Abstract
The design, synthesis, and biological evaluation of a novel class of C13-diversified bryostatin analogues are described. An innovative and general strategy based on a Prins macrocyclization-nucleophilic trapping cascade was used to achieve late-stage diversification. In vitro analysis of selected library members revealed that modification at the C13 position of the bryostatin scaffold can be used as a diversification handle to regulate biological activity.
Keywords: macrocycles, cyclizations, drugs, polyketides
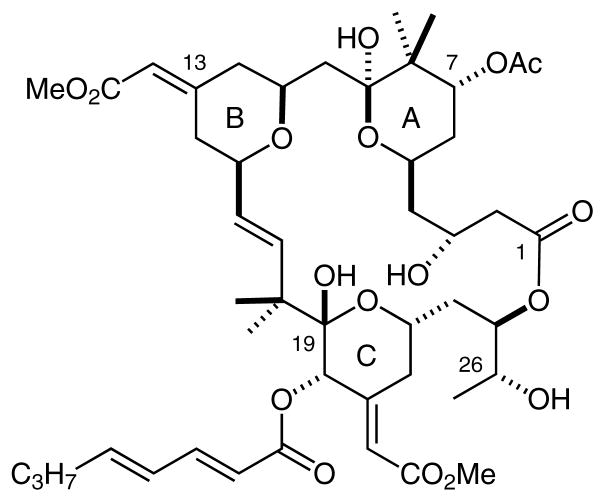
Bryostatin 1 is a marine-derived macrocyclic polyketide isolated from the bryozoan Bugula neritina (Figure 1).1 It exhibits clinically relevant, highly potent, and diverse biological activities,2 and is currently undergoing Phase I and Phase II clinical trials for treatment of a variety of cancers as well as Alzheimer’s disease.3 Bryostatin 1 has also been shown to reverse multidrug resistance,4 to promote immunostimulant effects,5 to enhance memory and learning in animal models,6 and to induce repair of neuronal damage resulting from stroke.7 More recently, bryostatin has also been shown to induce clearance of HIV viral reservoirs, an activity that could figure in the as yet unachieved goal of eradicating HIV/AIDS.8
Figure 1.
Bryostatin 1 (PKC binding affinity Ki = 1.4 nM)
The unique biological activities of this natural product might arise in part from its ability to target and modulate the protein kinase C (PKC) family of serine/threonine kinases. 9 PKCs are implicated in a wide range of cellular signal transduction cascades, including regulation of cell growth, modulation of cell membrane structure, and control of transcription. Dysfunction of certain PKC isozymes is implicated in a variety of pathologies.10 Although many ligands for PKC serve as kinase inhibitors at the ATP binding site, bryostatin 1 binds to the C1 regulatory domain, permitting up- or down-regulation of particular PKC isozymes. This additional activity of the natural product has many functional therapeutic ramifications, including induction of apoptosis in cancer cells, restoration of kinase activity in various disorders, and transcriptional regulation. In addition to interacting with PKC, bryostatin 1 also interacts with other C1-domain-containing proteins. For example, the natural product and its analogues have been shown to activate RasGRP1 in cultured T cells,11 and a recent study has linked RasGRP activation with apoptotic induction in Toledo non-Hodgkin’s lymphoma.12
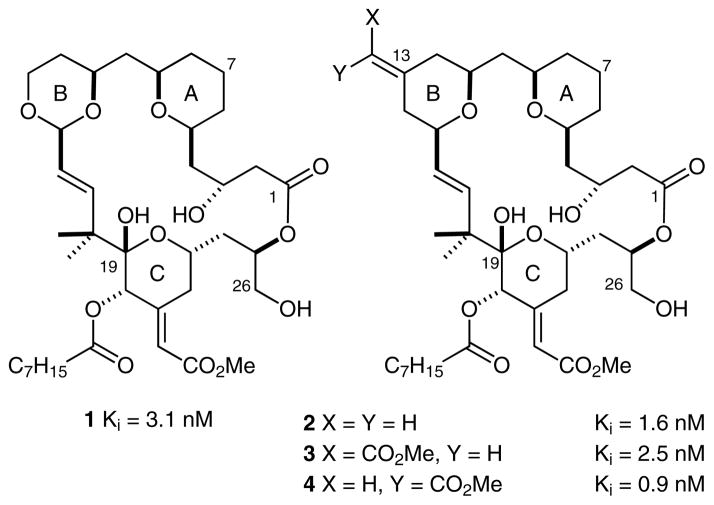
Despite its therapeutic potential, bryostatin 1, like many natural products, is isolated in variable low yields (1.4 × 10−4%) yields from its source.1 In addition, although several members of the bryostatin family (bryostatins 1,13 2,14 3,15 7,16 9,17 and 1618) have been prepared by total synthesis during the past two decades, from about 40 to as many as 89 steps have been required to produce the natural products. In 1988, anticipating the problems associated with obtaining a practical supply of bryostatin and with the view that bryostatin was not evolved for clinical use, we initiated an alternative approach to address problems associated with both its supply and its potential clinical performance.19 Through bioassay-informed computer modeling, our function-oriented synthesis (FOS) strategy20 sought to identify those structural features of the natural product that might affect its biological activity. The resulting hypotheses were then used to design new structures with potentially superior activities that might be produced in a practical manner.21 By using this computer-assisted synthesis-informed design approach, we reported in 1998 the first designed analogues of bryostatin (bryologs) with potencies that match that of bryostatin.22 We subsequently reported several analogues (Figure 2) whose potencies exceed that of the natural product while emulating its functional activity in various biological assays. Significantly, these analogues can be prepared in far fewer steps than required to synthesize the natural product (19 longest linear steps, 29 total steps).23 Importantly, this FOS strategy provides access to analogues that exhibit PKC isoform selectivities corresponding to that of the natural product, as well as access to other analogues that exhibit unique selectivities that are not observed with the natural products but which might be exploited as tools or leads in various preclinical studies.24
Figure 2.
PKC binding affinities of synthetic analogues of bryostatin 1
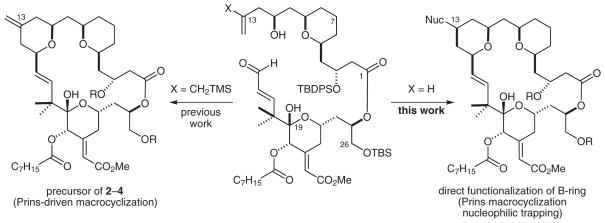
We recently showed that a Prins-driven macrocyclization reaction, a variant of our initially reported macrotransace-talization strategy, can be used to prepare a new class of B-ring tetrahydropyran (THP)-based bryostatin analogues. 25 This transformation is presumed to proceed through formation of an oxocarbenium ion (as inferred in our earlier work) that is subsequently captured by an allylsilane nucleophile to furnish a C13-substituted exocyclic olefin (Scheme 1).26 Deprotection of this compound with hydrogen fluoride·pyridine gives analogue 2, which can be elaborated by selective ozonolysis and Horner–Wadsworth–Emmons olefination, followed by global deprotection, to give bryostatin analogues 3 and 4. Importantly, the THP-based B-ring bryostatin analogues exhibit significant potencies that exceed the in vitro antiproliferative activity of 1 by up to two orders of magnitude. In addition, in terms of its PKC binding affinity, the natural C13-(Z)-enoate 4 is one of the most active analogues that has been discovered to date.
Scheme 1.
Synthetic strategies for preparing substituted THP-derived B-ring bryostatin analogues
Diversification at C13, which is needed to explore the role of this center in PKC activity, required cleavage of the C13 exo-alkene, ketone reduction, alcohol activation, and nucleophilic substitution. We reasoned that a Prins macrocyclization–nucleophilic trapping process would, in a single operation, form the macrocycle, assemble the B-ring, establish two new stereocenters, and permit incorporation of various functional groups at C13 (Scheme 1).27 Although the Prins macrocyclization reaction is emerging as a versatile synthesis strategy that has been used successfully in our work17,25a and in syntheses of kendomycin, 28 neopeltolide,29 and polycavernoside A,30 the reaction has not been used to diversify a lead scaffold, a strategy that might be exploited in advancing many natural hydropyranyl lead compounds.31 In the current study, we sought to explore the applicability of Prins macrocyclization–nucleophilic trapping methods in synthesizing bryostatin scaffolds, with the aims of establishing whether modifications to the THP-based B-ring confer advantageous biological activities, such as increased potency, efficacy, or therapeutic index, and of exploring the generality of this diversification strategy. These studies were also conducted to set the stage for positron emission tomography (PET) and NMR labeling studies on the structures and functions of PKC–ligand complexes. Here, we report the successful implementation of this strategy through the efficient and convergent synthesis of a novel class of C13-diversified bryostatin analogues, together with their preliminary biological evaluation.
