Abstract
The mammalian target of rapamycin (mTOR) pathway is associated with castration-resistant prostate cancer (CRPC). Thirty-nine taxane-treated CRPC patients were enrolled in a phase II trial assessing the safety and efficacy of targeted therapy with the mTOR inhibitor, Ridaforolimus (Merck & Co, Inc/ARIAD Pharmaceuticals, Inc). Treatment with Ridaforolimus was generally well tolerated. No objective responses were observed, but some patients experienced disease stabilization. Ridaforolimus may be an option in combination therapy.
Background
Few options are available after taxane-based therapy in men with CRPC. Genetic alterations involving the mTOR pathway have been associated with CRPC development, raising the hypothesis that blocking mTOR signaling may be an effective targeted approach to treatment.
Patients and Methods
In this open-label phase II study, the mTOR inhibitor Ridaforolimus was administered at a dose of 50 mg intravenous once weekly to 38 patients with taxane-treated CRPC. The primary end point was best overall response according to modified Response Evaluation Criteria in Solid Tumors guidelines. Serum prostate-specific antigen levels were prospectively monitored as a biomarker for cancer activity.
Results
No objective responses were observed, but 18 patients (47.4%) had stable disease as their best response. Based on progression-free survival analysis, median time to progression with Ridaforolimus was 28 days (95% confidence interval, 27–29). Eight patients (21.1%) had stable disease as their best overall prostate-specific antigen response. The median number of days from first to last dose was 109.5 days (range, 1–442 days). Ridaforolimus was generally well tolerated, with a safety profile similar to that observed in patients with advanced malignancies. The most common side effects were typically mild or moderate in severity.
Conclusions
Ridaforolimus was generally well tolerated. Treatment did not produce objective responses, but stable disease was observed in some patients with taxane-treated CRPC. Alternative treatment regimens, such as combination therapy with a taxane or in a maintenance treatment paradigm, should be considered for further evaluation in this patient population.
Keywords: Combination therapy, Disease stabilization, Phase II, Prostate-specific antigen, Targeted therapy
Introduction
Prostate cancer is the most common malignancy and the second leading cause of cancer death among men in the United States; in 2010, an estimated 217,730 new cases were diagnosed and 32,050 men were expected to die from the disease.1 Although most cases are diagnosed at a local stage, approximately 4% of patients present with metastatic disease.2 Many others ultimately develop metastases de spite previous surgery, radiation therapy, and androgen deprivation. The progression to castration-resistant prostate cancer (CRPC) marks a clinical acceleration of disease associated with a poor outcome. First-line treatment for CRPC typically consists of 3-week docetaxel in combination with prednisone.3 Other treatment regimens for CRPC patients include 3-week docetaxel and estramustine and 3-week mitoxantrone and prednisone.3 Despite high response rates to these regimens, patients eventually develop progressive disease (PD), with a median time to progression of 6 to 8 months and a median survival time of only 17 to 19 months.4 – 6 Cabazitaxel and abiraterone acetate are recommended as a second-line treatment for CRPC.3 However, no consensus exists regarding the best approach after docetaxel failure; current options include second-line hormonal therapy, use of a taxane, or immunotherapy. There continues to be a need for additional therapies in this setting, with targeted therapy representing a potentially viable option.
Multiple genetic alterations have been associated with the development of CRPC, including loss of the tumor suppressor phosphatase and tensin homolog (PTEN), amplification of protein kinase B (Akt), and, less frequently, mutations of phosphatidylinositol 3-kinase (PI3K).7–9 Preclinical studies show that mammalian target of rapamycin (mTOR) inhibitors suppress proliferation of aggressive CRPC cell lines and also act either additively or synergistically with taxanes and antiandrogens in prostate cancer models.10–17 The mTOR inhibitor RAD001 (everolimus) was shown to reverse Akt-dependent prostate intraepithelial neoplasia in a murine model in which human AKT1 was expressed in the ventral prostate.18 These observations suggest that agents targeting the PI3K/Akt/mTOR pathway might be effective in CRPC and might restore sensitivity to taxanes and/or antiandrogens.
Ridaforolimus (AP23573, MK-8669, formerly deforolimus; Merck & Co, Inc/ARIAD Pharmaceuticals, Inc) is an mTOR inhibitor that has been shown to reduce cancer cell proliferation in both in vitro and in vivo experimental models.19 In phase I trials, Ridaforolimus was well tolerated by patients with advanced malignancies.20,21 Results from these trials provided preliminary evidence of clinical benefit, typically reflected by disease stabilization. The present phase II trial was designed to assess the antitumor activity, safety, and tolerability of once-weekly Ridaforolimus in patients with taxane-treated CRPC.
