Summary
Background
Myeloablative chemoradiotherapy and immunomagnetically purged autologous bone marrow transplantation has been shown to improve outcome for patients with high-risk neuroblastoma. Currently, peripheral blood stem cells (PBSC) are infused after myeloablative therapy, but the effect of purging is unknown. We did a randomised study of tumour-selective PBSC purging in stem-cell transplantation for patients with high-risk neuroblastoma.
Methods
Between March 16, 2001, and Feb 24, 2006, children and young adults (<30 years) with high-risk neuroblastoma were randomly assigned at diagnosis by a web-based system (in a 1:1 ratio) to receive either nonpurged or immunomagnetically purged PBSC. Randomisation was done in blocks stratified by International Neuroblastoma Staging System stage, age, MYCN status, and International Neuroblastoma Pathology classification. Patients and treating physicians were not masked to treatment assignment. All patients were treated with six cycles of induction chemotherapy, myeloablative consolidation, and radiation therapy to the primary tumour site plus metaiodobenzylguanidine avid metastases present before myeloablative therapy, followed by oral isotretinoin. PBSC collection was done after two induction cycles. For purging, PBSC were mixed with carbonyl iron and phagocytic cells removed with samarium cobalt magnets. Remaining cells were mixed with immunomagnetic beads prepared with five monoclonal antibodies targeting neuroblastoma cell surface antigens and attached cells were removed using samarium cobalt magnets. Patients underwent autologous stem-cell transplantation with PBSC as randomly assigned after six cycles of induction therapy. The primary endpoint was event-free survival and was analysed by intention-to-treat. The trial is registered with ClinicalTrials.gov, number NCT00004188.
Findings
495 patients were enrolled, of whom 486 were randomly assigned to treatment: 243 patients to receive non-purged PBSC and 243 to received purged PBSC. PBSC were collected from 229 patients from the purged group and 236 patients from the non-purged group, and 180 patients from the purged group and 192 from the non-purged group received transplant. 5-year event-free survival was 40% (95% CI 33–46) in the purged group versus 36% (30–42) in the non-purged group (p=0·77); 5-year overall survival was 50% (95% CI 43–56) in the purged group compared with 51% (44–57) in the non-purged group (p=0·81). Toxic deaths occurred in 15 patients during induction (eight in the purged group and seven in the non-purged group) and 12 during consolidation (eight in the purged group and four in the non-purged group). The most common adverse event reported was grade 3 or worse stomatitis during both induction (87 of 242 patients in the purged group and 93 of 243 patients in the non-purged group) and consolidation (131 of 177 in the purged group vs 145 of 191 in the non-purged group). Serious adverse events during induction were grade 3 or higher decreased cardiac function (four of 242 in the purged group and five of 243 in the non-purged group) and elevated creatinine (five of 242 in the purged group and six of 243 non-purged group) and during consolidation were sinusoidal obstructive syndrome (12 of 177 in the purged group and 17 of 191 in the non-purged group), acute vascular leak (11 of 177 in the purged group and nine of 191 in the non-purged group), and decreased cardiac function (one of 177 in the purged group and four of 191 in the non-purged group).
Interpretation
Immunomagnetic purging of PBSC for autologous stem-cell transplantation did not improve outcome, perhaps because of incomplete purging or residual tumour in patients. Non-purged PBSC are acceptable for support of myeloablative therapy of high-risk neuroblastoma.
Funding
National Cancer Institute and Alex’s Lemonade Stand Foundation.
Introduction
High-risk neuroblastoma has a high rate of recurrence, most commonly in bone and bone marrow.1 Results from the Children’s Cancer Group (CCG)-3891 trial2 showed that myeloablative chemotherapy with rescue with immunomagnetic bead purged autologous bone marrow improved outcome compared with conventional dose chemotherapy. Immunocytology can detect neuroblastoma tumour cells in the bone marrow.3 Genetically labelled neuroblastoma cells infused from non-purged bone marrow can contribute to relapse after myeloablative therapy.4 These data supported immunomagnetically purging bone marrow to remove tumour detectable by immunocytology, which has a sensitivity of one tumour cell in 105 normal cells.3
Currently, autologous peripheral blood stem cells (PBSC) are used to restore haemopoiesis after myeloablative therapy for high-risk neuroblastoma. Blood has no or fewer neuroblastoma cells detectable by immunocytology even when bone marrow is positive.5 Quantitative real-time PCR (QrtPCR) can detect neuroblastoma mRNA in PBSC,6–8 although the effect of infusing these PBSC has not been defined. We postulated that immunomagnetic bead purging would decrease tumour burden in PBSC and improve outcome. We report the results of the randomised Children’s Oncology Group (COG) A3973 trial, which compared outcomes for high-risk neuroblastoma patients who received autologous purged versus non-purged PBSC after myeloablative chemotherapy. To our knowledge, this is the first randomised study in any cancer to evaluate the effect of tumour-selective PBSC purging.
Methods
Study design and participants
This phase 3 trial was open from Feb 9, 2001, to March 31, 2006, for children with high-risk neuroblastoma. Patients or parents or guardians provided written informed consent according to National Cancer Institute and local institutional review board guidelines.
Patients were enrolled from 95 COG member institutions in the USA (420) and Canada (66). Eligible patients had high-risk neuroblastoma according to the Children’s Oncology Group (COG) criteria, including previously untreated neuroblastoma in patients younger than 1 year with International Neuroblastoma Staging System9 (INSS) stage 3, 4, or 4S MYCN amplified tumours and, in children aged 1 year or older, stage 4 tumours, stage 3 tumours with either unfavourable histology or MYCN amplification, stage 2 tumours with unfavourable histology and MYCN amplification, and initially low-risk patients treated with surgery only who later progressed with metastatic disease.9 We excluded patients aged 12–18 months with stage 4 MYCN non-amplified tumours and favourable histology, and hyperdiploid tumours after an amendment in May, 2004.10 Additional eligibility criteria included age up to 30 years, no previous history of chemotherapy, registration on companion biology study, ability to tolerate PBSC collection, and adequate cardiac, liver, and renal function.
