Abstract
Background
Although several aspects of emotion appear intact in schizophrenia, there is emerging evidence that patients show an impaired ability to adaptively regulate their emotions. This ERP study examined whether schizophrenia is associated with impaired neural responses to appraisal frames – i.e., when negative stimuli are presented in a less negative context.
Methods
31 schizophrenia outpatients and 27 healthy controls completed a validated picture viewing task with three conditions: 1) Neutral pictures preceded by neutral descriptions (“Neutral”), 2) Unpleasant pictures preceded by negative descriptions (“Preappraised negative”), 3) Unpleasant pictures preceded by more neutral descriptions (“Preappraised neutral”). Analyses focused on the Late Positive Potential (LPP), an index of facilitated attention to emotional stimuli that is reduced following cognitive emotion regulation strategies, during four time windows from 300 – 2000 ms post picture onset.
Results
Replicating prior studies, controls showed smaller LPP in Preappraised neutral and Neutral vs. Preappraised negative conditions throughout 300 – 2000 ms. In contrast, patients showed (a) larger LPP in Preappraised neutral and Preappraised negative vs. Neutral conditions in the initial period (300 – 600 ms) and (b) an atypical pattern of larger LPP to Preappraised neutral vs. Preappraised negative and Neutral conditions in the 600–1500 ms epochs.
Conclusions
Modulation of neural responses by a cognitive emotion regulation strategy appears impaired in schizophrenia during the first two seconds after exposure to unpleasant stimuli.
Keywords: Schizophrenia, Event-Related Potentials (ERP), Late Positive Potential, Emotion regulation
1. Introduction
People with schizophrenia often demonstrate diminished emotional expression and engagement in pleasurable activities on clinical assessments of negative symptoms (Blanchard et al., 2011). However, laboratory studies using affective science methods have revealed that several components of emotion appear remarkably intact in schizophrenia. On tasks involving exposure to emotionally evocative stimuli (e.g., pictures, films, foods) people with schizophrenia report feeling pleasant emotions as strongly as unaffected people (Cohen and Minor, 2008). Similarly, they report feeling unpleasant emotions as strongly as, or sometimes more strongly than, people without schizophrenia. These intact responses extend beyond self-reported emotions as patients show normal emotion-modulated cardiovascular, electrodermal, and startle eyeblink responses to evocative stimuli (Kring and Moran, 2008).
People with schizophrenia also show largely normal Event Related Potentials (ERPs) during even the initial stages of processing emotional stimuli. A series of studies has focused on emotional modulation of the Late Positive Potential (LPP), an index of motivated attention that is believed to reflect increased allocation of attentional resources to, and sustained attentional processing of, motivationally relevant stimuli (Hajcak et al., 2010). In healthy adults, emotional modulation of the LPP begins about 300 ms following stimulus onset at midline central-parietal sites. The LPP can persist for up to several seconds during picture presentation and demonstrates somewhat different characteristics during early and later time windows. For example, the P300 and the early portion of the LPP appear quite similar in terms their temporal and spatial characteristics, as well as their sensitivity to emotional stimuli. In contrast to the transient P300, the LPP is sustained at central sites and is believed to reflect more elaborated processing related to the significance and meaning of stimuli (Hajcak et al., 2012). People with schizophrenia have shown largely normal patterns of LPP enhancement to emotional versus neutral pictures; this was true under experimental conditions in which subjects were explicitly instructed to attend to the pictures in a passive viewing paradigm and when the emotional pictures where incidental to task demands (Horan et al., 2010, Horan et al., 2012).
Although emotional responses to evocative stimuli appear largely intact in schizophrenia, adaptive functioning in daily life also requires the capacity to regulate one’s emotions to meet goals and manage arousal. Emotion regulation is the process by which people influence which emotion will be experienced, when it will be experienced, and how it will be experienced and expressed (Gross and Thompson, 2007). Gross and colleagues (Gross, 1998, Gross, 2003) distinguish between regulation strategies that are antecedent-focused, before emotion response tendencies have become fully activated to change behavioral and physiological responding, and response-focused, after the response tendencies have already been generated. Cognitive reappraisal, an extensively studied antecedent-focused strategy, is a form of cognitive change that involves construing a potentially emotion-eliciting situation in a way that changes its emotional impact (Hajcak and Nieuwenhuis, 2006, Ochsner and Gross, 2005, Gross, 2003, Lazarus, 1991). For example, during a stressful job interview, one might down-regulate negative emotions by viewing the experience as an opportunity to find out how much one is suited to a particular work environment rather than as a test of one’s worth. Reappraisal may be a particularly effective emotion regulation strategy because it serves to change the fundamental meaning and personal significance of an emotional stimulus prior to an unpleasant emotional response being elicited. Contrasted with less optimal response-focused strategies, such as suppression of emotional expression (e.g., maintaining a poker face), changing the personal significance of a unpleasant stimulus is efficient and effective, with few physiological or cognitive costs (Gross, 2003).
A number of studies have investigated the neural correlates of cognitive reappraisal in healthy adults. For example, fMRI studies examining the impact of reappraisal instructions (e.g., “Reappraise” vs. “Attend”) on emotional responses after unpleasant stimuli are presented have identified a key neural circuit that shows increased activity in prefrontal brain areas involved in cognitive control coupled with decreased activity in the amygdala and other brain areas involved in emotional responses (Ochsner and Gross, 2005). In addition, ERP paradigms have begun to shed light on time course of appraisal manipulations.
In a series of studies, Hajcak and colleagues (Foti and Hajcak, 2008, Macnamara et al., 2009, MacNamara et al., 2011) demonstrated that the magnitude of the LPP to unpleasant picture stimuli is sensitive to manipulations that impact how the stimuli are interpreted. These studies involved preappraisal in which auditory descriptions were presented before unpleasant and neutral pictures that framed the images in either a more negative or more neutral manner (Hajcak et al., in press). For example, a picture of a fierce looking snake might be preceded by either “This is a poisonous snake that is very dangerous” or “This is a snake that is completely harmless - it doesn’t even have teeth”; the latter description is designed to down-regulate the emotional response elicited by the unpleasant image. Preappraisal with verbal descriptions removes the potential confound of task difficulty (i.e., greater effort involved in “Reappraise” versus “Attend” conditions in many fMRI studies), such that each condition simply involves listening to descriptions and viewing pictures. Unpleasant images preceded by descriptions that framed them in a more neutral light elicited reliably smaller LPPs than unpleasant images preceded by more negative descriptions; LPPs to unpleasant images in both conditions were larger than those found for neutral pictures preceded by neutral descriptions. Modulation of the LPP began within 300 ms after stimulus onset and was sustained for at least several hundred milliseconds during picture presentation, indicating that shifts in the meaning due to preappraisal exert an early, sustained influence on emotion processing in the healthy adult brain. These findings have been extended to young children and applied to understanding emotion regulation processes associated with vulnerability to depression and anxiety disorders (Dennis and Hajcak, 2009, DeCicco et al., 2012, Mocaiber et al., 2009). Thus, it is possible to modulate early electrocortical responses to unpleasant pictures by preappraisal and this paradigm is well suited for use in clinical populations.
