Abstract
The field of mucosal immunology research has grown fast over the past few years, and our understanding on how mucosal surfaces respond to complex antigenic cocktails is expanding tremendously. With the advent of new molecular sequencing techniques, it is easier to understand how the immune system of vertebrates is, to a great extent, orchestrated by the complex microbial communities that live in symbiosis with their hosts. The commensal microbiota is now seen as the “extended self” by many scientists. Similarly, fish immunologist are devoting important research efforts to the field of mucosal immunity and commensals. Recent breakthroughs on our understanding of mucosal immune responses in teleost fish open up the potential of teleosts as animal research models for the study of human mucosal diseases. Additionally, this new knowledge places immunologists in a better position to specifically target the fish mucosal immune system while rationally designing mucosal vaccines and other immunotherapies. In this review, an updated view on how teleost skin, gills and gut immune cells and molecules, function in response to pathogens and commensals is provided. Finally, some of the future avenues that the field of fish mucosal immunity may follow in the next years are highlighted.
Keywords: teleost, mucosa, immune system, commensal, pathogen, evolution, gut, skin, gills
1. Introduction
Most infections start at or affect the mucosal epithelia of animals. Mucosal surfaces face many antigens while living in harmony with commensal microorganisms, known conjunctionally as microbiota. Over the last few years, the literature has been filled with many studies on how the microbiota shapes the host and its immune system [1–3]. Commensal colonization brings many physiological, metabolic and immunological benefits to the host. Some specific examples include harvesting of nutrients from food, providing essential vitamins and producing biofilms that block pathogen entrance [4]. The mucosal immune system of vertebrates comprises a unique array of innate and adaptive immune cells and molecules that act in concert to protect the host against pathogens (Figure 1). At the same time, the mucosal immune system has evolved to permit the colonization of mucosal surfaces with complex and diverse microbial communities [4, 5]. For example by developing lymphocytes with high specificity and memory capacities, the vertebrate mucosal immune system is capable of remembering commensals and pathogens. In a parallel way, commensals have evolved decreasing its pathogenicity in order to inhabit the advantageous and nutritious mucosal surfaces, like the gut, without being eliminated [4]. This represents an intricate example of co-evolution that scientists are slowly beginning to unravel.
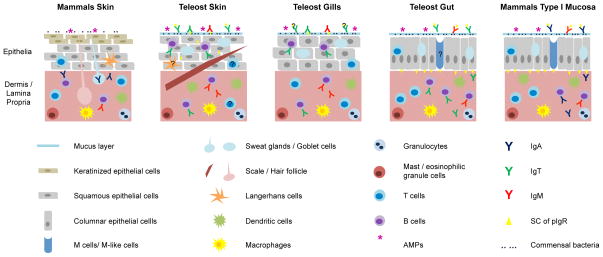
Fig. 1.
Esquematic representation of the similarities and differences between teleost fish skin, gills and gut, and mammalian skin and type I mucosal surfaces. Structural differences in the type and number of layers of epithelial cells, as well as the presence of keratine or mucus (and mucus producing cells) are displayed in the upper half of each diagram. In the bottom the connective tissue, named dermis in skin and lamina propria in gut and gills, is exhibited. Similarities in the celular components of the innate immune system (Langerhans cells, dendritic cells, macrophages, granulocytes and mast cells) are also displayed. Differences in the localization of B and T cells, the isotype of immunoglobulins and the prescence of the secretory component (SC) of the polymeric immonoglobulin receptor (pIgR) are represented as well. Finally, the presence of commensal bacteria and antimicrobial peptides (AMPs) is shown in the outer mucosal surface or over the keratin layer. Elements that are suspected to be present in a tissue, but have not been studied so far are marked as unknown (?).
Both pathogens and commensals share “microbe-associated molecular patterns” (MAMPs) recognized by the pattern recognition receptors (PRRs) of the immune system. This means that the immune system cannot distinguish, for instance, if the lipid A core binding motif of LPS that interacts with TLR4, in fact originates from a commensal or a pathogen [6]. Therefore, permitting commensal colonization requires precise homeostatic regulatory mechanisms from the host’s immune system. Commensals may nevertheless become harmful if homeostasis is breached and they access the host’s internal milieu [4].
Fish live in aquatic environments, which are an ideal medium for microorganism growth compared to air. These conditions may pose additional challenges to the mucosal immune system of aquatic vertebrates versus their terrestrial counterparts. As a consequence, some of the principles of mammalian mucosal immunity may not be necessarily applicable to aquatic vertebrates. In teleosts, the gut, the skin and the gills are the main mucosal surfaces and immune barriers. Lower vertebrates, like cartilaginous and teleost fish, are the oldest animals with an adaptive immune system based on antibodies, B cells and T cells [7]. Additionally, teleost fish are the most primitive vertebrates where dedicated antibodies specific to mucosal surfaces have been characterized [8]. These large and multifunctional surfaces represent the sites where the innate and adaptive immune systems first had to cooperate during the evolution of vertebrates to allow “good” while avoiding “bad” microorganisms.
As described later in this review, the gut, skin and gills of fish, despite having some functional and structural differences, all share many characteristics with type I mucosal surfaces of mammals [9] (Figure 1). Mammalian type I mucosal surfaces are represented by the intestine, the respiratory tract and the uterus, and they exert physiological functions in a similar way to those of teleost mucosal surfaces. Type I mucosal surfaces contain mucus-secreting cells generally arranged in a simple one-layered epithelium. Teleost mucosal surfaces also contain mucus-producing cells arranged in a simple columnar epithelium in the gut [10], one to four layers of cuboidal or squamous epithelial cells in the gills [11], and a stratified squamous epithelium in the skin [12]. In mammalian mucosal surfaces the main immunoglobulin is IgA, which is mostly produced by plasma cells present in the gut lamina propria. In a similar way, the teleost IgA homologue, IgT/IgZ, has a preponderant role in gut mucosal immunity [8]. Additionally, in mammals Igs are exported across epithelial barriers into the lumen via the polymeric immunoglobulin receptor (pIgR) expressed by epithelial cells. The pIgR is also expressed in the gut [8] and skin [13] of teleosts and it is responsible for the transport of IgM and IgT across mucosal barriers. Further, many other immunological elements of the adaptive and innate immune system, like the presence of T cells, macrophages, mast cells, dendritic cells and the coordinated expression of cytokines, are common to mucosal surfaces of mammals and teleost fish as illustrated in Figure 1. Thus, the study of fish mucosal immunity is not only exciting because of its interest to aquaculture researchers and evolutionary biologists but also because it offers a unique model to study unresolved aspects of mucosal immunity in mammals.
This review emphasizes the importance of investigating the mucosal immune system of teleosts and highlights the most recent advances (both basic and applied) in the field. Furthermore, the parallelisms and differences with the mammalian mucosal immune system and predictions on future discoveries in this research area are described. Finally, mucosal immunotherapy approaches, like probiotics and vaccines, which may help improving not only the mucosal, but also the overall immune response of fish, are discussed.
2. Innate immunity at teleost mucosal surfaces
The innate components of the immune system are the first barrier that the microorganisms have to confront in their contact with the host. Thereby these components are abundant at mucosal surfaces and their interaction with commensals is highly regulated to avoid hyper reaction. In this section, the humoral and cellular innate components present at mucosal surfaces of teleosts are reviewed and compared to their mammalian homologues.
2.1 Mucus
Amongst the innate defense mechanisms present at mucosal surfaces, the mucus is one of the most important ones. The predominant molecules present in mucus are the mucins. Mucins are high molecular weight glycoproteins that contain one or more protein domains with sites of extensive O-glycan attachment. Along with mucins, a complex mixture of other proteins, ions and lipids are also found in mucus, creating an ideal niche for microbial adherence and growth. Mucus composition determines its adhesiveness, viscoelasticity, transport and protective capacity.
In mammals, two different layers can be observed within the gut mucus: an outer “loose” mucus layer, rich in microbiota and diverse oligosaccharides of mucin glycoproteins, and an “inner” adherent mucus layer, largely devoid of bacteria [14]. The structure and composition of intestinal mucins were studied in carp (Cyprinus carpio) revealing their similarity to those found in mammals [15] although these two layers have not been described in teleosts to date. Both commensals and pathogens can adhere to mucus in fish as shown by an assessment of different bacterial strains conducted in Atlantic salmon (Salmo salar) [16]. However, certain pathogens may differ themselves from commensals, since one pathogenic strain, Vibrio anguillarum serotype O2, did not adhere to salmon mucus but to the mucosal tissues directly [16].
A few mucin-encoding genes (Muc) have been identified in teleosts [17–19]. In carp, two mucin genes have been characterized: Muc2 and Muc5B [18] showing a high similarity to their mammalian and avian counterparts. Carp Muc2 is mostly expressed in fish intestine as it is in mammals. Mammalian Muc2 is secreted from goblet cells residing in the epithelial lining into the lumen of the large intestine. Absence of Muc2 resulted in defective mucus layers in mice leading to increased bacterial adhesion to the surface epithelium, increased intestinal permeability, and enhanced susceptibility to colitis caused by dextran sodium sulfate (reviewed by Kim & Ho, 2010 [20]). This molecule disassociates both pathogenic and commensal bacteria from the colonic mucosa of mammals, highlighting the fact that innate immune components tend to eliminate any microorganism regardless of its nature [21]. In carp, Muc5B is mostly expressed in the skin and its expression in this tissue is up-regulated upon β-glucan administration [18]. In mammals, Muc5B is a major contributor to the lubricating and viscoelastic properties of whole saliva, normal lung mucus and cervical mucus [22].
Interestingly, carp skin mucus properties appear to shift in response to increases in the overall bacterial load in the water [23]. In particular, total glycosylation levels and acidic glycoconjugates increase whereas changes in the terminal presence of some sugars can be observed [23]. Similarly, the characteristics of seabream (Sparus aurata) gut mucus change in response to myxozoan parasite infection, with higher glycosylation levels and terminal glycosylation of mucus proteins in the posterior gut upon parasite infection [24]. These high molecular weight proteins of mucus from parasitized fish have lower adhesion of bacterial pathogens compared to controls [24]. In another study, the skin and gill mucus from three salmonids were studied in response to amoebic gill disease (AGD). From that study, it was concluded that Atlantic salmon and brown trout (Salmo trutta) both exhibit a whole-body mucus response to AGD, whereas rainbow trout (Oncorhynchus mykiss) exhibits only a local response in the gills [25]. Thus, it is possible that at least, the physicochemical properties of gill and skin mucus may shift coordinately in response to a gill parasite, although this may not be a general response in all hosts or to every disease agent. Yet, our understanding on how the fish host is able to regulate mucin gene expression, mucus glycosylation patterns and mucus composition in order to respond to pathogens and commensals, is far from complete.