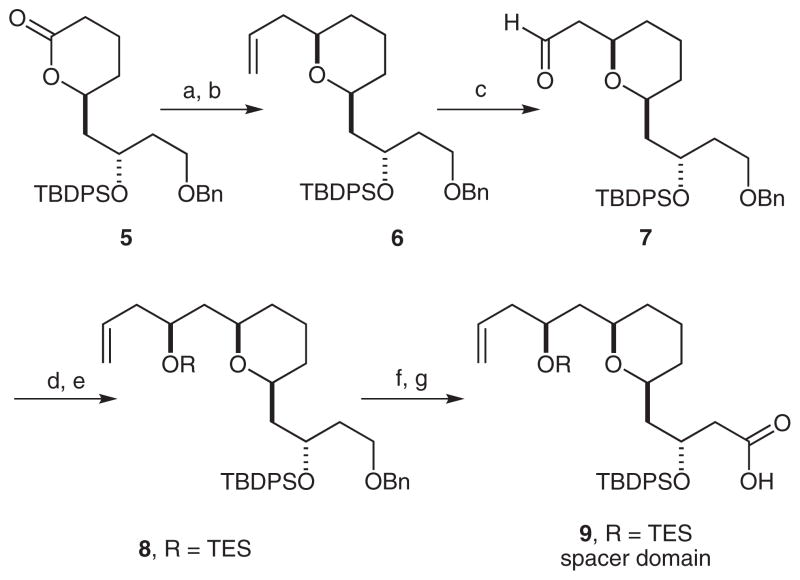
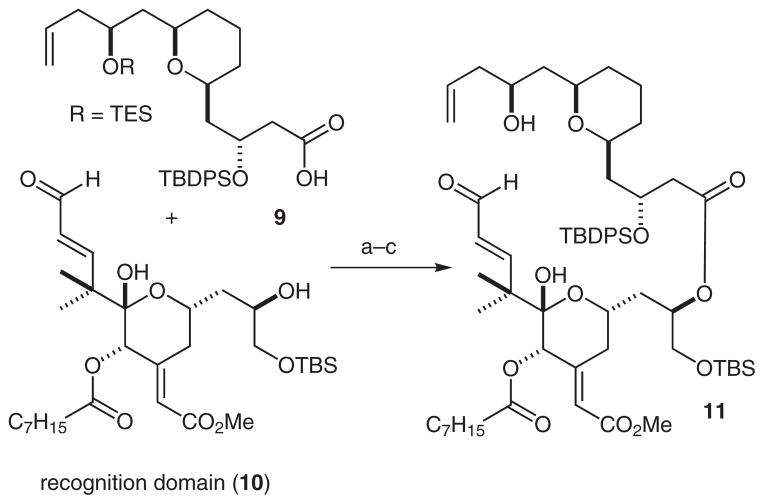
Our initial synthetic studies were aimed at a convergent preparation of the cyclization precursor 11. Allylation of the previously reported lactone 5,32 followed by reduction of the resulting hemiacetal gave the allyl derivative 6 in 55% yield (Scheme 2). The terminal olefin group was then oxidized with potassium osmate and sodium periodate to give the corresponding aldehyde 7 in 87% yield. Conversion of aldehyde 7 into the corresponding homoallylic alcohol was accomplished by an asymmetric Brown allylation, and the resulting secondary alcohol was protected by treatment with chloro(triethyl)silane (67% yield, two steps). To complete the synthesis of the spacer domain 9, the C1 benzyl group was removed by treatment with lithium naphthalenide (87% yield), and the resultant alcohol was oxidized to give the carboxylic acid in a two-step sequence (75% yield).
Scheme 2.
Synthesis of spacer domain 9. Reaction conditions: (a) AllMgBr, THF, −78 °C, 1.5 h; (b) TES, TFA, CH2Cl2, −30 °C, 1.5 h, 55%; (c) K2OsO4, NaIO4, 1,4-dioxane–H2O (3:1), 87%; (d) (−)-(+)-methoxy(diisopinocampheyl)borane, AllMgBr, Et2O, −78 °C, 3 h, then 30 % H2O2, 1 M aq NaOH, 1 h, 69%; (e) TESCl, imidazole, CH2Cl2, 1 h, 98%; (f) lithium naphthalenide, THF, −30 °C, 30 min, 87%; (g) (1) Pr4N+ RuO4 −, NMO, CH2Cl2, r.t., 30 min; (2) NaClO2, NaH2PO4, CH2Cl2, r.t., 30 min, 75% (two steps).
Spacer domain 9 was then coupled to the recognition domain 10 by means of a Yamaguchi esterification (Scheme 3). Upon TES deprotection, the resulting cyclization precursor 11 was produced in a 58% yield over three steps.
Scheme 3.
Synthesis of cyclization precursor, 11. Reaction conditions: (a) 9, 2,4,6-trichlorobenzoyl chloride, Et3N, toluene, r.t., 3 h; (b) 10, DMAP, toluene, r.t., 1 h; (c) PPTS, 20% H2O–THF, 20 h, 58% (three steps).
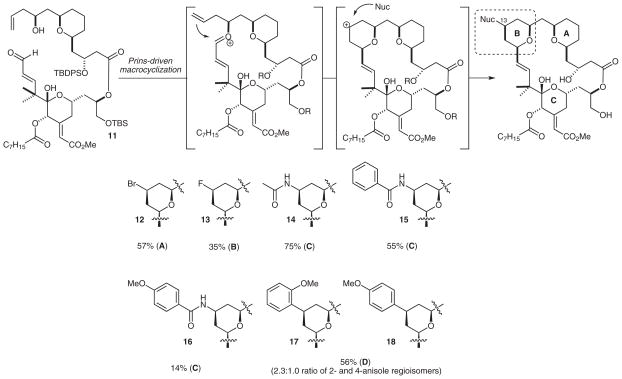
Next, we turned our attention to the evaluation of the Prins macrocyclization–nucleophilic trapping transformation with a halogen nucleophile (Scheme 4).33 Preliminary investigations indicated that enal 11 was unstable when exposed to various Lewis acids at 0 °C or above; optimizations of reaction conditions were therefore undertaken at lower temperatures. Although only modest conversion was detected when the halo-Prins macrocyclization was conducted with bromo(trimethyl)silane and iron(III) bromide at −78 °C (Scheme 4), the transformation was effective when performed at −40 °C and gave the C13 bromide in 57% yield (82% based on recovered starting material). Deprotection with hydrogen fluoride·pyridine resulted in clean production of analogue 12.
Scheme 4.
Synthesis of bryostatin analogues by Prins macrocyclization (only the B-rings are shown for products 12–18; the macrocyclization yields are given). Reaction conditions: (A, halide–Prins macrocyclization): (i) TMSBr, FeBr3, CH2Br2, −40 °C, 2 h; (ii) HF·pyridine, −78 °C to r.t.; (B, fluoro-Prins macrocyclization): HF·pyridine, −78 °C to r.t.; (C, Prins–Ritter macrocyclization): TMSOTf, nitrile, CH2Cl2, −40 °C, 3 h; H2O, −40 °C to r.t.; (D, Prins–Friedel–Crafts macrocyclization): (i) TMSOTf, arene (solvent), −40 °C, 2 h; (ii) HF·pyridine, −78 °C to r.t. In the case of B, C11 is the TES-protected alcohol in the starting material.
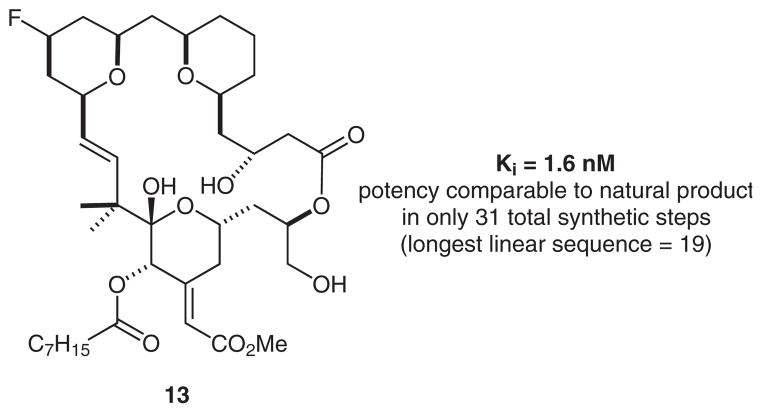
A key advantage of the Prins macrocyclization–nucleophilic trapping process is its ability to facilitate efficient synthesis of the THP-based B-ring. For example, upon coupling of fragments 9 and 10 by Yamaguchi esterification, the resulting product could be treated with hydrogen fluoride·pyridine to initiate directly a fluoro-Prins macrocyclization. This remarkable cascade assembles the macrocycle, introduces a fluorine atom at the C13 position, and achieves global removal all the silyl protecting groups to give the fluoro derivative 13 (Scheme 4). This transformation is only the second example of a hydrogen fluoride promoted Prins reaction,34 and only the second report of a fluoro-Prins macrocyclization.28 Although not yet optimized in terms reaction time, the reaction provides a one-step route to fluorine-labeled analogues. Importantly, the sequence to produce 13 represents the shortest synthesis (longest linear sequence 19, and 31 total steps) of a potent THP-derived B-ring bryostatin analogue (see below).
Because of the efficiency of the initial macrocyclization process, we next investigated the applicability of a range of nucleophiles, with the aim of accessing novel C13 substitution patterns. For example, employing a nitrile as the trapping agent in a tandem Prins–Ritter reaction would be expected to provide bryostatin analogues substituted with amide groups at the C13 position.35 Although few examples of nitrogen-centered nucleophiles have been reported to participate in the Prins reaction, they have been restricted to formation of simple THP moieties. The application of this method to complex polyfunctionalized molecular scaffolds has yet to be realized.
The tandem Prins–Ritter macrocyclization was initially examined by treating a solution of enal 11 in acetonitrile with trimethylsilyl triflate added dropwise at −40 °C (Scheme 4). Interestingly, this protocol resulted in the desired macrocyclization with a concomitant global deprotection to give analogue 14 directly. Although only a modest yield (22%) was obtained, the one-step reaction involves four distinct transformations and many more mechanistic steps. Further optimization of solvents, amounts of reagents, and their rate of addition did not result in any appreciable increase in the yield of the desired product. However, the order of addition proved to be crucial in improving the efficiency of the reaction. For example, when a solution of enal 11 in acetonitrile was added dropwise to a solution of trimethyl triflate in dichloromethane at −40 °C, 14 was isolated in a markedly improved yield of 75%. Furthermore, this reverse addition protocol could be applied to other nitriles. When benzonitrile was used as the trapping agent, a 55% yield of analogue 15 was obtained, whereas only a 20% yield was found under the initial standard conditions.
