Abstract
Mild cognitive impairment (MCI) is the intermediate stage between the cognitive changes of normal aging and dementia. Individuals with MCI show cognitive impairment greater than expected for their age, but otherwise are functioning independently and do not meet the criteria for dementia. MCI is important because it constitutes a high risk group for dementia. Ideally, prevention strategies should target individuals who are not even symptomatic. Indeed, the field is now moving towards identification of asymptomatic individuals who have underlying Alzheimer’s disease (AD) pathology that can be detected by using biomarkers and neuroimaging technologies. To this effect, the Alzheimer’s Association and the National Institute on Aging have developed a new classification scheme that has categorized AD into a preclinical phase (research category), MCI due to AD, and dementia of Alzheimer’s type. On the other hand, there are also ongoing researches to understand high risk groups for non-Alzheimer’s dementia as well.
Keywords: Aging, Mild cognitive impairment, Neuropsychiatric symptoms, Dementia, Dementia of Alzheimer’s type, Cognitive Impairment No dementia, Benign and malignant forgetfulness, Age-associated memory impairment, Age-associated cognitive decline, Mild neurocognitive decline, Age-associated memory impairment, Questionable dementia, Asymptomatic Alzheimer’s disease
Introduction
In the late 1980s, clinical researchers that were investigating aging and dementia noted that some elderly persons were neither demented nor cognitively normal. This means the investigators did not have an a priori hypothesis of defining and characterizing the gray zone between cognitive aging and dementia. Rather, they appear to have made a serendipitous observation of the intermediate stage between the cognitive changes of aging and dementia [1, 2]. The term mild cognitive impairment (MCI) was first used by Reisberg and colleagues of New York University [2]. They defined MCI in terms of the Global Deterioration Scale (GDS). The GDS measures cognitive and functional decline on a scale of 1 (cognitively normal) to 7 (severe dementia) with MCI defined as a GDS score of 3 [2, 3]. Interest in the gray zone between normal cognitive aging and dementia dates back to as early as 1962 at which time Kral described “benign and malignant forgetfulness” [4]. Several other research groups have described similar concepts such as age-associated memory impairment [5], aging-associated cognitive decline [6], mild neurocognitive decline [7], age-associated memory impairment [8], questionable dementia [9], etc. Additionally, the concept of subjective cognitive impairment has also been recently introduced and is defined as a transitional stage between normal cognition and MCI [10]. Further work is needed to empirically validate this construct.
Definition
MCI refers to the gray zone between the cognitive changes of normal aging and very early dementia [11, 12•]. Individuals with MCI show cognitive impairment greater than expected for their age, but otherwise are functioning independently and do not meet the commonly accepted criteria for dementia [1]. The original Mayo Clinic criteria for amnestic MCI are: 1) a memory complaint, preferably corroborated by an informant; 2) impaired memory for age on psychometric testing; 3) normal general cognitive function; 4) intact activities of daily living; 5) not demented [1]. Even though amnestic MCI is the most widely studied and empirically validated construct, the first international consensus on MCI has indicated that there are three additional subtypes [13].
Public Health Significance
The prevention of dementia is a public health priority [14]. In the US alone, Alzheimer’s disease (AD) is projected to afflict as many as 14 million people by the year 2050 [15, 16]. Dementia has several devastating consequences including substantial socioeconomic burdens. Even though life is priceless, the economic impact of AD is quite alarming. In 1993, the average cost of care for a patient with Alzheimer’s dementia in California was estimated to be over $40,000 per year [17]. Therefore, the time honored principles of the prevention of disease and promotion of health are particularly relevant to AD and dementia [18]. The prevention of a disease involves identifying high risk groups; indeed, MCI constitutes a high risk state for dementia, particularly for dementia of Alzheimer’s type [11]. Subjects with MCI constitute a high risk group because they develop dementia at 10% to15% per year as compared to the general population of 1% to 2% [19]. Delaying or preventing the onset of dementia by a mere 1 year alone could translate into 1 million fewer number of cases than predicted by the year 2050 [15]. Ideally, the prevention of disease and promotion of health should target individuals who are not even symptomatic. Indeed, the field of cognitive aging is now moving towards identification of asymptomatic individuals who have underlying AD pathology that can be detected by using biomarkers and neuroimaging technologies. To this effect, the Alzheimer’s Association and the National Institute on Aging (AA-NIA) have developed a new classification scheme that has categorized AD into a preclinical phase (research category) [20•], MCI due to AD [21•], and dementia of Alzheimer’s type [22•]. The ongoing work by the DSM-5 task force is anticipated to address the broader topic of neurocognitive disorders including Alzheimer’s dementia as well as non-AD dementia [23••].
