Abstract
The human voltage gated proton channel, hHV1, appears to exist mainly as a dimer. Teleologically, this is puzzling, because each protomer retains the main properties that characterize this protein: proton conduction that is regulated by conformational (channel opening and closing) changes that occur in response to both voltage and pH. The HV1 dimer is mainly linked by C terminal coiled-coil interactions. Several types of mutations produce monomeric constructs that open ~5 times faster than the WT dimeric channel, but with weaker voltage dependence. Intriguingly, the quintessential function of the HV1 dimer, opening to allow H+ conduction, occurs cooperatively. Both protomers undergo a conformational change, but both must undergo this transition before either can conduct. The teleological purpose of dimerization may be to steepen the voltage-dependence of channel opening, at least in phagocytes. In other cells, the purpose is not understood. Finally, several single-celled species have HV that are likely monomeric.
Keywords: proton channels, Hvcn1, respiratory burst, enhanced gating, cooperativity, pH, gating mechanisms, VSD, voltage sensors, gating kinetics, HV1, voltage sensing domain
I. INTRODUCTION
Ion channels comprise a large family of membrane proteins that regulate the passage of ions, usually one particular species of ion, across cell or organelle membranes. Most ion channels are multimeric, but dimeric channels do occur, albeit infrequently. The voltage gated proton channel (HV1) gene was identified in 2006 [1, 2], and its dimeric nature was discovered shortly thereafter [3–5]. The main focus of this review is to survey the consequences of dimerization for the molecular and biological functions of these channels. On the molecular scale, proton channels open and close, conduct only protons when they are open, and characteristically are regulated closely by the pH gradient [6]. Voltage-gated proton channels in humans (hHV1) have diverse functions in a variety of cells. They participate in pathogen killing by phagocytes [7–11], histamine secretion by basophils [12], surface pH regulation by airway epithelia [13], capacitation and motility of sperm prior to fertilization [14, 15], B lymphocyte signaling [16], and may exacerbate breast cancer metastasis [17] and brain damage in ischemic stroke [18].
II. DIMERIZATION OF MEMBRANE PROTEINS
A number of ion channels function only when assembled into the multimer that forms the ion conducting structure. For example, voltage-gated K+ channels are tetramers that use two transmembrane helices (S5–S6) from each of the four subunits to produce a single, central K+ selective pore (Fig. 1). In a few ion channels, including HV1, individual monomeric units appear to be fully functional, yet the native channel assembles as a dimer or oligomer with properties distinct from the monomer. The high frequency of membrane protein homodimers (and higher order homo-oligomers) argues that there is a strong evolutionary advantage for this kind of quaternary structure. Commonly accepted ‘reasons’ for dimerization of membrane (and soluble) proteins include increased stability, increased specificity of interaction with regulatory proteins, regulation or modulation of activity, and acquisition of cooperativity. HV1 activity is modulated by at least one protein kinase, although no evidence exists one way or the other for differential specificity of the kinase for monomeric or dimeric HV1. HV1 dimers do act cooperatively, which has implications for physiological interplay with NADPH oxidase function in human neutrophils. Although not definitely established, dimer assembly does not appear to be involved in enhanced gating, the best characterized functional modulation of HV1.
Figure 1.
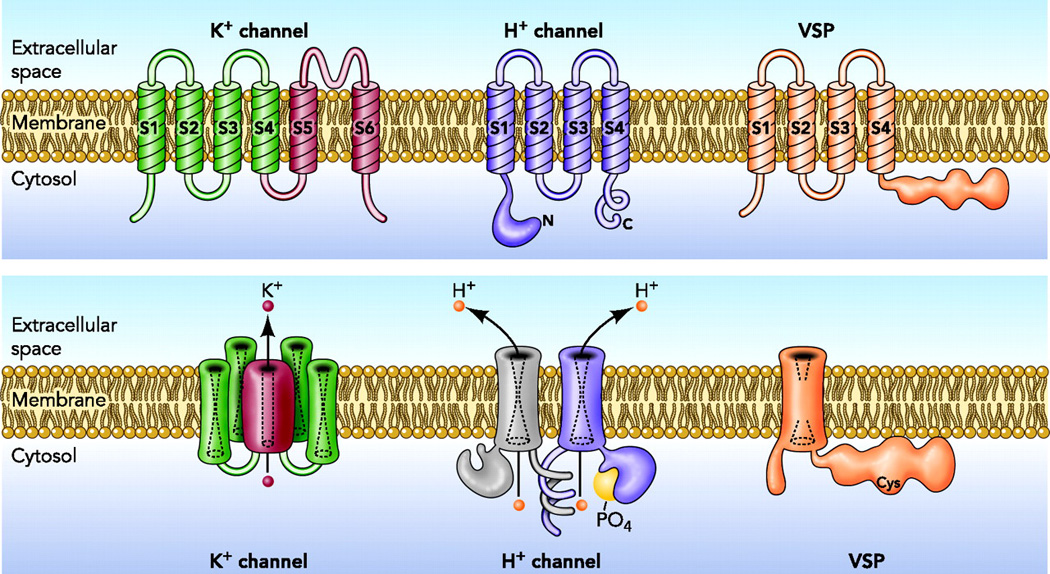
Architecture of three classes of VSD containing molecules. The top row shows the monomeric protein; the lower row shows the final assembled protein. The K+ channel assembles as a tetramer [89, 90], with four separate VSD elements connecting to a single central pore (the pathway taken by K+ as it permeates). The voltage gated H+ channel in many species, including mammals, assembles as a dimer [3–5], although when constrained to exist in monomeric form, it retains its key properties of proton specific conduction, voltage-gating (opening upon depolarization of the membrane potential), and ΔpH dependence, that strongly regulates the opening of the channel on the basis of pH. The VSP, a voltage sensitive phosphatase, is thought to exist and function as a monomer [49]. Reprinted from: DeCoursey TE. Voltage-gated proton channels find their dream job managing the respiratory burst in phagocytes. Physiology (Bethesda) 25, 27–40, copyright 2010.
III. EVIDENCE THAT PROTON CHANNELS EXIST AS DIMERS
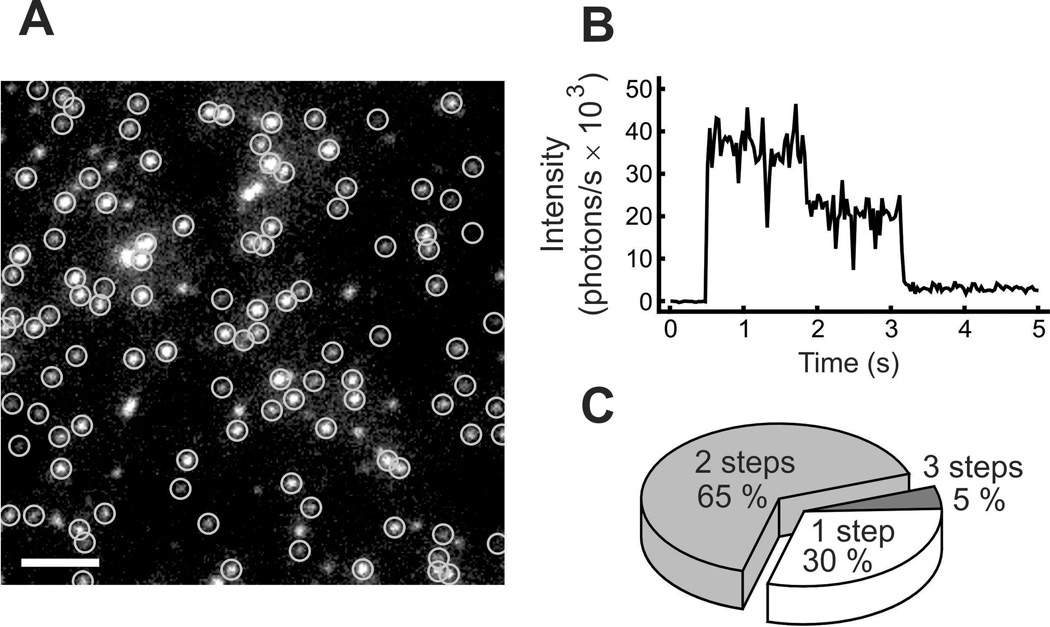
A variety of data supports the dimeric nature of the native HV1 in humans, mice, and Ciona intestinalis [3–5, 19–23]. The clearest and most direct evidence that hHV1 are dimers was provided by Tombola and colleagues [5], who attached green fluorescent protein (GFP) to the channel molecule, and then observed photobleaching of individual channel molecules. As illustrated in Fig. 2, the fluorescence intensity of most channels decayed in two discrete steps. Additional evidence that hHV1 is a dimer, and that each protomer has a separate conduction pathway, was provided by tandem dimers including an introduced Cys at a location that enables block of the channel by a methanethiosulfonate (MTS) reagent. WT-WT dimers were not blocked; WT-Cys dimers were half blocked, and Cys-Cys dimers were completely blocked [5]. Some impairment of dimerization was seen when the N-terminus was disrupted or replaced, but dimerization was completely prevented by C-terminus substitution [5].