The Prins macrocyclization–nucleophilic trapping process was further developed through the investigation of a tandem Prins–Friedel–Crafts macrocyclization.36 This method proved to be successful for the production of C13-aryl bryostatin analogues (Scheme 4). In this reaction, 11 was dissolved in the appropriate arene and then treated with trimethylsilyl triflate. Lower reaction temperatures were again necessary to prevent decomposition of the starting material. When anisole was used as the trapping agent, the corresponding C13-aryl products were isolated in 56% yield as a 2.3:1.0 ratio of the ortho- and para-regioisomers. The corresponding analogues 17 and 18 could then be obtained by hydrogen fluoride·pyridine mediated silyl deprotection. This process proved to be limited in scope to electron-rich arenes, as electron-deficient arenes gave little or no product. The reverse addition protocol did not improve the yield of the desired macrocyclization product.
To determine whether a representative example of this new C13-functionalized class of bryostatin analogues would effectively target PKC, we conducted a preliminary competitive binding assay with analogue 13. Importantly, this compound displayed a 1.6 nM affinity to rodent-brain-derived PKC (used to permit comparisons with previous studies) (Figure 3).25a This remarkably high potency toward the enzyme mixture was comparable to that of the parent natural product. However, 13 can be prepared in as few as 31 total transformations, and is therefore a more easily accessible target for synthesis. Furthermore, 13 represents one of the most potent bryostatin analogues that has been reported to date, and is therefore a superb candidate for PET and NMR studies.
Figure 3.
Binding affinity of a new C13-substituted bryostatin analogue for rat-brain PKC
The development of the Prins macrocyclization–nucleophilic trapping method provided a novel class of C13-substituted THP-based B-ring bryostatin analogues for biological evaluation. These compounds were further investigated for their growth inhibitory effects against the K562 human erythroleukemia cell line (Table 1). In our initial analysis, analogue 12 showed a promising level of activity (EC50 = 471 nM). Interestingly, in the examination of the corresponding C13-fluoride bryostatin analogue 13, we found a remarkable increase in potency (EC50 = 14.2 nM). A double-digit nanomolar in vitro activity was also observed for each member of the C13-amide-derived bryostatin analogue series 14–16. Furthermore, the C13-aryl-based bryostatin analogues 17 and 18 showed activity with potencies that were in the single-digit to low-double-digit nanomolar range. These results collectively show that, although the B-ring is positioned outside the proposed binding pocket of the enzyme, modifications to the spacer domain can result in significant changes in the biological profile of the corresponding bryostatin analogue, as would be expected from its potential role in translocation and membrane association. Thus, the C13 position appears to be a tunable element that can be utilized to regulate biological activity and pharmacological performance.
Table 1.
Effective Concentrations of C13-Functionalized Analogues against Human Leukemia Cell Line K562 after 48 Hours of Incubation: Average of Two Experiments.
| Analogue | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|
| EC50 (nM) | 471 | 14.2 | 55.0 | 30.0 | 45.2 | 5.8 | 23.3 |
In summary, we have described a novel Prins macrocyclization–nucleophilic trapping diversification strategy for accessing C13 halogen-, carbon-, and nitrogen-substituted THP-derived bryostatin analogues. This transformation was applicable to a range of nucleophiles, including halides, nitriles, and arenes, and it served as an efficient method for preparing a range of bryostatin analogues for further in vitro analysis. Notably, modifications at the C13 position of the bryostatin scaffold were found to affect the binding and potency of the analogues. Further biological evaluation of these analogues, including isozyme-selective activity, is currently underway and will be reported in due course.
Unless otherwise noted, all reactions were performed under N2 in flame- or oven-dried glassware. Mixtures were stirred by using Teflon-coated magnetic stirrer bars. Reactions were monitored by TLC on 0.25-mm silica gel 60F plates with a fluorescent indicator (Merck). Plates were visualized by treatment with an acidic p-anisaldehyde or KMnO4 stain and gentle heating. Products were purified by column chromatography on silica gel 60 (230–400 mesh) (EM) using the solvent systems indicated.
When necessary, solvents and reagents were purified before use. THF was distilled from sodium benzophenone ketyl. Et2O, CH2Cl2, and toluene were passed through a drying column of alumina (Solv-Tek Inc.) under N2 pressure. Anhyd DMF and DMSO were obtained from Acros Organics. EtOAc, PE, pentane, and MeOH were obtained from Fisher Scientific. DMSO used to prepare biological samples was obtained from Fisher BioReagents (Class III). DMSO used in the PKC and cellular assay protocols was purchased from Aldrich. TMSOTf was obtained from Aldrich and distilled under N2 in a Hickman apparatus immediately before use. Powdered 4-Å MS (<5 μm) were purchased from Aldrich and stored in a vacuum oven (140 °C, 90 mmHg). All other reagents were purchased from Aldrich and used as received without additional purification. (3H)phorbol dibutyrate [(3H)PDBu] was obtained from American Radiolabeled Chemicals, Inc. (St. Louis, MO) as a soln in acetone. Samples prepared for biological evaluation were purified by preparative HPLC with a H2O–MeCN gradient using a Varian Pro-Star (model 320) system equipped with an AllTech Alltima C18 column (10 μm, 10 × 250 mm).
NMR spectra were recorded on Varian INOVA 500 (1H at 500 MHz, 13C at 125 MHz), Varian 400 (1H at 400 MHz, 13C at 100 MHz), or Varian INOVA 600 MHz (1H at 500 MHz, 13C at 150 MHz) spectrometers, as noted. 1H chemical shifts are reported relative to the residual solvent peak (CHCl3; δ = 7.26 ppm, benzene; δ = 7.15 ppm). 13C chemical shifts are reported relative to the 13C signals for the deuterated solvent (CDCl3; δ = 77 ppm). IR spectra were recorded on a PerkinElmer 1600 Series Fourier-transform spectrometer. Optical rotation data were obtained by using a JASCO DIP. High-resolution mass spectra were recorded at the Stanford University Mass Spectrometry facility.
Protein Kinase C Isozyme Mixture Binding Assay
Preparation of assay materials
In a polypropylene vial, a buffer soln was prepared by diluting 1 M aq Tris-HCl (pH 7.4, 1 mL), 1 M aq KCl (2 mL), 0.1 M aq CaCl2 (30 μL), and bovine serum albumin (40 mg) with deionized H2O (20 mL). The buffer was stored on ice until required. Phosphatidyl serine (Avanti Polar Lipids, 10 mg/mL in CHCl3, 350 μL) was isolated by removing the CHCl3 under a stream of N2 and then suspended as vesicles by adding the prepared buffer soln (3.5 mL), followed by sonication (Branson Sonifier 250; power = 6; 40% duty cycle) for four 30-s periods with a 30 s rest between sonications. The resulting suspension was stored on ice until required. Assay Protein Kinase C (PKC) was prepared by dissolving an aliquot of a PKC rat-brain isozyme mixture (600 μL) in buffer soln (14 mL). This mixture was stored on ice for immediate use.
Preparation of dilutions of (3H)PDBu and test compounds
A commercial 1 mCi/mL soln of (3H)PDBu in acetone (American Radiolabeled Chemicals, Inc.) was diluted 10-fold in EtOH. This EtOH soln was further diluted by a factor of 16.7 (96 μL into 1504 μL) in DMSO for use in assays. The assay concentration of (3H)PDBu for any specific batch of dilutions was measured by competitive binding of (1H)PDBu [IC50 = assay (3H)PDBu concentration]. For the data presented above (Figure 3), the assay concentration of (3H)PDBu was 9.4 nM.
Dilutions of the test compounds were prepared in DMSO, with serially dilution from a high concentration of 2.0 μM (assay concentration 130 nM) by factors of three to a low concentration of 2.7 nM (assay concentration: 180 pM).
Assay protocol
Polypropylene assay vials (in triplicate) were charged with phosphatidyl serine vesicle suspension (60 μL), PKC soln (200 μL), and the diluted test compound (20 μL). Nonspecific (3H)PDBu binding was analyzed in triplicate by using analogue 4 (75 μM, 20 μL, assay concentration: 5 μM) instead of the test compound. Maximum (3H)PDBu binding was analyzed in triplicate by substitution of DMSO (20 μL) for the test compound. Finally, (3H)PDBu [diluted in DMSO (20 μL), assay concentration: 9.4 nM, determined by titration with (1H)PDBu] was added to all the vials. The solns were mixed with a vortexer, incubated at 37 °C for 90 min, and placed on ice for 15 min. Filters (Whatman GF/B 21 mm) were treated by soaking them for 90 min in 10 vol% aq poly(ethyleneamine) (6 mL) diluted in H2O (200 mL). A rinse buffer of 20 mM aq Tris (500 mL, pH 7.4) was prepared and cooled on ice for the duration of the incubation period and for the remainder of the assay. The contents of the assay vials were vacuum filtered through the poly(ethyleneamine)-soaked filter papers, washing the residual contents of the vial with additional rinse buffer (0.5 mL). The filters were then washed by dropwise addition of buffer (4.5 mL) and placed into scintillation vials. These vials were filled with Bio-Safe scintillation fluid (5 mL) and their radioactivity was immediately measured for 1 min each in a scintillation counter (Beckman LS 6000SC). Counts per minute (cpm) were averaged for each of the three triplicate dilutions. The data were then plotted [cpm versus log(concentration)] by using Prism software (GraphPad), and the value of the IC50 was calculated by using one-site-competition least-squares regression analysis. Ki values were then calculated by means of the equation: Ki = IC50/(1 + [(3H)PDBu])/[Kd of (3H)PDBu]. The Kd of (3H)PDBu was measured under these assay conditions to be 1.55 nM.