Patients and Methods
Patients
Male patients aged 18 years or older with histologically documented adenocarcinoma of the prostate were eligible if they were clinically refractory to hormone therapy (orchiectomy or luteinizing hormone-releasing hormone agonist/antagonist) and had progressive disease after a cytotoxic chemotherapy regimen. Eligibility also required metastatic disease with either measurable lesions or prostate-specific antigen (PSA) 5 ng/mL or greater; previous treatment with at least 1 taxane-containing regimen and not more than 3 additional cytotoxic drug regimens; and previous orchiectomy or maintenance of castrate levels of testosterone less than 50 mg/dL by luteinizing hormone-releasing hormone agonist/antagonist therapy. Patients who were not surgically sterile had to agree to use reliable birth control methods for the duration of the study until 30 days after the last dose of study drug. Patients were excluded if they had received previous therapy with rapamycin (a rapamycin analog or tacrolimus) or had ongoing toxicity associated with previous anticancer therapy. Patients were also excluded if they had received previous nonhormonal anticancer therapy or any investigational agent within 4 weeks of the first dose of Ridaforolimus (or within 6 weeks for nitrosourea or mitomycin). For patients treated with antiandrogens, a withdrawal period of at least 4 weeks for flutamide, or 6 weeks for bicalutamide or nilutamide, must have elapsed before study entry.
Study Design
This open-label, nonrandomized, single-arm, phase II trial was conducted at 4 centers in the United States (http://ClinicalTrials.gov identifier: NCT00110188; registration date May 4, 2005). Patients were screened for eligibility within 14 days of receiving their first dose of study medication. Eligible patients received Ridaforolimus once weekly at a fixed dose of 50 mg via a 30-minute intravenous infusion, with each treatment cycle defined as a 4-week period consisting of 4 weekly Ridaforolimus infusions. All patients were scheduled to receive at least 2 cycles of treatment in the absence of early disease progression or unacceptable toxicity and were allowed to continue treatment unless there was evidence of disease progression or other discontinuation criteria (eg, unacceptable toxicity, need for radiation therapy for pain management, grade 3 or 4 hyper-sensitivity, noncompliance with treatment or study procedures, withdrawn consent). Patients with increases in PSA as the only evidence of disease progression were allowed to continue treatment at the discretion of the investigator. Patients were followed for up to 24 months to collect survival data and ensure resolution of any adverse events (AEs). Follow-up visits were scheduled weekly during the treatment period and at 1, 3, 6, 12, 18, and 24 months after treatment completion.
Dose interruption or reduction was allowed in the event of AEs. The dose of Ridaforolimus was delayed for up to 2 weeks in the event of grade 1 or 2 AEs that could not be controlled by optimal supportive care, were associated with intolerable symptoms, or interfered with normal daily activities. In these instances, Ridaforolimus was resumed at a reduced dose of 25 mg weekly. For grade 3 or 4 AEs, the dose of Ridaforolimus was reduced to 25 mg or treatment was discontinued. Drug administration could be delayed for up to 2 weeks in order to allow AE symptoms to decrease to grade 1 or resolve. Increases in dose were not allowed when the dose had been reduced, and treatment was discontinued if further dose interruptions were necessary after dose reduction.
The study was conducted in accordance with the principles of the Declaration of Helsinki and its amendments and in compliance with International Conference on Harmonisation, Good Clinical Practices, and all applicable regulatory guidelines. The protocol, its amendments, and the patient informed consent form were reviewed and approved by an independent ethics committee or institutional review board at each site before patients were allowed to enroll at that site. All patients provided written informed consent before participating in this study.
Efficacy Assessments
The primary efficacy end point for the trial was the best overall response determined at the end of even-numbered cycles, assessed using the modified Response Evaluation Criteria in Solid Tumors 1.0 (RECIST).22 Secondary efficacy end points included time to tumor progression, duration of response, progression-free survival (PFS), overall survival (OS), and quality-of-life assessments. An additional end point was the best overall PSA response. Baseline PSA values were measured at screening (before the first dose of Ridaforolimus) and at the end of each treatment cycle; changes were measured at the beginning of every cycle. Results are reported according to the most recent consensus guidelines (Prostate Cancer Clinical Trials Working Group 2).23 All known sites of disease were assessed using appropriate radiologic procedures (computed tomography scan, magnetic resonance imaging, and/or bone scan) within 28 days of the first dose of study medication and by physical examination within 21 days for palpable lesions. Patients without documented disease progression at the 1-month follow-up visit continued to have disease assessments every 2 months until PD was documented or another anticancer therapy was initiated.
