Abstract
Light regulates a variety of behavioral and physiological processes, including activity rhythms and hormone secretory patterns. Seasonal changes in the proportion of light in a day (photoperiod) further modulate those functions. Recently, short (SP) versus long days (LP) were found to markedly increase light sensitivity for phase shifting in Syrian hamsters. To our knowledge, photoperiod effects on light sensitivity have not been studied in other rodents nor is it known if they generalize to other circadian responses. We tested whether photic phase shifting and melatonin suppression vary in Siberian hamsters maintained under LP or SP. Select irradiances of light were administered, and shifts in activity were determined. Photic sensitivity for melatonin suppression was examined in a separate group of animals via pulses of light across a 4 log-unit photon density range, with post-pulse plasma melatonin levels determined via RIA. Phase shifting and melatonin suppression were greater at higher irradiances for both LP and SP. The lower irradiance condition was below threshold for phase shifts in LP but not SP. Melatonin suppression did not vary by photoperiod, and the half saturation constant for fitted sigmoid curves was similar under LP and SP. Thus, photoperiodic modulation of light sensitivity for phase shifting is conserved across two hamster genera. The dissociation of photoperiod effects on photic phase shifting and melatonin suppression suggests modulation of sensitivity occurs downstream of the common retinal input pathway. Understanding the mechanistic basis for this plasticity may yield therapeutic targets for optimizing light therapy practices.
Keywords: light, photoperiod, melatonin, circadian, sensitivity, phase shift, hamster
Introduction
Circadian rhythms include a host of physiological and behavioral processes that maintain an endogenous period of approximately 24 hours in the absence of any environmental cues. Common examples of circadian rhythms in mammals include sleep-wake cycles, hormone fluctuations and core body temperature patterns. Under natural conditions, light resets the phase of the pacemaker in the suprachiasmatic nuclei (SCN) of the hypothalamus in order to maintain a stable relationship between circadian functions and the daily photocycle via a process called entrainment (Pittendrigh and Dann 1976; Pittendrigh 1981; Elliott and Tamarkin 1994). Such shifts reflect the differential effects of light on the SCN at different circadian phases, which are described experimentally in phase response curves (PRCs) (Johnson et al., 2003).
Acute exposure to light during the night resets the phase of circadian rhythms and suppresses nocturnal elevations of the pineal hormone melatonin. Both responses require ocular photoreception in mammals (Czeisler et al. 1995; Lockley et al., 1997, 1998; Wright et al., 2001), and similar spectral sensitivity further suggests a shared photoreceptor system (Provencio et al., 1995; Yoshimura et al., 1996; Hattar et al., 2003; Brainard et al., 2001; Thapan et al., 2001). Anatomical studies also support the existence of a common neural circuit (Klein et al., 1991; Moore, 1995; Panda et al., 2002). A well-established neural pathway conveys photic input for phase resetting and melatonin suppression, including phototransduction by melanopsin-containing ganglion cells (ipRGCs) that project directly to the SCN via the retinohypothalamic tract (RHT) (Klein et al., 1991; Panda et al., 2002). From the SCN, light information follows a multisynaptic pathway to the pineal gland for melatonin regulation, with intermediate connections in the paraventricular hypothalamus, the upper thoracic intermediolateral cell column, the superior cervical ganglion and post-ganglionic sympathetic fibers (reviewed in Moore, 1995). The precise point of divergence between the pathways for phase resetting and the acute suppressive effects of light on plasma melatonin remains unknown.
Because melatonin suppression by light shares similar properties to phase resetting, it has been a useful method for characterizing the physiology underlying the circadian system (Klein et al., 1991; Arendt, 1998; Brainard et al., 2001). Both demonstrate a characteristic dose-dependent function wherein a threshold amount of light is required to elicit a response; a steep rise in response occurs with increasing light intensities; and finally, a maximal saturating response is achieved, which cannot be surpassed with brighter light (Brainard et al., 1982, 2001; Neslon and Takahashi, 1991a; Zeitzer et al., 2000). Sensitivity is commonly indexed as the ED50, defined as the quanta of light required to produce half of the maximum response. Melatonin suppression has been shown to be relatively more sensitive to light than phase resetting, as indicated by the leftward displacement of the melatonin fluence response curve to lower irradiances relative to that for phase shifts (Nelson and Takahashi, 1991b; Zeitzer et al., 2000).
Not only is light the primary environmental cue for entraining rhythms to a 24 hour day, but the duration of light within a given 24-hour cycle (i.e. photoperiod) regulates the seasonality of myriad functions including reproduction, body weight, pelage, immuno-competence and behavior, to name a few (Goldman, 2001). Photoperiod also alters the 24 h waveform, or shape, of circadian rhythms. In both nocturnal and diurnal animals, the high nighttime levels of pineal melatonin are sustained for longer intervals in winter than in summer (Binkley et al., 1977; Rollag et al., 1980; Goldman and Elliott, 1988; Bartness et al., 1993; Lerchl and Schlatt, 1993; Wehr et al., 1993). Parallel lengthening of nocturnal activity under short days (Elliott and Tamarkin, 1994) along with alterations in waveform of SCN neuronal activity (vanderLeest et al., 2007) indicate that these photoperiod influences reflect changes in pacemaker network organization. With regard to phase resetting, under winter photoperiods, light-induced phase shifts occur across a broader fraction of the circadian cycle and the magnitude of the shifts is markedly greater (Pittendrigh et al., 1984; Millette and Turek, 1986; Puchalski and Lynch, 1988; Goldman and Elliott, 1988). These seasonal differences in circadian organization reflect entrainment effects and not sequela of immediate light history since they persist for many days after release into constant conditions (Pittendrigh and Daan, 1976).