The primary goal of the current study was to examine whether people with schizophrenia can modulate their electrophysiological responses (i.e., LPP) to unpleasant stimuli through preappraisal-based manipulations of stimulus meaning. We are unaware of prior studies that used ERP paradigms to study emotion regulation in schizophrenia, though the few relevant studies using alternative methods suggest impaired emotion regulation in this population. In the most directly relevant work, a recent fMRI study found that people with schizophrenia (n = 12) showed VLPFC hypoactivation, as well as decreased PFC – amygdala coupling, during downregulation of unpleasant emotion through cognitive reappraisal (Morris et al., in press). At a broader level, a number of studies indicate that people with schizophrenia demonstrate lower scores on the “Managing Emotions” branch of the Mayer-Salovey-Caruso Emotional Intelligence Test (Mayer et al., 2003), which assesses, in part, the ability to adaptively regulate one’s own emotions (e.g.,(Kee et al., 2009, Eack et al., 2008)). There have also been a few studies using self-report emotion regulation questionnaires in schizophrenia, though the findings are more mixed. Some studies found that patients reported lower habitual use of cognitive reappraisal (Livingstone et al., 2009, van der Meer et al., 2009) or greater use of suppression (van der Meer et al., 2009) than healthy controls, though others found no group differences on these measures (Henry et al., 2008, Badcock et al., 2011, Perry et al., 2011). The available evidence led us to predict that schizophrenia patients would show diminished modulation of the LPP to unpleasant pictures preceded by more neutral than more negative descriptions as compared to matched healthy controls.
A secondary goal was to explore relations among ERP indices from the emotion regulation task, scores on a self-report emotion regulation questionnaire assessing habitual use of cognitive reappraisal and suppression, and clinical symptoms within the patient group. The few prior studies that examined relations between indices of emotion regulation and symptoms are inconsistent, reporting relations to negative symptoms (Kee et al., 2009, Henry et al., 2007, Perry et al., 2011), positive symptoms (Badcock et al., 2011), or no relation to symptoms (Henry et al., 2008). We therefore did not have specific directional hypotheses for these analyses.
2. Methods
2.1 Participants
Thirty-one outpatients with schizophrenia and 27 healthy control subjects participated in this study. Schizophrenia patients were recruited from outpatient treatment clinics at the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS) and through presentations in the community. Patients met criteria for schizophrenia based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I (First et al., 1996)). None of the patients were in a depressed or manic episode at the time of testing. Additional exclusion criteria for patients included: substance abuse or dependence in the last six months; IQ < 70 based on chart reviews; a history of loss of consciousness for more than one hour; an identifiable neurological disorder; or insufficient fluency in English. All patients were clinically stable as defined by: no hospitalizations in the past three months, no changes in living situation in the past two months, and no medication changes in the past six weeks. Patients were medicated at clinically determined dosages, with 26 receiving atypical antipsychotics, 4 receiving typical antipsychotics, and 1 receiving both types of antipsychotic medication. The average antipsychotic medication dosage CPZ equivalent units (Andreasen et al., 2010) was 269.59 (SD = 160.07).
Healthy controls were recruited through flyers posted in local newspapers, websites, and posted advertisements. An initial screening interview excluded potential control participants with identifiable neurological disorder or head injury, had schizophrenia or other psychotic disorder in a first-degree relative, or were not sufficiently fluent in English. Potential controls were screened with the SCID-I and excluded for history of schizophrenia or other psychotic disorder, bipolar disorder, recurrent depression, lifetime history of substance dependence, or any substance abuse in the last 6 months. Potential controls were also administered portions of the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II (First et al., 1994)) and excluded if they had avoidant, paranoid, schizoid, or schizotypal personality disorder.
All SCID interviewers were trained through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC) to a minimum kappa of 0.75 for key psychotic and mood items (Ventura et al., 1998). All participants had the capacity to give informed consent and provided written informed consent after all procedures were fully explained in accordance with procedures approved by the Institutional Review Boards at UCLA and the VAGLAHS.
Demographic information for both groups and clinical data for the schizophrenia group are presented in Table 1. The groups did not significantly differ in sex, age, or ethnicity. The patients had lower personal education levels than controls but the groups did not differ in parental education. We intended to match the groups for parental education as a control for family socio-economic status because personal education can be influenced by the illness itself. The schizophrenia group had a typical age of onset, was chronically ill, and showed mild to moderate levels of clinical symptoms at the time of testing.
Table 1.
Demographic and Clinical Data
|
Schizophrenia (N = 31) |
Controls (N = 27) |
Statistic | |
|---|---|---|---|
| Sex (% male) | 75.0% | 77.4% | X2 (1,58) = .09 |
| Age (SD) | 47.8 (9.8) | 45.5 (6.7) | t(56) = 1.01 |
| Ethnicity | |||
| White | 54.8% | 74% | X2 (4,58) = 3.88 |
| African American | 35.5% | 26% | |
| Asian | 3.2% | 0% | |
| Hispanic | 3.2% | 0% | |
| Other | 3.2% | ||
| Education (SD) | 12.5 (1.9) | 14.93 (1.3) | t(56) = 5.52*** |
| Parental education (SD) | 13.0 (3.8) | 14.5 (3.1) | t(56) = 1.72 |
| Age of onset (SD) | 20.6 (5.7) | ||
| Duration of illness (SD) | 26.9 (11.8) | ||
| BPRS | |||
| Positive symptoms (SD) | 1.7 (0.7) | ||
| Negative symptoms (SD) | 2.0 (0.9) | ||
| Total (SD) | 36.7 (9.4) |
Notes:
p < .005
2.2 Symptom ratings
For all patients, psychiatric symptoms during the previous two weeks were rated using the expanded 24-item UCLA version of the Brief Psychiatric Rating Scale (BPRS (Overall and Gorham, 1962, Lukoff et al., 1986)) by a trained rater (Ventura et al., 1993). Ratings from the positive and negative symptom subscales, as well as total scores, were examined (Kopelowicz et al., 2008).
2.3 ERP paradigm
Stimulus Materials
The paradigm was modeled on prior ERP studies of preappraisal (Foti and Hajcak, 2008, Macnamara et al., 2009, Dennis and Hajcak, 2009). A total of 84 pictures were selected from the International Affective Picture System (IAPS; (Lang et al., 1999)); of these, 28 depicted neutral scenes and 56 depicted unpleasant scenes. The two categories differed on normative 1 (unpleasant) to 9 (pleasant) ratings of valence (M = 5.05, SD = 1.21, for neutral pictures; M = 2.82, SD = 1.64, for unpleasant pictures); additionally, the emotional pictures were reliably higher on normative 1 (low) to 9 (high) arousal ratings (M = 5.71, SD = 2.16, for unpleasant pictures; and M = 2.91, SD = 1.93, for neutral pictures). People with schizophrenia have consistently been found to report emotional responses to IAPS stimuli that are comparable to healthy control subjects (see (Cohen and Minor, 2008) for meta-analysis).
For the preappraisal manipulation, a brief auditory description (using established description of (Foti and Hajcak, 2008, Macnamara et al., 2009, Dennis and Hajcak, 2009)) of the upcoming picture was played through speakers, prior to the presentation of the picture. Every participant heard the same neutral description for the 25 neutral images (“Neutral” condition). For each of the 56 unpleasant images, participants heard one of two descriptions: for 28 of the unpleasant pictures, a neutral description preceded the image (“Preappraised neutral” condition), whereas a negative description preceded the other 28 (“Preappraised negative”condition). A complete list of the IAPS image number and associated descriptions are presented in Appendix A.
