Abstract
Phytoestrogens represent a diverse group of non-steroidal natural products, which seem to have some oestrogenic effects and are often marketed as food supplements. Population exposed to phytoestrogens is potentially increasing, in part because an unfavourable risk-benefit profile of Hormone Replacement Therapy (HRT) for prolonged treatments (e.g., osteoporosis prevention) highlighted by the publication of the Women Health Initiative (WHI) trial in 2002, but also because many post-menopausal women often perceived phytoestrogens in food supplements as a safer alternative than HRT. Despite of increasing preclinical and clinical studies in the past decade, appealing evidence is still lacking to support the overall positive risk-benefit profile of phytoestrogens. Their status as food supplements seems to discourage studies to obtain new evidence, and the chance to buy them by user’s initiative make it difficult to survey their prevalence and pattern of use. The aim of the present review is to: (a) outline the clinical scenario underlying the increased interest on phytoestrogens, by overviewing the evolution of the evidence on HRT and its main therapeutic goals (e.g., menopausal symptoms relief, chemoprevention, osteoporosis prevention); (b) address the chemical and pharmacological features (e.g. chemical structure, botanical sources, mechanism of action) of the main compounds (e.g., isoflavones, lignans, coumestans); (c) describe the clinical evidence on potential therapeutic applications; (d) put available evidence on their riskbenefit profile in a regulatory perspective, in light of the recent regulation on health claims of food supplements.
Keywords: Cardio-metabolic disorders, food supplements, menopausal symptoms, osteoporosis, phytoestrogens, vasomotor symptoms.
1. THE NEED FOR ADDITIONAL STRATEGIES FOR THE MANAGEMENT OF PERI- AND POSTMENOPAUSAL SYMPTOMS
1.1. The Habits in Hormone Replacement Therapy (HRT) Use Before 2002
Menopause is characterised by amenorrhea due to the cessation of ovarian function. Reduced circulating oestrogen levels can induce unacceptable symptoms that affect the women's health and well-being: at least in the first menopausal year, most women suffer from hot flushes, palpitations, night sweats and mood disturbances, with important implications in life habits.
Hormone Replacement Therapy (HRT) upon appearance of menopausal symptoms was very common, especially in Western Countries, up to 2002. With this approach, the decrease in endogenous synthesis of oestrogens were simply replaced by exogenous hormones in order to limit both short- and long-term consequences of decreased of oestrogen levels. Because of the risk of cell proliferation, especially for some tissues (uterus), combination with progestins was recommended. The availability of different formulations (oral, transdermal, intrauterine) allowed high compliance and consequent wide population coverage. Frequently, women continued treatment, even after the acute phase of symptoms, to obtain supposed advantages on known risks of advanced age, especially osteoporosis and major cardiovascular events.
1.2. HRT Benefits and Risks Known Before 2002
Actual benefits, well perceived by women already after short-term use of HRT, were certainly represented by relief in vasomotor symptoms and vaginal discomfort. These effects, especially relief from hot flushes, vaginal dryness, sexual dysfunction and general improvement in quality of life in peri-menopausal symptomatic women were convincingly demonstrated by large Randomized Controlled Trial (RCT) and authoritative reviews [1, 2]. Concerning long-term effects of prolonged use, some additional benefits were already suggested, whereas no alarming data were published before 2002. The role of HRT in prevention of osteoporosis and consequently occurrence of fracture was largely demonstrated [3, 4].
However, as regards cardiovascular benefits, observational studies provided evidence supporting them [5, 6], not confirmed by clinical trials [7-9]. Although there was no support from clinical trials, HRT was considered protective for cardiovascular events on the basis of published data and a patho-physiological relationship between oestrogen levels and atherosclerosis. Venous thromboembolic events already represented an exception: the risk of this event from HRT was well known and previous events or risk factors represented contraindication for HRT use. Another known strong contraindication was previous breast cancer, to avoid oestrogen receptor agonism in this condition.
1.3. Publications of Women Health Initiative (WHI) Trial and its Consequences
In July 2002, the first publication of WHI trial results appeared on JAMA [10] and many other original articles followed it [11-13] to describe the large amount of data obtained from that study. More than 16,000 post-menopausal women with an intact uterus were recruited and randomly assigned to continuous, combined, conjugated equine oestrogen with medroxyprogesterone acetate or placebo, planning 8.5 years of treatment. Evidence for statistically significant increase in cases of breast cancer (8 additional cases per 10,000 person-years) and cardiovascular events (8 additional cases of stroke, 8 of pulmonary embolism and 7 of coronary heart disease) prompted sudden changes in recommendations concerning HRT. The study, however, showed reduced risk for hip fractures (5 saved cases) and colorectal cancer (6 saved cases).
The results of HERS II study (Estrogen/progestin Replacement Study), also published in July 2002, strengthen the lack of cardiovascular benefits of prolonged HRT, in particular for women with previous coronary heart disease [14].
After the publication of WHI result, many specific warnings appeared around the world [15, 16] and in fact the use of HRT drastically decreased [17]. The most common recommendation was to use HRT only for the short term relief of vasomotor and vaginal symptoms just for a few months (or a few years) from amenorrhea, whereas no prolonged use was recommended because of the unfavourable risk-benefit profile. Post-menopausal women, apart from short-term discomfort, are considered healthy subjects and no risk of prolonged oestrogen exposure should be accepted: prevention of osteoporotic fractures does not justify acceptance of HRT-related adverse reactions, in the light of their incidence and severity.
There was a fast decline in HRT use since the third quarter of 2002, both in prevalence and incidence [18, 19], with differences in rate decrease depending from social variables (i.e., ethnicity, income, education) [20, 21].
1.4. Current Knowledge on HRT and Relevant Recommendations
Nowadays, 10 years after the WHI trial publication, a lot of evidence has become available on the risk-benefit profile of HRT, by considering different doses and preparations, specific populations (e.g., > vs. <65-year-old women, intact uterus vs. hysterectomised patients) and comparison with possible alternatives [22, 23]. However, recommendations have not significantly changed in comparison with the version hastily published in July 2002.
Current guidelines suggest that patient history is vital in determining and assessing menopausal symptoms, and patient education is often sufficient to help women cope with their menopausal symptoms. Medication is infrequently required, as is the case of unacceptable vasomotor symptoms or urogenital atrophy; treatment should be individualised and the lowest dose of oestrogen providing relief should be used for the shortest period of time, especially in those women with an intact uterus. Treatment should be reviewed regularly.
Most systemic hormonal preparations increase the risk of venous thromboembolism, cardiovascular disease and breast cancer. Thus, before prescribing HRT, the cardiovascular risk profile and risk of venous thromboembolism and breast cancer should be assessed [24, 25]. Also abnormal vaginal bleeding, impairment in liver function, migraine, history of endometrial or ovarian cancer and gallbladder disease represent conditions requiring caution in HRT use.
Local hormonal treatment can be indicated for vaginal symptoms. Urinary incontinence may be improved with the use of local oestrogen treatment. However, there was little evidence from the trials on the period after oestrogen treatment had finished and no information about the long-term effects of this therapy was given. Conversely, systemic HRT may worsen incontinence.
Finally, HRT is not indicated for primary or secondary prevention of dementia, nor for preventing deterioration of cognitive function in postmenopausal women. Although considered effective for the prevention of postmenopausal osteoporosis, it is generally recommended as an option only for women at significant risk, for whom non-oestrogen therapies are unsuitable [26].
1.5. Current Alternative Options to HRT and Relevant Level of Evidence
HRT is considered the most effective treatment for unacceptable vasomotor symptoms and therefore, alternatives for this specific aim should represent only a second line in case of contraindication for HRT use (see above). Some antidepressants (i.e., Selective Serotonin Reuptake Inhibitors and Serotonin–Norepinephrine Reuptake Inhibitors), clonidine and gabapentin showed a mild to moderate effect on reducing hot flushes in women with history of breast cancer [27], but specific contraindications exists also for this option. Also relaxation therapy provided similar encouraging data.
As already cited, local hormone preparations can have a favourable risk-benefit profile if vaginal discomfort prevails. Current recommendation for prevention of osteoporosis and relevant fracture suggests pharmacological treatment only when bone mass density is strongly decreased in comparison with physiological values. In these cases, bisphosphonates, SERMs (Selective Estrogen Receptor Modulators) or parathormone analogues represent the main option of therapy in decreasing order of preference. Their critical risk-benefit profile does not allow to use them in case of low risk. Prolonged HRT use represents an alternative only in hysterectomised women.
Middle aged women frequently resort to nutritional supplements also according with a general trend towards increased use of alternative medicines to improve the general wellness; relief of menopausal symptoms and prevention of its long-term consequences belong to reasons for using natural remedies and, in particular, phytoestrogens. Their similarity to oestrogens and the thought advantage of vegetal sources caused the rapid increase of their consumption especially in the last decade, no matter what supporting scientific evidence was available on their biological effects.
Subsequent sections will describe the main aspects of phytoestrogens, starting from chemical, pharmacological characteristics, to evidence on clinical effects and regulatory status.
2. CHEMICAL FEATURES AND BOTANICAL SOURCES OF PHYTOESTROGENS
Phytoestrogen is a general term used to define classes of compounds that are non-steroidal and are either of plant origin or derived from the in vivo metabolism of precursors present in several plants eaten by human beings. Phytoestrogens are secondary metabolites that induce biological responses in vertebrates and can mimic or modulate the actions of endogenous oestrogens, usually by binding to Estrogen Receptors (ERs) [28]. Oestrogens influence the growth and functioning of female and male reproductive tissues, maintain both the skeletal and central nervous systems, and provide cardioprotective effects in the cardiovascular system [29]. Considering the numerous effects oestrogens have on the human body, it is not surprising to consider the potential of phytoestrogens for human health.
The ability of plant substances to cause estrus in animals was documented in the mid-1920s.
The recognition of ‘clover disease’ in Australian sheep in the 1940s led to the investigation of oestrogenic activity of isoflavones. The sheep whose diet was predominately subterranean clover (Trifolium subterraneum L., Fabaceae) suffered from a reproductive disorder that reduced the lambing rates and involved abnormal lactation, changes in the sex organs, and permanent infertility [30, 31]. Indeed, the phytoestrogens are present in green clover and are not present at senescence.
Exposure to phytoestrogens for humans is mainly through diet and we are daily exposed to highly variable amounts of these substances. The oestrogenic plant compounds are widespread in food, including herbs and seasonings (garlic, parsley), grains (wheat, rice), vegetables (soybeans, beans, carrots, potatoes), fruits (date, pomegranates, cherries, apples), and drinks (coffee). Asian populations generally eat large quantities of soy products compared to Western populations, and recent research found that Asian populations have lower rates of hormone-dependent breast and endometrial cancers [32] and lower incidences of menopausal symptoms and osteoporosis than Westerners. On the other hand, Asian immigrants living in Western nations also have increased risk of these maladies as they “Westernize” their diets to include more protein and fat and reduce their fibre and soy intake [33].
The 3 major classes of phytoestrogens found in typical human diets are isoflavones, which are concentrated in soybeans and soy products, but are also found in other legumes; lignans, which are distributed in seeds, whole grains, berries, fruit, vegetables, and nuts; and coumestans, which are found in broccoli and sprouts. Although their medical importance has been less extensively studied, lignans occur in higher concentrations in US and European diets than do isoflavones.
2.1. Isoflavones
Isoflavones make up the most common and well known form of phytoestrogens. Isoflavones are found in a variety of plants, including fruits and vegetables, but they are predominantly found in leguminous plants and are especially abundant in soy (Glycine max). They play important roles in plant defense, exhibit various health-promoting activities, and have attracted great interest from the pharmaceutical and nutraceutical industries as drug target and dietary supplements. Reinli and Block have compiled reference data on the levels of isoflavones found in a variety of food items [34]. In addition, the contents of isoflavones in different soy products (e.g. tofu and soy protein concentrates) vary substantially. For example, processed soy products, such as soy hot dogs and tofu yogurt, may contain only 1/10th the isoflavone content of whole soy beans (0.2– 0.3 vs. 2–4 mg isoflavone/g) [35].
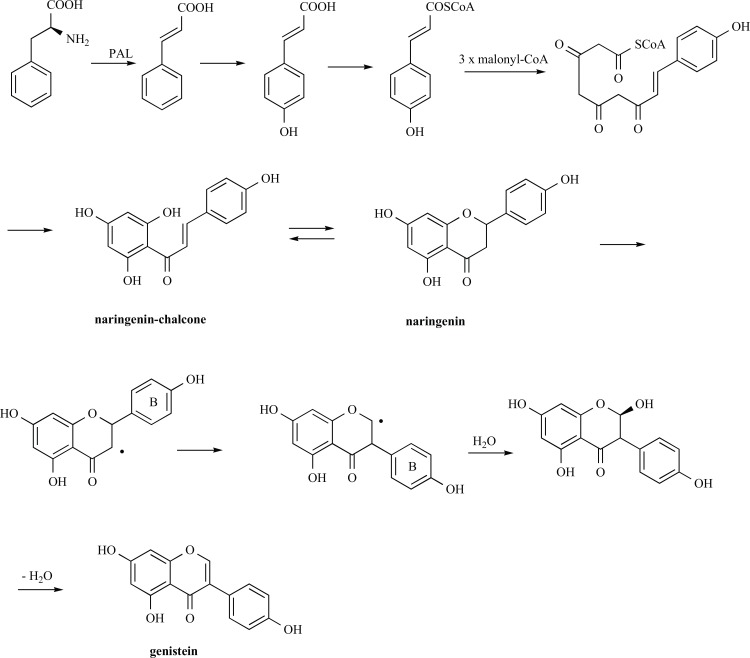
Isoflavones are generated via the phenylpropanoid pathway from the amino acid L-phenylalanine and are generally recognised to be stored as conjugates in the vacuole [36, 37] of soybeans, and then mobilised as required by the tissue in responses to biological events such as germination or infection.
