Abstract
Upon activation, a subset of mature human CD8+ T cells re-expresses CD4 dimly. This CD4dimCD8bright T cell population is genuine and enriched in antiviral CD8+ T cell responses. The signaling pathway that leads to CD4 re-expression on mature CD8+ T cells is not clear. Given that Wnt/β-catenin signaling plays a critical role in the transition of CD4−CD8− to CD4+CD8+ thymocytes, we determined whether β-catenin mediates CD4 expression on mature CD8+ T cells. We demonstrate that active β-catenin expression is 20-fold higher on CD4dimCD8bright than CD4−CD8+ T cells. Activation of β-catenin signaling, through LiCl or transfection with a constitutively active construct of β-catenin, induced CD4 on CD8+ T cells by ~10-fold. Conversely, inhibition of β-catenin signaling through transfection with a dominant-negative construct for T cell factor-4, a downstream effector of β-catenin signaling, diminished CD4 expression on CD8+ T cells by 50% in response to T cell activation. β-catenin–mediated induction of CD4 on CD8+ T cells is transcriptionally regulated, as it induced CD4 mRNA, and T cell factor/lymphoid enhancer factor sites were identified within the human CD4 promoter. Further, β-catenin expression induced the antiapoptotic factor BcL-xL, suggesting that β-catenin may mediate protection against activation-induced cell death. Collectively, these data demonstrate that β-catenin is critical in inducing CD4 expression on mature CD8+ T cells, suggesting that it is a common pathway for CD4 upregulation among thymocytes and mature CD8+ T cells.
The majority of human peripheral T cells express either CD4 or CD8 on their surface, defining Th and cytotoxic T cells, respectively. Yet, considerable evidence from our laboratory (1–3) and that of others (4–9) demonstrates that CD4 can be expressed on a subset of mature CD8+ T cells. In the periphery, 1–3% of lymphocytes and 3–5% of CD8+ T cells express CD4 on their surface (10, 11). Expression of CD4 on CD8+ T cells is lower than that on conventional CD4+ Th cells, and thus, this population is often designated CD4dimCD8bright T cells. In response to T cell activation, such as stimulation by anti-CD3/CD28 Abs or super-Ag staphylococcal enterotoxin B (SEB), de novo CD4 is induced on purified CD8+ T cells by 30–60% (1). This finding suggests that upregulation of CD4 on CD8+ T cells is a normal response to T cell activation.
The importance of CD4dimCD8bright T cells in antiviral immunity is emerging. CD4 expression on CD8+ T cells enhances CD8+ T cell responses (2, 6, 7, 12). We have demonstrated that CD4dimCD8bright T cells are enriched in potent HIV- and CMV-specific responses (13). Others have shown that CD4dimCD8bright T cells have higher IFN-γ responses postligation of the CD4 molecule (6). In CD4 knockout mice, which lack CD4 expression on CD8+ T cells, CD8+ T cell responses to lymphocytic choriomeningitis virus infection are diminished (7). Despite the emerging importance of CD4dimCD8bright T cells in antiviral immunity (2, 6, 7, 12), the signaling pathway that leads to re-expression of CD4 on mature CD8+ T cells is not clear.
The Wnt signaling pathway is highly conserved among species. In humans, Wnt is a family of 19 soluble secreted glycoproteins involved in signal transduction pathways that regulate the transcriptional activity of hundreds of genes that impact cell differentiation, communication, apoptosis/survival, and proliferation. Wnt/β-catenin signaling is initiated by binding of Wntprotein to the seven transmembrane Frizzled family of receptors. When the Wnt signal is inactive, the destruction complex (consisting of glycogen synthase kinase 3β [GSK-3β], adenomatosis polyposis coli, and axin) phosphorylates β-catenin, allowing for its ubiquination and proteosomal degradation. Binding of Wnt to Frizzled often requires the recruitment of low-density lipoprotein receptor-related protein 5/6. An intact Wnt signal inhibits this destruction complex from phosphorylating and tagging β-catenin for degradation. Hypophosphorylated β-catenin can function as a transcriptional coactivator, or it can bind to cadherins to provide structural support for adhesion. As a transcriptional coactivator, β-catenin binds to the transcriptional factor T cell factor/lymphoid enhancer factor (TCF/LEF), leading to target gene transcription.
The canonical Wnt/β-catenin pathway plays a significant role in mediating the transition of thymocytes from the double-negative (CD4−CD8−) to the double-positive (DP; CD4+CD8+) stage (14). Continued activation of β-catenin, however, causes a developmental block in the transition from DP to single-positive (SP) thymocytes (15). These findings indicate that β-catenin is important in generation of DP thymocytes but that the pathway must be turned off prior to T cell maturation and release from the thymus.
Although Wnt/β-catenin signaling is thought to be silenced post thymocyte development, accumulating evidence points to reactivation of this signaling pathway in mature T and B lymphocytes in nononcogenic states (16–19). Given that β-catenin plays a role in the transcriptional upregulation of CD4 on thymocytes, we evaluated whether expression of active β-catenin induces CD4 on mature CD8+ T cells. We used both gain and loss of function studies to determine the role of β-catenin on induction of the CD4dimCD8bright T cell phenotype and determined the biologic relevance of an intact Wnt/β-catenin signal in T cells.
Materials and Methods
Reagents
LiCl and SEB were purchased from Sigma-Aldrich (St. Louis, MO). Anti-active β-catenin was purchased from U.S. Biological (Swampscott, MA). Goat anti-mouse secondary Abs (FITC and allophycocyanin) were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Constitutively active β-catenin construct (pcDNA3-S33Y β-catenin plasmid 19286) was purchased from Addgene (Cambridge, MA) (and is described in Ref. 20). Anti-CD3 (clone UCHT1, AF700, PE-Cy7), anti-CD8 (clone SK1, PerCP–Cy5.5, APC-H7), anti-CD4 (clone SK3, APC), anti–Bcl-2 (clone 6C8, FITC), anti-CD19 (clone SJ25C1, APC-H7), and anti-CD14 (clone M5E2, Pacific Blue) were purchased from BD Pharmingen (San Diego, CA). Anti-CD4 (clone S3.5, PE-TR) and Aqua Live/Dead viability dye were purchased from Invitrogen (Carlsbad, CA). Anti–Bcl-xL (clone 2H12, PE) was purchased from Beckman Coulter (Fullerton, CA). The TCF-4 DN mutant construct has a DNA binding domain but lacks the N terminus required for β-catenin binding (21) and was a gift from James O’Kelly (University of California, Los Angeles, Los Angeles, CA).
Lymphocyte isolation, immunofluorescence staining, and flow cytometric analysis
Venous blood was obtained from healthy donors in accordance with institutional and U.S. government guidelines on human research. PBMCs were isolated from blood using lymphocyte separation medium (Lonza Biologics, Portsmouth, NH) and density centrifugation. In some experiments, CD8+ T cells were isolated by negative immunoselection (Invitrogen Dynal CD8 Negative Isolation Kit, Invitrogen). The cells were subsequently cultured for 1, 3, or 6 d, depending on the experiment and mode of stimulation. Poststimulation, cells were collected via centrifugation and stained with appropriate Abs using conventional surface and/or intracellular staining methods. Cells were subsequently fixed with 2% formaldehyde. An LSRII flow cytometer with FACSDiva software (BD Biosciences, San Jose, CA) was used for data collection and analysis. Side scatter area versus Aqua Live/Dead staining and forward scatter area versus forward scatter height were employed to exclude dead cells and doublets, respectively.