Safety Assessments
Patients were monitored throughout the study for the occurrence of AEs, which were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3, and characterized according to their relationship to study medication by the investigator. Safety assessments also included physical examinations, vital signs, 12-lead electrocardiogram, chest x-ray, slit-lamp ophthalmologic examinations, and clinical laboratory testing. The safety evaluations were repeated when patients discontinued study treatment and at the 1-month follow-up visit.
Statistical Analyses
Given the absence of established phase II trial designs in the CRPC patient population, the sample size for this study was primarily based on clinical rather than statistical considerations. With a null hypothesis that the response rate is 5% (ie, treatment is not effective), a sample size of 30 evaluable patients was chosen to provide a statistical power of 0.82 for detecting a 22% response rate in a 1-sided Fisher's exact test at a significance level of 0.05.
The overall response rate was determined for all patients and in the subset of evaluable patients who received at least 2 cycles of study drug and completed at least 1 response evaluation or otherwise discontinued early because of disease progression. Prostate-specific antigen responses were evaluated in patients with baseline PSA of 5 ng/mL or greater. The Kaplan-Meier method was used to estimate time-to-event parameters, including time to disease progression, duration of response, PFS, and OS. All statistical tests were performed using 2-sided tests controlling for type I error at the 0.05 level. Safety parameters were evaluated in all patients who received at least 1 dose of Ridaforolimus. Adverse events were coded according to body system and preferred term using the Medical Dictionary for Regulatory Activities (MedDRA, version 8.1) and then analyzed using descriptive statistics.
Results
Patient Baseline Characteristics and Disposition
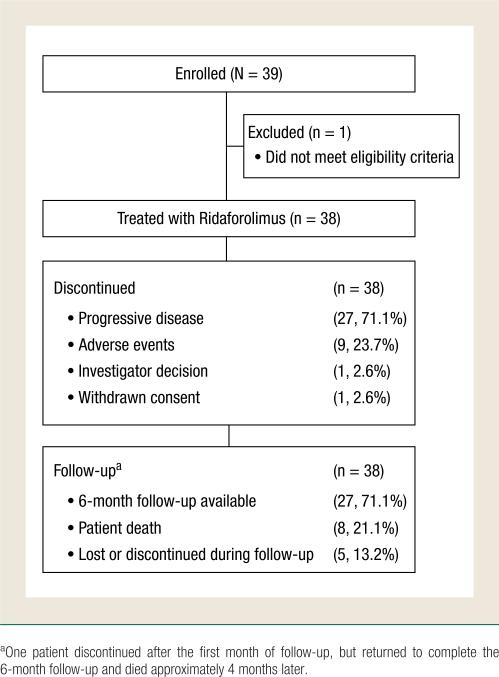
A total of 39 patients were enrolled and 38 received treatment with Ridaforolimus; 1 patient was not treated because he had an Eastern Cooperative Oncology Group performance status greater than 2 and did not meet eligibility criteria (Figure 1). Patient demographic and baseline clinical characteristics are presented in Table 1. Most patients in the study cohort were white (34, 89.5%) and included 23 patients (60.5%) 65 years or older. All patients had metastatic disease, including 35 patients (92.1%) with bone metastases and 16 (42.1%) with lymph node metastases. All patients had received previous chemotherapy; other previous treatments included surgery (36, 94.7%), radiation therapy (25, 65.8%), hormone therapy (36, 94.7%), antiandrogens (29, 76.3%), and immunotherapy (11, 28.9%). Twenty-seven patients (71.1%) discontinued treatment because of disease progression, 9 patients (23.7%) discontinued because of AEs, 1 patient discontinued because of the investigator's decision based on 3 consecutive rising PSA values, and 1 patient withdrew consent.
Figure 1.
Patient Disposition
Table 1.
Demographic and Clinical Characteristics
| Characteristic | Patients (n = 38) |
|---|---|
| Age, Years | |
| Mean (SD) | 66.5 (8.37) |
| Range | 45–84 |
| Race, n (%) | |
| White | 34 (89.5) |
| Black/African American | 4 (10.5) |
| ECOG Performance Status, n (%) | |
| 0 | 24 (63.2) |
| 1 | 14 (36.8) |
| Gleason Score at Diagnosis, n (%) | |
| 4 | 1 (2.6) |
| ≥6 | 36 (94.7) |
| Unknown | 1 (2.6) |
| Metastatic Sites, n (%) | |
| Bone | 35 (92.1) |
| Lymph Node | 16 (42.1) |
| Lung | 3 (7.9) |
| Liver | 3 (7.9) |
| Other sites | 3 (7.9) |
| Previous Therapy | |
| Surgery | 36 (97.4) |
| Radiation therapy | 25 (65.8) |
| Chemotherapy | 38 (100) |
| Hormone therapy | 36 (94.7) |
| Antiandrogens | 29 (76.3) |
| Immunotherapy | 11 (28.9) |
| Small-molecular targeted therapy | 7 (18.4) |
| Monoclonal antibody therapy | 2 (5.3) |
| Other | 14 (36.8) |
Abbreviation: ECOG = Eastern Cooperative Oncology Group; SD = standard deviation.