MCI Subtypes
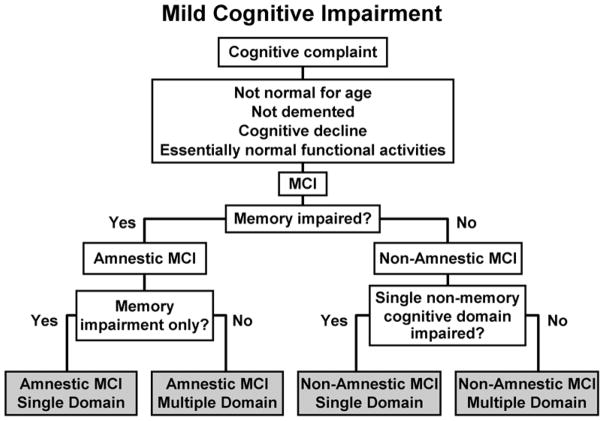
Most of the empirical work regarding MCI has primarily focused on the amnestic type of MCI [1]; however, many investigators have argued that MCI is a heterogeneous entity [13]. Thus, three more MCI subtypes have been proposed [13]. The classification is based on presence or absence of memory domain involvement as well as the number of cognitive domains involved, thus resulting in four categories: 1) MCI-single domain, memory type; 2) MCI-single domain, non-memory type; 3) multi-domain, including memory domain; and 4) multi-domain without memory involvement. Figure 1 depicts the diagnostic algorithm that can be pursued to arrive at a diagnosis of a particular subtype of MCI. The process essentially boils down to two major steps, the first one being establishment of the diagnosis of MCI, while the second pertains to identification of the type and number of cognitive domains involved.
Figure 1.
Flow chart of decision process for making diagnosis of subtypes of mild cognitive impairment.
The putative etiology of MCI should be taken into consideration when considering the potential progression of MCI. For example, recent AA-NIA criteria have defined MCI in terms of etiology, ie, MCI due to AD [21•] is considered to be the prodromal stage of Alzheimer’s dementia. In contrast, the other subtypes emphasizing impairments in non-memory domains, such as comportment-executive function and visuospatial skills, may have a higher likelihood of progressing to a non-AD dementia, such as frontotemporal dementia in the former or dementia with Lewy bodies in the case of the latter [24]. Therefore, the combination of clinical subtypes and putative etiologies may be useful in some people in predicting the ultimate type of dementia to which these diseases may progress.
The Epidemiology of MCI
The main focus of this section is to summarize the prevalence and incidence of MCI as well as the conversion rate of MCI to dementia.
The incidence of MCI ranges from 1% to 6% per year while prevalence estimates range from 3% to 22% per year [25–28••]. The factors that may account for this variability can be attributed to sampling and measurement bias [29]. The former pertains to issues of study design and sampling, eg, recruiting research participants by using an advertisement can introduce non-respondent/volunteer bias [29] while the latter pertains to variability in the measurement of MCI. One good example to illustrate measurement bias would be studies that retrofit MCI criteria into a cohort. The ideal research design that is well-suited to compute epidemiological indices such as prevalence and incidence would be a population-based study that prospectively employs the operational criteria of MCI in elderly individuals [30, 31].
One of the first population-based studies that estimated the prevalence of MCI subtypes was that of the Cardiovascular Health Study. As the name implies, this cohort was assembled to examine cardiovascular risk factors [32]; hence, the investigators retrofitted the criteria for amnestic and multi-domain MCI to the cohort and reported an overall prevalence of MCI to be 22%, with amnestic MCI accounting for 6% and multi-domain MCI representing 16% [32]. An Australian research group estimated prevalence in a probability sample of elderly individuals in the age range of 60- to 64-years-old; they reported a much smaller prevalence rate than the Cardiovascular Health Study, ie, 3.8% and 3.1% for MCI and aging-associated cognitive decline, respectively [33]. Even though their study design was optimal, limiting the sample to the relatively younger age group is likely to have biased their findings toward underestimation of the prevalence of MCI.
The Prevalence and Incidence of MCI Differs by Sex
Recently, the population based Mayo Clinic Study of Aging reported that both the prevalence [30] and incidence [28••] of MCI differ by sex. The investigators followed 1,450 cognitively normal persons, aged 70 to 89, forward in time for a median of 3.4 years. They reported that 296 of the study participants developed incident MCI. Thus the overall age- and sex-standardized incidence rate of MCI was 6.36% and the incidence rate was higher in men (7.24%) than women (5.73%). The incidence of a-MCI was 3.77 and that of non-amnestic MCI was 1.47 %. Additionally, the incidence rate of amnestic MCI was also higher in men than women (4.39% and 3.25% respectively). Similarly, the incidence of non-amnestic MCI was higher in men (2.00%) than in women (1.09%). The incidence of MCI in subjects with ≤ 12 years of education was two times higher than in participants with >12 years of education (2.03% vs 1.02%).