2.2 Humoral innate immunity
It is well-known that fish mucosal secretions carry a wide variety of innate immune molecules including complement proteins, lysozyme, proteases, esterases and AMPs (for previous reviews see Esteban, 2012 [26]). Mucosal innate defense mechanisms even if intended to eliminate pathogens need to be permissive towards commensals. This permission does not mean inactivity, since the production of biologically active innate components like lipids, antimicrobial peptides, cytokines, and chemokines, as well as oxidative and nitrosylated moieties, are essential for microbes and host cells to form a symbiosis [6]. Currently, our knowledge on how teleosts modulate innate immunity to tolerate commensal colonization is scant.
Studies on a germ-free zebrafish (Danio rerio) model have revealed that protection of fish larvae until the onset of adaptive immunity is dependent on innate immune mechanisms that are directly regulated upon commensal colonization [27]. The results from this study have important implications for the aquaculture sector since different aquarium environments will lead to a different commensal community colonizing the larvae mucosa. Therefore, the commensals first colonizing the fish larvae may ultimately decide the larval ability to resist viral and bacterial infections. As a consequence, manipulating microbial colonization in hatcheries has the potential to modulate the host’s innate immune response. This point is further discussed later in this review.
The relative contribution of each one of the innate components to the total immune response appears to be variable amongst different teleost species. Interestingly, differences in the levels of innate immune molecules in the mucus may also reflect certain ecological strategies. For example skin mucus enzyme activities have been reported high in two bottom dweller freshwater species, and low in clean water inhabiting species [28]. The same study found that serine and metalloproteases were the major mucus proteases in all six fish species investigated. Aquatic vertebrates appear to have high concentrations of free sugars on the skin surface, which could possibly be a mechanism to protect the integrity of the epidermis against the attacks of commensal skin micro inhabitants, including bacteria and fungi [29].
With respect to cytokines, the current paradigm in mucosal immunity in mammals is that commensals induce the expression of anti-inflammatory cytokines such as TGF-β whereas pathogens trigger pro-inflammatory responses such as IL-1β and IL-17 [30]. Moreover, the cytokine IL-22 is able to drive the anatomical containment of gut commensals in mammals [31]. It is thought that analogous mechanisms must exist in aquatic vertebrates although these are yet to be discovered.
This review mostly focuses on complement and AMPs, due to the importance of the first in bridging innate and adaptive immunity and of the latter in regulating commensals and pathogens. The complement system is present both in vertebrates and invertebrates. In mammals, the complement is responsible for several functions including the modulation of adaptive immune responses, the promotion of inflammatory reactions, the elimination of apoptotic and necrotic cells and most importantly, the destruction of pathogens. Full-complement component lytic activity was measured in human midcycle cervical mucus, its activity was 11.5% of the activity of complement in an equal volume of undiluted human serum [32], revealing the importance of the complement in mucosal immunity. More importantly, the complement protein C3 is essential for the regulation of intestinal tolerance and thereby for the establishment of commensals in mammals [33]. All three complement activation pathways (classical, lectin and alternative pathway) as well as the cytolytic pathway are present in teleosts [34, 35]. The presence of several complement components has been demonstrated in mucosal tissues of teleost fish. At the transcript level, C7 expression can be detected in the skin and intestine of grass carp (Ctenopharyngodon idella) [36], C9 in the gills and intestine of ayu (Plecoglossus altivelis) [37], C3-1, C3-3, C3-4, C4, C5, C7, factor B and D in the intestine of rainbow trout [38] and C7, factor P and D in skin of carp [39]. At the protein level, C3 was found in the gills of Atlantic salmon [40], the intestine of Atlantic halibut (Hippoglossus hippoglossus) [41] and gills and intestine of Atlantic cod (Gadus morhua) [42], while mannan-binding lectins (MBL) have been detected in the anterior intestine of rainbow trout [43]. Expression of complement genes is up-regulated in teleost mucosal surfaces in response to mucosal pathogens. For example, the skin of carp infected with Ichthyophthirius multifiliis (Ich) showed a ~250-fold increase in the expression of a factor B-homologue [39, 44]. Ich infections also increased the expression of C3 in the gills of rainbow trout [45]. In a similar way, expression of some complement genes was slightly induced in the skin (C3-2, C8b, B/C2-A1, B/C2-B, MASP2, I) and gills (C1q, C4, C3, C6, C7, B/C2-A1, B/C2-B) of zebrafish after stimulation with poly I:C [46]. C3, C8, C9 and factor B were induced in the skin of the same species after infection with the bacteria Citrobacter freundii [47]. C6 expression was up-regulated in the gut but down-regulated in the skin of grass carp after challenge with Aeromonas hydrophila [48]. Thus far, studies addressing the role of complement in teleost mucosal sites are lacking, although an important function of complement in the killing of pathogens in these surfaces is suspected.
The study of AMPs represents one of the fastest growing fields in mucosal immunity. Fish, similar to mammals, produce many different AMPs (Figure 1) with antibacterial, anti-viral and anti-fungal activities (reviewed by Rajanbabu & Chen, 2011 [49]). Importantly, AMPs shape the composition of the microbial communities associated with mucosal surfaces in mammals and limit the extent of microorganism colonization [50]. Due to the importance of AMPs in mucosal immunity and in controlling commensals and pathogens, the current status on fish AMPs in the context of mucosal immune tissues is presented here. Table 1 summarizes the AMPs from teleosts that have been found in the gut, skin and gills. Assuming that homogenous sampling efforts have been undertaken, this table shows that teleost skin is a major source of AMPs with approximately ~70% of all AMPs expressed in this mucosal tissue, compared to ~52% and ~29% expressed in the gills and the gut, respectively (Table 1). Amphibian skin is the largest source of AMPs of all vertebrate animals. Thus, it appears that skin may rely more heavily than other mucosal barriers on AMP function. The molecular and structural studies of teleost AMPs are starting to be coupled to investigations on their specific role in mucosal immunity and their effects on commensals and pathogens. For instance, bath exposure of Atlantic cod to a commensal bacterium results in a downregulation of the expression of beta defensin in the gills, whereas the same AMP is upregulated in the skin. Bath challenge with live pathogen upregulated cathelicidin expression mainly in gills, and hepcidin in gills, skin and rectum [51]. Additionally, the “extended self” (commensals) may secrete their own repertoire of antimicrobial defenses (bacteriocins) as summarized by Gallo & Nakatsuji, 2011 [52]. Commensal-derived antimicrobial substances can also indirectly modulate the host AMP activity in vertebrates [53], but examples in fish are currently lacking. From an applied point of view, fish AMPs can have important applications as antimicrobial and antitumoral agents, vaccine adjuvants and inactivated vaccines [49]. For instance, some AMPs may be useful immunotherapeutics and enhance the effects of some drugs as demonstrated by Zahran & Noga, 2010 [54]. Yet, caution must be applied since AMP delivery to farmed fish may lead to dysbiosis, i.e. microbial imbalance in the mucosa, similar to what happens when antibiotics are delivered to fish or mammals. Thus, more research in the field of fish-derived and microbial-derived AMPs will be very useful in understanding the host-commensal-pathogen interactions and to potentiate the immune responses in the host.
Table 1.
Antimicrobial peptides (AMPs) found in mucosal tissues of teleost fish.
| AMP | Tissue/cells | Host species | References |
|---|---|---|---|
| AJN-10 | Skin | Japanese eel | Liang (2011) [135] |
| Apolipoprotein A-I and A-II | Skin | Carp | Concha (2003) [136] |
| AS-hepcidin 2 and 6 | Gut, skin and gills | Black porgy | Yang (2011)[137] |
| Cathelicidin-1 and 2, hepcidin, LEAP-2 | Gut, skin and gills | Rainbow trout | Casadei (2013) [138] |
| Chionodracine | Gill mast cells | Icefish | Buonocore (2012) [139] |
| Chrysophsin | Gills | Red sea bream | Iijima (2003) [140] |
| Congerin | Club cells in gut, skin and gills | Japanese conger eel | Nakamura (2001) [141] |
| β-defensin 1 and 2 | Skin and gills | Carp | Marel (2012) [18] |
| Efap | Skin | Brown-spotted grouper | Zhang (2008) [142] |
| Epinecidin-1 | Gut, skin and gills | Grouper | Pan (2007) [143] |
| Gaduscidin-1 and -2 | Gills | Cod | Browne (2011) [144] |
| Hbb P-1 | Skin and gills. Malpighian cells and alarm cells | Channel catfish | Ullal (2008) [145] |
| Hipposin | Skin | Halibut | Birkemo (2003) [146] |
| Moronedicin | Gills | Hybrid striped seabass | Lauth (2002) [147] |
| omDB-2, -3, 4 | Gut, skin and gills | Rainbow trout | Casadei (2009) [148] |
| Oncorhycin III | Skin | Rainbow trout | Fernandes (2003) [149] |
| Parasin I | Skin | Catfish | Park (1998) [150] |
| Pelteobagrin | Skin | Yellow catfish | Su (2011) [151] |
| Piscidin | Gut, skin and gills. Mast cells and rodlet cells | 7 perciform species | Silphaduang (2006) [152] |
| Piscidin 1 | Ubiquitous | Cod | Ruangsri (2012) [153] |
| Piscidin 1 and 2, β-defensin, hepcidin, cathelicidin 1 | Skin, gills and gut | Atlantic cod | Ruangsri (2013) [51] |
| Piscidin 3 | Gill mast cells | Seabream | Dezfuly (2011) [66] |
| Piscidin 4 | Gills | Striped bass, white bass seabass seabream red drum, barramundi | Corrales (2010) [154] |
| Pleurocidin | Gut and skin goblet cells | Winter flounder | Cole (2000) [155] |
| TP1-5 | Gut (TP4), Skin (TP2-3), Gills (TP3) | Nile tilapia | Peng (2012) [156] |
| YFGAP | Skin | Yellowfin tuna | Seo (2012) [157] |
2.3 Cellular innate immunity
The main cellular components of the mammalian innate immune system are natural killer cells, mast cells, eosinophils, basophils and phagocytic cells including macrophages, neutrophils and dendritic cells. This section will focus on the cell types that are especially relevant in mucosal tissues and have equivalents in teleost fish. Due to their importance in mucosal defense, the role of epithelial cells as first sensors of commensals and pathogens is also discussed.