Randomisation and masking
Patients were randomly assigned to treatment at study enrolment using the COG’s online remote data entry system, which assigned treatment group in real-time based on the balance existing at that time, within blocks of size four. The method was random until such time as a random assignment exceeded the prespecified margin of two within a block, and only then did the method become deterministic. Patients were randomly assigned (ratio 1:1) to receive either purged PBSC or non-purged PBSC at study entry; patients and treating physicians were not masked to this assignment. Randomisation was stratified into blocks by International Neuroblastoma Staging System (INSS) stage,9 age at diagnosis (<365 days or ≥365 days), MYCN gene status (amplified or non-amplified), and International Neuroblastoma Pathology Classification (INPC; unfavourable or favourable).11
Procedures
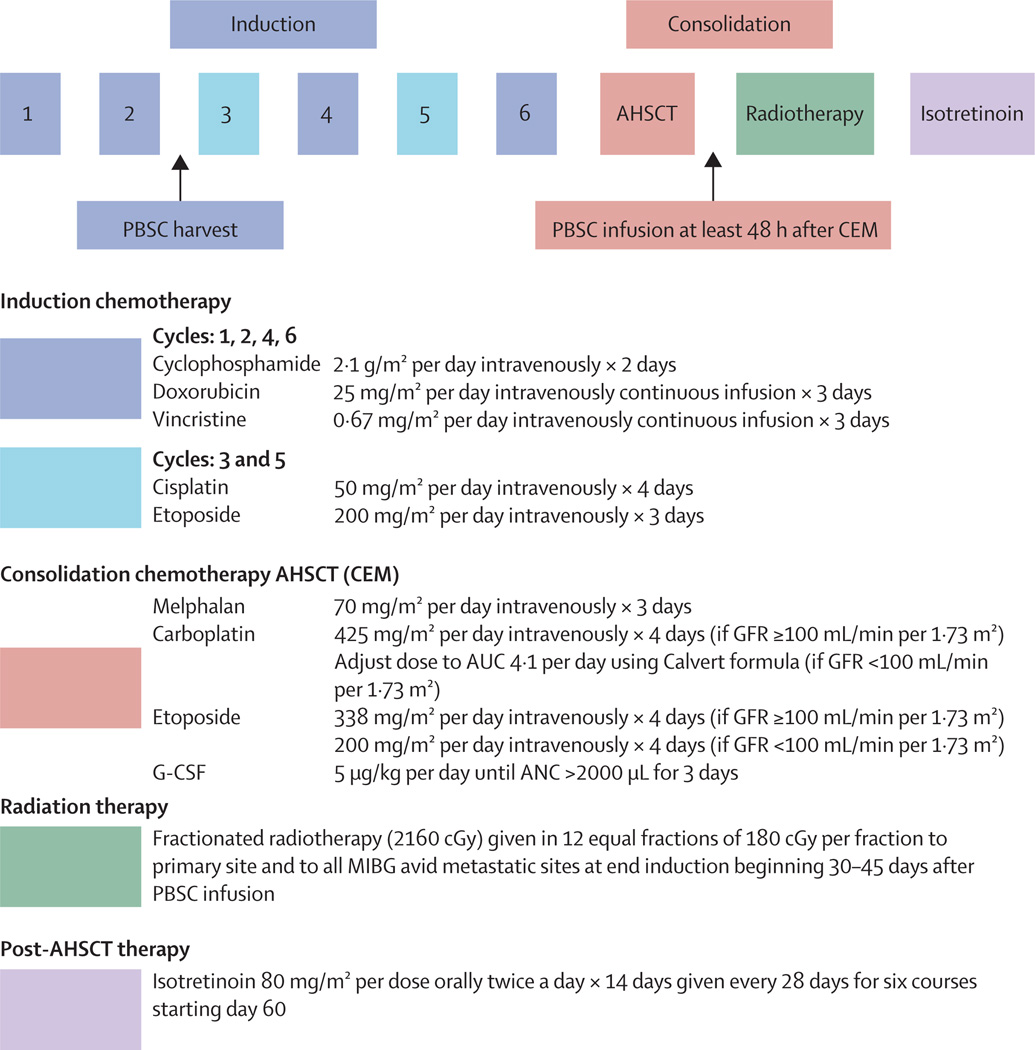
All patients were prescribed identical chemotherapy, radiotherapy, and post-myeloablative isotretinoin (figure 1). Patients without progressive disease and adequate organ function received myeloablative carboplatin, etoposide, and melphalan,12 with dose adjustment for glomerular filtration rate (GFR) lower than 100 mL/min per 1·73 m2.13 After local irradiation, patients could enrol in the COG ANBL0032 trial of isotretinoin plus chimeric anti-GD2 monoclonal antibody ch14.18, interleukin 2, and GM-CSF versus isotretinoin alone,14 with randomisation stratified by assigned arm of this study. We assessed response using the International Neuroblastoma Response Criteria.9 We graded adverse events using the National Cancer Institute’s Common Toxicity Criteria, version 2.15 Reporting was required for all grade 3–5 toxic effects (non-targeted). To monitor safety during the study, the protocol required reporting of all grades of specific organ function and infectious toxic effects (targeted).
Figure 1. Treatment schema.
PBSC=peripheral blood stem cells. AHSCT=autologous haemopoietic stem-cell transplantation. CEM=carboplatin, etoposide, and melphalan. GFR=glomerular filtration rate. AUC=area under the curve. G-CSF=granulocyte colony-stimulating factor. ANC=absolute neutrophil count. MIBG=123I or 131I- meta-iodobenzylguanidine.
PBSC were collected after two induction cycles regardless of histological tumour content in the bone marrow. Requirements to proceed to consolidation were: immunocytology-negative PBSC with minimum of 1·5×106 CD34 cells per kg for non-purged PBSC or minimum of 1×106 viable CD34 cells per kg for purged PBSC. We determined viability on a thawed purged PBSC aliquot using trypan blue.16 Patients with insufficient purged PBSC could receive non-purged PBSC (or purged bone marrow if non-purged PBSC also insufficient) meeting protocol criteria. Patients with immunocytology-positive non-purged PBSC could be recollected and purged. Purged PBSC were shipped frozen to the transplant institution and thawed immediately before reinfusion. We defined neutrophil engraftment as the first of three consecutive absolute neutrophil counts of more than 500 cells per µL; and platelet engraftment as the first of three consecutive platelet counts of more than 20 000 without transfusion.
Non-purged PBSC were cryopreserved at collecting sites. For purged PBSC, heparinised PBSC were transported overnight at ambient temperature to a centralised laboratory and purged the day after leukapheresis according to US Food and Drug Administration (FDA) IDE# BB-IDE 2259. PBSC were mixed with 200–300 mg of carbonyl iron per 106 total cells to remove phagocytic cells and decrease the number of immunomagnetic beads required. Carbonyl iron and attached cells were removed using samarium cobalt magnets. Remaining cells were mixed with immunomagnetic beads using five monoclonal antibodies targeting neuroblastoma cell surface antigens (459, HSAN 10·2, BA-1, HNK-1, and 126-4; appendix). Magnetic beads and attached cells were removed using samarium cobalt magnets.16 Purged samples were suspended in L15 containing human serum albumin 10% volume/volume (Central Lab, Blood transfusion Service, Swiss Red Cross, Bern, Switzerland), hetastarch 1·5% weight/volume, and DMSO 10% volume/volume (Cryoserv, Ben Venue Laboratories Inc, Bedford, OH, USA) in Cryocyte Freezing bags (Baxter-Fenwal, Deerfield, NJ, USA). Stem-cell samples were also cryopreserved in 2 mL Cryovials (Baxter-Fenwal). All products were frozen with a rate-controlled programmed freezer (Cryomed) to −80°C and stored in liquid nitrogen vapour (150°C). Quality control of purged product required for release included immunocytology to detect neuroblastoma, CD34 viable content on post-purge test thaw sample, and endotoxin testing. Cryopreserved products were shipped to transplant centres in MVE LN2 dry shippers with constant temperature monitoring by overnight air delivery.
