Abstract
Purpose
Chemotherapy for relapsed medulloblastoma has been inadequate, and most patients succumb to disease.
Methods
We retrospectively reviewed nine cases of relapsed medulloblastoma treated with bevacizumab, irinotecan, ±temozolomide. Patients received one to three prior therapeutic regimens. Five patients received 10 mg/kg bevacizumab and 125–150 mg/m2 irinotecan IV every 2 weeks, with temozolomide, starting at a median dose of 150 mg/m2 orally for 5 days monthly. Two patients received bevacizumab and irinotecan, but not temozolomide, due to provider preference. Two of nine patients received 15 mg/kg bevacizumab IV, 90 mg/m2 irinotecan orally for five consecutive days, 100 mg/m2/day temozolomide IV for 5 days, and 1.5 mg/m2 vincristine IV, each administered every 21 days.
Results
Median time to progression was 11 months. Median overall survival was 13 months. Objective tumor response at 3 months was 67 %, including six patients with partial response (PR) and three patients with stable disease (SD). At 6 months, objective response was 55 %, with two patients with PR and three with complete response. Additionally, one patient had SD and three had PD. Two patients remain alive and progression free at 15 and 55 months; another is alive with disease at 20 months. Toxicities included two patients with grade III neutropenia, two with grade III thrombocytopenia, one with grade III elevation of liver function tests, and one patient with grade III diarrhea.
Conclusions
The combination of bevacizumab and irinotecan, with or without temozolomide, produces objective responses with minimal toxicity in children with recurrent medulloblastoma. Prospective clinical trials are needed to evaluate the efficacy of this strategy.
Keywords: Medulloblastoma, Relapse, Bevacizumab, Irinotecan, Temozolomide
Introduction
Over the last 20 years, the prognosis for medulloblastoma, the most common malignant brain tumor of childhood, has improved significantly. Treatment for the current standard-risk group includes maximal surgical resection followed by craniospinal irradiation (CSI) with a boost to the involved field and chemotherapy with vincristine, platinum-, and nitrosourea-containing regimens. This approach has resulted in 5-year event-free (EFS) and overall survival (OS) rates greater than 80 % in the standard-risk group [1]. Unfortunately, up to 20 % of children experience tumor progression or recurrence, for which no curative therapies exist [2].
Some factors that correlate with prognosis at the time of initial diagnosis include: metastatic disease, young age (less than 3 years old), and evidence of residual disease following surgical debulking [3-5]. Eberhart et al. have shown that moderate to severe anaplasia in medulloblastoma cells is also associated with poor survival [6]. Additionally, large-scale genomic changes such as isochromosome 17q, with loss of chromosome 17p and gain of 17q, present in 30–50 % of medulloblastomas, are associated with poor prognosis [7, 8].
In the USA, the incidence of recurrence or progression for standard-risk medulloblastoma is 20 %. The incidence of recurrence in the posterior fossa (PF) alone was reported in one study as 6.4 %: 25 (8 %) patients developed medulloblastoma recurrence outside the PF; 16 (5.1 %) cases recurred in both the PF and distant sites [1]. Most relapses occurred within 36 months of diagnosis, with a median time to recurrence of 1.2 years. Recurrence in the PF occurred at a median of 0.8 years; recurrence in the spine or cerebrospinal fluid (CSF) occurred at a median of 1.2 years from diagnosis. Sixty percent of non-metastatic (M0) patients who relapsed recurred simultaneously within and outside the PF. Patients staged with metastatic disease (M+) at diagnosis were more likely to recur in the spine or CSF. The 5-year cumulative incidence of recurrence in the spine or CSF increased from 14.8 % in M0 patients to 40.2 % in patients with M3 disease [9].
Historically, medulloblastoma relapse has been associated with a 2-year post-recurrence OS of approximately 25 %. Bowers et al. reported a 5-year probability of OS of 26.3 % upon recurrence or progression of medulloblastoma. In this study, the only factor associated with prolonged survival was recurrence that was restricted to the primary site. Median survival following progression was 1.8 years. Younger patients were more likely than older patients to experience recurrence, and patients with diffuse meningeal disease at recurrence had a dismal prognosis [10].
To date, there is no single treatment that has demonstrated superior results and hence no standard of treatment in the setting of disease relapse. Salvage treatment modalities include re-resection, re-irradiation [11], high-dose chemotherapy (HDC) with autologous stem cell rescue (ASCR) [12], and treatment with biologically targeted agents, such as angiogenesis inhibitors [13]. Ongoing phase I/II clinical trials are investigating the role of promising chemotherapeutics and biologically targeted therapies for medulloblastoma relapse.
Our group has previously reported on the results of treatment of two patients with relapsed medulloblastoma with bevacizumab, irinotecan, ±temozolomide. The first patient was progression-free for >30 months. The second patient had a near complete response that was sustained for 18 months. The regimen was well tolerated with minimal toxicity and provided prolonged progression-free survival in these two patients [13]. In this retrospective review, we report on the response and toxicity profile of salvage therapy with bevacizumab, irinotecan, ±temozolomide in nine patients with medulloblastoma relapse.