Procedure
After a brief description of the experiment, electroencephalograph (EEG) electrodes were attached. Participants were told that they would be viewing pictures and that they would hear a brief description played over the speakers prior to each picture that would describe the upcoming picture. Prior to each picture, a white fixation cross was presented on a black screen for 6 sec. During this period, a brief (3–5 sec) description of the upcoming picture was presented. Subjects then passively viewed the pictures, which were in color, displayed for 6 sec, and occupied the entire monitor (48.26 cm). Viewing distance was approximately 1 meter. After each picture, there was a variable 5 – 7 sec delay until the next trial. To ensure that subjects understood the task, 6 practice trials were administered to all subjects and were repeated with additional instruction if necessary. As in prior studies using a similar paradigm in children (Dennis and Hajcak, 2009, DeCicco et al., 2012) self-reported emotional responses were not collected in this initial ERP study of emotion regulation in schizophrenia. After the practice trials, all participants performed the 84 task trials with breaks after every 14 trials. Order of trials and the descriptions were determined randomly for each subject. The task was administered using Eprime software (PST Technologies, Pittsburgh, PA) to control the presentation and timing of all stimuli.
2.4 EEG recording and processing
Participants had their EEG activity continuously recorded from 64 electrodes based on the 10/20 system placed in an electrode cap (Cortech Solutions, Wilmington, North Carolina, USA) and the Active Two BioSemi system (BioSemi, Amsterdam, Netherlands). The signal was preamplified at the electrode with a gain of one; the EEG was digitized at 24-bit resolution with a sampling rate of 512 Hz with a bandpass of 0–100 Hz using a low-pass fifth order sinc filter with a half-power cutoff of 102.4 Hz. Recordings were taken from the 64 electrodes, as well as two electrodes placed on the left and right mastoids. The electrooculogram was recorded from four facial electrodes: two 1 cm above and below the left eye, one 1 cm to the left of the left eye, and one 1 cm to the right of the right eye. Each electrode was measured online with respect to a common mode sense electrode that formed a monopolar channel.
Off-line analysis was performed using Brain Vision Analyzer software (Brain Products, Munich, Germany). All EEG data were re-referenced to the average of all electrodes and band-pass filtered with cutoffs of 0.1 and 30 Hz. The EEG was segmented for each image beginning 200 ms before each stimulus and continuing for 2000 ms post stimulus onset (total of 2200 ms). Each EEG segment was corrected for blinks and eye movements using the method developed by Gratton and colleagues (Gratton et al., 1983). Specific channels were rejected in each trial using a semi-automated procedure, with physiological artifacts identified by the following criteria: a step of more than 50 µV between sample points, a maximum difference of less than 0.5 µV within 100-ms intervals, and an amplitude that exceeded +/− 75 µV. Two patients and two controls were excluded because the quality of the ERP data was poor (less than 50% artifact free trials) and one patient was excluded due to ERP values that differed more than 3 standard deviations from the patient group means. The final sample in this report consisted of 58 individuals (31 patients, 27 controls).
Based on the topographical maps from this sample, we selected a set of six electrodes (C1, C2, Cz, CPz, CP1, CP2) where the LPP was maximal for analyses. Based on prior studies (Foti and Hajcak, 2008, Dennis and Hajcak, 2009) and the morphology of the waveforms from the current study, we evaluated the LPP across four time epochs: 1) 300–600 ms; 2) 600–1000 ms; 3) 1000–1500 ms; 4) 1500–2000 ms. ERP’s were constructed by separately averaging segments of the three experimental conditions (Neutral, Preappraised neutral, Preappraised negative). In each case, the average activity in the 200-ms window prior to picture onset served as the baseline. The LPP was quantified as the mean activity during each epoch across the six electrode sites for each participant.
2.5. Emotion regulation questionnaire
All participants completed the Emotion Regulation Questionnaire (ERQ; (Gross and John, 2003)). On this 10-item questionnaire, six items focus on cognitive reappraisal (e.g., “When I want to feel more positive emotion, I change the way I'm thinking about the situation”) and four focus on suppression (e.g., “I keep my emotions to myself”). Subjects were asked to indicate to what extent the statements apply to them from 1 (strongly disagree) to 7 (strongly agree). Higher scores reflect the more frequent use of a particular strategy. The ERQ has been shown to be a reliable and valid measure of emotion regulation strategies in healthy subjects (Gross and John, 2003) and people with schizophrenia (e.g., (van der Meer et al., 2009, Henry et al., 2008, Perry et al., 2011)).
2.6 Data analyses
For demographic data, group differences for continuous variables were evaluated with t-tests and for categorical variables with chi-square tests. Preliminary analyses of the distributional properties of the demographic, clinical, ERP, and self-report measures indicated that parametric statistical tests were appropriate. Primary analyses were conducted in three stages. First, for the ERP data, separate 3 (condition: Neutral, Preappraised neutral, Preappraised negative) × 2 (group: patient, control) repeated measures analyses of variance (ANOVAs) were conducted for each of the four time epochs. Greenhouse–Geisser epsilon corrections were used for repeated-measures analyses with more than one degree of freedom. In these cases, we report the uncorrected degrees of freedom and the corrected p values. Second, group differences on the self-report emotion regulation scales were evaluated with t-tests. Third, within the schizophrenia group, Pearson correlation coefficients examined associations among ERP, symptom, and self-report emotion regulation variables. For the ERPs in these correlational analyses, LPP difference wave scores during each epoch for the Preappraised negative minus Preappraised neutral conditions were used as the electrophysiological index of emotion regulation.
3. Results
3.1 LPP results
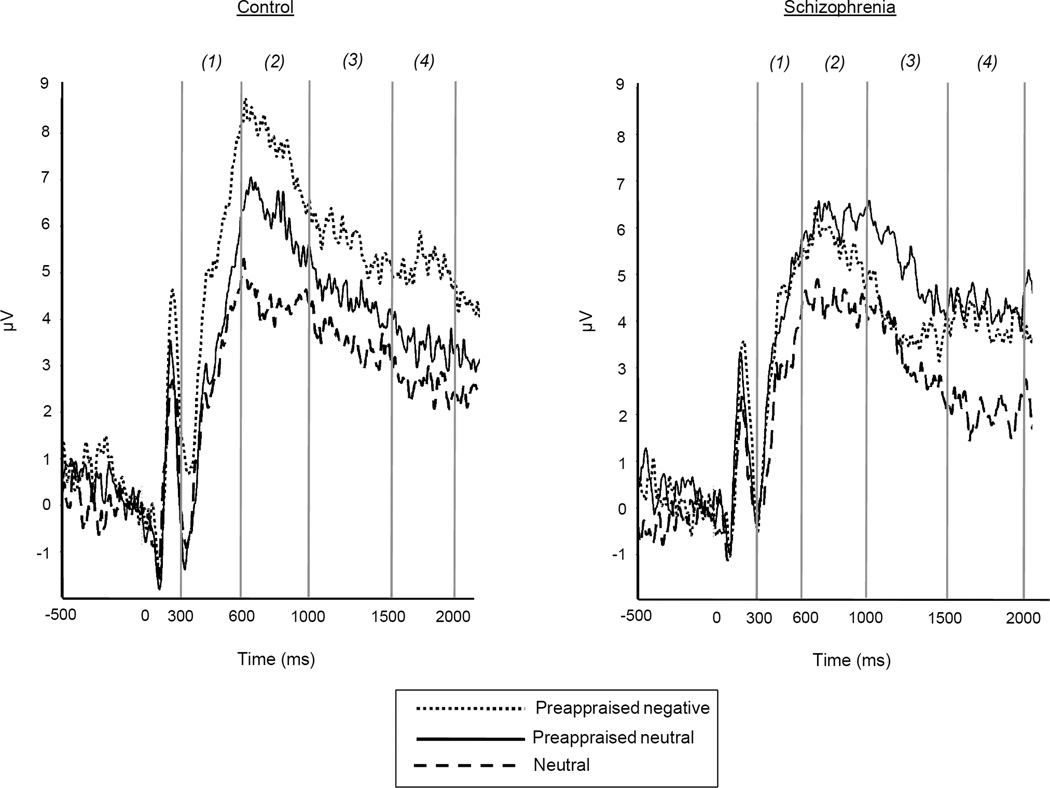
Grand average ERPs for the control and schizophrenia groups are presented in Figure 11. Table 2 displays results of the RM-ANOVA’s along with corresponding follow-up within-group comparisons during each LPP epoch.