Efficacy
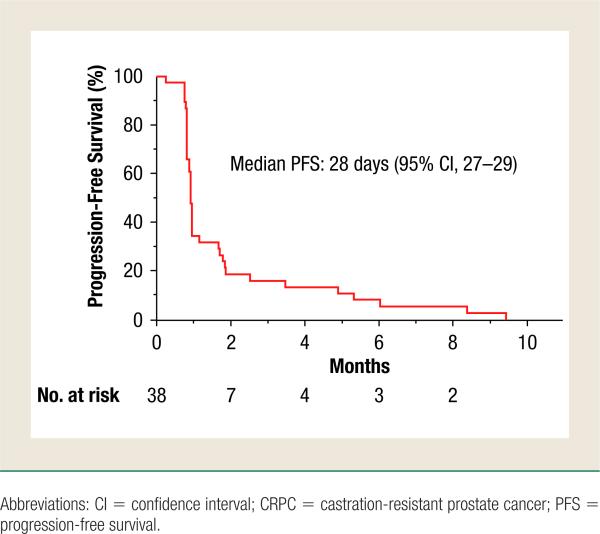
For the primary efficacy end point, best overall response rate, all 38 patients who received at least 1 dose of Ridaforolimus were considered for assessment (Table 2). Fourteen patients (36.8%) could not be assessed for best overall response because they did not have target lesions that met measurable criteria. No objective responses were observed: 18 patients (47.4%) had stable disease as their best overall response, whereas 5 patients (13.2%) demonstrated PD and 1 patient was not evaluable. Patients assessed by computed tomography scan, magnetic resonance imaging, and/or bone scans were determined to have stable disease if the criteria were met after a minimum interval of 8 weeks from the start of treatment. Progression-free survival, analyzed as 1 of the secondary end points, demonstrated a median PFS of 28 days (95% confidence interval, 27–29) in the cohort of patients in this study treated with Ridaforolimus (Figure 2). Of note, for PFS analysis, patients were considered to have progressed based on either radiologic evidence assessed by RECIST, or increases in PSA levels.
Table 2.
Treatment Responses by Modified RECIST Criteria
| Treatment Response | Ridaforolimus (N = 38) Number of Patients, n (%) |
|---|---|
| Complete Response | 0 (0) |
| Partial Response | 0 (0) |
| Stable Disease | 18 (47.4) |
| Progressive Disease | 5 (13.2) |
| Unable to Evaluate | 1 (2.6) |
| Not Assessed | 14 (36.8) |
Abbreviation: RECIST = Response Evaluation Criteria in Solid Tumors.
Figure 2.
Progression-Free Survival of Taxane-Treated CRPC Patients Treated With Ridaforolimus. Kaplan-Meier Analysis of 38 Patients Treated With Ridaforolimus Showed a Median Time to Progression of 28 Days (95% CI, 27–29)
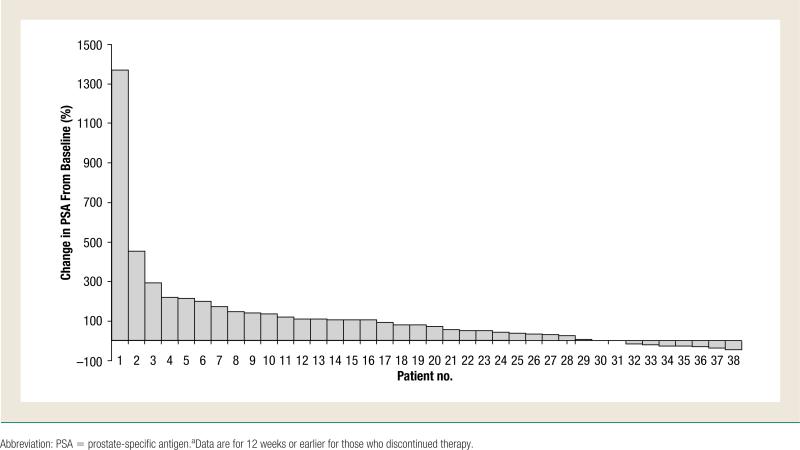
Changes in serum PSA were also analyzed as a secondary efficacy end point for all 38 patients enrolled in the trial with baseline PSA of 5 ng/mL or greater (Table 3). Eight patients (21.1%) had stable disease as their best overall PSA response, whereas 23 patients (60.5%) had PSA progression. PSA responses could not be evaluated or assessed in the other 7 patients. Figure 3 presents a waterfall plot showing the percentage change in PSA levels for each individual patient.