Methods
Participants
Our analysis of the medical records of patients with relapsed medulloblastoma treated with bevacizumab (BV) and irinotecan (IRI), with or without temozolomide (TMZ), from 2006 until 2011 was approved by the institutional IRB. Children were less than 18 years old at initial diagnosis. Initial treatments included maximal surgical resection followed by chemotherapy with or without CSI. Medulloblastoma recurrence or progression was demonstrated by MRI of the brain and/or spine, and/or positive CSF cytology (Tables 1 and 2).
Table 1.
Patient characteristics
| All patients | |
|---|---|
| Variables | N |
| Age (years at diagnosis) | |
| <3 | 3 |
| 3–18 | 6 |
| Gender | |
| Male | 6 |
| Female | 3 |
| Chang staging at diagnosis | |
| M0 | 4 |
| M+ | 5 |
| Histology | |
| Desmoplastic | 1 |
| Classic | 4 |
| Anaplastic/large cell | 4 |
| Craniospinal irradiation | |
| Yes | 8 |
| No | 1 |
| High-dose chemotherapy/autologous stem cell rescue | |
| No | 8 |
| Yes (triple) | 1 |
| Number of previous failed regimens | |
| 1 | 6 |
| 2 | 1 |
| 3 | 2 |
M0 no evidence of metastasis, M+ evidence of metastatic disease
Table 2.
Treatment description
| Pt | Sex | Age | Histology | Prev Rx | Regimens | Rx | Doses | Rx duration (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 4 | Classic | 1 | ACNS0331 | BIT | BV 10 mg/kg q2weeks IV IRI 125 mg/m2
q2weeks IV TMZ 200 mg/m2 × 5 days qMo |
6 |
| 2 | M | 6 | LCA | 1 | ACNS0332 | BIT | BV 10 mg/kg q2weeks IV IRI 125 mg/m2
q2weeks IV TMZ 200 mg/m2 × 5 days qMo |
14a |
| 3 | M | 2 | LCA | 3 | ACNS0334 CSI Cis-retinoic acid |
BIT | BV 10 mg/kg q2weeks IV IRI 125 mg/m2
q2weeks IV TMZ 135 mg/m2 × 5 days qMo |
15b |
| 4 | F | 2 | Desmo | 2 | ACNS0334 4 drug metronome |
BIT | BV 10 mg/kg q2weeks IV IRI 125 mg/m2
q2 weeks IV TMZ 100 mg/m2 × 5 days qMo |
11 |
| 5 | M | 7 | LCA | 3 | ACNS0331 ICE 4 drug metronome |
BI | BV 10 mg/kg q2weeks IV IRI 125 mg/m2
q2 weeks IV |
6 |
| 6 | F | 4 | LCA | 1 | CCG99701 | BIT | BV 10 mg/kg q2W IV IRI 125–150 mg/m2
q2weeks IV TMZ 150 mg/m2 × 5 days qMo |
55b |
| 7 | M | 2 | Classic | 1 | CCG99701 | BI | BV 10 mg/kg q2weeks IV IRI 125 mg/m2
q2 weeks IV |
18 |
| 8 | F | 6 | Classic | 1 | ACNS0331 | BITV | BV 10 mg/kg q2weeks IV IRI 90 mg/m2 × 5 days PO q3 weeks TMZ 100 mg/m2 × 5 days qMo Vincristine 1.5 mg/m2 |
10 |
| 9 | M | 6 | Classic | 1 | CCG-A9961 | BITV | BV 10 mg/kg q2weeks IV IRI 90 mg/m2 × 5 days PO q3 weeks TMZ 100 mg/m2 × 5 days qMo Vincristine 1.5 mg/m2 |
4 |
Pt patient number, Prev previous, Rx treatment, M male, F female, qMo every month, IV intravenous, PO oral, LCA large cell/anaplastic, Desmo desmoplastic, BI bevacizumab+irinotecan, BIT BI+temozolomide, BITV BIT+vincristine, BV bevacizumab, IRI irinotecan, TMZ temozolomide, CSI craniospinal irradiation, ICE ifosfamide+carboplatinum+etoposide
Alive with disease progression
Alive and progression-free
Prior treatment regimens included the Children’s Oncology Group (COG) standard-risk medulloblastoma protocol ACNS0331 (three patients), high-risk medulloblastoma protocol ACNS0332 (one patient), CCG-99701 (two patients), infant medulloblastoma protocol ACNS0334 (two patients), and CCG-A9961 (one patient). Eight of nine patients received prior CSI; one of nine patients received HDC with ASCR. Six patients had one prior relapse treatment, one patient received two prior regimens, and two patients received three prior regimens. Treatments utilized at time of previous recurrences included HDC (one patient), a four-drug metronomic regimen (two patients), cis-retinoic acid for 1 year (two patients), and/or combination chemotherapy with ifosfamide, carboplatin, and etoposide (one patient) and CSI (one patient). Five patients received treatment with BV, IRI, and TMZ as follows: BV 10 mg/kg IV with IRI 125–150 mg/m2 IV every 2 weeks and TMZ starting at 100–200 mg/m2/day (median dose, 150 mg/m2) orally for five consecutive days every month. Two patients received BV 10 mg/kg IV with IRI 125–150 mg/m2 IV every 2 weeks. These heavily pretreated patients did not receive TMZ due to provider preference because of previous delays in bone marrow recovery. Two of nine patients received 15 mg/kg BV IV, IRI 90 mg/m2 orally for five consecutive days, TMZ 100 mg/m2/day for 5 days, and vincristine 1.5 mg/m2 IV, each administered every 21 days (Table 2).