Phenylpropanoids consist of metabolites with the basic building unit C6-C3, including an aromatic benzene ring (C6) and a three-carbon lateral linear chain (C3, a propane), and arise from a common precursor (the essential aromatic amino acid phenylalanine) through a reaction catalysed by the enzyme phenylalanine ammonia-lyase, PAL. PAL catalyses the conversion of phenylalanine into cinnamate (Fig. 1), then cinnamate 4-hydroxylase, a cytochrome P450 enzyme, introduces a hydroxyl group at the para position of the phenyl ring of cinnamate and produces 4-coumarate. 4-coumarate CoA-ligase catalyses the final step of general phenylpropanoids pathway, producing the corresponding CoA thiol esters, the p-coumaroyl-CoA. Then, the ubiquitous plant enzyme, chalcone synthase, catalyzes the first step of flavanoid pathway, the condensation of one p-coumaroyl-CoA and three malonyl-CoA molecules to form the chalcone scaffold (naringenin-chalcone, Fig. 1); chalcone isomerase carries out the cyclisation of the chalcone to form the flavanoid core, in particular the flavanone naringenin. Through a legume-specific pathway, isoflavone synthase converts the flavanone to isoflavanone by performing an aryl-ring migration to transfer the aromatic B-ring from position C-2 to C-3, likely with a radical mechanism. After hydroxylation in position C-2, the dehydration of 2-hydroxyisoflavanone occurs to generate the isoflavones [37].
Fig. (1).
Biosynthesis of isoflavones.
Two of the major isoflavones found in humans are genistein (4′,5,7-trihydroxyisoflavone) and daidzein (4′,7-dihydroxyisoflavone). Genistein and daidzein are parent compounds, which are obtained from their plant precursors 4′-methyl ethers derivatives, biochanin A and formononetin, respectively. Formononetin and biochanin A are also the main isoflavones of red clover (Trifolium pratense) (Table 1). In plants, isoflavones are inactive when present in the bound form as glycosides (genistin and daidzin, respectively), but when the sugar residue is removed these compounds become activated and only the aglycones can be absorbed. Consequently, the aglycones show higher and more rapid absorption in the gastrointestinal tract than their glycosides in humans. Despite the good permeability of isoflavones, their oral bioavailability is usually low, and marked first pass metabolism, extensive conjugation and efflux transporter have been cited as the main reasons responsible for their low oral exposure (for reviews on ADME studies see [38, 39]).
Table 1.
The Structures of Isoflavones.
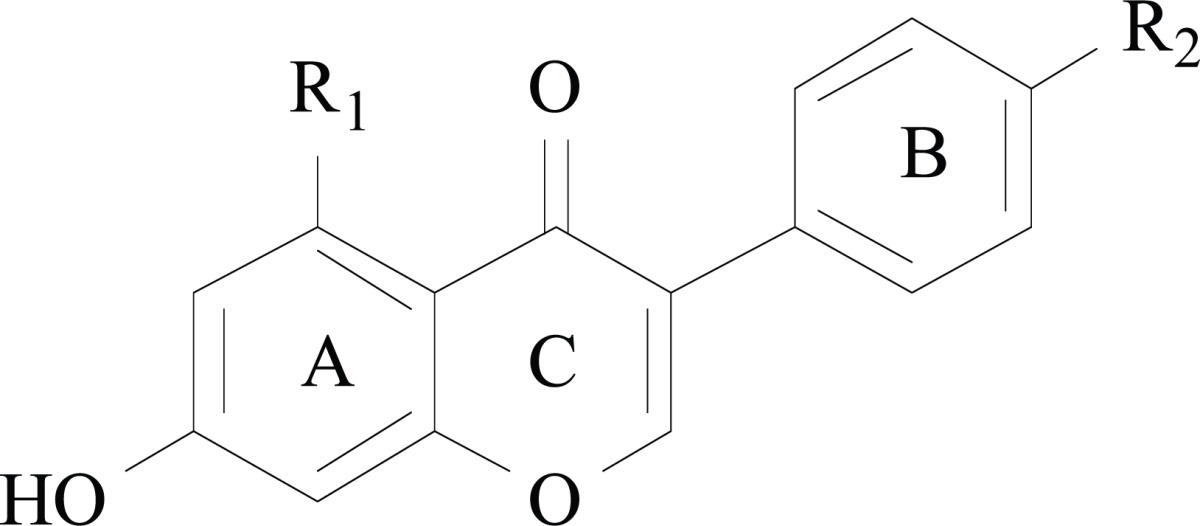
| isoflavone | R1 | R2 |
|---|---|---|
| Biochanin A | OH | OCH3 |
| Genistein | OH | OH |
| Formononetin | H | OCH3 |
| Daidzein | H | OH |
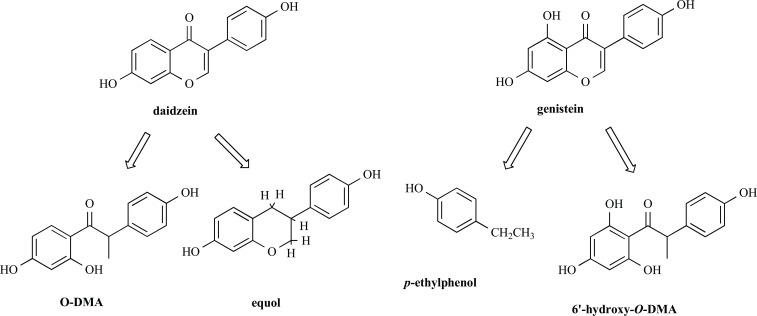
After mammals consume isoflavones, daidzein and genistein are extensively transformed by intestinal bacteria into a variety of metabolites which may exert enhanced or decreased biological activity. In the colonic microflora, daidzein may be metabolised to O-desmethylangolensin (O-Dma) and equol, and genistein may be metabolised to 6′-hydroxy-O-DMA and hormonally inert p-ethylphenol (Fig. 2) [40]. Daidzein, genistein, equol, and O-DMA are the major phytoestrogens detected in the blood and urine of humans and animals and may have different biological effects than the original isoflavones digested. For example, the conversion of daidzein into equol affords a 30 times more active metabolite.
Fig. (2).
Structures of metabolized isoflavones.
Equol is not equally metabolised in all humans and the ability to produce it varies between individuals [41, 42]. For example, equol is notably present in the blood and urine in some individuals and absent in others. Noteworthy, the prevalence of equol producers among Western adults is 20 to 35 % compared with 50 to 55 % among Asian adults [43].
Several investigators have reported that individual variability in colonic microflora plays an important role in determining the preferred pathways of isoflavone metabolism and the bioavailability of isoflavones [44, 45].
2.2. Lignans
Lignans are widely distributed in the plant kingdom, and play a role in plant growth. Still more important, they play a role in the Western diet, and act as antioxidants in human metabolism. They are present in a wide variety of plant foods, including seeds (flax, pumpkin, sunflower, poppy, sesame), whole grains (rye, oats, barley), bran (wheat, oat, rye), beans, fruits (particularly berries), and cruciferous vegetables such as broccoli and cabbage [46]. Tea and coffee also have some lignans. Flaxseed (Linum usatissimum) is by far the richest dietary source of plant lignans, and lignan bioavailability can be improved by crushing or milling flaxseed [47]. Much lower amounts are contained in sesame seeds, the second most lignan rich food.
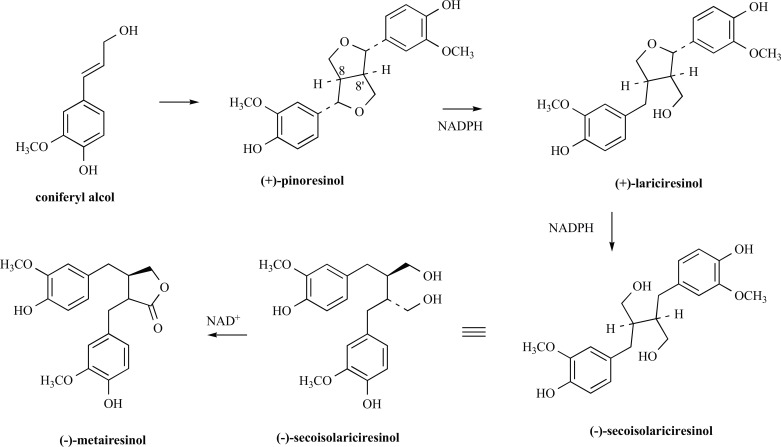
Plant lignans are polyphenolic substances derived from phenylalanine via dimerisation of substituted cinnamic alcohols, mostly coniferyl alcohol (Fig. 3). This reaction is catalysed by oxidative enzymes, and one-electron oxidation followed by free radical resonance distribution leads to oxidative phenol coupling products, where the phenylpropane units are linked by the central carbon (C8) of their side chains. Accordingly, the entry point involves stereoselective coupling of two E-coniferyl alcohol molecules to afford (+)-pinoresinol [48]. Afterward, (+)-pinoresinol is reduced via (+)-lariciresinol to (-)-secoisolariciresinol by NADPH dependent pinoresinol-lariciresinol reductase and subsequently oxidised to (-)-matairesinol. The key enzyme involved in the synthesis of (-)-matairesinol is secoisolariciresinol dehydrogenase, which converts (-)-secoisolariciresinol into (-)-matairesinol by means of the coenzyme nicotinamide adenine dinucleotide (NAD+) in a hydride transfer process [49].
Fig. (3).
Biosynthesis of lignans.
Secoisolariciresinol and matairesinol were among the first lignan precursors identified in the human diet and are therefore the most extensively studied. Glycosilation of secoisolariciresinol is accomplished by secoisolariciresinol diglucosyl transferase that appears to be mainly localised in the seed [50] (for example the principal lignan precursor found in flaxseed is secoisolariciresinol diglucoside, SDG).
Pinoresinol and lariciresinol are more recently identified plant lignans that contribute substantially to the total dietary lignan intake. Typically, they contribute about 75% to the total lignan intake whereas secoisolariciresinol and matairesinol contribute only about 25%.
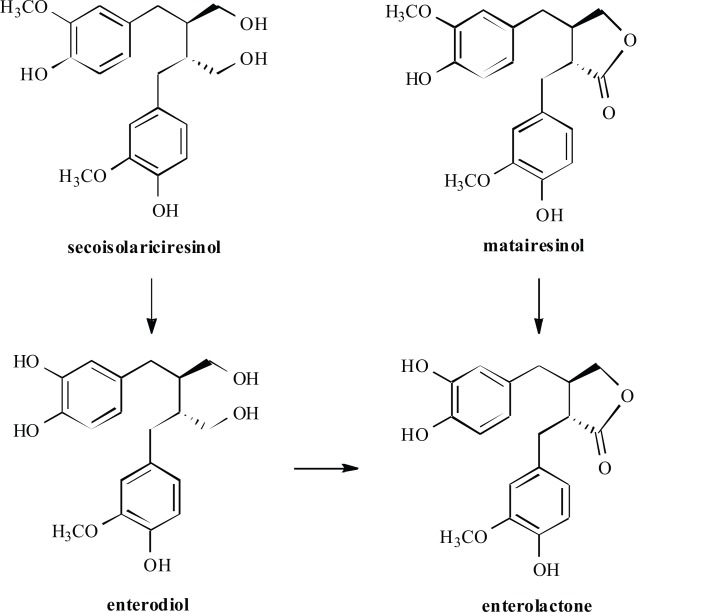
The plant lignans, such as pinoresinol, lariciresinol, secoisolariciresinol, matairesinol, hydroxymatairesinol, syri-ngaresinol and sesamin, are converted in the body to the mammalian lignans known as enterolactone and enterodiol (Fig. 4), by normally occurring intestinal bacteria [51].
Fig. (4).
Structures of metabolized lignans.
The concentration of SDG in flaxseeds is 75–800 times higher than that in other foods, and thus intake of flaxseed causes the highest mammalian lignan production [52].
The biological activity of flax and other plant lignans depends on the presence of certain bacteria in the gut. Some humans appear to lack either the right type or a sufficient number of gut bacteria to convert SDG and other lignans to mammalian lignans. The use of antibiotics may abolish the ability of intestinal flora to produce active phytoestrogen metabolites for several weeks [53]. Smoking and obesity are also associated with a reduction in enterolactone production, while coffee, tea, and of course fibre intake are noted to enhance the production of enterolactone [54].
The main flax lignan SDG is also an antioxidant. It scavenges for certain free radicals like the hydroxyl ion (•OH) [55].
2.3. Coumestans
Coumestans are another group of plant phenols that show oestrogenic activity, possess a coumarin structure, and are biosynthetically related to isoflavones (Fig. 5) [56]. Coumestans are produced predominantly during germination of legume seeds, but also occur in fodder crops [57]. Although a large number of coumestans have been isolated from
Fig. (5).
Biosynthesis of coumestans.
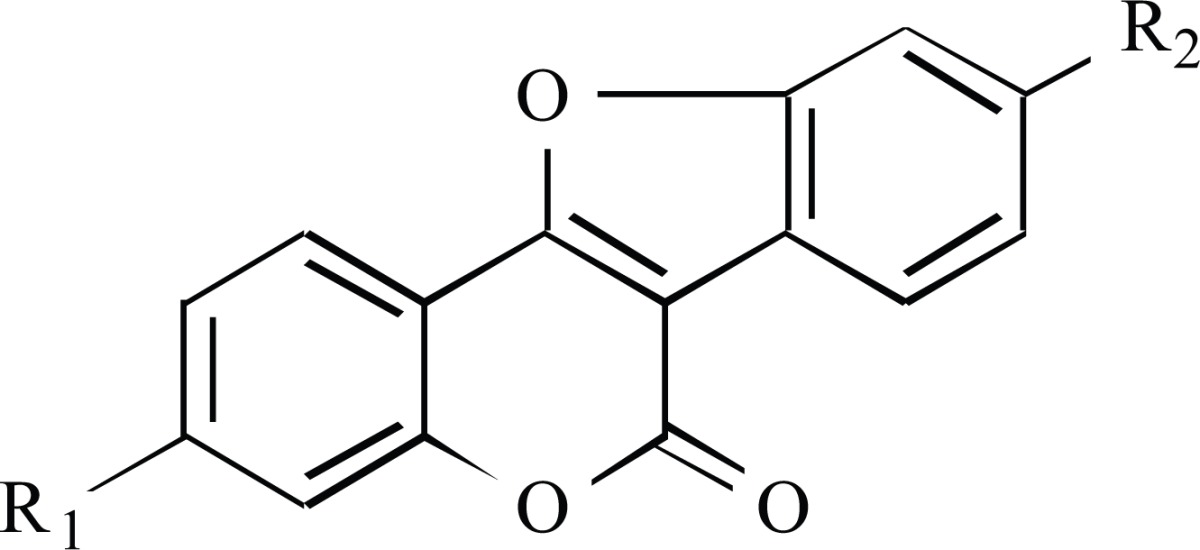
plants, only a small number have shown oestrogenic activity, predominantly coumestrol (7,12-dihydroxycoumestan) and trifoliol (4’-methoxycoumestrol) [58] (Table 2).