“Conversion” Rates of MCI
There are several studies that have estimated the progression rate of MCI to dementia [3, 34–36]. Their findings vary depending upon the study design and measurement instrument utilized [29]. For example, researchers from Harvard University recruited study participants via advertisement. They then prospectively followed the cohort of subjects with MCI and reported a conversion rate of 6% per year [36] whereas a recent multi-center randomized, double blind, placebo-controlled clinical trial reported a conversion rate of 16% per year [34]. Prior to that, Mayo Clinic and other researchers have also reported a conversion rate in the range of 10% to 15% [1]. Hence, the rather smaller rate reported by the Harvard group could be attributed to non-respondent/volunteer bias [29]. One important point that all studies highlight is that individuals with MCI develop dementia at a higher rate than the general population. It is this consistent finding that makes MCI a potential target for clinical trials.
One topic of debate and discussion is the “instability” of the MCI construct [27, 37]. Larrieu and colleagues reported a reversion rate (ie, from MCI back to normal) to be as high as 40% over 2 to 3 years of follow-up. However, they defined MCI based on only one single memory measurement, ie, Benton Visual Retention Test [38]. A recent international consensus panel on MCI did emphasize the importance of progressive decline rather than entirely relying on poor performance at a cross-sectional point in time, which may help to minimize the “instability” of the construct [13].
Clinical Evaluation of MCI
Clinical Vignette
A right-handed male patient aged 72 years presents with forgetfulness for recent events and future engagements. Family members and close friends also notice these changes. The patient feels that the onset of these symptoms is rather of insidious onset and gradually progressing over a period of 2 to 3 years. Otherwise, he is living independently and has no difficulty carrying out activities of daily living, such as handling finances, cooking, and driving. He denies profound depression, stress, or other complicating medical issues. He requests an appointment with a physician in order to determine if his memory problem should be pursued further. The clinical evaluation, ie, meticulous history and physical examination including bed side cognitive screening using the Short Test of Mental Status was suggestive of cognitive impairment but not severe enough to warrant the diagnosis of dementia. Hence, a clinical diagnosis of MCI is made. Investigations including psychometric testing and magnetic resonance imaging (MRI) are ordered. The neuropsychological testing confirms the clinical diagnosis. Furthermore, it reveals memory impairment, particularly on measures of learning and delayed recall beyond what is expected to be normal for age; however, other cognitive domains such as language and visuospatial skills are relatively intact. MRI of the head reveals mild hippocampal atrophy. The above clinical scenario is probably indicative of amnestic MCI. The patient is becoming slightly more forgetful, and this is noticeable to his family and friends. The most salient feature of the history concerns forgetfulness of insidious onset that gradually progressed over a year or so. All other cognitive domains, ie, language, comportment-executive function, visuospatial skills were intact. The individual is functioning independently. This likely represents an early disease process involving the medial temporal lobe since meaningful information could no longer be stored in an efficient manner, nor is it recalled well.
There are four variables that the clinician should bear in mind when collecting clinical data from a patient with suspected cognitive impairment: 1) exploring cognitive domains; 2) day-to-day functioning; 3) disease course; and 4) neuropsychiatric symptoms.
Cognitive Domains
Sometimes, it may be helpful for the clinician to bear in mind the major cognitive domains while eliciting history, ie, inquire about memory, language, visuospatial, executive-comportment function, and human face- and object-recognition networks [39]. Such an approach will ensure whether cognitive domains other than memory are significantly impaired in the patient. For example, after inquiring about recent events, the clinician may ask about any loss of sense of direction, such as getting lost in one’s neighborhood or having difficulty finding the bathroom in one’s own house.
Day-to-Day Functions
In addition to gathering clinical data on cognitive domains, the clinician should make sure that the patient is presently functioning independently. This is accomplished by inquiring about instrumental activities of daily living such as balancing a checkbook or making travel reservations.
Temporal Course of the Illness
Finally, the clinician should determine the temporal profile of the illness. If the memory problem is of insidious onset and gradual progression, then one should think of possible underlying neurodegenerative processes whereas if there has been a rather acute onset of cognitive changes then vascular or infectious contributions may be considered.
From a cognitive domain perspective, the clinician should inquire about forgetfulness for recent events and future engagements (memory domain), problems of comprehension and expression (language), problems with sense of direction, and behavior changes such as disinhibited behavior or apathy. One may want to ask about major events such as severe weather or other widely discussed political, cultural, or sporting events, depending on the patient’s cultural background. The clinician can get an idea about the patient’s baseline interests and hobbies from the patient and family and make inquiries based on recent experiences. For example, if the patient is a known sports fan, then inquiring about recent games might be informative. If the patient maintains an interest in current events and politics, then inquiring about recent happenings may be helpful. Also, the clinician should inquire about recent important engagements, such as a family gathering or a critical doctor appointment, that were missed due to forgetfulness.