Table 3.
Treatment Response by PSA Levels
| Treatment Response | Ridaforolimus (N = 38) Number of Patients, n (%) |
|---|---|
| PSA Response | 0 (0) |
| PSA Stable Disease | 8 (21.1) |
| PSA Progression | 23 (60.5) |
| Unable to Evaluate | 4 (10.5) |
| Not Assessed | 3 (7.9) |
Abbreviation: PSA = prostate-specific antigen.
Figure 3.
Percentage Change in PSA from Baseline to 12 Weeks,a or the Maximum Decline in PSA That Occurred at Any Point After Treatment for Each Patient
Safety
The median duration of Ridaforolimus treatment, calculated from the day of the first dose to the day of the last dose, was 109.5 days (range, 1–442 days). Ridaforolimus was administered for a median of 16 doses (range, 1–64 doses) and the median cumulative dose was 725 mg (range, 50–3200 mg). Three patients discontinued after 1 cycle of treatment: 1 patient because of PD, 1 patient because of a treatment-related AE (mucosal inflammation), and 1 patient because of a non–treatment-related AE (respiratory failure).
Treatment-emergent AEs (TEAEs) were reported by all 38 patients who received at least 1 dose of Ridaforolimus. The most common were anemia, thrombocytopenia, neutropenia, fatigue, mucosal inflammation, nausea, peripheral edema, rash, diarrhea, and constipation (Table 4). Grade 3 to 4 hematologic (eg, thrombocytopenia, neutropenia) and nonhematologic (eg, fatigue, hypertriglyceridemia, hyperglycemia, hypokalemia) TEAEs were also reported with Ridaforolimus treatment. Potential mTOR inhibitor class effects, including oral-related side effects, skin disorders, metabolic events, and pneumonitis, were analyzed separately. Oral AEs consisted of mucosal inflammation (22, 57.9%), mouth ulceration (8, 21.1%), stomatitis (7, 18.4%), and tongue ulceration (2, 5.3%), all of which were grade 1 or 2 except for 1 patient with grade 3 mucosal inflammation. All oral side effects were considered to be treatment-related, and all events resolved without sequelae. Skin disorders were reported in 26 patients (68.4%), most commonly as rash (17, 44.7%), nail disorder (8, 21.1%), or dry skin (5, 13.2%). Metabolic disorders reported by Ridaforolimus-treated patients included hypertriglyceridemia (8, 21.1%), hyperglycemia (7, 18.4%), and hypercholesterolemia (4, 10.5%). Grade 1 pneumonitis was reported in 1 patient.
Table 4.
Treatment-Emergent Adverse Events Regardless of Causalitya
| Adverse Event | Ridaforolimus (N = 38) Number of Patients, n (%) | ||
|---|---|---|---|
| Total | Grade 3 | Grade 4 | |
| Hematologic | |||
| Anemia | 17 (44.7) | 0 (0) | 0 (0) |
| Thrombocytopenia | 8 (21.1) | 2 (5.3) | 0 (0) |
| Neutropenia | 6 (15.8) | 3 (7.9) | 0 (0) |
| Nonhematologic | |||
| Fatigue | 31 (81.6) | 2 (5.3) | 0 (0) |
| Mucosal inflammation | 22 (57.9) | 1 (2.6) | 0 (0) |
| Rash | 17 (44.7) | 0 (0) | 0 (0) |
| Peripheral edema | 17 (44.7) | 0 (0) | 0 (0) |
| Nausea | 17 (44.7) | 0 (0) | 0 (0) |
| Diarrhea | 16 (42.1) | 1 (2.6) | 0 (0) |
| Constipation | 14 (36.8) | 1 (2.6) | 0 (0) |
| Vomiting | 11 (28.9) | 1 (2.6) | 0 (0) |
| Anorexia | 11 (28.9) | 0 (0) | 0 (0) |
| Arthralgia | 11 (28.9) | 0 (0) | 0 (0) |
| Dyspnea | 10 (26.3) | 1 (2.6) | 0 (0) |
| Cough | 10 (26.3) | 0 (0) | 0 (0) |
| Back pain | 10 (26.3) | 0 (0) | 0 (0) |
| Pyrexia | 9 (23.7) | 0 (0) | 0 (0) |
| Hypertriglyceridemia | 8 (21.1) | 0 (0) | 1 (2.6) |
| Pain in extremity | 8 (21.1) | 1 (2.6) | 0 (0) |
| Nail disorder | 8 (21.1) | 0 (0) | 0 (0) |
| Hyperglycemia | 7 (18.4) | 2 (5.3) | 1 (2.6) |
| Stomatitis | 7 (18.4) | 0 (0) | 0 (0) |
| Decreased appetite | 7 (18.4) | 0 (0) | 0 (0) |
| Upper respiratory tract infection | 7 (18.4) | 0 (0) | 0 (0) |
| Epistaxis | 6 (15.8) | 0 (0) | 0 (0) |
| Hypokalemia | 4 (10.5) | 2 (5.3) | 0 (0) |
| Pruritis | 4 (10.5) | 1 (2.6) | 0 (0) |
Adverse events occurring in 15% of patients or more, and all grade 3 or 4 adverse events.