Nucleofection
Cells were nucleofected with DN TCF-4, constitutively active β-catenin, GFP plasmids, or mock nucleofected using a nucelofection protocol for nonstimulated T cells, according to the manufacturer’s instructions (Lonza Biologics). Postnucleofection, cells were rested in complete media (RPMI 1640, 10% heat-inactivated FBS, 200 mM l-glutamine, and 1% penicillin/streptomycin) for 4 h, then treated as described for each experiment or left untreated.
Real-time RT-PCR
RNA was isolated using TRIzol reagent (Invitrogen), according to the manufacturer’s recommendations. Subsequently, cDNA was synthesized using the Quantitect reverse transcription kit (Qiagen, Valencia, CA). Real-time RT-PCR was performed using a Quantitect SYBR green PCR kit (Qiagen) in a 7900HT Fast Real-Time PCR system (Applied Biosystems, Foster City, CA). Melting curve analysis was performed to ensure that the primers amplified the desired amplicon and that primer-dimers were absent. Primers used were: Bcl-xL mRNA forward 5′-GGAAAGCGTAGACAAGGAGATG-3′ and reverse 5′-TCCACAAAAGTATCCCAGCC-3; CD4 mRNA forward 5′-GCCCTTGAAGCGAAAACAG-3′ and reverse 5′-CTCCTTGTTCTCCAGTTTCAAAC-3′; and GAPDH mRNA forward 5′-CTTCAACGACCACTTTGT-3′ and reverse 5′-TGGTCCAGGGGTCTTACT-3′. Fold change in RNA expression was calculated by relative quantification using the comparative cycle threshold method. GAPDH expression was used as endogenous control.
EMSA
dsDNA probes with or without biotin conjugation at the 5′ end were purchased from IDT DNA Technologies (San Diego, CA). Sequence of the TCF binding probe was 5′-GTGTCAAAGATTACA-3′ and the scrambled probe was 5′-GTGTGGCCTCTTACA-3′. Nuclear extract was prepared from U251 astrocyte cells, a rich source of TCF expression, according to instructions provided by the NE-PER Nuclear and Cytoplasmic Extraction Reagent kit (Thermo Fisher Scientific, Waltham, MA). Protein content was determined by the Pierce BCA protein assay kit (Thermo Fisher Scientific). For EMSA, 50 ng rTCF4 protein (Abnova, Taipei, Taiwan) and 100 ng probe was incubated for 15 min at room temperature in a total volume of 10 µl binding buffer (10 mM Tris [pH 7.5], 50 mM NaCl, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, and 4% glycerol). The DNA–protein complexes were run on 6% native gel and detected with SYBR green dye using the EMSA kit, as recommended by the manufacturer (Invitrogen).
Immunoprecipitation and Western blot
A total of 100 µg nuclear extract was incubated with 200 ng biotinylated TCF or scrambled probe for 5 min at room temperature with mild mixing. Streptavidin-conjugated agarose beads were added, and incubation was continued for another 30 min with slow mixing. Beads were then pelleted, washed three times with TNE buffer (10 mM Tris [pH 7.5], 1 mM EDTA, and 300 mM NaCl), suspended in SDS loading buffer, and equal volumes run on a 10% SDS electrophoresis gel. The gel was then transferred onto a nitrocellulose membrane and immunoblotted with a mouse anti-TCF4 Ab (Cell Signaling Technology, Beverly, MA).
Statistical analyses
Statistical analyses were performed using GraphPad Prism software version 4.03 (GraphPad, La Jolla, CA). Two-tailed unpaired t tests were used for data evaluation. p values <0.05 were considered significant.
Results
CD4dimCD8bright T cells express higher levels of basal and inducible active β-catenin than other mature (CD4−CD8+ and CD4+CD8−) T cells
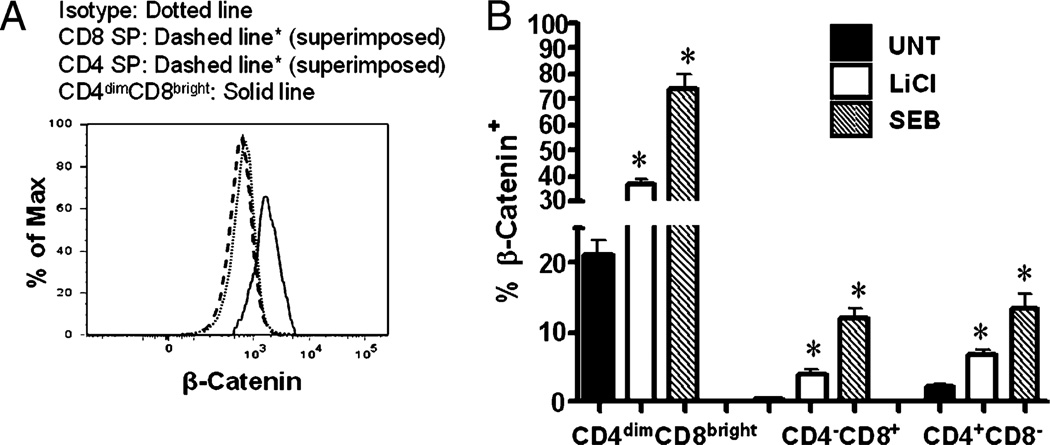
To determine basal and inducible expression of β-catenin in CD4−CD8+, CD4+CD8−, and CD4dimCD8bright T cells, PBMCs were isolated from healthy donors and used fresh, stimulated with SEB, LiCl, or left untreated for 6 d. LiCl is a well-known activator of β-catenin expression through inhibition of GSK-3β (22), and SEB stimulation of PBMCs results in maximum induction of CD4 on CD8+ T cells by day 6 (1). Therefore, cells were stained on day 6 for intracellular β-catenin. Basal expression of β-catenin was ~20-fold higher in CD4dimCD8bright T cells than in both CD4−CD8+ and CD4+CD8− T cells (Fig. 1; 21 versus 0.4% and 2.2%, respectively; p < 0.0001). LiCl and SEB treatments resulted in a significant increase of active β-catenin expression in all T cell subsets (CD4dimCD8bright, CD4−CD8+, and CD4+CD8−), with CD4dimCD8bright T cells demonstrating a dramatic upregulation of β-catenin (Fig. 1B). In CD4dimCD8bright T cells, β-catenin expression was enhanced from ~21% in untreated cultures to 37% for LiCl (p < 0.005) and 74% for SEB-treated cells (p < 0.005). In CD4−CD8+ T cells, the level of β-catenin increased from 0.4 to 4% and 12% post LiCl (p < 0.005) and SEB (p < 0.0001) treatments, respectively. In CD4+CD8− T cells, β-catenin expression increased from a baseline of 2.2–6.8% post LiCl (p < 0.005) and 13.5% post-SEB treatment (p < 0.005). These data demonstrate that although resting CD4−CD8+ and CD4+CD8− have relatively low levels of active β-catenin, resting CD4dimCD8brightT cells have significantly higher levels of β-catenin. Further, β-catenin expression is induced in all T cell subsets in response to stimuli that are known to activate β-catenin signaling (LiCl) or result in T cell activation (SEB), with the most robust induction occurring within the CD4dimCD8bright T cell population.