Response
Response was assessed by measuring maximal change in bidirectional area of tumor on MRI of the brain and spine, performed at the time of initiation of treatment with BV, IRI, and TMZ, and every 3 months thereafter until tumor progression. Enhancing tumor was measured on post-contrast T1 images or FLAIR images. T2 FLAIR images were used for assessment of tumor response in patients with more subtle leptomeningeal disease. Tumor was measured as per COG criteria as follows: complete response (CR): complete disappearance of tumor signal on MRI; partial response (PR): ≥50 % reduction in bi-directional tumor area on MRI; stable disease (SD): 0 to <25 % increase or decrease in tumor area on MRI; progressive disease (PD): ≥25 % increase in tumor area on MRI. Patients were also considered to have PD if they had clear neurologic deterioration secondary to tumor, even without definitive evidence of tumor growth on MRI of the brain and spine. Assessment of the safety of salvage therapy was determined according to the NCI common toxicity criteria using the CTCAE version 4.0 guidelines.
Results
The objective tumor response, which included six PRs at 3 months following initiation of therapy, was 67 %. Three patients had SD, but no CRs were seen at 3 months. Conversely, 3 months into therapy, none of the patients had PD. By 6 months, the total objective response (CR+PR) was 55 %, including three patients (33 %) with CR and two (22 %) with continuing PR. At 6 months, one patient (11 %) had SD and three (33 %) developed PD. Patients who responded to therapy with BV, IRI, ±TMZ, did so by 3 months (Fig. 1). The median PFS following initiation of therapy was 11 months (95 % confidence interval (CI), 4–18 months). The median OS following therapy with BV, IRI, ±TMZ was 13 months (95 % CI, 6–not estimable months).
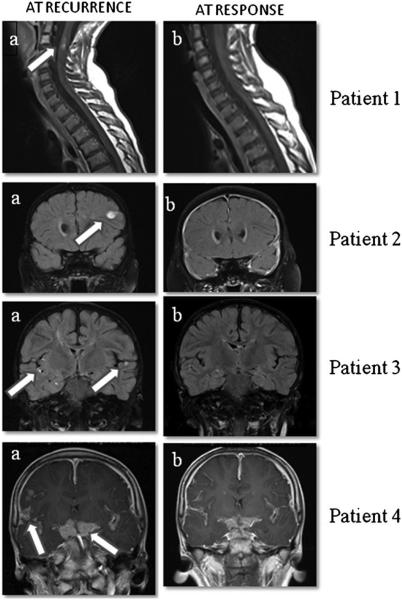
Fig. 1.
MRI images from four patients at the time of medulloblastoma recurrence and at response. Sagittal T1 post-contrast images of the cervicothoracic spine of patient 1 (top row) demonstrate enhancing intramedullary drop metastases (arrow in a at the level of C2–3, that resolve (b) after treatment. Coronal post-contrast T2 FLAIR images of patient 2 (second row) demonstrate an enhancing lesion in the left frontal lobe at recurrence (arrow in a) that resolves (b) after treatment. Coronal post-contrast T2 FLAIR images of patient 3 (third row) demonstrate leptomeningeal metastases in the bilateral sylvian fissures (arrows in a) and adjacent to the right hippocampus at recurrence that resolve (b) after treatment. Post-contrast T1 coronal images of patient 4 (fourth row) demonstrate thick leptomeningeal metastases in the suprasellar cistern and sylvian fissures (arrows in a) at the time of recurrence that significantly improve (b) following treatment
Seven out of nine patients with relapsed medulloblastoma developed PD. The two patients who did not receive TMZ developed PD at 6 and 18 months. The median time (±standard deviation) to death from stopping treatment with BV, IRI, ±TMZ was 2.5 (±0.8)months. Following tumor progression, treatment was stopped in six of seven patients due to families’ preference for palliation and symptom control. Following tumor progression, one patient has demonstrated SD for 8 months while receiving treatment with BV and metronomic agents. Currently, three patients are alive. Two remain progression-free at 15 and 55 months (Table 3). A multivariate analysis failed to identify significant variables such as age or number of prior treatment regimens as prognostic of response to salvage therapy with BV, IRI, ±TMZ.
Table 3.