Figure 2.
Evidence that the human proton channel, hHV1, is a dimer. A shows GFP (green fluorescent protein) tagged hHV1 channels visualized under fluorescence microscopy. Circled spots were followed over time, as illustrated in B, where the fluorescence intensity of one spot can be seen to decay in two distinct steps. The pie chart in C shows the frequency that tagged channels decayed with the indicated number of steps. Reprinted from: Tombola F, Ulbrich MH, and Isacoff EY. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron 58, 546–556, copyright 2008, with permission from Elsevier.
Koch et al [3] demonstrated the multimeric nature of the mouse HV1, mHV1 (which they called mVSOP), by coimmunoprecipitation of the channel labeled with two different tags. They further used FRET (fluorescence resonance energy transfer) to determine the distance between tagged residues in an extracellular loop of Ciona intestinalis HV1 (CiHV1 or CiVSOP) protomers to be 42 Å, also consistent with their being multimers. They showed that the preferred multimeric configuration was a dimer by creating a linked heterodimer including a single introduced Cys residue. Lack of FRET in this construct showed that only two subunits are involved. Gonzalez et al [19] demonstrated FRET in full-length CiHV1, which disappeared in the N- and C-terminal truncated channel, showing that this construct was monomeric.
Another kind of evidence is provided by the crosslinker disuccinimidyl suberate (DSS), which produced a distinct band at the dimer position on western blots [3, 4, 23]. In one study there were weak bands at positions corresponding to higher oligomers [4], in the other not [3]. The higher order oligomer bands could be nonspecific artifacts, as suggested by Lee and coauthors, but at least some could also represent natural, weaker interactions. Overexpression, cross-linking, and Western blotting all suffer from artifacts, so results must be interpreted with caution; still, these two studies used different HV1 proteins, cell lines, and methods, greatly increasing the probability that they represent normal interactions. Further increase in confidence is provided by the demonstration of the dimeric nature of native hHV1 in situ in human neutrophils [23] using western blots. Faint bands at the dimer level, which were greatly enhanced by DSS treatment, were seen in lysates from human neutrophils, eosinophils, and monocytes.
Not all HV1 are likely to be dimers
Direct evidence exists for the dimeric tendencies of HV1 in humans, mice, and Ciona intestinalis. However, because the most important determinant of dimeric status is C terminal coiled-coil interaction [3–5, 19–23], HV1 lacking this property may exist as monomers. Of confirmed or predicted HV1, those in the dinoflagellate Karlodinium veneficum [24] and in the diatoms Phaeodactylum tricornutum [25] and Thalassiosira, lack predicted coiled-coil domains and are presumed to be monomeric. HV1 in coccolithophores, which are also single celled eukaryotes, contain predicted C-terminal coiled-coil domains, which precludes any generalization regarding distinctions between oligomerization in multicellular and unicellular organisms. At present, there is no evidence that any accessory proteins are involved in or required for dimerization. The proton conduction that is the biological function of hHV1 can be demonstrated when the purified protein is incorporated into membrane vesicles [26]. Further characterization of the physiological roles of HV1 in various organisms will be needed to understand any distinction in the roles of dimeric vs. monomeric channels.
IV. COMPARISON OF THE PROPERTIES OF MONOMERIC AND DIMERIC CONSTRUCTS OF HV1
Exploring the differences in behavior between monomeric and dimeric constructs of HV1 is of intrinsic interest, but may also provide clues to the evolutionary “purpose” of dimerization. A word of caution must be proffered before accepting the following comparison. Because (most) HV1 normally exist as dimers, unnatural and invariably extreme measures must be adopted to force it into monomeric status. For example, truncation of the N- and C- termini must be considered drastic measures. These manipulations themselves may have consequences other than simply producing monomeric channels.
What does “cooperative gating” mean for ion channels?
Cooperative gating of HV1 has been discussed with the idea that the two protomers do not function independently. In order to understand the evidence that exists for cooperative gating in HV1, we will first consider the idea of cooperativity as it relates to ion channels. Cooperative binding of substrates to proteins that have multiple binding sites is a straightforward concept. A well-known example of (positive) cooperativity is that the first O2 to bind the tetrameric hemoglobin molecule increases the O2 affinity of the three remaining binding sites. A classic description of cooperativity in ion channels was provided by Hodgkin and Huxley.
Hodgkin and Huxley observed that both Na+ and K+ currents in squid axons activated (turned on) with a delay [27], which they could explain by postulating multiple identical voltage-sensing elements, all of which must move before the conductance appears. A mathematical representation of this idea is to assume identical conformational changes in each element, each a simple first-order transition between a resting state (R) and an activated state (A):
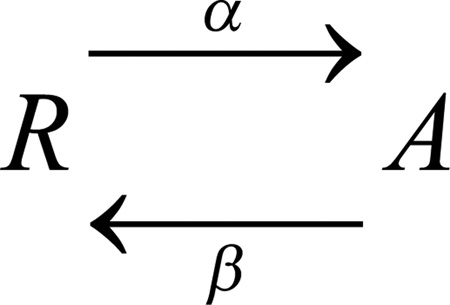 |
Scheme 1 |
where the forward and backward rate constants are α and β. On average, the movement of one element (protomer) after a step change of voltage follows an exponential time course, with time constant, τact = 1/(α + β). Both rate constants are typically voltage dependent, and the steady state probability of A, the activated state, at a given voltage is α/(α + β). If we assume that the channel can conduct only after both voltage sensor domains (VSDs) in a dimeric channel reach state A, then the opening process can be shown as follows:
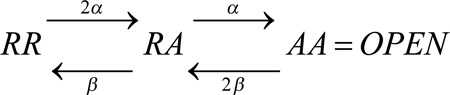 |
Scheme 2 |
This scheme describes the Hodgkin-Huxley (HH) model for a two-component channel. In this scheme, the current will turn on with a sigmoid time course, and upon repolarization, will turn off with an exponential time course, because once either protomer moves to its R state, conductance ends.
Turning to HV1, we have discussed evidence for dimers consisting of two identical protomers, each with a distinct conduction pathway and some means of responding to membrane potential changes. In the simplest case, movement of charged groups within the transmembrane (TM) helices of the protein would produce a conformational change that directly results in conductance (“channel opening”). In this case, as soon as the first protomer “opened” there would be current. Non-independence in this scenario could reflect interactions between protomers, such that opening of one facilitates (or inhibits) the opening of the other, exhibiting positive (or negative) cooperativity, in a sense similar to O2 binding in hemoglobin.
A different class of non-independence would arise if interactions within the HV1 dimer resulted in conductance occurring only after both protomers “open.” In the sense that conductance requires both members of the dimer to perform, this can be considered cooperative, but this phenomenon can also occur if each protomer moves independently of the other in response to voltage changes. The cooperativity in this example results not from interactions during the initial response to voltage, but during a subsequent step during which the conductance appears [28]. This is the kind of cooperativity modeled by Hodgkin and Huxley [29].
A slightly more complicated model was proposed by Gonzalez et al. [19]. They postulated an “allosteric” mechanism, in which the R→A transition does not immediately result in conductance, even in a monomer, but instead an additional transition must occur: R→A→O, where O is the open (conducting) state. Here the R→A transition (horizontal steps) reflects the movement of the fourth TM helix (S4) in response to voltage into an activated configuration, and the A→O transition (vertical steps) reflects a conformational change that results in conductance (channel opening). For the HV1 dimer, a simplified version of the allosteric model is:
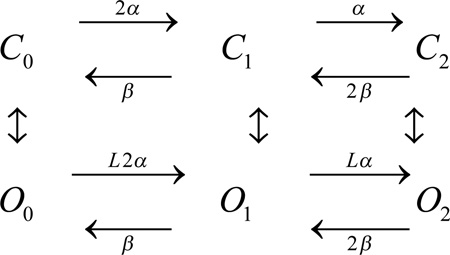 |
Scheme 3 |
Here the Cn states are closed (nonconducting) and the On states are open (conducting). The allosteric factor L (which also appears in the vertical transitions, but is not shown here) determines how much interaction exists between protomers. Aside from additional complexity, the allosteric model differs in one significant respect from the HH model. This point requires a preliminary discussion of gating charge.
What is gating charge and how is it measured?