K562 Cellular Assay: MTT Cellular Viability Readout
K562 cells were cultured in RPMI medium 1640 (Gibco) supplemented with fetal bovine serum( FBS) (10% v/v), penicillin (100 units/mL), and streptomycin (50 units/mL) at 37 °C, under 5% CO2 in air in a humidified incubator. Cells were subcultured every 72–96 h once the cellular concentration had reached > 1,000,000 cells/mL, to a starting concentration of 100,000 cells per mL. The cell concentration was determined with a hemocytometer. To assay the compounds, cells were plated in plasma-treated 96-well polystyrene plates at a density of 10,000–14,000 cells/well in 50 μL of culture medium, and incubated for 1 h while compound dilutions were prepared. Compounds were diluted from a 4 mM DMSO stock soln with culture medium to a concentration of 20 μM. Triplicate serial dilutions were then prepared in culture medium from 20 μM to 80 pM across 10 wells of a 96-well plate (diluted by factors of 4). Plates containing cells were dosed with the compound (50 μL/well) to afford final assay concentrations of 10 μM to 40 pM. The maximum DMSO concentration was 0.25%. Cells were then incubated at 37 °C under 5% CO2–air in a humidified incubator for 48 h. A 5 mg/mL soln of thiazolyl blue tetrazolium bromide (MTT; Aldrich) in cell culture medium (10 μl) was then added to each well. The cells were allowed to incubate for an additional 2.5 h then lysed with 100 μL of a detergent solvent [Triton-X 100 (Aldrich) (20 mL) dissolved in 0.1 M HCl in i-PrOH (180 mL)]. After thorough mixing, the plates were analyzed with a VERSAmax tunable microplate reader (Molecular Devices) using SOFTmax Pro software (version 3.1.1), reading at 570 nm and subtracting at 690 nm. The sigmoidal dose–response curves generated by plotting the corrected signal versus the log(drug concentration) were analyzed by least-squares regression using Prism software (GraphPad) to generate the EC50 values.
[(2R,6S)-6-((2R)-4-(Benzyloxy)-2-{[tert-butyl(diphenyl)silyl] oxy}butyl)tetrahydro-2H-pyran-2-yl]acetaldehyde (7)
An oven-dried round-bottomed flask equipped with stirrer bar was charged with terminal olefin 637 (468 mg, 0.862 mmol) and the flask was evacuated, backfilled with N2. A 3:1 mixture of 1,4-dioxane and H2O (16 mL) followed by 2,6-lutidine (0.200 mL, 0.276 mmol) were added at r.t by syringe through a septum. K2OsO4·2H2O (1.5 mg, 1.72 mmol) and NaIO4 (736 mg, 3.44 mmol) were added directly to the flask under N2, and the mixture was stirred at r.t. for 15 h. The reaction was quenched with sat. aq NaHCO3 (25.0 mL) and diluted with Et2O (30 mL). The mixture was then extracted with Et2O (3 × 30 mL) and the organic layers were combined, dried (MgSO4), filtered, and concentrated in vacuo. The resulting crude product was purified by flash chromatography (10% EtOAc–PE) to give an oil; yield: 410 mg (87%); [α]D 24.2 +13.7 (c 1.65, CH2Cl2); Rf = 0.50 (15% EtOAc–PE) (stained with KMnO4).
IR (thin film): 3069, 2931, 2856, 1726, 1427, 1110, 821, 737, 702 cm−1.
1H NMR (500 MHz, CDCl3): δ = 9.48 (t, 3 Hz, 1 H), 7.66 (d, 8 Hz, 4 H), 7.24–7.42 (m, 11 H), 4.35 (s, 2 H), 4.12 (pent, J = 7 Hz, 1 H), 3.47 (t, J = 7 Hz, 2 H), 3.42 (m, 1 H), 3.32 (m, 1 H), 2.23–2.36 (m, 2 H), 1.78 (m, 2 H), 1.70 (m, 1 H), 1.56 (m, 2 H), 1.48 (m, 1 H), 1.25–1.34 (m, 3 H), 1.09 (m, 1 H), 1.03 (s, 9 H).
13C NMR (125 MHz, CDCl3): δ = 201.9, 138.5, 135.9, 134.4, 134.3, 129.5 (×2), 128.2, 127.5, 127.4 (×2), 127.3, 74.5, 72.7, 72.5, 68.2, 66.8, 49.9, 44.4, 37.7, 31.4, 31.3, 30.9, 27.0, 23.3, 19.4.
HRMS (ES+): m/z calcd for C34H44NaO4Si: 567.2901; found: 567.2906.
(2S)-1-[(2R,6S)-6-((2R)-4-(Benzyloxy)-2-{[tert-butyl(diphenyl) silyl]oxy}butyl)tetrahydro-2H-pyran-2-yl]pent-4-en-2-ol (8; R = H)
(−)-(+)-Methoxy(diisopinocampheyl)borane (460 mg, 1.45 mmol) was weighed into a flask in a dry box. Et2O (15.0 mL) was added and the soln was cooled to −78 °C. A 1.0 M soln of CH2=CHCH2MgBr in Et2O (1.21 mL, 1.21 mmol) was added and the mixture stirred for 5 min at −78 °C then for 1 h at r.t. The mixture was cooled to −78 °C, a soln of aldehyde 7 (440 mg, 0.808 mmol) in Et2O (5 mL) was added, and the mixture was stirred for 2.5 h. The reaction was quenched with 30% H2O2 (1 mL) and 1 M aq NaOH (400 μL), and the mixture was stirred at 30 °C for 30 min. The soln was then extracted with Et2O (3 × 30 mL) and the organic layers were combined, dried (MgSO4), filtered, and concentrated in vacuo. The crude product was dissolved in MeOH (3 × 10 mL) and concentrated in vacuo to remove the remaining borane. The resulting product was purified by flash chromatography (10% EtOAc–PE) to give an oil; yield: 55 mg (69%); [α]D 24.2 −2.4 (c 4.0, CH2Cl2); Rf = 0.40 (10% EtOAc–PE) [stained with anisaldehyde (red), UV active].
IR (thin film): 3497, 3070, 2932, 2857, 1640, 1589, 1427, 1106, 702 cm−1.
1H NMR (500 MHz, CDCl3): δ = 7.68 (d, 8 Hz, 4 H), 7.24–7.42 (m, 11 H), 5.85 (m, 1 H), 5.07–5.12 (m, 2 H), 4.36 (s, 2 H), 4.08 (dt, J = 2, 6 Hz, 1 H), 3.68 (m, 1 H), 3.64 (s, 1 H), 3.47 (dt, J = 3, 7 Hz, 2 H), 3.34 (m, 1 H), 3.27 (m, 1 H), 2.10–2.26 (m, 2 H), 1.78 (q, J = 7 Hz, 2 H), 1.62–1.70 (m, 2 H), 1.55–1.60 (m, 1 H), 1.39–1.49 (m, 3 H), 1.24–1.32 (m, 2 H), 1.10–1.20 (m, 1 H), 1.04 (s, 9 H).
13C NMR (125 MHz, CDCl3): δ = 138.5, 135.9, 135.8, 135.2, 134.3, 134.2, 129.5 (×2), 128.2, 127.6, 127.5 (×2), 127.3, 116.8, 78.6, 74.8, 72.7, 70.9, 68.4, 66.7, 44.5, 42.2, 41.9, 37.5, 31.8, 31.3, 26.9, 23.2, 19.4.
[((1S)-1-{[(2R,6S)-6-((2R)-4-(Benzyloxy)-2-{[tert-butyl(diphenyl) silyl]oxy}butyl)tetrahydro-2H-pyran-2-yl]methyl}but-3-en-1-yl)oxy](triethyl)silane (8, R = TES)
Imidazole (22.5 mg, 0.331 mmol) was added to a soln of alcohol 8 (R = H; 55 mg, 0.094 mmol) in CH2Cl2 (1.0 mL) under ambient air. Upon dissolution of the solids, TMSCl (28 μL, 0.166 mmol) was added in one portion to give a white suspension. The mixture was stirred for 15 min and then diluted with sat. aq NH4Cl (5 mL) and Et2O (5 mL). The organic and aqueous layers were separated and the aqueous layer was extracted with Et2O (3 × 5 mL). The organic phases were combined, dried (MgSO4), filtered, and concentrated. The crude product was purified chromatography (silica gel, 5% EtOAc–PE) to give a clear colorless oil; yield: 65 mg (98%); [α]D 24.2 +15.2 (c 3.35, CH2Cl2); Rf = 0.50 (5% EtOAc–pentane) [stained with anisaldehyde (green), UV active].
IR (thin film): 3071, 2932, 2875, 2857, 1641, 1589, 1455, 1427, 1106, 1005, 736, 707 cm−1.
1H NMR (500 MHz, CDCl3): δ = 7.71 (m, 4 H), 7.24–7.42 (m, 11 H), 5.84 (m, 1 H), 5.06 (s, 1 H), 5.03 (d, J = 4 Hz, 1 H), 4.37 (s, 2 H), 4.13 (dt, J = 2, 5 Hz, 1 H), 3.86 (p, J = 6 Hz, 1 H), 3.48 (dt, J = 3, 7 Hz, 2 H), 3.29 (m, 2 H), 2.17–2.29 (m, 2 H), 1.79 (q, J = 7 Hz, 2 H), 1.45–1.75 (m, 6 H), 1.28–1.38 (m, 2 H), 1.07 (s, 9 H), 1.02 (m, 2 H), 0.97 (t, J = 8 Hz, 9 H), 0.61 (q, J = 8 Hz, 6 H).
13C NMR (125 MHz, CDCl3): δ = 138.6, 135.8, 135.0, 134.4 (×2), 129.4 (×2), 128.2, 127.5 (×2), 127.4, 127.3, 116.9, 74.4, 74.3, 72.7, 68.7, 68.5, 66.9, 44.8, 43.5, 42.0, 37.7, 31.9, 31.3, 27.0, 23.6, 19.4, 6.9, 5.0.