To detect neuroblastoma cells in PBSC, immuno cytology and TLDA assays were done on a PBSC aliquot from day 1 of leukapheresis on all patients before purging. Immunocytology after purging was done with mononuclear cells isolated by separation with Ficoll-Hypaque density media. Immunocytology used monoclonal antibodies against cell surface antigens (126-4, 390, 459, HSAN1·2, and BW575).17,18 The TLDA assay quantified CHGA, DCX, DDC, PHOX2B, and TH mRNA expression. We deemed results to be detectable if one or more of the five genes had a cycle threshold (CT) value lower than 40 and to be undetectable if no signal was detected for any genes after 40 cycles (CT=40). We did a second analysis of the same data using only PHOX2B and TH mRNA expression to define detectable samples (appendix).
Statistical analysis
Analyses were done by intention to treat. We targeted an enrolment of 486 patients, which would provide 80% power for a one-sided log-rank test of superiority of the purged group over the non-purged group at a level of 0·05, able to detect a 9% improvement in 2-year eventfree survival (38% vs 47%).
We did an intention-to-treat sequential monitoring of the trial, and considered early stopping if the groups proved19 or would never prove20 to be significantly different, or if the conditional power fell under 20%. We calculated the relative risk as the ratio of non-purged to purged using the planning variables for 2-year event-free survival, and under the alternative hypothesis, it would equal 1·33. The Fleming-Harrington-O’Brien20 lower (futility) boundary was equivalent to repeated testing of the alternative hypothesis at p=0·005 for a cumulative α level of 0·05. The total expected information was 341 events. Only the COG data safety monitoring committee and study statistician were aware of interim efficacy monitoring results. Trial efficacy results remained masked until release by the data safety monitoring committee after all patients had completed protocol therapy.
The primary endpoint was event-free survival, for which the time to event was calculated from study enrolment and randomisation until first occurrence of relapse, progressive disease, secondary malignancy, death, or until last contact with the patient if no event occurred. For overall survival, the time to event was calculated from study enrolment until death, or until last contact with the patient. Post-hoc, we also calculated event-free survival and overall survival from the time of transplant. We generated Kaplan-Meier survival curves.21 We report 5-year point estimates with 95% CI.22
With the exception of the sequential monitoring, we deemed p values lower than 0·05 significant. We did the statistical analyses using SAS version 9.2 and Stata version 12.1. This trial is registered with ClinicalTrials.gov, number NCT00004188.
Role of the funding source
The NCI contributed to study design through scientific review. Alex’s Lemonade Stand had no role in study design or reporting. Neither of these two funders had a role in data collection, data analysis, data interpretation, or writing of the report. All authors participated in writing the report and had full access to all the data in the study. The corresponding author had final responsibility for the decision to submit for publication.
Results
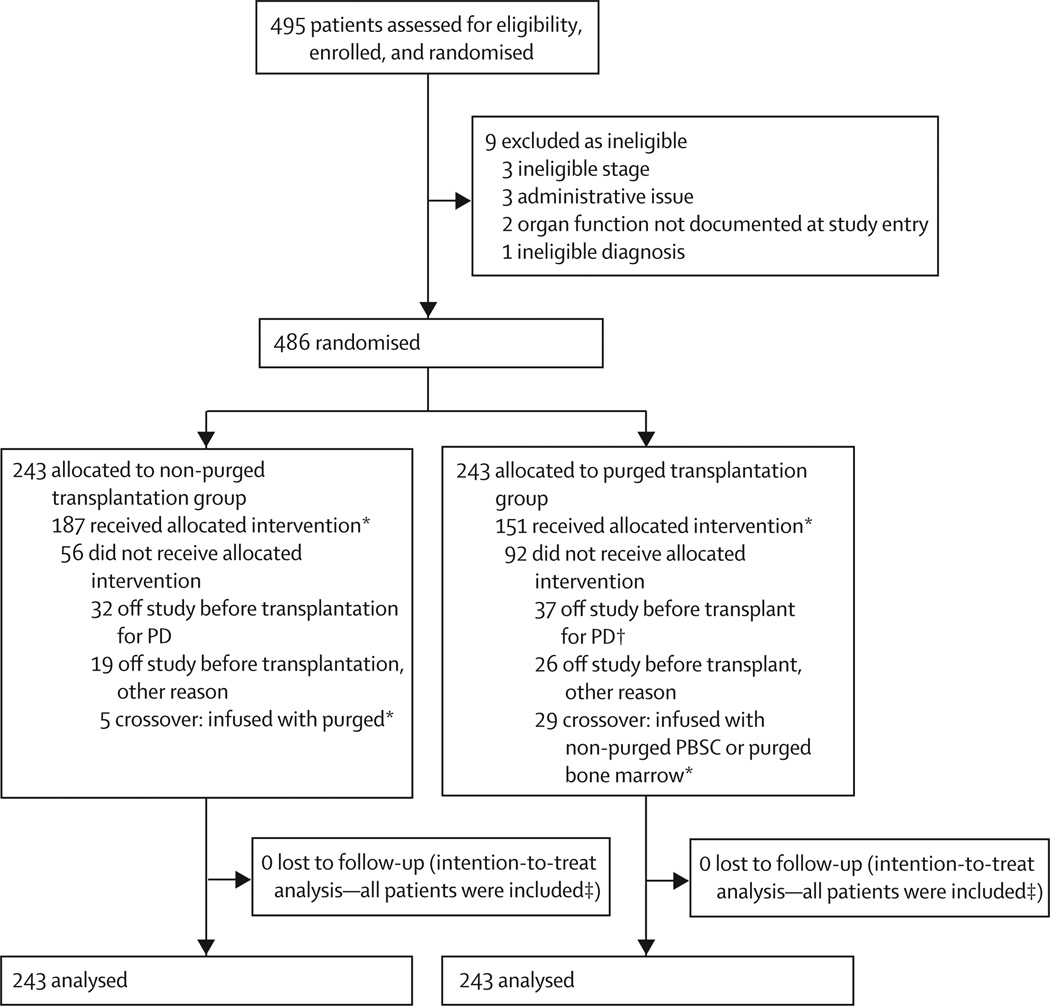
The trial ended after 495 patients had been enrolled (patients were enrolled from March 16, 2001, to Feb 24, 2006). 486 patients were eligible for randomisation; 243 were randomly assigned to receive purged PBSC and 243 to receive non-purged PBSC (figure 2). Baseline characteristics were much the same in each group and seemed similar to the COG overall high risk cohort (table 1).2 Median age of the patients was 3·1 years (range 0·2–29·1 years). Of the 486 eligible for randomisation, 137 (28%) patients subsequently enrolled in the COG ANBL0032 trial after transplantation, and 78 (16%) were assigned to the ch14.18 antibody group (36 of 243 in the purged group and 42 of 243 in the non-purged group).
Figure 2. Trial profile.
*372 patients (192 non-purged, 180 purged) received a transplant, including those who received the PBSC product to which they were allocated (randomised) and those who were crossovers. †One additional patient who received a transplant was retrospectively (post-transplant) determined by the treating institution to have had progressive disease at the end of induction; thus table 1 presents a total of 38 patients in the purged group with end of induction progressive disease. ‡Patients alive at last contact were censored in survival analysis.
Table 1.