A voltage-dependent ion channel opens in response to a change in membrane potential. This occurs because there are net charges within the membrane spanning regions of the protein that move in response to voltage changes. For example, the S4 region of most voltage gated ion channels contains several positively charged Arg (or Lys) residues, and when the membrane is depolarized (by making the inside of the cell more positive), these positive charges are pushed outward. “Gating charge” refers to the number of elementary charges (e0) in each channel molecule that must be transferred from one side of the membrane to the other during the opening process. If one charge travels across only half the membrane potential field, this would count as 0.5 e0. One additional complication is that the membrane potential does not change uniformly, as was assumed by Goldman in his “constant field theory” [30], but is focused at the narrowest part of the pore [31–34]. A standard method for estimating the effective gating charge is called the “limiting slope” method. The steady-state conductance (which is assumed to reflect approximately the open probability of the channel, Popen), is plotted semilogarithmically against voltage. Almers showed that the slope at the most negative voltage range that can be detected is a good estimate of the effective gating charge for any gating model in which the channel traverses an arbitrary number of closed states to arrive at a single open state [35]. More detailed analysis of which kinds of models follow or deviate from this guideline is provided by Sigg & Bezanilla [36]. For Na+ [37] and Shaker K+ channels, the total effective gating charge estimated by this method is 12–14 e0 [38–41]. To a rough approximation, this estimate corresponds well with the physical manifestation expected if four Arg residues from each of the four VSDs of the tetrameric channel, crossed most of the membrane electrical field. Returning to the models described above, the limiting slope method applies to the HH model (Scheme 2), but not strictly to the allosteric model (Scheme 3). The reason for this is that the allosteric model permits (a) channel opening before all the VSDs have moved, and (b) transitions between open states. If these transitions involve movement of gating charge, then the limiting slope will underestimate the true gating charge [36]. An excellent example of this phenomenon is the BK channel, whose g-V relationship at moderately negative voltages first becomes steeper, but at larger negative voltages becomes much shallower [42]. An allosteric model described the gating of this channel well, and explained the anomalous limiting slope data, because channels can open before all the voltage sensors have moved, and also because charge movement can occur during transitions between open states [43]. The error introduced by using the limiting slope approach depends on the degree of allosteric interaction; at one extreme the allosteric model degenerates into a linear model (in which all channels follow the C0→C1→C2→O2 pathway in Scheme 3), in which case the limiting slope gives the correct value.
If we consider the K+ channel in Fig. 1, the physical embodiment of the HH model (with movement of all four VSDs required before opening occurs) is clear. There are four identical VSDs, each of which must undergo a conformational change before the central pore is opened [27, 44, 45]. For HV1, the physical interpretation is less obvious, because there are two separate pores.
Evidence that gating of the two protomers in HV1 is “cooperative” (not independent)
Evidence already discussed shows that each protomer in HV1 contains a separate conduction pathway. It has become clear, however, that the gating (opening and closing in response to voltage changes) of the two protomers does not occur independently. One line of evidence comes from co-expressing roughly equal amounts of WT hHV1 and a mutant, E153C, that has the property that its proton conductance-voltage (gH-V) relationship is shifted −50 mV compared with WT. Independent gating of the protomers should result in a component of negatively shifted channels. On the other hand, if both protomers must undergo a conformational change that precedes conduction of either, channels should open at the more restrictive (more positive) regime. When the experiment was performed, the resulting gH-V relationship practically superimposed on that of the WT channel, which has the more positive voltage requirement. Linked heterodimers shared the nearly WT gH-V relationship seen in the co-expression studies. A puzzling feature of these results was the lack of a shoulder on the gH-V relationship in the co-expression studies, which would be expected from the fraction of double-mutant dimers.
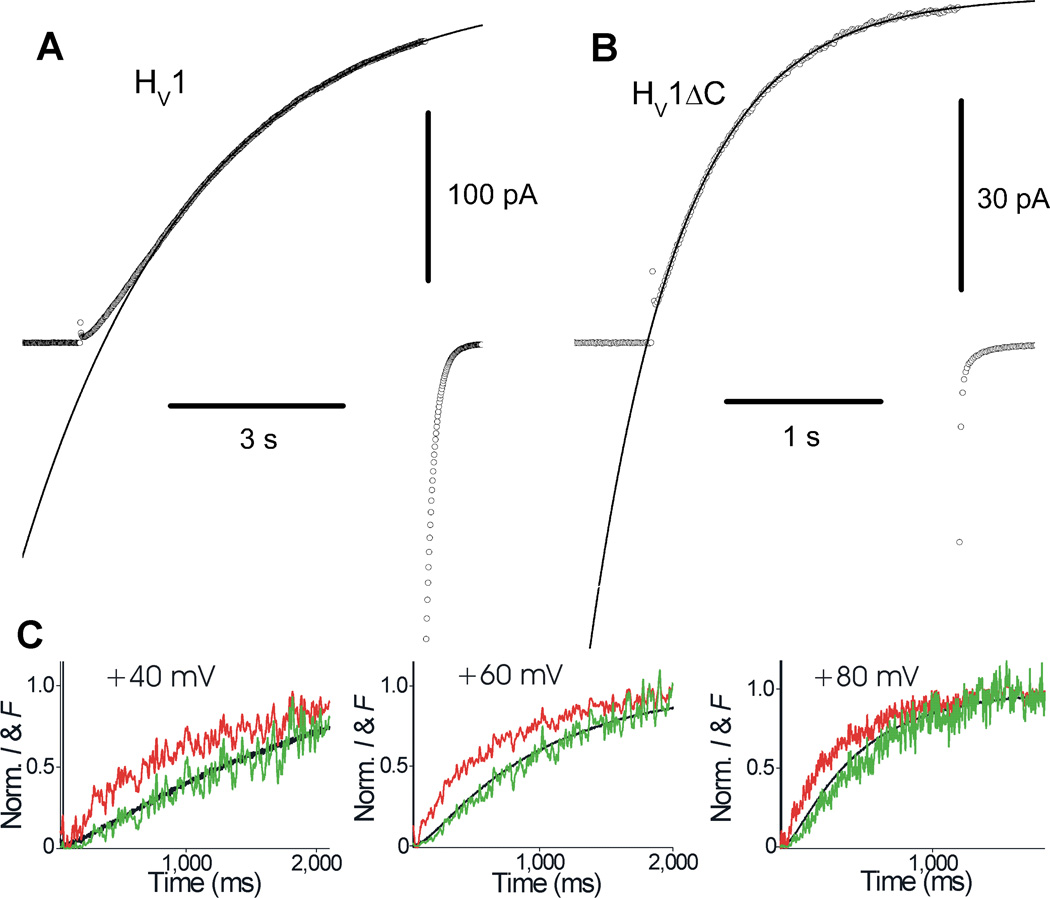
Gonzalez et al [19] also produced elegant evidence for cooperative gating (Fig. 3C). They labeled the outer end of the S4 domain with a fluorophore and used voltage-clamp fluorometry to observe S4 movement simultaneously with proton currents during voltage pulses. The fluorescence signal reflecting S4 movement (red) increased exponentially, whereas the proton current (black) increased with a sigmoid time course. In the traditional Hodgkin-Huxley [29] formalism of Scheme 2, the time course of the fluorescence signal raised to the second power (green) will predict the time course of the current if the two protomers gate independently, and if both must undergo a conformational change before either conducts. It is a happy event when data cooperate so nicely with theoretical expectations!
Figure 3.
Gating kinetics of monomeric and dimeric hHV1 and CiHV1. The sigmoid activation of WT dimeric hHV1 channels (A) is contrasted with the more rapid and exponential turn-on of C-truncated channels (B), presumed to be monomeric. The curves are single exponential fits. In C the kinetics of current activation of CiHV1 at three voltages (black) is compared with the normalized fluorescence signal (red) emitted by a fluorophore tag attached to the top of the S4 domain. When the fluorescence signal is squared (see Scheme 2), it practically superimposes onto the current, consistent with a Hodgkin-Huxley-type gating mechanism in which both protomers must “activate” before either can conduct. A, B; Reprinted with permission from: Musset B, Smith SME, Rajan S, Cherny VV, Sujai S, Morgan D, and DeCoursey TE. Zinc inhibition of monomeric and dimeric proton channels suggests cooperative gating. Journal of Physiology 588, 1435–1449, copyright 2010. C; Reprinted by permission from Macmillan Publishers Ltd, Gonzalez C, Koch HP, Drum BM and Larsson HP. Strong cooperativity between subunits in voltage-gated proton channels. Nature Structural Molecular Biology 17, 51–56, copyright 2010.
Further evidence supporting this form of cooperative gating emerged when the Larsson group determined the effective gating charge of hHV1. WT HV1 channels have an effective gating charge of ~6 e0 in rat [46, 47] and human [48]. In CiHV1, the WT channel had 5.9 e0 but the monomeric construct had 2.7 e0 [19]. This result strongly supports the idea that both protomers must move before either can conduct. A recent study of the mouse Hv1, mHV1, produced identical phenomenology: the dimeric channel had twice the effective gating charge as seen in monomeric constructs [22]. In this species, the gating charge in WT channels was 4 e0, with 2 e0 seen in monomeric constructs.
Activation kinetics differs between monomer and dimer
Koch et al [3] produced monomeric mHV1 (mouse) channels by truncating the C terminus, and also by truncating both C- and N-termini. The C-truncated channel (ΔC) opened 2.5 times faster and the doubly truncated channel (ΔNΔC) opened 5 times faster. If we interpret the double truncation as more efficiently producing monomeric channels (i.e., that some dimers remain even with C-truncation), then the monomer opens 5 times faster. In a later study, C truncation of mHV1 accelerated channel activation, τact, by ~6 fold (Fujiwara et al, 2012).