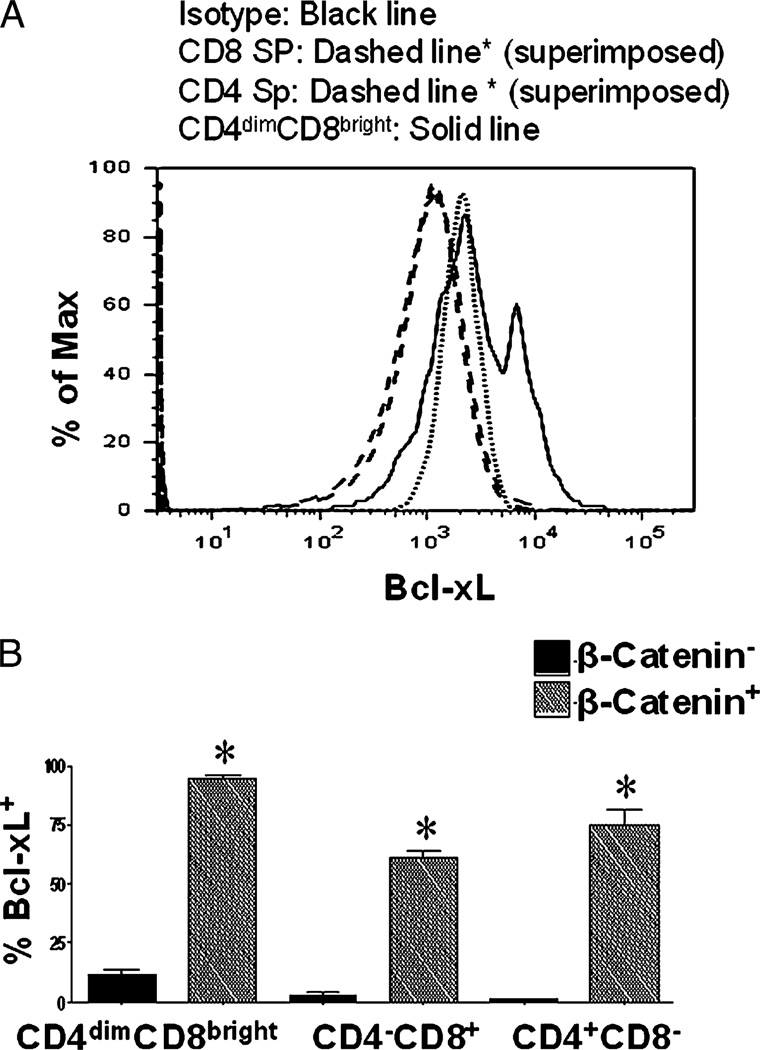
FIGURE 1.
Basal β-catenin is higher in CD4dimCD8bright T cells than CD4−CD8+ and CD4+CD8− T cells and is inducible in all three T cell subsets. A, PBMCs from healthy donors were immunostained for active β-catenin, CD3, CD4, and CD8 in conjunction with a live/dead stain. A representative histogram of active β-catenin expression in CD4dimCD8bright, CD8+ SP, and CD4+ SP T cells is shown. B, PBMCs from healthy donors were stimulated with LiCl (5 mM), SEB (1 µg/ml), or left untreated (UNT) for 6 d. Expression of active β-catenin on the three T cell subsets (x-axis) was evaluated by conventional flow cytometry. Data represent a minimum of three experiments from three healthy donors. *p < 0.005. UNT, untreated.
Stimuli that induce β-catenin expression cause upregulation of CD4 on CD8+ T cells
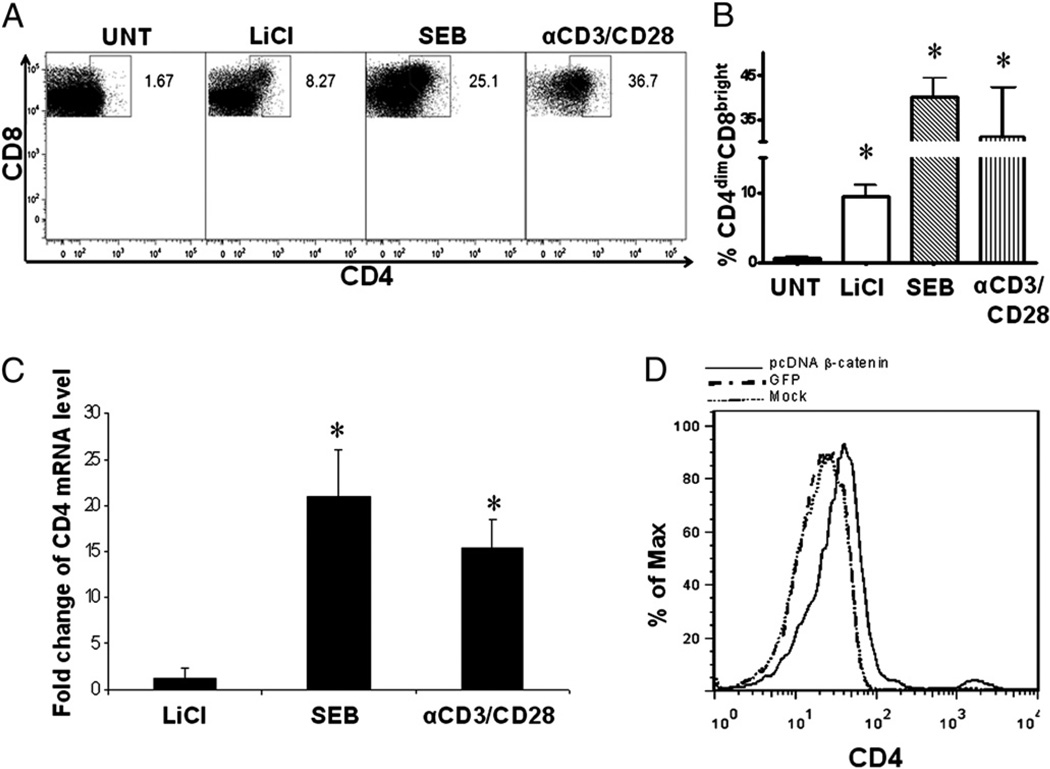
To determine the impact of β-catenin expression on induction of CD4 expression in CD8+ T cells, CD8+ T cells were isolated from blood of healthy donors by negative immunoselection as previously described (>99% purity) (2). Purified CD8+ T cells were then treated with LiCl (5 mM), a well-described inducer of β-catenin expression (23), SEB (1 µg/ml), or anti-CD3/CD28 (1 µg/ml each) or left untreated. Post 6 d of culture, CD4 expression in response to these treatments was evaluated by flow cytometry (Fig. 2A). LiCl induced CD4 expression on CD8+ T cells by ~16-fold in comparison with untreated cultures (9.5 versus 0.58%; p < 0.005) (Fig. 2B). SEB and anti-CD3/CD28 costimulation, as expected, induced CD4 expression on CD8+ T cells by ~69-fold and 50-fold in comparison with untreated cultures (p < 0.0001), respectively (Fig. 2B), which is consistent with our previously published data (1). Levels of CD4 mRNA were evaluated by real-time RT-PCR in response to these three treatments. Both SEB and anti-CD3/CD28 treatments resulted in a 15–20-fold induction of CD4 mRNA in comparison with untreated cultures (Fig. 2C). LiCl induced a modest change in CD4 mRNA expression, which may reflect the stronger ability of SEB or anti-CD3/CD28 to induce CD4 expression on CD8+ T cells.