In 6 (15.8%) of the 9 patients (23.7%) who discontinued treatment as a result of TEAEs, the events were determined by the investigator to be related to study drug. In the other 3 patients, AEs associated with treatment discontinuation were considered unrelated to study medication; specific AEs leading to discontinuation and their relationship to study medication are summarized in Table 5. Two patients had dose reduction (1 with grade 2 and 1 with grade 3 mucosal inflammation) and 2 patients had their dose delayed (1 with grade 2 mucosal inflammation and 1 with grade 2 mouth ulceration). Eight patients who were enrolled in the study died, including 1 patient within 30 days of the last dose of study treatment. All deaths were due to disease progression, and none were considered to be treatment-related. Nine patients (23.7%) experienced serious AEs, but in only 2 cases were these events considered to be at least possibly related to treatment. One patient had grade 4 hypertriglyceridemia that resolved after a dose reduction and delay and grade 3 hyperglycemia that resolved with sequelae after no action was taken. The other patient had grade 1 orthostatic hypotension that resolved without treatment. No safety issues were identified from physical examinations, vital signs, 12-lead electrocardiogram, and slit-lamp ophthalmologic evaluations. One patient had inflammation at the infusion site, which occurred once during cycle 22 and was reported as grade 1 erythema.
Table 5.
Summary of Treatment-Emergent Adverse Events Leading to Study Discontinuation
| Adverse Event | Grade | Relationship to Study Drug | Outcome After Discontinuation |
|---|---|---|---|
| Mucosal Inflammation | 2 | Definitely related | Resolved |
| Thrombocytopenia | 3 | Probably related | Resolved |
| Weight Decrease | 1 | Probably related | Continuing effect |
| Pneumonitis | 1 | Possibly related | Resolved |
| Neutropenia | 3 | Probably related | Resolved |
| Urinary Bladder Hemorrhage | 3 | Probably not related | Resolved |
| Fatigue | 3 | Probably not related | Resolved |
| Pulmonary Embolism | 3 | Not related | Resolved, with sequelae |
Discussion
This phase II study evaluated single-agent therapy with Ridaforolimus in heavily pretreated patients with metastatic CRPC that had progressed after previous taxane-based therapy. Patients enrolled in the trial had received no more than 3 other anticancer therapies, including other cytotoxic chemotherapy, hormonal therapy, antiandrogens, small-molecule targeted therapy, and monoclonal antibodies. The results of this trial demonstrate that Ridaforolimus may delay disease progression in some patients with taxane-treated CRPC. Ridaforolimus was generally well tolerated; the most common side effects—all typically mild or moderate in severity—included fatigue, mucositis, nausea, peripheral edema, rash, and diarrhea. The safety profile observed in this patient population is consistent with that reported in a phase I trial of patients with advanced malignancies, in which Ridaforolimus was administered using a once-weekly regimen similar to the schedule used in the current phase II trial.21 After initiation of this trial, an oral formulation of Ridaforolimus at a dose of 40 mg once daily 5 times per week was selected for testing in a large phase III study in sarcoma patients. The oral dose of Ridaforolimus has not been tested in taxane-treated CRPC patients; therefore, it is yet to be established whether this formulation would produce better treatment responses in this patient population.