Epithelial barriers isolate the underlying tissues from potentially noxious determinants of the environment. These barriers are formed by epithelial cells, mucus-producing cells, neuroendocrine cells and an intrinsic immune system (Figure 1). Epithelial cells are not the mere bricks that form a palisade, but active orchestrators of homeostasis, commensal colonization, innate and adaptive immune responses [2]. Epithelial cells interact directly with pathogens and commensals. They express PRRs including lectins, nod-like receptors (NLRs) and toll-like receptors (TLRs). In fish, as in mammals, intestinal epithelial cells express intestinal alkaline phosphatase. In zebrafish, this enzyme was shown to detoxify LPS and to prevent intestinal inflammation in response to the resident microbiota [55]. Whereas the array of PRRs expressed by mammalian epithelial cells is well characterized, studies on teleost fish are not available except for the case of NOD1/NOD2, which are expressed by zebrafish gut epithelial cells [56]. Intestinal epithelial cells (enterocytes) are known to be responsible for antigen uptake in teleosts (reviewed by Rombout et al., 2011 [57]) and play a critical role in the transport of mucosal Igs by expressing pIgR, as explained later in this review.
As an example of the interaction between fish epithelial cells and microorganisms, resident intestinal bacteria enhance the stability of β-catenin in intestinal epithelial cells and promote cell proliferation in the developing zebrafish intestine [58]. High epithelial turnover is also an effective way to clear pathogens. On the other hand, pathogens such as viruses are known to induce apoptosis in fish epithelial cells [59, 60].
Most mammalian studies use the cell line CACO-2 as a model to study gut epithelial cells (see review by Bailey et al., 1996 [61] as an example). Similarly, a gut epithelial cell line from rainbow trout, RTgutGC, was recently developed [62] and epithelial cell lines from other origins (non-intestinal) are available from a number of teleost species. Primary skin epithelial cell cultures from rainbow trout were instrumental to reveal the ability of this bacterial pathogen to evade endocytosis by epithelial cells [63]. Since the skin (and gills) of aquatic vertebrates lacks keratinization, they secrete mucus, and they are formed by living cells (Figure 1), the epithelial cells of these tissues are likely to carry important immune functions as it is the case in the gut.
Mast/eosinophilic granule cells (EGCs) are tissues-resident cells found throughout the body, particularly in association with structures such as blood vessels and nerves, and in proximity to surfaces that interface the external environment. In fish, mast cells, sometimes referred to as EGCs, are most abundant in the gills, gut and skin. Functionally, teleost EGCs show close similarity to the mast cells of mammals. Their tissue distribution in fish species from a certain genus appears to be conserved [64]. In mammals it has been shown that recruitment of mast cells to mucosal tissues is dependent on the presence of commensal microorganisms [65]. Recruitment of mast cells/EGCs to sites of inflammation is a common feature in many teleost mucosal tissues (reviewed by Reite & Evensen, 2006 [64]). As shown in Table 1, mast cells from mucosal tissues have been studied with respect to their AMP content. Additionally, the number of mast cells and their AMP content have been quantified in striped trumpeter (Latris lineata) and seabream (Sparus aurata) in response to copepode parasite infections [66, 67]. However, apart from their localization and AMP content, little is known about the biology or function of these innate immune cells in mucosal immunity. For instance, the receptors they express, the Igs they bind (or not) and their role in Th2 immunity are yet to be investigated.
Mucosal dendritic cells (DCs) are one of the most important components of the mucosal immune system of mammals (Figure 1). They represent a population of DCs when compared to systemic DCs and they specifically imprint mucosal homing molecules on B and T lymphocytes [68, 69]. Mucosal DCs are instrumental for the containment of mucosal immune responses avoiding systemic immune responses. In mammals, mucosal DCs are able to directly sample antigens from the gut lumen and uptake both commensals and pathogens [70]. Moreover, mucosal DCs can retain small numbers of live commensals for several days [71]. Recently, dendritic-like cells have been characterized in zebrafish and rainbow trout [72, 73]. It is interesting that DCs are scarce within the gut, spleen, and kidney of adult zebrafish but account for approximately 15% of the mononuclear phagocytes in the skin [74]. In fact, it has been postulated that zebrafish skin contains DCs that are equivalent to mammalian Langerhans cells [72]. Future studies may reveal how fish mucosal DCs orchestrate innate and adaptive immunity in response to pathogens and commensals.
Macrophages and granulocytes are also present in all three teleost mucosal lymphoid tissues (Figure 1). Within the gut of some species, regional differences exist with regards to the abundance of these innate immune cell types. For instance, the rectum of cod (Gadus morhua) appears to harbor higher numbers of granulocytes and macrophages than the distal gut (hindgut) [75]. The paradigm in mammalian immunology is that mucosal macrophages are essential for local homeostasis and in keeping a balance with the commensal microbiota. Intestinal macrophages are partially inert in the healthy gut and cannot promote inflammation despite constant exposure to bacteria and other stimuli [76]. In vitro studies in rainbow trout comparing mucosal versus non mucosal leucocyte activities show some parallelism with mammalian mucosal macrophages [77]. Intestinal phagocytes are 4–10 times less activated by yeast cells than head-kidney phagocytes. However, when they are activated they can ingest as many yeast cells as their HK counterparts. The zebrafish model offers a unique opportunity to study these two cells types in the different mucosal surfaces since there are available lines where macrophages and granulocytes can be visualized by fluorescence microscopy thanks to GFP-labeling of specific markers. The Tg(mpx::eGFPi114) zebrafish larvae have neutrophils expressing eGFP [78]. The lysC::EGFP and lysC::DsRED2 are lysozyme C reporter lines where macrophages can be visualized [79]. Using these tools, it was demonstrated that homeostatic numbers of neutrophils in the intestinal epithelium of zebrafish are established by the microbiota [55].
3. Adaptive immunity at teleost mucosal surfaces
Adaptive immunity first emerged when the earlier vertebrates (agnathans) appeared approximately 500 million years ago. One of the current evolutionary hypotheses is that adaptive immunity may have been driven mainly by the microbial colonization of mucosal surfaces [4, 80]. In this section the presence and roles of immunoglobulins (Igs), and B and T lymphocytes in mucosal surfaces of teleost fish are discussed in the context of commensals and pathogens.
3.1 Humoral adaptive immunity
The principal components of the humoral adaptive immune response are the Igs. Due to the particular characteristics of mucosal surfaces, vertebrates have specialized Igs in their mucosal surfaces. In mammals, IgA is the main Ig involved in mucosal immune responses and immune exclusion of commensals. Low-affinity IgA has enough capacity to protect the host from the immune activation induced by commensals, but affinity maturation of IgA is required to protect the host from more invasive microorganisms [81]. In contrast to mammals (five classes of Igs present), only three Ig isotypes have been described in teleosts so far: IgM, IgD and IgT. IgM represents the main Ig in the plasma of teleosts and the main player in systemic immune responses. IgM is also present in mucosal secretions and is involved in responses against several pathogens (reviewed in Salinas et al., 2011 [82]). For instance, while at low levels, IgM has been reported to be present in mucosal areas of several species [82], including the gut of rainbow trout [8], skin of channel catfish (Ictalurus punctatus) [83] and gills of rainbow trout [84] and brook trout [85, 86] (Figure 1). Moreover, recently it has been demonstrated that the IgM heavy chain of fugu acts as a N-acetyl-glucosamine (GlcNac) binding protein having a potent inhibitory effect on the growth of many kinds of bacteria [87], and thereby possibly controlling commensals. IgD is found in all vertebrates although its expression was lost in avians and in some groups of mammals [88]. IgD is known to be expressed in all immune tissues at the transcript level, and at the protein level IgD is detected at very low concentration in the rainbow trout plasma, while its function still remains enigmatic [89]. The last discovered Ig in teleosts is IgT [90] also called IgZ in some species [91]. IgT has been found in all teleosts species examined so far, except for channel catfish [92]. IgT, similar to mammalian IgA, is the only teleost Ig isotype with a specialized mucosal function as demonstrated in the gut of rainbow trout [8]. More specifically, it was shown that IgT-specific responses against a gut parasite are only detected in the gut mucus, while IgM-specific responses are only found in the serum [8]. This indicates a compartmentalization of IgT and IgM responses in mucosal and systemic sites respectively. Supporting the idea that IgT plays a key role in gut immunity, this Ig coats gut commensal bacteria in a similar way than IgA does in mammals [8]. Thus far, IgT represents the most ancient immunoglobulin specialized in gut mucosal immunity in the vertebrate lineage. In addition, IgT is also the prevalent Ig isotype in the skin mucosa of rainbow trout and an specific anti-Ich IgT-response is found in the skin mucus of survivor fish [93](Figure 1). Also, comparable to mammalian mucosal surfaces, teleosts express pIgR and its secretory component (SC) associates with IgT and IgM in rainbow trout gut mucus [8] and with IgM in fugu (Takifugu rubripes) skin mucus [13] (Figure 1). The pIgR is directly involved in the transport of secreted Igs across the gut mucosal epithelium of mammals, and thereby plays an important role in immune exclusion. Moreover, its association to Igs in teleosts reveals an evolutionary conservation, and thus, the importance of these two elements in the mucosal immune system of vertebrates.
3.2 Cellular adaptive immunity
There are two main arms in the adaptive immune system of any gnathostome vertebrate: B cells and T cells. Among other functions, the main role of B cells in adaptive immunity appears to be the recognition of antigens in their native form and the production of Igs against those antigens. Some teleosts like the channel catfish possess three B cell subsets IgM+/IgD+, IgM+/IgD−, IgM−/IgD+ [88], whereas two populations (IgD+/IgM+/IgT− and IgD−/IgM−/IgT+ B cells) have been characterized in rainbow trout [8]. Additionally, IgM+ B cell populations have been found in many other teleost species [94–97]. For a detailed review of their presence and distribution in gut, skin and gills of several teleost species see Salinas et al., 2011 [82]. In rainbow trout, IgM+ B cells represent the major population of B cells in most immunological tissues, but in the gut IgT+ B cells account for ~54 % of all B cells [8] (Figure 1). Furthermore, the percentage of IgT+ B cells, but not IgM+ cells, increased in the lamina propria of the gut after a parasite infection [8], indicating a preponderant role for IgT and IgT+ B cells in gut mucosal immunity. Interestingly, rainbow trout IgM+ and IgT+ B cells have phagocytic and bactericidal capacities [8, 98], proving that these cells not only produce antibodies, but also may act directly in the elimination of pathogens in systemic and probably mucosal areas in fish. In a similar way, IgT+ B cells are located in the gill epithelium whereas IgM+ B cells are located in the gill arterioles and capillaries of rainbow trout after infection with Ich, supporting the idea of a separation between mucosal and systemic immunity, respectively [45] (Figure 1). Channel catfish skin contains IgM secreting plasma cells with numbers increasing after Ich infection [83]. Importantly, recent results in our laboratory indicate that IgT+ B cells are also the main B cell population involved in adaptive immunity in the rainbow trout skin epithelia [93] (Figure 1). IgD secreting plasma cells are present in the gills of rainbow trout, occurring in similar proportions to IgM secreting cells [89]. However, B cells accounted for only ~1% of the total gill leukocytes in that study [89].