Corroborating data from the family is helpful, not only in determining comparison with baseline function of the patient, but also in validating the history provided by the patient. Patients with memory problems may give little detail about recent events and tend to be vague.
Physical Examination
The physical examination often renders important clinical data regarding the etiology of cognitive problems. For instance, rigidity, bradykinesia, and tremor in an individual complaining of “memory” problems could be indicative of Parkinson’s disease or any other Parkinsonian syndrome. Aphasia and right hemiparesis in a right-handed person presenting with acute “memory” complaint could be indicative of left-sided cerebrovascular accident. Fever, nuchal rigidity, and seizure could be suggestive of Herpes Simplex encephalitis in someone presenting with subacute memory loss.
Mental Status Examination
The mental status examination is a critical part of the clinical evaluation which plays an important role in the diagnostic process. This evaluation also aids the clinician in considering the need for more detailed neuropsychological evaluations. The clinician can use any of the standard bedside tests, such as the Mini-Mental State Examination or the Kokmen Short Test of Mental Status [40]. The clinician has to be familiar with the limitations of these tests as well. These tests use the learning of three or four words, with a relatively short recall interval. The Short Test of Mental Status has the advantage of assessing learning by taking into account the number of trials the subject requires to learn the four words accurately [41].
It is important that bedside mental status examinations screen for problems with attention or language. If observation of affect is suggestive of significant depression or anxiety, the mental status examination should be augmented by screening for psychiatric symptoms using a psychiatric inventory such as the Geriatric Depression Scale, Hamilton Depression/Anxiety Rating Scale, Beck Depression/Anxiety Inventory, or similar screening instruments.
Investigations
Psychometric Testing
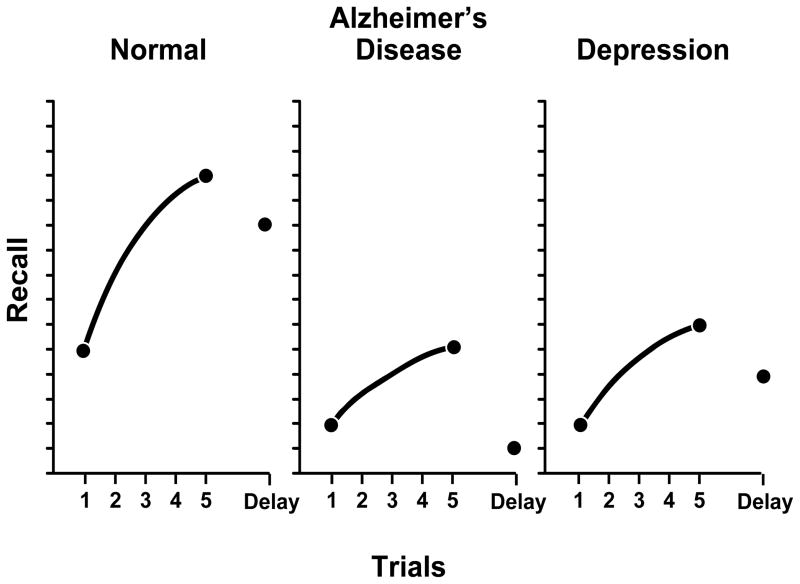
A meticulous history and examination may indicate the need for neuropsychological testing standardized for age and education level. Psychometric testing is essentially an extension of the bedside mental status exam. Both verbal and nonverbal functions should be addressed to effectively evaluate a memory problem. One example, the Rey Auditory Verbal Learning Test, is often used in such patients. In this test, a patient is given 5 trials to learn 15 unrelated words. The score of each trial is recorded, which later will be used to generate the learning curve of the patient. After 30 minutes, the patient is asked to recall the learned material and should be able to recall 50% or more of the material acquired. This test evaluates the patient’s ability to encode learned material and subsequently transfer this from a working memory to recent memory. The learning curves generated from such a test have a characteristic pattern, eg, in patients with AD, the learning curve will be flat, ie, a curve with a slope approaching zero. Alternatively, in cognitively normal individuals, the learning curve will have a positive slope indicating the ability of an individual to learn more with each successive trial. Patients with depression display cognitive inefficiency; such that they are able to learn but require more effort than normal individuals. The learning curve is somewhat flat and falls to the right of a normal curve (Fig. 2).
Figure 2.