Although objective responses were not observed, 8 patients (47%) treated with Ridaforolimus had no disease progression based on RECIST guidelines, and 8 patients (21%) maintained stable PSA levels. Changes in PSA values may depend on multiple factors, including the nature of previous interventions, and therefore may not correlate with radiologic assessments.24 This may be particularly true for cytostatic agents, which may modulate PSA levels independently of influencing tumor cell growth or survival.23 In addition, clinical studies have shown a direct effect of rapamycin on PSA levels in men without prostate cancer.25 Interestingly, evidence suggests that PSA levels are mediated by an mTOR-dependent increase in PSA transcription.26,27 Ridaforolimus may trigger a similar mechanism, which may explain the elevated PSA levels observed in patients treated with Ridaforolimus in this trial. Moreover, mTOR inhibitors differ from cytotoxic chemotherapeutic drugs in that they typically produce cytostatic effects.10 Consequently, mTOR inhibitors are expected to stabilize disease rather than induce early tumor shrinkage. Therefore, clinical benefit, defined as objective response plus stable disease that lasts 16 weeks or longer, may be a more appropriate measure of the antitumor activity of mTOR inhibitors rather than classical responses, based on RECIST, that were developed to evaluate cytotoxic agents.28
Based on available evidence, several potential options can be considered to further evaluate Ridaforolimus in CRPC. Ridaforolimus can be evaluated in combination with a taxane to determine if it restores chemosensitivity. In experimental studies using prostate cancer cells and xenografts, the combination of an mTOR inhibitor with docetaxel was found to be more effective than either agent alone.10,14,16 Preclinical studies showing promising results with Ridaforolimus in combination with a taxane would further support its potential use in combination therapy. In other solid tumors, such as breast cancer and non–small-cell lung cancer, patients with no disease progression after first-line chemotherapy may receive maintenance therapy to extend the time to disease progression. Analogous to sarcoma, Ridaforolimus could be evaluated in a maintenance paradigm, in which patients without disease progression after a fixed number of cycles of taxane-based therapy would be started on Ridaforolimus. Temsirolimus, another mTOR inhibitor, is currently being evaluated in patients with CRPC as maintenance therapy for patients with responses to first-line docetaxel-based therapy.29
Conclusion
Results from this trial demonstrate that treatment with Ridaforolimus (50-mg dose) administered intravenously once weekly did not produce objective responses. Stable disease was observed in some patients with taxane-treated CRPC. Ridaforolimus was generally well tolerated in this patient population, with a safety profile similar to that observed in patients with advanced malignancies. By blocking mTOR signaling in the PI3K/Akt/mTOR pathway, patients treated with Ridaforolimus may achieve only stable disease. Future clinical trials in patients with CRPC could assess the benefit and safety profile of Ridaforolimus in combination with other chemotherapies (eg, taxanes or targeted agents). Trials exploring new treatment paradigms for CRPC may also be considered with mTOR inhibitors, such as Ridaforolimus.
Clinical Practice Points.
Limited treatment options are available for patients with advanced prostate cancer disease because of acquired resistance or intolerance to established therapies.
Molecular studies indicate that genetic aberrations involving phosphatase and tensin homolog, PI3K, and Akt may play a role in the development of CRPC, suggesting that the PI3K/Akt/mTOR pathway may be a therapeutic target.
Results from this trial demonstrate that some patients with taxane-treated CRPC experienced disease stabilization after treatment with Ridaforolimus, an mTOR inhibitor that has demonstrated antitumor activity in patients with solid tumors.
Intravenous administration of Ridaforolimus was safe, with an acceptable toxicity profile in this patient population.
Although objective responses were not seen, the tolerability of Ridaforolimus suggests that maintenance or combination therapy trials may be considered for patients with recurrent or taxane-treated CRPC.
Acknowledgments
This study was sponsored by Merck & Co, Inc and ARIAD Pharmaceuticals, Inc. Medical writing and editorial assistance was provided by Joseph J. Abrajano, PhD, and Kakuri M. Omari, PhD, of Integrus Scientific, a division of Medicus International New York, New York, NY. This assistance was funded by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Whitehouse Station, NJ. The authors were fully responsible for all content and editorial decisions and received no financial support or other compensation related to the development of this report.
Footnotes
This trial is registered at http://ClinicalTrials.gov (NCT00110188; registration date May 4, 2005)
Disclosure
John Loewy and Frank Haluska were employees at Ariad Pharmaceuticals, Inc at the time the trial was conducted and during preparation of the manuscript. Robert J. Amato, George Wilding, Glenn Bubley, and Mitchell E. Gross have no conflicts of interest.
References
- 1.American Cancer Society . Cancer Facts and Figures 2010. American Cancer Society; Atlanta: 2010. [Google Scholar]
- 2.Surveillance Epidemiology and End Results Program [May 29, 2012];SEER Stat Fact Sheets: Prostate. Available at: http://seer.cancer.gov/statfacts/html/prost.html.
- 3.National Comprehensive Cancer Network [May 29, 2012];NCCN clinical practice guidelines in oncology: prostate cancer. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#prostate.