In the Syrian hamster, the known effects of photoperiod on circadian function have recently been extended to include the modulation of photic sensitivity. The ED50 is approximately 40-fold lower in hamsters entrained to a short photoperiod than in a long photoperiod (Glickman et al., 2012). Furthermore, those behavioral differences are preceded by differential expression of SCN activation, with a much greater amount of light being required to induce similar levels of expression of pERK, PER1 and cFOS in animals previously maintained under long versus short days (Glickman et al., 2012). Importantly, the increased sensitivity of the short day pacemaker appears to be specific to light inputs since enhanced responsiveness is not found with non-photic resetting cues (Evans et al., 2004). Thus, the enhanced short day sensitivity may be specific to the light input pathway.
In view of the potential utility of enhancing sensitivity to light for treatment of circadian and affective disorders, it is important to identify whether photoperiod effects generalize to melatonin suppression, a response that is often used as a proxy for studies of circadian regulation by light in humans. Limited previous works suggests that photic sensitivity for melatonin suppression by light may indeed be altered by seasonal changes in photoperiod (Thompson et al., 1990; Owen and Arendt, 1992). However, the influence of photoperiod on light sensitivity for melatonin suppression has not previously been studied in an animal model wherein light history can be rigorously controlled and monitored. Siberian hamsters are a particularly good model for studies of circadian function as they demonstrate rhythms in locomotor activity and melatonin secretion, both of which also show robust and predictable changes in waveform as a function of photoperiod (Milette and Turek, 1986; Puchalski and Lynch, 1986; Goldman and Elliott, 1988). In order to determine whether photoperiod history alters sensitivity to light for melatonin suppression, we constructed complete fluence-response curves for light-induced melatonin suppression in Siberian hamsters previously maintained under short versus long days. We also tested the generality of photoperiod influences on photic sensitivity to phase shifts in this species. Increased sensitivity to light for both responses under shorter days would suggest a common mechanism of action is serving to alter light sensitivity as a function of photoperiod. Alternatively, a photoperiod difference in light-induced phase shifts, but not melatonin suppression, would indicate seasonal modulation of photic response is targeting mechanisms unique to phase resetting.
Methods
Male Siberian hamsters (Phodopus sungorus), 4–6 weeks old, were selected from a breeding colony established in 1994. In both studies, animals for each photoperiod condition were maintained in separate large ventilated, light-tight, matte white interior chambers. On test nights, cages were temporarily transferred to a separate matte white interior pulsing cabinet (43 cm × 36 cm × 46 cm) for the 15-min light exposure regime. For the phase shift experiment, animals were individually housed in polypropylene cages (27 cm × 20 cm × 15 cm) equipped with 12 cm diameter wheels from when they first entered their randomly assigned photoperiod entrainment condition until the completion of the study. Animals in the melatonin experiment were group housed in polypropylene cages (48 cm × 27 cm × 20 cm) until the test night, when each animal was transferred to the smaller polypropylene cage (27 cm × 20 cm × 15 cm) immediately before placement in the pulsing cabinet. Food and water were available ad libitum. All procedures were approved by UCSD Institutional Animal Care and Use Committee.
Phase shifting experiment
General protocol
An Aschoff type II randomized within-subjects design was employed in a group of Siberian hamsters that were entrained to LD14:10 (LP, 14 h light, 10 h dark; n =10) or LD10:14 (SP, n = 10) for 6 weeks. To examine photosensitivity associated with phase delay shifts, light pulses were administered 2 h into the dark (ZT14; with ZT12 representing the time of lights out) for both LP and SP. Each animal received three 15-min short wavelength light pulse at 0 (dark control), 0.11 and 3.14 μW/cm2, with each test night separated by 21 days and each photoperiod group counterbalanced for the order of irradiance condition. Animals were exposed to constant darkness beginning at the normal time of lights-off on the night of each test pulse, remained under constant conditions for 10 days following the pulse, and were then re-entrained to their original photoperiod for 11 days. Cages were changed on day 1 of re-entrainment near the expected time of activity onset, under dim red illumination.
Assessment of wheel-running activity
Each one-half wheel revolution generated a switch closure signal that was recorded via VitalView software (Mini-Mitter, Bend, OR). Actograms were analyzed via Clocklab software (Actimetrics, Evanston, IL). Activity onsets and offsets were determined in Clocklab, with adjustments to automated selections being made via eye-fit by raters who were blind to pulsing condition. As a check of proper entrainment to photoperiod condition, activity duration (α) was determined as the mean difference between regression lines fitted to the onsets and offsets for the two weeks preceding the first test pulse. Due to significantly greater variance in offsets versus onsets in baseline entrainment data, the more reliable activity onset was the phase marker used for calculation of phase shifts. Phase shifts were calculated as the difference between the time of activity onset on the day of the light pulse (as determined by a regression line fitted to the onsets of the 7 days prior to the pulse) and the time of the activity onset on the day after the pulse (as predicted by the post-pulse regression line, calculated through activity onsets for days 4–10 following the light pulse). Two animals were eliminated from our analysis due to recording issues on days of constant conditions, making an accurate assessment of phase shift difficult. Phase-shift scores were expressed relative to each animal’s phase shift in response to the sham control condition. All group values are expressed as means ± SEM and analyzed with analysis of variance (ANOVA).
Melatonin suppression experiment
General protocol
A separate cohort of hamsters (n=106 total) was maintained on LP or SP for 6–7 weeks prior to melatonin suppression tests. Each animal was pulsed individually for 15-min with short wavelength light at ZT18 (LP) or ZT20 (SP) at one of the following prescribed intensities: 0, 0.003, 0.03, 0.3, 1.31, 4.8 or 68 uW/cm2 (n=6–8 per irradiance for each photoperiod condition). The timing of the light pulse was chosen to occur approximately 60% of the way through the scotophase (60% for LP, 57% for SP) at a time when circulating melatonin is at its peak under each photoperiod in Siberian hamsters (Darrow and Goldman, 1986; Lerchl and Schlatt, 1993). Precisely one hour following the start time of each light pulse, animals were anesthetized with isofluorane and blood was collected via the retro-orbital sinus using heparinized caraway micro collecting tubes (Fisherbrand). Animals were then euthanized. Blood was transferred to tubes containing 40 uL of heparin, centrifuged, and plasma was stored at −70°C until assay.