HRMS (ES+): m/z calcd for C43H64NaO4Si2: 723.4235; found: 723.4246.
(2S)-1-[(2R,6S)-6-((2R)-2-{[tert-Butyl(diphenyl)silyl]oxy}-4-hydroxybutyl) tetrahydro-2H-pyran-2-yl]pent-4-en-2-ol
A ~1 M soln of lithium naphthalenide in THF was prepared by dissolving Li metal (high sodium content; 347 mg, 50 mmol) and naphthalene (7.69 g, 60 mmol) in THF (50 mL) with sonication under N2 at r.t. The resulting deep green soln was stored under N2 in a Schlenk flask at −20 °C until required. Solns of this reagent could be stored for several weeks with only slight loss of activity.
A soln of 8 (R = TES; 307 mg, 0.438 mmol) in THF (7.0 mL) was cooled in a CO2/MeCN bath (external temperature −25 °C) and then the ~1.0 M soln of lithium naphthalenide in THF (5.39 mL, 5.39 mmol) was added dropwise in 200-μL portions at 1 min intervals. When addition of the lithium naphthalenide reagent was complete, the resulting opaque green mixture was stirred for 10 min until the starting material was completely consumed (TLC). The reaction was then quenched with sat. aq NH4Cl (5 mL), which caused the color to disappear immediately. The biphasic mixture was diluted with H2O (10 mL) and Et2O (20 mL). The organic and aqueous layers were separated and the aqueous layer was extracted with Et2O (2 × 20 mL). The organic layers were combined, dried (MgSO4), filtered, and concentrated. The residue was purified by chromatography (silica gel, 10 to 20% EtOAc–PE) to give a clear, colorless oil; yield: 232 mg (87%); [α]D 24.2 +10.7 (c 1.1, CH2Cl2); Rf = 0.20 (10% EtOAc–pentane) [stained with anisaldehyde (green), UV active].
IR (thin film): 3443, 2932, 2876, 2857, 1642, 1427, 1110, 1068, 1046, 702 cm−1.
1H NMR (500 MHz, CDCl3): δ = 7.69 (m, 4 H), 7.36–7.45 (m, 6 H), 5.79 (m, 1 H), 5.02 (d, J = 2 Hz, 1 H), 4.98 (dd, J = 2, 7 Hz, 1 H), 4.08 (p, J = 5 Hz, 1 H), 3.75–3.81 (m, 2 H), 3.69 (m, 1 H), 3.19 (m, 1 H), 2.99 (m, 2 H), 2.31 (br s, 1 H), 2.09–2.24 (m, 2 H), 1.81–1.89 (m, 1 H), 1.59–1.76 (m, 4 H), 1.39–1.56 (m, 3 H), 1.16–1.29 (m, 2 H), 1.07 (s, 9 H), 1.02 (m, 1 H), 0.94 (t, J = 8 Hz, 9 H), 0.57 (q, J = 8 Hz, 6 H).
13C NMR (125 MHz, CDCl3): δ = 135.9, 135.8, 134.9, 134.0, 133.8, 129.7, 129.6, 127.7, 127.6, 116.9, 75.2, 74.6, 71.0, 68.5, 59.6, 43.7, 43.4, 41.8, 39.1, 31.9, 31.3, 27.0, 23.4, 19.3, 6.9, 5.0.
HRMS (ES+): m/z calcd for C36H58NaO4Si2: 633.3766; found: 633.3775.
(3S)-3-{[tert-Butyl(diphenyl)silyl]oxy}-4-((2S,6R)-6-{(2S)-2-[(triethylsilyl)oxy]pent-4-en-1-yl}tetrahydro-2H-pyran-2-yl)butanoic acid (9; Spacer Domain)
An oven-dried vial was charged with the alcohol prepared as described above (30 mg, 0.049 mmol) and powdered 4-Å MS (250 mg). The vessel was purged twice with N2 and then a soln of NMO (22 mg, 0.188 mmol) in CH2Cl2 (0.50 mL) was added. A soln of Pr4N+ RuO4 − (2.2 mg, 0.00625 mmol) in CH2Cl2 (0.50 mL) was added and the resulting green mixture was stirred at r.t. for 10 min until the starting material was completely consumed (TLC). After 15 min, the mixture was loaded directly onto a short pad of silica gel and eluted with 20% EtOAc–pentane. The filtrate was concentrated to give a colorless oil that was used directly in the next step.
The oil was dissolved in t-BuOH (1.20 mL) under air, and H2O (600 μL) and 2-methylbut-2-ene (450 μL) were added successively. The resulting cloudy mixture was stirred vigorously and cooled in an ice water bath. The suspension was treated with sequentially with NaH2PO4 (75 mg, 0.625 mmol) and NaClO2 (35 mg, 0.384 mmol), then stirred for 25 min. The reaction was then quenched with a 1:1 mixture of sat. aq NaCl and sat. aq Na2S2O3 (3 mL). The resulting mixture was diluted with H2O (10 mL) and Et2O (10 mL), and the layers were separated. The aqueous layer was extracted with Et2O (3 × 10 mL) and the organic phases were combined, dried (MgSO4), filtered, and concentrated. The resulting oil was purified by chromatography (silica gel, 30% EtOAc–PE) to give the carboxylic acid 9 as a viscous colorless oil; yield: 23 mg (75%, two steps); [α]D 24.2 +9.0 (c 0.95, CH2Cl2); Rf = 0.20 (20% EtOAc–PE) [stained with anisaldehyde (green), UV active].
IR (thin film): 3072, 2931, 1712, 1641, 1590, 1428, 1111, 1084, 1006, 702 cm−1.
1H NMR (500 MHz, CDCl3): δ = 7.68 (m, 4 H), 7.36–7.45 (m, 6 H), 5.77 (m, 1 H), 5.02 (s, 1 H), 4.98 (dd, J = 2, 7 Hz, 1 H), 4.25 (p, J = 5 Hz, 1 H), 3.78 (p, J = 6 Hz, 1 H), 3.24 (m, 1 H), 3.12 (m, 1 H), 2.55 (m, 2 H), 2.19 (m, 2 H), 1.50–1.74 (m, 5 H), 1.45 (m, 1 H), 1.18–1.32 (m, 3 H), 1.04 (s, 9 H), 1.00 (m, 1 H), 0.93 (t, J = 8 Hz, 9 H), 0.57 (q, J = 8 Hz, 6 H); the CO2H proton was not observed.
13C NMR (125 MHz, CDCl3): δ = 174.6, 135.9, 135.8, 134.7, 133.8, 133.3, 129.8, 129.7, 127.7, 127.6, 117.1, 74.7 (×2), 68.7, 68.5, 43.9, 43.3, 42.6, 42.0, 31.8, 31.1, 26.9, 23.4, 19.3, 6.9, 5.0.
HRMS (ES+): m/z calcd for C36H56NaO5Si2: 647.3558; found: 647.3569.
Cyclization Precursor 11
Carboxylic acid 9 (126 mg, 0.0202 mmol) was dissolved in toluene (6.00 mL) under N2, and the soln was treated sequentially with Et3N (163 μL, 1.17 mmol) and 2,4,6-trichlorobenzoyl chloride (60 μL, 0.383 mmol). The resulting soln was stirred for 3 h at r.t., by which time the formation of salts was observed. A soln of recognition domain 10 (80 mg, 0.133 mmol) in toluene (500 μL) was added through a cannula with the aid of 2 × 150 μL washes. A soln of DMAP (85 mg, 0.695 mmol) in toluene (200 μL) was then immediately added and the resulting mixture was stirred for 1 h then loaded directly onto a silica gel column and eluted with 20% EtOAc–pentane. Fractions containing the product were collected and concentrated in vacuo to afford a residue that was used directly in the next step.
The residue was dissolved in a soln of TsOH (10.6 mg, 0.042 mmol) in 20% H2O–THF (25 mL) and the soln was stirred at r.t. under air for 21 h, then diluted with Et2O (50 mL) and sat. aq NaHCO3 (50 mL). The organic and aqueous layers were separated, and the aqueous layer was extracted with Et2O (3 × 50 mL). The organic layers were combined, dried (MgSO4), filtered, and concentrated, and the residue was purified by chromatography (silica gel, 20% EtOAc–PE) to afford 11 as a colorless oil; yield: 84.7 mg (58%, two steps); [α]D 24.2 −39.1 (c 1.24, CH2Cl2); Rf = 0.45 (15% EtOAc–PE) [stained with anisaldehyde (black spot), UV active].
IR (thin film): 3497, 3072, 3049, 2931, 2857, 2721, 1723, 1689, 1471, 1429, 1388, 1257, 1229, 1156, 1106, 837, 704 cm−1.
1H NMR (500 MHz, CDCl3): δ = 9.56 (d, J = 8 Hz, 1 H), 7.66 (m, 4 H), 7.34–7.43 (m, 7 H), 5.97 (s, 1 H), 5.93 (dd, J = 8, 9 Hz, 1 H), 5.78 (m, 1 H), 5.21 (m, 1 H), 5.09 (s, 1 H), 5.07 (d, J = 4 Hz, 1 H), 5.03 (s, 1 H), 4.32 (m, 1 H), 3.77 (t, J = 9 Hz, 1 H), 3.64 (s, 3 H), 3.52–3.63 (m, 5 H), 3.21 (t, J = 9 Hz, 1 H), 3.10 (m, 1 H), 2.54 (dd, J = 5, 15 Hz, 1 H), 2.44 (dd, J = 5, 15 Hz, 1 H), 2.20 (m, 1 H), 1.96–2.10 (m, 6 H), 1.88 (t, J = 11 Hz, 1 H), 1.54–1.75 (m, 6 H), 1.34–1.52 (m, 9 H), 1.17–1.28 (m, 14 H), 1.19 (s, 3 H), 1.14 (s, 3 H), 1.01 (s, 9 H), 0.89 (s, 3 H), 0.86 (t, J = 7 Hz, 1 H), 0.05 (s, 3 H), 0.04 (s, 3 H).