Table 2.
The Structures of Coumestans.
| Coumestan | R1 | R2 |
|---|---|---|
| coumestrol | OH | OH |
| trifoliol | OH | OCH3 |
Coumestrol was first reported in 1957 by Bickoff and coworkers as a new phytoestrogen that was isolated from ladino clover (Trifolium repens L., Fabaceae), strawberry clover (Trifolium fragiferum L., Fabaceae) and alfalfa or lucerne (Medicago sativa L., Fabaceae) [31, 59].
Coumestans are less common in the human diet than isoflavones and lignans. The main dietary sources of coumestans are legume shoots and sprouts, mainly clover and alfalfa, however low levels have been reported in brussel sprouts and spinach [60]. Legumes such as split peas, pinto beans, lima beans, and soybean sprouts also contain small amounts of coumestrol. Furthermore, it has been observed that coumestrol concentrations in legumes increase after insect and fungal attack [61].
2.4. Mechanism of Action
The ERs bind a large number of compounds that exhibit remarkably diverse structural features. In fact, the ER is probably unique among the steroid receptors in its ability to interact with a wide variety of compounds [62]. ERs belong to the family of nuclear receptors and modulate transcriptions of target genes in a ligand dependent way, which makes them very attractive drug targets. The endogenous oestrogens bind to ERs under physiological conditions, and are associated with a wide range of physiological and pathological pathways [63]. The ER ligands bind to the ligand binding domain (LBD) and induce conformational changes, mainly of the flexible C-terminal helix 12. The rat, mouse and human ER exists as two subtypes, ERα and ERβ, which differ in the C-terminal LBD and in the N-terminal transactivation domain.
The phytoestrogens are believed to work by binding to ERs on cell membranes, much like the body’s own steroid oestrogens do. Coumestrol and genistein have been shown to be more potent than any other known phytoestrogen when their in vitro oestrogenic activity was compared with that of the primary female hormone, 17β-estradiol (E2) [64]. Genistein is one of the well-studied phytoestrogens and for it crystallographic analyses and molecular modelling studies were performed.
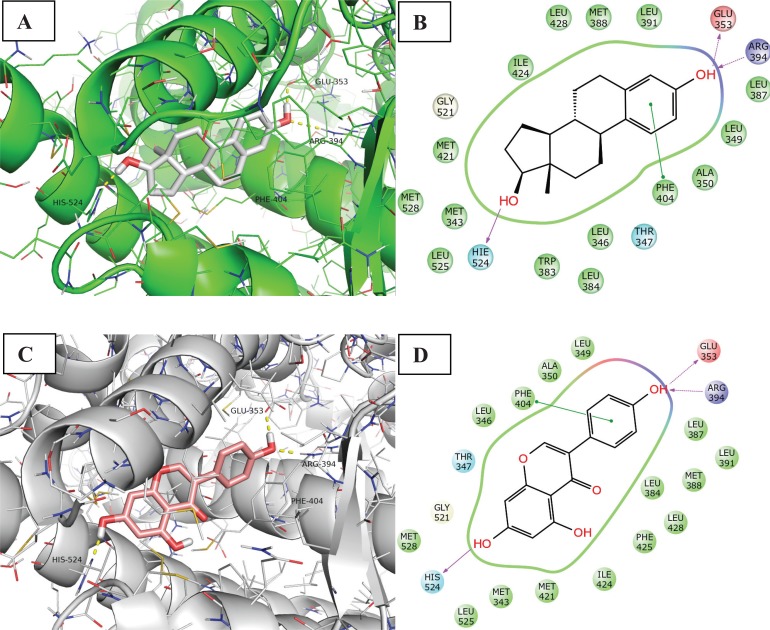
The ERα ligands E2 and genisteine are classified as full and partial agonists, respectively, and stabilize helix 12 in the agonist conformation. The agonists bind to the LBD through H-bonding and van der Waals contacts. Besides the hydrophobic features of the pocket, ligands with a high affinity to ERα usually form H-bonds primarily with the side chains of Glu353, Arg394, and His524, as illustrated in Fig. 6. His524 exerts a pivotal role in maintaining the protein structure in the agonist−LBD conformer. Both His524 and Leu525 are geometrically in contact with the agonist ligands in the crystal structures, and are furthermore located on H11, which is closely linked to the essential helix 12. Hence, His524 and Leu525 are involved in ligand binding and allostery, which are intimately involved in the transcriptional activation of ERα agonists [65].
Fig. (6).
Binding mode and ligand interaction diagram of Estradiol (A and B respectively) and Genestein (C and D respectively) in ERα (PDB code: 1A52 and 1X7R). In A and C the protein is represented as line and cartoon, the ligand as stick. H-bonds are represented as yellow dotted lines. In B and D the interactions are represented as: violet arrow (H-bond), green arrow (Π-Π stacking), ciano sphere (polar), green sphere (Hydrophobic), red sphere (Charged positive) and blue sphere (Charged negative).
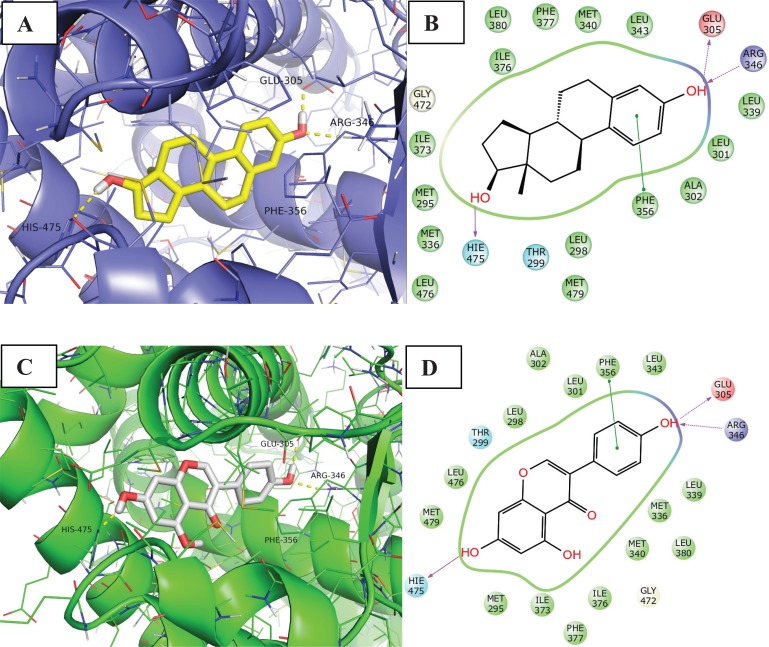
Unlike E2, which displays relatively equivalent potency at both ER subtypes, genistein is a 40-fold ERb-selective ligand [66]. As regards interactions made by genisteine with the two isoforms, the phenol group mimics the E2 “A ring”, with the phenolic hydroxyl (4’-OH) involved in a hydrogen bonding network between ERα residues Glu353 and Arg394 (ERβ residues Glu305 and Arg346), and a highly ordered water molecule. Another hydrogen bond is formed between the isoflavone 7-OH and N of ERα His524 (ERβ His475). The core scaffold fills the remainder of the primarily hydrophobic pocket.
The results of a study from Manas et al. suggest that the interaction of the genisteine B ring with ERa Leu384 relative to ER( Met336, and to some extent the interaction of the genisteine 5-OH group with ERa Met421 relative to ERb Ile373 are capable of contributing significantly to the observed ERb selectivity of genisteine (Fig. 7) [66].
Fig. (7).
Binding mode and ligand interaction diagram of Estradiol (A and B respectively) and Genestein (C and D respectively) in ERβ (PDB code: 3OLS and 1X7J). In A and C the protein is represented as line and cartoon, the ligand as stick. H-bonds are represented as yellow dotted lines. In B and D the interactions are represented as: violet arrow (H-bond), green arrow (Π-Π stacking), ciano sphere (polar), green sphere (Hydrophobic), red sphere (Charged positive) and blue sphere (Charged negative).
Another study shows the efficacy of activation of the two receptors from the most to the least effective compound. The sequence and the EC(50) were as follows: E2 (0.03 µM) > coumestrol (0.2 µM) > equol (3.5 µM) > genistein (15 µM) > daidzein (>300 µM) for ERa and E2 (0.01 µM) > coumestrol (0.025 µM) > genistein (0.03 µM) > daidzein (0.35 µM) > equol (0.4 µM) for ERb [67].
In particular, the fact that ERb is widely expressed, but not the primary ER in, for example, the uterus (where oestrogenic effects are mediated by ERa) opens up the possibility of targeting other tissues while avoiding certain classical oestrogenic effects.
Most phytoestrogens are found to offer benefits for menopausal symptoms and bone density without carrying the risks of heart disease, coronary artery damage, or peripheral vascular issues [68].
In addition to their function as phytoestrogens, isoflavones act as potent antioxidants and help in neutralizing the harmful effects of free radicals in tissues. Furthermore, genistein possesses a number of biochemical features that may have influence on cancer cells. In particular, it inhibits protein tyrosine kinase, thereby disrupting signal transduction and inducing cell differentiation, and topoisomerase II, leading to DNA fragmentation and apoptosis. These anti-cancer properties of genistein were also considered in 1995 by the National Cancer Institute (NCI) that recommended genestein for clinical development as a cancer chemopreventive agent. Recently, Klein and King claimed that the concentrations at which such effects occurred were often much higher than the physiologically relevant doses achievable by dietary or pharmacologic intake of soy foods or supplements [69]. The inhibitory activities of intracellular enzymes displayed by genistein are not expressed by other isoflavones. This seems to be due to the absence of the hydroxyl group in position 5, which is present in the structure of genistein and appears to be essential for inhibitory activity [70].
The mammalian lignans are not as potent as the endogenous ligand in their estrogenic activities, they can act as either weak oestrogens or they can oppose the actions of oestrogen, depending on the presence of stronger oestrogens like estradiol. During women’s reproductive years, when blood levels of endogenous oestrogens are at their highest, the lignans can bind to the ER and block the actions of endogenous oestrogens. In this case, they act as antagonists. After menopause, the levels of endogenous oestrogens in the blood naturally decrease because the ovaries release less natural oestrogens. In this case, the lignans act like weak oestrogens [71].
Many phytoestrogens such as lignans, coumestrol, and isoflavonoids are known to be aromatase inhibitors [72]. Aromatase is a member of the cytochrome P450 enzyme family that converts androgens (androstenedione and testosterone) into oestrogens (estrone and estradiol, respectively); high levels of this enzyme are associated with breast, adrenal, and prostate cancers.
2.5. Other Oestrogenic Compounds
2.5.1. Flavonoids
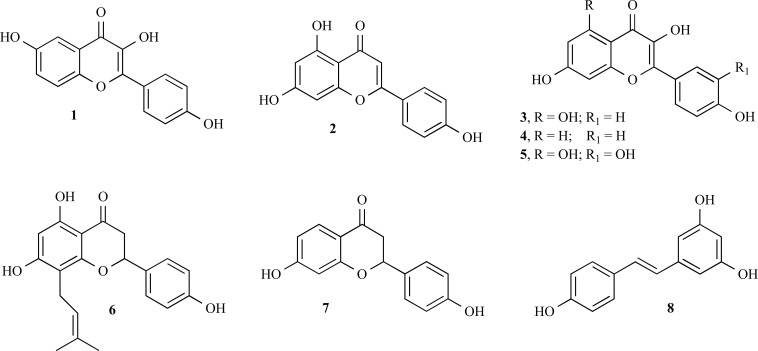
Flavonoids are widely distributed in plants as glycosylated derivatives. Recent studies on oestrogenic activity of flavonoids revealed that their oestrogenic potency is 102 to 105 fold less than E2. The binding of flavonoids to ERs is known to be determined by the position of the OH groups attached to rings A and C that can mimic the OH groups of E2. Therefore, the active molecules reflect the essential basic features that determine oestrogenic activity, that is the exact O-O distance. The OH groups in positions 6 and 4’ of flavones, such as in 3,6,4’-trihydroxyflavone (structure 1, Fig. 8), match the OH groups of E2, while flavonoids with OH groups in positions 7 and 4’, such as apigenin (structure 2, Fig. 8), kaempferol (structure 3, Fig. 8) and fisetin (structure 4, Fig. 8), show the lowest activity and quercetin (structure 5, Fig. 8) is not active. However, 8-prenylnaringenin (structure 6, Fig. 8), also named hopein, a recently discovered flavanone from hops (Humulus lupulus) and beer, demonstrated a higher oestrogenic activity than coumestrol and isoflavones [73].
Fig. (8).
Structures of other estrogenic compounds.
Mersereau et al. isolated an ERβ-selective compound, liquiritigenin (structure 7, Fig. 8), from the root of Glycyrrhiza uralensis [74].