Tombola et al [5] produced monomeric chimerae by attaching the C terminus alone or both the N- and C-termini from the CiVSP, a phosphatase from Ciona intestinalis thought to exist as a monomer [49], onto hHV1. A minor effect of swapping the N terminus alone was also observed. The chimera with both N- and C-termini from CiVSP grafted onto the hHV1 transmembrane domains activated substantially more rapidly, although no quantitative results were provided. The gH-V relationship for this monomeric construct was 30% less steep and its midpoint voltage about 10 mV more positive.
Musset and colleagues [21] produced presumed monomeric hHV1 by C-truncation (hHV1ΔC). Although independent confirmation that C truncation produces mainly monomers was not obtained, the activation kinetics were well fitted by a single exponential function (Fig. 3B). Activation of the C-truncated channel was 6.6 times faster than the WT dimer, measured in inside-out membrane patches at pHo 7.5, pHi 7.5. The difference was less pronounced in whole-cell data at pHo 7.0, pHi 6.5, with the hHV1ΔC about 3 times faster than WT [50]. Consistent with the report of Tombola et al [5], the gH-V relationship was somewhat less steep in the monomeric construct, with the midpoint shifted 10–15 mV more positive than WT. The hHV1ΔC construct not only opened more rapidly, but the time course of turn-on of current was exponential (Fig. 3B), in contrast with the distinctly sigmoidal time course of WT currents (Fig. 3A) [21]. This behavior is consistent with the Hodgkin-Huxley-type model discussed above (Scheme 2) in which each protomer must activate before either can conduct. In summary, gating kinetics supports the idea of cooperative gating.
A mechanism for cooperative gating of mHV1 was proposed recently by Fujiwara et al [22]. A number of mutations were carried out in the C terminal domain. The short region between the C-terminal (intracellular) end of S4 and the start of the coiled-coil region was deleted, extended, or replaced with different amino acids. The resulting mutants fell into two groups: one group (including the monomeric ΔC) opened several times more rapidly and had an effective gating charge of 2 e0 based on limiting slope measurements, half that of 4 e0 in the other group (including WT channels). Cooperative gating is consistent with the observation that the slower opening phenotype had twice the gating charge. Several conclusions can be drawn. In general, coiled-coil dimer assembly inhibits activation of HV1, in the sense that the dimer opens more slowly. Second, the mere fact that the channel was dimeric did not ensure cooperative gating, because a flexible linker (GGG) inserted between S4 and the C terminus prevented cooperativity. Similarly, a dimer comprising two C-truncated protomers failed to exhibit cooperativity. A related conclusion is that interactions occurring within the transmembrane regions evidently are not sufficient to ensure cooperativity, because these constructs were shown to be dimeric, yet exhibited no signs of cooperativity.
The Okamura group [22] proposed that the C-termini that are known to be linked in the dimer by coiled-coil interactions [4, 20] directly modulate S4 movement that results in channel opening. The C termini are considered to be part of a rigid rod extending through the S4 helix, so that movement of S4 in one protomer would be linked mechanically via the C termini to the second S4 domain. It is not precisely clear what degree of independence is allowed in this model. Thus, if both entire C-terminus-S4 domain assemblies were completely rigid, both protomers would be expected to move simultaneously. If the result of this concerted movement were direct activation of the conductance (opening of the channels), then the time course of current turn-on would be exponential, not sigmoid. Given that the movement of S4 observed directly by Gonzalez et al [19] had an exponential time course, but the current has a sigmoid turn-on (Fig. 3C), one possibility is that a certain degree of flexibility in the structure allows a delay between the movement of the two S4 domains. Alternatively, there must be one or more additional steps that couple S4 movement to opening of the conduction pathway that would account for the delayed turn-on of the current. The Larsson group [19] could model their results either with the assumption (a) that the two protomers move independently and both must move before conduction occurs in either, or (b) that there is a strong allosteric interaction that favors coordinated movement of both S4 domains over the movement of a single protomer. The latter model was also proposed by Tombola et al [51].
Distinguishing these two classes of models may be difficult, especially when there is a sufficiently high degree of allosteric coupling, because the allosteric mechanism becomes indistinguishable from the HH model. Indirect evidence that seems to support the allosteric model comes from attempts to measure single channel current amplitude in HV1. Unitary H+ currents are ~103 smaller than those of most ion channels, and consequently are very difficult to resolve directly. However, under favorable conditions (absence of other conductances, extremely high resistance seals (up to 5 TΩ) which minimize noise [52], maximized conductance induced by using low pHi), Cherny and colleagues [53] were just able to resolve what appeared to be unitary currents, of ~10 fA amplitude. An alternative method of measuring unitary currents involves analyzing current fluctuations resulting from stochastic opening and closing of channels [54]. Current variance was well resolved in excised membrane patches from human eosinophils, and provided reproducible estimates of single channel conductance [53]. However, the values derived from current fluctuations were consistently about two-fold smaller than those from direct observation. Concerted opening of both protomers would result in channel openings with a conductance double that of each monomer, while allosteric interaction would allow two separate steps. The current fluctuation results can be reconciled with the apparent double-sized unitary current events if allosteric interaction results in both protomers opening in rapid succession, so that the current appears to comprise a single step, rather than two steps. Because the unitary current amplitude was just on the borderline of detectability, caution should be exercised before putting too much weight on this kind of interpretation.
V. PROPOSALS FOR THE HV1 DIMER INTERFACE
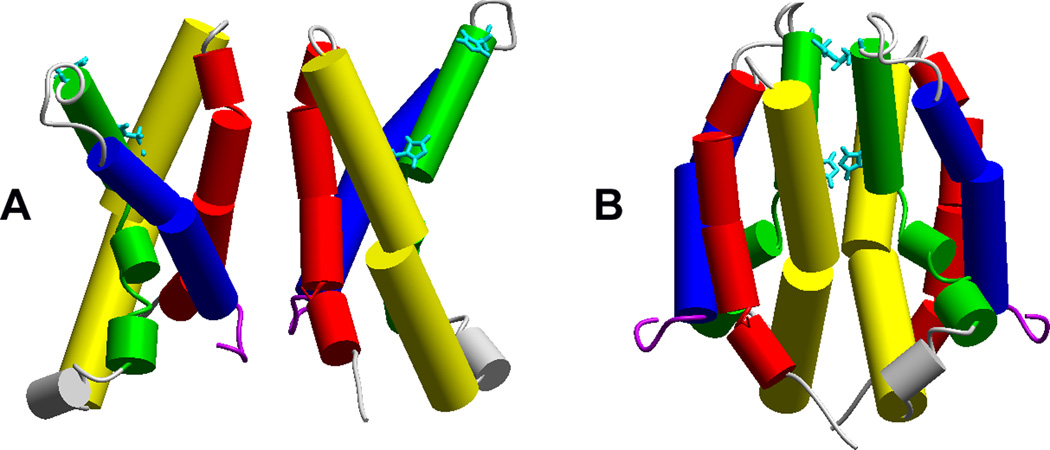
The first proposal [4] for the orientation of the hHV1 dimer is shown in Fig. 4A. Cys residues were introduced at numerous locations, and cross bridge formation was evaluated. Several cross bridges were detected at the extracellular end of S1 (red cylinders, extracellular is towards top of Fig. 4) and in the S1–S2 linker; this was proposed to be the main interface region, in addition to the predicted coiled-coil interaction of the C termini [4]. This dimer orientation is supported by FRET data obtained from Ciona Hv1 [3] that indicated a distance of 42 Å between Cys243 on each protomer. Cys243 of CiHv1 is on the S2/S3 linker (connecting green and blue helices in Fig. 4), predicted to be far apart in a dimer connected at the S1 helices.
Figure 4.
Two proposed dimer interfaces. Crosslinking studies indicate several points of attachment at the top (outer) end of S1 (red) [4]. The binding of Zn2+ to various hHV1 mutants with His140 or His193 (aqua) replaced by Ala suggested that high-affinity bidentate Zn2+ binding occurs at the dimer interface [21]. Reprinted with permission from: Musset B, Smith SME, Rajan S, Cherny VV, Sujai S, Morgan D, and DeCoursey TE. Zinc inhibition of monomeric and dimeric proton channels suggests cooperative gating. Journal of Physiology 588, 1435–1449, copyright 2010.
On the other hand, data consistent with more than one dimer interface also exist. In the Lee et al. [4] study, distinct crosslinks at hHV1 position 194, also in the S2/S3 linker, were observed, indicating that this position attains close proximity a measurable fraction of the time, which would be unexpected if the only possible interface were through the S1 helices.