13C NMR (125 MHz, CDCl3): δ = 194.8, 171.8, 171.5, 167.0, 166.5, 135.8, 135.1, 133.8, 133.7, 129.7 (×2), 127.6, 127.2, 120.1, 116.9, 99.4, 78.5, 74.3, 72.8, 71.0, 70.9, 67.6, 66.3, 64.8, 51.1, 45.7, 44.7, 43.0, 41.7, 37.9, 34.5, 31.7, 31.6, 31.2, 31.1, 28.9 (×2), 26.9, 25.8, 24.4, 23.1, 23.0, 22.5, 20.0, 19.4, 18.2, 14.1, −5.3 (×2).
HRMS (ES+): m/z calcd for C61H94O13Si2Na: 1113.6125; found: 1113.6128.
Bryostatin Analogue 12
Cyclization precursor 11 (7.6 mg, 0.0070 mmol) was dissolved in CH2Br2 (0.250 mL) under N2 and the resulting soln was cooled in a CO2/MeCN bath. After 10 min, 50 μL of a stock soln of FeBr3 (1.1 mg, 0.00367 mmol) and TMSBr (4.8 μL, 0.0367 mmol) in CH2Br2 (0.20 mL) was then added dropwise and the resulting mixture was stirred for 3 h. The reaction was quenched with H2O (0.50 mL) and the mixture was diluted with Et2O (1.0 mL) and H2O (1 mL). The organic and aqueous layers were separated and the aqueous layer was extracted with Et2O (3 × 1 mL). The organic layers were combined, dried (MgSO4), filtered, and concentrated to give a yellow oil. This oil was purified by chromatography (silica gel, 25% EtOAc–PE) to give the cyclized, C26 TBS-deprotected product as a clear colorless viscous residue; yield: 4.1 mg (57% yield); Rf = 0.40 (25% EtOAc–PE).
The crude oily product was dissolved in THF (5.0 mL) under N2 in a polypropylene vial and the resulting soln was cooled in an ice water bath. HF·pyridine (70% HF; 1.50 mL) was added dropwise over 2 min and the mixture was stirred for 10 min. The cold bath was then removed and the soln was stirred for an additional 24 h at r.t. The reaction was then quenched with sat. aq NaHCO3 (2 mL) and the mixture was diluted with Et2O (5 mL). The organic and aqueous layers were separated and the aqueous layer was extracted with Et2O (3 × 5 mL). The organic phases were combined, dried (MgSO4), filtered, and concentrated. The crude residue was purified by chromatography (silica gel, 60% EtOAc–PE) to give 12; yield: 2.1 mg (37%, two steps). This product was further purified by C18 reverse-phase HPLC (gradient 60% MeCN–H2O to 100% MeCN) to give an amorphous white solid; Rf = 0.30 (60% EtOAc–PE) [stained with anisaldehyde (red)].
IR (thin film): 3395, 2955, 2919, 2850, 2360, 1720, 1656, 1570, 1540, 1464, 1413, 1377, 1261, 1113, 1023 cm−1.
1H NMR (500 MHz, CDCl3): δ = 5.99 (s, 1 H), 5.74 (d, J = 16 Hz, 1 H), 5.32–5.37 (m, 2 H), 5.26 (dd, J = 8, 15 Hz, 1 H), 5.13 (s, 1 H), 5.13 (s, 1 H), 4.44 (d, J = 11 Hz, 1 H), 4.16–4.24 (m, 2 H), 4.06 (t, J = 11 Hz, 1 H), 3.97 (t, J = 9 Hz, 1 H), 3.85 (dd, J = 2, 11 Hz, 1 H), 3.64–3.71 (m, 4 H), 3.55 (t, J = 10 Hz, 1 H), 3.52 (t, J = 11 Hz, 1 H), 3.40 (t, J = 11 Hz, 1 H), 2.22–2.38 (m, 3 H), 1.70–2.13 (m, 3 H), 2.26–2.30 (m, 2 H), 2.00–2.12 (m, 5 H), 1.77–1.82 (m, 3 H), 1.40–1.65 (m, 7 H), 1.18–1.33 (m, 9 H), 1.09 (s, 3 H), 0.98 (s, 3 H), 0.86 (t, J = 7 Hz, 3 H).
HRMS (ES+): m/z calcd for C39H61BrNaO12: 823.3239; found: 823.3231.
Bryostatin Analogue 13
The C11-TES protected cyclization precursor 11 (10.6 mg, 0.0089 mmol) was dissolved in THF (7.0 mL) under N2 in a polypropylene vial and the soln was cooled to −78 °C. HF·pyridine (70% HF; 2.00 mL) was added dropwise over 1 min and the mixture was stirred at −78 °C for 3 h and then allowed to warm gradually to r.t. over 24 h without removal of the cooling bath. The reaction was quenched with sat. aq NaHCO3 (10 mL) and the mixture was diluted with Et2O (10 mL). The organic and aqueous layers were separated and the aqueous layer was extracted with EtOAc (3 × 10 mL). The organic phases were combined, dried (MgSO4), filtered, and concentrated. The crude residue was purified by chromatography (silica gel, 60% EtOAc–PE) to give a colorless oil; yield: 2.3 mg (35%); Rf = 0.40 (60% EtOAc–PE) [stained with anisaldehyde (red)].
IR (thin film): 3426, 2919, 2850, 1663, 1449, 1422, 1377, 1263, 1167, 1086, 1031, 704 cm−1.
1H NMR (500 MHz, CDCl3): δ = 5.99 (s, 1 H), 5.75 (d, J = 16 Hz, 1 H), 5.29–5.34 (m, 2 H), 5.17 (s, 1 H), 5.14 (s, 1 H), 4.72 (m, 1 H), 4.47 (d, J = 10 Hz, 1 H), 4.18 (m, 1 H), 4.07 (t, J = 12 Hz, 1 H), 3.95 (t, J = 12 Hz, 1 H), 3.84 (m, 1 H), 3.68 (s, 3 H), 3.64–3.71 (m, 4 H), 3.50–3.57 (m, 2 H), 3.43 (t, J = 12 Hz, 1 H), 2.44–2.51 (m, 2 H), 2.22–2.31 (m, 2 H), 1.94–2.05 (m, 5 H), 1.76–1.80 (m, 3 H), 1.39–1.60 (m, 8 H), 1.18–1.33 (m, 10 H), 1.12 (s, 3 H), 1.00 (s, 3 H), 0.87 (t, J = 7 Hz, 3 H).
HRMS (ES+): m/z calcd for C39H61FNaO12: 763.4039; found: 763.4045.
Bryostatin Analogue 14
TMSOTf (1.0 μL) was dissolved in CH2Cl2 (0.100 mL) under N2 in an oven-dried vial and the soln was cooled in a CO2/MeCN bath for about 10 min. A soln of 11 (3.0 mg, 0.0028 mmol) in MeCN (0.100 mL) was then added dropwise over 5 min and the resulting mixture was stirred for 2 h. The reaction was quenched with H2O (0.300 mL) and the mixture was allowed to warm to r.t. over 30 min then diluted with Et2O (0.50 mL). The aqueous layer was extracted with Et2O (3 × 0.50 mL) and the organic layers were combined, dried (MgSO4), filtered, and concentrated. The resulting oil was purified by chromatography (silica gel, EtOAc to 5% MeOH–EtOAc) to give a colorless oil; yield: 1.6 mg (75%); Rf = 0.40 (5% MeOH–EtOAc) [stained with anisaldehyde (red)].
IR (thin film): 3442, 3356, 2922, 2852, 1721, 1658, 1463, 1435, 1377, 1259, 1167, 1086, 1031 cm−1.
1H NMR (500 MHz, CDCl3): δ = 5.99 (s, 1 H), 5.74 (d, J = 16 Hz, 1 H), 5.34 (m, 1 H), 5.25 (dd, J = 8, 16 Hz, 1 H), 5.17 (s, 1 H), 5.13 (s, 1 H), 4.47 (d, J = 12 Hz, 1 H), 4.04–4.18 (m, 3 H), 3.85 (m, 1 H), 3.67 (s, 3 H), 3.59–3.75 (m, 4 H), 3.52–3.63 (m, 5 H), 3.53 (t, J = 12 Hz, 1 H), 3.41 (t, J = 12 Hz, 1 H), 2.49–2.63 (m, 2 H), 2.29 (q, J = 7 Hz, 1 H), 2.18 (s, 1 H), 1.88–2.19 (m, 5 H), 1.76–1.80 (m, 3 H), 1.39–1.60 (m, 7 H), 1.18–1.33 (m, 12 H), 1.10 (s, 3 H), 0.97 (s, 3 H), 0.88 (t, J = 7 Hz, 3 H).
HRMS (ES+): m/z calcd for C41H65NNaO13: 802.4348; found: 802.4354.
Bryostatin Analogue 15
TMSOTf (1.3 μL) was dissolved in CH2Cl2 (0.100 mL) under N2 in an oven-dried vial and the soln was cooled in a CO2/MeCN bath for about 10 min. A soln of 11 (4.0 mg, 0.0037 mmol) in PhCN (0.100 mL) was added dropwise over 5 min, and the resulting mixture was stirred for 2 h. The reaction was then quenched with H2O (0.300 mL) and the mixture was allowed to warm to r.t. over 30 min, then diluted with Et2O (0.50 mL). The aqueous layer was extracted with Et2O (3 × 0.50 mL) and the organic layers were combined, dried (MgSO4), filtered, and concentrated. The resulting oil was purified by chromatography (silica gel, EtOAc to 5% MeOH–EtOAc) to give a colorless oil; yield: 1.7 mg (55%); Rf = 0.50 (5% MeOH–EtOAc) [stained with anisaldehyde (red)].