Melatonin assay
Melatonin was extracted from samples, and concentrations were determined via an adaptation of the Buhlmann melatonin radioimmunoassay (RIA) kit (01-RK-MDI) from ALPCO (Salem, NH). Extraction included the following, with centrifuging of columns in between each step: conditioning with methanol and water, loading with sample, washing with 10% methanol and hexane, eluting melatonin with methanol, and evaporating to dryness. Extracted material was then reconstituted and diluted using the kit’s zero calibrator, resulting in a functional sensitivity of 2.6 pg/ml. Standard curve calibrators (1–81 pg/ml), experimental and control samples were incubated with the Kennaway anti-melatonin antibody and 125I-melatonin for 18–24 hours at 2–8° C. Second antibody was then added, and samples were incubated for 15 minutes at 2–8° C. After the subsequent addition of water, samples were centrifuged, unbound supernatant was aspirated, and antibody-bound precipitate was counted with a Gamma-counter (Titertek Instruments, Inc., Hunstsville, AL). The gamma counter software utilizes the standard curve to determine relative potency estimates of melatonin levels in pg/ml. Intra- and inter-assay coefficients of variation of this RIA were estimated to be 6.7% and 10.4%, respectively.
Analyses
Melatonin data were analyzed as concentrations (pg/ml) and percent control-adjusted melatonin suppression (((mean melatonin for control − mean melatonin at each irradiance)/mean melatonin for control) × 100; %CA melatonin suppression). Adjustment for dark control values is commonly employed for studies of melatonin suppression to account for baseline differences in melatonin concentrations as would be expected of no pulse controls groups maintained under different baseline conditions (Brainard et al., 1984, 2001; Nelson and Takahashi, 1991; Owen and Arendt, 1992). SPSS (version 13.0.0) was used to perform ANOVA and post hoc tests. Effect size estimates were determined via eta-squared (η2) for ANOVA and Cohen’s d for pairwise comparisons. Eta- squared was calculated as the between group sum of squares/total sum of squares. Cohen’s d was calculated as the difference between two group means divided by the average of the standard deviations for those means. Effect size for within-subjects data was also corrected in order to make direct comparisons to estimates for between-subjects studies (Morris and DeShon, 2002). GraphPad Prism software (version 5; GraphPad Software, San Diego, CA) was used for curve fitting of the fluence-response functions, constraining the minimum to 0 but allowing other parameters to remain unconstrained. Statistical differences between groups were considered significant if p<0.05, with Bonferroni correction for post-hoc comparisons.
Light Sources
From the time of gestation until entry into the study, environmental lighting conditions consisted of 24 hour periods of cycled light and darkness, with 14 hours of light and 10 hours of darkness (i.e. LD14:10). The LP groups remained on the same room photocycle, and the photoperiod for SP animals was achieved by extending the time of lights off by 4 hours. During the entrainment phase of both experiments, photophases were illuminated with broad-spectrum white fluorescent bulbs (F4T5) (100–150 mW/cm2). Dark phases (scotophases) were dimly illuminated by narrowband light-emitting diodes (LEDs) affixed to the shelves of each chamber (560 nm, 23 nm half-peak bandwidth; 7.9 × 10−6 μW/cm2, ~0.01 lux) during both entrainment and post-pulse free-run periods. This dim scotopic illumination is comparable in irradiance to natural ambient light at night and represents a very small fraction (~1/380) of our lowest irradiance test pulse. Dim light scotophases have been shown to facilitate rapid and full entrainment to SP and reduce the incidence of short day non-responder Siberian hamsters (i.e. animals who fail to adopt the more typical winter phenotype, including expansion of activity duration, gonadal regression and weight loss) (Gorman and Elliott, 2004). Experimental light pulses were administered in separate pulsing cabinets that were optimized for a homogenous, highly controlled exposure. Test pulses consisted of a 480 nm (23 nm half-peak bandwidth) 8-LED lamp source with diffuser, positioned in a standardized location at the center top of each cage lid. Irradiance levels of these lamps were obtained using neutral density filters, except for the highest intensity condition (68 μW/cm2), which was administered via a 24-LED lamp with the same spectral composition as the 8-LED lamp source. Spectral power distributions and half-peak bandwidth of the LED lamps were characterized with an Ocean Optics spectral radiometer (model USB2000; Dunedin, FL). Irradiance measures were determined with an IL1700 radiometer (International Light, Inc., Newburyport, MA), with the sensor head positioned 5 cm from the center floor of the cage (approximating the hamster’s eye level). Irradiance was measured in μW/cm2 and converted to photon density (photons/cm2/sec) based on the energy per photon for 480 nm.
Results
Phase shifting study
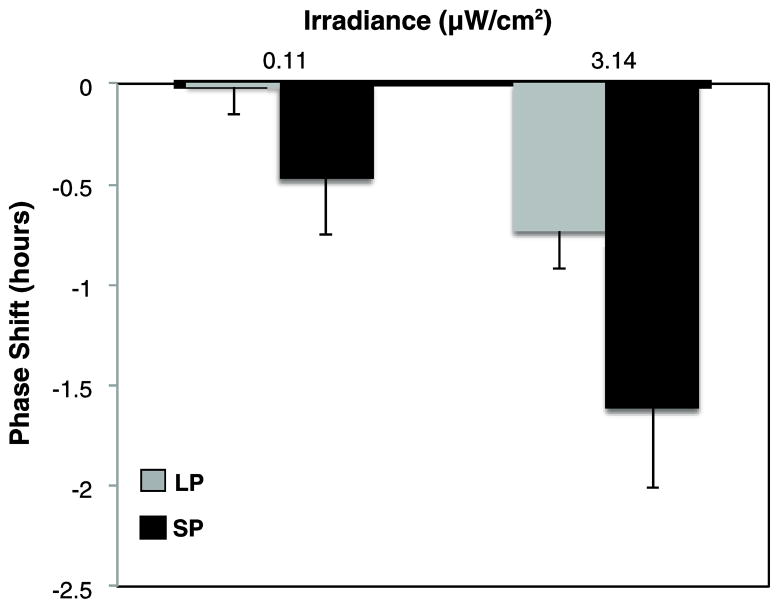
Light pulses of increasing irradiance elicited greater phase delays (main effect repeated-measures ANOVA, F1,18= 25.50, p < 0.001, η2= 0.28). Phase shifting also varied by photoperiod condition (F1,18= 6.91, p<0.02, η2= 0.19), with greater delays in SP versus LP at both tested irradiances (p<0.001; Figures 1 and 2). In addition, LP response to the lower irradiance condition was not different from 0, whereas all other conditions produced significant phase delays. The mean phase shift induced by the sham dark control was not statistically different from 0 for either photoperiod condition (95% CI for LP and SP is [−0.280, 0.248] and [−0.528, 0.272], respectively), confirming that the practice of administering the pulse (independent of the light) was minimally disruptive to the animals. The duration of wheel-running activity (α) differed between long and short day conditions, verifying that animals were appropriately entrained to their respective photoperiods (mean ± SE α for LP: 9.03 ± 0.22 hours and SP: 13.07 ± 0.23 hours).