Figure 1.
LPP grand averages at pooled electrodes C1, Cz, C2, CP1, CPz, and CP2 in Preappraised negative, Preappraised neutral, and Neutral conditions for control (left) and schizophrenia (right) groups. Stimulus onset occurred at 0 ms. Positive is plotted upward. The four epoch numbers appear in parentheses.
Table 2.
Group Comparisons On the Late Positive Potential (LPP)
| ANOVA results | Group means | Within-group t-tests | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group F(1,56) |
Condition F(2,56) |
Group × Condition F(2,56) |
Neutral | Preappraised neutral |
Preappraised negative |
Neutral vs. Preappraised neutral |
Neutral vs. Preappraised negative |
Preappraised neutral vs. Preappraised negative |
||
| Epoch 1: 300–600 ms | .06 | 8.51*** | 4.29* | |||||||
| Control | 2.67 (3.68) | 3.07 (4.87) | 5.02 (4.11) | −0.59 | −4.00*** | 2.52* | ||||
| Scz | 2.38 (4.47) | 4.11 (3.67) | 3.55 (3.88) | −2.90* | −2.30* | −1.14 | ||||
| Epoch 2: 601–1000 ms | 1.26 | 13.32*** | 5.82** | |||||||
| Control | 4.42 (3.50) | 6.23 (4.56) | 7.80 (3.74) | −2.81** | −5.43*** | 2.83** | ||||
| Scz | 4.21 (4.22) | 6.23 (3.89) | 5.03 (3.54) | −2.64* | −1.33 | −2.12* | ||||
| Epoch 3: 1001–1500 ms | .86 | 2.73† | 3.58* | |||||||
| Control | 3.62 (3.88) | 4.60 (3.75) | 5.74 (3.33) | −1.19 | −2.82*** | 1.97† | ||||
| Scz | 3.42 (4.39) | 5.12 (3.35) | 3.25 (4.50) | −1.7† | 0.18 | −2.64* | ||||
| Epoch 4: 1501–2000 ms | .21 | 3.40* | 1.51 | |||||||
| Control | 2.65 (3.77) | 3.52 (3.80) | 5.06 (3.19) | −1.03 | −2.79** | 1.94† | ||||
| Scz | 2.45 (5.06) | 4.35 (3.11) | 3.48 (4.81) | −1.66 | −0.83 | −1.06 | ||||
Notes:
p < .10;
p < .05;
p<.01;
p < .005;
p<.001.
Scz = schizophrenia. Standard deviations appear in parentheses.
3.1.1 LPP during epoch 1 (300–600 ms)
There were significant condition and interaction effects during the earliest LPP epoch. For controls, preappraisal effects were evident – the LPP was significantly larger during the Preappraised negative condition compared to both the Preappraised neutral and Neutral conditions, which did not significantly differ from each other. In contrast, for patients the LPP was significantly larger during both the Preappraised negative and Preappraised neutral conditions compared to the Neutral condition, and the two Preappraise conditions did not differ from each other. Thus, the patients showed larger LPPs to unpleasant than neutral pictures, but no dampening of the LPP for the Preappraised neutral condition during this initial epoch.
3.1.2 LPP during epoch 2 (600–1000 ms)
There were again significant condition and interaction effects in this epoch. Controls continued to show a significant preappraisal effect evidenced by significant differences among the three conditions. The LPP amplitude showed a linear decrease across conditions, with the LPP largest during the Preappraised negative, intermediate during the Preappraised neutral, and smallest during the Neutral condition. Patients showed a strikingly different pattern. Their LPP was significantly larger during the Preappraised neutral condition than during both the Preappraised negative and Neutral conditions, which did not differ from each other. The patients, therefore, demonstrated an atypical pattern of relatively enhanced LPP for the Preappraised neutral condition.
3.1.3 LPP during epoch 3 (1000–1500 ms)
During the third epoch, a trend-level condition effect was accompanied by a significant interaction. The controls continued to show separation among the conditions in line with the expected preappraisal influence. The LPP was larger in the Preappraised negative versus Neutral condition while there was no difference between the Preappraised neutral and Neutral conditions; there was also a trend for larger LPP during the Preappraised negative versus Preappraised neutral conditions. For patients, the pattern was again different. Their LPP during the Preappraised neutral condition was significantly larger than the Preappraised negative condition and different from the Neutral condition at a trend level. There was no difference between the Preappraised negative and Neutral conditions, again indicating an enhanced LPP during the Preappraised neutral condition.
3.1.4 LPP during epoch 4 (1500–2000 ms)
During the final epoch, there was a significant condition effect, but the interaction effect was not significant. Across the two groups, the LPP was larger for the Preappraised negative versus Neutral conditions, t (56) = −2.17, p < .05, and there was a trend for larger LPP in the Preappraised negative versus Preappraised neutral conditions, t (56) = −1.96, p = .09. For consistency, within group t-tests are also presented in Table 2. The control group continued to show a pattern similar to the previous epoch (the LPP was larger in the Preappraised negative versus Neutral condition and no difference between the Preappraised neutral and Neutral conditions; also a trend for larger LPP during the Preappraised negative versus Preappraised neutral conditions). There were no significant differences across condition within the patient group.
3.2 Group comparisons on self-reported emotion regulation strategies
The groups differed on both types of self-reported habitual emotion regulation strategies. Cognitive reappraisal scores were significantly higher in controls (M = 5.3; SD = 1.0) than patients (M = 4.5; SD = 1.3), t(56) = −2.45, p <. 05. In contrast, suppression scores were significantly lower in controls (M = 3.2; SD = .9) than patients (M = 3.9; SD = 1.6), t(56) = 2.27, p < .05.
3.3 Exploratory correlations within the schizophrenia group
First, we examined whether the four LPP difference wave scores (Preappraised negative minus Preappraised neutral) significantly correlated with self-reported habitual emotion regulation strategies (reappraisal, suppression) and clinical symptoms (positive, negative, total). Of the forty correlations, only one was significant and direction of the correlation was counter-intuitive. Higher difference scores (i.e., greater preappraisal effect) during the 300–600 ms epoch correlated with higher positive symptoms, r (31) = .37, p < .05. There were also no significant correlations with CPZ equivalent units. (There were no significant correlations between ERPs and self-reported emotion regulation strategies in the control group). Second, we examined correlations between the self-reported emotion regulation and clinical symptom variables. Higher levels of suppression significantly correlated with higher positive, r (31) = .36, p < .05, negative, r (31) = .45, p < .05, and total r (31) = .41, p < .05, symptom levels. Reappraisal levels did not significantly correlate with symptoms.