Patient characteristics
| Purged transplantation group (n=243) |
Non-purged transplantation group (n=243) |
|
|---|---|---|
| Age | ||
| <18 months | 33/243 (14%) | 31/243 (13%) |
| ≥18 months | 210/243 (86%) | 212/243 (87%) |
| INSS stage at diagnosis | ||
| Stage 1* | 2/243 (1%) | 1/243 (0·4%) |
| Stage2a/2b | 1/243 (<1%) | 5/243 (2%) |
| Stage 3 | 29/243 (12%) | 29/243 (12%) |
| Stage 4 | 208/243 (86%) | 206/243 (85%) |
| Stage 4S | 3/243 (1%) | 2/243 (1%) |
| Tumour MYCN status | ||
| Amplified | 85/194 (44%) | 87/196 (44%) |
| Non-amplified | 109/194 (56%) | 109/196 (56%) |
| Unknown | 49 | 47 |
| Tumour histology | ||
| Favourable | 7/171 (4%) | 5/173 (3%) |
| Unfavourable | 164/171 (96%) | 168/173 (97%) |
| Unknown | 72 | 70 |
| Tumour ploidy | ||
| Hyperdiploid | 84/189 (44%) | 91/196 (46%) |
| Diploid | 105/189 (56%) | 105/196 (54%) |
| Unknown | 54 | 47 |
| Bone marrow morphology | ||
| After induction cycle 2 | ||
| Negative | 107/168 (64%) | 118/177 (67%) |
| Positive | 61/168 (36%) | 59/177 (33%) |
| Unknown | 75 | 66 |
| At the end of induction | ||
| Negative | 168/208 (81%) | 181/216 (84%) |
| Positive | 40/208 (19%) | 35/216 (16%) |
| Unknown | 35 | 27 |
| PBSC day 1 immunocytology | ||
| Positive | 1/219 (<1%) | 4/220 (2%) |
| Negative | 218/219 (>99%) | 216/220 (98%) |
| No harvest | 14 | 7 |
| Unknown day 1 immunocytology† | 10 | 16 |
| TLDA analysis of PBSC from day 1 of leukapheresis‡ | ||
| Tumour detectable | 68/129 (53%) | 54/116 (47%) |
| Tumour undetectable | 61/129 (47%) | 62/116 (53%) |
| No TLDA specimen obtained or specimen of insufficient quality | 114 | 127 |
| Overall response at the end of induction | ||
| Complete response | 52/236 (22%) | 55/241 (23%) |
| Very good partial response | 62/236 (26%) | 73/241 (30%) |
| Partial response | 68/236 (29%) | 65/241 (27%) |
| Stable disease | 16/236 (7%) | 16/241 (7%) |
| Progressive disease | 38/236 (16%)§ | 32/241 (13%) |
| Unknown | 7 | 2 |
| MIBG scan at the end of induction | ||
| Complete response | 107/191 (56%) | 100/197 (51%) |
| Less than complete response | 84/191 (44%) | 97/197 (49%) |
| Unknown | 52 | 46 |
| Transplantation | ||
| Number proceeding to stem-cell transplantation | 180/243 (74%) | 192/243 (79%) |
| Number for whom any stem-cell product infused was not the product randomised | 35/180 (19%) | 5/192 (3%) |
| Number for whom back-up PBSC infusion was given | 5/180 (3%) | 6/192 (3%) |
| Number receiving post-stem-cell transplantation anti-GD2 antibody | 36/180 (20%) | 42/192 (22%) |
| Post-induction GFR | ||
| ≥100 mL/min per 1·73 m2 (normal GFR) | 163/194 (84%) | 156/201 (78%) |
| <100 mL/min per 1·73 m2 (low GFR) | 31/194 (16%) | 45/201 (22%) |
| Unknown | 49 | 42 |
Proportions have been calculated excluding patients with unknown values. INSS=international neuroblastoma staging system. PBSC=peripheral blood stem cell. TLDA=TaqMan low density array. MIBG=meta-iodobenzylguanidine. GFR=glomerular filtration rate.
Stage 1 at diagnosis and progressed to stage 4 without interval chemotherapy.
All but two patients without day 1 immunocytological data had immunocytology testing on a separate stem-cell sample before stem cell-infusion for transplantation; treating physicians chose to infuse non-immunocytology tested products in those two patients.
Percentages calculated on the basis of the number of patients who were harvested and who had a specimen of sufficient quality (129 purged, 116 non-purged, 245 overall).
One additional patient who received a transplant was retrospectively (post-transplant) determined by the treating institution to have had progressive disease at the end of induction.
We obtained PBSC from 465 (96%) of 486 randomised patients: 229 in the purged group and 236 in the nonpurged group. Transplantation was completed in 372 patients (180 randomly assigned to purged and 192 to non-purged groups; figure 2). Reasons patients were not transplanted included progressive disease (28 from purged group and 25 from non-purged group) or death during induction (eight from purged group and seven from non-purged group), organ toxic effects (seven from purged group and five from non-purged group), withdrawal from protocol (three from purged group), insufficient PBSC (five from purged group and one from non-purged group), insufficient response (two from purged group and four from non-purged group), and other (ten from purged group and nine from non-purged group). 29 patients randomly assigned to purged PBSC could not comply because of insufficient PBSC yield for purging (12 patients) or after purging (eight patients), regulatory or technical issues (six patients), positive microbial culture of PBSC (one patient), and parental refusal (two patients). Five (1%) of 439 patients with immunocytology from day 1 of leukapheresis before purging had detectable tumour (table 1). Four of these patients were randomly assigned to receive non-purged PBSC and underwent subsequent purged PBSC collection. The fifth patient, also assigned to receive non-purged PBSC, had a negative sample from day 1 immunocytology, but the pooled PBSC collection had positive immunocytology before purging. All five patients had negative immunocytology after purging.