Treatment responses
| Regimen | Time to progression (months) |
Response (3 months) |
Response (6 months) |
Status | Death from stopping regimen (months) |
Follow-up (months) |
|
|---|---|---|---|---|---|---|---|
| Patient | |||||||
| 1 | BIT | 6 | SD | PD | Dead | 2 | |
| 2 | BIT | 14 | PR | CR | AWDa | N/A | 20 |
| 3 | BIT | N/A | PR | PR | AWD PF | N/A | 15 |
| 4 | BIT | 11 | PR | CR | Dead | 2 | |
| 5 | BI | 6 | PR | PD | Dead | 4 | |
| 6 | BIT | N/A | SD | SD | AWD PF | N/A | 55 |
| 7 | BI | 18 | PR | CR | Dead | 3 | |
| 8 | BITV | 10 | PR | PR | Dead | 3 | |
| 9 | BITV | 4 | SD | PD | Dead | 2 |
Bl bevacizumab+irinotecan, BIT BI+temozolomide, BITV BIT+vincristine, N/A not applicable, AWD alive with disease, CR complete response, PD progressive disease, PF progression free, PR partial response, SD stable disease
Progressed after 14 months of salvage therapy, but remains AWD for 18 months
Toxicity
Treatment with BV, IRI, ±TMZ was well tolerated by seven of nine patients allowing them to have a subjective excellent quality of life including regular school attendance and participation in social activities with peers. Toxicities included three patients with grade III neutropenia, two with grade III thrombocytopenia, one with grade III elevation of AST and ALT, one patient with grade III diarrhea, and two patients with grade II diarrhea. Due to grade III thrombocytopenia within 2 months of salvage therapy, TMZ was reduced from 170 to 140 mg/m2 in one patient, and from 200 to 160 mg/m2 in another patient with grade III neutropenia within 4 months of starting therapy. One patient developed grade II mucositis. One patient developed invasive infections including two episodes of cellulitis around the gastrostomy tube site, one episode of grade II colitis, one episode of grade IV colitis, and one event of neutropenic fever. Other reported toxicities included grade I pancreatitis, fatigue, alopecia, epistaxis, and new-onset seizures in a patient with leptomeningeal disease. One patient developed grade I proteinuria. Another developed cavernous malformations of the brain and spine 9 months following the end of salvage therapy. We did not observe any episodes of intracranial bleeding or thromboembolism.
Discussion
The biology of medulloblastoma recurrence remains poorly understood. It is therefore not surprising that the effective treatment of relapsed disease is controversial and challenging. Here, we report the results of our experience in treating nine children with relapsed medulloblastoma with a combination of BV, IRI, ±TMZ. Our hypothesis was that combination therapy with agents that target different aspects of tumor biology would be more effective against progressive or relapsed disease than treatment with a single agent that targets a narrow spectrum of mechanisms of carcinogenesis. Overall, our study demonstrated an early objective tumor response of 67 % in relapsed medulloblastoma. No children were removed from therapy due to toxicity. Most toxicities were hematologic and gastrointestinal. Importantly, three children with relapsed medulloblastoma remain alive between 15 and 55 months following salvage therapy. Two are progression free. These results suggest an important role for combination chemotherapy in medulloblastoma relapse and await the results of larger clinical trials.
Since medulloblastoma is known to be radiosensitive at diagnosis, others have examined the role of re-irradiation in relapse. For recurrent CNS malignancies including medulloblastoma, prior studies have reported median survivals of 11.5–17.3 months following re-treatment with external beam irradiation [14, 15]. A more recent retrospective analysis of 13 patients who underwent at least one course of re-irradiation for local or disseminated medulloblastoma reported 5-year EFS and OS after first recurrence of 48 and 65 %, respectively. The median CSI dose at the time of re-irradiation was 35 Gy, with a median cumulative dose of 89 Gy. Of those without gross evidence of disease at re-irradiation, 83 % had no evidence of disease (NED) at a median follow-up of 92 months. In contrast, only 14 % of those with evidence of residual disease at re-irradiation had NED, with a median follow-up of 7 months [11]. Patients in our cohort did not undergo re-irradiation since most presented with widely disseminated disease in a short interval from the time of initial irradiation.
Prior publications have reported objective radiographic responses of recurrent medulloblastoma to re-treatment with chemotherapeutic agents, including cisplatinum, carboplatinum, and cyclophosphamide. Others have documented responses to treatment with oral etoposide [16]. In one study, five of eight patients with locally recurrent medulloblastoma responded to treatment with 2 cycles of oral etoposide at a dose of 50 mg/m2/day for 21 days. The regimen was well tolerated, but the median duration of response or stable disease was 6 months. Furthermore, this cohort consisted entirely of patients with non-disseminated disease. And none of the responses were sustained [17]. In contrast, the patients in our study were higher risk due to the presence of M+ disease in >50 % at diagnosis and in all patients at recurrence.