T cells play an essential role in cell-mediated immunity and as they interact with the bacteria present in mucosal surfaces it seems they are very important in creating tolerance or immunity against the commensal microbiota [30]. There are different T cell subsets in mammals (cytotoxic, Th1, Th2, Treg and Th17) and some of them have also been described in teleost fish (for tissue distribution in mucosal surfaces see Figure 1). The most studied mucosal tissue in teleost is the gut where the presence of a T cell-like population has been shown in a number of species including sea bass (Dicentrarchus labrax), carp, Atlantic salmon (reviewed in Rombout et al., 2011 [57]) and recently rainbow trout [99]. Specifically, gut intraepithelial lymphocytes (IELs) in seabass account for 55% of the total leukocytes in the gut [100] as assessed by immunostaining with the DLT15 mAb [101]. In rainbow trout an anti-CD8α mAb has shown that cytotoxic T lymphocytes (CTLs) constitute ~55% and 25% of all lymphocytes from gut and gills, respectively [99]. Additionally CD3ε+ T cells are found in the gut and abundantly in the inter branchial tissue within the gills of Atlantic salmon [102]. The presence of a putative CD8+ T cell population has been found in the intraepithelial lymphoid tissue of rainbow trout at the base of gill filaments, and it accumulates in that area after Ich infection [45]. Moreover, several studies have investigated the expression of T cell markers in gut and gill T cells [99, 103, 104]. The summary from those studies shows that teleost mucosal T cells express CD3ε, TCRα, TCRβ, TCRγ, CD4, CD28, TCRζ, CD8α and β and RAG-1. Which T cell subsets actually express the aforementioned markers remains to be investigated. Moreover, functional studies on teleost T cells in mucosal areas are scarce.
Teleost skin T cells have not been studied in detail so far. At the transcript level, expression analyses suggest a transient increase of TCRα (CD4-1) in Atlantic salmon skin following salmon louse infection [105] but a down-regulation of some T cell markers [105] (i.e., CD3, CD8, CD28, CD99 and TCR) and also tyrosine kinases (BTK and LCK) after cortisol treatment [106]. It is therefore likely that putative T cells present in fish skin participate in skin immune responses although this hypothesis is yet to be tested.
To date, no studies have investigated the cooperation between B and T cells during mucosal immune responses in teleost fish, probably because only few antibodies recognizing T cells are available. Such studies will be critical in order to understand the relationships between commensals, pathogens and the host immune system.
4. Exploiting mucosal immunity for immunotherapy
The control and eradication of mucosal pathogens requires targeted immunotherapies that specifically protect local mucosal sites. Several new mucosal delivery approaches are being developed and optimized for use in mammals. The aquaculture industry will benefit from these technological advances by exploiting the strengths of the fish mucosal immune system.
4.1 Manipulating the fish host microbiota
With the advent of deep sequencing, the microbiomes of many organisms including fish are becoming available to the scientific community [107, 108]. However, the great biological diversity found in teleosts is likely to result in significant differences in the microbial communities associated with their mucosal surfaces. This diversity is not fully characterized yet. Moreover, it is clear that the environmental microbial composition in fish farms differs from that of the natural aquatic environment. Since the microbiome is known to control the development and function of the mucosal immune system, any manipulation of the microbial composition will affect how the fish host will mount mucosal immune responses. Microbial intervention in aquaculture is a powerful way to increase water quality, inhibit pathogens and boost the host immune response [109].
It is presumed that those microorganisms transferred from the broodstock to the eggs (maternal transmission), form the pioneer microbiota of the developing larvae, which may influence the future actions of the immune system. Aquaculture often involves artificial spawning and fertilization of fish eggs. It is likely, therefore, that the pioneer microbiome of hatchery fish is largely different to the one of wild specimens, although this remains to be investigated. In aquaculture, eggs are kept in incubators with a microflora that differs considerably from that in the sea, and become heavily overgrown with bacteria within hours after fertilization [110]. Eggs may be sterilized by various techniques such as UV-irradiation, ozonization, membrane filtration, and antibiotics. All of them are commonly used in intensive larviculture, disturbing the balance of microbial communities and favoring bacterial growth (reviewed by Olafsen, 2001 [110]). Overall, removal of the egg epiflora will reduce the microbial heterogeneity and this is likely to impact the development of the mucosal immune system. After hatching, bacteria present in the water and food may colonize the gut, gills and skin of the fish. In aquaculture, fish larvae are kept in incubators with hatching eggs and debris, resulting in a 1000-fold increase in bacterial counts of the ambient water through hatching [111]. In the case of marine fish larvae, drinking begins before the yolk sac is consumed and, thus, bacteria enter the digestive tract before the period of active feeding commences [110]. Older larvae may also ingest bacteria by grazing on suspended particles and egg debris.
Fish commensal bacteria are thought to remain in the external surfaces, associated with the mucus. However, mammalian studies have revealed an active role of epithelial cells in sensing commensals via a number of innate immune receptors as well as presence of commensals inside of gut dendritic cells and Peyer’s Patches [71]. The composition of fish commensal communities as well as their metabolic and immunological roles have been poorly characterized. It is likely that resident commensals shape and control mucosal immune responses of fish in unique ways we do not yet understand. In the case of commensals translocating across the epithelium (in a controlled manner or not), it is likely that the fish host will develop specific antibodies against these bacteria. Those antibodies can be secreted to the mucus and coat commensals, keeping them in the external environment in a process known as immune exclusion [112].
Although there are many definitions found in the scientific literature, probiotics can be defined as “one or more microorganisms with beneficial effects for the host, able to persist in the digestive tract because of its tolerance to acid and bile salts” [113]. Over the recent years, there is great interest in the application of probiotics in aquaculture [114] including the manipulation of the fish microbiota. This field has grown considerably over the past ten years and currently there are several commercial preparations of probiotics that contain one or more live microorganisms for use in aquaculture. Probiotics can be delivered in the food or added directly to the water tank. Bacterial and fungal strains isolated from the skin and gut of different fish species have been delivered orally resulting in increased systemic immunity [115], mucosal immunity [116, 117] as well as resistance to fish pathogens [118], respectively. If the probiotic delivered is able to colonize the host mucosal surface, the modification of the microbiota may be permanent. If the microorganisms delivered are present only as long as they keep being delivered to the host, the effects on the immune system may be just temporary [119]. The latter appears to be the most common case both in fish and mammals. Yet, temporary effects may be critical and still shape the development of immunity during early larval stages. Interestingly, it seems that site-specific commensals are critical to protect the mucosal site where they usually reside [120]. This is illustrated by comparisons of skin and gut commensals in mammals and the unique properties of skin commensals to specifically protect against skin pathogens [120]. Thus, further efforts must be directed to characterize gut-specific, gill-specific and skin-specific commensals in fish that can uniquely control pathogens that enter or affect those sites. Ultimately, mucosal antibodies may have a critical effect on whether any microorganism delivered to farmed fish will colonize the mucosal epithelia or translocate and cause and inflammatory systemic immune response. Additionally, it cannot be ignored that after initial delivery of a strain not previously recognized as self by the host, tolerance may be generated and therefore subsequent treatments will no longer elicit the same responses. Overall, our basic understanding of fish mucosal Igs and memory responses needs to be used to expand and support the rational design of probiotic-based immunotherapies.
Immunostimulants are commonly used in aquaculture to boost the natural immune response of fish. Oral immunostimulant delivery aims to enhance local gut and systemic immune defenses. Many of the immunostimulant formulations for fish are substances that the commensal bacteria use as a source of nutrients and energy. For instance, Protec Trout contains β-glucan, nucleotides and vitamins to improve gut health whereas AquaVac Ergosan contains extracts of Laminaria digitata and Ascophyyllum nodosum. For this reason, it is likely that immunostimulants have an impact on the fish microbiome. However, few studies have addressed this question and the 16s ribosomal DNA has not been sequenced in this context. For a review on this topic see [121]. The ability of orally-delivered immunostimulants to enhance innate immunity seems to be different in the gut, compared to other mucosal surfaces. This is illustrated by a recent study where mannan was orally administered to seabass and gut but not skin mucus innate immune functions were significantly modulated [122]. On the other hand, feeding ergosan or fermented Saccharomyces cerevisiae to rainbow trout enhances skin mucus innate immune parameters, indicating some connection between both compartments [123, 124]. Thus, more studies in the area of immunostimulants are required to understand how effective they are at multiple mucosal sites and whether or not specific stimulants are required for each mucosal surface. For a current review of fish immunostimulants see Tafalla et al., 2013 [125].
4.2 The potential of mucosal vaccination
Mucosal vaccines are the most promising strategy to protect fish from the wide variety of mucosal pathogens. Vaccination targets adaptive immunity and relies on the generation of better and stronger responses following primary immunization. In addition, mucosal vaccines offer many advantages to the aquaculture industry, mostly related to the reduced labor required when mass vaccinating fingerlings by immersion. Despite these advantages, the majority of the vaccines for use in aquaculture are delivered by injection, which is by far the most effective vaccination route implemented to date [126]. However, it is likely that the route of delivery will be critical for the generation of efficient and long-lasting mucosal immune responses in fish, as it is known in mammals. Fish mucosal vaccines are delivered by immersion or orally. For a detailed review on advances in fish vaccine delivery see [126]. Currently, the vast majority of research efforts are directed to the development of novel oral vaccines and oral delivery technologies for fish. For instance, Artemia, chitosan microspheres, alginate microparticles or Poly (D, L-lactic-co-glycolic acid) (PLGA) nanoparticles have been used to encapsulate a variety of vaccine formulations, both bacterial and viral [127–130]. Many of these encapsulation methods are directed to the delivery of DNA vaccines usually injected intramuscularly. For a complete review see Tafalla et al., 2013 [125]. The overall conclusion from these studies is that even if it is possible to induce an immune response using an orally delivered DNA vaccine, the current oral delivery systems needs improvement. To our knowledge, no studies have evaluated the changes that take place in the fish microbiome due to mucosal vaccine delivery. It is possible that the limited success of mucosal vaccines for use in aquaculture is indirectly mediated by shifts in the commensal communities caused by the immersion or oral delivery.