2.5.2. Stilbenes
Resveratrol (structure 8, Fig. 8), the most common and most studied stilbene, is found in a variety of plants and functions as a phytoalexin to protect against fungal infections. The most notable sources of resveratrol are the skin of grapes (Vitis vinifera), red wine and other brightly pigmented fruit juices. Peanuts (Arachis), particularly the papery skin around the nut, and pistachios also contain resveratrol. While resveratrol is most often discussed in terms of its vascular effects (similar to flavonoids), it has been shown to possess some phytoestrogenic activity as well, only reported for the trans isomers of this compound [75].
3. PRECLINICAL AND CLINICAL EVIDENCE ON THE EFFECTS OF PHYTOESTROGENS
3.1. Search Strategy and Literature Mapping Approach
In this section, we explore and summarise the available literature on phytoestrogens by highlighting past and future potential areas of research on the basis of a critical evaluation of the evidence. Our literature review was performed in the MEDLINE database through a broad search strategy using the following MESH search terms: "Phytoestrogens"[Mesh] OR "Isoflavones"[Mesh] OR "coumestan" Supplementary Concept) OR "Lignans"[Mesh]. Retrieved articles were then characterized between “Clinical” and “Preclinical” studies by using relevant MESH descriptors. Subsequently, we mapped the literature to highlight the most important therapeutic uses of phytoestrogens. Thus, on the basis of an automated analysis, keywords assigned to relevant abstracts were grouped in terms of investigational areas. Considering the vast amount of experimental research, we decided to collapse key findings by focusing the literature analysis on systematic reviews and meta-analyses of RCTs when phytoestrogens are investigated in postmenopausa and its clinical consequences. To this aim, the following MESH search strategy was used: Postmenopause"[Mesh] AND "Phytoestrogens"[Mesh] OR "Isoflavones"[Mesh] OR "coumestan" Supplementary Concept) OR "Lignans"[Mesh]). Finally, we focused the literature by critically analyzing RCTs published in the last 5 years.
3.2. Eligible Studies
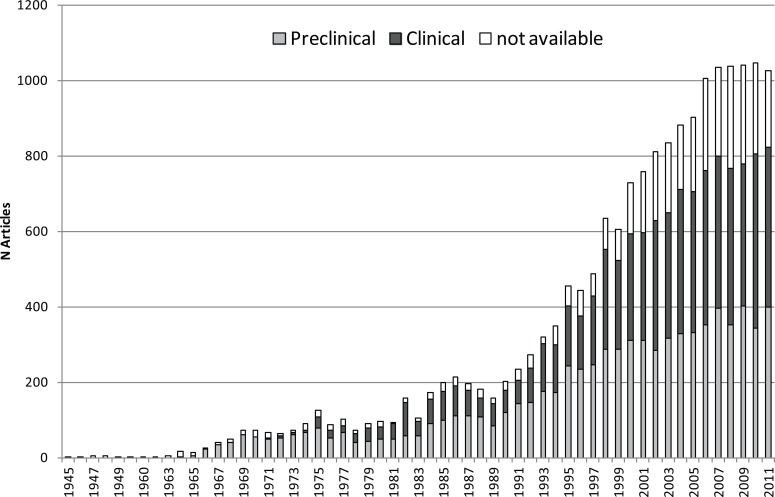
As of December 23rd, 2012, 18,474 articles were retrieved. The interest in phytoestrogens exponentially increased during the last two decades, as denoted by the number of articles published since 1945 (Fig. 9). Notably, a remarkable increase clearly started from 1990 (200 articles per year) and reached a plateau in the period 2006-2011. The characterisation of the articles between “Clinical” and “Preclinical” studies highlighted a shift in the interest towards human investigations, although basic research (i.e., in vivo/in vitro studies) is still fervent in this area. As expected, in the 60-70’s, only pre-clinical research was carried out; starting in the 80’s, the proportion of clinical studies steadily increased. In the last decade, the publication trend is equally distributed between clinical and pre-clinical evidence.
Fig. (9).
Publication trend (from 1945 to 2011) for articles concerning phytoestrogens, classified for preclinical and clinical studies. Data concerning 2012 are not showed because they are incomplete at the time the search strategy was performed.
The reasons subtending the growing interest on phytoestrogens are many-fold and may be summarised as follows: (a) the treatment of menopausa still represents an unmet clinical need both for women and clinicians. Indeed, more than 80% of women who may benefit from hormone therapy are unwilling or unable to start treatment due to various medical reasons [76]; in addition, up to 70% of them experienced vasomotor symptoms during the peri-menopausal period (up to 20 daily episodes) [77]; (b) biochemical similarities between phytoestrogens and SERMs with resulting favourable clinical implications (see below); (c) the WHI trial [10] was prematurely interrupted in 2002 for unfavourable risk-benefit profile of hormone therapy, thus causing an increased perception of risk of the available pharmacological treatment (see section 1); (d) phytoestrogens theoretically do not raise the same safety concerns associated with HRT as they are commonly administered without the addition of progestin. Therefore, women have recently resorted to the so-called very popular “natural” alternatives (especially after 2002) [78] and research is now exploring further areas of investigation.
As regards the most important therapeutic uses of phytoestrogens, oncology (47%), cardio-metabolic diseases (18%), menopausal disorders (17%) were the most investigated therapeutic areas, followed by cognitive diseases (4%). When focusing on the systematic reviews and meta-analysis in postmenopausa, 56 studies were initially collected: 19 of them were excluded after manual assessment of full texts/abstracts (e.g., out of scope, inability to obtain definite author’s assessment); 39 publications were finally considered eligible and scrutinised in terms of clinical outcomes. Table 3 showed the distribution of these outcomes and provided an overall summary of the potential effect of phytoestrogens (for details see Table 1S (197.1KB, pdf) in supplementary material, available on the publisher’s website).
Table 3.
Published Reviews and Meta-Analyses on Clinical Outcomes of Phytoestrogens. In the Table "+" Indicates a Potential Benefit, "?" an Uncertain Benefit, and "-" No Benefit.
| Reference | Year | intervention | Cardio-Metabolic Disorders | Aging | Menopausal Symptoms |
Bone Disorders |
Cognitive Disorders |
Neoplasm prevention |
|---|---|---|---|---|---|---|---|---|
| Horn-Ross [122] | 1995 | Phytoestrogens | + | |||||
| Herrington [123] | 2000 | Phytoestrogens | + | |||||
| Kim [124] | 2000 | Genistein | + | |||||
| Arjmandi [125] | 2001 | Phytoestrogens | + | |||||
| Glazier [126] | 2001 | Phytoestrogens | + | + | + | ? | ||
| Ariyo [127] | 2002 | Phytoestrogens | + | |||||
| Ewies [128] | 2002 | Phytoestrogens | + | + | + | + | ||
| Lord [129] | 2002 | Lignans | + | |||||
| Messina [130] | 2002 | Soybeans | + | + | + | |||
| Phipps [131] | 2002 | Isoflavones | + | ? | ? | ? | ? | |
| Jacquot [132] | 2003 | Phytoestrogens | ? | ? | ? | ? | ||
| Altavilla [133] | 2004 | Genistein | + | |||||
| Limer [134] | 2004 | Phytoestrogens | + | |||||
| McCue [135] | 2004 | Soy isoflavones | + | + | + | |||
| Messina [136] | 2004 | Soy isoflavones | + | |||||
| Nandur [137] | 2004 | Phytoestrogens | + | |||||
| Gikas [138] | 2005 | Phytoestrogens | ? | |||||
| Mahady [139] | 2005 | Soy isoflavones | + | |||||
| Viereck [140] | 2005 | Actaea racemosa extracts | + | + | - | |||
| Cassidy [141] | 2006 | Phytoestrogens | + | + | ? | ? | ? | |
| Geller [142] | 2006 | Isoflavones | + | + | + | |||
| Howes [143] | 2006 | Isoflavones | + | |||||
| Qin [144] | 2006 | Soy isoflavones | + | |||||
| Usui [145] | 2006 | Phytoestrogens | + | ? | ? | ? | - | |
| Vafeiadou [146] | 2006 | Isoflavones | - | |||||
| Duffy [147] | 2007 | Phytoestrogens | - | |||||
| Messina [148] | 2008 | Soy isoflavones | ? | |||||
| Hooper [149] | 2009 | Soy isoflavones | - | |||||
| Velentzis [150] | 2009 | Lignans | + | |||||
| Buck [151] | 2010 | Lignans | + | |||||
| Cano [152] | 2010 | Isoflavones | + | |||||
| Hooper [153] | 2010 | Isoflavones | ? | |||||
| Li SH [154] | 2010 | Isoflavones | ? | |||||
| Ricci [155] | 2010 | Soy isoflavones | - | |||||
| Ricci [156] | 2010 | Soy isoflavones | - | |||||
| Dong [157] | 2011 | Soy isoflavones | ? | |||||
| SalariSharif [158] | 2011 | Phytoestrogens | + | |||||
| Williamson [159] | 2011 | Isoflavones | ? | ? | ||||
| Zaineddin [160] | 2012 | Lignans | + |
As regards recently published RCTs, after the exclusion of 8 studies (conducted on pharmacokinetic aspects), 62 RCTs were finally analysed for clinical outcomes. Relevant PICO information (i.e., Patient/Population, Intervention, Comparison, Outcome) are provided as supplementary material (Table 2S (197.1KB, pdf) in supplementary material, available on the publisher’s website). The majority of RCTs (22/62) were performed on metabolic diseases, followed by aging (12/62), menopausal symptoms (11/62) and bone disorders (7/62). These findings underline that menopausa-related issues still carry important research interest. Notably, more than half of the studies (33/62) investigated diet soy supplements, whereas soy extracts in tablets or topical preparations were explored in 9 out of 62 RCTs. Genistein and red clover were investigated in 8 and 6 studies, respectively.
The most intriguing findings pertain to the fact that phytoestrogens are mostly, if not exclusively, investigated against the use of placebo (including Mediterranean diet, physical exercise life or the additional of milk protein and calcium/vitamin D supplements). Therefore, the question arises whether future ad hoc studies should be designed to compare head-to-head phytoestrogens with HRT. In addition, issues related to the dose and the superiority of a given phytoestrogen (e.g., soy supplements, red clover) warrant clarification.
3.3. Key Findings: Areas of Interest and Potential Effectiveness
Before drawing any conclusion, it must be acknowledged that effectiveness of phytoestrogens in all therapeutic areas is difficult to assess because the majority of studies were not designed against an active comparator, namely hormone therapy or any other standard therapy for a given disorder. In addition, individual ability to effectively metabolise phytoestrogens could also contribute to generate conflicting data. By using the same therapeutic groups identified through overall mapping, Table 3 highlighted 6 key areas of research: cardio-metabolic diseases, aging, menopausal symptoms, bone diseases, cognitive diseases and neoplasms/oncology prevention. Notably, the interest appeared to move from menopausal symptoms and cardio-metabolic diseases (2000-2006) towards the oncological and aging areas. The term “aging” was introduced to distinguish the clear cardio-metabolic effect from the antioxidant properties of phytoestrogens that are also evaluated through alterations of inflammatory biomarkers such as C-reactive protein.
As regards the oncological area, the chemo-protective properties of phytoestrogens have been recently advocated: most studies analysed reduction in breast cancer occurrence in population with high soyfood intake. Among specific compounds, the most consistent protective effects were observed for lignans and breast cancer risk [79]. The common view ascribed the potential protective effects of phytoestrogens against breast cancer to the preference for binding the ERβ isoform, which predominates in the normal mammary epithelium. By contrast, the α isoform is mainly expressed in tumor cells. However, the epidemiological data together with the latest evidence [80] raised doubts on this oversimplified concept: phytoestrogens interfere with a large panel of regulatory proteins and are implicated in epigenetic activity thus causing both promotion and antagonism of carcinogenesis. It is still too early to consider phytoestrogens as a therapeutic choice in oncology; first, we need to fill the gap of knowledge on the mechanism by which these compounds may exert chemo-protective effect. More appropriately, they should be considered as a tool to guide drug development of novel ligands for the treatment of endocrine disrupting diseases including breast cancer.
The interest in the cardio-metabolic area does not appear to be so attractive at the moment, although reviews and meta-analysis demonstrated an overall potential benefit. In particular, a number of mechanisms of action have been recently proposed that may support interest in a potential preventing role of phytoestrogens on metabolic syndrome: down-regulation of pro-inflammatory cytokines, increase in reverse cholesterol transport, increase in insulin activity, energy expenditure and lipolisis [81]. So far, only surrogate markers were included in the clinical studies, such as decrease in lipoprotein blood levels or glycaemia.
Reviews on cognitive diseases are promising, although actual neuro-protective properties are still far to be defined. Indeed, isoflavones could have a dual influences on cognitive functions (i.e. neuronal injury prevention or induction of neuronal apoptosis), therefore further studies are required to establish their actual effect in cognitive diseases [82].
As regards menopausal symptoms, reviews and meta-analyses disappointingly agreed on the lack of clear benefit of phytoestrogens, recognizing heterogeneity of studies and also a considerable “placebo effect”. Eligible literature addressed different issues that may be directly or indirectly related to this paraphysiological status, including vasomotor symptoms (e.g., hot flushes, vaginal atrophy), bone health and sleep disturbances. In particular, the latter is starting to be considered; though limited and preliminary, existing data have raised potential beneficial effect of phytoestrogens on insomnia and cognitive function. It should be recognised that post-menopausal women are particularly affected by sleep disturbances as compared to pre-menopausal phase; in addition, many menopausal symptoms may be the result of actual sleep deprivation. Based on our assessment and considering the latest published evidence [83-85], there is still insufficient evidence to support the effectiveness of phytoestrogens (as compared to HRT) in the management of vasomotor symptoms. This is also in line with the 2007 Cochrane review [86]. Nonetheless, hot flushes and vaginal atrophy still represent unmet clinic needs for women because they have a negative impact on quality of life. The magnitude of the problem is important form an epidemiological standpoint: flushes, though more frequent and severe in the peri-menopausal and early post-menopausal period, are still a concern in 14.6% and 8.6% of women in their sixties and seventies, respectively [87]. In addition, a large proportion of patients undergoing therapy with tamoxifen or aromatase inhibitors may suffer from vasomotor symptoms and should select a symptom-relieving treatment that should not theoretically compromise the effectiveness of anticancer drugs. Several important research issues emerged and may help in guiding future investigations. For instance, the actual endpoint should be clarified when evaluating hot flushes: studies that focused on the reduction of the intensity rather than frequency are more consistent and suggest that phytoestrogens are effective at attenuating rather than alleviating vasomotor symptoms in post-menopausal women. The relative intensity of hot flushes represents an important aspect that is strongly perceived by women. The overall effect on vulvovaginal atrophy requires further investigation, as illustrated by the case of Femarelle® (see section 4).