FIGURE 2.
Induction of β-catenin signaling regulates CD4 expression on purified CD8+ T cells. CD8+ T cells were isolated from healthy donors by negative immunoselection then treated with LiCl (5 mM), SEB (1 µg/ml), or anti-CD3/CD28 (1 µg/ml each) or left untreated. On day 6, CD4 protein expression was evaluated by flow cytometry, for which a representative dot plot of the upregulation of CD4 on CD8+ T cells is shown in A, and cumulative data of mean percent expression of CD4dimCD8bright T cells are shown in B. CD4 mRNA fold change, at day 6, was evaluated by real-time RT-PCR (C). D, PBMCs were nucleofected with an irrelevant construct (GFP, GFP) or a stable β-catenin construct (pCDNA–β-catenin) or not DNA transfected. At day 6, CD4 expression on CD8+ T cells was evaluated by conventional flow cytometry. *p < 0.05.
Although at the dose used in our studies, LiCl induces β-catenin by inhibiting GSK-3β, lithium may have effects that are independent of β-catenin. To directly assess the role of β-catenin signaling in inducing CD4 expression on CD8+ T cells, we used a molecular approach to upregulate β-catenin. PBMCs were transfected with a constitutively active construct of β-catenin (pcDNA–β-catenin). This construct has a missense mutation of tyrosine for serine at codon 33 (S33Y), resulting in stabilized expression of β-catenin and induction of strong TCF/LEF-mediated signaling (20). Expression of stabilized β-catenin led to a significant increase in CD4dim expression on CD8+ T cells (Fig. 2D). Collectively, these data demonstrate that signals that induce β-catenin (lithium or stabilized β-catenin) lead to the induction of CD4 on CD8+ T cells.
Induction of CD4 expression on CD4dimCD8bright and conventional CD4+ T cells is dependent on β-catenin signaling
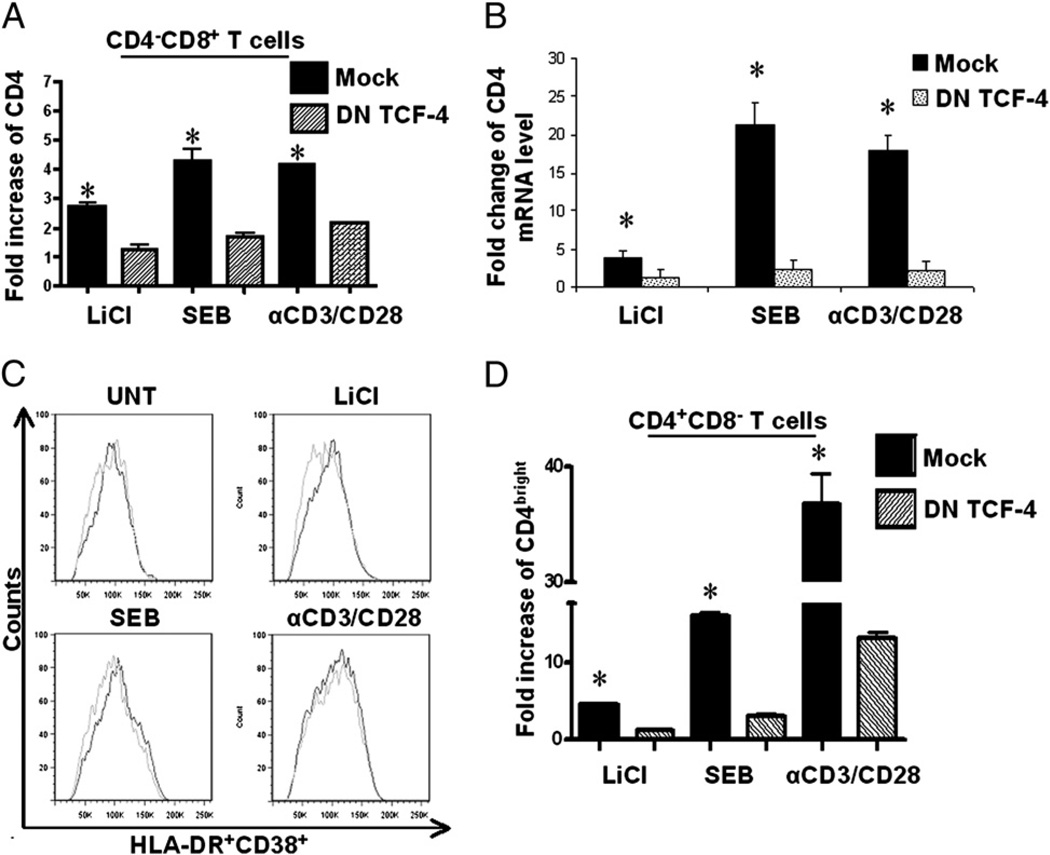
To determine whether LiCl, SEB, and anti-CD3/CD28–mediated induction of CD4 expression on CD8+ T cells is β-catenin dependent, we performed loss of function studies. PBMCs were transfected with a DN mutant construct of TCF-4 or mock transfected. TCF-4 is a downstream effector of Wnt/β-catenin signaling. The TCF-4 DN construct contains the DNA binding domain but lacks the N terminus, which is required for β-catenin binding. This construct blocks β-catenin signaling (24, 25). Nucleofected PBMCs were treated with LiCl (5 mM), SEB (1 µg/ml), or anti-CD3/CD28 (1 µg/ml each) or left untreated for 3 d. CD4 protein expression on CD8+ T cells induced by LiCl, SEB, and anti-CD3/CD28 stimulation was reduced by ~50% in response to nuclefoection with DN TCF-4 (Fig. 3A). Likewise, CD4 mRNA expression was dramatically reduced in cells nucleofected with DN TCF-4 (Fig. 3B). Because interruption of T cell activation may interfere with induction of CD4 expression, we evaluated the impact of the DN TCF-4 construct on T cell activation, as measured by coexpression of HLA-DR and CD38. The DN TCF construct had no significant effect on T cell activation (Fig. 3C). Further, given that these treatments also induce a higher/brighter-level of CD4 expression on conventional CD4+ T cells (CD4+CD8− cells), we evaluated the impact of β-catenin signaling in mediating this induction on conventional CD4+ T cells. As observed for CD4dimCD8bright T cells, inhibiting β-catenin signaling also significantly reduced CD4 expression on conventional CD4+ T cells in response to LiCl, SEB, and anti-CD3/CD28 treatments (Fig. 3D). These data are especially remarkable considering that the nucleofection efficiency was ~50% based on analysis of GFP expression (data not shown). Collectively, these data demonstrate that the ability of lithium, SEB, and anti-CD3/CD28 to induce CD4 expression on CD8+ T cells or conventional CD4+ T cells is β-catenin signaling dependent.
FIGURE 3.