HDC followed by ASCR has also demonstrated efficacy against relapsed medulloblastoma. A recent review suggests that age, extent of disease at the time of recurrence, and a history of prior irradiation are important factors in the outcome of HDC [18]. Finlay et al. demonstrated that children with recurrent brain tumors, especially those with minimal residual disease following tumor resection, can be salvaged with HDC followed by ASCR [19]. Unfortunately, this regimen was associated with a mortality rate of 10–15 %. A study of younger patients with relapsed medulloblastoma who were salvaged with HDC reported 36-month EFS and OS post-ASCR of 34 and 46 %, respectively [20]. Patients who relapsed having received upfront CSI had an inferior outcome (25 % EFS at 36 months), compared to those who received chemotherapy alone prior to relapse (43 % EFS at 36 months). The results of a more recent study examining the efficacy of HDC with carboplatin, thiotepa, and etoposide for treatment of recurrent medulloblastoma are far less encouraging. The 10-year EFS and OS were 24 % with a median OS of 26.8 months [12]. Toxicity was substantial in all patients, and three patients (12 %) died due to toxicity associated with HDC. Considering all eight clinical trials in children older than 3 years, reviewed by Gajjar et al., only 15–20 % of children who received irradiation prior to relapse could be salvaged with HDC [18]. Therefore, HDC appears to be a treatment strategy beneficial to a small subgroup of patients with relapsed medulloblastoma.
A phase II COG trial of TMZ in children with recurrent CNS tumors reported an overall response rate of 16 %, with a CR in 1 and PR in 3 of 25 medulloblastoma patients [21]. A recent study of eight children with recurrent brain tumors, treated with TMZ at a dose of 150 mg/m2/day, for 5 days in a 28-day cycle, reported only one CR and PFS of 26 months, in a patient with medulloblastoma [22]. Thus, TMZ exhibits measurable activity as a single agent against recurrent medulloblastoma.
Evidence suggests that combination therapy with oral TMZ and etoposide is also well tolerated and effective against recurrent medulloblastoma. TMZ was administered up to the maximal tolerated dose of 150 mg/m2 daily for 5 days; etoposide was administered at 50 mg/m2 daily for 10 days. Two of 12 evaluable patients demonstrated a treatment response by MRI (one CR and one PR). Seven of the 12 patients had SD; three had PD [23]. In comparison, we had higher risk patients, based on the number of prior recurrences and evidence of metastasis in all our patients, at the time of salvage.
Irinotecan (IRI) is a topoisomerase I inhibitor that has demonstrated activity against pediatric brain tumor xenografts, including medulloblastoma [24]. Due to promising preclinical results, Turner et al. examined the single-agent activity of IRI against 22 high-grade pediatric brain tumors, including three recurrent medulloblastomas or primitive neuroectodermal tumors. Two patients experienced SD for 19 months; the third patient had PD. All three died of disease [25]. A subsequent COG phase II trial treated 181 patients with treatment-refractory solid tumors, including 27 patients with recurrent medulloblastoma. Patients received irinotecan at 50 mg/m2 for 5 days every 21 days. The overall response rate was 5 %. Four of 25 patients with recurrent medulloblastoma demonstrated a PR (16 %) [26]. This suggests clinical efficacy of IRI in the treatment of recurrent medulloblastoma.
Molecularly targeted therapies are also being studied in the setting of relapsed disease. One example is the Sonic Hedgehog (SHH) pathway inhibitor GDC-0449, an orally administered drug that binds the smoothened receptor and prevents downstream SHH signaling, which is active in up to 30 % of human medulloblastomas. An initial report of 13 children with refractory medulloblastoma treated with GDC-0449 demonstrated few side effects, without any grade 4 toxicities. Responses included two children with confirmed SHH activation in their tumors: one remains on-study without progression after 391 days of follow-up; the other progressed 6 months into therapy with GDC-0449 [27].
Angiogenesis is one of the hallmarks that distinguish high-from low-grade brain tumors. Increased microvascular density (MVD) has been reported in medulloblastoma, compared to surrounding normal cerebellum [28]. However, inter-tumor variability was high, and MVD failed to correlate with metastatic status or patient survival [29]. A subsequent study found expression of numerous angiogenic factors, including VEGF165, PDGF-A, and VEGF-B, in 93 % of human medulloblastomas [30]. We have previously identified increased angiogenesis by IHC and upregulation of Vegfa, Flt1 (Vegfr1), and Hbegf in a Shh-activated, Pten-deficient mouse model of medulloblastoma [31]. Given the findings of increased expression of angiogenic factors in human tissues and mouse models of medulloblastoma, inhibition of the VEGF signaling pathway represents a potential option for treatment of recurrent medulloblastoma where few effective options exist.
Bevacizumab (Avastin, Genentech, San Francisco, CA) is a monoclonal antibody against VEGFA. Based on its ability to improve peri-tumoral edema and PFS in patients with recurrent glioblastoma multiforme (GBM), it has been FDA-approved for use in patients with GBM recurrence [32]. A report has described an adult with a multiply-recurrent medulloblastoma who exhibited a sustained CR to treatment with single agent BV (5 mg/kg IV every 14–21 days) for 7–8 months. Increased frequency of BV administration produced a partial response in a cervical spine lesion within 3 months, before the patient died of causes unrelated to his tumor [33]. Unfortunately, single-agent therapy with BV does not appear to significantly prolong OS in other patients with a recurrent brain tumor [32].