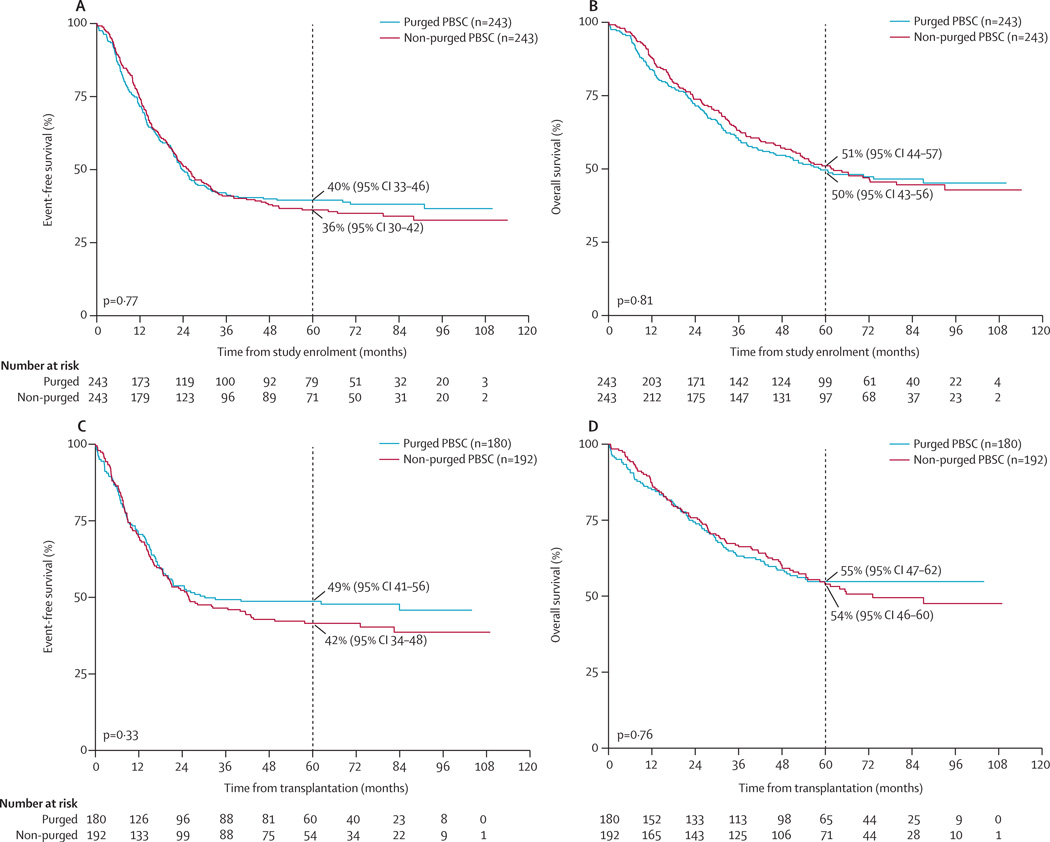
For the whole study population, 5-year event-free survival from enrolment was 38% (95% CI 34–42) and 5-year overall survival was 50% (95% CI 46–55), with median follow-up for patients without an event of 6·2 years (IQR 5·2–7·8). Neither event-free survival nor overall survival from enrolment differed between the purged and non-purged groups (figure 3A, B). We also noted no difference in event-free survival or overall survival from the time of transplantation between the purged and non-purged groups who completed transplantation (figure 3C, D). Post-hoc analysis by treatment actually received showed no difference in event-free survival (p=0·81) or overall survival (p=0·89) from study enrolment between groups; similar results were noted when event-free survival (p=0·15) and overall survival (p=0·23) were measured from time of transplantation (data not shown). In the 354 patients with stage 4 disease older than 18 months, event-free survival (p=0·32) or overall survival (p=0·77) from enrolment did not differ between the purged and non-purged groups. Outcome was similar for individuals with protocol-defined low compared with normal GFRs.
Figure 3. Event-free survival and overall survival.
(A) Event-free survival for intention-to-treat population from time of enrolment or randomisation. (B) Overall survival for intention-to-treat population from time of enrolment or randomisation.
(C) Event-free survival for comparison of patients randomly assigned to purged treatment group versus patients randomly assigned to non-purged treatment group, from time of transplantation.
(D) Overall survival for comparison of patients randomly assigned to purged treatment group versus patients randomly assigned to non-purged treatment group, from time of transplantation.
Although potentially underpowered and done post-hoc, analyses suggested that 5-year event-free and overall survival for the 270 patients with stage 4 disease with invaded bone marrow at diagnosis did not significantly differ between groups (p=0·20 for event-free survival and p=0·50 for overall survival). Similarly, for the 120 patients who were stage 4 with invaded bone marrow after two cycles of chemotherapy (at the time of PBSC collection), there was no difference between groups in terms of 5-year event-free or overall survival (p=0·22 for event-free survival and p=0·52 for overall survival).
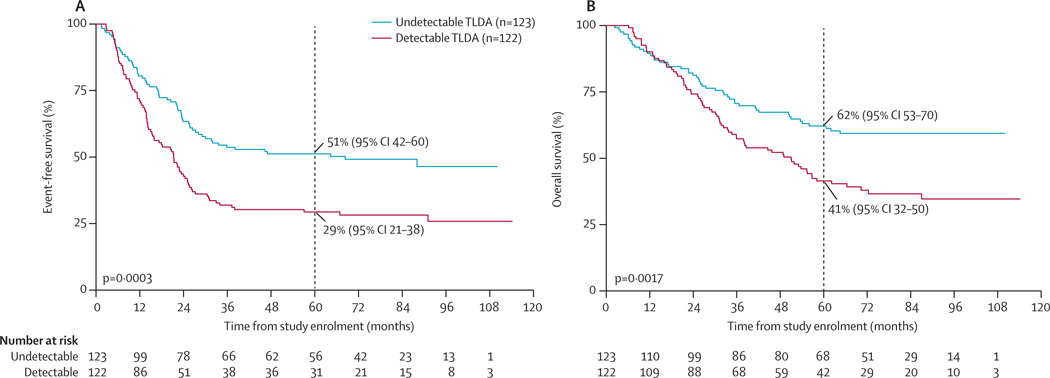
The 245 patients with TLDA results from day 1 of leukapheresis (before purging) were representative of all 486 patients in terms of clinical and prognostic characteristics (data not shown). Of these 245 patients, 122 (50%) had detectable tumour mRNA by the five-gene TLDA: 68 (53%) of 129 patients in the purged group and 54 (47%) of 116 patients in the non-purged group (table 1). Patients with detectable TLDA had lower event-free survival (at 5 years 29%, 95% CI 21–38) and overall survival (at 5 years 41%, 95% CI 32–50) than did patients with undetectable TLDA (5-year event-free survival 51%, 95% CI 42–60; p=0·0003; and 5-year overall survival 62%, 95% CI 53–70; p=0·0017; figure 4). When we analysed the same data using only expression of TH and PHOX2B, 62 (25%) patients had detectable TLDA (34 in the purged group and 28 in the non-purged group), with lower event-free survival (at 5 years 26%, 95% CI 16–37) and overall survival (at 5 years 35%, 95% CI 23–47) than those with undetectable TLDA (5-year event-free survival 45%, 95% CI 38–52; p=0·005; and 5-year overall survival 58%, 95% CI 50–65; p=0·01). 60 (33%) of 183 PBSC with undetectable two-gene TLDA (PHOX2B and TH; 34 in the purged group and 26 in the non-purged group) were detectable using five genes.
Figure 4. Event-free survival and overall survival by TLDA test results.
(A) Event-free survival. (B) Overall survival. TLDA=TaqMan low density array. PBSC=peripheral blood stem cells.
Sufficient numbers of CD34 cells per protocol criteria were obtained in 443 of 465 patients: 221 of 229 in the purged group and 222 of 236 in the non-purged group. The median number of CD34 cells per kg infused was significantly greater for non-purged versus purged PBSC (5·6 [IQR 3·7–10·7] vs 3·7 [2·1–6·3] million; p<0·0001). Patients receiving non-purged PBSC had shorter median time to neutrophil engraftment (11 [IQR 10–12] vs 12 [10–13] days; p=0·007) and platelet engraftment (19 [14–36] vs 28 [16–40] days; p=0·006) than did those receiving purged PBSC, with no evidence of a difference in infection rates.
Five patients (three in the purged group, two in the non-purged group) required additional PBSC infusions for delayed neutrophil engraftment; all subsequently engrafted. Six patients (two in the purged group, four in the non-purged group) with initial neutrophil engraftment received additional PBSC infusion because of secondary neutropenia or thrombocytopenia.
