), levetiracetam (40/161, 24.8%), and lamotrigine (37/161, 23.0%).
Table 1.
Patient characteristics
The median 8-week seizure rate during the 8-week baseline phase was 13.4 in the 600 mg/d group and 11.3 in the 150 mg/d group (seizure frequency 6.7 and 5.7 per 4 weeks, respectively). During baseline, the most frequent seizure types were simple and complex partial seizures, occurring in 62.8% and 64.9% of patients, respectively. A summary of the baseline seizure types and frequency is shown in table e-1 on the Neurology® Web site at www.neurology.org.
The median duration of pregabalin therapy during the study was 144 days in the 600 mg/d group and 110 days in the 150 mg/d group.
Efficacy analyses.
The primary efficacy analysis, based on whether the trial was stopped early for efficacy, was conducted for the interim analysis cohort for the pregabalin 600 mg/d group. All other efficacy and safety analyses were performed for the full study population.
Primary endpoint.
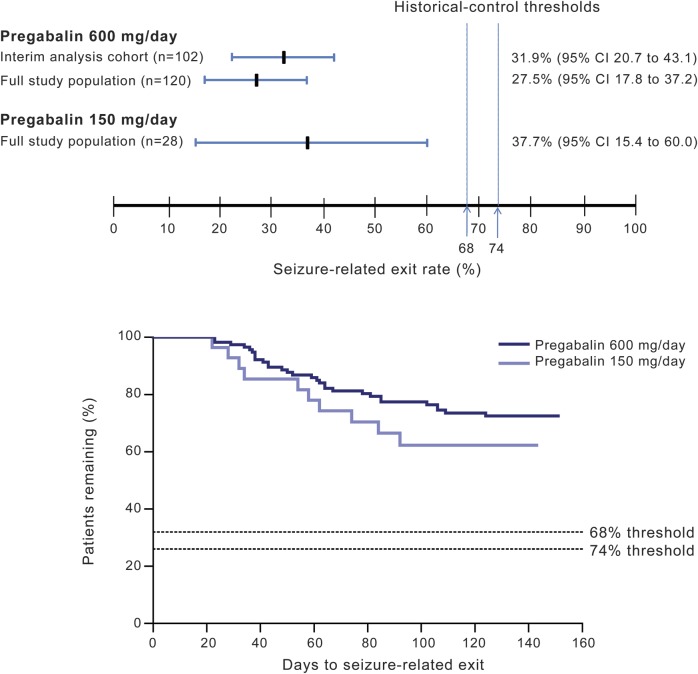
Of the 102 pregabalin 600 mg/d–treated patients included in the interim analysis, 29 (28.4%) met at least one exit criteria. The resulting Kaplan-Meier–estimated seizure-related exit rate in the interim analysis was 31.9% (95% CI, 20.7%–43.1%) (figure 3). As per predefined efficacy criteria, the upper bound of the 2-sided 95% CI was below the historical-control threshold of 74% (p < 0.001) and also below the more stringent threshold of 68% (p < 0.001).
Figure 3. Seizure-related exit rates and comparison with 74% and 68% thresholds, and Kaplan-Meier plot for patients (efficacy evaluable population; n = 148) meeting seizure-related exit criteria during the double-blind treatment phase.
The exit rate was calculated as (1 − Kaplan-Meier product limit estimate for survival function) × 100%. CI = confidence interval.
Of the 120 pregabalin 600 mg/d treated patients in the full study population, 30 (25%) met at least one exit criteria. The resulting Kaplan-Meier–estimated seizure-related exit rate was 27.5% (95% CI, 17.8%–37.2%; p < 0.001 vs the 74% and 68% thresholds). The pregabalin 150 mg/d exit rate was 37.7% (95% CI, 15.4%–60.0%; p ≤ 0.001 vs the 74% and 68% thresholds) (figure 3A, table e-2).
The observed percentages meeting seizure-related exit criteria were 25.0% (30/120) in the 600 mg/d group and 35.7% (10/28) in the 150 mg/d group. The proportion meeting individual exit criteria is shown in table e-2.