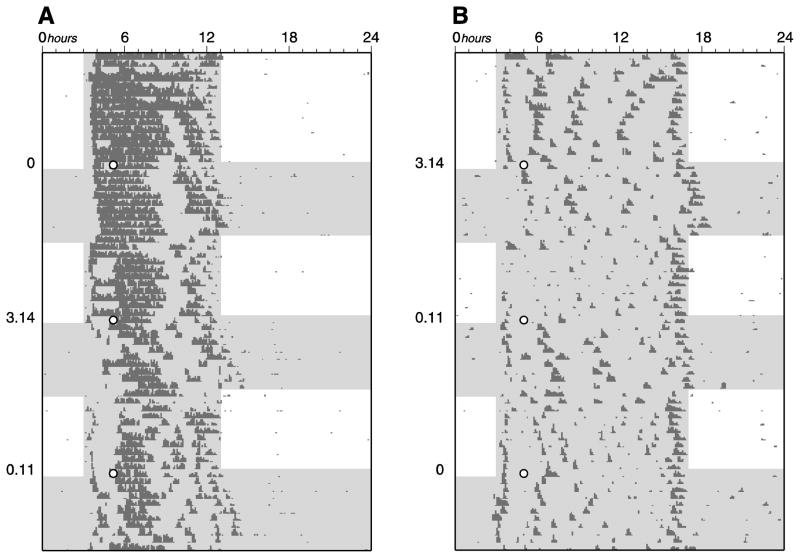
Figure 1.
Representative wheel-running actograms of Siberian hamsters tested for phase-shifting responses following entrainment to A) long photoperiod (LP) and B) short photoperiod (SP). Animals were repeatedly entrained to LP or SP, exposed to a brief light pulse of three different irradiances- 0, 0.11 and 3.14 μW/cm2 (as indicated along the left of each actogram), and allowed to free-run in constant conditions for assessment of phase-shifting. Each line represents a 24-h day. Unshaded and shaded areas represent times of bright light and relative dark, respectively. The 15-min short wavelength light pulses after release into constant conditions are indicated with white circles.
Figure 2.
Dependence of phase-delay magnitude on photoperiod entrainment and irradiance. Mean ± SE phase delays (h) for long photoperiod (LP; gray) versus short photoperiod (SP; black) (n = 10 per condition). Phase-shift scores are expressed relative to each animal’s phase shift on the dark control night. Phase delays differed by both irradiance (p<0.001) and photoperiod (p<0.02).
Melatonin suppression study
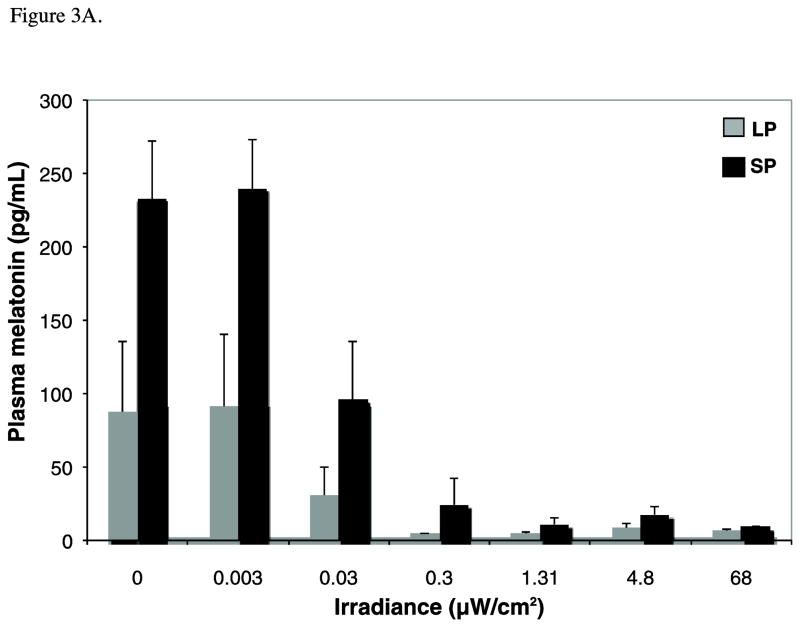
There was a significant effect of both irradiance (F7,92=7.49, p<0.001, η2=0.33) and photoperiod (two-way between subjects ANOVA, F1,92=10.51, p<0.005, η2=0.16) on melatonin concentrations. Figure 3A shows the mean +/− SEM melatonin for the two photoperiod conditions (mean range, 4.9 to 277.7 pg/ml). Melatonin concentrations did not differ between the dark control and 0.003 μW/cm2 exposure conditions (p=1.00, d=0.09) but levels in both groups were higher compared to irradiances >0.03 μW/cm2 (p≤0.05, d ≥0.61). Mean melatonin at 0.03 μW/cm2 was also greater than the remaining higher irradiance conditions (p<0.005, d≥0.81). Finally, melatonin levels at irradiances ≥1.31 μW/cm2 were not statistically different, reflecting saturating responses in both LP and SP. Post-hoc tests showed higher melatonin in SP versus LP animals at all but the highest irradiance condition.