4. Discussion
This ERP study evaluated whether people with schizophrenia alter their neurophysiological responses to unpleasant stimuli following appraisal-based manipulations of stimulus meaning. Consistent with prior studies (Dennis and Hajcak, 2009, Macnamara et al., 2009, Foti and Hajcak, 2008, DeCicco et al., 2012), healthy controls showed a pattern in which the LPP was significantly when unpleasant pictures were preappraised in less negative terms. Specifically, the LPP in the Preappraised neutral condition did not differ from the Neutral condition during the earliest epoch (300–600 ms) and fell between the Neutral and Preappraised negative conditions during the middle two epochs (601 – 1500 ms). In contrast, patients failed to down regulate their LPP responses, instead showing (a) larger LPP in response to both preappraise conditions vs. Neutral conditions in the initial epoch and (b) the opposite pattern from controls in the middle periods with larger LPP to Preappraised neutral vs. Preappraised negative and Neutral conditions. The patients also reported lower habitual use of cognitive reappraisal and higher expressive suppression than controls, though there were no significant correlations between these self-reports and LPP variables. This study provides the first electrophysiological evidence of impaired sensitivity to preappraisal manipulations of emotional stimulus meaning in schizophrenia.
The patients’absence of LPP preappraisal effects converges with a handful of prior studies of cognitive reappraisal in schizophrenia using self-report, behavioral, and fMRI paradigms (e.g.,(Kee et al., 2009, Eack et al., 2008, Livingstone et al., 2009, van der Meer et al., 2009)). A likely explanation for this impairment is a disturbance in top down cognitive control of emotional responses. Disturbances in cognitive control and associated prefrontal cortical circuitry are well documented in schizophrenia (Lesh et al., 2011, Barch and Braver, 2005) and cognitive control plays a central role in cognitive reappraisal (Ochsner and Gross, 2005). This explanation converges with a recent fMRI study in schizophrenia in which subjects were instructed to voluntarily decrease, maintain, or increase their emotional responses to unpleasant pictures (Morris et al., in press). When attempting to decrease their responses, patients displayed hypoactivation of prefrontal regions and significantly less cortico-limbic coupling than healthy controls, consistent with impaired cognitive control. A key aspect of the fMRI study that affects interpretation was that subjects had to generate their own reinterpretations in the decrease condition. This leaves open the possibility that the patients’ impairment merely reflected increased cognitive demands associated with generating alternative interpretations after unpleasant stimuli are presented, rather than an inability to benefit from a shift in meaning of the stimuli. The current ERP paradigm eliminated these differences in cognitive demands across conditions by presenting subjects with descriptions before each type of trial. In this way, the shifts in meaning occurred in a more implicit manner (see (Foti and Hajcak, 2008, Macnamara et al., 2009) for further discussion). Even under these task conditions, schizophrenia patients showed impaired modulation of the neurophysiological responses.
We previously found that patients and controls showed comparable increases in LPP amplitudes for unpleasant versus neutral pictures across tasks in a passive viewing paradigm (Horan et al., 2010) and when emotional pictures where incidental to task demands (Horan et al., 2012). In contrast, patients in the current study showed a smaller LPP difference between the Preappraised negative and Neutral conditions than controls; this dampening was true during even the first epoch (t [56] = 2.80, p < .005). Because the recruitment process and sample characteristics were similar across studies, the most likely explanation for this discrepancy is differences in the experimental tasks. An important difference is that the current study provided descriptive sentences before each trial whereas the previous studies did not provide any such information. The sentence frames essentially provide a context in which the images can be perceived. Thus, the patients’ atypical LPP pattern in the current preappraisal manipulation may be associated with impaired context processing, an aspect of cognitive control that has also received considerable investigation in schizophrenia (MacDonald et al., 2005, Hemsley, 2005, Park et al., 2003).
Although preappraisal modulated the LPP in the control group, it did not affect the patients in a similar way. In the initial epoch, patients showed a larger LPP to unpleasant pictures in both the Preappraised negative and Preappraised neutral conditions compared to the Neutral condition. Although this indicates some early sensitivity to the emotional significance of unpleasant pictures, no preappraisal modulation was evident. During the two middle epochs, the sentence frames appeared to have a paradoxical effect in the patients – they showed larger LPP amplitude in the Preappraised neutral condition versus Preappraised negative and Neutral conditions, suggesting that the Preappraised neutral condition actually resulted in enhanced, sustained neural processing. Perhaps the relative incongruity between the context provided by the more neutral frames and the evocative unpleasant pictures was more difficult or effortful for patients to process than the other conditions in which the sentence and picture pairings were congruous. One might consider whether this effect is impacted by N400, a centro-posteriorly distributed negative-going waveform found to be larger (more negative) to words that are semantically incongruous (vs. congruous) with preceding words or sentences (Van Petten and Luka, 2006). If N400 accounted for the patients’ unusual pattern one would expect a more negative LPP in the Preappraised neutral than the Preappraised negative condition in this group. However, the patients showed the opposite pattern. Furthermore, prior research in healthy subjects suggests that LPP preappraisal modulation in this paradigm is not driven by N400 (MacNamara et al.,2009). Thus, patients failed to show an adaptive benefit from the meaning shift typically associated with the contextual frames. Context is a complex, multi-faceted construct (Hemsley, 2005, Park et al., 2003, MacDonald et al., 2005) and four behavioral studies that examined socio-emotional context processing in schizophrenia have found both impaired (Huang et al., 2009, Green et al., 2007) and intact (Chung and Barch, 2011, Lee et al., in press) context effects. Further investigations will help to clarify the factors that influence how contextual information impacts emotional responses in schizophrenia.
This study also examined self-reported habitual use of emotion regulation strategies. In addition to an atypical LPP pattern, the schizophrenia group reported less frequent use of cognitive reappraisal and more frequent expressive suppression than healthy controls. This pattern is consistent with some (Livingstone et al., 2009, van der Meer et al., 2009) though not all (Henry et al., 2008, Badcock et al., 2011, Perry et al., 2011) prior studies. Although there were no significant correlations between self-reported emotion regulation strategies and LPPs, higher levels of suppression were associated with higher levels of positive, negative, and overall symptoms. Again, some prior studies found suppression related to higher negative or positive symptoms (Kee et al., 2009, Henry et al., 2007, Badcock et al., 2011), as well as worse social functioning (Perry et al., 2011), though others did not (Henry et al., 2008). It is unclear why results have been inconsistent across studies, though differences in sample characteristics (e.g., some included mixed samples of outpatients and inpatients, patients were selected for high levels of hallucinations in one study) and symptom assessment measures may be relevant. The current findings are consistent with the notion that expressive suppression is associated with greater levels of psychopathology (Werner and Gross, 2010), even among people with schizophrenia.