Secondary endpoints.
The time to seizure-related exit in the pregabalin 600 and 150 mg/d groups is shown in figure 3B. The mean time on pregabalin monotherapy was 78.0 days (range, 2–128) in the 600 mg/d group and 73.8 days (range, 5–119) in the 150 mg/d group.
In the 600 mg/d group, 12.5% (15/120) remained seizure-free during the last 28 days of the monotherapy phase and 6.7% (8/120) during the entire monotherapy phase. In the 150 mg/d group, 17.9% (5/28) remained seizure-free during the last 28 days of monotherapy and 7.1% (2/28) during the entire monotherapy phase. Two patients (1.7%) in the 600 mg/d, and none in the 150 mg/d group, were seizure-free for the entire double-blind phase.
Efficacy in the 600 mg/d group was also assessed according to the number of prior AEDs at screening (exploratory analysis): seizure-related exit rate was 22.8% (95% CI, 11.6%–34.0%) for patients receiving 1 AED (n = 83) and 39.1% (95% CI, 19.2%–59.0%) for those receiving 2 AEDs (n = 36).
Safety analyses.
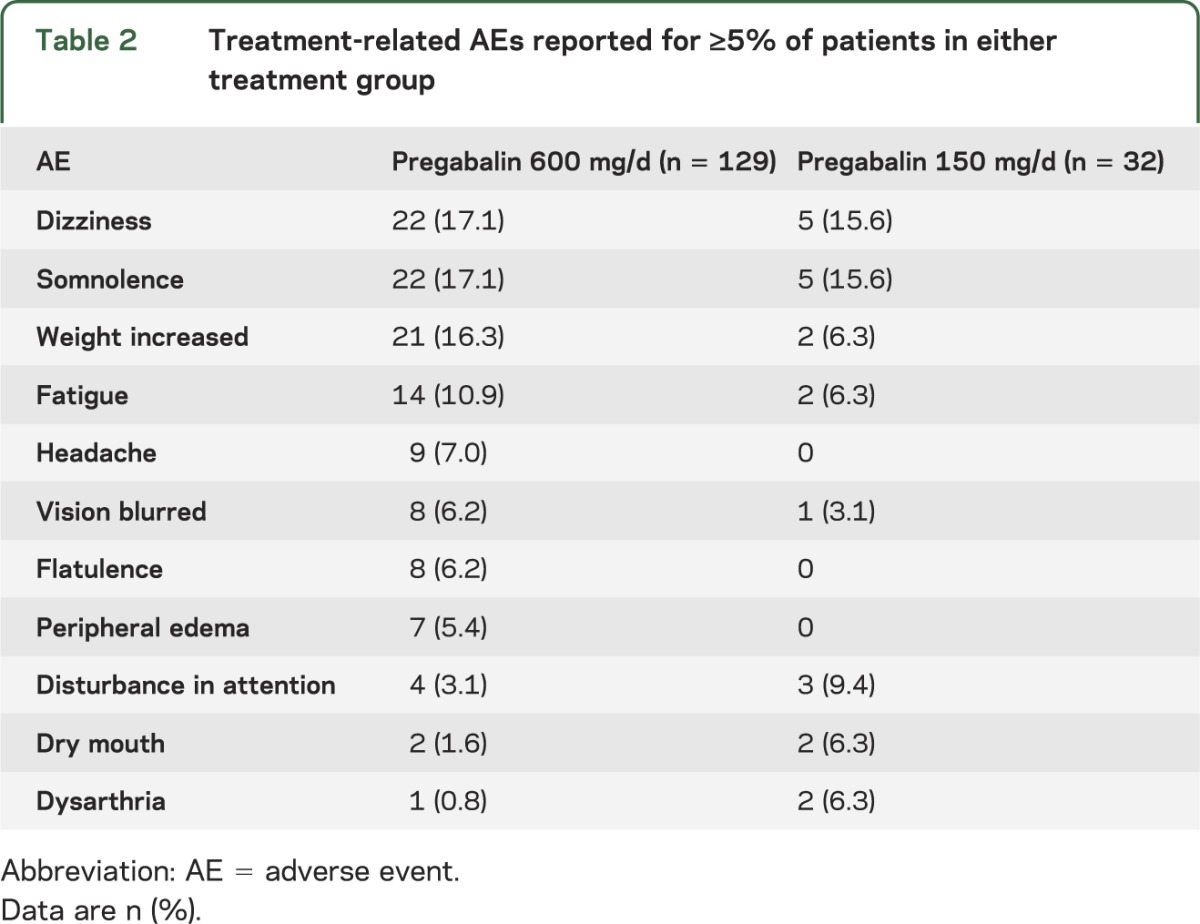
In the 600 mg/d group, 79.1% (102/129) of safety evaluable patients experienced all-causality treatment-emergent AEs and 62.8% (81/129) AEs were considered related to treatment (in the investigator's judgment). In the 150 mg/d group, 71.9% (23/32) experienced all-causality treatment-emergent AEs and 53.1% (17/32) treatment-related AEs. The most frequent treatment-related AEs in both groups were dizziness and somnolence. Increased weight was more common in the 600 mg/d group (table 2). The majority of AEs were considered mild or moderate in severity. Eighteen patients (1 in the 150 mg/d and 17 in the 600 mg/d group) reported a total of 20 serious treatment-emergent AEs, of which approximately half were considered seizure-related. Four patients in the 600 mg/d group had ≥1 treatment-related serious AE. Twenty-five patients withdrew from the study because of AEs: 3 (9.4%) in the 150 mg/d group and 22 (17.1%) in the 600 mg/d