At the end of induction, 242 (51%) of 477 patients attained an overall complete response or very good partial response (table 1), 207 (53%) of 388 had a complete response by 131I or 123I-meta-iodobenzylguanidine (MIBG) scan and 349 (82%) of 424 had no tumour detectable in bone marrow by standard morphology (table 1). 70 (15%) of 477 patients progressed during induction (table 1).
For brevity, the summary of toxic effects (table 2) is limited to all protocol-required (targeted) toxic effects and any non-haematological toxic effects (non-targeted) that occurred in 5% of patients or more. Ototoxic effects requiring amplification (grade 3–4) occurred in 33 (7%) patients following induction (table 2). Systemic fungal infection during induction occurred in 40 (8%) of 485 patients (18 in the purged group; 22 in the non-purged group). There was no difference in consolidation toxic effects between the two randomised groups or the low versus normal GFR groups. 12 (7%) patients in the purged group and 17 (9%) of patients in the non-purged group had sinusoidal obstructive syndrome of grade 3 or higher. Sinusoidal obstructive syndrome was reported as severe in 15 (4%) patients (10 of 177 in the purged group, five of 191 in the non-purged group), life-threatening in 13 (3%) patients (one of 177 in the purged group, 12 of 191 in the non-purged group), or fatal in one (<1%) patient in the purged group.
Table 2.
Summary of protocol-required targeted toxic effects, and non-hematological toxic effects that occurred in 5% of patients or more
| Purged transplantation group* | Non-purged transplantation group* | |||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | |
| Induction† | ||||||||
| Targeted toxic effects‡ | ||||||||
| Hearing loss | 70/242 (29%) | 16/242 (7%) | 0 | 0 | 75/243 (31%) | 17/243 (7%) | 0 | 0 |
| Cardiac left ventricular function | 13/242 (5%) | 3/242 (1%) | 1/242 (<1%) | 0 | 14/243 (6%) | 3/243 (1%) | 2/243 (1%) | 0 |
| Stomatitis/pharyngitis | 80/242 (33%) | 50/242 (21%) | 37/242 (15%) | 0 | 81/243 (33%) | 52/243 (21%) | 41/243 (17%) | 0 |
| Serum creatinine | 18/242 (7%) | 4/242 (2%) | 0 | 1/242 (<1%) | 20/243 (8%) | 5/243 (2%) | 1/243 (<1%) | 0 |
| Non-targeted toxic effects§ | ||||||||
| Infection or febrile neutropenia: catheter related infection | 0 | 32/242 (13%) | 4/242 (2%) | 0 | 1/243 (<1%) | 28/243 (12%) | 6/243 (2%) | 0 |
| Consolidation¶ | ||||||||
| Targeted toxic effects | ||||||||
| Acute vascular leak | 6/177 (3%) | 7/177 (4%) | 4/177 (2%) | 0 | 5/191 (3%) | 5/191 (3%) | 4/191 (2%) | 0 |
| Cardiac left ventricular function | 11/177 (6%) | 1/177 (<1%) | 0 | 0 | 5/191 (3%) | 2/191 (1%) | 2/191 (1%) | 0 |
| Weight gain | 7/177 (4%) | 9/177 (5%) | 1/177 (<1%) | 0 | 9/191 (5%) | 4/191 (2%) | 12/191 (6%) | 0 |
| Stomatitis or pharyngitis | 28/177 (16%) | 94/177 (53%) | 37/177 (21%) | 0 | 30/191 (16%) | 104/191 (54%) | 40/191 (21%) | 1/191 (<1%) |
| Bilirubin | 34/177 (19%) | 16/177 (9%) | 4/177 (2%) | 0 | 35/191 (18%) | 15/191 (8%) | 6/191 (3%) | 0 |
| Hepatic enlargement | 0 | 31/177 (18%) | 0 | 0 | 0 | 28/191 (15%) | 0 | 0 |
| Stem-cell infusion complications | 19/177 (11%) | 7/177 (4%) | 1/177 (<1%) | 0 | 18/191 (9%) | 7/191 (4%) | 2/191 (1%) | 0 |
| Sinusoidal obstruction syndrome | 11/177 (6%) | 10/177 (6%) | 1/177 (<1%) | 1 (<1%) | 16/191 (8%) | 5/191 (3%) | 12/191 (6%) | 0 |
| Serum creatinine | 8/177 (5%) | 2/177 (1%) | 0 | 0 | 13/191 (7%) | 2/191 (1%) | 1/191 (<1%) | 0 |
| Non-targeted toxic effects | ||||||||
| Renal failure | 0 | 1/177 (<1%) | 1/177 (<1%) | 0 | 0 | 8/191 (4%) | 3/191 (2%) | 0 |
| Infection or febrile neutropenia: catheter-related infection | 0 | 13/177 (7%) | 0 | 0 | 0 | 15/191 (8%) | 2/191 (1%) | 0 |
| Isotretinoin‖ | ||||||||
| Targeted toxic effects | ||||||||
| Hypertension | 5/98 (5%) | 1/98 (1%) | 0 | 0 | 3/94 (3%) | 3/94 (3%) | 0 | 0 |
| Haematuria | 14/98 (14%) | 0 | 0 | 0 | 15/94 (16%) | 1/94 (1%) | 1/94 (1%) | 0 |
| Serum creatinine | 10/98 (10%) | 1/98 (1%) | 0 | 0 | 12/94 (13%) | 0 | 1/94 (1%) | 0 |
| Proteinuria | 7/98 (7%) | 1/98 (1%) | 0 | 0 | 14/94 (15%) | 1/94 (1%) | 1/94 (1%) | 0 |
A given patient is counted once for a given toxicity at the highest grade within a treatment period.
One patient in the purged transplantation group died on day of enrolment, never received any treatment, and was therefore excluded from toxicity analysis.
Targeted toxic effects: selected organ or infectious toxic effects with mandatory reporting of all grades.
Non-targeted toxic effects: required reporting of all grade 3–5 toxic effects.
Data were not reported regarding toxicity for three patients in the purged transplantation group and one patient in the non-purged transplantation group.
There were no non-targeted toxic effects of grades 3–5 that were noted in more than 5% of patients.
The 15 (3%) deaths that occurred during induction were due to infection (four in the purged group and one in the non-purged group), tumour bleeding (three in the purged group and one in the non-purged group), tumour-related organ compromise (one in the purged group and two in the non-purged group), multi-organ failure (one death in non-purged group), unrelated event (one death in the non-purged group), and central venous line placement (one death in the purged group). Infectious deaths were from typhlitis (one death in the non-purged group), or fungal (two deaths in the purged group) and viral (two deaths in the purged group) causes. In the 12 (2%) deaths during consolidation (eight in the purged group and four in the non-purged group), causes included infection (three from adenovirus, one from cytomegalovirus, two from candida, one from aspergillus, and two bacterial infections), sinusoidal obstructive syndrome (one death), clinical sepsis with negative cultures (one death), and multi-organ failure (one death). In addition to the primary cause of death, five patients also had sinusoidal obstructive syndrome as a contributing factor in their death.