The development of better mucosal vaccines for finfish needs to be based on our knowledge of mucosal adaptive immunity and mucosal memory responses. These studies are yet to be conducted. In particular, IgT plasma cells and memory cells may be the key to induce long-lasting gut mucosal immune responses. Combining mucosal vaccines with mucosal immunostimulants and probiotics could potentially boost both innate and adaptive mucosal immune responses. In fact, the potential of probiotics as mucosal vaccine carriers and as vaccine adjuvants is already acknowledged in human medicine [131]. Nanotechnological breakthroughs continue to open up new avenues in mucosal vaccinology [132] and aquaculture mucosal vaccines will benefit from these new technologies.
5. Concluding remarks
The mucosal immune system of vertebrates is one of the most sophisticated examples of evolution found in nature. During vertebrate evolution, increasingly complex body structures implied higher metabolic rates and created an evolutionary pressure for new metabolic abilities. Vertebrates thus may have allowed commensals to colonize their mucosa for the benefits of new genetic material rather than coding for those new metabolic abilities themselves [133]. The metabolome is therefore defined as the composite product of both the host genome and the microbiome. The microbiome’s highly adaptive metabolic engine, in addition to providing essential non-nutrient factors, also substantially increases the host’s ability to harvest nutrients from food [4]. Thus, the greater the diversity of an animal’s microbiome, the greater its metabolic capacity. This symbiosis occurs at the mucosal epithelia, where not only commensals, but also pathogens co-exist. The caveat to being permissive towards commensals is the need for effective immune regulatory mechanisms in the mucosal epithelia. In this review common themes shared between fish and mammalian mucosal immune systems as well as those themes that are unique to teleosts, have been highlighted. The mucosal immune system of teleosts faces similar obstacles to those encountered by terrestrial vertebrates, with the added challenge of living in a medium where microorganisms thrive more than in the air.
In mammals, the normal intestinal microbiota influences numerous biological processes in the healthy host, including organ morphogenesis, immune system and gastrointestinal tract development and maturation, intestinal vascularization, tissue regeneration, carcinogenesis, bone homeostasis, metabolism and behavior [1]. Although we are still far from understanding how commensals influence the physiological functions of fish, it can be speculated that they carry broad and critical roles both in health and disease status. During intensive fish farming, the microbial composition of the environment and the fish host are heavily manipulated, impacting the mucosal immunity. Thus, both the “extended self” and the fish host should be the target of aquaculture immunotherapies during broodstock management, egg hatching, larval rearing, ongrowing phases as well as diet design, stress management and product quality assessment. Thinking of the fish host and its microbiome as one unit and understanding how mucosal immunity works will have a critical impact for the development of fish vaccines and other immunotherapeutics. As already witnessed, teleost models such as rainbow trout and zebrafish have the potential to advance our current knowledge on mucosal immunity and mucosa-related diseases [134]. Therefore, the field of fish mucosal immunology is likely to grow even further over the next few years. New breakthroughs in this field will be applicable not only to the aquaculture industry, but also to human-based research.
Acknowledgments
This work was supported by the National Science Foundation (NSF-MCB-0719599 to J.O.S.), National Institutes of Health (R01GM085207-01 to J.O.S.) and CETI COBRE grant (P20GM103452 to I.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sommer F, Bäckhed F. The gut microbiota - masters of host development and physiology. Nat Rev Microbiol. 2013 doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 2.Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2009;28:623–67. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamada N, Nunez G. Role of the gut microbiota in the development and function of lymphoid cells. J Immunol. 2013;190:1389–95. doi: 10.4049/jimmunol.1203100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–41. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow J, Lee SM, Shen Y, Khosravi A, Mazmanian SK. Chapter 8: Host-bacterial symbiosis in health and disease. In: Fagarasan S, Cerutti A, editors. Adv Immunol. San Diego: Academic Press; 2010. pp. 243–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nussbaum JC, Locksley RM. Infectious (non)tolerance-frustrated commensalism gone awry? Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet. 2010;11:47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang YA, Salinas I, Li J, Parra D, Bjork S, Xu Z, et al. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat Immunol. 2010;11:827–35. doi: 10.1038/ni.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 10.Al-Hussaini A. On the functional morphology of the alimentary tract of some fish in relation to differences in their feeding habits: anatomy and histology. Quarterly Journal of Microscopical Science. 1949;3:109–39. [PubMed] [Google Scholar]
- 11.Wilson JM, Laurent P. Fish gill morphology: inside out. J Exp Zool. 2002;293:192–213. doi: 10.1002/jez.10124. [DOI] [PubMed] [Google Scholar]
- 12.Zaccone G, Kapoor B, Fasulo S, Ainis L. Structural, histochemical and functional aspects of the epidermis of fishes. Adv Mar Biol. 2001;40:253–348. [Google Scholar]
- 13.Hamuro K, Suetake H, Saha NR, Kikuchi K, Suzuki Y. A teleost polymeric Ig receptor exhibiting two Ig-like domains transports tetrameric IgM into the skin. J Immunol. 2007;178:5682–9. doi: 10.4049/jimmunol.178.9.5682. [DOI] [PubMed] [Google Scholar]
- 14.Johansson ME, Larsson JMH, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. Proc Natl Acad Sci USA. 2011;108:4659–65. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuhaus H, van der Marel M, Caspari N, Meyer W, Enss ML, Steinhagen D. Biochemical and histochemical study on the intestinal mucosa of the common carp Cyprinus carpio L., with special consideration of mucin glycoproteins. J Fish Biol. 2007;70:1523–34. [Google Scholar]
- 16.Knudsen G, Sorum H, Press CM, Olafsen JA. In situ adherence of Vibrio spp. to cryosections of Atlantic salmon, Salmo salar L., tissue. J Fish Dis. 1999;22:409–18. [Google Scholar]
- 17.Lang T, Alexandersson M, Hansson GC, Samuelsson T. Bioinformatic identification of polymerizing and transmembrane mucins in the puffer fish Fugu rubripes. Glycobiology. 2004;14:521–7. doi: 10.1093/glycob/cwh066. [DOI] [PubMed] [Google Scholar]
- 18.van der Marel M, Adamek M, Gonzalez SF, Frost P, Rombout JH, Wiegertjes GF, et al. Molecular cloning and expression of two β-defensin and two mucin genes in common carp (Cyprinus carpio L.) and their up-regulation after β-glucan feeding. Fish Shellfish Immunol. 2012 doi: 10.1016/j.fsi.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Micallef G, Bickerdike R, Reiff C, Fernandes JM, Bowman AS, Martin SA. Exploring the Transcriptome of Atlantic Salmon (Salmo salar) Skin, a Major Defense Organ. Mar Biotechnol. 2012;14:559–69. doi: 10.1007/s10126-012-9447-2. [DOI] [PubMed] [Google Scholar]
- 20.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–30. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergstrom KSB, Kissoon-Singh V, Gibson DL, Ma CX, Montero M, Sham HP, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Path. 2010:6. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickstrom C, Davies JR, Eriksen GV, Veerman ECI, Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochem J. 1998;334:685–93. doi: 10.1042/bj3340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Marel M, Caspari N, Neuhaus H, Meyer W, Enss M, Steinhagen D. Changes in skin mucus of common carp, Cyprinus carpio L., after exposure to water with a high bacterial load. J Fish Dis. 2010;33:431–9. doi: 10.1111/j.1365-2761.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 24.Estensoro I, Jung-Schroers V, Álvarez-Pellitero P, Steinhagen D, Sitjà-Bobadilla A. Effects of Enteromyxum leei (Myxozoa) infection on gilthead sea bream (Sparus aurata)(Teleostei) intestinal mucus: glycoprotein profile and bacterial adhesion. Parasitol Res. 2013;112:567–76. doi: 10.1007/s00436-012-3168-3. [DOI] [PubMed] [Google Scholar]
- 25.Roberts SD, Powell MD. The viscosity and glycoprotein biochemistry of salmonid mucus varies with species, salinity and the presence of amoebic gill disease. J Comp Physiol, B. 2005;175:1–11. doi: 10.1007/s00360-004-0453-1. [DOI] [PubMed] [Google Scholar]
- 26.Esteban MÁ. An overview of the immunological defenses in fish skin. ISRN Immunology. 2012;2012:1–29. [Google Scholar]
- 27.Galindo-Villegas J, García-Moreno D, de Oliveira S, Meseguer J, Mulero V. Regulation of immunity and disease resistance by commensal microbes and chromatin modifications during zebrafish development. Proc Natl Acad Sci USA. 2012;109:E2605–E14. doi: 10.1073/pnas.1209920109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nigam AK, Kumari U, Mittal S, Mittal AK. Comparative analysis of innate immune parameters of the skin mucous secretions from certain freshwater teleosts, inhabiting different ecological niches. Fish Physiol Biochem. 2012;38:1245–56. doi: 10.1007/s10695-012-9613-5. [DOI] [PubMed] [Google Scholar]
- 29.Meyer W, Seegers U, Schnapper A, Neuhaus H, Himstedt W, Toepfer-Petersen E. Possible antimicrobial defense by free sugars on the epidermal surface of aquatic vertebrates. Aquatic Biology. 2007;1:167–75. [Google Scholar]
- 30.Nutsch KM, Hsieh CS. T cell tolerance and immunity to commensal bacteria. Curr Opin Immunol. 2012;24:385–91. doi: 10.1016/j.coi.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–5. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price R, Boettcher B. The presence of complement in human cervical mucus and its possible relevance to infertility in women with complement-dependent sperm-immobilizing antibodies. Fertil Steril. 1979;32:61–6. doi: 10.1016/s0015-0282(16)44117-8. [DOI] [PubMed] [Google Scholar]