group. One patient in the 600 mg/d group died during the study (on day 28, after escalation to a fixed dose but before completion of the conversion phase) due to cardiac arrest (probable sudden unexplained death in epilepsy) that was considered unrelated to study treatment. Changes from baseline in vital signs and laboratory measures were generally small and not considered clinically significant.
Table 2.
Treatment-related AEs reported for ≥5% of patients in either treatment group
DISCUSSION
The utility and clinical applicability of the historical-control design has been questioned because most monotherapy treatment is prescribed to newly diagnosed patients, while the design requires drug-resistant patients. The European Medicines Agency requires noninferiority studies in newly diagnosed patients. Nevertheless, the historical-control design has been used because it is consistent with FDA requirements. There are ongoing discussions with regulatory agencies regarding these differences in requirements. This historical-controlled study supports the efficacy of pregabalin monotherapy for partial-onset seizures. In the full study population, the exit rate for pregabalin 600 mg/d was 27.5% (95% CI, 17.8%–37.2%); because the upper bound of the CI was below the 74% historical-control threshold, efficacy was declared. Efficacy was also declared for pregabalin 600 mg/d compared with the more rigorous threshold of 68%. Pregabalin safety was consistent with its known profile as adjunctive therapy for partial-onset seizures.12,13
For this study, the highest pregabalin dose approved for adjunctive use in partial seizures (600 mg/d) was selected for the primary analysis. Pregabalin 150 mg/d (the lowest approved dose) was also included to meet the requirement of the historical-control methodology for a second arm to allow for blinding and randomization. However, unequal (4:1) randomization resulted in a small 150 mg/d group, and results should be interpreted with caution.