Metronomic therapy that targets angiogenesis in recurrent brain tumors has previously been reported [34]. In the setting of trying to delay CSI following HDC, use of metronomic chemotherapy has been reported in ten infants with medulloblastoma. Treatment consisted of daily etoposide, cyclophosphamide, and TMZ, alternating with celecoxib and isotretinoin. Eight infants, including six with metastatic disease, exhibited SD at a mean of 20 months from diagnosis. Two of ten evaluable patients had PD [35]. More recently, metronomic therapy with BV, thalidomide, celecoxib, etoposide, fenofibrate, and cyclophosphamide along with intraventricular therapy with etoposide and liposomal cytarabine was used in 16 patients with recurrent embryonal brain tumors. For seven of eight patients with relapsed medulloblastoma in the study, OS and EFS were 100, 85, and 68 % at 6, 12, and 24 months after the start of therapy, respectively [36]. Unfortunately, the patient numbers were small, and numerous patients in these studies underwent re-resection and/or irradiation in addition to metronomic therapy. These interventions as well as the use of intrathecal chemotherapy in relapse distinguish these patients from the patients in our report and make comparisons difficult.
Ongoing clinical trials are examining the toxicity and efficacy of BV in combination with other cytotoxic therapies in patients with recurrent brain tumors, including medulloblastoma. We recently reported the results of treatment of two patients with refractory/recurrent medulloblastoma with BV plus IRI every 14 days. Responses included SD and PFS for 30 months, as well as a near CR that was sustained for 18 months. The regimen demonstrated minimal toxicity. Both patients exhibited a qualitative subjective good quality of life during salvage therapy [13]. Currently, there is an ongoing clinical trial through Children’s Oncology Group that evaluates the benefit of BV on the backbone of treatment with IRI and TMZ (ClinicalTrials.gov #: NCT01217437).
In summary, we have shown that salvage chemotherapy for relapsed medulloblastoma with BV, IRI, ±TMZ achieved an objective response rate of 55 % at 6 months from the start of therapy. These results suggest an important role for combination therapy with anti-angiogenic, replication-inhibiting, and alkylating agents, even in the setting of a multiply-recurrent brain tumor. Several questions remain to be answered, such as the length of therapy required to maintain a durable response and the potential long-term side effects of this treatment regimen. Additionally, little information is available about use of BV, IRI, ±TMZ, followed by treatments such as HDC with ASCR, to improve response or achieve CR. Part of the difficulty in answering these questions is our lack of understanding of the biology underlying medulloblastoma progression or relapse, and the lack of other durable salvage regimens. Nevertheless, results from our retrospective review are encouraging. We await results from prospective clinical trials in larger patient populations to answer questions about the efficacy and safety of therapy with BV, IRI, ±TMZ in recurrent medulloblastoma.
Acknowledgements
This work was supported by grants from St. Baldrick’s Foundation (R.C.C.), CURE Childhood Cancer Foundation (R.C.C.), Southeastern Brain Tumor Foundation (R.C.C.), and the Emory Egleston Children’s Research Center (R.C.C.)
References
- 1.Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, Bayer L, LaFond D, Donahue BR, Marymont MH, Muraszko K, Langston J, Sposto R. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 2.Torres CF, Rebsamen S, Silber JH, Sutton LN, Bilaniuk LT, Zimmerman RA, Goldwein JW, Phillips PC, Lange BJ. Surveillance scanning of children with medulloblastoma. N Engl J Med. 1994;330:892–895. doi: 10.1056/NEJM199403313301303. [DOI] [PubMed] [Google Scholar]
- 3.Chang CH, Housepian EM, Herbert C., Jr An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93:1351–1359. doi: 10.1148/93.6.1351. [DOI] [PubMed] [Google Scholar]
- 4.Evans AE, Jenkin RD, Sposto R, Ortega JA, Wilson CB, Wara W, Ertel IJ, Kramer S, Chang CH, SL Leikin, et al. The treatment of medulloblastoma. Results of a prospective randomized trial of radiation therapy with and without CCNU, vincristine, and prednisone. J Neurosurg. 1990;72:572–582. doi: 10.3171/jns.1990.72.4.0572. [DOI] [PubMed] [Google Scholar]