Learning curves: Normal vs. AD vs. Depression
Neuroimaging of the Brain
A computerized tomography (CT) or MRI scan of the brain may be done in order to visualize the medial temporal lobe structures that are involved in MCI. An MRI of the brain will be more sensitive in imaging medial temporal lobe structures. A CT scan of the head may have a number of artifacts, since the medial temporal lobe structures are located near the calvarium. Techniques for the detection of MCI or AD through neuroimaging have improved in recent years. Automated imaging techniques have been shown to be highly useful in the evaluation of MCI and AD [42].
In selected cases, one should consider using single-photon emission computed tomography (SPECT) or positron emission tomography (PET) scans. In the early stages of the disease, an MRI of the head may not show any gross abnormality, whereas a decreased blood flow pattern on SPECT or decreased glucose utilization on PET could be noted in areas of the brain reported as normal on MRI. Since these tests are expensive, they should be reserved for special circumstances.
Molecular Imaging
Pittsburgh Compound B (PiB) imaging
Several agents have been developed in recent years but PiB is the most intensely investigated. PiB is a tracer used in PET scans that labels fibrillar amyloid and allows for the assessment of cerebral amyloid burden in living persons; therefore, it may prove to be very useful in imaging amyloid in normal cognitive aging and dementia [43]. More information is necessary about PiB imaging before it is used in clinical settings. Several groups around the world are investigating PiB among selected convenience samples, and most of these studies are cross-sectional associations. Current data suggest that 20% to 30% of cognitively normal subjects have positive PiB scans while about 60% of MCI subjects have PiB-positive scans [44]. A few studies have begun examining the serial properties of PiB imaging over time [45, 46]. Investigators from Washington University in St. Louis, Missouri reported the outcome of following 159 cognitively normal persons that had PiB imaging at baseline and were followed for a mean (SD) of 2.4 (1.3) years [47]. Twenty-three subjects progressed from 0 to 0.5 on the Clinical Dementia Rating Scale, and nine of these subjects developed dementia of Alzheimer’s type. They concluded that preclinical AD as measured by PiB may predict symptomatic AD. However, given the small AD events and shorter follow-up of their study, larger studies with longer follow-up duration are indicated.
Treatment of Mild Cognitive Impairment
As the focus of AD research moves toward prevention, numerous clinical trials designed to determine pharmacotherapy options for MCI are underway [48].
Clinical trials involving MCI patients are promising because such trials will likely uncover new information in the detection and intervention of the disease while it is still in a transitional clinical stage. Trials currently underway or recently completed are outlined in Table 1. The therapeutic agents being tested are similar to those under consideration for the treatment of AD, namely cholinesterase inhibitors, antioxidants, anti-inflammatories, nootropics, and glutamate receptor modulators. There is no specific medication treatment for MCI. Nonetheless, it is important for a clinician working with MCI subjects to be aware of AD treatment options that may be of some benefit in MCI patients; hence, these medications are discussed below.
Most medications that are used to treat AD are cholinomimetics (reversible inhibitors of acetylcholinesterase enzyme activity), and were developed based on the rationale that the cholinergic system is involved in learning processes. The first FDA-approved cholinomimetic agent was tacrine (Cognex®) [49]. It is rarely used currently because of its hepatotoxic side effect profile. Currently, there are three FDA-approved cholinomimetics: 1) donepezil (Aricept®); 2) rivastigmine (Exelon®); and 3) galantamine (Razadyne®) [50–55]. These medications are not curative but they have been shown to minimize morbidity in AD. These medications may also be helpful in managing some neuropsychiatric and behavioral symptoms in AD patients [53]. Side effects of cholinomimetics can be understood by recalling the multiple systemic functions of the cholinergic system. Through their primary action of increased cholinergic activity, these medications can lead to bradycardia, increased gastric acid secretions, and increased gastrointestinal motility. Further considerations pertain to drug-drug interactions, eg, cholinomimetics are known to interact with some anesthetics.
Memantine (Namenda®) is an FDA-approved N-methyl-D-aspartate receptor antagonist of glutamate activity, used in patients with moderate to severe dementia. Clinical trials to determine the efficacy of memantine in such patients were conducted based on the rationale that glutamate contributes to neurodegenerative disorders by overstimulating the N-methyl-D-aspartate receptor [54, 55].
Apart from the above agents, other medications targeting the monoaminergic neurotransmitter system, such as selective serotonin reuptake inhibitors, adrenergic agents, peptides and nootropics [56], have been considered for symptomatic treatment of cognitive impairment. Limited data have suggested that nicotine treatment may reduce symptoms of MCI, and clinical trials are being conducted to explore this treatment option [57]. Another group that has attracted research interest is the anti-oxidants. At least one randomized, placebo-controlled, double-blind multi-center trial indicates that vitamin E may delay the progression of moderate to severe AD [34]. However, more recent studies have indicated that vitamin E is not efficacious in the treatment or prevention of AD or MCI [58]. Further, a meta-analysis indicated that high dose vitamin E may increase all-cause mortality and thus should be avoided [59].