Concerning bone health, the SERM-like mechanism of action of phytoestrogens has been advocated as key factor in the potential positive effect on menopausal bone loss. Indeed, a number of molecular effects (inhibition of the osteoclastogenesis and promotion of osteoblastogenesis) have been demonstrated by in vitro/in vivo studies [88, 89]. Our literature analysis, together with the latest evidence [84], showed that, although favorable effects have been demonstrated on Bone Mass Density and bone turnover markers after six-month treatment in postmenopausal women, the actual effects on fractures and the long-term safety of phytoestrogens need to be established. Based on the available literature, it is impossible to make an accurate estimate of a treatment effect and to make treatment recommendations at this time [88]. Further clinical studies are needed to assess the influence on clinical endpoint such as fractures, factors affecting the magnitude of the beneficial effects and possible interactions with anti-osteoporosis drugs [90]. Therefore, at the current state of knowledge, evidence is insufficient to make recommendations on the actual effect of phytoestrogens on bone health.
3.4. Key Findings: Potential Safety Issues
Before drawing definite recommendations on phytoestrogen use, a careful review of their safety profile must be provided. As SERMs, the effects of phytoestrogens on the endometrium warrant vigilant scrutiny. As a matter of fact, the risk of endometrial hyperplasia and carcinoma appeared increased from clinical trials [91]. The most updated meta-analysis performed on 174 RCTs was designed to compare side effects of phytoestrogens with placebo or no treatment.
It was shown that phytoestrogen supplements had only a moderately elevated rates of gastrointestinal side effects such as abdominal pain as well as myalgia and sleepiness [92]. Regional difference was found, with Asian studies showing higher side effect rates than western studies. Side effects were also more common in women over age 55. No association emerged between duration of study and incidence of side effects suggesting no cumulative dose effect with time. Although these key findings are reassuring, the long-term safety profile is still largely unknown. In addition to routine reporting of suspected adverse reactions, active pharmacovigilance activities should be encouraged to clarify the pattern of use and perception of both benefit and risks by women.
3.5. Conclusion and Gaps in Knowledge
In summary, we scanned the available evidence on potential therapeutic roles of phytoestrogens, by providing a general direction of clinical research in this field. No attempt was carried out to assess the quality of studies. In addition, a formal systematic review was not conducted because, as already pointed out by several recent systematic analysis, differences in study design (i.e., study population, duration, route, dose of administration, types of phytoestrogens and endpoint) do not allow firm conclusions. Despite a plethora of studies, our literature review highlighted gaps in knowledge and the persistence of unmet clinical needs for women in post-menopausa (e.g., proper treatment of vasomotor symptoms) and researchers (e.g., whether differences exist among products and doses). Moreover, additional therapeutic roles have been recently proposed such as chemoprevention in breast cancer, but require effort in research. Until results from dedicated head-to-head comparative effectiveness studies and long-term safety investigations are not public, we believe that phytoestrogens should be considered only as second-line treatment in post-menopausal women that inadequately respond or do not tolerate to conventional pharmacological therapy.
4. THE REGULATORY CLASSIFICATION OF PHYTOESTROGENS
Herbal products can be marketed in several products: differences among countries depend mainly on the way a medicinal product is defined. In the European Union and in the United States, definitions of a medicinal product are very similar and a similar regulatory framework is applied.
Differences in the way herbal products are regulated regard mainly food supplements because: 1 (197.1KB, pdf) ) some regulatory authorities have defined positive and negative lists (i.e. lists of botanicals allowed and prohibited in food supplements); 2 (197.1KB, pdf) ) the procedure to inform the regulatory authority before placing the product on the market is variable (usually simple notification rather than authorisation is required). This review will refer mainly to current European legislation as an example of the complexity involved in regulating herbal products and will focus on specific issues related to phytoestrogens and their health claims so far submitted to the European Food Safety Authority (EFSA).
In the European Union, herbal preparations can be marketed in medicinal products, food supplements, foods, cosmetics, and so on, and it essentially up to the manufacturer to decide which legal framework to apply depending on the intended use. Products that are presented as having the ability to treat or prevent disease should always be regulated under medicinal law, because therapeutic or prophylactic claims must be proved by appropriate evidence and must obtain marketing authorisation. However, the way herbal medicinal products are licensed and marketed in Europe has changed after the approval of Directive 2004/24/EC [93], amending Directive 2001/83/EC [94] as regards Traditional Herbal Medicinal Products. Most individual herbal medicinal products continue to be licensed nationally by Member States, but the process for licensing and information on herbal substances/preparations is increasingly harmonised across the European Union.
A subcategory of herbal medicinal products, the so-called ‘traditional herbal medicinal products’, is introduced. For these, safety needs to be shown, but efficacy does not. Some of them have a long tradition of use: the legislation classifies under this subcategory those herbal medicinal products used for at least 30 years, including at least 15 years within the EU, and intended to be used without the supervision of a medical practitioner and are not administered by injection. An example of herbals used in traditional herbal medicinal products is Mentha piperita L. [95]; the complete EU list, established by Committee for Herbal Medicinal Products, can be found at the EMA website [96]. At present, to the best of our knowledge, no product containing phytoestrogens intended for use in menopause is included here.
Directive 2004/24/EC was clearly adopted to facilitate marketing of traditional herbal medicinal products through a simpler and less costly registration procedure, while providing the necessary guarantees of quality and safety. The long tradition of these products makes it possible to reduce the need for clinical trials, replaced by documentation indicating that the product is not harmful in specified conditions of use and that its efficacy is plausible on the basis of long-standing experience. Clearly, even long-standing traditional use does not rule out safety concerns and competent national authorities can ask for additional data, when necessary. It is the responsibility of national authorities to decide, on a case-by-case basis, whether a herbal product fulfils the definition of medicinal product. Where a herbal medicinal product is not registered or authorised by 1 May 2011, the product may not be on the EU market [95].
However, herbal products may be classified and marketed as food supplements, provided that they do not fulfil the definition of medicinal products and comply with Directive 2002/46/EC [97] on food supplements and EC Regulation 1924/2006 on nutrition and health claims made on foods [98]. In other words, products used to restore, correct or modify physiological functions by exerting a pharmacological, immunological or metabolic action should be considered as medicinal products when used to treat, prevent or diagnose a disease; beneficial properties to health in general are not sufficient to define a medicinal product, because this is the case also for food supplements and products with “health claims” (this term should not be confused with the “indication” of a medicinal product).
In the European Union, any health claim must be pre-approved before it can be used: approval can be obtained via its submission to the EFSA in the generic procedure that is intended to produce a list of claims (the so-called list of claims under article 13.1 of the Regulation [98]), such as those regarding the role of a nutrient or other substance in growth, development and body functions or psychological and behavioural functions. These claims do not include those related to child development or health or disease risk reduction and are based on generally accepted scientific evidence well understood by the average consumer. Most claims for plant food supplements have been introduced under article 13.1. However, it must be acknowledged that the evidence so far provided was essentially based on traditional knowledge and not on scientific studies and that this type of evidence is accepted also under the provisions of the traditional herbal medicinal product legislation for the approval of medicinal botanical products. Thus, under current legislation, comparable products containing the same botanicals can be submitted under a fundamentally different procedure, an issue still under discussion by the European Commission.
Examples of scientific opinions of the EFSA Panel on Dietetic Products, Nutrition and Allergies on soy isoflavones under article 13.1 include: a) in 2012, a scientific opinion on health claims related to soy isoflavones and maintenance of bone mineral density and reduction of vasomotor symptoms associated with menopause [99]; b) in 2011, a scientific opinion on health claims related to soy isoflavones and protection of DNA, proteins and lipids from oxidative damage, maintenance of normal blood LDL-cholesterol concentrations, reduction of vasomotor symptoms associated with menopause, maintenance of normal skin tonicity, contribution to normal hair growth, “cardiovascular health”, treatment of prostate cancer and “upper respiratory tract” [100]. In all cases, the EFSA Panel concluded that there was insufficient evidence to substantiate the above claims.
EC Regulation 1924/2006 [98] also considers other types of health claims: a) those under article 13.5, which are based on newly developed scientific evidence and/or for which protection of proprietary data is requested: for these health claims, authorisation is required on a case-by-case basis, following the submission of a scientific dossier to the European Food Safety Authority (EFSA) for assessment; b) those under article 14, which refer to the reduction of disease risk or to children's development or health. EFSA has now adopted its first series of opinions on health claims under Article 14 and these provide information on their scientific substantiation to the European Commission and Member States, which will decide whether to authorise these claims.
Several products from a botanical source (including phytoestrogen) have been already submitted to the EFSA pursuant to article 14. Examples are: 1) in 2008, the EFSA Panel assessed the scientific substantiation of a health claim related to Femarelle® (mixture of DT56a soy derivative and ground flaxseed at a ratio of 3:1, for oral administration; each capsule contains 344 mg soy and 108 mg flaxseed – altogether 430mg powder) and “induces bone formation and increases bone mineral density reducing the risk for osteoporosis and other bone disorders”. The Panel concluded that “a cause and effect relationship has not been established between the consumption of Femarelle® and increased BMD, increased bone formation, or decreased risk of osteoporosis or other bone disorders in post-menopausal women” [101]; 2) in 2010, the EFSA Panel was asked an opinion on a health claim related to soy protein and reduction of blood cholesterol concentrations. The Panel concluded that “a cause and effect relationship has not been established between the consumption of soy protein and the reduction of LDL-cholesterol concentrations” [102]; 3) finally, in 2012, the scientific opinion on a health claim of isolated soy protein and reduction of blood LDL-cholesterol concentrations was, again, negative and stated that “a cause and effect relationship has not been established between the consumption of isolated soy protein (as defined by the applicant) and a reduction in blood LDL-cholesterol concentrations” [103].
As regards the United States of America, the Food and Drug Administration (FDA) is the regulatory authority for both foods/food supplements and medicinal products and the regulatory framework is similar to the European one. Medical food and food supplements have to meet the standards specified in guidance documents published on the FDA website. These are beyond the scope of this review and the reader is referred to the following documents: a) Draft Guidance for Industry: Dietary Supplements: New Dietary Ingredient Notifications and Related Issues [104] b) Guidance for Industry: Evidence-Based Review System for the Scientific Evaluation of Health Claims –Final [105].
Thus, as in most European countries, food supplements are not required to seek approval for their products or to register them [106, 107]. The responsibility for ensuring safety is up to the manufacturer and there is no requirement to prove effectiveness. The FDA is responsible to take actions when needed for two basic aspects: a) when safety issues arise after the product has reached the market; b) when the food supplement is claimed by the manufacturer to have properties that make it a medicinal product (the product is promoted as intended for the cure, mitigation, treatment, or prevention of a disease). This has happened recently several times for phytoestrogen-containing products, which prompted FDA intervention through the so-called cyber letters [109]: for example, with red-clover-containing products [109, 110] or soy-derived products [111, 112].
5. ONGOING RESEARCH INITIATIVES AND PERSPECTIVES
From this review, it clear that the potential of phytoestrogens in menopause is of great interest for both patients, clinicians and manufacturers, although there are several key areas deserving attention.
First, the hypothesis that phytoestrogens behave as SERM requires close experimental scrutiny. Affinity for oestrogen receptors provisionally supports the hypothesis that their risk profile for mammary and uterine tissue is lower than that of HRT [62, 68]. Namely, the fact that ERβ is widely expressed, but, for example, not in the uterus (where oestrogenic effects are mediated by ERα), opens up the possibility of targeting other tissues while avoiding certain classical oestrogenic effects. Accordingly, some phytoestrogens appear to offer some benefit for menopausal symptoms and bone density without carrying the risks of heart disease, coronary artery damage, or peripheral vascular issue [68].
Second, pharmaceutical and pharmacokinetic aspects deserve specific attention because botanical preparations must be offered to the end-user as standardised as possible as regards their content in phytoestrogens. Bioavailability, dose regimen and duration of treatment should be considered according to the patient’s specific clinical needs. As previously discussed, the effectiveness of phytoestrogens is highly variable among individuals, especially when investigating primary clinical endpoints: pharmaceutical and pharmacokinetic properties may largely explain this variability, in particular absorption and distribution to target tissues [113-115]. As a matter of fact, isoflavones exist primarily in soybeans and in most of soy foods as a complex mixture of glucoside conjugates that are not bioavailable in this form. After ingestion, these glucosides are hydrolysed by both intestinal mucosal and bacterial β-glucosidases, thus releasing the aglycones [116, 117]. Therefore, gut bacteria play a key role in the bioactivation of these compounds. S-equol is by far the most abundant active metabolite of daidzein; in humans, its activation is quite variable among adult individuals, with Caucasians producing significantly lower amount of S-equol as compared to Asian populations. Phytoestrogens strongly interact with plasma proteins such as albumin, but only slightly with lipoproteins [118]. This suggests that they may interfere, in the context of poly-therapies, with drug distribution and clearance. Remarkably, because fatty tissues are a major site of storage, unpredictable release of phytoestrogens may occur, especially in women with overweight or lipid excess.