Additional evidence for another dimer interface comes from the use of Zn2+, the most potent inhibitor of HV channels [55–58], which acts by preventing channel opening. Although sensitivity to Zn2+ has been examined almost religiously as a pathognomonic feature that must be confirmed in any newly discovered proton conductance in cells or species not previously studied, Zn2+ sensitivity is extremely sensitive to pH [55]. The sensitivity of the channel to externally applied Zn2+ becomes quite weak at low pHo, strongly suggestive of competition between H+ and Zn2+ for the binding site where Zn2+ exerts its inhibitory effects. By mathematically modeling the competition between H+ and Zn2+ at different pHo, Cherny & DeCoursey concluded that Zn2+ must be multiply coordinated, and that the pKa of the coordinating groups was roughly 6–7, suggesting that two or more His residues formed the Zn2+ binding site [55].
When the hHV1 gene was identified, and two externally accessible His residues (His140 and His193) were shown to account for the inhibitory effects of Zn2+ [1], the obvious conclusion was that these two His residues most likely coordinated Zn2+ at its site of action. However, in a molecular model of the open state monomer of hHV1, His140 and His193 were too far apart to coordinate Zn2+ plausibly [21]. Protein:protein docking of the model structure indicated the possibility that His pairs from different protomers could approach close enough for Zn2+ coordination to occur in a dimer whose interface was between the S2/S3 helices.
Several types of experiments were carried out to test the hypothesis of inter-protomer Zn2+ coordination. The first prediction of this hypothesis is that the affinity of Zn2+ for the dimeric channel should be greater than for the monomer, which would lack the possibility of bidentate coordination. Indeed, C-terminally truncated constructs of both human and mouse HV1 (presumed to be monomeric – see above) exhibited significantly diminished response to Zn2+, consistent with the prediction. This result conflicts with a report using a different HV1 monomer construct [51]. Because this report was based on measurements under one set of conditions at one pH at a single voltage [51], a chance combination of separate Zn2+ effects may have canceled each other out. Zn2+ has complex pharmacological effects, including profoundly slowing activation, shifting the gH-V relationship positively, and probably also decreasing gH,max [55]. It is also possible that the different monomeric constructs responded differently to Zn2+.
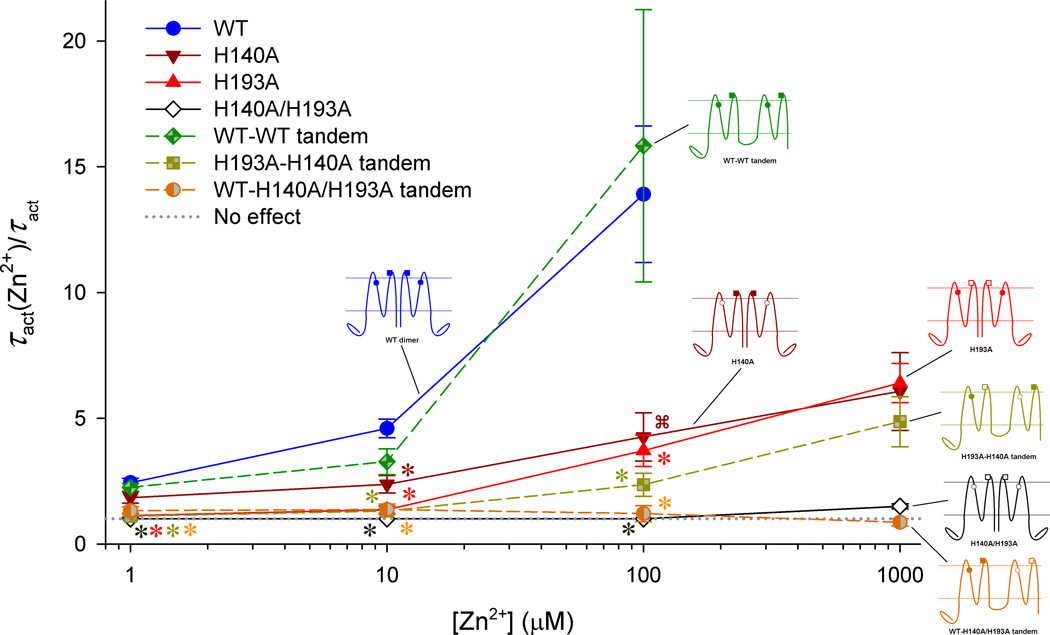
Another set of experiments involved testing mutants and tandem constructs in which alanine replaced the histidines in several combinations. Fig. 5 illustrates the constructs tested and their relative Zn2+ sensitivity, using the slowing of channel opening as the parameter of interest. A similar pattern was observed when the shift of the gH-V relationship by Zn2+ was the parameter evaluated. As previously shown by Ramsey et al [1], mutation of His140 or His193 alone attenuated but did not eliminate the Zn2+ sensitivity, demonstrating that both residues contribute to WT Zn2+ sensitivity. The double mutant lacked any slowing up to 1 mM Zn2+, suggesting that no other groups on the channel contribute significantly. Control tandem dimers in which both protomers possessed both histidines showed Zn2+ sensitivity indistinguishable from WT (wild-type) (Fig. 5, blue vs. green symbols) as well as sigmoidal activation kinetics suggestive of cooperative gating. A tandem dimer in which one protomer lacked His140 and the other protomer lacked His193 (“H193A-H140A tandem”) exhibited Zn2+ sensitivity similar to single mutants in the WT background (Fig. 5). A surprising result was observed for a tandem dimer in which one protomer was WT, with both His present, while the other protomer lacked both His. Just as for the double His mutant (lacking both His), Zn2+ did not slow opening in the tandem dimer in which both histidines were present in one protomer, while the other protomer lacked His. In summary, slowing of channel opening by Zn2+ was observed only when there was at least one His in each protomer. Taken together, these data indicated that Zn2+ is most likely coordinated between protomers, which implies a dimer interface involving S2 and S3 helices (Fig. 4B).
Figure 5.
Evidence that Zn2+ binds with high affinity at the interface between protomers in the hHV1 dimer. The slowing of proton current activation by Zn2+ is plotted for several hHV1 constructs. The constructs listed in the inset are illustrated with His140 shown as a circle and His193 as a square. Solid symbols indicate His at the indicated position, open symbols indicate replacement with Ala. Reprinted with permission from: Musset B, Smith SME, Rajan S, Cherny VV, Sujai S, Morgan D, and DeCoursey TE. Zinc inhibition of monomeric and dimeric proton channels suggests cooperative gating. Journal of Physiology 588, 1435–1449, copyright 2010.
The apparent discrepancy between the two suggested dimer interfaces could be explained in at least two ways. In one, the transmembrane domains of the two protomers, although tethered by coiled-coil interactions within the C-termini, may be able to rotate around the tether and adopt different orientations relative to each other. This possibility appears to be less likely in light of the proposed rigidity of the linker between S4 and the coiled-coil region [22]. Nevertheless, a tandem WT-WT dimer, linked with a short (6 AA) connection between the C-terminus of one monomer and the N terminus of the second, functioned almost identically to the WT channel, with the exception of somewhat slower opening kinetics [21, 50]. This result might indicate that the required C-terminal interaction can occur almost normally despite the proximity of an attached N-terminus. Another explanation posits the formation of a tetramer consisting of a dimer-of-dimers; this complex maybe stabilized by Zn2+ binding at an interface at the S2/S3 helices. Wherever Zn2+ binds, it clearly stabilizes the closed conformation, because it slows channel opening, and shifts the gH-V relationship in the positive direction [55].
It might be objected that the molecular model that gave rise to the hypothesis of Zn2+ binding between protomers is presumed to be an open-state model, whereas the general interpretation of Zn2+ effects presumes that Zn2+ binds to the closed channel. The relative positions of His140 and His193 were estimated in the predicted closed state, based on the positions of the corresponding residues in molecular models of closed KV1.2 voltage sensor domains [59]. The estimated closed state positions do not differ greatly from those in the open state – in particular, they still do not appear to approach closely enough to coordinate Zn2+. This is not unexpected, considering that neither His is located on the S4 helix, which is thought to be the main ‘moving part’ of the HV1. In any case, the results obtained do not depend on perfect accuracy of the molecular model. When the crystal structure of the closed hHV1 molecule has been obtained, this direct evidence should resolve many questions that at present can only be addressed indirectly.
VI. PHYSIOLOGICAL CONSEQUENCES OF DIMERIZATION OF HV1
The most consistent difference between monomeric and dimeric HV1 is in gating kinetics. However, this difference, while clear, is not dramatic. In some situations, the 3–6 fold faster monomeric opening rate might seem to be an important distinction, but considering that among species, HV1 activation kinetics varies over several orders of magnitude [60], it would appear that a species with a need for quickly opening proton channels would be better served in making a channel that is intrinsically fast. HV in snail neurons open within a few milliseconds [61], whereas mammalian HV1 require seconds [62]. From this perspective, fundamental kinetics would most logically be determined genetically, not by adjusting multimerization. On the other hand, if it were possible for a cell to switch its HV1 between monomer and dimer status, then a 5-fold change in gating kinetics might have important consequences. In fact, Koch et al [3] initially suggested that this kind of mechanism might be responsible for the “enhanced gating mode” of proton channels in phagocytes.
Does the enhanced gating mode in phagocytes reflect dimer-to-monomer conversion of HV1?