Discussion
To our knowledge, this is the only randomised study of tumour-selective PBSC purging in any cancer (panel). Similar outcomes for the purged and non-purged groups establish that non-purged PBSC are acceptable for support of myeloablative therapy of high-risk neuroblastoma. Despite not requiring morphologically tumour-negative bone marrow before PBSC collection, only 1% of PBSC samples had tumour detectable by immunocytology; therefore, immunocytological testing of PBSC has been eliminated in COG studies.
The proportion of patients who achieved complete or very good partial response following induction chemotherapy in this trial was 51%, similar to other groups24,25 compared with 87·5% as originally reported (with small patient cohort at one institution).26 Response and progressive disease rates were similar to the less intensive CCG 3891 induction, which had fewer induction deaths.2
This trial escalated carboplatin, etoposide, and melphalan doses from the CCG 3891 regimen and omitted total body radiation (TBI), while maintaining a 5-year event-free survival similar to that reported in the myeloablative chemotherapy plus isotretinoin group from CCG 3891.2 TBI was replaced with irradiation to the primary tumour site and post-induction MIBG avid metastatic sites. TBI is associated with short stature, cataracts, dental abnormalities, thyroid dysfunction, and radiation pneumonitis.27 The toxic effects from the escalated chemotherapy regimen were tolerable, with sinusoidal obstructive syndrome of grade 3 or higher occurring in roughly the same proportion of patients as with the CCG 3891 CEM-TBI regimen,2 where it led to death in 3% of patients. Similar results were obtained in a pilot study of tandem high dose chemotherapy using cyclophosphamide and thiotepa followed by CEM with PBSC rescue.12
Immunomagnetic purging of PBSC did not improve outcome, possibly because of incomplete purging or due to residual tumour in patients. In preclinical modelling, immunomagnetic purging removed 3–4 logs of tumour cells from bone marrow when starting with 10–20% tumour cells.16 All five PBSC products with tumour detected by immunocytology from the sample of day 1 became undetectable after purging, which supports a purging effect. The number of randomised patients achieving complete response were insufficient to resolve the issue of whether residual tumour in patients was a possible cause for the failure of purging to improve outcome. All patients received isotretinoin and similar numbers of patients from both groups received post-consolidation ch14.18 antibody plus cytokines. This post-consolidation therapy might have eliminated tumour cells infused in the stem-cell product, which could obscure an effect of purging.
Apheresis was planned after cycle two of induction to obtain adequate CD34 cell per kg yield and avoid stem-cell exposure to topoisomerases to decrease secondary leukaemia risk.26 Results from a previous study5 showed a very low incidence of immunocytology-detectable tumour in PBSC even when bone marrow contained residual neuroblastoma at the time of pheresis.5 This result was confirmed in our current study, with only 1·2% of PBSC products having immunocytology-detectable tumour.
We assessed TLDA on an aliquot of PBSC from day 1 of leukapheresis to assess the prognostic significance of TLDA before any manipulation of PBSC for all patients. Our analysis showed a detectable signal by five-gene TLDA was associated with a worse outcome. We did an additional analysis of our TLDA data using only TH and PHOX2B expression to compare with other studies.6–8 Our patients with a detectable signal with either TH or PHOX2B also had significantly worse outcome compared with those patients with an undetectable signal. A higher number of patients had a detectable signal with the five-gene than with the two-gene analysis, providing more sensitive or less specific, or both, tumour mRNA detection. Thus, TLDA analysis of PBSC provides novel prognostic information that might provide early identification of patients requiring alternative therapy. Smaller series have shown conflicting results regarding the prognostic value of minimum tumour detection in PBSC.6–8 Data from other groups support the prognostic significance of QrtPCR detection of neuroblastoma mRNA in bone marrow.28,29 An international task force is currently assessing QrtPCR methodologies to reach a consensus for implementing this technology.30 Multivariable analysis of significance of TLDA compared with other prognostic factors is ongoing. Further analyses that are still in progress include the detection of tumour mRNA by five-gene TLDA in bone marrow and peripheral blood and multivariate analysis of TLDA, MIBG score, bone marrow morphology, and overall clinical response.
The study was designed to assess the effect of purging in patients with high-risk neuroblastoma as defined by the COG. It was not powered to assess outcome in the subset of patients with stage 4 disease aged 18 months or older. However, because of the insufficient evidence for an outcome difference in the treatment groups, this limitation became a non-issue. Another potential limitation is the lack of data from TLDA analyses before and after purging. Although we measured this in a subset of patients, the purging methodology might have caused technical interference with interpretation of the TLDA assay, which would prevent accurate quantification of the tumour mRNA reduction after purging. As such, we have not presented these data.
In conclusion, our results support the use of non-purged PBSC products following myeloablative therapy of high-risk neuroblastoma.
Supplementary Material
Panel: Research in context.
Systematic review
We searched PubMed for all publications with the terms “neuroblastoma”, “randomized”, “transplant”, “peripheral blood stem cells” and “purging”. We had no language or date restrictions. We did not find any randomised trial testing the impact of purging on outcome in neuroblastoma. We identified one randomised trial23 of ex-vivo CD34 cell selected or unselected PBSC transplantation in patients with multiple myeloma in which response and progression-free survival did not differ between groups.
Interpretation
In our trial, purging did not improve outcome in high-risk neuroblastoma patients receiving dose intensive induction and consolidation with autologous PBSC transplantation followed by isotretinoin with or without anti-GD2 antibody. Patients with PBSC having TLDA-detectable tumour mRNA expression had a lower event-free survival and overall survival. These findings support the use of non-purged PBSC for autologous transplantation for high-risk neuroblastoma in future trials. It will be important to assess the prognostic significance of TLDA analysis of PBSC products using a multivariable analysis with other prognostic factors.
Acknowledgments
This work was supported by U10-CA98543 COG Group Chair’s grant, COG Statistics and Data Center grants: U10-CA98413, U10-CA37379, U10- CA30969, and U10-CA29139; R33-CA152809-01, Alex’s Lemonade Stand Foundation. Immunomagnetic bead purging of PBSC was done under a US Food and Drug Administration (FDA) Investigational New Device exemption (IDE) BB-IDE 2259 with periodic review done as required by law. Third party payers were responsible for the cost of PBSC purging as allowed under IDE BB-IDE 2259. We thank the Children’s Hospital of Los Angeles staff in the hematopoietic stem cell processing/purging laboratory (Carolyn Billups, Jin-Hua Min, Maybelle Sim, and Robert Torres), the immunocytology laboratory (Rich Gallego, Alfonso Parra), and the TLDA laboratory (Cathy Wei Yao Liu and Betty Liu) for their essential contributions, and Peter Wakamatsu for his statistical and database efforts. We also thank the COG staff, CRA, nursing and pharmacy committee members for their contributions: Dina Willis (COG Statistics and Data Center), Sally Jones (Washington University School of Medicine, MO, USA), Casey Hook (University of Minnesota Medical Center, Fairview, MN, USA), and John T Wiernikowski (McMaster University, ON, Canada) for providing technical and pharmaceutical assistance.