IR (thin film): 3351, 2923, 2852, 1723, 1691, 1664, 1642, 1586, 1462, 1328, 1260, 1158, 1084 cm−1.
1H NMR (500 MHz, CDCl3): δ = 7.73 (t, J = 7 Hz, 2 H), 7.49 (t, J = 7 Hz, 1 H), 7.42 (t, J = 7 Hz, 2 H), 5.98 (s, 1 H), 5.83 (m, 1 H), 5.78 (d, J = 15 Hz, 1 H), 5.27–5.34 (m, 2 H), 5.19 (s, 1 H), 5.14 (s, 1 H), 4.50 (d, J = 11 Hz, 1 H), 4.33 (br s, 1 H), 4.15 (m, 1 H), 4.10 (m, 1 H), 3.86 (m, 1 H), 3.68 (s, 3 H), 3.64–3.74 (m, 3 H), 3.52–3.63 (m, 5 H), 3.55 (t, J = 12 Hz, 1 H), 3.45 (t, J = 12 Hz, 1 H), 2.52–2.60 (m, 2 H), 2.21–2.35 (m, 2 H), 1.91–2.10 (m, 5 H), 1.77–1.84 (m, 3 H), 1.40–1.65 (m, 7 H), 1.18–1.33 (m, 9 H), 1.11 (s, 3 H), 0.99 (s, 3 H), 0.87 (t, J = 7 Hz, 3 H).
HRMS (ES+): m/z calcd for C46H67NNaO13: 864.4505; found: 864.4487.
Bryostatin Analogue 16
Cyclization precursor 11 (3.0 mg, 0.0028 mmol) and 4-methoxy-benzonitrile (9.2 mg, 0.069 mmol) were dissolved in CH2Cl2 (0.200 mL) under N2, and the resulting soln was cooled in a CO2/MeCN for about 10 min. TMSOTf (1.0 μL) was then added in one portion and the mixture was stirred for 4 h. The reaction was then quenched with H2O (0.300 mL) and the mixture was allowed to warm to r.t. over 30 min then diluted with Et2O (0.50 mL). The separated aqueous layer was extracted with Et2O (3 × 0.50 mL) and the organic layers were combined, dried (MgSO4), filtered, and concentrated. The resulting oil was purified by chromatography (silica gel, EtOAc) to give a colorless oil; yield: 0.3 mg (14%); Rf = 0.250 (EtOAc) [stained with anisaldehyde (red)].
IR (thin film): 3425, 2950, 2919, 2850, 1641, 1547, 1463, 1379, 1261, 1033 cm−1.
1H NMR (500 MHz, CDCl3): δ = 7.78 (d, J = 8 Hz, 2 H), 6.94 (d, J = 8 Hz, 1 H), 5.98 (s, 1 H), 5.83 (m, 1 H), 5.72–5.78 (m, 2 H), 5.31–5.38 (m, 2 H), 5.28 (d, J = 7, 15 Hz, 1 H), 5.18 (s, 1 H), 5.13 (s, 1 H), 4.51 (d, J = 13 Hz, 1 H), 4.27 (m, 1 H), 4.18 (m, 1 H), 4.12 (t, J = 10 Hz, 1 H), 4.07 (t, J = 8 Hz, 1 H), 3.86 (s, 3 H), 3.66 (s, 3 H), 3.61–3.78 (m, 3 H), 3.54 (t, J = 12 Hz, 1 H), 3.44 (t, J = 12 Hz, 1 H), 2.52–2.60 (m, 2 H), 2.17–2.36 (m, 3 H), 1.97–2.06 (m, 4 H), 1.77–1.84 (m, 3 H), 1.40–1.65 (m, 6 H), 1.18–1.33 (m, 14 H), 1.11 (s, 3 H), 0.98 (s, 3 H), 0.86 (t, J = 7 Hz, 3 H).
HRMS (ES+): m/z calcd for C47H69NO14Na: 894.4610; found: 894.4613
Bryostatin Analogues 17 and 18
Cyclization precursor 12 (9.0 mg, 0.0083 mmol) was dissolved in PhOMe (0.225 mL) under N2 and the resulting soln was cooled in a CO2/MeCN bath for about 10 min. TMSOTf (1.8 μL, 0.0099 mmol) was then added in one portion, and the resulting mixture was stirred for 1.5 h. The reaction was then quenched with NaHCO3 (0.50 mL) and the mixture was diluted with Et2O (1.5 mL) and H2O (1 mL). The organic and aqueous layers were separated and the aqueous layer was extracted with Et2O (3 × 2 mL). The organic phases were combined and concentrated to give a yellow oil that was purified via chromatography (silica gel, 25% EtOAc–PE) to give the cyclized C26-TBS-deprotected product as a clear colorless viscous residue; yield: 4.9 mg (56%); Rf = 0.35 (25% EtOAc–PE)
This product was dissolved in THF (7.0 mL) under N2 in a polypropylene vial and the soln was cooled in an ice water bath. HF·pyridine (70% HF; 1.82 mL) was added dropwise over 1 min, and the mixture was stirred for 10 min. The cold bath was then removed and the soln was stirred for an additional 25 h at r.t. The reaction was quenched with sat. aq NaHCO3 (2 mL) and the mixture was diluted with EtOAc (5 mL). The organic and aqueous layers were separated and the aqueous layer was extracted with EtOAc (3 × 5 mL). The organic phases were combined, dried (Na2SO4), filtered, and concentrated. The crude residue was purified by chromatography (silica gel, 60% EtOAc–PE) to give a 7:3 mixture of the C13-(2-methoxyphenyl) analogue 17 and the C13-(4-methoxyphenyl) analogue 18 as a white solid; yield: 1.9 mg (27%); Rf = 0.40 (60% EtOAc–PE) [stained with anisaldehyde (red)]. The pure analogues were obtained by C18 reverse-phase HPLC (60% MeCN–H2O to 100% MeCN) as amorphous white solids.
17
IR (thin film): 3426, 2922, 2852, 1722, 1663, 1463, 1429, 1376, 1260, 1232, 1158, 1109, 704 cm−1.
1H NMR (500 MHz, CDCl3): δ = 7.13–7.18 (m, 2 H), 6.91 (dt, J = 2, 7 Hz, 1 H), 6.84 (dd, J = 7, 8 Hz, 1 H), 5.99 (s, 1 H), 5.73 (d, J = 15 Hz, 1 H), 5.32–5.37 (m, 2 H), 5.27 (s, 1 H), 5.14 (s, 1 H), 4.64 (d, J = 11 Hz, 1 H), 4.21 (m, 1 H), 4.10 (m, 1 H), 3.88 (m, 1 H), 3.82 (s, 3 H), 3.68 (s, 3 H), 3.66–3.74 (m, 3 H), 3.52–3.63 (m, 5 H), 3.54 (m, 1 H), 3.45 (t, J = 12 Hz, 1 H), 3.38 (t, J = 12 Hz, 1 H), 2.52–2.64 (m, 2 H), 2.26–2.30 (m, 2 H), 2.00–2.12 (m, 5 H), 1.77–1.82 (m, 3 H), 1.40–1.65 (m, 7 H), 1.18–1.33 (m, 9 H), 1.09 (s, 3 H), 0.98 (s, 3 H), 0.86 (t, J = 7 Hz, 3 H).
HRMS (ES+): m/z calcd for C46H68NaO13: 851.4572; found: 851.4552.
18
IR (thin film): 3426, 2922, 2852, 1722, 1663, 1463, 1429, 1376, 1260, 1232, 1158, 1109, 704 cm−1.
1H NMR (500 MHz, CDCl3): δ = 7.08 (d, J = 8 Hz, 1 H), 6.83 (dt, J = 7, 8 Hz, 2 H), 5.99 (s, 1 H), 5.74 (d, J = 15 Hz, 1 H), 5.32–5.37 (m, 2 H), 5.22 (s, 1 H), 5.14 (s, 1 H), 4.60 (d, J = 11 Hz, 1 H), 4.21 (m, 1 H), 4.10 (m, 2 H), 3.88 (m, 1 H), 3.78 (s, 3 H), 3.68 (s, 3 H), 3.66–3.74 (m, 3 H), 3.52–3.63 (m, 5 H), 3.54 (t, J = 12 Hz, 1 H), 3.45 (t, J = 12 Hz, 1 H), 3.38 (t, J = 12 Hz, 1 H), 2.52–2.64 (m, 2 H), 2.26–2.30 (m, 2 H), 2.00–2.12 (m, 5 H), 1.77–1.82 (m, 3 H), 1.40–1.65 (m, 7 H), 1.18–1.33 (m, 9 H), 1.09 (s, 3 H), 0.98 (s, 3 H), 0.86 (t, J = 7 Hz, 3 H).
HRMS (ES+): m/z calcd for C46H68NaO13: 851.4572; found: 851.4575.
Acknowledgments
Support of this work through a grant (CA31845) provided by the NIH to P.A.W. is gratefully acknowledged.
Footnotes
To a friend and scholar in recognition of his towering contributions to education and to mechanistic and synthetic chemistry.
References
- 1.Pettit GR, Herald CL, Doubek DL, Herald DL, Arnold E, Clardy J. J Am Chem Soc. 1982;104:6846. [Google Scholar]
- 2.For overviews of the chemistry and biology of the bryostatins, see: Wender PA, Baryza JL, Hilinski MK, Horan JC, Kan C, Verma VA. In: In Drug Discovery Research: New Frontiers in the Post-Genomic Era. Huang Z, editor. Chap 6. Wiley; Hoboken: 2007. p. 127.Hale KJ, Hummersone S, Manaviazar MG, Frigerio M. Nat Prod Rep. 2002;19:413. doi: 10.1039/b009211h.Hale KJ, Manaviazar S. Chem Asian J. 2010;5:704. doi: 10.1002/asia.200900634.