- 5.Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, Allen JC, Stevens KR, Stanley P, Li H, Wisoff JH, Geyer JR, McGuire-Cullen P, Stehbens JA, Shurin SB, Packer RJ. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17:832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 6.Eberhart CG, Kepner JL, Goldthwaite PT, Kun LE, Duffner PK, Friedman HS, Strother DR, Burger PC. Histopathologic grading of medulloblastomas: a pediatric oncology group study. Cancer. 2002;94:552–560. doi: 10.1002/cncr.10189. [DOI] [PubMed] [Google Scholar]
- 7.Pan E, Pellarin M, Holmes E, Smirnov I, Misra A, Eberhart CG, Burger PC, Biegel JA, Feuerstein BG. Isochromosome 17q is a negative prognostic factor in poor-risk childhood medulloblastoma patients. Clin Cancer Res. 2005;11:4733–4740. doi: 10.1158/1078-0432.CCR-04-0465. [DOI] [PubMed] [Google Scholar]
- 8.Rossi MR, Conroy J, McQuaid D, Nowak NJ, Rutka JT, Cowell JK. Array CGH analysis of pediatric medulloblastomas. Genes Chromosome Cancer. 2006;45:290–303. doi: 10.1002/gcc.20292. [DOI] [PubMed] [Google Scholar]
- 9.Yao MS, Mehta MP, Boyett JM, Li H, Donahue B, Rorke LB, Zeltzer PM. The effect of M-stage on patterns of failure in posterior fossa primitive neuroectodermal tumors treated on CCG-921: a phase III study in a high-risk patient population. Int J Radiat Oncol Biol Phys. 1997;38:469–476. doi: 10.1016/s0360-3016(97)00010-2. [DOI] [PubMed] [Google Scholar]
- 10.Bowers DC, Gargan L, Weprin BE, Mulne AF, Elterman RD, Munoz L, Giller CA, Winick NJ. Impact of site of tumor recurrence upon survival for children with recurrent or progressive medulloblastoma. J Neurosurg. 2007;107:5–10. doi: 10.3171/PED-07/07/005. [DOI] [PubMed] [Google Scholar]
- 11.Bakst RL, Dunkel IJ, Gilheeney S, Khakoo Y, Becher O, Souweidane MM, Wolden SL. Reirradiation for recurrent medulloblastoma. Cancer. 2011;117(21):4977–4982. doi: 10.1002/cncr.26148. [DOI] [PubMed] [Google Scholar]
- 12.Dunkel IJ, Gardner SL, Garvin JH, Jr, Goldman S, Shi W, Finlay JL. High-dose carboplatin, thiotepa, and etoposide with autologous stem cell rescue for patients with previously irradiated recurrent medulloblastoma. Neuro Oncol. 2010;12:297–303. doi: 10.1093/neuonc/nop031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguilera DG, Goldman S, Fangusaro J. Bevacizumab and irinotecan in the treatment of children with recurrent/refractory medulloblastoma. Pediatr Blood Cancer. 2011;56:491–494. doi: 10.1002/pbc.22868. [DOI] [PubMed] [Google Scholar]
- 14.Milker-Zabel S, Zabel A, Thilmann C, Zuna I, Hoess A, Wannenmacher M, Debus J. Results of three-dimensional stereotactically-guided radiotherapy in recurrent medulloblastoma. J Neurooncol. 2002;60:227–233. doi: 10.1023/a:1021184400053. [DOI] [PubMed] [Google Scholar]
- 15.Bauman GS, Sneed PK, Wara WM, Stalpers LJ, Chang SM, McDermott MW, Gutin PH, Larson DA. Reirradiation of primary CNS tumors. Int J Radiat Oncol Biol Phys. 1996;36:433–441. doi: 10.1016/s0360-3016(96)00315-x. [DOI] [PubMed] [Google Scholar]
- 16.Ashley DM, Meier L, Kerby T, Zalduondo FM, Friedman HS, Gajjar A, Kun L, Duffner PK, Smith S, Longee D. Response of recurrent medulloblastoma to low-dose oral etoposide. J Clin Oncol. 1996;14:1922–1927. doi: 10.1200/JCO.1996.14.6.1922. [DOI] [PubMed] [Google Scholar]
- 17.Chamberlain MC, Kormanik PA. Chronic oral VP-16 for recurrent medulloblastoma. Pediatr Neurol. 1997;17:230–234. doi: 10.1016/s0887-8994(97)00098-2. [DOI] [PubMed] [Google Scholar]
- 18.Gajjar A, Pizer B. Role of high-dose chemotherapy for recurrent medulloblastoma and other CNS primitive neuroectodermal tumors. Pediatr Blood Cancer. 2010;54:649–651. doi: 10.1002/pbc.22378. [DOI] [PubMed] [Google Scholar]
- 19.Finlay JL, Goldman S, Wong MC, Cairo M, Garvin J, August C, Cohen BH, Stanley P, Zimmerman RA, Bostrom B, Geyer JR, Harris RE, Sanders J, Yates AJ, Boyett JM, Packer RJ. Pilot study of high-dose thiotepa and etoposide with autologous bone marrow rescue in children and young adults with recurrent CNS tumors. The Children’s Cancer Group. J Clin Oncol. 1996;14:2495–2503. doi: 10.1200/JCO.1996.14.9.2495. [DOI] [PubMed] [Google Scholar]
- 20.Dunkel IJ, Boyett JM, Yates A, Rosenblum M, Garvin JH, Jr, Bostrom BC, Goldman S, Sender LS, Gardner SL, Li H, Allen JC, Finlay JL. High-dose carboplatin, thiotepa, and etoposide with autologous stem-cell rescue for patients with recurrent medulloblastoma. Children’s Cancer Group. J Clin Oncol. 1998;16:222–228. doi: 10.1200/JCO.1998.16.1.222. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson HS, Kretschmar CS, Krailo M, Bernstein M, Kadota R, Fort D, Friedman H, Harris MB, Tedeschi-Blok N, Mazewski C, Sato J, Reaman GH. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children’s Oncology Group. Cancer. 2007;110:1542–1550. doi: 10.1002/cncr.22961. [DOI] [PubMed] [Google Scholar]