Figure 3.
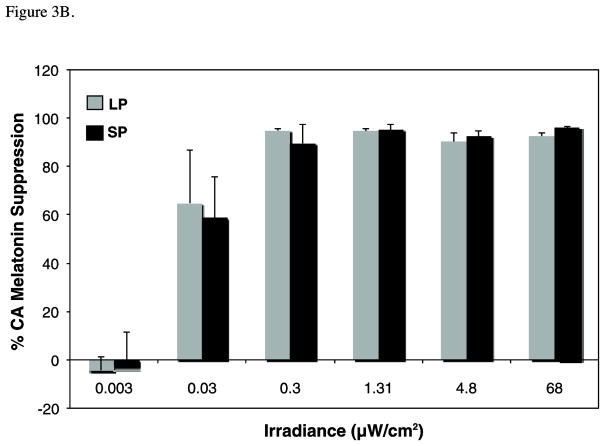
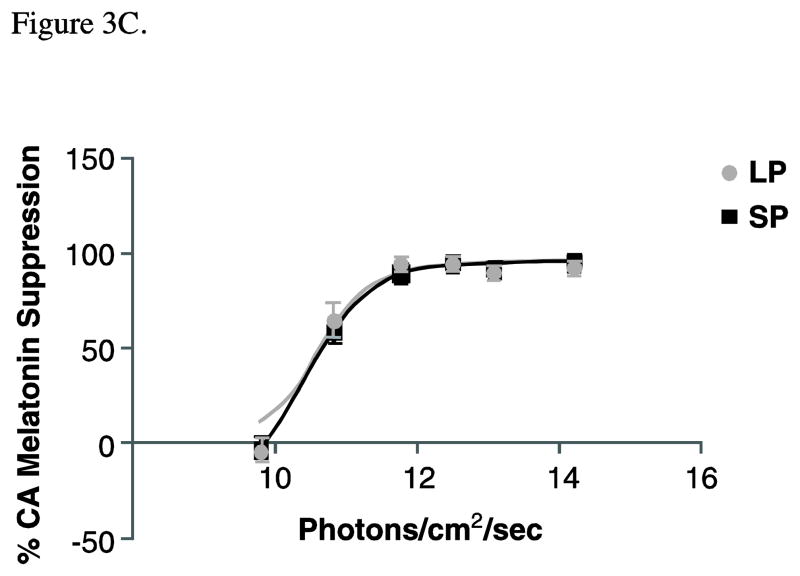
Melatonin data are illustrated in the following figures: A) Raw mean melatonin concentrations (pg/mL) for long photoperiod (LP; gray) versus short photoperiod (SP; black) (n=6–8 per irradiance for each photoperiod condition). Two-way between-subjects ANOVA demonstrates a significant effect of both irradiance (p<0.001) and photoperiod (p<0.005) on melatonin concentrations. Note, however, the significant photoperiod differences for the dark control condition. B) In order to account for the photoperiod differences in baseline levels, these means reflect the percent change in melatonin from the dark control for the photoperiod-matched condition under long (LP; grey) and short (SP; black) days. Suppression scores varied with irradiance (p<0.001) but not by photoperiod (p= .954). C) Fluence-response curves for the same suppression scores are illustrated for animals previously entrained to LP (grey circles) or SP (black squares). Data for both conditions were well fit to this sigmoid function, as demonstrated by high coefficients of correlation (R2 for SP= 0.97 and LP= 0.95). However, there are no photoperiod differences in ED50 (p=0.74) or maximum response (p=0.99).
Melatonin suppression scores varied with irradiance (F6,84=4.96, p<0.001, η2=0.26) but not by photoperiod (F1,84=0.003, p= 0.95, η2<0.00, Figure 3B). All intensities above 0.03 μW/cm2 suppressed melatonin more than 0.003 μW/cm2 (p< 0.05, d≥1.26). In addition, irradiances ≥ 1.31 μW/cm2 reduced circulating melatonin to a greater extent than the two lowest irradiances (p<0.05, d≥1.39). Percent control adjusted data were converted to a best-fit sigmoidal fluence-response curve, with melatonin suppression plotted as a function of photon density on a log linear plot (Figure 3C). The formula for the curve is: Y=Minimum + (Maximum−Minimum)/(1+10^((LogED50-X)*p))(where p estimates the slope of the curve between the minimum and maximal response dose). The data for both photoperiod conditions were well fit to this sigmoid function, as demonstrated by high coefficients of correlation (R2 for SP= 0.97 and LP= 0.95). However, there were no photoperiod differences in ED50 (p=0.74) or maximum response (p=0.99).
Discussion
Here we demonstrate increased photic sensitivity for phase resetting but not melatonin suppression under short photoperiods in Siberian hamsters, suggesting a functional divergence in photoperiod modulation of these two light responses. In addition, our combined study findings are consistent with reports in other species, where lower levels of light are required for melatonin suppression as compared to phase shifting (Nelson and Takahashi, 1991b). Indeed, the lower irradiance in the phase shifting study, which surpassed threshold for SP but not LP, falls on the saturating portion of the melatonin suppression response curves for both photoperiod conditions.
The lower threshold for photic phase delays under SP versus LP in both Siberian and Syrian hamsters suggests a general enhancement of light sensitivity for phase shifting that is common to both species. Because quantitative data on circadian light sensitivity of Siberian hamsters were lacking, irradiances for these studies were selected on the basis of similar experiments in Syrian hamsters, where SP animals show a 40-fold increase in photic sensitivity (Glickman et al., 2012). The range of irradiances employed for the melatonin suppression experiment corresponded to that used in constructing fluence-response for phase advances in Syrian hamsters. The two select irradiances of the phase shifting experiment were calculated from those same fluence-response curves, representing the doses required to elicit an equivalent one hour phase shift under long and short days (which were also used in our Syrian hamster phase delay and SCN experiments). In the present phase shifting study of Siberian hamsters, the lower of the two selected irradiances elicited a delay under short but not under long daylengths whereas a higher irradiance induced shifts under both photoperiods, closely mirroring patterns of behavioral and molecular data from previous Syrian hamster studies (Glickman et al., 2012).