- 3.For current information, see the US National Institutes of Health Clinical Trials database at http://clinicaltrials.gov
- 4.(a) Spitaler M, Utz I, Hilbe W, Hofmann J, Grunicke HH. Biochem Pharmacol. 1998;56:861. doi: 10.1016/s0006-2952(98)00107-5. [DOI] [PubMed] [Google Scholar]; (b) Alkatib AM, Smith MR, Kamanda WS, Pettit GR, Hamdan M, Mohamed AN, Chelladurai B, Mohammad RM. Clin Cancer Res. 1998;4:1305. [PubMed] [Google Scholar]
- 5.(a) Oz HS, Hughes WT, Rehg JE, Thomas EK. Microb Pathog. 2000;29:187. doi: 10.1006/mpat.2000.0374. [DOI] [PubMed] [Google Scholar]; (b) Scheid C, Prendiville J, Jayson G, Crowther D, Fox B, Pettit GR, Stern PL. Cancer Immunol Immunother. 1994;39:223. doi: 10.1007/BF01525985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Sun MK, Alkon DL. Eur J Pharmacol. 2005;512:43. doi: 10.1016/j.ejphar.2005.02.028. [DOI] [PubMed] [Google Scholar]; (b) Alkon DL, Epstein H, Kuzirian A, Bennett MC, Nelson TJ. Proc Natl Acad Sci U S A. 2005;102:16432. doi: 10.1073/pnas.0508001102. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kuzirian AM, Epstein HT, Gagliardi CJ, Nelson TJ, Sakakibara M, Taylor C, Scioletti AB, Alkon DL. Biol Bull. 2006;210:201. doi: 10.2307/4134558. [DOI] [PubMed] [Google Scholar]
- 7.(a) Sun MK, Hongpaisan J, Nelson TJ, Alkon DL. Proc Natl Acad Sci U S A. 2008;105:13620. doi: 10.1073/pnas.0805952105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sun MK, Hongpaisan J, Alkon DL. Proc Natl Acad Sci U S A. 2009;106:14676. doi: 10.1073/pnas.0907842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehla R, Bivalkar-Mehla S, Zhang R, Handy I, Albrecht H, Giri S, Nagarkatti P, Nagarkatti M, Chauhan A. PLoS One. 2010;5:e11160. doi: 10.1371/journal.pone.0011160.For a recent report on the use of synthetically accessible bryostatin analogues to induce latent HIV reservoirs, see: DeChristopher BA, Loy BA, Marsden MD, Schrier AJ, Zack JA, Wender PA. Nature Chem. 2012;4:705. doi: 10.1038/nchem.1395.
- 9.(a) Rosse C, Linch M, Kermorgant S, Cameron AJM, Boeckeler K, Parker PJ. Nat Rev Mol Cell Biol. 2010;11:103. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]; (b) Newton AC. Chem Rev. 2001;101:2353. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 10.(a) Dekker LV, editor. Protein Kinase C. 2. Springer; New York: 2004. [Google Scholar]; (b) Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. Science (Washington D C) 2002;298:1912. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 11.Stone JC, Stang SL, Zheng Y, Dower NA, Brenner SE, Baryza JL, Wender PA. J Med Chem. 2004;47:6638. doi: 10.1021/jm0495069. [DOI] [PubMed] [Google Scholar]
- 12.Stang SL, Lopez-Campistrous A, Song X, Dower NA, Blumberg PM, Wender PA, Stone JC. Exp Hematol. 2009;37:122. doi: 10.1016/j.exphem.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keck GE, Poudel YB, Cummins TJ, Rubra A, Covel JA. J Am Chem Soc. 2011;133:744. doi: 10.1021/ja110198y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans DA, Carter PH, Carreira EM, Charette AB, Prunet JA, Lautens M. J Am Chem Soc. 1999;121:7540. [Google Scholar]
- 15.Ohmori K, Ogawa Y, Obitsu T, Ishikawa Y, Nishiyama S, Yamamura S. Angew Chem Int Ed. 2000;39:2290. [PubMed] [Google Scholar]
- 16.(a) Kageyama M, Tamura T, Nantz MH, Roberts JC, Somfai P, Whritenour DC, Masamune S. J Am Chem Soc. 1990;112:7407. [Google Scholar]; (b) Lu Y, Woo SK, Krische MJ. J Am Chem Soc. 2011;133:13876. doi: 10.1021/ja205673e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wender PA, Schrier AJ. J Am Chem Soc. 2011;133:9228. doi: 10.1021/ja203034k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trost BM, Dong G. Nature (London) 2008;456:485. doi: 10.1038/nature07543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wender PA, Cribbs CM, Koehler KF, Sharkey NA, Herald CL, Komano Y, Pettit GR, Blumberg PM. Proc Natl Acad Sci U S A. 1988;85:7197. doi: 10.1073/pnas.85.19.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wender PA, Verma VA, Paxton TJ, Pillow TH. Acc Chem Res. 2008;41:40. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]
- 21.Wender PA, Loy BA, Schrier AJ. Isr J Chem. 2011;51:453. doi: 10.1002/ijch.201100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wender PA, De Brabander JK, Harran PG, Jimenez JM, Koehler MFT, Lippa B, Park CM, Siedenbiedel C, Pettit GR. Proc Natl Acad Sci U S A. 1998;95:6624. doi: 10.1073/pnas.95.12.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wender PA, Baryza JL, Bennett CE, Bi FC, Brenner SE, Clarke MO, Horan JC, Kan C, Lacote E, Lippa B, Nell PG, Turner TM. J Am Chem Soc. 2002;124:13648. doi: 10.1021/ja027509+. [DOI] [PubMed] [Google Scholar]
- 24.Wender PA, Baryza JL, Brenner SE, DeChristopher BA, Loy BA, Schrier AJ, Verma VA. Proc Natl Acad Sci U S A. 2011;108:6721. doi: 10.1073/pnas.1015270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wender PA, DeChristopher BA, Schrier AJ. J Am Chem Soc. 2008;131:6658. doi: 10.1021/ja8015632.For a seminal study on the intermolecular silyl-terminated Prins process, see: Marko IE, Bayston DJ. Tetrahedron Lett. 1993;34:6595.and references cited therein. (c) Keck has reported applications of the intermolecular process to bryostatin analogues (see ref. 13 and references cited therein).
- 26.For recent examples of the application of silyl-terminated Prins cyclizations, see: Ogawa Y, Painter PP, Tantillo DJ, Wender PA. J Org Chem. 2013;78:104. doi: 10.1021/jo301953h.Gesinski MR, Rychnovsky SD. J Am Chem Soc. 2011;133:9727. doi: 10.1021/ja204228q.Zhu K, Panek JS. Org Lett. 2011;13:4652. doi: 10.1021/ol201863b.Ghosh AK, Cheng X. Org Lett. 2011;13:4108. doi: 10.1021/ol201626h.Wrona IE, Gozman A, Taldone T, Chiosis G, Panek JS. J Org Chem. 2010;75:2820. doi: 10.1021/jo1000109.
- 27.Olier C, Kaafarani M, Gastaldi S, Bertrand MP. Tetrahedron. 2010;66:413. [Google Scholar]
- 28.Bahnck KB, Rychnovsky SD. J Am Chem Soc. 2008;130:13177. doi: 10.1021/ja805187p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.(a) Custar DW, Zabawa TP, Scheidt KA. J Am Chem Soc. 2008;130:804. doi: 10.1021/ja710080q. [DOI] [PubMed] [Google Scholar]; (b) Woo SK, Kwon MS, Lee E. Angew Chem Int Ed. 2008;47:3242. doi: 10.1002/anie.200800386. [DOI] [PubMed] [Google Scholar]
- 30.Woo SK, Lee E. J Am Chem Soc. 2010;132:4564. doi: 10.1021/ja1009579. [DOI] [PubMed] [Google Scholar]
- 31.For a recent review, see: Crane AE, Scheidt KA. Angew Chem Int Ed. 2010;49:8316. doi: 10.1002/anie.201002809.For previous work, see: Gesinski MR, Tadpetch K, Rychnovsky SD. Org Lett. 2009;11:5342. doi: 10.1021/ol9022062.Custar DE, Zabawa TP, Hines J, Crews CM, Scheidt KA. J Am Chem Soc. 2009;131:12406. doi: 10.1021/ja904604x.Yadav JS, Krishana GG, Kumar SN. Tetrahedron. 2010;66:480.
- 32.Wender PA, Mayweg AVW, VanDeusen CL. Org Lett. 2003;5:277. doi: 10.1021/ol0272390. [DOI] [PubMed] [Google Scholar]
- 33.Miranda PO, Carballo RM, Martín VS, Padrón JI. Org Lett. 2009;11:357. doi: 10.1021/ol802593u. [DOI] [PubMed] [Google Scholar]
- 34.(a) Kishi Y, Nagura H, Inagi S, Fuchigami T. Chem Commun (Cambridge) 2008:3876. doi: 10.1039/b806389c. [DOI] [PubMed] [Google Scholar]; (b) Kishi Y, Nagura H, Inagi S, Fuchigami T. Eur J Org Chem. 2009:103. [Google Scholar]
- 35.(a) Albizati KF, Perron F. J Org Chem. 1987;52:4128. [Google Scholar]; (b) Epstein OL, Rovis T. J Am Chem Soc. 2006;128:16480. doi: 10.1021/ja066794k. [DOI] [PubMed] [Google Scholar]
- 36.Reddy UC, Bondalapati S, Saikia AK. J Org Chem. 2009;74:2605. doi: 10.1021/jo802531h. [DOI] [PubMed] [Google Scholar]
- 37.Wender PA, Verma VA. Org Lett. 2006;8:1893. doi: 10.1021/ol060457z. [DOI] [PubMed] [Google Scholar]