The clearest function of proton channels in phagocytes (neutrophils, macrophages, and eosinophils) is to facilitate and sustain NADPH oxidase (or “Nox2”) activity. Detection of a foreign invader (e.g., a bacterium) by the phagocyte triggers a complex series of biochemical and cellular process culminating in phagocytosis of the detected material. A rapid biochemical consequence of the detection mechanism is the assembly, from its several components, of Nox2 in the phagosome membrane. Nox2 uses intracellular NADPH as a source of electrons, which it translocates across the phagosome membrane to produce reactive oxygen species (ROS) that are released into the forming or mature phagosome. Electron translocation by Nox2 depolarizes the membrane potential [63], while NADPH catabolism, which releases NADP+ and H+ [8, 64], decreases the intracellular pH [63, 65–68]. Proton efflux through HV1 compensates for both the membrane depolarization and the pH change [10, 11, 62, 63, 66, 67, 69–71].
Another consequence of the detection of foreign entities by phagocytes is a radical change in the gating kinetics of HV1, which turns out to be a significant factor contributing to the activation of H+ efflux. Four properties of hHV1 change: opening occurs more quickly, closing slows, the maximum conductance gH,max increases, and the gH-V relationship shifts negatively by 40 mV [69, 72–75]. This transformation of HV1 properties is termed the “enhanced gating mode.” As a result of the four changes in HV1 behavior, each of which promotes H+ efflux, during enhanced gating the channel opens sooner and thereby improves the efficiency of NADPH oxidase activity by 15–20% [76]. Is it possible that enhanced gating reflects conversion of the normally dimeric hHV1 to monomeric status? This suggestion [3] has not yet been resolved beyond doubt, but the bulk of evidence suggests that this is not the case [77].
Monomeric HV1 and HV1 in enhanced gating mode share one property: monomeric HV1 open several times faster than dimeric channels [3, 5, 21, 50]. Similarly, during enhanced gating, HV1 opens 4–6 times faster than in resting cells [12, 69, 72–75, 78, 79]. Three other properties evident in the enhanced gating mode differ from those of monomeric constructs. 1) The position of the gH-V relationship appears to be 10–15 mV more positive in monomeric constructs than WT dimeric channels [50, 51]. In enhanced gating mode, the gH-V relationship shifts in the opposite direction, negatively by ~40 mV [69, 72–75, 78, 79]. 2) During enhanced gating, the Zn2+ sensitivity of hHV1 is not changed [50] whereas the Zn2+ sensitivity of the C-truncated construct was weaker than that of the dimer [21]. 3) During enhanced gating, activation of hHV1 continues to have a sigmoid time course, in contrast to the exponential activation of the monomer [21]. On balance, the differences in channel properties during enhanced gating mode compared to monomeric constructs appear to outweigh the similarities. In another supporting piece of evidence, Petheő and colleagues [23] did not observe any difference in DSS crosslinking potential when they pretreated human neutrophils with PMA, which effectively induces enhanced gating mode.
An alternative explanation is that phosphorylation of hHV1 [80], specifically at Thr29 [81] is responsible for converting the resting channel into enhanced gating mode. The PKC activator PMA produces enhanced gating [12, 15, 16, 72–75, 78, 80–82] that is prevented or reversed by PKC inhibitors such as staurosporine or GF109203X (GFX) [12, 15, 74, 75, 80–82]. Other activators of enhanced gating including arachidonic acid and LPS (lipopolysaccharide) act at least partially through PKC [79, 80, 82].
Might the differences in properties of monomeric and dimeric constructs provide clues to the functional importance of dimerization?
The main differences between WT dimeric HV1 and monomeric constructs include four properties: (a) activation (channel opening) is ~5 times slower in WT dimeric channels, (b) WT channel opening occurs with a sigmoid rather than exponential time course, (c) the WT gH-V relationship is 10–15 mV more positive, and (d) the WT voltage dependence is twice steeper. We may for now discount the difference in opening time course (b) and the relatively subtle change in the position of the gH-V relationship (c). Although there are certainly situations in which these changes could have functional ramifications -- in excitable cells like cardiac muscle, for example, a 10 mV shift of a g-V curve has profound physiological significance [83] -- in the absence of detailed knowledge about the precise situations in which HV1 operate in most of the many cells where they exist, it seems pedantic to focus on small differences. The faster opening (a) and steeper voltage dependence (d) do however seem important enough to warrant speculation.
As discussed above, phagocytes, especially neutrophils and eosinophils, use proton channels during the respiratory burst, when Nox2 is active. The best understood role of HV1 in this situation is to compensate charge [63], to prevent excessive depolarization that would otherwise result from the activity of the electrogenic Nox2. Nox2 activity tends to occur with a delay of several seconds or even minutes, depending upon the stimulus, and may continue for hours [84]. Thus, it may be that the ability of HV1 to open rapidly may not have much value to these cells. The phagocyte “cares” only that enough HV1 open as soon as Nox2 is active to limit the depolarization. From the vantage point of the phagocyte, there is no rush, because Nox2 turns on slowly and remains active for a long while. In fact, in these non-excitable cells, proton channel opening is quite slow, compared with the kinetics of gating of most ion channels, and compared with the opening of HV in other species, such as snail neurons [61, 85, 86].
On the other hand, HV1 opening plays a major role in limiting the extent of the depolarization that occurs when Nox2 is active; membrane depolarization, especially beyond +50 mV, directly inhibits Nox2 activity [10]. To ‘help’ Nox2, HV1 should open within a relatively negative voltage range. The enhanced gating mode, which shifts Hv1’s gH-V relationship negatively by 40 mV, clearly facilitates this goal. Enhanced gating improves the efficiency of Nox2 by 15–20%, because to open enough channels to compensate Nox2, requires 24–30 mV less depolarization in human neutrophils and eosinophils than would be needed if there were no enhanced gating mode [76]. We have already seen that enhanced gating mode does not seem to represent a change in the dimerization state of HV1.
The steep voltage dependence of HV1 also facilitates Nox2 activity. Cooperative gating increases the steepness of the voltage dependence of ion channels [87]. The cooperative gating of the hHV1 dimer results in a doubling of the voltage dependence compared to the monomer [19]. The result is that substantially less depolarization is required to open a given number of proton channels. One might argue that the cell could enable sufficient activation of gH simply by shifting the gH-V relationship negatively, but once enhanced gating is in effect, Vthreshold is already perilously close to EH. The cell “wants” HV1 to turn on rapidly once there is an outward electrochemical gradient for H+, but it definitely does not want HV1 to open when the gradient is inward. The main function of HV1 in most cells is acid extrusion – cells continually produce metabolic acid that they must eliminate; this is the “central problem of pHi regulation” [88]. For this reason, it would be self-destructive to have a constitutively active gH, because this would act as a proton leak that would flood the cell with unwanted H+. The most optimal possible design is to poise Vthreshold near EH and then to have strong dependence of Popen upon depolarization above this point. Nature has produced the most efficient possible mechanism.
It would be interesting to know why HV in some species appear to be monomeric. However, because we know the functions and the situations in which the channels are active only in the most general terms, it seems premature to attempt to predict why monomeric behavior would be preferable.
CONCLUSIONS
In most of the species where it has been identified, HV1 exists as a dimer. Dimerization is driven and enforced mainly through coiled-coil interactions at the C terminus; HV1 sequences from a few single-celled organisms do not have a predicted coiled-coil region and so may exist as monomers. Dimerization in HV1 provides the opportunity for a cooperative gating mechanism that gives rise to a steeply voltage dependent conductance. This property appears to be important in the most intensively studied function of HV1, which exists in human phagocytes, where steep voltage dependence provides optimum compensation for Nox activity and phagocyte function. A number of questions regarding HV1 dimerization remain open, including the nature of the dimer interface, the mechanism of cooperativity, the impact of multimeric state on trans-acting factors (e.g., kinases) of HV1, possible effects of transitions between monomeric and multimeric states, and implications of cooperative gating in cell types other than phagocytes.
ACKNOWLEDGMENTS
This work was supported by NSF award MCB-0943362 to TED and SMES; and NIH R01-GM087507 to TED. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institute of General Medical Sciences or the National Institutes of Health.