Footnotes
Contributors
SGK, RCS, KKM, MPL, DAH-K, JRP, SLC, JMM, CPR, and JGV were involved in the design and development of the study. All authors were involved with writing or reviewing the protocol. SGK, RCS, CPR, WBL, and JGV were responsible for data collection. WBL, RS, and AB did the biostatistical analysis. All authors were involved in data interpretation. SGK and JGV wrote the first draft of the report. All authors have seen and reviewed the final manuscript draft.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a Children’s Oncology Group study. J Clin Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seeger RC, Reynolds CP, Gallego R, et al. Quantitative tumor cell content of bone marrow and blood as a predictor of outcome in stage IV neuroblastoma: a Children’s Cancer Group Study. J Clin Oncol. 2000;18:4067–4076. doi: 10.1200/JCO.2000.18.24.4067. [DOI] [PubMed] [Google Scholar]
- 4.Rill DR, Santana VM, Roberts WM, et al. Direct demonstration that autologous bone marrow transplantation for solid tumors can return a multiplicity of tumorigenic cells. Blood. 1994;84:380–383. [PubMed] [Google Scholar]
- 5.Bensimhon P, Villablanca JG, Sender LS, et al. Peripheral blood stem cell support for multiple cycles of dose intensive induction therapy is feasible with little risk of tumor contamination in advanced stage neuroblastoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2010;54:596–602. doi: 10.1002/pbc.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambon F, Tchirkov A, Pereira B, Rochette E, Demeocq F, Kanold J. Molecular assessment of minimal residual disease in PBSC harvests provides prognostic information in neuroblastoma. Pediatr Blood Cancer. 2013 doi: 10.1002/pbc.24538. published online March 21. [DOI] [PubMed] [Google Scholar]
- 7.Burchill SA, Kinsey SE, Picton S, et al. Minimal residual disease at the time of peripheral blood stem cell harvest in patients with advanced neuroblastoma. Med Pediatr Oncol. 2001;36:213–219. doi: 10.1002/1096-911X(20010101)36:1<213::AID-MPO1052>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Corrias MV, Haupt R, Carlini B, et al. Peripheral blood stem cell tumor cell contamination and survival of neuroblastoma patients. Clin Cancer Res. 2006;12:5680–5685. doi: 10.1158/1078-0432.CCR-06-0740. [DOI] [PubMed] [Google Scholar]
- 9.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 10.London WB, Castleberry RP, Matthay KK, et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. J Clin Oncol. 2005;23:6459–6465. doi: 10.1200/JCO.2005.05.571. [DOI] [PubMed] [Google Scholar]
- 11.Shimada H, Ambros IM, Dehner LP, et al. The International Neuroblastoma Pathology Classification (the Shimada system) Cancer. 1999;86:364–372. [PubMed] [Google Scholar]
- 12.Seif AE, Naranjo A, Baker DL, et al. A pilot study of tandem high-dose chemotherapy with stem cell rescue as consolidation for high risk neuroblastoma: Children’s Oncology Group study ANBL00P1. Bone Marrow Transplant. 2013 doi: 10.1038/bmt.2012.276. published online Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvert Ah, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7:1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 14.Yu AL, Gilman AL, Ozkaynak F, et al. Chimeric anti-GD2 antibody with GM-CSF, IL2 and 13-cis-retinoic acid for high-risk neuroblastoma: a Children’s Oncology Group (COG) phase 3 study. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. [accessed Feb 9, 2001];CTC version 2. http://ctep.cancer.gov/protocolDevelopment/electronic_application/docs/ctcv20_4-30-992.pdf.
- 16.Reynolds CP, Black AT, Woody JN. A sensitive method for detecting viable cells seeded into bone marrow. Cancer Research. 1986;46:5878–5881. [PubMed] [Google Scholar]
- 17.Reynolds CP, Seeger RC, Vo DD, Black AT, Wells J, Ugelstad J. Model system for removing neuroblastoma cells from bone marrow using monoclonal antibodies and magnetic immunobeads. Cancer Res. 1986;46:5882–5886. [PubMed] [Google Scholar]
- 18.Wu ZL, Schwartz E, Seeger RC, Ladisch S. Expression of GD2 ganglioside by untreated primary human neuroblastomas. Cancer Res. 1986;46:440–443. [PubMed] [Google Scholar]
- 19.Lan KKG, Demets DL. Discrete sequential boundaries for clinical-trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 20.Fleming TR, Harrington DP, O’Brien PC. Designs for group sequential tests. Controlled Clin Trials. 1984;4:348–361. doi: 10.1016/s0197-2456(84)80014-8. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric-estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Greenwood M. Report on Public Health and Medical Subjects No 33. London, UK: Her Majesty’s Stationery Office; 1926. The natural duration of cancer; pp. 1–26. [Google Scholar]
- 23.Bourhis JH, Bouko Y, Koscielny S, et al. Relapse risk after autologous transplantation in patients with newly diagnosed myeloma is not related with infused tumor cell load and the outcome is not improved by CD34+ cell selection: long term follow-up of an EBMT phase III randomized study. Haematologica. 2007;92:1083–1090. doi: 10.3324/haematol.10535. [DOI] [PubMed] [Google Scholar]
- 24.Kohler JA, Ellershaw C, Machin D, et al. Response to N7 induction chemotherapy in children more than one year of age diagnosed with metastatic neuroblastoma treated in UKCCSG centers. Pediatr Blood Cancer. 2007;49:234–239. doi: 10.1002/pbc.21139. [DOI] [PubMed] [Google Scholar]
- 25.Valteau-Couanet D, Michon J, Boneu A, et al. Results of induction chemotherapy in children older than 1 Year with a stage 4 neuroblastoma treated with the NB 97 French Society of Pediatric Oncology (SFOP) protocol. J Clin Oncol. 2005;23:532–540. doi: 10.1200/JCO.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 26.Kushner BH, Kramer K, LaQuaglia MP, et al. Reduction from seven to five cycles of intensive induction chemotherapy in children with high risk neuroblastoma. J Clin Oncol. 2004;22:2888–2892. doi: 10.1200/JCO.2004.02.101. [DOI] [PubMed] [Google Scholar]
- 27.Flandin I, Hartmann O, Michon J, et al. Impact of TBI on late effects in children treated by megatherapy for Stage IV neuroblastoma. A study of the French Society of Pediatric Oncology. Int J Radiat Oncol Biol Phys. 2006;64:1424–1431. doi: 10.1016/j.ijrobp.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Stutterheim J, Zappeij-Kannegieter L, Versteeg R, et al. The prognostic value of fast molecular response of marrow disease in patients aged over 1 year with stage 4 neuroblastoma. Eur J Cancer. 2011;47:1193–1202. doi: 10.1016/j.ejca.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Cheung IY, Lo Piccolo MS, Kushner BH, Cheung NKV. Early molecular response of marrow disease to biologic therapy is highly prognostic in neuroblastoma. J Clin Oncol. 2003;21:3853–3858. doi: 10.1200/JCO.2003.11.077. [DOI] [PubMed] [Google Scholar]
- 30.Beiske K, Burchill SA, Cheung IY, et al. Consensus criteria for sensitive detection of minimal neuroblastoma cells in bone marrow, blood and stem cell preparations by immunocytology and QRTPCR: recommendations by the International Neuroblastoma Risk Group Task Force. Br J Cancer. 2009;100:1627–1637. doi: 10.1038/sj.bjc.6605029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.