In review articles, Geda and Petersen, and others [11, 60–62] discussed several clinical trials on MCI, including one conducted by the Alzheimer’s Disease Cooperative Study (ADCS), and other pharmaceutical company-sponsored trials. The ADCS completed a randomized, double-blind, placebo-controlled study involving three arms to assess the safety and efficacy of high dose vitamin E and donepezil [34]. The objective was to assess the safety and efficacy of vitamin E (2000 IU per day) and donepezil (10 mg per day), and was powered to decrease the conversion rate of MCI to AD from the anticipated 45% down to 30% over the course of 3 years. Seven hundred sixty-nine subjects were randomized in the trial and the annual conversion rate from MCI to AD was approximately 16% per year. Over the course of the study, donepezil reduced the risk of progressing to AD for the first 18 months of the trial while vitamin E had no therapeutic effect. The secondary cognitive measures supported the overall group progression rates. No unexpected adverse events were observed. This trial was an important first step in elucidating techniques to delay progression from MCI to AD. Furthermore, a meta-analysis pooled the data from four randomized clinical trials in MCI. There were 3,574 subjects with MCI, of these, 1,784 were treated with cholinesterase inhibitors (CheI) and 1,790 were given placebo. Two hundred seventy-five (15.4%) of those treated with CheI progressed to AD while 366 (20.4%) of those treated with placebo progressed to AD (relative risk [95% confidence interval]=0.75 [0.66–0.87]; p<0.001) [62]. These results indicate that the CheI treatment may have some role in delaying the progression of MCI to AD.
Conclusion
The study of aging and dementia has led to the description of MCI. The construct of MCI, particularly amnestic MCI, has stimulated research as it is a high risk group for dementia. In the past one decade, there is an exponential increase in the epidemiology, clinical, neuroimaging, and interventional research targeting MCI. The field of cognitive aging is now moving towards identification of asymptomatic individuals who have underlying AD pathology that can be detected by using biomarkers and neuroimaging technologies. To this effect, the AA-NIA has developed a new classification scheme that has categorized AD into a preclinical phase (research category), MCI due to AD, and dementia of Alzheimer’s type. The AA-NIA criteria will catalyze the research work to prevent Alzheimer’s dementia. Similarly, non-amnestic MCI may be a precursor of non-Alzheimer’s dementia; thus, several investigations are underway to identify high risk groups for Lewy body dementia and other non-AD dementias.
Acknowledgments
I would like to thank Barbara J. Balgaard for her superb secretarial assistance. Preparation of this chapter was supported by K01 MH68351, U01 AG06786, P50 AG16574, and U01 AG10483.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Reisberg B, Ferris SH, de Leon MJ, Crook T. Global Deterioration Scale (GDS) Psychopharmacol Bull. 1988;24(4):661–3. [PubMed] [Google Scholar]
- 3.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41(7):1006–9. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 4.Kral VA. Senescent forgetfulness: benign and malignant. Can Med Assoc J. 1962;86:257–60. [PMC free article] [PubMed] [Google Scholar]
- 5.Crook T, Bartus RT, Ferris SH, Whitehouse P, Cohen GD, Gershon S. Age-associated memory impairment: proposed diagnostic criteria and measures of clinical change — report of a National Institute of Mental Health work group. Developmental Neuropsychology. 1986;2(4):261–76. doi: 10.1080/87565648609540348. [DOI] [Google Scholar]
- 6.Levy R. Aging-associated cognitive decline. Working Party of the International Psychogeriatric Association in collaboration with the World Health Organization. Int Psychogeriatr. 1994;6(1):63–8. [PubMed] [Google Scholar]
- 7.American Psychiatric Association, American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. Task Force on DSM-IV. [Google Scholar]
- 8.Blackford RC, La Rue A. Criteria for diagnosing age-associated memory impairment: proposed improvements from the field. Developmental Neuropsychology. 1989;5(4):295–306. doi: 10.1080/87565648909540440. [DOI] [Google Scholar]
- 9.Devanand DP, Folz M, Gorlyn M, Moeller JR, Stern Y. Questionable dementia: clinical course and predictors of outcome. J Am Geriatr Soc. 1997;45(3):321–8. doi: 10.1111/j.1532-5415.1997.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 10.Reisberg B, Prichep L, Mosconi L, John ER, Glodzik-Sobanska L, Boksay I, et al. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement. 2008;4(1 Suppl 1):S98–S108. doi: 10.1016/j.jalz.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61(1):59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 12•.Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364(23):2227–34. doi: 10.1056/NEJMcp0910237. This reference is the most up-to-date review, particularly on advances made in the area of biomarkers and neuroimaging research pertinent to MCI and AD. [DOI] [PubMed] [Google Scholar]