Intestinal conjugation and subsequent excretion is dependent by type I/II enzymes, with subsequent efflux through transporters such as multidrug resistance-associated proteins. Recently, daidzein and genistein have been found to increase function and expression of P-glycoprotein in human intestinal epithelial cells, thus raising concern on potential interactions with concomitant drugs binding P-glycoprotein in intestine [119]. Renal clearance represents another important aspect that carries clinical implications: because kidney is the major elimination route of phytoestrogens, patients with renal disease show increased half-lives of these compounds.
Bioavailability is also influenced by the formulation used: for instance, it was reported that soy isoflavone aglycones are absorbed faster and in higher amount as compared to glucosides. It was also hypothesised that inulin-stimulated bacterial growth induces soybean breakdown and facilitates isoflavone absorption. As a consequence, a variety of pharmaceutical strategies have been developed to improve the solubility and bioavailability of isoflavones (e.g., lipid-incorporation in self-microemulsifying formulations or complexation with ciclodextrins) [103].
Finally, ongoing discussion on regulatory status should clarify those conditions for which a product must comply with legislation of medicinal products (in our opinion, any specific indication should be documented in clinical trials) from other situations when a health claim, according to the food supplement regulation, could be more appropriate. In any case, product development requires clarification of key chemical, pharmacological and pharmaceutical aspects. Several current initiatives worldwide consider as possible targets of phytoestrogens bone loss, atherosclerosis, metabolic syndrome, breast cancer: see, for instance, the large number of studies recruiting or already completed on phytoestrogens (either as food or pure compounds) listed in clinicaltrials.gov [120]. There are also consortia systematically addressing key interdisciplinary aspects of botanical preparations in general, such as the PlantLIBRA project (acronym of PLANT food supplements: Levels of Intake, Benefit and Risk Assessment), co-financed in the context of the 7th EU Framework Program [121]; this project aims to foster the safe use of botanical food supplements, by increasing science-based decision-making by regulators and food chain operators. This is further proof that only through international cooperation can the quality, efficacy and safety of herbal products be ensured.
ACKNOWLEDGEMENTS
The study was supported by funds from the University of Bologna.
The authors thank Dr. Federico Falchi and the Pharmacy Student Benedetta Vitamia for their support in editing Figures and in preparing Tables, respectively.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers web site along with the published article.
LIST OF ABBREVIATIONS
- E2
= 17β-estradiol
- EFSA
= European Food Safety Authority
- EMA
= European Medicines Agency
- ER
= Estrogen Receptor
- FDA
= Food and Drug Administration
- HRT
= Hormone Replacement Therapy
- LBD
= Ligand Binding Domain
- RCT
= Randomised Controlled Trial
- SDG
= Secoisolariciresinol Diglucoside
- SERM
= Selective Estrogen Receptor Modulator
- WHI trial
= Women Health Initiative trial
REFERENCES
- 1.Maclennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev. 2004;(4):CD002978. doi: 10.1002/14651858.CD002978.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welton AJ, Vickers MR, Kim J, Ford D, Lawton BA, Maclennan AH, Meredith SK, Martin J, Meade TW. Health related quality of life after combined hormone replacement therapy randomised controlled trial.doi: 10.1136/bmj.a1190. a1190. BMJ. 2008;337:a1190. doi: 10.1136/bmj.a1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiel DP, Felson DT, Anderson JJ, Wilson PW, Moskowitz MA. Hip fracture and the use of estrogens in postmenopausal women.The Framingham Study. N. Engl. J Med. 1987;317(19):1169–1174. doi: 10.1056/NEJM198711053171901. [DOI] [PubMed] [Google Scholar]
- 4.Cauley JA, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR. Estrogen replacement therapy and fractures in older women.Study of Osteoporotic Fractures Research Group. Ann. Intern. Med. 1995;122(1):9–16. doi: 10.7326/0003-4819-122-1-199501010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann. Intern. Med. 2000;133(12):933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 6.Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, Ernster VL, Cummings SR. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann. Intern. Med. 1992;117(12):1016–1037. doi: 10.7326/0003-4819-117-12-1016. [DOI] [PubMed] [Google Scholar]
- 7.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women.Heart and Estrogen progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 8.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen replacement therapy after ischemic stroke. N. Engl. J. Med. 2001;345(17):1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 9.Cherry N, Gilmour K, Hannaford P, Heagerty A, Khan MA, Kitchener H, McNamee R, Elstein M, Kay C, Seif M, Buckley H. Oestrogen therapy for prevention of reinfarction in postmenopausal women a randomised placebo controlled trial. Lancet. 2002;360(9350):2001–2008. doi: 10.1016/s0140-6736(02)12001-0. [DOI] [PubMed] [Google Scholar]
- 10.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 11.Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, Wallace RB, Jackson RD, Pettinger MB, Ridker PM. Inflammatory biomarkers hormone replacement therapy and incident coronary heart disease prospective analysis from the Women's Health Initiative observational study. JAMA. 2002;288(8):980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- 12.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van HL, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 13.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 14.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. Cardiovascular disease outcomes during 6. years of hormone therapy. Heart and Estrogen progestin Replacement Study follow-up (HERS II). JAMA. 2002;288(1):49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Josefson D. FDA issues advice to women taking hormone replacement therapy. Accessed February 25. 2013 [Google Scholar]
- 16.Medicines and Healthcare products Regulatory Agency (MHRA). Risks and benefits of HRT message to health professionals from Dr Pat Troop Deputy Chief Medical Officer. Department of Health and the Committee on Safety of Medicines press release. Accessed February 25. 2013 [Google Scholar]
- 17.Majumdar SR, Almasi EA, Stafford RS. Promotion and prescribing of hormone therapy after report of harm by the Women's Health Initiative. JAMA. 2004;292(16):1983–1988. doi: 10.1001/jama.292.16.1983. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC, Mamdani MM, Tu K, Jaakkimainen L. Prescriptions for estrogen replacement therapy in Ontario before and after publication of the Women's Health Initiative Study. JAMA. 2003;289(24):3241–3242. doi: 10.1001/jama.289.24.3241. [DOI] [PubMed] [Google Scholar]
- 19.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy annual trends and response to recent evidence. JAMA. 2004;291(1):47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 20.Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use results from the National Health and Nutrition Examination Survey 1999-2010. Obstet. Gynecol. 2012;120(3):595–603. doi: 10.1097/AOG.0b013e318265df42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang WF, Tsai YW, Hsiao FY, Liu WC. Changes of the prescription of hormone therapy in menopausal women an observational study in Taiwan. BMC Public Health. 2007;7:56. doi: 10.1186/1471-2458-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hickey M, Davis SR, Sturdee DW. Treatment of menopausal symptoms: what shall we do now. Lancet. 2005;366(9483):409–421. doi: 10.1016/S0140-6736(05)66519-1. [DOI] [PubMed] [Google Scholar]
- 23.Hickey M, Elliott J, Davison SL. Hormone replacement therapy. doi: 10.1136/bmj.e763. e763. BMJ. 2012;344:e763. doi: 10.1136/bmj.e763. [DOI] [PubMed] [Google Scholar]
- 24.Nelson HD, Walker M, Zakher B, Mitchell J. Menopausal hormone therapy for the primary prevention of chronic conditions a systematic review to update the U. . Preventive Services Task Force recommendations. Ann. Intern. Med. 2012;157(2):104–113. doi: 10.7326/0003-4819-157-2-201207170-00466. [DOI] [PubMed] [Google Scholar]
- 25.Moyer VA. Menopausal Hormone Therapy for the Primary Prevention of Chronic Conditions U. . Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2012:10–158. doi: 10.7326/0003-4819-158-1-201301010-00553. [DOI] [PubMed] [Google Scholar]
- 26.Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Datab. Syst. Rev. doi:10.1002/14651858.CD004143.pub4. CD004143. 2012;7:CD004143. doi: 10.1002/14651858.CD004143.pub4. [DOI] [PubMed] [Google Scholar]
- 27.Rada G, Capurro D, Pantoja T, Corbalan J, Moreno G, Letelier LM, Vera C. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane. Database. Syst. Rev. 2010;(9):CD004923. doi: 10.1002/14651858.CD004923.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Setchell KD. Phytoestrogens the biochemistry physiology and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998;68(6 Suppl):1333S–1346S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- 29.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N. Engl. J. Med. 2002;346(5):340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 30.Kingsbury JM. Poisonous Plants of the United States and Canada ed. Prentice-Hall Englewood Cliffs New York. 1964 [Google Scholar]
- 31.Ososki AL, Kennelly EJ. Phytoestrogens a review of the present state of research. Phytother. Res. 2003;17(8):845–869. doi: 10.1002/ptr.1364. [DOI] [PubMed] [Google Scholar]
- 32.HornRoss PL, John EM, Lee M, Stewart SL, Koo J, Sakoda LC, Shiau AC, Goldstein J, Davis P, Perez-Stable EJ. Phytoestrogen consumption and breast cancer risk in a multiethnic population the Bay Area Breast Cancer Study. Am. J. Epidemiol. 2001;154(5):434–441. doi: 10.1093/aje/154.5.434. [DOI] [PubMed] [Google Scholar]
- 33.Kao PC, P'eng FK. How to reduce the risk factors of osteoporosis in Asia. Zhonghua Yi. Xue. Za Zhi. (Taipei). 1995;55(3):209–213. [PubMed] [Google Scholar]
- 34.Reinli K, Block G. Phytoestrogen content of foods a compendium of literature values. Nutr. Cancer. 1996;26(2):123–148. doi: 10.1080/01635589609514470. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Murphy PA. Isoflavone Content in Commercial Soybean Foods. J. Agric. Food Chem. 1994;42(8):1666–1673. [Google Scholar]
- 36.Yu O, Shi J, Hession AO, Maxwell CA, McGonigle B, Odell JT. Metabolic engineering to increase isoflavone biosynthesis in soybean seed. Phytochemistry. 2003;63(7):753–763. doi: 10.1016/s0031-9422(03)00345-5. [DOI] [PubMed] [Google Scholar]
- 37.Wang X. Structure function and engineering of enzymes in isoflavonoid biosynthesis. Funct. Integr. Genomics. 2011;11(1):13–22. doi: 10.1007/s10142-010-0197-9. [DOI] [PubMed] [Google Scholar]
- 38.Yang Z, Kulkarni K, Zhu W, Hu M. Bioavailability and pharmacokinetics of genistein: mechanistic studies on its ADME. Anticancer Agents Med Chem. 2012;12(10):1264–1280. doi: 10.2174/187152012803833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WahajuddinTaneja I, Arora S, Raju KS, Siddiqui N. Disposition of pharmacologically active dietary isoflavones in biological systems. Curr. Drug Metab. 2013;14(4):369–380. doi: 10.2174/1389200211314040002. [DOI] [PubMed] [Google Scholar]
- 40.Kurzer MS, Xu X. Dietary phytoestrogens. Annu. Rev. Nutr. 1997;17:353–81. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- 41.Kelly GE, Joannou GE, Reeder AY, Nelson C, Waring MA. The variable metabolic response to dietary isoflavones in humans. Proc. Soc. Exp. Biol. Med. 1995;208(1):40–43. doi: 10.3181/00379727-208-43829. [DOI] [PubMed] [Google Scholar]
- 42.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002;132(12):3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 43.Yuan JP, Wang JH, Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora implications for health. Mol. Nutr. Food Res. 2007;51(7):765–781. doi: 10.1002/mnfr.200600262. [DOI] [PubMed] [Google Scholar]
- 44.Tham DM, Gardner CD, Haskell WL. Clinical review 97 Potential health benefits of dietary phytoestrogens a review of the clinical epidemiological and mechanistic evidence. J Clin. Endocrinol. Metab. 1998;83(7):2223–2235. doi: 10.1210/jcem.83.7.4752. [DOI] [PubMed] [Google Scholar]
- 45.Heinonen S, Wahala K, Adlercreutz H. Identification of isoflavone metabolites dihydrodaidzein dihydrogenistein 6'-OH-O-dma and cis-4-OH-equol in human urine by gas chromatography mass spectroscopy using authentic reference compounds. Anal. Biochem. 1999;274(2):211–219. doi: 10.1006/abio.1999.4279. [DOI] [PubMed] [Google Scholar]
- 46.Meagher LP, Bentz EK. Assessment of data on the lignan content of foods. J Food Compos Anal. 2000;13(6):935–947. [Google Scholar]
- 47.Kuijsten A, Arts IC, van't VP, Hollman PC. The relative bioavailability of enterolignans in humans is enhanced by milling and crushing of flaxseed. J Nutr. 2005;135(12):2812–2816. doi: 10.1093/jn/135.12.2812. [DOI] [PubMed] [Google Scholar]
- 48.Davin LB, Lewis NG. Dirigent proteins and dirigent sites explain the mystery of specificity of radical precursor coupling in lignan and lignin biosynthesis. Plant Physiol. 2000;123(2):453–462. doi: 10.1104/pp.123.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moinuddin SG, Youn B, Bedgar DL, Costa MA, Helms GL, Kang C, Davin LB, Lewis NG. Secoisolariciresinol dehydrogenase mode of catalysis and stereospecificity of hydride transfer in Podophyllum peltatum. Org. Biomol. Chem. 2006;4(5):808–816. doi: 10.1039/b516563f. [DOI] [PubMed] [Google Scholar]
- 50.Ford JD, Huang KS, Wang HB, Davin LB, Lewis NG. Biosynthetic pathway to the cancer chemopreventive secoisolariciresinol diglucoside-hydroxymethyl glutaryl ester-linked lignan oligomers in flax (Linum usitatissimum) seed. J Nat. Prod. 2001;64(11):1388–1397. doi: 10.1021/np010367x. [DOI] [PubMed] [Google Scholar]
- 51.Clavel T, Borrmann D, Braune A, Dore J, Blaut M. Occurrence and activity of human intestinal bacteria involved in the conversion of dietary lignans. Anaerobe. 2006;12(3):140–147. doi: 10.1016/j.anaerobe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 52.ChungJa J, VasanthaRupasinghe H. Tables of Isoflavone, Coumestan, and Lignan Data. In Phytoestrogens and Health ed. Edited by Anderson J.J.B. Sarwar Gilani G. AOCS Publishing. 2002 [Google Scholar]
- 53.Raffaelli B, Hoikkala A, Leppala E, Wahala K. Enterolignans. J Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;777(1-2):29–43. doi: 10.1016/s1570-0232(02)00092-2. [DOI] [PubMed] [Google Scholar]
- 54.Stansbury JE. Phytoestrogen Review. Accessed April 9. 2013 [Google Scholar]