- 33.Pekkarinen PT, Vaali K, Jarva H, Kekalainen E, Hetemaki I, Junnikkala S, et al. Impaired intestinal tolerance in the absence of a functional complement system. J Allergy Clin Immunol. 2013;131:1167–75. doi: 10.1016/j.jaci.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Nakao M, Tsujikura M, Ichiki S, Vo TK, Somamoto T. The complement system in teleost fish: Progress of post-homolog-hunting researches. Dev Comp Immunol. 2011;35:1296–308. doi: 10.1016/j.dci.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Boshra H, Li J, Sunyer JO. Recent advances on the complement system of teleost fish. Fish Shellfish Immunol. 2006;20:239–62. doi: 10.1016/j.fsi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Shen YB, Zhang JB, Xu XY, Fu JJ, Li JL. Expression of complement component C7 and involvement in innate immune responses to bacteria in grass carp. Fish Shellfish Immunol. 2012;33:448–54. doi: 10.1016/j.fsi.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Kong C-J, Huang Z-A, Chen J, Shi Y-H, Lu X-J. Molecular cloning, sequence analysis and expression of ayu complement component C9 gene. Zool Res. 2012;33:151–7. doi: 10.3724/SP.J.1141.2012.02151. [DOI] [PubMed] [Google Scholar]
- 38.Lovoll M, Kilvik T, Boshra H, Bogwald J, Sunyer JO, Dalmo RA. Maternal transfer of complement components C3-1, C3-3, C3-4, C4, C5, C7, Bf, and Df to offspring in rainbow trout (Oncorhynchus mykiss) Immunogenetics. 2006;58:168–79. doi: 10.1007/s00251-006-0096-3. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez SF, Chatziandreou N, Nielsen ME, Li WZ, Rogers J, Taylor R, et al. Cutaneous immune responses in the common carp detected using transcript analysis. Mol Immunol. 2007;44:1664–79. doi: 10.1016/j.molimm.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Lovoll M, Johnsen H, Boshra H, Bogwald J, Sunyer JO, Dalmo RA. The ontogeny and extrahepatic expression of complement factor C3 in Atlantic salmon (Salmo salar) Fish Shellfish Immunol. 2007;23:542–52. doi: 10.1016/j.fsi.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Lange S, Bambir S, Dodds AW, Magnadottir B. An immunohistochemical study on complement component C3 in juvenile Atlantic halibut (Hippoglossus hippoglossus L.) Dev Comp Immunol. 2004;28:593–601. doi: 10.1016/j.dci.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Lange SR, Bambir S, Dodds AW, Magnadottir B. The ontogeny of complement component C3 in Atlantic cod (Gadus morhua L.) - an immunohistochemical study. Fish Shellfish Immunol. 2004;16:359–67. doi: 10.1016/j.fsi.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Kania PW, Sorensen RR, Koch C, Brandt J, Kliem A, Vitved L, et al. Evolutionary conservation of mannan-binding lectin (MBL) in bony fish: Identification, characterization and expression analysis of three bona fide collectin homologues of MBL in the rainbow trout (Onchorhynchus mykiss) Fish Shellfish Immunol. 2010;29:910–20. doi: 10.1016/j.fsi.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez SF, Buchmann K, Nielsen ME. Complement expression in common carp (Cyprinus carpio L.) during infection with Ichthyophthirius multifiliis. Dev Comp Immunol. 2007;31:576–86. doi: 10.1016/j.dci.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Olsen MM, Kania PW, Heinecke RD, Skjoedt K, Rasmussen KJ, Buchmann K. Cellular and humoral factors involved in the response of rainbow trout gills to Ichthyophthirius multifiliis infections: molecular and immunohistochemical studies. Fish Shellfish Immunol. 2011;30:859–69. doi: 10.1016/j.fsi.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 46.Vo KT, Tsujikura M, Somamoto T, Nakao M. Expression responses of the complement components in zebrafish organs after stimulation with poly I:C, mimicry of viral infection. Journal of the Faculty of Agriculture Kyushu University. 2009;54:389–95. [Google Scholar]
- 47.Lu AJ, Hu XC, Xue J, Zhu JR, Wang Y, Zhou GZ. Gene expression profiling in the skin of zebrafish infected with Citrobacter freundii. Fish Shellfish Immunol. 2012;32:273–83. doi: 10.1016/j.fsi.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 48.Shen YB, Zhang JB, Xu XY, Li JL. Molecular cloning, characterization and expression analysis of the complement component C6 gene in grass carp. Vet Immunol Immunopathol. 2011;141:139–43. doi: 10.1016/j.vetimm.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Rajanbabu V, Chen J-Y. Applications of antimicrobial peptides from fish and perspectives for the future. Peptides. 2011;32:415–20. doi: 10.1016/j.peptides.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Otte JM, Vordenbaumen S. Role of antimicrobial peptides in inflammatory bowel disease. Polymers. 2011;3:2010–7. [Google Scholar]
- 51.Ruangsri J, Lokesh J, Fernandes JM, Kiron V. Transcriptional regulation of antimicrobial peptides in mucosal tissues of Atlantic cod Gadus morhua L. in response to different stimuli. Aquacult Res. 2013 [Google Scholar]
- 52.Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. The Journal of Investigative Dermatology. 2011;131:1974–80. doi: 10.1038/jid.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li DQ, Lei H, Li ZH, Li HQ, Wang Y, Lai YP. A novel lipopeptide from skin commensal activates TLR2/CD36-p38 MAPK signaling to increase antibacterial defense against bacterial infection. PLoS One. 2013:8. doi: 10.1371/journal.pone.0058288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zahran E, Noga EJ. Evidence for synergism of the antimicrobial peptide piscidin 2 with antiparasitic and antioomycete drugs. J Fish Dis. 2010;33:995–1003. doi: 10.1111/j.1365-2761.2010.01205.x. [DOI] [PubMed] [Google Scholar]
- 55.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2:371–82. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oehlers SH, Flores MV, Hall CJ, Swift S, Crosier KE, Crosier PS. The inflammatory bowel disease (IBD) susceptibility genes NOD1 and NOD2 have conserved anti-bacterial roles in zebrafish. Dis Model Mech. 2011;4:832–41. doi: 10.1242/dmm.006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rombout JH, Abelli L, Picchietti S, Scapigliati G, Kiron V. Teleost intestinal immunology. Fish Shellfish Immunol. 2011;31:616–26. doi: 10.1016/j.fsi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Cheesman SE, Neal JT, Mittge E, Seredick BM, Guillemin K. Epithelial cell proliferation in the developing zebrafish intestine is regulated by the Wnt pathway and microbial signaling via Myd88. Proc Natl Acad Sci USA. 2011;108:4570–7. doi: 10.1073/pnas.1000072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bjorklund HV, Johansson TR, Rinne A. Rhabdovirus-induced apoptosis in a fish cell line is inhibited by a human endogenous acid cysteine proteinase inhibitor. J Virol. 1997;71:5658–62. doi: 10.1128/jvi.71.7.5658-5662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong JR, Huang LJ, Wu JL. Aquatic birnavirus induces apoptosis through activated caspase-8 and-3 in a zebrafish cell line. J Fish Dis. 2005;28:133–40. doi: 10.1111/j.1365-2761.2004.00604.x. [DOI] [PubMed] [Google Scholar]
- 61.Bailey CA, Bryla P, Malick AW. The use of the intestinal epithelial cell culture model, Caco-2, in pharmaceutical development. Adv Drug Del Rev. 1996;22:85–103. [Google Scholar]
- 62.Kawano A, Haiduk C, Schirmer K, Hanner R, Lee LEJ, Dixon B, et al. Development of a rainbow trout intestinal epithelial cell line and its response to lipopolysaccharide. Aquacult Nutr. 2011;17:E241–E52. [Google Scholar]
- 63.Lindell K, Fahlgren A, Hjerde E, Willassen N-P, Fällman M, Milton DL. Lipopolysaccharide O-antigen prevents phagocytosis of Vibrio anguillarum by rainbow trout (Oncorhynchus mykiss) skin epithelial cells. PLoS One. 2012;7:e37678. doi: 10.1371/journal.pone.0037678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reite OB, Evensen O. Inflammatory cells of teleostean fish: A review focusing on mast cells/eosinophilic granule cells and rodlet cells. Fish Shellfish Immunol. 2006;20:192–208. doi: 10.1016/j.fsi.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Kunii J, Takahashi K, Kasakura K, Tsuda M, Nakano K, Hosono A, et al. Commensal bacteria promote migration of mast cells into the intestine. Immunobiology. 2011;216:692–7. doi: 10.1016/j.imbio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 66.Dezfuli BS, Giari L, Lui A, Lorenzoni M, Noga EJ. Mast cell responses to Ergasilus (Copepoda), a gill ectoparasite of sea bream. Fish Shellfish Immunol. 2011;30:1087–94. doi: 10.1016/j.fsi.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 67.Andrews M, Battaglene S, Cobcroft J, Adams M, Noga E, Nowak B. Host response to the chondracanthid copepod Chondracanthus goldsmidi, a gill parasite of the striped trumpeter, Latris lineata (Forster), in Tasmania. J Fish Dis. 2010;33:211–20. doi: 10.1111/j.1365-2761.2009.01107.x. [DOI] [PubMed] [Google Scholar]
- 68.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 69.Mora JR, Iwata M, Eksteen B, Song S-Y, Junt T, Senman B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 70.Rescigno M, Rotta G, Valzasina B, Ricciardi-Castagnoli P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology. 2001;204:572–81. doi: 10.1078/0171-2985-00094. [DOI] [PubMed] [Google Scholar]
- 71.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–5. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 72.Lugo-Villarino G, Balla KM, Stachura DL, Banuelos K, Werneck MBF, Traver D. Identification of dendritic antigen-presenting cells in the zebrafish. Proc Natl Acad Sci USA. 2010;107:15850–5. doi: 10.1073/pnas.1000494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bassity E, Clark TG. Functional identification of dendritic cells in the teleost model, rainbow trout (Oncorhynchus mykiss) PLoS One. 2012:7. doi: 10.1371/journal.pone.0033196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wittamer V, Bertrand JY, Gutschow PW, Traver D. Characterization of the mononuclear phagocyte system in zebrafish. Blood. 2011;117:7126–35. doi: 10.1182/blood-2010-11-321448. [DOI] [PubMed] [Google Scholar]