Inhibition of β-catenin signaling reduces CD4 upregulation on CD8+ T cells. PBMCs were nucleofected with either a DN construct of TCF-4 or mock nucleofected. The cells were then stimulated with LiCl, SEB, or anti-CD3/CD28 Ab or left untreated. On day 6, CD4 protein expression on CD8+ (A) and CD4+ (D) T cells was evaluated by flow cytometry, and CD4 mRNA levels were evaluated by real-time RT-PCR (B). Fold change is determined by value of each treatment (Mock or DN TCF-4) divided by the value of the untreated condition. C, Co-expression of HLA-DR/CD38 was evaluated by flow cytometry between mock and DN-TCF nucleofected PBMCs. Gray lines represent DN TCF-4 nucleofected cultures, and black lines represent mock nucleofected cultures. Data are based on at least three independent experiments. *p < 0.005, except in D, where *p < 0.05.
Human CD4 promoter binds TCF
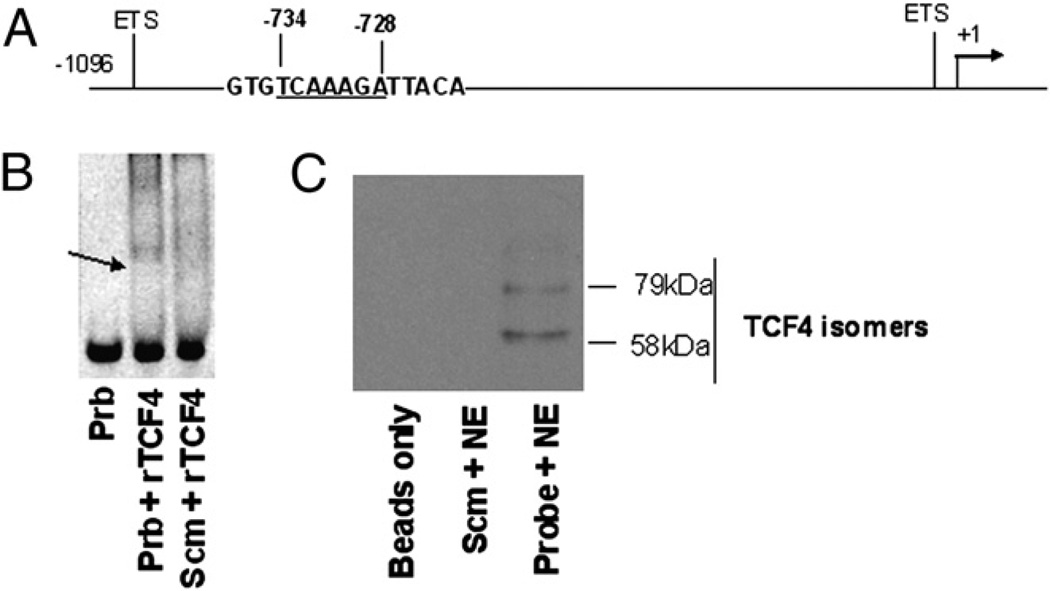
Using the DNA Sequencher program, we identified a TCF/LEF binding consensus sequence (TCAAAGA) at nt −734 to −728 from the transcription start site on the human CD4 promoter partial sequence (Gene Bank accession number U01066; www.ncbi.nlm.nih.gov/genbank/) (Fig. 4A). By gel shift assay as well as immunoprecipitation (IP)/Western blot, we show that this putative TCF binding site within the human CD4 promoter binds a member of the TCF/LEF family (TCF-4) (Fig. 4B, 4C). Taken together, these data demonstrate that β-catenin signaling is a critical component of CD4 induction on CD8+ T cells that is regulated at the transcriptional level.
FIGURE 4.
Identification of a TCF/LEF binding site within human CD4 promoter that binds TCF-4. A, Schematic diagram of TCF/LEF binding consensus sequence (TCAAAGA) at nt −734 to −728 from the transcription initiation site of the human CD4 promoter. ETS transcription factor binding sites are shown as a reference point (31). B, EMSA was performed using double-stranded biotin-conjugated probe of the consensus sequence of TCF/LEF binding site (5′-GTGTCAAAGATTACA-3′) or a scrambled probe (5′-GTGTGGCCTCTTACA-3′) and rTCF-4 or nuclear extracts isolated from a rich source of TCF-4 (U251 cells). The DNA–protein complexes were resolved on 6% native gel and detected with SYBR green dye. Arrow indicates binding of TCF/LEF DNA to rTCF-4. C, IP followed by Western blot was performed using 100 µg nuclear extract and 200 ng biotinylated TCF/LEF or scrambled probe, and streptavidin-conjugated agarose beads were used for IP of the complex, which was resolved on 10% SDS gel, transferred to a cellulose membrane, and immunoblotted with a mouse anti–TCF-4 Ab. ETS, E-twenty-six.
β-catenin–positive T cells express high levels of Bcl-xL
To determine the biologic significance of β-catenin expression within CD8+ and CD4+ mature T cells, we evaluated the relationship between β-catenin expression and Bcl-xL. Bcl-xL is an anti-apoptotic protein and a target gene for the β-catenin signaling pathway (26). By gating on β-catenin+ and β-catenin− T cell subsets (within the CD4−CD8+, CD4+CD8−, and CD4dimCD8bright T cell populations), we demonstrate that all three populations that are β-catenin+ overwhelmingly expressed Bcl-xL (>60%), whereas the β-catenin− cells expressed low levels of Bcl-xL (1–13%, depending on the T cell subset) (Fig. 5).
FIGURE 5.
Bcl-xL is preferentially expressed in β-catenin+ T cells. PBMCs from healthy donors were immunostained for CD3, CD4, CD8, β-catenin, and Bcl-XL. Using polychromatic flow cytometry, expression of the antiapoptotic factor Bcl-xL in β-catenin+ and β-catenin− subsets of CD4−CD8+, CD4+CD8−, and CD4dimCD8bright T cells was measured. A, Representative histogram showing the expression of Bcl-xL in CD4dimCD8bright, CD4+CD8−, and CD4−CD8+ T cells. B, Cumulative data of Bcl-xL expression in β-catenin+ and β-catenin− T cells. Data are based on three experiments. *p < 0.005.