For a historical-controlled study, it is critical that a number of methodologic criteria be consistent with the studies contributing to the historical-control dataset. Overall, we believe this study matches well with the historical-control trials. The study used a randomized, double-blind, parallel-group design, consistent with the 8 trials comprising the historical control. Other key characteristics that were similar between this and the historical-control trials included the choice of primary endpoint (seizure-related exit rate) and definition of exit criteria, requirements for the number of baseline seizures (≥2 to ≥4 per 4 weeks in the historical-control trials) and prior AEDs (≥1 for all historical-control trials), diagnosis of partial epilepsy, and exclusion for primary generalized seizures and status epilepticus.8
Key differences included the fact that the current study allowed conversion from 1 or 2 prior AEDs, without further qualification for the second AED. In contrast, only 5 of the 8 historical-control studies allowed 2 AEDs at baseline, of which 4 required 1 AED to be <50% of therapeutic concentration or minimum therapeutic dose. Notably, the exit rate for pregabalin 600 mg/d when patients were converted from 2 AEDs was higher than for 1 AED (while still meeting criteria for efficacy vs the historical control), although the smaller sample with 2 AEDs should be considered. The historical-control threshold applied was slightly higher for the current study (74%) compared with the white paper upon which the historical-control methodology was based (65%)8 because of a change in assumptions after FDA approval. However, the primary endpoint would still have been significant if the 65% threshold was used. Where the studies were conducted also differed slightly, with the historical-control studies conducted essentially exclusively in the United States while the current study was conducted in 3 additional countries. The enrolled patient population was broadly comparable to the historical-control population (table 1), although the percentage with complex partial seizures during baseline was slightly lower (65% vs 83%–95% in the 4 historical-control trials for which data were available).
We hope that this study, together with the previously reported lamotrigine XR historical-controlled trial,9 might aid the design of future historical-control studies for AED monotherapy. For example, considering the results and sample sizes in these studies could provide insight into the approximate sample size needed for comparison with the historical-control dataset. Enrollment in contemporary epilepsy conversion-to-monotherapy trials is often a significant challenge (the current study enrolled only 161 patients over 3.75 years). This may be attributable to a perception that efficacy is assessed by patients not worsening rather than a reduction in seizures (i.e., improvement) as would be expected for a trial of adjunctive treatment. Thus, it is particularly valuable for these studies to limit the sample size to the smallest possible. The current study demonstrated efficacy in the interim analysis for 102 patients in the 600 mg/d group. Together, these results suggest a required sample size of approximately 100 patients per group. In the lamotrigine XR study,9 there was a tendency toward underreporting of exits by investigators; we aimed to address this in the current study by calculating exit criteria relating to seizure frequency automatically at each visit based on seizure diary data.
Overall, we believe the historical-control group comparison to be a valuable approach to assess efficacy and safety for AED monotherapy. However, there are several limitations of this design that should be acknowledged. In particular, although it is a requirement that the study conduct and entry criteria are as closely matched to the historical-control dataset as possible, there will inevitably be some differences relating to factors such as differing study sites and investigators, differences in standard practice between the time of the study and the historical-control trials, and differences among the original historical-control trials (meaning that it is not possible to align all criteria with all of the studies). It has been suggested that contemporary conversion-to-monotherapy studies are likely to recruit patients with less severe epilepsy than the historical-control studies, although further investigation would be required to confirm whether this is the case. In addition, although the current study was blinded as to the dose of pregabalin received, all patients were aware they would receive active treatment, which may have affected their expectations.
Pregabalin 600 mg/d was shown to be safe and efficacious as monotherapy for patients with partial-onset seizures inadequately controlled on 1 or 2 prior AEDs, using a historical-controlled conversion-to-monotherapy study design. This information will be important for physicians who wish to convert their patients from existing therapy to pregabalin monotherapy.
Supplementary Material
GLOSSARY
- AE
adverse event
- AED
antiepileptic drug
- CI
confidence interval
- FDA
US Food and Drug Administration
- IDMC
Independent Data Monitoring Committee
- XR
extended release
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
J. French, P. Kwan, T. Fakhoury, V. Pitman, L. Knapp, and L. Yurkewicz contributed to the design/conduct of the study. S. DuBrava was responsible for the statistical analysis of the data. All authors contributed to the interpretation of the data, and all authors contributed to the drafting/revision of the manuscript and approved the final version.
STUDY FUNDING
This study was funded by Pfizer Inc. Editorial support with the collation of author comments and proofreading of the manuscript was provided by Elizabeth Harvey, PhD, of Engage Scientific Solutions, and funded by Pfizer Inc.