- 75.Inami M, Taverne-Thiele AJ, Schroder MB, Kiron V, Rombout J. Immunological differences in intestine and rectum of Atlantic cod (Gadus morhua L.) Fish Shellfish Immunol. 2009;26:751–9. doi: 10.1016/j.fsi.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 76.Mowat AM. Mucosal macrophages in intestinal homeostasis and inflammation. J Innate Immun. 2011;3:550–64. doi: 10.1159/000329099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin E, Trichet VV, Legrand-Frossi C, Frippiat JP. Comparison between intestinal and non-mucosal immune functions of rainbow trout, Oncorhynchus mykiss. Fish Shellfish Immunol. 2012;33:1258–68. doi: 10.1016/j.fsi.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 78.Renshaw SA, Loynes CA, Trushell DM, Elworthy S, Ingham PW, Whyte MK. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108:3976–8. doi: 10.1182/blood-2006-05-024075. [DOI] [PubMed] [Google Scholar]
- 79.Hall C, Flores MV, Storm T, Crosier K, Crosier P. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev Biol. 2007;7:42. doi: 10.1186/1471-213X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–73. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Slack E, Balmer ML, Fritz JH, Hapfelmeier S. Functional flexibility of intestinal IgA–broadening the fine line. Front Immunol. 2012;3:1–10. doi: 10.3389/fimmu.2012.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salinas I, Zhang YA, Sunyer JO. Mucosal immunoglobulins and B cells of teleost fish. Dev Comp Immunol. 2011;35:1346–65. doi: 10.1016/j.dci.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao X, Findly RC, Dickerson HW. Cutaneous antibody-secreting cells and B cells in a teleost fish. Dev Comp Immunol. 2008;32:500–8. doi: 10.1016/j.dci.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 84.von Gersdorff Jørgensen L, Heinecke R, Skjødt K, Rasmussen K, Buchmann K. Experimental evidence for direct in situ binding of IgM and IgT to early trophonts of Ichthyophthirius multifiliis (Fouquet) in the gills of rainbow trout, Oncorhynchus mykiss (Walbaum) J Fish Dis. 2011;34:749–55. doi: 10.1111/j.1365-2761.2011.01291.x. [DOI] [PubMed] [Google Scholar]
- 85.Lumsden J, Ostland V, MacPhee D, Ferguson H. Production of gill-associated and serum antibody by rainbow trout (Oncorhynchus mykiss) following immersion immunization with acetone-killed Flavobacterium branchiophilum and the relationship to protection from experimental challenge. Fish Shellfish Immunol. 1995;5:151–65. [Google Scholar]
- 86.Lumsden J, Ostland V, Byrne P, Ferguson H. Detection of a distinct gill-surface antibody response following horizontal infection and bath challenge of brook trout Salvelinus fontinalis with Flavobacterium branchiophilum, the causative agent of bacterial gill disease. Dis Aquat Org. 1993;16:21–7. [Google Scholar]
- 87.Tsutsui S, Ariji T, Sato A, Yoshida T, Yamamura N, Odaka T, et al. Serum GlcNAc-binding IgM of fugu (Takifugu rubripes) suppresses the growth of fish pathogenic bacteria: A novel function of teleost antibody. Dev Comp Immunol. 2013;41:20–6. doi: 10.1016/j.dci.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 88.Edholm E-S, Bengten E, Wilson M. Insights into the function of IgD. Dev Comp Immunol. 2011;35:1309–16. doi: 10.1016/j.dci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 89.Ramirez-Gomez F, Greene W, Rego K, Hansen JD, Costa G, Kataria P, et al. Discovery and characterization of secretory IgD in rainbow trout: secretory IgD is produced through a novel splicing mechanism. J Immunol. 2012;188:1341–9. doi: 10.4049/jimmunol.1101938. [DOI] [PubMed] [Google Scholar]
- 90.Hansen JD, Landis ED, Phillips RB. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. Proc Natl Acad Sci USA. 2005;102:6919. doi: 10.1073/pnas.0500027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Danilova N, Bussmann J, Jekosch K, Steiner LA. The immunoglobulin heavy-chain locus in zebrafish: identification and expression of a previously unknown isotype, immunoglobulin Z. Nat Immunol. 2005;6:295–302. doi: 10.1038/ni1166. [DOI] [PubMed] [Google Scholar]
- 92.Bengtén E, Quiniou S, Hikima J, Waldbieser G, Warr G, Miller N, et al. Structure of the catfish IGH locus: analysis of the region including the single functional IGHM gene. Immunogenetics. 2006;58:831–44. doi: 10.1007/s00251-006-0139-9. [DOI] [PubMed] [Google Scholar]
- 93.Xu Z, Parra D, Gómez D, Salinas I, Zhang YA, von Gersdorff Jørgensen L, et al. Teleost skin, an ancient mucosal type-I surface that elicits gut-like mucosal immune responses. Proc Natl Acad Sci USA. 2013;110:13097–102. doi: 10.1073/pnas.1304319110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rombout JHWM, Taverne N, van de Kamp M, Taverne-Thiele AJ. Differences in mucus and serum immunoglobulin of carp (Cyprinus carpio L.) Dev Comp Immunol. 1993;17:309–17. doi: 10.1016/0145-305x(93)90003-9. [DOI] [PubMed] [Google Scholar]
- 95.Rombout JHWM, Taverne-Thiele AJ, Villena MI. The gut-associated lymphoid tissue (GALT) of carp (Cyprinus carpio L.): An immunocytochemical analysis. Dev Comp Immunol. 1993;17:55–66. doi: 10.1016/0145-305x(93)90015-i. [DOI] [PubMed] [Google Scholar]
- 96.Fournier-Betz V, Quentel C, Lamour F, LeVen A. Immunocytochemical detection of Ig-positive cells in blood, lymphoid organs and the gut associated lymphoid tissue of the turbot (Scophthalmus maximus) Fish Shellfish Immunol. 2000;10:187–202. doi: 10.1006/fsim.1999.0235. [DOI] [PubMed] [Google Scholar]
- 97.Parker S, La Flamme A, Salinas I. The ontogeny of New Zealand groper (Polyprion oxygeneios) lymphoid organs and IgM. Dev Comp Immunol. 2012;38:215–23. doi: 10.1016/j.dci.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 98.Li J, Barreda DR, Zhang YA, Boshra H, Gelman AE, LaPatra S, et al. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol. 2006;7:1116–24. doi: 10.1038/ni1389. [DOI] [PubMed] [Google Scholar]
- 99.Takizawa F, Dijkstra JM, Kotterba P, Korytář T, Kock H, Köllner B, et al. The expression of CD8α discriminates distinct T cell subsets in teleost fish. Dev Comp Immunol. 2011;35:752–63. doi: 10.1016/j.dci.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 100.Romano N, Caccia E, Piergentili R, Rossi F, Ficca AG, Ceccariglia S, et al. Antigen-dependent T lymphocytes (TcRβ+) are primarily differentiated in the thymus rather than in other lymphoid tissues in sea bass (Dicentrarchus labrax, L.) Fish Shellfish Immunol. 2011;30:773–82. doi: 10.1016/j.fsi.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 101.Scapigliati G, Mazzini M, Mastrolia L, Romano N, Abelli L. Production and characterisation of a monoclonal antibody against the thymocytes of the sea bass Dicentrarchus labrax(L.) (Teleostea, Percicthydae) Fish Shellfish Immunol. 1995;5:393–405. [Google Scholar]
- 102.Koppang EO, Fischer U, Moore L, Tranulis MA, Dijkstra JM, Kollner B, et al. Salmonid T cells assemble in the thymus, spleen and in novel interbranchial lymphoid tissue. J Anat. 2010;217:728–39. doi: 10.1111/j.1469-7580.2010.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bernard D, Six A, Rigottier-Gois L, Messiaen S, Chilmonczyk S, Quillet E, et al. Phenotypic and functional similarity of gut intraepithelial and systemic T cells in a teleost fish. J Immunol. 2006;176:3942–9. doi: 10.4049/jimmunol.176.7.3942. [DOI] [PubMed] [Google Scholar]
- 104.Boschi I, Randelli E, Buonocore F, Casani D, Bernini C, Fausto AM, et al. Transcription of T cell-related genes in teleost fish, and the European sea bass (Dicentrarchus labrax) as a model. Fish Shellfish Immunol. 2011;31:655–62. doi: 10.1016/j.fsi.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 105.Tadiso TM, Krasnov A, Skugor S, Afanasyev S, Hordvik I, Nilsen F. Gene expression analyses of immune responses in Atlantic salmon during early stages of infection by salmon louse (Lepeophtheirus salmonis) revealed bi-phasic responses coinciding with the copepod-chalimus transition. BMC Genomics. 2011;12:141. doi: 10.1186/1471-2164-12-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krasnov A, Skugor S, Todorcevic M, Glover KA, Nilsen F. Gene expression in Atlantic salmon skin in response to infection with the parasitic copepod Lepeophtheirus salmonis, cortisol implant, and their combination. BMC Genomics. 2012;13:130. doi: 10.1186/1471-2164-13-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, et al. Evidence for a core gut microbiota in the zebrafish. ISME J. 2011;5:1595–608. doi: 10.1038/ismej.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–51. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Panigrahi A, Azad IS. Microbial intervention for better fish health in aquaculture: The Indian scenario. Fish Physiol Biochem. 2007;33:429–40. [Google Scholar]
- 110.Olafsen JA. Interactions between fish larvae and bacteria in marine aquaculture. Aquaculture. 2001;200:223–47. [Google Scholar]
- 111.Hansen GH, Olafsen JA. Bacterial colonization of cod (Gadus morhua L.) and halibut (Hippoglossus hipoglossus) eggs in marine aquaculture. Appl Environ Microbiol. 1989;55:1435–46. doi: 10.1128/aem.55.6.1435-1446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stokes C, Soothill J, Turner M. Immune exclusion is a function of IgA. Nature. 1975;255:745–6. doi: 10.1038/255745a0. [DOI] [PubMed] [Google Scholar]
- 113.Irianto A, Austin B. Probiotics in aquaculture. J Fish Dis. 2002;25:633–42. [Google Scholar]
- 114.Martínez Cruz P, Ibáñez AL, Monroy Hermosillo OA, Saad R, Hugo C. Use of probiotics in aquaculture. ISRN Microbiology. 2012;2012 doi: 10.5402/2012/916845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Díaz-Rosales P, Salinas I, Rodríguez A, Cuesta A, Chabrillón M, Balebona MC, et al. Gilthead seabream (Sparus aurata L.) innate immune response after dietary administration of heat-inactivated potential probiotics. Fish Shellfish Immunol. 2006;20:482–92. doi: 10.1016/j.fsi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 116.Picchietti S, Mazzini M, Taddei AR, Renna R, Fausto AM, Mulero V, et al. Effects of administration of probiotic strains on GALT of larval gilthead seabream: Immunohistochemical and ultrastructural studies. Fish Shellfish Immunol. 2007;22:57–67. doi: 10.1016/j.fsi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 117.Salinas I, Abelli L, Bertoni F, Picchietti S, Roque A, Furones D, et al. Monospecies and multispecies probiotic formulations produce different systemic and local immunostimulatory effects in the gilthead seabream (Sparus aurato L.) Fish Shellfish Immunol. 2008;25:114–23. doi: 10.1016/j.fsi.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 118.Andani HRR, Tukmechi A, Meshkini S, Sheikhzadeh N. Antagonistic activity of two potential probiotic bacteria from fish intestines and investigation of their effects on growth performance and immune response in rainbow trout (Oncorhynchus mykiss) J Appl Ichthyol. 2012;28:728–34. [Google Scholar]