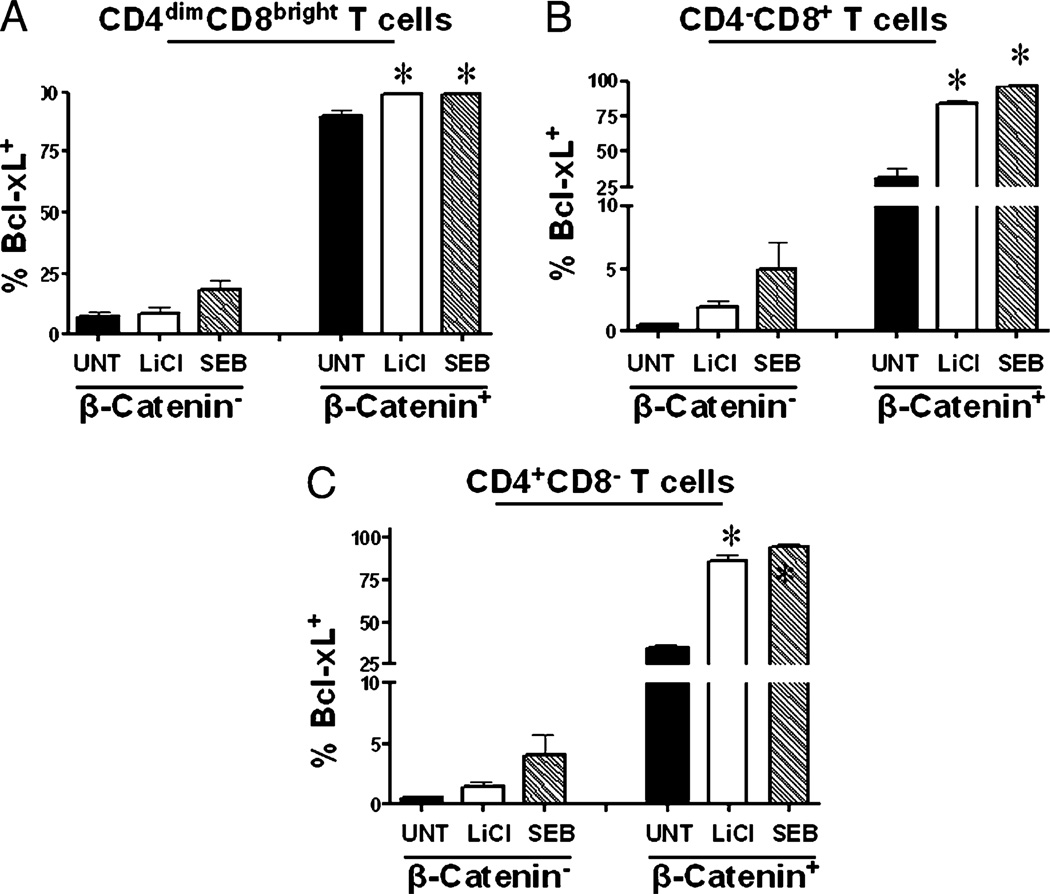
To determine whether activation of β-catenin can induce expression of Bcl-xL in T cells, PBMCs were stimulated with LiCl or SEB or left untreated. On day 6, expression of Bcl-xL was evaluated within β-catenin+ and β-catenin− CD4−CD8+, CD4+CD8−, and CD4dimCD8bright T cell populations using multiparameter flow cytometry (Fig. 6). Within β-catenin− subsets of CD4−CD8+, CD4+CD8−, and CD4dimCD8bright T cell populations, neither LiCl nor SEB treatment significantly induced Bcl-xL expression. However, within the β-catenin+ subsets, both LiCl and SEB significantly induced Bcl-xL expression by 15–60%, depending on the subset (Fig. 6). Further, anti-CD3/CD28 treatment increased Bcl-xL expression to ~100% in all T cell subsets (data not shown). These data indicate that upon T cell stimulation, the majority of β-catenin+ cells become Bcl-xL+, whereas the β-catenin− cells do not significantly increase their levels of Bcl-xL. This finding demonstrates that mature T cells can re-express β-catenin post thymic development and suggests that that Bcl-xL is regulated, at least in part, by β-catenin.
FIGURE 6.
Bcl-xL is inducible by T cell stimuli that induce active β-catenin. PBMCs were isolated and stimulated with LiCl or SEB or left untreated. On day 6, β-catenin+ and β-catenin− T cells were evaluated for their expression of Bcl-xL within CD4dimCD8bright (A), CD4−CD8+ (B), and CD4+CD8− (C) T cells, as measured by flow cytometry. Data are representative of a minimum of three experiments. *p < 0.005.
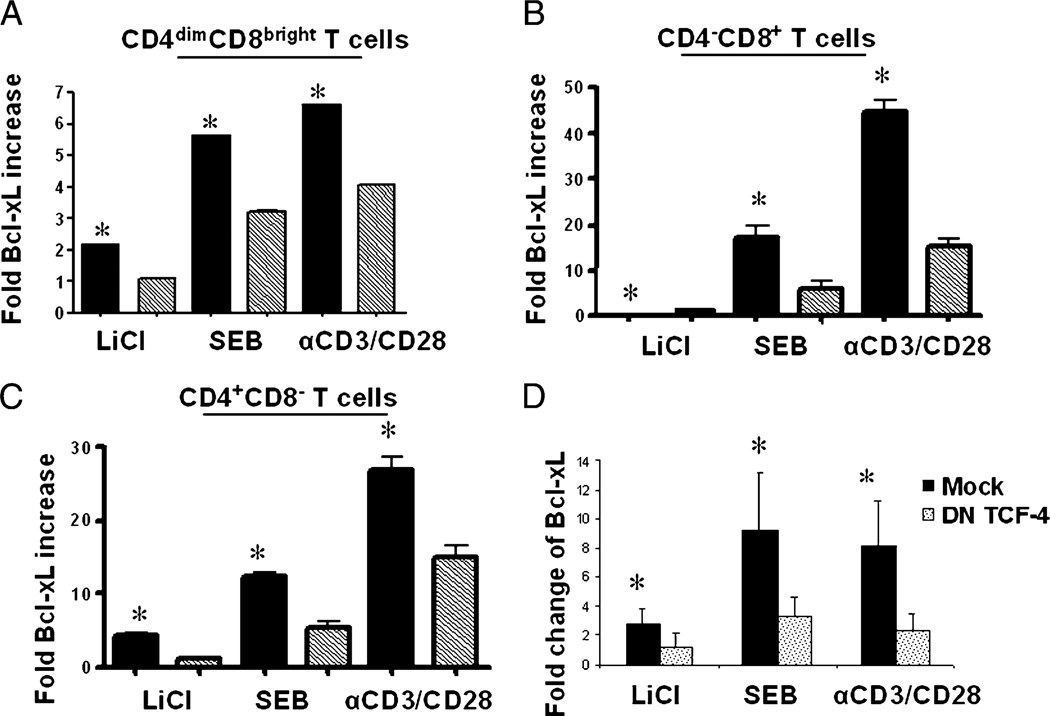
Induction of Bcl-xL in T cell subsets is mediated by β-catenin
To determine if the observed induction of BcL-xL is mediated by β-catenin, we inhibited β-catenin signaling by nucleofecting the cells with DN TCF-4 construct or an irrelevant construct prior to treatment with LiCl, SEB, or anti-CD3/CD28. Inhibiting β-catenin– mediated signaling reduced Bcl-xL protein expression by at least 50% in CD4dimCD8bright, CD4−CD8+, and CD4+CD8− T cells (Fig. 7A–C). Bcl-XL mRNA was also dramatically reduced in PBMCs nucleofected with DN TCF-4 in comparison with mock nucleofection or untreated controls (Fig. 7D). These data demonstrate that induction of β-catenin in mature T cells leads to BcL-xL upregulation, which may be responsible for preventing activation-induced cell death.
FIGURE 7.
β-catenin is required for Bcl-xL upregulation in T cell subsets. PBMCs were nucleofected as previously described with a DN construct of TCF-4 or mock nucleofected. The cells were then stimulated for 6 d with LiCl, SEB, or anti-CD3/CD28 Ab or left untreated. Relative fold change of Bcl-xL protein over untreated cultures in CD4dimCD8bright (A), CD8+CD4− (B), and CD4+CD8− (C) T cells is shown. Relative Bcl-XL mRNA expression is shown in D. Data are based on at least three experiments. *p < 0.005, except in D, where *p < 0.05.