REFERENCES
- 1.Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- 3.Koch HP, Kurokawa T, Okochi Y, Sasaki M, Okamura Y, Larsson HP. Multimeric nature of voltage-gated proton channels. Proc Natl Acad Sci USA. 2008;105:9111–9116. doi: 10.1073/pnas.0801553105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SY, Letts JA, Mackinnon R. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc Natl Acad Sci USA. 2008;105:7692–7695. doi: 10.1073/pnas.0803277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tombola F, Ulbrich MH, Isacoff EY. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron. 2008;58:546–556. doi: 10.1016/j.neuron.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherny VV, Markin VS, DeCoursey TE. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J Gen Physiol. 1995;105:861–896. doi: 10.1085/jgp.105.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demaurex N. Functions of proton channels in phagocytes. Wiley Interdisciplinary Reviews: Membrane Transport and Signaling. 2012;1:3–15. doi: 10.1002/wmts.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson LM, Chappel JB. NADPH oxidase of neutrophils. Biochim Biophys Acta. 1996;1273:87–107. doi: 10.1016/0005-2728(95)00140-9. [DOI] [PubMed] [Google Scholar]
- 9.DeCoursey TE. Voltage-gated proton channels find their dream job managing the respiratory burst in phagocytes. Physiology (Bethesda) 2010;25:27–40. doi: 10.1152/physiol.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeCoursey TE, Morgan D, Cherny VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–534. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- 11.Morgan D, Capasso M, Musset B, Cherny VV, Ríos E, Dyer MJS, DeCoursey TE. Voltage-gated proton channels maintain pH in human neutrophils during phagocytosis. Proc Natl Acad Sci USA. 2009;106:18022–18027. doi: 10.1073/pnas.0905565106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musset B, Morgan D, Cherny VV, MacGlashan DW, Jr, Thomas LL, Ríos E, DeCoursey TE. A pH-stabilizing role of voltage-gated proton channels in IgE-mediated activation of human basophils. Proc Natl Acad Sci USA. 2008;105:11020–11025. doi: 10.1073/pnas.0800886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer H. Function of proton channels in lung epithelia. WIRES Interdisciplinary Reviews: Membrane Transport and Signaling. 2011 [Google Scholar]
- 14.Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 2010;140:327–337. doi: 10.1016/j.cell.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 15.Musset B, Clark RA, DeCoursey TE, Petheo GL, Geiszt M, Chen Y, Cornell JE, Eddy CA, Brzyski RG, El Jamali A. NOX5 in human spermatozoa: expression, function and regulation. J Biol Chem. 2012;287:9376–9388. doi: 10.1074/jbc.M111.314955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capasso M, Bhamrah MK, Henley T, Boyd RS, Langlais C, Cain K, Dinsdale D, Pulford K, Khan M, Musset B, Cherny VV, Morgan D, Gascoyne RD, Vigorito E, DeCoursey TE, MacLennan ICM, Dyer MJS. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat Immunol. 2010;11:265–272. doi: 10.1038/ni.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Li SJ, Wu X, Che Y, Li Q. Clinicopathological and biological significance of human voltage-gated proton channel Hv1 over-expression in breast cancer. J Biol Chem. 2012;287:13877–13888. doi: 10.1074/jbc.M112.345280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu LJ, Wu G, Sharif MR, Baker A, Jia Y, Fahey FH, Luo HR, Feener EP, Clapham DE. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat Neurosci. 2012;15:565–573. doi: 10.1038/nn.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez C, Koch HP, Drum BM, Larsson HP. Strong cooperativity between subunits in voltage-gated proton channels. Nat Struct Mol Biol. 2010;17:51–56. doi: 10.1038/nsmb.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li SJ, Zhao Q, Zhou Q, Unno H, Zhai Y, Sun F. The role and structure of the carboxyl-terminal domain of the human voltage-gated proton channel Hv1. J Biol Chem. 2010;285:12047–12054. doi: 10.1074/jbc.M109.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musset B, Smith SM, Rajan S, Cherny VV, Sujai S, Morgan D, DeCoursey TE. Zinc inhibition of monomeric and dimeric proton channels suggests cooperative gating. J Physiol. 2010;588:1435–1449. doi: 10.1113/jphysiol.2010.188318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujiwara Y, Kurokawa T, Takeshita K, Kobayashi M, Okochi Y, Nakagawa A, Okamura Y. The cytoplasmic coiled-coil mediates cooperative gating temperature sensitivity in the voltage-gated H+ channel Hv1. Nat Commun. 2012;3:816. doi: 10.1038/ncomms1823. [DOI] [PubMed] [Google Scholar]
- 23.Petheő GL, Orient A, Baráth M, Kovács I, Réthi B, Lányi A, Rajki A, Rajnavölgyi E, Geiszt M. Molecular and functional characterization of HV1 proton channel in human granulocytes. PLoS One. 2010;5:e14081. doi: 10.1371/journal.pone.0014081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SME, Morgan D, Musset B, Cherny VV, Place AR, Hastings JW, DeCoursey TE. Voltage-gated proton channel in a dinoflagellate. Proc Natl Acad Sci USA. 2011;108:18162–18168. doi: 10.1073/pnas.1115405108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor AR, Brownlee C, Wheeler GL. Proton channels in algae: reasons to be excited. Trends Plant Sci. 2012;17:675–684. doi: 10.1016/j.tplants.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Lee SY, Letts JA, MacKinnon R. Functional reconstitution of purified human Hv1 H+ channels. J Mol Biol. 2009;387:1055–1060. doi: 10.1016/j.jmb.2009.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodgkin AL, Huxley AF. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952;116:473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horn R. Uncooperative voltage sensors. J Gen Physiol. 2009;133:463–466. doi: 10.1085/jgp.200910236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldman DE. Potential, impedance, and rectification in membranes. J Gen Physiol. 1943;27:37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahern CA, Horn R. Focused electric field across the voltage sensor of potassium channels. Neuron. 2005;48:25–29. doi: 10.1016/j.neuron.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Asamoah OK, Wuskell JP, Loew LM, Bezanilla F. A fluorometric approach to local electric field measurements in a voltage-gated ion channel. Neuron. 2003;37:85–97. doi: 10.1016/s0896-6273(02)01126-1. [DOI] [PubMed] [Google Scholar]
- 33.Starace DM, Bezanilla F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature. 2004;427:548–553. doi: 10.1038/nature02270. [DOI] [PubMed] [Google Scholar]
- 34.Grabe M, Lecar H, Jan YN, Jan LY. A quantitative assessment of models for voltage-dependent gating of ion channels. Proc Natl Acad Sci USA. 2004;101:17640–17645. doi: 10.1073/pnas.0408116101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almers W. Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol. 1978;82:96–190. doi: 10.1007/BFb0030498. [DOI] [PubMed] [Google Scholar]
- 36.Sigg D, Bezanilla F. Total charge movement per channel. The relation between gating charge displacement and the voltage sensitivity of activation. J Gen Physiol. 1997;109:27–39. doi: 10.1085/jgp.109.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirschberg B, Rovner A, Lieberman M, Patlak J. Transfer of twelve charges is needed to open skeletal muscle Na+ channels. J Gen Physiol. 1995;106:1053–1068. doi: 10.1085/jgp.106.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Islas LD, Sigworth FJ. Voltage sensitivity and gating charge in Shaker and Shab family potassium channels. J Gen Physiol. 1999;114:723–742. doi: 10.1085/jgp.114.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noceti F, Baldelli P, Wei X, Qin N, Toro L, Birnbaumer L, Stefani E. Effective gating charges per channel in voltage-dependent K+ and Ca2+ channels. J Gen Physiol. 1996;108:143–155. doi: 10.1085/jgp.108.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoppa NE, McCormack K, Tanouye MA, Sigworth FJ. The size of gating charge in wild-type and mutant Shaker potassium channels. Science. 1992;255:1712–1715. doi: 10.1126/science.1553560. [DOI] [PubMed] [Google Scholar]
- 41.Seoh SA, Sigg D, Papazian DM, Bezanilla F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron. 1996;16:1159–1167. doi: 10.1016/s0896-6273(00)80142-7. [DOI] [PubMed] [Google Scholar]
- 42.Horrigan FT, Cui J, Aldrich RW. Allosteric voltage gating of potassium channels I. Mslo ionic currents in the absence of Ca2+ J Gen Physiol. 1999;114:277–304. doi: 10.1085/jgp.114.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horrigan FT, Aldrich RW. Allosteric voltage gating of potassium channels II. Mslo channel gating charge movement in the absence of Ca2+ J Gen Physiol. 1999;114:305–336. doi: 10.1085/jgp.114.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horn R, Ding S, Gruber HJ. Immobilizing the moving parts of voltage-gated ion channels. J Gen Physiol. 2000;116:461–476. doi: 10.1085/jgp.116.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gagnon DG, Bezanilla F. A single charged voltage sensor is capable of gating the Shaker K+ channel. J Gen Physiol. 2009;133:467–483. doi: 10.1085/jgp.200810082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeCoursey TE, Cherny VV. Effects of buffer concentration on voltage-gated H+ currents: does diffusion limit the conductance? Biophys J. 1996;71:182–193. doi: 10.1016/S0006-3495(96)79215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeCoursey TE, Cherny VV. Deuterium isotope effects on permeation and gating of proton channels in rat alveolar epithelium. J Gen Physiol. 1997;109:415–434. doi: 10.1085/jgp.109.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musset B, Cherny VV, Morgan D, Okamura Y, Ramsey IS, Clapham DE, DeCoursey TE. Detailed comparison of expressed and native voltage-gated proton channel currents. J Physiol. 2008;586:2477–2486. doi: 10.1113/jphysiol.2007.149427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kohout SC, Ulbrich MH, Bell SC, Isacoff EY. Subunit organization and functional transitions in Ci-VSP. Nat Struct Mol Biol. 2008;15:106–108. doi: 10.1038/nsmb1320. [DOI] [PubMed] [Google Scholar]