- 22.Wang CH, Hsu TR, Wong TT, Chang KP. Efficacy of temozolomide for recurrent embryonal brain tumors in children. Childs Nerv Syst. 2009;25:535–541. doi: 10.1007/s00381-008-0781-7. [DOI] [PubMed] [Google Scholar]
- 23.Ruggiero A, Rizzo D, Attina G, Lazzareschi I, Mastrangelo S, Maurizi P, Migliorati R, Bertolini P, Pastore M, Colosimo C, Riccardi R. Phase I study of temozolomide combined with oral etoposide in children with recurrent or progressive medulloblastoma. Eur J Cancer. 2010;46:2943–2949. doi: 10.1016/j.ejca.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Vassal G, Boland I, Santos A, Bissery MC, Terrier-Lacombe MJ, Morizet J, Sainte-Rose C, Lellouch-Tubiana A, Kalifa C, Gouyette A. Potent therapeutic activity of irinotecan (CPT-11) and its schedule dependency in medulloblastoma xenografts in nude mice. Int J Cancer. 1997;73:156–163. doi: 10.1002/(sici)1097-0215(19970926)73:1<156::aid-ijc24>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 25.Turner CD, Gururangan S, Eastwood J, Bottom K, Watral M, Beason R, McLendon RE, Friedman AH, Tourt-Uhlig S, Miller LL, Friedman HS. Phase II study of irinotecan (CPT-11) in children with high-risk malignant brain tumors: the duke experience. Neuro Oncol. 2002;4:102–108. doi: 10.1093/neuonc/4.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bomgaars LR, Bernstein M, Krailo M, Kadota R, Das S, Chen Z, Adamson PC, Blaney SM. Phase II trial of irinotecan in children with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol. 2007;25:4622–4627. doi: 10.1200/JCO.2007.11.6103. [DOI] [PubMed] [Google Scholar]
- 27.Gajjar AJ, Stewart CF, Ellison DW, Curran T, Phillips P, Goldman S, Packer R, Kun LE, Boyett JM, Gilbertson RJ. A phase I pharmacokinetic trial of sonic hedgehog (SHH) antagonist GDC-0449 in pediatric patients with recurrent of refractory medulloblastoma: A Pediatric Brain Tumor Consortium study (PBTC 25) J Clin Oncol. 2010;28 (suppl; abstr CRA9501) [Google Scholar]
- 28.Li VW, Folkerth RD, Watanabe H, Yu C, Rupnick M, Barnes P, Scott RM, Black PM, Sallan SE, Folkman J. Microvessel count and cerebrospinal fluid basic fibroblast growth factor in children with brain tumours. Lancet. 1994;344:82–86. doi: 10.1016/s0140-6736(94)91280-7. [DOI] [PubMed] [Google Scholar]
- 29.Grotzer MA, Wiewrodt R, Janss AJ, Zhao H, Cnaan A, Sutton LN, Rorke LB, Phillips PC. High microvessel density in primitive neuroectodermal brain tumors of childhood. Neuropediatrics. 2001;32:75–79. doi: 10.1055/s-2001-13872. [DOI] [PubMed] [Google Scholar]
- 30.Huber H, Eggert A, Janss AJ, Wiewrodt R, Zhao H, Sutton LN, Rorke LB, Phillips PC, Grotzer MA. Angiogenic profile of childhood primitive neuroectodermal brain tumours/medulloblastomas. Eur J Cancer. 2001;37:2064–2072. doi: 10.1016/s0959-8049(01)00225-8. [DOI] [PubMed] [Google Scholar]
- 31.Castellino RC, Barwick BG, Schniederjan M, Buss MC, Becher O, Hambardzumyan D, Macdonald TJ, Brat DJ, Durden DL. Heterozygosity for pten promotes tumorigenesis in a mouse model of medulloblastoma. PLoS One. 2010;5:e10849. doi: 10.1371/journal.pone.0010849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14:1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 33.Privitera G, Acquaviva G, Ettorre GC, Spatola C. Antiangiogenic therapy in the treatment of recurrent medulloblastoma in the adult: case report and review of the literature. J Oncol. 2009;2009:247873. doi: 10.1155/2009/247873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kieran MW, Turner CD, Rubin JB, Chi SN, Zimmerman MA, Chordas C, Klement G, Laforme A, Gordon A, Thomas A, Neuberg D, Browder T, Folkman J. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J Pediatr Hematol Oncol. 2005;27:573–581. doi: 10.1097/01.mph.0000183863.10792.d4. [DOI] [PubMed] [Google Scholar]
- 35.Choi LM, Rood B, Kamani N, La Fond D, Packer RJ, Santi MR, Macdonald TJ. Feasibility of metronomic maintenance chemotherapy following high-dose chemotherapy for malignant central nervous system tumors. Pediatr Blood Cancer. 2008;50:970–975. doi: 10.1002/pbc.21381. [DOI] [PubMed] [Google Scholar]
- 36.Peyrl A, Chocholous M, Kieran MW, Azizi AA, Prucker C, Czech T, Dieckmann K, Schmook MT, Haberler C, Leiss U, Slavc I. Antiangiogenic metronomic therapy for children with recurrent embryonal brain tumors. Pediatr Blood Cancer. 2012;59:511–517. doi: 10.1002/pbc.24006. [DOI] [PubMed] [Google Scholar]