- 55.Prasad K. Hydroxyl radical-scavenging property of secoisolariciresinol diglucoside (SDG) isolated from flax-seed. Mol. Cell Biochem. 1997;168(1-2):117–123. doi: 10.1023/a:1006847310741. [DOI] [PubMed] [Google Scholar]
- 56.Martin M, Dewick PM. Biosynthesis of pterocarpan isoflavan and coumestan metabolites of Medicago sativa. The role of an isoflav-3-ene. Phytochemistry. 1980;19:2341–2346. [Google Scholar]
- 57.Price KR, Fenwick GR. Naturally occurring oestrogens in foods a review. Food Addit. Contam. 1985;2(2):73–106. doi: 10.1080/02652038509373531. [DOI] [PubMed] [Google Scholar]
- 58.Harbourne JB, Mabry TJ, Mabry H, Wong E. The Isoflavonoids. In The Flavonoids ed. Edited by Chapman & Hall London. 1975:780–784. [Google Scholar]
- 59.Bickoff EM, Booth AN, Lyman RL, Livingston AL, Thompson CR, Deeds F. Coumestrol a new estrogen isolated from forage crops. Science. 1957;126(3280):969–970. doi: 10.1126/science.126.3280.969-a. [DOI] [PubMed] [Google Scholar]
- 60.Franke AA, Custer LJ, Cerna CM, Narala K. Rapid HPLC analysis of dietary phytoestrogens from legumes and from human urine. Proc. Soc. Exp. Biol. Med. 1995;208(1):18–26. doi: 10.3181/00379727-208-43826. [DOI] [PubMed] [Google Scholar]
- 61.Fristsche S, Steinhart H. Occurrence of Hormonally Active Compounds in Food. A Review. Eur. Food Res. Techn. 1999;209:153–179. [Google Scholar]
- 62.Lorand T, Vigh E, Garai J. Hormonal action of plant derived and anthropogenic non-steroidal estrogenic compounds phytoestrogens and xenoestrogens. Curr. Med Chem. 2010;17(30):3542–3574. doi: 10.2174/092986710792927813. [DOI] [PubMed] [Google Scholar]
- 63.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 64.Milligan SR, Kalita JC, Heyerick A, Rong H, De CL, De KD. Identification of a potent phytoestrogen in hops (Humulus lupulus L. and beer. J Clin. Endocrinol. Metab. 1999;84(6):2249–2252. doi: 10.1210/jcem.84.6.5887. [DOI] [PubMed] [Google Scholar]
- 65.Gao L, Tu Y, Agren H, Eriksson LA. Characterization of Agonist Binding to His524 in the Estrogen Receptor alpha Ligand Binding Domain. J Phys. Chem. B. 2012 doi: 10.1021/jp300895g. [DOI] [PubMed] [Google Scholar]
- 66.Manas ES, Xu ZB, Unwalla RJ, Somers WS. Understanding the selectivity of genistein for human estrogen receptor-beta using X-ray crystallography and computational methods. Structure. 2004;12(12):2197–2207. doi: 10.1016/j.str.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 67.Kostelac D, Rechkemmer G, Briviba K. Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element. J Agric. Food Chem. 2003;51(26):7632–7635. doi: 10.1021/jf034427b. [DOI] [PubMed] [Google Scholar]
- 68.Oseni T, Patel R, Pyle J, Jordan VC. Selective estrogen receptor modulators and phytoestrogens. Planta Med. 2008;74:1656–1665. doi: 10.1055/s-0028-1088304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klein CB, King AA. Genistein genotoxicity critical considerations of in vitro exposure dose. Toxicol. Appl. Pharmacol. 2007;224(1):1–11. doi: 10.1016/j.taap.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 70.Polkowski K, Mazurek AP. Biological properties of genistein.A review of in vitro and in vivo data. Acta Pol. Pharm. 2000;57(2):135–155. [PubMed] [Google Scholar]
- 71.Hutchins AM, Slavin JL. In Flaxseed in Human Nutrition . 2nd ed. Champaign Illinois.: Edited by Thompson L.U.C.S.C. AOCS Press. ; 2003. Effects of flaxseed on sex hormone metabolism. pp. 126–149. [Google Scholar]
- 72.Karkola S, Wahala K. The binding of lignans flavonoids and coumestrol to CYP450 aromatase a molecular modelling study. Mol. Cell Endocrinol. 2009;301(1-2):235–244. doi: 10.1016/j.mce.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Overk CR, Yao P, Chadwick LR, Nikolic D, Sun Y, Cuendet MA, Deng Y, Hedayat AS, Pauli GF, Farnsworth NR, vanBreemen RB, Bolton JL. Comparison of the in vitro estrogenic activities of compounds from hops (Humulus lupulus) and red clover (Trifolium pratense). J Agric. Food Chem. 2005;53(16):6246–6253. doi: 10.1021/jf050448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mersereau JE, Levy N, Staub RE, Baggett S, Zogovic T, Chow S, Ricke WA, Tagliaferri M, Cohen I, Bjeldanes LF, Leitman DC. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol. Cell Endocrinol. 2008;283(1-2):49–57. doi: 10.1016/j.mce.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bagchi D, Das DK, Tosaki A, Bagchi M, Kothari SC. Benefits of resveratrol in women's health. Drugs Exp. Clin. Res. 2001;27(5-6):233–248. [PubMed] [Google Scholar]
- 76.Johannes CB, Crawford SL, Posner JG, McKinlay SM. Longitudinal patterns and correlates of hormone replacement therapy use in middle-aged women. Am. J Epidemiol. 1994;140(5):439–452. doi: 10.1093/oxfordjournals.aje.a117266. [DOI] [PubMed] [Google Scholar]
- 77.Kronenberg F. Hot flashes epidemiology and physiology. Ann. N. Y. Acad. Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. [DOI] [PubMed] [Google Scholar]
- 78.Newton KM, Buist DS, Keenan NL, Anderson LA, LaCroix AZ. Use of alternative therapies for menopause symptoms results of a population based survey. Obstet. Gynecol. 2002;100(1):18–25. doi: 10.1016/s0029-7844(02)02005-7. [DOI] [PubMed] [Google Scholar]
- 79.Miller PE, Snyder DC. Phytochemicals and cancer risk a review of the epidemiological evidence. Nutr. Clin. Pract. 2012;27(5):599–612. doi: 10.1177/0884533612456043. [DOI] [PubMed] [Google Scholar]
- 80.Leclercq G, Jacquot Y. Interactions of isoflavones and other plant derived estrogens with estrogen receptors for prevention and treatment of breast cancer.Considerations concerning related efficacy and safety. J. Steroid Biochem. Mol. Biol. 2012 doi: 10.1016/j.jsbmb.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 81.Jungbauer A, Medjakovic S. Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas. 2012;71(3):227–239. doi: 10.1016/j.maturitas.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 82.Lee YB, Lee HJ, Sohn HS. Soy isoflavones and cognitive function. J Nutr. Biochem. 2005;16(11):641–649. doi: 10.1016/j.jnutbio.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 83.Bedell S, Nachtigall M, Naftolin F. The pros and cons of plant estrogens for menopause. J. Steroid Biochem. Mol. Biol. 2012 doi: 10.1016/j.jsbmb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 84.Lagari VS, Levis S. Phytoestrogens for menopausal bone loss and climacteric symptoms. J. Steroid Biochem. Mol. Biol. 2012 doi: 10.1016/j.jsbmb.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Eden JA. Phytoestrogens for menopausal symptoms a review. Maturitas. 2012;72(2):157–159. doi: 10.1016/j.maturitas.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 86.Lethaby AE, Brown J, Marjoribanks J, Kronenberg F, Roberts H, Eden J. Phytoestrogens for vasomotor menopausal symptoms. Cochrane. Database. Syst. Rev. 2007;(4):CD001395. doi: 10.1002/14651858.CD001395.pub3. [DOI] [PubMed] [Google Scholar]
- 87.Villaseca P. Non-estrogen conventional and phytochemical treatments for vasomotor symptoms what needs to be known for practice. Climacteric. 2012;15(2):115–124. doi: 10.3109/13697137.2011.624214. [DOI] [PubMed] [Google Scholar]
- 88.Atmaca A, Kleerekoper M, Bayraktar M, Kucuk O. Soy isoflavones in the management of postmenopausal osteoporosis. Menopause. 2008;15(4 Pt 1):748–757. doi: 10.1097/gme.0b013e31815c1e7f. [DOI] [PubMed] [Google Scholar]
- 89.Poulsen RC, Kruger MC. Soy phytoestrogens impact on postmenopausal bone loss and mechanisms of action. Nutr. Rev. 2008;66(7):359–374. doi: 10.1111/j.1753-4887.2008.00046.x. [DOI] [PubMed] [Google Scholar]
- 90.Taku K, Melby MK, Nishi N, Omori T, Kurzer MS. Soy isoflavones for osteoporosis an evidence-based approach. Maturitas. 2011;70(4):333–338. doi: 10.1016/j.maturitas.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 91.Andres S, Abraham K, Appel KE, Lampen A. Risks and benefits of dietary isoflavones for cancer. Crit Rev. Toxicol. 2011;41(6):463–506. doi: 10.3109/10408444.2010.541900. [DOI] [PubMed] [Google Scholar]
- 92.Tempfer CB, Froese G, Heinze G, Bentz EK, Hefler LA, Huber JC. Side effects of phytoestrogens a meta-analysis of randomized trials. Am. J. Med. 2009;122(10):939–946. doi: 10.1016/j.amjmed.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 93.The european parliament and the council of the european union. directive 2004/24/ec of the european parlia-ment and of the council. Accessed February 25. 2013 [Google Scholar]
- 94.The european parliament and the council of the european union. directive 2001/83/ec of the european parlia-ment and of the council. Accessed February 25. 2013 [Google Scholar]
- 95.Q&A Registration of Traditional Herbal Medicinal Products. Accessed April 9. 2013 [Google Scholar]
- 96.European Medicines Agency EMA. Herbal medicines for human use. Accessed February 25. 2013 [Google Scholar]
- 97.The european parliament and the council of the european union.directive 2002/46/ec of the european parlia-ment and of the council. Accessed February 25. 2013 [Google Scholar]
- 98.The european parliament and the council of the european union. regulation (ec) no 1924/2006 of the european parliament and of the council. Accessed February 25. 2013 [Google Scholar]
- 99.EFSA Panel on Dietetic Products N..A.N. . Scientific Opinion on the substantiation of health claims related to soy isoflavones and maintenance of bone mineral density (ID 1655) and reduction of vasomotor symptoms associated with menopause (ID 1654. 1704. 2140. 3093. 3154. 3590) (further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. Accessed February 25. 2013 [Google Scholar]
- 100.EFSA Panel on Dietetic Products N..A.N. . Scientific Opinion on the substantiation of health claims related to soy isoflavones and protection o DNA proteins and lipids from oxidative damage (ID 1286. 4245). maintenance of normal blood LDL-cholesterol concentrations (ID 1135. 1704a, 3093a). reduction of vasomotor symptoms associated with menopause (ID 1654. 1704b. 2140. 3093b. 3154. 3590). maintenance of normal skin tonicity (ID 1704a) contribution to normal hair growth (ID 1704a 4254) "cardiovascular health" (ID 3587) treatment of prostate cancer (ID 3588) and "upper respiratory tract" (ID 3589) pursuant to Article 13(1) of Regulation. (EC) No 1924/20061. Accessed February 25. 2013 [Google Scholar]
- 101.Panel on Dietetic Products N..A. . Scientific substantiation of a health claim related to "Femarelle®" and "induces bone formation and increases bone mineral density reducing the risk for osteoporosis and other bone disorders" pursuant to Article 14 of the Regulation (EC) No 1924/2006. Accessed February 25. 2013 [Google Scholar]
- 102.EFSA Panel on Dietetic Products N..A. Scientific Opinion on the substantiation of a health claim related to soy protein and reduction of blood cholesterol concentrations pursuant to Article 14 of the Regulation (EC) No 1924/2006. Accessed February 25. 2013 [Google Scholar]
- 103.EFSA Panel on Dietetic Products N..A. . Scientific Opinion on the substantiation of a health claim related to isolated soy protein and reduction of blood LDL cholesterol concentrations ursuant to Article 14 of Regulation (EC) No 1924/2006. Accessed February 25. 2013 [Google Scholar]
- 104.U..Department of Health and Human Services Food and Drug Administration. Center for Food Safety and Applied Nutrition. Draft Guidance for Industry Dietary Supplements. New Dietary Ingredient Notifications and Related Issues. Accessed February 25. 2013 [Google Scholar]
- 105.U..Department of Health and Human Services Food and Drug Administration Center for Food Safety and Applied Nutrition. Guidance for Industry Evidence-Based Review System for the Scientific Evaluation of Health Claims Final. Accessed February 25. 2013 [Google Scholar]
- 106.Reichenbach S, Juni P. Medical food and food supplements not always as safe as generally assumed. Ann. Intern. Med. 2012;156(12):894–5. doi: 10.7326/0003-4819-156-12-201206190-00012. [DOI] [PubMed] [Google Scholar]
- 107.Vatistas TJ, Samuels JG. The regulation of dietary supplements in the United States advocating for a reasonable approach protecting patient safety and the role of nursing. Policy Polit. Nurs. Pract. 2012;13(2):113–116. doi: 10.1177/1527154412456706. [DOI] [PubMed] [Google Scholar]
- 108.U.. Food and Drug Administration FDA. Cyber Letters. Accessed February 25. 2013 [Google Scholar]
- 109.U.. Department of Health and Human Services Food and Drug Administration. Center for Food Safety and Applied Nutrition. Ref. No. CL-05-HFS-810-155. Accessed February 25. 2013 [Google Scholar]
- 110.U.. Department of Health and Human Services Food and Drug Administration. Center for Food Safety and Applied Nutrition. Letter to Elliott Goodman (IntraCell Nutrition Inc) - October 12. 2005 Ref. No. CL-05-HFS-810-203. Accessed February 25. 2013 [Google Scholar]
- 111.U.. Department of Health and Human Services Food and Drug Administration. Center for Food Safety and Applied Nutrition. WARNING LETTER to Tip Top Vitamins November 9. 2005. Accessed February 25. 2013 [Google Scholar]
- 112.U.. Department of Health and Human Services Food and Drug Administration. Center for Food Safety and Applied Nutrition. WARNING LETTER To Roger Howard and JoAnn Howar (Healthy Days Inc.) November 9. 2005. Accessed February 25. 2013 [Google Scholar]
- 113.Vitale DC, Piazza C, Melilli B, Drago F, Salomone S. Isoflavones estrogenic activity biological effect and bioavailability. Eur. J. Drug Metab Pharmacokinet. 2012 doi: 10.1007/s13318-012-0112-y. [DOI] [PubMed] [Google Scholar]
- 114.de CP, This P, Leclercq G, Jacquot Y. Controversies concerning the use of phytoestrogens in menopause management bioavailability and metabolism. Maturitas. 2010;65(4):334–339. doi: 10.1016/j.maturitas.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 115.Larkin T, Price WE, Astheimer L. The key importance of soy isoflavone bioavailability to understanding health benefits. Crit Rev. Food Sci. Nutr. 2008;48(6):538–552. doi: 10.1080/10408390701542716. [DOI] [PubMed] [Google Scholar]
- 116.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132(12):3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 117.Setchell KD, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, Kirschner AS, Heubi JE. Evidence for lack of absorption of soy isoflavone glycosides in humans supporting the crucial role of intestinal metabolism for bioavailability. Am. J Clin. Nutr. 2002;76(2):447–453. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]
- 118.Boulton DW, Walle UK, Walle T. Extensive binding of the bioflavonoid quercetin to human plasma proteins. J Pharm. Pharmacol. 1998;50(2):243–249. doi: 10.1111/j.2042-7158.1998.tb06183.x. [DOI] [PubMed] [Google Scholar]
- 119.Okura T, Ibe M, Umegaki K, Shinozuka K, Yamada S. Effects of dietary ingredients on function and expression of P-glycoprotein in human intestinal epithelial cells. Biol. Pharm. Bull. 2010;33(2):255–259. doi: 10.1248/bpb.33.255. [DOI] [PubMed] [Google Scholar]
- 120. clinicaltrial.ov. Accessed March 1. 2013 [Google Scholar]
- 121.PlantLIBRA (PLANT food supplements Levels of Intake Benefit and Risk Assessment). www.plantlibra.eu. Accessed March 1. 2013.