Discussion
Wnt/β-catenin signaling is important for the transition of thymocytes from the double-negative to DP stage and for the survival of DP thymocytes (14). In the nucleus, β-catenin binds to TCF/LEF transcription factors and docks on TCF/LEF sites to induce CD4 expression in thymocytes. Gene transcription of CD4 by a β-catenin–dependent mechanism, however, is thought to be terminated post thymocyte maturation. To the contrary, we demonstrate in this study that β-catenin signaling is not silenced post T cell development. In response to activation (via SEB stimulation or anti-CD3/CD28 costimulation), β-catenin expression is induced on several T lymphocyte populations (CD4+CD8− T cells, CD8+CD4− T cells, and CD4dimCD8brightT cells). Induction of β-catenin drives the generation of CD4dimCD8bright T cells by inducing the de novo expression of CD4. This observation is also not unique to the CD4dimCD8bright T cell population, as higher intensity of CD4 on T cells in response to activation is also mediated by a β-catenin–dependent mechanism. Our observation that the human CD4 promoter contains putative TCF/LEF binding sites is consistent with those from a mouse CD4 promoter (27) and points to a transcriptionally dependent mechanism by which β-catenin signaling promotes CD4 expression on mature CD8+ T cells.
A number of cell types, other than conventional CD4+ T cells, express CD4 on their surface, including monocytes and CD8+ T cells. Even eosinophils express CD4 during one stage of their development (28). However, the functional relevance of CD4 expression on these cells is not clear. Traditionally, the CD4 molecule functions as a coreceptor by binding to MHC class II on APCs to enhance T cell recognition of cognate Ag. CD4 enhancement of T cell–APC interaction is mediated by enhancing the avidity of the T cell–APC interaction and/or increasing T cell signaling in response to this interaction. CD4 also binds the chemotactic factor IL-16, which enhances chemotaxis of CD4-expressing cells (5). Thus, CD4 expression may enhance immunologic responses by additional mechanisms independent of CD4–MHC class II interaction in immune cells that express CD4 either transiently or permanently.
A growing body of evidence points to a role for CD4dimCD8bright T cells in enhanced antiviral immunity. In comparison with conventional CD8+ T cells (those that do not express CD4 on their surface), CD4dimCD8bright T cells have potent response against CMV (2), HIV (13), lymphocytic choriomeningitis virus (7), and hepatitis C virus (12). We recently demonstrated that CD4dimCD8bright T cells are polyfunctional and expanded in HIV long-term nonprogressors (13). The CD4 molecule on CD4dimCD8bright T cells is functional and is linked to Src kinase (8). Upon ligation of the CD4 molecule on CD4dimCD8bright T cells, IFN-γ and Fas ligand expression is increased (6). The finding of increased Fas ligand expression coupled with the finding that these cells are more activated than CD4−CD8+ T cells (1) suggests that they may be more susceptible to apoptosis. Yet CD4dimCD8bright T cells are actually less susceptible to apoptosis (2). A contributing factor is their higher level of endogenous β-catenin in both their resting and activated states, which allows for higher levels of the antiapoptotic protein Bcl-xL, but not Bcl-2 (data not shown). β-catenin has been previously reported to regulate Bcl-xL expression in thymocytes, but expression of β-catenin is thought to be largely silenced post thymocyte development. To the contrary, we show in this study that β-catenin expression is inducible on conventional lymphocytes (CD4+CD8− and CD8+CD4+ T cells) and is endogenously high in CD4dimCD8bright T cells.
Although Wnt/β-catenin is synonymous with early developmental events, including T cell development, its role post T cell development is emerging. In CD8+ T cells, β-catenin signaling is active, but its role in immunity is not clear. We have demonstrated that β-catenin signaling can also be induced post SEB, anti-CD3/CD28 costimulation, or lithium treatment of human PBMCs. Induction of β-catenin expression by retroviral transduction enhances regulatory T cell survival and function but promotes anergy in nonregulatory CD4+ T cells (29). It is likely that when β-catenin is transduced into T cells, it leads to robust/constitutive expression of β-catenin, the consequences of which seem to result in anergy as opposed to perhaps protection against activation-induced cell death when β-catenin is not overly expressed (29). In our study, β-catenin was upregulated by using LiCl. LiCl is an inhibitor of a negative regulator of β-catenin, GSK-3β. Inhibition of GSK-3β allows for β-catenin to translocate to the nucleus, where it can bind to TCF/LEF proteins and impact target gene transcription. This approach is a transient approach for β-catenin induction.
Recently, induction of β-catenin in CD8+ T cells was demonstrated to generate a unique population of CD8+ T cells, termed T memory stem cells (30). This population has the capability of self-renewal, retention of IL-2 and IFN-γ secretion, potent proliferation in response to cognate Ag, and clearance of tumors (30). Given that these cells were generated using pharmacological agents that upregulate β-catenin through inhibition of GSK-3β, it is possible that these cells described in mice may be the same and/or similar to the CD4dimCD8bright T cells described in humans. Our demonstration that CD4dimCD8bright T cells are generated by signals that activate β-catenin and that these cells have potent antiviral immune responses against a number of chronic viruses points to a role of β-catenin in T cell immunity (2, 13) (6, 7, 12). Harnessing β-catenin to induce CD4dimCD8bright T cells for maintenance of long-term memory may have therapeutic potential for vaccine strategies and/or adoptive immunotherapy.
Acknowledgments
We thank the Jane B. Pendleton Charitable Trust for purchase of the BD LSR II flow cytometer (BD Biosciences). We also thank Jeff Martinson for technical assistance with the LSRII.
This work was supported by grants to L.A. from the National Institutes of Health (R01 NS060632 and R21 A1077329) and the Campbell Foundation (Fort Lauderdale, FL). Support was also provided by the Chicago Developmental Center for AIDS Research (P30 AI 082151), a National Institutes of Health-funded program supported by the National Institutes of Allergy and Infectious Diseases, National Cancer Institute, National Institute of Mental Health, National Institute on Drug Abuse, National Institute of Child Health and Human Development, National Heart, Lung, and Blood Institute, and National Center for Complementary and Alternative Medicine.