Some limitations of this study should be considered. First, this study focused on an ERP index of emotional regulation and did not include self-reported levels of valence or arousal after each trial. Future studies can examine the coherence between ERP’s and self-reported emotions, as well as other aspects of emotional responding such as facial EMG (Wu et al., In press). Second, this study only examined preappraisal of unpleasant images. We focused on this emotion regulation process to eliminate differences in difficulty across task conditions and focus on contextual modulation of the LPP. It remains an open question whether similar LPP results would be seen for other types of regulation strategies (e.g., cognitive reappraisal, expressive suppression) or for pleasant stimuli. Third, patients were taking various antipsychotic medications at clinically determined dosages and the impact of these medications on the LPP are unknown. Although there were no significant correlations with CPZ equivalents and atypical antipsychotics have been, if anything, to improve some aspects of emotional processing (Juckel et al., 2006, Schlagenhauf et al., 2008) further research on antipsychotic medication effects and in unmedicated samples is needed. Fourth, the patients were clinically stable, predominantly male, and chronically ill, which may limit the generalizability of these findings. Research in other types of samples, such as patients in the early phases of schizophrenia, can help address these limitations.
Although recent research has highlighted several areas of intact emotional responding in schizophrenia, the current findings contribute to emerging evidence of disturbances in the regulation of emotion in this disorder. Impairments in emotion regulation may show linkages to clinical symptoms and could also contribute to social cognitive impairments associated with poor functional outcome (Green and Horan, 2010). For example, emotion regulation has been described as a key component of empathy (Decety and Lamm, 2006), a core social cognitive processes that appears to be disturbed in schizophrenia (e.g., (Smith et al., 2012)). Difficulties in emotion regulation have been associated with various forms of psychopathology and empirically supported interventions have been developed to treat them (Aldao et al., 2010). The current results support recent suggestions that emotion regulation interventions may also be useful in treating people with schizophrenia (Bassam and LeCompte, 2012).
Acknowledgements
Support for this study came from a VA Career Development Award (PI: William P. Horan, Ph.D.) and NIMH Grant MH065707, MH43292 (PI: Michael F. Green, PhD). The authors wish to thank Junghee Lee, Amanda Bender, Michelle Dolinsky, Crystal Gibson, Cory Tripp, and Katherine Weiner for assistance in data collection.
Appendix 1
Descriptions for IAPS Pictures
| IAPS picture # |
Picture valence |
Negative interpretations | Neutralizing interpretations |
|---|---|---|---|
| 2716 | Negative | This man is addicted to crack cocaine. | This man is an actor in a movie about addiction. |
| 9800 | Negative | This is a photo of a German Nazi. | This is an actor in a movie about neo-Nazis. |
| 6312 | Negative | This woman is being abducted by a rapist | This is an actress in a self-defense training video |
| 6831 | Negative | This is a police officer investigating the scene of a murder. | This is the set of a 1960s crime show. |
| 2691 | Negative | This is a protester during a riot where 50 people were killed. | This is a scene from a movie about a riot in the Middle East. |
| 9480 | Negative | People have come to see their families' bones; they make them very sad. | These are bones of ancient people; scientists are studying them to learn about the past. |
| 1120 | Negative | This is a snake that is about to attack and bite another animal. | This snake has a very large mouth; he is begging for a snack. |
| 9425 | Negative | This man has just been taken hostage by terrorists. | This is a scene from a movie called “The Terrorists.” |
| 9921 | Negative | The firefighters do not save this woman in time. | The firefighters get this woman to safety just in time. |
| 9600 | Negative | This ship sinks and no one survives. | This is a scene from a movie much like “Titanic.” |
| 2683 | Negative | This is a bloody clash between police and protestors. | These are actors in a movie about tension in the Middle East. |
| 9470 | Negative | This building collapsed on the people who were sleeping in it; they were badly hurt | This old building was knocked down; everyone was happy to see it go |
| 1201 | Negative | A poisonous tarantula is about to bite this man | This is a harmless pet tarantula sitting on his owner's shoulder |
| 1930 | Negative | This is a shark that attacked and killed a diver | This is a mechanical shark from the movie "Jaws" |
| 9901 | Negative | The victims in this accident could not be saved in time. | No one was in this car when it was totaled at a construction site. |
| 9911 | Negative | The driver in this accident was killed before help could arrive. | This is contrived scene from an educational film about drunk driving. |
| 3220 | Negative | This man is dying in a hospital. | This man is recovering from illness in a hospital. |
| 6370 | Negative | This person is dangerous and is hiding his face because he is a criminal. | This person is visiting his friend; he is wearing a mask because it is cold. |
| 9042 | Negative | This man has been punished by his tribe. | This tradition is a rite of passage and is actually not painful. |
| 1300 | Negative | This is a wild dangerous dog that will attack anyone. | This dog just went to the dentist – look how clean her teeth are. |
| 2661 | Negative | This premature baby may not live more than a couple of days. | Thanks to early care this baby develops into a healthy toddler. |
| 3168 | Negative | This man suffers from a number of deformities since birth | The costume worn in this horror film won an Academy award in 1982 |
| 9050 | Negative | This is a terrible plane crash in which many people were killed. | This plane veered off the runway but no one was seriously hurt. |
| 9920 | Negative | Two people died in this horrendous car crash. | No one was seriously injured in this car accident. |
| 9611 | Negative | All passengers were killed in this plane crash. | This fake plane crash was put together for a movie. |
| 2700 | Negative | These women are mourning the loss of their close friend. | These women are overwhelmed with joy at a friend’s wedding. |
| 6190 | Negative | This woman is about to pull the trigger on her husband. | This is a picture from a training video on gun safety. |
| 2130 | Negative | This woman is very angry and might hit people around her. | This woman had her picture taken right when she was about to sneeze. |
| 3301 | Negative | This child was severely injured in a car accident. | This child was injured but makes a full recovery. |
| 2141 | Negative | This woman just found her mother dead | These are actresses for a movie called "The Funeral" |
| 2710 | Negative | This man was found dead from an overdose in a halfway house. | This is an actor from the 1970s film called “Drug Smuggle.” |
| 2688 | Negative | The poacher is shooting the bear to sell its fur. | A vet is tranquilizing this bear to give him medicine. |
| 2120 | Negative | This man is very angry and might become violent and dangerous. | This man has held his breath to try to make a red face. |
| 2399 | Negative | This woman suffers from intense migraine headaches. | This is an actress posing for an aspirin commercial. |
| 5970 | Negative | This terrible storm has destroyed many people's homes. | This storm is dying away; it is harmless and everyone is safe. |
| 9635 | Negative | This man was set on fire during a civil war. | This daredevil sets himself on fire as a stunt. |
| 2900 | Negative | This boy is very upset and sad because he found out that his pet ran away. | This boy's dad picked him up after he fell; he got scared but is totally okay. |
| 2750 | Negative | This is a homeless man who lives under a bridge in London. | This is an actor who is playing the role of a homeless man. |
| 6830 | Negative | This man is preparing to rob a bank. | This is an actor in bank robbery film. |
| 9584 | Negative | This man is undergoing painful dental surgery. | The man is having a routine dental checkup. |
| 3022 | Negative | This person is very scared and is trapped and cannot get out. | This person is on a fun ride and he is excited. |