- 122.Horn-Ross PL. Phytoestrogens body composition and breast cancer. Cancer Causes Control. 1995;6(6):567–573. doi: 10.1007/BF00054166. [DOI] [PubMed] [Google Scholar]
- 123.Herrington D. Role of estrogens selective estrogen receptor modulators and phytoestrogens in cardiovascular protection. Can. J. Cardiol. 2000;16(Suppl E):5E–9E. [PubMed] [Google Scholar]
- 124.Kim H, Xia H, Li L, Gewin J. Attenuation of neurodegeneration-relevant modifications of brain proteins by dietary soy. Biofactors. 2000;12(1-4):243–250. doi: 10.1002/biof.5520120137. [DOI] [PubMed] [Google Scholar]
- 125.Arjmandi BH. The role of phytoestrogens in the prevention and treatment of osteoporosis in ovarian hormone deficiency. J. Am. Coll. Nutr. 2001;20(5 Suppl):398S–402S. doi: 10.1080/07315724.2001.10719175. [DOI] [PubMed] [Google Scholar]
- 126.Glazier MG, Bowman MA. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch. Intern. Med. 2001;161(9):1161–1172. doi: 10.1001/archinte.161.9.1161. [DOI] [PubMed] [Google Scholar]
- 127.Ariyo AA, Villablanca AC. Estrogens and lipids.Can HRT designer estrogens and phytoestrogens reduce cardiovascular risk markers after menopause. Postgrad. Med. 2002;111(1):23–30. doi: 10.3810/pgm.2002.01.1085. [DOI] [PubMed] [Google Scholar]
- 128.Ewies AA. Phytoestrogens in the management of the menopause up-to-date. Obstet. Gynecol. Surv. 2002;57(5):306–313. doi: 10.1097/00006254-200205000-00023. [DOI] [PubMed] [Google Scholar]
- 129.Lord RS, Bongiovanni B, Bralley JA. Estrogen metabolism and the diet-cancer connection rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern. Med. Rev. 2002;7(2):112–129. [PubMed] [Google Scholar]
- 130.Messina MJ. Soy foods and soybean isoflavones and menopausal health. Nutr. Clin. Care. 2002;5(6):272–282. doi: 10.1046/j.1523-5408.2002.05602.x. [DOI] [PubMed] [Google Scholar]
- 131.Phipps WR, Duncan AM, Kurzer MS. Isoflavones and postmenopausal women a critical review. Treat. Endocrinol. 2002;1(5):293–311. doi: 10.2165/00024677-200201050-00003. [DOI] [PubMed] [Google Scholar]
- 132.Jacquot Y, Rojas C, Refouvelet B, Robert JF, Leclercq G, Xicluna A. Recent advances in the development of phytoestrogens and derivatives an update of the promising perspectives in the prevention of postmenopausal diseases. Mini. Rev. Med. Chem. 2003;3(5):387–400. doi: 10.2174/1389557033488006. [DOI] [PubMed] [Google Scholar]
- 133.Altavilla D, Crisafulli A, Marini H, Esposito M, D'Anna R, Corrado F, Bitto A, Squadrito F. Cardiovascular effects of the phytoestrogen genistein. Curr. Med. Chem. Cardiovasc. Hematol. Agents. 2004;2(2):179–186. doi: 10.2174/1568016043477297. [DOI] [PubMed] [Google Scholar]
- 134.Limer JL, Speirs V. Phyto-oestrogens and breast cancer chemoprevention. Breast Cancer Res. 2004;6(3):119–127. doi: 10.1186/bcr781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McCue P, Shetty K. Health benefits of soy isoflavonoids and strategies for enhancement a review. Crit Rev. Food Sci. Nutr. 2004;44(5):361–367. doi: 10.1080/10408690490509591. [DOI] [PubMed] [Google Scholar]
- 136.Messina M, Ho S, Alekel DL. Skeletal benefits of soy isoflavones a review of the clinical trial and epidemiologic data. Curr. Opin. Clin. Nutr. Metab Care. 2004;7(6):649–658. doi: 10.1097/00075197-200411000-00010. [DOI] [PubMed] [Google Scholar]
- 137.Nandur R, Kumar K, Villablanca AC. Cardiovascular actions of selective estrogen receptor modulators and phytoestrogens. Prev. Cardiol. 2004;7(2):73–79. doi: 10.1111/j.1520-037x.2006.2527.x. [DOI] [PubMed] [Google Scholar]
- 138.Gikas PD, Mokbel K. Phytoestrogens and the risk of breast cancer a review of the literature. Int. J. Fertil. Womens Med. 2005;50(6):250–258. [PubMed] [Google Scholar]
- 139.Mahady GB. Do soy isoflavones cause endometrial hyperplasia. Nutr. Rev. 2005;63(11):392–397. doi: 10.1111/j.1753-4887.2005.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 140.Viereck V, Emons G, Wuttke W. Black cohosh just another phytoestrogen. Trends Endocrinol. Metab. 2005;16(5):214–221. doi: 10.1016/j.tem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 141.Cassidy A, Albertazzi P, Lise NI, Hall W, Williamson G, Tetens I, Atkins S, Cross H, Manios Y, Wolk A, Steiner C, Branca F. Critical review of health effects of soyabean phyto-oestrogens in post menopausal women. Proc. Nutr. Soc. 2006;65(1):76–92. doi: 10.1079/pns2005476. [DOI] [PubMed] [Google Scholar]
- 142.Geller SE, Studee L. Soy and red clover for mid-life and aging. Climacteric. 2006;9(4):245–263. doi: 10.1080/13697130600736934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Howes LG, Howes JB, Knight DC. Isoflavone therapy for menopausal flushes a systematic review and meta-analysis. Maturitas. 2006;55(3):203–211. doi: 10.1016/j.maturitas.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 144.Qin LQ, Xu JY, Wang PY, Hoshi K. Soyfood intake in the prevention of breast cancer risk in women a meta analysis of observational epidemiological studies. J. Nutr. Sci. Vitaminol. (Tokyo). 2006;52(6):428–436. doi: 10.3177/jnsv.52.428. [DOI] [PubMed] [Google Scholar]
- 145.Usui T. Pharmaceutical prospects of phytoestrogens. Endocr. J. 2006;53(1):7–20. doi: 10.1507/endocrj.53.7. [DOI] [PubMed] [Google Scholar]
- 146.Vafeiadou K, Hall WL, Williams CM. Does genotype and equol-production status affect response to isoflavones.Data from a pan-European study on the effects of isoflavones on cardiovascular risk markers in post-menopausal women. Proc. Nutr. Soc. 2006;65(1):106–115. doi: 10.1079/pns2005483. [DOI] [PubMed] [Google Scholar]
- 147.Duffy C, Perez K, Partridge A. Implications of phytoestrogen intake for breast cancer. CA Cancer J. Clin. 2007;57(5):260–277. doi: 10.3322/CA.57.5.260. [DOI] [PubMed] [Google Scholar]
- 148.Messina MJ, Wood CE. Soy isoflavones estrogen therapy and breast cancer risk analysis and commentary.doi: 10.1186/1475-2891-7-17. 17. Nutr. J. 2008;7:17. doi: 10.1186/1475-2891-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, Cassidy A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre and post-menopausal women a systematic review and meta-analysis. Hum. Reprod. Update. 2009;15(4):423–440. doi: 10.1093/humupd/dmp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Velentzis LS, Cantwell MM, Cardwell C, Keshtgar MR, Leathem AJ, Woodside JV. Lignans and breast cancer risk in pre- and post-menopausal women meta-analyses of observational studies. Br. J. Cancer. 2009;100(9):1492–1498. doi: 10.1038/sj.bjc.6605003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Buck K, Zaineddin AK, Vrieling A, Linseisen J, Chang-Claude J. Meta-analyses of lignans and enterolignans in relation to breast cancer risk. Am. J. Clin. Nutr. 2010;92(1):141–153. doi: 10.3945/ajcn.2009.28573. [DOI] [PubMed] [Google Scholar]
- 152.Cano A, Garcia-Perez MA, Tarin JJ. Isoflavones and cardiovascular disease. Maturitas. 2010;67(3):219–226. doi: 10.1016/j.maturitas.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 153.Hooper L, Madhavan G, Tice JA, Leinster SJ, Cassidy A. Effects of isoflavones on breast density in pre and post-menopausal women a systematic review and meta-analysis of randomized controlled trials. Hum. Reprod. Update. 2010;16(6):745–760. doi: 10.1093/humupd/dmq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li SH, Liu XX, Bai YY, Wang XJ, Sun K, Chen JZ, Hui RT. Effect of oral isoflavone supplementation on vascular endothelial function in postmenopausal women a meta-analysis of randomized placebo-controlled trials. Am. J. Clin. Nutr. 2010;91(2):480–486. doi: 10.3945/ajcn.2009.28203. [DOI] [PubMed] [Google Scholar]
- 155.Ricci E, Cipriani S, Chiaffarino F, Malvezzi M, Parazzini F. Soy isoflavones and bone mineral density in perimenopausal and postmenopausal Western women a systematic review and meta-analysis of randomized controlled trials. J. Womens Health (Larchmt ). 2010;19(9):1609–1617. doi: 10.1089/jwh.2010.2021. [DOI] [PubMed] [Google Scholar]
- 156.Ricci E, Cipriani S, Chiaffarino F, Malvezzi M, Parazzini F. Effects of soy isoflavones and genistein on glucose metabolism in perimenopausal and postmenopausal non-Asian women a meta-analysis of randomized controlled trials. Menopause. 2010;17(5):1080–1086. doi: 10.1097/gme.0b013e3181dd05a9. [DOI] [PubMed] [Google Scholar]
- 157.Dong JY, Wang P, He K, Qin LQ. Effect of soy isoflavones on circulating C-reactive protein in post-menopausal women meta-analysis of randomized controlled trials. Menopause. 2011;18(11):1256–1262. doi: 10.1097/gme.0b013e31821bfa24. [DOI] [PubMed] [Google Scholar]
- 158.Salari SP, Nikfar S, Abdollahi M. Prevention of bone resorption by intake of phytoestrogens in postmenopausal women a meta-analysis. Age (Dordr). 2011;33(3):421–431. doi: 10.1007/s11357-010-9180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Williamson G, Coppens P, Serra-Majem L, Dew T. Review of the efficacy of green tea, isoflavones and aloe vera supplements based on randomised controlled trials. Food Funct. 2011;2(12):753–759. doi: 10.1039/c1fo10101c. [DOI] [PubMed] [Google Scholar]
- 160.Zaineddin AK, Vrieling A, Buck K, Becker S, Linseisen J, Flesch-Janys D, Kaaks R, Chang-Claude J. Serum enterolactone and postmenopausal breast cancer risk by estrogen progesterone and herceptin 2 receptor status. Int. J. Cancer. 2012;130(6):1401–1410. doi: 10.1002/ijc.26157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publishers web site along with the published article.