In contrast to results for phase resetting, melatonin suppression does not demonstrate analogous photoperiod modulation. Instead, response curves for the two photoperiod conditions are virtually indistinguishable and do not vary in terms of threshold, sensitivity, or maximum response. The absolute change in melatonin may give the illusion of increased photic sensitivity under shorter days; however, statistical equivalence of control-adjusted scores suggests that this apparent photoperiod effect is being driven by baseline differences (see Figure 3). Photoperiod-dependent disparities in raw melatonin values confirm several but not all previous reports in this species (Hoffman et al., 1981, 1985; Illnerova et al., 1984; Darrow and Goldman, 1986; Hoffman and Illnerova, 1986; Lerchl and Schlatt, 1993; Niklowitz et al., 1994). The fluence response curves for suppression in LP and SP show statistically similar ED50s of 4.78 × 1010 and 4.98 × 1010 photons/cm2/sec, respectively. In terms of threshold, both LP and SP animals require at least 0.03 μW/cm2 in order to significantly reduce circulating melatonin levels. Maximum melatonin suppression is also statistically similar regardless of photoperiod history. This is to be expected since there is an obvious ceiling effect that renders it a less robust comparison measure than the threshold sensitivity or ED50. Ultimately, no matter how the two conditions are compared, photoperiod history does not appear to modulate melatonin suppression by light in the Siberian hamster.
To our knowledge, these are the first published fluence response curves for photic responses in this species. Melatonin suppression by light in Siberian hamsters is well fit to the sigmoid function obtained in other model systems. Dose response curves for melatonin suppression have been described in Syrian hamsters under LD14:10- but not shorter photoperiods- and yield comparable ED50s to that reported here for Siberian hamsters entrained to the same photoperiod (Brainard et al., 1984; Nelson and Takahashi, 1991b). We also do not know of published studies examining photoperiod effects on light-induced melatonin suppression in other mammalian models. In pilot work with Syrian hamsters, we found a sharp and short-lived melatonin peak, particularly under LP, that limited the utility of that model system (personal observations).
In humans, dimensions of photic history other than entrainment photoperiod have been shown to alter melatonin suppression by light (Thompson et al., 1990; Owen and Arendt, 1992; Hebert et al., 2002; Jasser et al., 2006). Specifically, prior exposure to higher daytime light intensities over a one-week period decreases the melatonin suppressing effects of test pulses at night (Hebert et al., 2002). Even just two hours of 18 lux light versus complete dark pre-adaptation results in an attenuated melatonin suppression response (Jasser et al., 2006). Under more naturalistic seasonal conditions, humans demonstrate increased light-induced melatonin suppression during the winter versus summer (Thompson et al., 1990; Owen and Arendt, 1992); however, daylight exposure patterns of the seasonal conditions in those studies were not explicitly monitored or controlled, so it is difficult to know if photoperiod can account for the results. Separate reports have not consistently found a seasonal variation in the distribution of daily light exposure in humans (Cole et al., 1995; Hebert et al., 1998).
In any study administering single light pulses under different photoperiods, two potential confounds to be acknowledged are the amount of time in darkness prior to a test pulse and the circadian timing of the stimulus. With regard to possible dark adaptation effects, both melanopsin-containing ipRGCs and SCN neurons demonstrate hours-long dark-adaptation kinetics (Aggelopoulos and Meissl, 2000; Wong et al., 2005), so it is possible that animals were not completely dark-adapted prior to the administration of light pulses. Because the timing of the light pulse for the melatonin study sampled time points representing similar proportions of the subjective night, animals under short days were exposed to darkness for 2 hours more than those in long days. Notably, in that experiment there was no discernible photoperiodic effect on light sensitivity. In contrast, photic sensitivity was influenced by photoperiod in the phase-resetting experiment in which the immediate duration of darkness was equivalent for the two groups. Therefore, dark adaptation effects cannot account for the increased response to light for phase resetting under shorter days.
With regard to equivalence of circadian phase, it is not possible to match the phase of two different waveforms with single time points. For example, if two different photoperiods are aligned based on one phase indicator (e.g. activity onset), they are rendered misaligned with respect to another (e.g. activity offset). Therefore, within each reported experiment, we cannot claim that our selected pulse times represent identical circadian phases for the SP and LP conditions. In addition, the melatonin suppression data were derived using light stimuli in the late subjective night (a time that reliably elicits phase advancing), whereas the phase shifting study employed phase delaying stimuli administered in the early subjective night. We cannot, therefore, rule out the possibility that photoperiod modulation of sensitivity to light may be partly due to circadian phase in Siberian hamsters. However, there is no evidence indicating the timing of a light pulse contributes significantly to threshold response or absolute sensitivity to light in hamsters. On the contrary, full dose response curves to light for phase shifting of activity rhythms at two selected phases do not reveal differences in sensitivity (i.e. ED50) (Nelson and Takahashi, 1991). Furthermore, photoperiod effects have not been limited to a particular circadian phase in Syrian hamsters, where the greater photic phase shifts under SP versus LP are maintained at different times in the subjective night (Evans et al., 2004; Glickman et al., 2012).
Constant dim illumination throughout all scotophases was employed to be consistent with our previous work, wherein dimly lit nights serve to enhance photoperiod entrainment but do not induce robust shifts in rhythms on their own (Evans et al., 2007). Dim light of similar irradiance has also been relatively ineffective at suppressing melatonin in Syrian hamsters (Brainard et al., 1982, 1984; Nelson and Takahashi, 1991b). Consistent with those findings, our melatonin study does not show levels of the hormone to differ between the lowest irradiance pulse and the sham condition for either photoperiod group, suggesting that the dim light alone is not potent enough to elicit a neuroendocrine response in Siberian hamsters.