| 9582 | Negative | This person is having surgery on his teeth and it hurts a lot! | The doctor just finished working on his teeth; he feels all better. |
| 2205 | Negative | This man has just lost his wife to cancer. | This man’s wife was ill but is fully recovering. |
| 7380 | Negative | These disgusting bugs are crawling all over the food and ruining it. | These bugs are eating the delicious leftover pizza. |
| 9250 | Negative | Something terrible has happened to this person; she has been hurt badly. | These are the doctors who have saved the woman's life. |
| 6571 | Negative | This man is having his car stolen by a thief. | This is a scene from a movie about an undercover cop. |
| 6020 | Negative | This is an electric chair used to execute prisoners on death row. | This is a prop from a movie about a man who is on death row. |
| 3280 | Negative | This boy is having a cavity filled; he is in a lot of pain. | This boy is having his teeth cleaned; it feels funny but doesn't hurt. |
| 2810 | Negative | This boy suffers from intense anger problems. | This boy is yelling “Ready or not here I come.” |
| 3230 | Negative | This man is very sick; he is worried that he might never get better. | This man was sick but he is okay now and on his way to getting all better. |
| 9520 | Negative | These abandoned children are near a nuclear reactor. | These children are actors in a movie about poverty. |
| 6300 | Negative | This person is waiting around the corner and wants to stab someone with the knife. | This man is using the tool to fix the wall. |
| 8230 | Negative | This boxer is being sent into a coma. | This is a scene from the movie about boxing. |
| 1050 | Negative | This is a poisonous snake that is very dangerous. | This is a snake that is completely harmless; it doesn't even have teeth. |
| 9594 | Negative | This person hates to give blood – the needle scares him! | This person gives blood all the time because it helps people who are sick. |
| 1321 | Negative | This is a mean bear that is angry and could attack people. | This is a friendly and harmless bear that is yawning. |
| 2102 | Neutral | This man reads the stock report every morning. | |
| 7595 | Neutral | These types of cars were popular in the 1970s. | |
| 7130 | Neutral | This truck has been used by five different companies. | |
| 7217 | Neutral | This coat rack is used by three people. | |
| 7010 | Neutral | This woven basket was made to hold fruit. | |
| 7090 | Neutral | This book was written in 1950. | |
| 5530 | Neutral | This is an edible mushroom. | |
| 2593 | Neutral | This cafe´ has outdoor seating. | |
| 7030 | Neutral | This iron is used to press shirts and dresses. | |
| 7037 | Neutral | These trains transport commercial goods. | |
| 2393 | Neutral | This man and woman are working on an assembly line. | |
| 7950 | Neutral | These tissues sell for 99 cents. | |
| 5740 | Neutral | This plant is common to the northern United States. | |
| 2575 | Neutral | This propeller will be used on a small cargo ship. | |
| 7056 | Neutral | This tool is often used by electricians for wiring. | |
| 7150 | Neutral | This is a blue umbrella. | |
| 7211 | Neutral | This clock is in the lobby of an office building. | |
| 7175 | Neutral | This lamp takes a 60-Watt bulb. | |
| 7002 | Neutral | This towel was used to clean the floor. | |
| 7550 | Neutral | This man is working on an old engineering program. | |
| 7140 | Neutral | This bus travels a route from Boston to Atlanta. | |
| 7700 | Neutral | This is a poster from a work-training video. | |
| 7705 | Neutral | This cabinet can hold up to 500 file folders. | |
| 7491 | Neutral | This building was used in a TV sitcom. | |
| 7050 | Neutral | This hairdryer is sitting on a laundry basket in a bathroom. | |
| 7004 | Neutral | This spoon is from a 1970s collection. | |
| 7500 | Neutral | This is the office of a large law firm. | |
| 2580 | Neutral | These men play chess three times a week. |
Footnotes
Financial disclosure
Dr. Green reports having received consulting fees from Abbott Laboratories, Amgen, Cypress, Lundbeck, and Teva. He has received speaking fees from Otsuka and Sunovion. The rest of the authors report no biomedical financial interests or potential conflicts of interest.
We also evaluated whether the positive deflection seen before 300 ms in Figure 1 revealed any effects involving group. Mean amplitude was evaluated in the time window from 165 – 215 ms (centered around a peak at 190 ms) and this positive deflection was maximal in the same six central/parietal electrodes used in the primary analyses - we therefore based the mean on a pooling of these six electrodes. Notably, this deflection appears to reflect the beginning of the LPP rather than an independent, earlier ERP component such P1 or P2, an interpretation that is consistent with prior research (Foti et al., 2009; Hajcak et al., 2009). A significant main effect of condition F(2,56) = 6.44, p < .02, indicated a significant difference between the Unregulated versus Neutral conditions, t(57) = −4.00, p < .001, but not between the other conditions (Regulated versus Neutral, t(57) = −1.72, p = .09; Unregulated versus Regulated, t(57) = −1.68, p = .10). However, the group, F(1,56) = .37, p > .05, and group × condition interaction, F(2,56) = 1.02, p > .05, were not significant. There were no significant correlations between the Unregulated minus Regulated difference score and self-reported regulation strategies or symptoms in either group.
Contributor Information
William P. Horan, VA Greater Los Angeles Healthcare System, University of California, Los Angeles
Greg Hajcak, Stony Brook University.
Jonathan K. Wynn, VA Greater Los Angeles Healthcare System, University of California, Los Angeles
Michael F. Green, University of California, Los Angeles, VA Greater Los Angeles Healthcare System
References
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clinical Psychology Review. 2010;30:217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Entipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. BIological Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badcock JC, Paulik G, Maybery MT. The role of emotion regulation in auditory hallucinations. Psychiatry Research. 2011;185:303–308. doi: 10.1016/j.psychres.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS. Cognitive control and schizophrenia: psychological and neural mechanisms. In: Engle RW, Sedek G, von Hecker U, McIntosh DN, editors. Cognitive Limitations in Aging and Psychopathology. Cambridge: Cambridge University Press; 2005. pp. 122–159. [Google Scholar]
- Bassam K, Lecompte T. Emotion regulation and schizophrenia. International Journal of Cognitive Therapy. 2012;5:67–76. [Google Scholar]
- Blanchard JJ, Kring AM, Horan WP, Gur R. Toward the next generation of negative symptom assessments: the collaboration to advance negative symptom assessment in schizophrenia. Schizophrenia Bulletin. 2011;37:291–299. doi: 10.1093/schbul/sbq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YS, Barch DM. The effect of emotional context on facial emotion ratings in schizophrenia. Schizophrenia Research. 2011;103:235–241. doi: 10.1016/j.schres.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Minor KS. Emotional experience in patients with schizophrenia re-revisited: meta-analysis of laboratory studies. Schizophrenia Bulletin. 2008;36:143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Lamm C. Human empathy through the lens of social neuroscience. Scientific World Journal. 2006;6:1146–1163. doi: 10.1100/tsw.2006.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decicco J, Solomon B, Dennis TA. Neural correlates of cognitive reappraisal in children: an erp study. Developmental Cognitive Neuroscience. 2012;2:70–80. doi: 10.1016/j.dcn.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, Hajcak G. The late positive potential: a neurophysiological marker for emotion regulation in children. Journal of Child Psycholology & Psychiatry. 2009;50:1373–1383. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Greeno CG, Pogue-Geile MF, Newhill CE, Hogarty GE, Keshavan MS. Assessing social-cognitive deficits in schizophrenia with the mayer-salovey-caruso emotional intelligence test. Schizophrenia Bulletin. 2008;36:370–380. doi: 10.1093/schbul/sbn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Patient Edition. New York: Biometrics Research; 1996. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, Benjamin l. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (version 2.0) New York: New York State Psychiatric Institute; 1994. [Google Scholar]