- 4.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–5. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 7.Garcia JA, Danielpour D. Mammalian target of rapamycin inhibition as a therapeutic strategy in the management of urologic malignancies. Mol Cancer Ther. 2008;7:1347–54. doi: 10.1158/1535-7163.MCT-07-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rai JS, Henley MJ, Ratan HL. Mammalian target of rapamycin: a new target in prostate cancer. Urol Oncol. 2010;28:134–8. doi: 10.1016/j.urolonc.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung AS, Wu L, Tannock IF. Concurrent and sequential administration of chemo-therapy and the mammalian target of rapamycin inhibitor temsirolimus in human cancer cells and xenografts. Clin Cancer Res. 2009;15:5389–95. doi: 10.1158/1078-0432.CCR-08-3007. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh PM, Malik SN, Bedolla RG, et al. Signal transduction pathways in androgen-dependent and -independent prostate cancer cell proliferation. Endocr Relat Cancer. 2005;12:119–34. doi: 10.1677/erc.1.00835. [DOI] [PubMed] [Google Scholar]
- 12.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–64. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi T, Shimizu Y, Terada N, et al. Regulation of androgen receptor transactivity and mTOR-S6 kinase pathway by rheb in prostate cancer cell proliferation. Prostate. 2010;70:866–74. doi: 10.1002/pros.21120. [DOI] [PubMed] [Google Scholar]
- 14.Morgan TM, Pitts TE, Gross TS, et al. RAD001 (everolimus) inhibits growth of prostate cancer in the bone and the inhibitory effects are increased by combination with docetaxel and zoledronic acid. Prostate. 2008;68:861–71. doi: 10.1002/pros.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Mikhailova M, Bose S, et al. Regulation of androgen receptor transcriptional activity by rapamycin in prostate cancer cell proliferation and survival. Oncogene. 2008;27:7106–17. doi: 10.1038/onc.2008.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu L, Birle DC, Tannock IF. Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res. 2005;65:2825–31. doi: 10.1158/0008-5472.CAN-04-3137. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Zhu J, Efferson CL, et al. Inhibition of tumor growth progression by antiandrogens and mTOR inhibitor in a PTEN-deficient mouse model of prostate cancer. Cancer Res. 2009;69:7466–72. doi: 10.1158/0008-5472.CAN-08-4385. [DOI] [PubMed] [Google Scholar]
- 18.Majumder PK, Febbo PG, Bikoff R, et al. MTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 19.Mita M, Sankhala K, Abdel-Karim I, et al. Deforolimus (AP23573) a novel mTOR inhibitor in clinical development. Expert Opin Investig Drugs. 2008;17:1947–54. doi: 10.1517/13543780802556485. [DOI] [PubMed] [Google Scholar]
- 20.Mita MM, Mita AC, Chu QS, et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J Clin Oncol. 2008;26:361–7. doi: 10.1200/JCO.2007.12.0345. [DOI] [PubMed] [Google Scholar]
- 21.Hartford CM, Desai AA, Janisch L, et al. A phase I trial to determine the safety, tolerability, and maximum tolerated dose of deforolimus in patients with advanced malignancies. Clin Cancer Res. 2009;15:1428–34. doi: 10.1158/1078-0432.CCR-08-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scher HI, Morris MJ, Kelly WK, et al. Prostate cancer clinical trial end points: “RECIST”ing a step backwards. Clin Cancer Res. 2005;11:5223–32. doi: 10.1158/1078-0432.CCR-05-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chamie K, Ghosh PM, Koppie TM, et al. The effect of sirolimus on prostate-specific antigen (PSA) levels in male renal transplant recipients without prostate cancer. Am J Transplant. 2008;8:2668–73. doi: 10.1111/j.1600-6143.2008.02430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cinar B, De Benedetti A, Freeman MR. Post-transcriptional regulation of the androgen receptor by mammalian target of rapamycin. Cancer Res. 2005;65:2547–53. doi: 10.1158/0008-5472.CAN-04-3411. [DOI] [PubMed] [Google Scholar]
- 27.Sharef S, Jac J, Khan M, et al. Rapamycin for androgen independent prostate cancer (AIPC). 2006 ASCO Annual Meeting Proceedings Part I. J Clin Oncol. 2006;24(18S) Abstract 14584. [Google Scholar]
- 28.Ohorodnyk P, Eisenhauer EA, Booth CM. Clinical benefit in oncology trials: is this a patient-centred or tumour-centred end-point? Eur J Cancer. 2009;45:2249–52. doi: 10.1016/j.ejca.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 29.Emmenegger U, Sridhar SS, Booth CM, et al. A phase II study of maintenance therapy with temsirolimus (TEM) after response to first-line docetaxel (TAX) chemotherapy in castration-resistant prostate cancer (CRPC). J Clin Oncol. 2010;28(15s):TPS246. [Google Scholar]