In summary, these studies suggest that photic responses of phase resetting and melatonin suppression are modulated differently by photoperiod history. In short daylengths, the increased photic sensitivity for the phase-resetting function of the SCN without comparable changes to melatonin suppression suggests a mechanism downstream of their common retinal input. This divergence may take place at the level of the SCN and/or involve alternate paths to the pineal (e.g. intergeniculate leaflet (Mikkelsen and Møller, 1990)). It has been proposed that light induces per1 mRNA expression and suppresses melatonin via separate synaptic mechanisms as NMDA receptor activation in the SCN is required for the former but not the latter (Paul et al., 2003). Since photoperiod modulation of photic sensitivity is evident very early in the signal transduction cascade of SCN neurons (Glickman et al., 2012) but not for melatonin suppression (as reported here), we suggest that only the pathway involving NMDA receptor activation is photoperiod sensitive. Better understanding the mechanistic basis for the profound impact of photoperiod on light response may ultimately lead to new strategies for optimizing light therapy for circadian sleep disturbances and affective disorders.
Highlights.
Photic thresholds for circadian responses were assessed in Siberian hamsters
Light sensitivity for phase shifting is enhanced in short versus long daylengths
Light sensitivity for melatonin suppression is identical in long versus short days
Modulation of light sensitivity occurs downstream of common retinal input pathway
Photoperiod effects on circadian resetting are conserved across hamster species
Acknowledgments
The authors gratefully acknowledge the animal care provided by Antonio Mora. Special thanks are also extended to Aaron Steckelberg and Jonathan Sun for technical assistance. This work was supported by grants NICHD36460, NS30235, and NS067934 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggelopoulos NC, Meissl H. Responses of neurons of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. J Physiol. 2000;523:211–222. doi: 10.1111/j.1469-7793.2000.t01-1-00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev Reprod. 1998;3:13–22. doi: 10.1530/ror.0.0030013. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery – what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses. J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Binkley S, Stephens J, Riebman J, Reilly K. Regulation of pineal rhythms in chickens: photoperiod and dark-time sensitivity. Gen Comp Endocrinol. 1977;32:411–416. doi: 10.1016/0016-6480(77)90222-2. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Ricahrdson B, Peterborg L, Reiter R. The effect of different light intensities on pineal melatonin content. Brain Res. 1982;233:75–81. doi: 10.1016/0006-8993(82)90931-3. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Richardson BA, Hurlbut EC, Steinlechner S, Matthews SA, Reiter RJ. The influence of various irradiances of artificial light, twilight and moonlight on the suppression of pineal melatonin content in the Syrian hamster. J Pineal Res. 1984;1(2):105–119. doi: 10.1111/j.1600-079x.1984.tb00202.x. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RJ, Kripke DF, Wisbey J, Mason WJ, Gruen W, Hauri PJ, Juarez S. Seasonal variation in human illuminaton exposure at two different latitudes. J Biol Rhythms. 1995;10:324–334. doi: 10.1177/074873049501000406. [DOI] [PubMed] [Google Scholar]
- Czeisler C, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF. Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- Darrow JM, Goldman BD. Circadian regulation of pineal melatonin and reproduction in the Djungarian hamster. J Biol Rhythms. 1986;1:39–54. doi: 10.1177/074873048600100106. [DOI] [PubMed] [Google Scholar]
- Elliott JA, Tamarkin L. Complex circadian regulation of pineal melatonin and wheel-running in Syrian hamsters. J Comp Physiol A. 1994;174:469–484. doi: 10.1007/BF00191713. [DOI] [PubMed] [Google Scholar]
- Evans JA, Elliott JA, Gorman MR. Photoperiod differentially modulates photic and nonphotic phase response curves of hamsters. Am J Physiol Regul Integr Comp Physiol. 2004;286:R539–R546. doi: 10.1152/ajpregu.00456.2003. [DOI] [PubMed] [Google Scholar]
- Evans JA, Elliott JA, Gorman MR. Circadian effects of light no brighter than moonlight. J Biol Rhythms. 2007;22:356–367. doi: 10.1177/0748730407301988. [DOI] [PubMed] [Google Scholar]
- Glickman G, Webb IC, Elliott JA, Baltazar RM, Reale ME, Lehman MN, Gorman MR. Photic sensitivity for circadian response to light varies with photoperiod. J Biol Rhythms. 2012;27:308–318. doi: 10.1177/0748730412450826. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Goldman BD, Elliott JA. Photoperiodism and seasonality in hamsters: role of the pineal gland. In: Stetson MH, editor. Processing of Enrvironmental Information In Vertebrates. New York: Springer-Verlag; 1988. pp. 203–218. [Google Scholar]
- Gorman MR, Elliott JA. Dim nocturnal illumination alters coupling of circadian pacemakers in Siberian hamsters, Phodopus sungorus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190(8):631–639. doi: 10.1007/s00359-004-0522-7. [DOI] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofman F, Foster RG. Melaopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert M, Dumont M, Pacquet J. Seasonal and diurnal patterns of human illumination in natural conditions. Chronobiol Int. 1998;15:59–70. doi: 10.3109/07420529808998670. [DOI] [PubMed] [Google Scholar]
- Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Illnerova H, Vanecek J. Effect of photoperiod and of one minute light at night-time on the pineal rhythm on N-acetyltransferase activity in the Djungarian Hamster Phodopus sungorus. Biology of Reproduction. 1981;24:551–556. doi: 10.1095/biolreprod24.3.551. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Illnerova H, Vanecek J. Comparison of pineal melatonin rhythms in young adult and old djungarian hamsters (phodopus sungorus) under long and short photoperiods. Neurosci Lett. 1985;56:39–43. doi: 10.1016/0304-3940(85)90437-9. [DOI] [PubMed] [Google Scholar]