- 13.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–6. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 14.Williams JW United States. Agency for Healthcare Research and Quality., Duke University Evidence-based Practice Center. Evidence report/technology assessment. Vol. 193. Rockville, MD: Agency for Healthcare Research and Quality; 2010. Preventing Alzheimer’s disease and cognitive decline. [Google Scholar]
- 15.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88(9):1337–42. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3(3):186–91. doi: 10.1016/j.jalz.2007.04.381. S1552-5260(07)00475-X [pii] [DOI] [PubMed] [Google Scholar]
- 17.Rice DP, Fox PJ, Max W, Webber PA, Lindeman DA, Hauck WW, et al. The economic burden of Alzheimer’s disease care. Health Aff (Millwood) 1993;12(2):164–76. doi: 10.1377/hlthaff.12.2.164. [DOI] [PubMed] [Google Scholar]
- 18.Geda YE, Negash S, Petersen RC. Memory Disorders. In: Agronin ME, Maletta GJ, editors. Principles and Practice of Geriatric Psychiatry. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 19.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 20•.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. This paper describes AA-NIA research criteria for asymptomatic AD. Very useful in a research setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9. doi: 10.1016/j.jalz.2011.03.008. This paper descirbes the criteria for MCI due to AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. This paper describes the AA-NIA criteria for the diagnosis of dementia due to AD. Involvement of memory domain does not have a central place in the diagnostic criteria for dementia of Alzheimer’s type. This is in sharp contracst with the DSM-IV criteria for dementia of Alzheimer’s type. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Ganguli M, Blacker D, Blazer DG, Grant I, Jeste DV, Paulsen JS, et al. Classification of neurocognitive disorders in DSM-5: a work in progress. Am J Geriatr Psychiatry. 2011;19(3):205–10. doi: 10.1097/jgp.0b013e3182051ab4. This is a very important reference in order to understand the ongoing work of the DSM-5 task force on neurocognitive disorders including MCI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molano J, Boeve B, Ferman T, Smith G, Parisi J, Dickson D, et al. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain: a journal of neurology. 2010;133(Pt 2):540–56. doi: 10.1093/brain/awp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63(1):115–21. doi: 10.1212/01.wnl.0000132523.27540.81. 63/1/115 [pii]. [DOI] [PubMed] [Google Scholar]
- 26.Hanninen T, Hallikainen M, Tuomainen S, Vanhanen M, Soininen H. Prevalence of mild cognitive impairment: a population-based study in elderly subjects. Acta Neurol Scand. 2002;106(3):148–54. doi: 10.1034/j.1600-0404.2002.01225.x. [DOI] [PubMed] [Google Scholar]
- 27.Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Le Carret N, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59(10):1594–9. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 28••.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology. 2012;78(5):342–51. doi: 10.1212/WNL.0b013e3182452862. This reference contains the most recent population-based estimates of the incidence of MCI. The paper has reported unexpected differences in incidence of MCI by sex ie, the incidence of MCI is higher in men than in women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32(1–2):51–63. doi: 10.1016/0021-9681(79)90012-2. [DOI] [PubMed] [Google Scholar]
- 30.Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75(10):889–97. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60(10):1385–9. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 33.Kumar R, Dear KB, Christensen H, Ilschner S, Jorm AF, Meslin C, et al. Prevalence of mild cognitive impairment in 60- to 64-year-old community-dwelling individuals: The Personality and Total Health through Life 60+ Study. Dement Geriatr Cogn Disord. 2005;19(2–3):67–74. doi: 10.1159/000082351. [DOI] [PubMed] [Google Scholar]
- 34.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–88. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 35.Tierney MC, Szalai JP, Snow WG, Fisher RH, Tsuda T, Chi H, et al. A prospective study of the clinical utility of ApoE genotype in the prediction of outcome in patients with memory impairment. Neurology. 1996;46(1):149–54. doi: 10.1212/wnl.46.1.149. [DOI] [PubMed] [Google Scholar]
- 36.Daly E, Zaitchik D, Copeland M, Schmahmann J, Gunther J, Albert M. Predicting conversion to Alzheimer disease using standardized clinical information. Arch Neurol. 2000;57(5):675–80. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- 37.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56(1):37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 38.Benton A, Hamsher K. Multilingual Aphasia Examination Manual. Iowa City, IA: University of Iowa; 1978. [Google Scholar]