DISCLOSURE
J. French serves as the president of The Epilepsy Study Consortium, a nonprofit organization. NYU receives a fixed amount from the Epilepsy Study Consortium toward Dr. French's salary. The money is for work performed by Dr. French on behalf of The Epilepsy Study Consortium, for consulting, and clinical trial–related activities. Dr. French receives no personal income for these activities. Within the past year, The Epilepsy Study Consortium received payments from Biotie, Cyberonics, Eisai Medical Research, Entra Pharmaceuticals, GlaxoSmithKline, Icagen, Inc., Johnson & Johnson, Mapp Pharmaceuticals, Marinus, Neurotherapeutics, NeuroPace, NeuroVista Corporation, Ono Pharma USA, Inc., Lundbeck, Pfizer Inc., Sepracor, Sunovion, SK Life Science, Supernus Pharmaceuticals, UCB Inc./Schwarz Pharma, Upsher-Smith, Valeant, and Vertex. P. Kwan's institution has received research support from Pfizer Inc. T. Fakhoury has served as a member of a speakers bureau for Pfizer Inc. and his institution has received research grants from Pfizer Inc. V. Pitman, S. DuBrava, L. Knapp, and L. Yurkewicz are employees of Pfizer Inc. and own stock options in Pfizer Inc. Go to Neurology.org for full disclosures.
REFERENCES
- 1.French JA, Kugler AR, Robbins JL, Knapp LE, Garofalo EA. Dose-response trial of pregabalin adjunctive therapy in patients with partial seizures. Neurology 2003;60:1631–1637 [DOI] [PubMed] [Google Scholar]
- 2.Arroyo S, Anhut H, Kugler AR, et al. Pregabalin add-on treatment: a randomized, double-blind, placebo-controlled, dose-response study in adults with partial seizures. Epilepsia 2004;45:20–27 [DOI] [PubMed] [Google Scholar]
- 3.Beydoun A, Uthman BM, Kugler AR, Greiner MJ, Knapp LE, Garofalo EA. Safety and efficacy of two pregabalin regimens for add-on treatment of partial epilepsy. Neurology 2005;64:475–480 [DOI] [PubMed] [Google Scholar]
- 4.Kwan P, Brodie MJ, Kalviainen R, Yurkewicz L, Weaver J, Knapp LE. Efficacy and safety of pregabalin versus lamotrigine in patients with newly diagnosed partial seizures: a phase 3, double-blind, randomised, parallel-group trial. Lancet Neurol 2011;10:881–890 [DOI] [PubMed] [Google Scholar]
- 5.Perucca E. Designing clinical trials to assess antiepileptic drugs as monotherapy: difficulties and solutions. CNS Drugs 2008;22:917–938 [DOI] [PubMed] [Google Scholar]
- 6.Sachdeo R. Monotherapy clinical trial design. Neurology 2007;69:S23–S27 [DOI] [PubMed] [Google Scholar]
- 7.Gilliam FG. Limitations of monotherapy trials in epilepsy. Neurology 2003;60:S26–S30 [DOI] [PubMed] [Google Scholar]
- 8.French JA, Wang S, Warnock B, Temkin N. Historical control monotherapy design in the treatment of epilepsy. Epilepsia 2010;51:1936–1943 [DOI] [PubMed] [Google Scholar]
- 9.French JA, Temkin NR, Shneker BF, Hammer AE, Caldwell PT, Messenheimer JA. Lamotrigine XR conversion to monotherapy: first study using a historical control group. Neurotherapeutics 2012;9:176–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia 1981;22:489–501 [DOI] [PubMed] [Google Scholar]
- 11.Yurkewicz Y, Kwan P, Fakhoury T, Pitman V, Knapp L. Long-term safety and efficacy of pregabalin monotherapy in patients with partial onset seizures: an open-label, extension study. Presented at the 66th Annual Meeting of the American Epilepsy Society; November 30–December 4, 2012; San Diego; Poster 3.237
- 12.Lyrica (pregabalin) [prescribing information]. New York: Pfizer Inc.; 2011. Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=561. Accessed April 10, 2012 [Google Scholar]
- 13.Lyrica summary of product characteristics. Sandwich, UK: Pfizer Ltd; Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000546/WC500046602.pdf. Accessed April 10, 2012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.