- Foti D, Hajcak G. Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience. 2008;20:977–988. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology. 2009;46:521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of occular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Green MF, Horan WP. Social cognition in scihzophrenia. Current Directions in Psychological Science. 2010;19:243–248. [Google Scholar]
- Green MJ, Waldron JH, Coltheart M. emotional context processing is impaired in schizophrenia. Cognitive Neuropsychiatry. 2007;12:259–280. doi: 10.1080/13546800601051847. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent – and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: conceptual foundations. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 3–23. [Google Scholar]
- Hajcak G, Macnamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental Neuropsychology. 2010;35:129–155. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, Foti D. Motivated and controlled attention to emotion: Time-course of the late positive potential. Clinical Neurophysiology. 2009;120:505–510. doi: 10.1016/j.clinph.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, Macnamara A, Foti D. ERPs and the study of emotion. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of ERP Components. New York: Oxford University Press; 2012. pp. 441–474. [Google Scholar]
- Hajcak G, Dunning J, Foti D, Weinberg A. Temporal dynamics of emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation (2nd edition) New York: Guilford Publications; pp. 441–474. (in press). [Google Scholar]
- Hemsley DR. The schizophrenic experience: taken out of context? Schizophrenia Bulletin. 2005;31:43–53. doi: 10.1093/schbul/sbi003. [DOI] [PubMed] [Google Scholar]
- Henry JD, Green MJ, de Lucia A, Restuccia C, Mcdonald S, O'Donnell M. Emotion dysregulation in schizophrenia: reduced amplification of emotional expression is associated with emotional blunting. Schizophenia Research. 2007;95:197–204. doi: 10.1016/j.schres.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Henry JD, Rendell PG, Green MJ, Mcdonald S, O'Donnell M. emotion regulation in schizophrenia: affective, social, and clinical correlates of suppression and reappraisal. Journal of Abnormal Psychology. 2008;117:473–478. doi: 10.1037/0021-843X.117.2.473. [DOI] [PubMed] [Google Scholar]
- Horan WP, Foti D, Hajcak G, Wynn JK, Green MF. Intact motivated attention in schizophrenia: evidence from event-related potentials. Schizophrenia Research. 2012;135:95–99. doi: 10.1016/j.schres.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Wynn JK, Kring AM, Simons RF, Green MF. Electrophysiological correlates of emotional responding in schizophrenia. Journal of Abnormal Psychology. 2010;119:18–30. doi: 10.1037/a0017510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Chan RC, Lu X, Tong Z. Emotion categorization perception in schizophrenia in conversations with different social contexts. The Australian and New Zealand Journal of Psychiatry. 2009;43:438–445. doi: 10.1080/00048670902817646. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wustenberg T, Villringer A, Knutson B, Kienast T, Gallinat J, Wrase J, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology. 2006;187:222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- Kee KS, Horan WP, Salovey P, Kern RS, Sergi MJ, Fiske AP, Lee J, Subotnik KI, Nuechterlein K, Sugar CA, Green MF. Emotional intelligence in schizophrenia. Schizophrenia Research. 2009;107:61–68. doi: 10.1016/j.schres.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Kopelowicz A, Ventura J, Liberman RP, Mintz J. consistency of brief psychiatric rating scale factor structure across a broad spectrum of schizophrenia patients. Psychopathology. 2008;41:77–84. doi: 10.1159/000111551. [DOI] [PubMed] [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophrenia Bulletin. 2008;34:819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system: instruction manual and affective ratings. University of Florida: Technical Report a-4, The Center for Research in Psychophysiology; 1999. [Google Scholar]
- Lee J, Kern RS, Harvey PO, Horan WP, Kee KS, Ochsner K, Penn DL, Green MF. An intact social cognitive process in schizophrenia: situational context effects on perception of facial affect. Schizophrenia Bulletin. doi: 10.1093/schbul/sbs063. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meanings. Neuropsychopharmacology. 2011;36:216–318. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone K, Harper S, Gillanders D. An exploration of emotion regulation in psychosis. Clinical Psychology and Psychotherapy. 2009;16:418–430. doi: 10.1002/cpp.635. [DOI] [PubMed] [Google Scholar]
- Lukoff D, Nuechterlein KH, Ventura J. Manual for the expanded brief psychiatric rating scale. Schizophrenia Bulletin. 1986;12:578–602. doi: 10.1093/schbul/12.4.578. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, Stenger VA, Cohen JD. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. American Journal of Psychiatry. 2005;162:475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Foti D, Hajcak G. Tell me about it: neural activity elicited by emotional pictures and preceding descriptions. Emotion. 2009;9:531–543. doi: 10.1037/a0016251. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Ochsner KN, Hajcak G. Previously reappraised: the lasting effects of description type on picture-elicited electrocortical activity. Social Cognitive and Affective Neuroscience. 2011;6:348–358. doi: 10.1093/scan/nsq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR, Sitarenios G. Measuring emotional intelligence with the msceit v2.0. Emotion. 2003;3:97–105. doi: 10.1037/1528-3542.3.1.97. [DOI] [PubMed] [Google Scholar]
- Mocaiber I, Pereira MG, Erthal FS, Figueira I, Machado-Pinheiro W, Cagy M, Volchan E, de Oliveira l. Regulation of negative emotions in high trait anxious individuals: an ERP study. Psychology & Neuroscience. 2009;2:211–217. [Google Scholar]
- Morris RW, Sparks A, Mitchell PB, Weickert CS, Green MJ. Lack of cortico-limbic coupling in bipolar disorder and schizophrenia during emotion regulation. Translational Psychiatry. doi: 10.1038/tp.2012.16. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Park S, Lee J, Folley B, Kim J. Schizophrenia: putting context in context. Behavior & Brain Research. 2003;26:98–99. [Google Scholar]
- Perry Y, Henry JD, Grisham JR. The habitual use of emotion regulation strategies in schizophrenia. British Journal of Clinical Psychology. 2011;50:217–222. doi: 10.1111/j.2044-8260.2010.02001.x. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Juckel G, Koslowski M, Kahnt T, Knutson B, Dembler T, Kienast T, Gallinat J, Wrase J, Heinz A. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology. 2008;196:673–684. doi: 10.1007/s00213-007-1016-4. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Horan WP, Karpouzian TM, Abram SV, Cobia DJ, Csernansky JG. Self-reported empathy deficits are uniquely associated with poor functioning in schizophrenia. Schizophrenia Research. 2012;137:196–202. doi: 10.1016/j.schres.2012.01.012. [DOI] [PubMed] [Google Scholar]
- van der Meer I, van’Twout M, Aleman A. Emotion regulation strategies in patients with schizophrenia. Psychiatry Research. 2009;170:108–113. doi: 10.1016/j.psychres.2009.07.010. [DOI] [PubMed] [Google Scholar]
- van Petten C, Luka BJ. Neural localization of semantic context effects in electromagnetic and hemodynamic studies. Brain & Language. 2006;97:279–293. doi: 10.1016/j.bandl.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the brief psychiatric rating scale: 'the drift busters'. International Journal of Methods in Psychiatric Research. 1993;3:221–224. [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A. Training and quality assurance with the Structured Clinical Interview for DSM-IV. Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Werner K, Gross JJ. Emotion regulation and psychopathology: a conceptual framework. In: Kring A, Sloan DS, editors. Emotion regulation and Psychopathology. New York: Guilford Press; 2010. pp. 13–37. [Google Scholar]
- Wu I, Winkler MH, Andreatta M, Hajcak G, Pauli P. Appraisal frames of pleasant and unpleasant pictures alter emotional responses as reflected in self-report and facial electromyographic activity. International Journal of Psychophysiology. doi: 10.1016/j.ijpsycho.2012.04.010. (in press). [DOI] [PubMed] [Google Scholar]