- 39.Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–52. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 40.Kokmen E, Naessens JM, Offord KP. A Short Test of Mental Status: description and preliminary results. Mayo Clin Proc. 1987;62(4):281–8. doi: 10.1016/s0025-6196(12)61905-3. [DOI] [PubMed] [Google Scholar]
- 41.Tang-Wai DF, Knopman DS, Geda YE, Edland SD, Smith GE, Ivnik RJ, et al. Comparison of the Short Test of Mental Status and the Mini-Mental State Examination in mild cognitive impairment. Arch Neurol. 2003;60(12):1777–81. doi: 10.1001/archneur.60.12.1777. [DOI] [PubMed] [Google Scholar]
- 42.Ferrarini L, Frisoni GB, Pievani M, Reiber JH, Ganzola R, Milles J. Morphological hippocampal markers for automated detection of Alzheimer’s disease and mild cognitive impairment converters in magnetic resonance images. J Alzheimers Dis. 2009;17(3):643–59. doi: 10.3233/JAD-2009-1082. [DOI] [PubMed] [Google Scholar]
- 43.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 44.Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–52. doi: 10.1212/01.wnl.0000228230.26044.a4. 67/3/446 [pii] [DOI] [PubMed] [Google Scholar]
- 45.Engler H, Forsberg A, Almkvist O, Blomquist G, Larsson E, Savitcheva I, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129(Pt 11):2856–66. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- 46.Klunk WE, Mathis CA, Price JC, Lopresti BJ, DeKosky ST. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129(Pt 11):2805–7. doi: 10.1093/brain/awl281. [DOI] [PubMed] [Google Scholar]
- 47.Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66(12):1469–75. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petersen RC. Mild cognitive impairment clinical trials. Nat Rev Drug Discov. 2003;2(8):646–53. doi: 10.1038/nrd1155. [DOI] [PubMed] [Google Scholar]
- 49.Knapp MJ, Knopman DS, Solomon PR, Pendlebury WW, Davis CS, Gracon SI. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer’s disease. The Tacrine Study Group.[see comment] JAMA. 1994;271(13):985–91. [PubMed] [Google Scholar]
- 50.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group.[see comment] Neurology. 1998;50(1):136–45. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 51.Rosler M, Anand R, Cicin-Sain A, Gauthier S, Agid Y, Dal-Bianco P, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial.[see comment][comment][erratum appears in BMJ 2001 Jun 16;322(7300):1456] BMJ. 1999;318(7184):633–8. doi: 10.1136/bmj.318.7184.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised controlled trial. Galantamine International-1 Study Group. BMJ. 2000;321(7274):1445–9. doi: 10.1136/bmj.321.7274.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cummings JL, Schneider L, Tariot PN, Kershaw PR, Yuan W. Reduction of behavioral disturbances and caregiver distress by galantamine in patients with Alzheimer’s disease. A– J Psychiatry. 2004;161(3):532–8. doi: 10.1176/appi.ajp.161.3.532. [DOI] [PubMed] [Google Scholar]
- 54.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348(14):1333–41. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 55.Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–24. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 56.Lockhart BP, Lestage PJ. Cognition enhancing or neuroprotective compounds for the treatment of cognitive disorders: why? when? which? Exp Gerontol. 2003;38(1–2):119–28. doi: 10.1016/s0531-5565(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 57.Hayes TL, Larimer N, Adami A, Kaye JA. Medication adherence in healthy elders: small cognitive changes make a big difference. J Aging Health. 2009;21(4):567–80. doi: 10.1177/0898264309332836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lloret A, Badia MC, Mora NJ, Pallardo FV, Alonso MD, Vina J. Vitamin E paradox in Alzheimer’s disease: it does not prevent loss of cognition and may even be detrimental. J Alzheimers Dis. 2009;17(1):143–9. doi: 10.3233/JAD-2009-1033. [DOI] [PubMed] [Google Scholar]
- 59.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 60.Geda YE, Petersen RC. Clinical Trials in Mild Cognitive Impairment. In: Gauthier S, Cummings J, editors. Alzheimer’s Disease and Related Disorders. 2. London: Martin Dunitz; 2001. [Google Scholar]
- 61.Salloway S, Ferris S, Kluger A, Goldman R, Griesing T, Kumar D, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology. 2004;63(4):651–7. doi: 10.1212/01.wnl.0000134664.80320.92. [DOI] [PubMed] [Google Scholar]
- 62.Diniz BS, Pinto JA, Jr, Gonzaga ML, Guimaraes FM, Gattaz WF, Forlenza OV. To treat or not to treat? A meta-analysis of the use of cholinesterase inhibitors in mild cognitive impairment for delaying progression to Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 2009;259(4):248–56. doi: 10.1007/s00406-008-0864-1. [DOI] [PubMed] [Google Scholar]