- 50.Musset B, Smith SM, Rajan S, Cherny VV, Morgan D, DeCoursey TE. Oligomerization of the voltage gated proton channel. Channels (Austin) 2010;4:260–265. doi: 10.4161/chan.4.4.12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tombola F, Ulbrich MH, Kohout SC, Isacoff EY. The opening of the two pores of the Hv1 voltage-gated proton channel is tuned by cooperativity. Nat Struct Mol Biol. 2010;17:44–50. doi: 10.1038/nsmb.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levis RA, Rae JL. The use of quartz patch pipettes for low noise single channel recording. Biophys J. 1993;65:1666–1677. doi: 10.1016/S0006-3495(93)81224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cherny VV, Murphy R, Sokolov V, Levis RA, DeCoursey TE. Properties of single voltage-gated proton channels in human eosinophils estimated by noise analysis and by direct measurement. J Gen Physiol. 2003;121:615–628. doi: 10.1085/jgp.200308813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates, Inc.; 2001. [Google Scholar]
- 55.Cherny VV, DeCoursey TE. pH-dependent inhibition of voltage-gated H+ currents in rat alveolar epithelial cells by Zn2+ and other divalent cations. J Gen Physiol. 1999;114:819–838. doi: 10.1085/jgp.114.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahaut-Smith MP. The effect of zinc on calcium and hydrogen ion currents in intact snail neurones. J Exp Biol. 1989;145:455–464. doi: 10.1242/jeb.145.1.455. [DOI] [PubMed] [Google Scholar]
- 57.Thomas RC, Meech RW. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature. 1982;299:826–828. doi: 10.1038/299826a0. [DOI] [PubMed] [Google Scholar]
- 58.DeCoursey TE, Cherny VV. Pharmacology of voltage-gated proton channels. Curr Pharm Des. 2007;13:2400–2420. doi: 10.2174/138161207781368675. [DOI] [PubMed] [Google Scholar]
- 59.Pathak MM, Yarov-Yarovoy V, Agarwal G, Roux B, Barth P, Kohout S, Tombola F, Isacoff EY. Closing in on the resting state of the Shaker K+ channel. Neuron. 2007;56:124–140. doi: 10.1016/j.neuron.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 60.DeCoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 61.Byerly L, Meech R, Moody W., Jr Rapidly activating hydrogen ion currents in perfused neurones of the snail, Lymnaea stagnalis. J Physiol. 1984;351:199–216. doi: 10.1113/jphysiol.1984.sp015241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DeCoursey TE, Cherny VV. Potential, pH, arachidonate gate hydrogen ion currents in human neutrophils. Biophys J. 1993;65:1590–1598. doi: 10.1016/S0006-3495(93)81198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henderson LM, Chappell JB, Jones OTG. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem J. 1987;246:325–329. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Babior BM, Curnutte JT, McMurrich BJ. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976;58:989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gabig TG, Lefker BA, Ossanna PJ, Weiss SJ. Proton stoichiometry associated with human neutrophil respiratory-burst reactions. J Biol Chem. 1984;259:13166–13171. [PubMed] [Google Scholar]
- 66.Henderson LM, Chappell JB, Jones OTG. Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem J. 1988;255:285–290. [PMC free article] [PubMed] [Google Scholar]
- 67.Henderson LM, Chappell JB, Jones OTG. Internal pH changes associated with the activity of NADPH oxidase of human neutrophils. Further evidence for the presence of an H+ conducting channel. Biochem J. 1988;251:563–567. doi: 10.1042/bj2510563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molski TF, Naccache PH, Volpi M, Wolpert LM, Sha'afi RI. Specific modulation of the intracellular pH of rabbit neutrophils by chemotactic factors. Biochem Biophys Res Commun. 1980;94:508–514. doi: 10.1016/0006-291x(80)91260-7. [DOI] [PubMed] [Google Scholar]
- 69.Bánfi B, Schrenzel J, Nüsse O, Lew DP, Ligeti E, Krause KH, Demaurex N. A novel H+ conductance in eosinophils: unique characteristics and absence in chronic granulomatous disease. J Exp Med. 1999;190:183–194. doi: 10.1084/jem.190.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El Chemaly A, Okochi Y, Sasaki M, Arnaudeau S, Okamura Y, Demaurex N. VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification. J Exp Med. 2010;207:129–139. doi: 10.1084/jem.20091837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramsey IS, Ruchti E, Kaczmarek JS, Clapham DE. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc Natl Acad Sci USA. 2009;106:7642–7647. doi: 10.1073/pnas.0902761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DeCoursey TE, Cherny VV, DeCoursey AG, Xu W, Thomas LL. Interactions between NADPH oxidase-related proton and electron currents in human eosinophils. J Physiol. 2001;535:767–781. doi: 10.1111/j.1469-7793.2001.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeCoursey TE, Cherny VV, Morgan D, Katz BZ, Dinauer MC. The gp91phox component of NADPH oxidase is not the voltage-gated proton channel in phagocytes, but it helps. J Biol Chem. 2001;276:36063–36066. doi: 10.1074/jbc.C100352200. [DOI] [PubMed] [Google Scholar]
- 74.Musset B, Cherny VV, DeCoursey TE. Strong glucose dependence of electron current in human monocytes. Am J Physiol Cell Physiol. 2012;302:C286–C295. doi: 10.1152/ajpcell.00335.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mori H, Sakai H, Morihata H, Kawawaki J, Amano H, Yamano T, Kuno M. Regulatory mechanisms and physiological relevance of a voltage-gated H+ channel in murine osteoclasts: phorbol myristate acetate induces cell acidosis and the channel activation. J Bone Miner Res. 2003;18:2069–2076. doi: 10.1359/jbmr.2003.18.11.2069. [DOI] [PubMed] [Google Scholar]
- 76.Murphy R, DeCoursey TE. Charge compensation during the phagocyte respiratory burst. Biochim Biophys Acta. 2006;1757:996–1011. doi: 10.1016/j.bbabio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Musset B, DeCoursey TE. Biophysical properties of the voltage-gated proton channel HV1. Wiley Interdisciplinary Reviews: Membrane Transport and Signaling. 2012;1:605–620. doi: 10.1002/wmts.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeCoursey TE, Cherny VV, Zhou W, Thomas LL. Simultaneous activation of NADPH oxidase-related proton and electron currents in human neutrophils. Proc Natl Acad Sci USA. 2000;97:6885–6889. doi: 10.1073/pnas.100047297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cherny VV, Henderson LM, Xu W, Thomas LL, DeCoursey TE. Activation of NADPH oxidase-related proton and electron currents in human eosinophils by arachidonic acid. J Physiol. 2001;535:783–794. doi: 10.1111/j.1469-7793.2001.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morgan D, Cherny VV, Finnegan A, Bollinger J, Gelb MH, DeCoursey TE. Sustained activation of proton channels and NADPH oxidase in human eosinophils and murine granulocytes requires PKC but not cPLA2α activity. J Physiol. 2007;579:327–344. doi: 10.1113/jphysiol.2006.124248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Musset B, Capasso M, Cherny VV, Morgan D, Bhamrah M, Dyer MJS, DeCoursey TE. Identification of Thr29 as a critical phosphorylation site that activates the human proton channel Hvcn1 in leukocytes. J Biol Chem. 2010;285:5117–5121. doi: 10.1074/jbc.C109.082727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Szteyn K, Yang W, Schmid E, Lang F, Shumilina E. Lipopolysaccharide sensitive H+ current in dendritic cells. Am J Physiol Cell Physiol. 2012;303:C204–C212. doi: 10.1152/ajpcell.00059.2012. [DOI] [PubMed] [Google Scholar]
- 83.Liao Z, Lockhead D, Larson ED, Proenza C. Phosphorylation and modulation of hyperpolarization-activated HCN4 channels by protein kinase A in the mouse sinoatrial node. J Gen Physiol. 136:247–258. doi: 10.1085/jgp.201010488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DeCoursey TE, Ligeti E. Regulation and termination of NADPH oxidase activity. Cell Mol Life Sci. 2005;62:2173–2193. doi: 10.1007/s00018-005-5177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doroshenko PA, Kostyuk PG, Martynyuk AE. Transmembrane outward hydrogen current in intracellularly perfused neurones of the snail Helix pomatia. Gen Physiol Biophys. 1986;5:337–350. [PubMed] [Google Scholar]
- 86.Mahaut-Smith MP. Separation of hydrogen ion currents in intact molluscan neurones. J. Exp. Biol. 1989;145:439–454. doi: 10.1242/jeb.145.1.439. [DOI] [PubMed] [Google Scholar]
- 87.Sigworth FJ. Voltage gating of ion channels. Q Rev Biophys. 1994;27:1–40. doi: 10.1017/s0033583500002894. [DOI] [PubMed] [Google Scholar]
- 88.Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- 89.MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350:232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- 90.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]