- Illnerova H, Hoffman K, Vanecek J. Adjustment of pineal melatonin and n-acetyltransferase rhythms to change from long to short photoperiod in the Djungarian hamster Phodopus sungorus. Neuroendocrinol. 1984;38:226–231. doi: 10.1159/000123895. [DOI] [PubMed] [Google Scholar]
- Jasser SA, Hanifin JP, Rollag MD, Brainard GC. Dim light adaptation attenuates acute melatonin suppression in humans. J Biol Rhythms. 2006;21(5):394–404. doi: 10.1177/0748730406292391. [DOI] [PubMed] [Google Scholar]
- Johnson C, Elliott J, Foster R. Entrainment of circadian programs. Chronobiology Intl. 2003;20(5):741–774. doi: 10.1081/cbi-120024211. [DOI] [PubMed] [Google Scholar]
- Klein D, Moore R, Reppert S, editors. Suprachiasmatic Nucleus: The Mind’s Clock. Oxford: Oxford University Press; 1991. [Google Scholar]
- Lerchl A, Schlatt S. Influence of photoperiod on pineal melatonin synthesis, fur color, body weight and reproductive function in the female Djungarian hamster. Phodopus sungorus. Neuroendocrinol. 1993;57:359–364. doi: 10.1159/000126380. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, Arendt J, Tabandeh H, Bird AC, Defrace R. Relationship between melatonin rhythms and visual loss in blind. J Clin Endocrinol Metab. 1997;82:3763–3770. doi: 10.1210/jcem.82.11.4355. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, Thapan K, English J, Ribeiro D, Haimov I, Hampton S, Middleton B, von Schantz M, Arendt J. Extraocular light exposure does not suppress plasma melatonin in humans. J Clin Endocrinol Metab. 1998;83:3369–3372. doi: 10.1210/jcem.83.9.5244. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Møller M. A direct connexion from the intergeniculate leaflet of the lateral geniculate nucleus to the deep pineal gland demonstrated with Phaseolus vulgaris leucoagglutinin (PHA-L) in the rat. Brain Res. 1990;520:342–347. doi: 10.1016/0006-8993(90)91727-x. [DOI] [PubMed] [Google Scholar]
- Millette JJ, Turek FW. Circadian and photoperiod effects of brief light pulses in male Djungarian hamsters. Biol Reprod. 1986;35(2):327–335. doi: 10.1095/biolreprod35.2.327. [DOI] [PubMed] [Google Scholar]
- Moore RY. Organization of the mammalian circadian system. Ciba Found Symp. 1995;183:88–99. [PubMed] [Google Scholar]
- Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological Methods. 2002;7:105–125. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus) J Physiol. 1991a;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Comparison of visual sensitivity for suppression of pineal melatonin and circadian phase shifting in the golden hamster. Brain Res. 1991b;554:272–277. doi: 10.1016/0006-8993(91)90200-f. [DOI] [PubMed] [Google Scholar]
- Niklowitz P, Lerchl A, Nieschlag E. Photoperiodic responses in Djungarian hamsters (Phodopus sungorus): Importance of light history for pineal and serum melatonin profiles. Biol Reprod. 1994;51:714–724. doi: 10.1095/biolreprod51.4.714. [DOI] [PubMed] [Google Scholar]
- Owen J, Arendt J. Melatonin suppression in human subjects by bright and dim light in Antarctica: time and season dependent changes. Neurosci Lett. 1992;137:181–184. doi: 10.1016/0304-3940(92)90399-r. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato T, Castrucci A, Rollag MD, DeGrip W, Hogenesch J, Provencio I, Kay S. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Sci. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Paul KN, Fukuhara C, Tosini G, Albers HE. Transduction of light in the suprachiasmatic nucleus: evidence for two different neurochemical cascades regulating the levels of Per1 mRNA and pineal melatonin. Neurosci. 2004;119(1):137–144. doi: 10.1016/s0306-4522(03)00098-8. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS. Circadian systems: entrainment. In: Achoff J, editor. Handbook of Behavioral Neurobiology: Biological Rhythms. IV. New York: Plenum; 1981. pp. 95–124. [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents: IV. Entrainment: pacemaker as clock. J Comp Physiol. 1976;106:291–351. [Google Scholar]
- Pittendrigh CS, Elliott JA, Takamura T. The circadian component in photoperiodic induction. CIBA Found Symp. 1984;104:26–47. [Google Scholar]
- Provencio I, Foster R. Circadian rhythms in mice can be regulated by photoreceptors with cone-like characteristics. Brain Res. 1995;694:183–190. doi: 10.1016/0006-8993(95)00694-l. [DOI] [PubMed] [Google Scholar]
- Puchalski W, Lynch GR. Evidence for differences in the circadian organization of hamsters exposed to short day photoperiod. J Comp Physiol. 1986;159(1):7–11. doi: 10.1007/BF00612490. [DOI] [PubMed] [Google Scholar]
- Rollag MD, Panke E, Reiter RJ. Pineal melatonin content in male hamsters throughout the seasonal reproductive cycle. Proc Soc Exp Biol Med. 1980;165:330–334. doi: 10.3181/00379727-165-40981. [DOI] [PubMed] [Google Scholar]
- Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;53:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C, Stinson D, Smith A. Seasonal affective disorder and season dependent abnormalities of melatonin suppression by light. Lancet. 1990;336:703–706. doi: 10.1016/0140-6736(90)92202-s. [DOI] [PubMed] [Google Scholar]
- vanderLeest H, Houben T, Michel S, Deboer T, Albus H, Vansteensel M, Block G, Meijer J. Seasonal encoding by the circadian pacemaker of the SCN. Current Biol. 2007;17:468–473. doi: 10.1016/j.cub.2007.01.048. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Moul DE, Barbato G, Giesen HA, Seidel JA, Barker C, Bender C. Conservation of photoperiod responsive mechanisms in humans. Am J Physiol. 1993;265:R846–R857. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- Wong K, Dunn F, Berson D. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:1001–1010. doi: 10.1016/j.neuron.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Ebihara S. Spectral sensitivity of photoreceptors mediating phase-shifts of circadian rhythms in retinally degenerate CBA/J (rd/rd) and normal CBA/N (+/+) mice. J Comp Physiol (A) 1996;178:797–802. doi: 10.1007/BF00225828. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeizler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]