Abbreviations used in this paper
- DN
dominant-negative
- DP
double-positive
- ETS
E-twenty-six
- GSK-3β
glycogen synthase kinase 3β
- IP
immunoprecipitation
- LEF
lymphoid enhancer factor
- SEB
staphylococcal enterotoxin B
- SP
single-positive
- TCF
T cell factor
- UNT
untreated
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Sullivan YB, Landay AL, Zack JA, Kitchen SG, Al-Harthi L. Upregulation of CD4 on CD8+ T cells: CD4dimCD8bright T cells constitute an activated phenotype of CD8+ T cells. Immunology. 2001;103:270–280. doi: 10.1046/j.1365-2567.2001.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zloza A, Sullivan YB, Connick E, Landay AL, Al-Harthi L. CD8+ T cells that express CD4 on their surface (CD4dimCD8bright T cells) recognize an antigen-specific target, are detected in vivo, and can be productively infected by T-tropic HIV. Blood. 2003;102:2156–2164. doi: 10.1182/blood-2002-07-1972. [DOI] [PubMed] [Google Scholar]
- 3.Zloza A, Al-Harthi L. Multiple populations of T lymphocytes are distinguished by the level of CD4 and CD8 coexpression and require individual consideration. J. Leukoc. Biol. 2006;79:4–6. doi: 10.1189/jlb.0805455. [DOI] [PubMed] [Google Scholar]
- 4.Kitchen SG, Korin YD, Roth MD, Landay A, Zack JA. Costimulation of naive CD8(+) lymphocytes induces CD4 expression and allows human immunodeficiency virus type 1 infection. J. Virol. 1998;72:9054–9060. doi: 10.1128/jvi.72.11.9054-9060.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitchen SG, LaForge S, Patel VP, Kitchen CM, Miceli MC, Zack JA. Activation of CD8 T cells induces expression of CD4, which functions as a chemotactic receptor. Blood. 2002;99:207–212. doi: 10.1182/blood.v99.1.207. [DOI] [PubMed] [Google Scholar]
- 6.Kitchen SG, Jones NR, LaForge S, Whitmire JK, Vu BA, Galic Z, Brooks DG, Brown SJ, Kitchen CM, Zack JA. CD4 on CD8(+) T cells directly enhances effector function and is a target for HIV infection. Proc. Natl. Acad. Sci. USA. 2004;101:8727–8732. doi: 10.1073/pnas.0401500101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitchen SG, Whitmire JK, Jones NR, Galic Z, Kitchen CM, Ahmed R, Zack JA. The CD4 molecule on CD8+ T lymphocytes directly enhances the immune response to viral and cellular antigens. Proc. Natl. Acad. Sci. USA. 2005;102:3794–3799. doi: 10.1073/pnas.0406603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flamand L, Crowley RW, Lusso P, Colombini-Hatch S, Margolis DM, Gallo RC. Activation of CD8+ T lymphocytes through the T cell receptor turns on CD4 gene expression: implications for HIV pathogenesis. Proc. Natl. Acad. Sci. USA. 1998;95:3111–3116. doi: 10.1073/pnas.95.6.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang LP, Riley JL, Carroll RG, June CH, Hoxie J, Patterson BK, Ohshima Y, Hodes RJ, Delespesse G. Productive infection of neonatal CD8+ T lymphocytes by HIV-1. J. Exp. Med. 1998;187:1139–1144. doi: 10.1084/jem.187.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blue ML, Daley JF, Levine H, Schlossman SF. Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometry. J. Immunol. 1985;134:2281–2286. [PubMed] [Google Scholar]
- 11.Ortolani C, Forti E, Radin E, Cibin R, Cossarizza A. Cytofluorimetric identification of two populations of double positive (CD4+,CD8+) T lymphocytes in human peripheral blood. Biochem. Biophys. Res. Commun. 1993;191:601–609. doi: 10.1006/bbrc.1993.1260. [DOI] [PubMed] [Google Scholar]
- 12.Nascimbeni M, Shin EC, Chiriboga L, Kleiner DE, Rehermann B. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood. 2004;104:478–486. doi: 10.1182/blood-2003-12-4395. [DOI] [PubMed] [Google Scholar]
- 13.Zloza A, Schenkel J, Tenorio A, Martinson JA, Jeziorczak P, Al-Harthi L. Potent HIV-specific responses are enriched in a unique subset of CD8+ T cells that co-expresses CD4 on its surface. Blood. 2009;114:3841–3853. doi: 10.1182/blood-2009-02-202481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ioannidis V, Beermann F, Clevers H, Held W. The beta-catenin—TCF-1 pathway ensures CD4(+)CD8(+) thymocyte survival. Nat. Immunol. 2001;2:691–697. doi: 10.1038/90623. [DOI] [PubMed] [Google Scholar]
- 15.Guo Z, Dose M, Kovalovsky D, Chang R, O’Neil J, Look AT, von Boehmer H, Khazaie K, Gounari F. Beta-catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation. Blood. 2007;109:5463–5472. doi: 10.1182/blood-2006-11-059071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hossain MZ, Yu Q, Xu M, Sen JM. ICAT expression disrupts beta-catenin-TCF interactions and impairs survival of thymocytes and activated mature T cells. Int. Immunol. 2008;20:925–935. doi: 10.1093/intimm/dxn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willinger T, Freeman T, Herbert M, Hasegawa H, McMichael AJ, Callan MF. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J. Immunol. 2006;176:1439–1446. doi: 10.4049/jimmunol.176.3.1439. [DOI] [PubMed] [Google Scholar]
- 19.Christian SL, Sims PV, Gold MR. The B cell antigen receptor regulates the transcriptional activator beta-catenin via protein kinase C-mediated inhibition of glycogen synthase kinase-3. J. Immunol. 2002;169:758–769. doi: 10.4049/jimmunol.169.2.758. [DOI] [PubMed] [Google Scholar]
- 20.Kolligs FT, Hu G, Dang CV, Fearon ER. Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol. Cell. Biol. 1999;19:5696–5706. doi: 10.1128/mcb.19.8.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie D, Yin D, Tong X, O’Kelly J, Mori A, Miller C, Black K, Gui D, Said JW, Koeffler HP. Cyr61 is overexpressed in gliomas and involved in integrin-linked kinase-mediated Akt and beta-catenin-TCF/Lef signaling pathways. Cancer Res. 2004;64:1987–1996. doi: 10.1158/0008-5472.can-03-0666. [DOI] [PubMed] [Google Scholar]
- 22.Hedgepeth CM, Conrad LJ, Zhang J, Huang HC, Lee VM, Klein PS. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev. Biol. 1997;185:82–91. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Zloza A, Moon RT, Watts J, Tenorio AR, Al-Harthi L. Active beta-catenin signaling is an inhibitory pathway for human immunodeficiency virus replication in peripheral blood mononuclear cells. J. Virol. 2008;82:2813–2820. doi: 10.1128/JVI.02498-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung EJ, Hwang SG, Nguyen P, Lee S, Kim JS, Kim JW, Henkart PA, Bottaro DP, Soon L, Bonvini P, et al. Regulation of leukemic cell adhesion, proliferation, and survival by beta-catenin. Blood. 2002;100:982–990. doi: 10.1182/blood.v100.3.982. [DOI] [PubMed] [Google Scholar]
- 25.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 26.Xie H, Huang Z, Sadim MS, Sun Z. Stabilized beta-catenin extends thymocyte survival by up-regulating Bcl-xL. J. Immunol. 2005;175:7981–7988. doi: 10.4049/jimmunol.175.12.7981. [DOI] [PubMed] [Google Scholar]
- 27.Huang Z, Xie H, Ioannidis V, Held W, Clevers H, Sadim MS, Sun Z. Transcriptional regulation of CD4 gene expression by T cell factor-1/beta-catenin pathway. J. Immunol. 2006;176:4880–4887. doi: 10.4049/jimmunol.176.8.4880. [DOI] [PubMed] [Google Scholar]
- 28.Rand TH, Cruikshank WW, Center DM, Weller PF. CD4-mediated stimulation of human eosinophils: lymphocyte chemoattractant factor and other CD4-binding ligands elicit eosinophil migration. J. Exp. Med. 1991;173:1521–1528. doi: 10.1084/jem.173.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat. Med. 2008;14:162–169. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- 30.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salmon P, Giovane A, Wasylyk B, Klatzmann D. Characterization of the human CD4 gene promoter: transcription from the CD4 gene core promoter is tissue-specific and is activated by Ets proteins. Proc. Natl. Acad. Sci. USA. 1993;90:7739–7743. doi: 10.1073/pnas.90.16.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]