- 119.Verschuere L, Rombaut G, Sorgeloos P, Verstraete W. Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev. 2000;64:655–71. doi: 10.1128/mmbr.64.4.655-671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–9. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ringø E, Olsen JE, Vecino JLG, Wadsworth S, Song S-Y. Use of immunostimulants and nucleotides in aquaculture: a review. J Marine Sci Res Development. 2011:1. [Google Scholar]
- 122.Torrecillas S, Makol A, Benítez-Santana T, Caballero MJ, Montero D, Sweetman J, et al. Reduced gut bacterial translocation in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides (MOS) Fish Shellfish Immunol. 2011;30:674–81. doi: 10.1016/j.fsi.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 123.Sheikhzadeh N, Heidarieh M, Pashaki AK, Nofouzi K, Farshbafi MA, Akbari M. Hilyses (R), fermented Saccharomyces cerevisiae, enhances the growth performance and skin non-specific immune parameters in rainbow trout (Oncorhynchus mykiss) Fish Shellfish Immunol. 2012;32:1083–7. doi: 10.1016/j.fsi.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 124.Sheikhzadeh N, Pashaki AK, Nofouzi K, Heidarieh M, Tayefi-Nasrabadi H. Effects of dietary Ergosan on cutaneous mucosal immune response in rainbow trout (Oncorhynchus mykiss) Fish Shellfish Immunol. 2012;32:407–10. doi: 10.1016/j.fsi.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 125.Tafalla C, Bøgwald J, Dalmo RA. Adjuvants and immunostimulants in fish vaccines: Current knowledge and future perspectives. Fish Shellfish Immunol. 2013 doi: 10.1016/j.fsi.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 126.Plant KP, LaPatra SE. Advances in fish vaccine delivery. Dev Comp Immunol. 2011;35:1256–62. doi: 10.1016/j.dci.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 127.Lin CC, Lin JHY, Chen MS, Yang HL. An oral nervous necrosis virus vaccine that induces protective immunity in larvae of grouper (Epinephelus coioides) Aquaculture. 2007;268:265–73. [Google Scholar]
- 128.Kumar SR, Ahmed VPI, Parameswaran V, Sudhakaran R, Babu VS, Hameed ASS. Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in Asian sea bass (Lates calcarifer) to protect from Vibrio (Listonella) anguillarum. Fish Shellfish Immunol. 2008;25:47–56. doi: 10.1016/j.fsi.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 129.de las Heras AI, Saint-Jean SR, Perez-Prieto SI. Immunogenic and protective effects of an oral DNA vaccine against infectious pancreatic necrosis virus in fish. Fish Shellfish Immunol. 2010;28:562–70. doi: 10.1016/j.fsi.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 130.Adomako M, St-Hilaire S, Zheng Y, Eley J, Marcum RD, Sealey W, et al. Oral DNA vaccination of rainbow trout, Oncorhynchus mykiss (Walbaum), against infectious haematopoietic necrosis virus using PLGA Poly(D,L-Lactic-Co-Glycolic Acid) nanoparticles. J Fish Dis. 2012;35:203–14. doi: 10.1111/j.1365-2761.2011.01338.x. [DOI] [PubMed] [Google Scholar]
- 131.Licciardi P, Tang M. Vaccine adjuvant properties of probiotic bacteria. Discov Med. 2011;12:525–33. [PubMed] [Google Scholar]
- 132.Chadwick S, Kriegel C, Amiji M. Nanotechnology solutions for mucosal immunization. Adv Drug Del Rev. 2010;62:394–407. doi: 10.1016/j.addr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 133.Gill SR, Pop M, DeBoy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–9. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sunyer JO. Perspective article: Fishing for new paradigms in teleost fish immune systems. Nat Immunol. 2013 doi: 10.1038/ni.2549. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liang Y, Guan R, Huang W, Xu T. Isolation and Identification of a Novel Inducible Antibacterial Peptide from the Skin Mucus of Japanese Eel, Anguilla japonica. Protein J. 2011;30:413–21. doi: 10.1007/s10930-011-9346-9. [DOI] [PubMed] [Google Scholar]
- 136.Concha MI, Molina S, Oyarzun C, Villanueva J, Amthauer R. Local expression of apolipoprotein A-I gene and a possible role for HDL in primary defence in the carp skin. Fish Shellfish Immunol. 2003;14:259–73. doi: 10.1006/fsim.2002.0435. [DOI] [PubMed] [Google Scholar]
- 137.Yang M, Chen B, Cai J-J, Peng H, Yuan J-J, Wang K-J. Molecular characterization of hepcidin AS-hepc2 and AS-hepc6 in black porgy (Acanthopagrus schlegelii): Expression pattern responded to bacterial challenge and in vitro antimicrobial activity. Comp Biochem Physiol B: Biochem Mol Biol. 2011;158:155–63. doi: 10.1016/j.cbpb.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 138.Casadei E, Bird S, Gonzalez Vecino JL, Wadsworth S, Secombes CJ. The effect of peptidoglycan enriched diets on antimicrobial peptide gene expression in rainbow trout (Oncorhynchus mykiss) Fish Shellfish Immunol. 2013;34:529–37. doi: 10.1016/j.fsi.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 139.Buonocore F, Randelli E, Casani D, Picchietti S, Belardinelli MC, de Pascale D, et al. A piscidin-like antimicrobial peptide from the icefish Chionodraco hamatus (Perciformes: Channichthyidae): Molecular characterization, localization and bactericidal activity. Fish Shellfish Immunol. 2012;33:1183–91. doi: 10.1016/j.fsi.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 140.Iijima N, Tanimoto N, Emoto Y, Morita Y, Uematsu K, Murakami T, et al. Purification and characterization of three isoforms of chrysophsin, a novel antimicrobial peptide in the gills of the red sea bream, Chrysophrys major. Eur J Biochem. 2003;270:675–86. doi: 10.1046/j.1432-1033.2003.03419.x. [DOI] [PubMed] [Google Scholar]
- 141.Nakamura O, Watanabe T, Kamiya H, Muramoto K. Galectin containing cells in the skin and mucosal tissues in Japanese conger eel, Conger myriaster: an immunohistochemical study. Dev Comp Immunol. 2001;25:431–7. doi: 10.1016/s0145-305x(01)00012-x. [DOI] [PubMed] [Google Scholar]
- 142.Zhang Y-x, Zou A-h, Manchu R-g, Zhou Y-c, Wang S-f. Purification and antimicrobial activity of antimicrobial protein from brown-spotted grouper, Epinephelus fario. Zool Res. 2008;6:627–32. [Google Scholar]
- 143.Pan CY, Chen JY, Cheng YSE, Chen CY, Ni IH, Sheen JF, et al. Gene expression and localization of the epinecidin-1 antimicrobial peptide in the grouper (Epinephelus coioides), and its role in protecting fish against pathogenic infection. DNA Cell Biol. 2007;26:403–13. doi: 10.1089/dna.2006.0564. [DOI] [PubMed] [Google Scholar]
- 144.Browne MJ, Feng CY, Booth V, Rise ML. Characterization and expression studies of Gaduscidin-1 and Gaduscidin-2; paralogous antimicrobial peptide-like transcripts from Atlantic cod (Gadus morhua) Dev Comp Immunol. 2011;35:399–408. doi: 10.1016/j.dci.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 145.Ullal AJ, Litaker RW, Noga EJ. Antimicrobial peptides derived from hemoglobin are expressed in epithelium of channel catfish (Ictalurus punctatus, Rafinesque) Dev Comp Immunol. 2008;32:1301–12. doi: 10.1016/j.dci.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 146.Birkemo GA, Lüders T, Andersen Ø, Nes IF, Nissen-Meyer J. Hipposin, a histone-derived antimicrobial peptide in Atlantic halibut (Hippoglossus hippoglossus L.) Biochim Biophys Acta: Proteins Proteomics. 2003;1646:207–15. doi: 10.1016/s1570-9639(03)00018-9. [DOI] [PubMed] [Google Scholar]
- 147.Lauth X, Shike H, Burns JC, Westerman ME, Ostland VE, Carlberg JM, et al. Discovery and characterization of two isoforms of moronecidin, a novel antimicrobial peptide from hybrid striped bass. J Biol Chem. 2002;277:5030–9. doi: 10.1074/jbc.M109173200. [DOI] [PubMed] [Google Scholar]
- 148.Casadei E, Wang TH, Zou J, Vecino JLG, Wadsworth S, Secombes CJ. Characterization of three novel beta-defensin antimicrobial peptides in rainbow trout (Oncorhynchus mykiss) Mol Immunol. 2009;46:3358–66. doi: 10.1016/j.molimm.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 149.Fernandes JM, Saint N, Kemp GD, Smith VJ. Oncorhyncin III: a potent antimicrobial peptide derived from the non-histone chromosomal protein H6 of rainbow trout, Oncorhynchus mykiss. Biochem J. 2003;373:621–8. doi: 10.1042/BJ20030259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Park IY, Park CB, Kim MS, Kim SC. Parasin I, an antimicrobial peptide derived from histone H2A in the catfish, Parasilurus asotus. FEBS Lett. 1998;437:258–62. doi: 10.1016/s0014-5793(98)01238-1. [DOI] [PubMed] [Google Scholar]
- 151.Su Y. Isolation and identification of pelteobagrin, a novel antimicrobial peptide from the skin mucus of yellow catfish (Pelteobagrus fulvidraco) Comp Biochem Physiol B: Biochem Mol Biol. 2011;158:149–54. doi: 10.1016/j.cbpb.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 152.Silphaduang U, Colorni A, Noga E. Evidence for widespread distribution of piscidin antimicrobial peptides in teleost fish. Dis Aquat Org. 2006;72:241–52. doi: 10.3354/dao072241. [DOI] [PubMed] [Google Scholar]
- 153.Ruangsri J, Fernandes JM, Rombout JH, Brinchmann MF, Kiron V. Ubiquitous presence of piscidin-1 in Atlantic cod as evidenced by immunolocalisation. BMC Vet Res. 2012;8:46. doi: 10.1186/1746-6148-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Corrales J, Mulero I, Mulero V, Noga EJ. Detection of antimicrobial peptides related to piscidin 4 in important aquacultured fish. Dev Comp Immunol. 2010;34:331–43. doi: 10.1016/j.dci.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 155.Cole AM, Darouiche RO, Legarda D, Connell N, Diamond G. Characterization of a fish antimicrobial peptide: gene expression, subcellular localization, and spectrum of activity. Antimicrob Agents Chemother. 2000;44:2039–45. doi: 10.1128/aac.44.8.2039-2045.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Peng KC, Lee SH, Hour AL, Pan CY, Lee LH, Chen JY. Five different piscidins from Nile tilapia, Oreochromis niloticus: Analysis of their expressions and biological functions. PLoS One. 2012:7. doi: 10.1371/journal.pone.0050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Seo JM, Lee MJ, Go HJ, Park TH, Park NG. Purification and characterization of YFGAP, a GAPDH-related novel antimicrobial peptide, from the skin of yellowfin tuna, Thunnus albacares. Fish Shellfish Immunol. 2012;33:743–52. doi: 10.1016/j.fsi.2012.06.023. [DOI] [PubMed] [Google Scholar]