Abstract
BACKGROUND
Following the release of the 2002 report of the Women’s Health Initiative (WHI) trial of estrogen plus progestin, the use of menopausal hormone therapy in the United States decreased substantially. Subsequently, the incidence of breast cancer also dropped, suggesting a cause-and-effect relation between hormone treatment and breast cancer. However, the cause of this decrease remains controversial.
METHODS
We analyzed the results of the WHI randomized clinical trial — in which one study group received 0.625 mg of conjugated equine estrogens plus 2.5 mg of medroxy-progesterone acetate daily and another group received placebo — and examined temporal trends in breast-cancer diagnoses in the WHI observational-study cohort. Risk factors for breast cancer, frequency of mammography, and time-specific incidence of breast cancer were assessed in relation to combined hormone use.
RESULTS
In the clinical trial, there were fewer breast-cancer diagnoses in the group receiving estrogen plus progestin than in the placebo group in the initial 2 years of the study, but the number of diagnoses increased over the course of the 5.6-year intervention period. The elevated risk decreased rapidly after both groups stopped taking the study pills, despite a similar frequency of mammography. In the observational study, the incidence of breast cancer was initially about two times as high in the group receiving menopausal hormones as in the placebo group, but this difference in incidence decreased rapidly in about 2 years, coinciding with year-to-year reductions in combined hormone use. During this period, differences in the frequency of mammography between the two groups were unchanged.
CONCLUSIONS
The increased risk of breast cancer associated with the use of estrogen plus progestin declined markedly soon after discontinuation of combined hormone therapy and was unrelated to changes in frequency of mammography.
The Women’s Health Initiative (whi) trial of conjugated equine estrogens plus medroxyprogesterone acetate was stopped when health risks were shown to exceed the benefits of combined hormone therapy.1 The incidence of breast cancer was higher in the hormone-therapy group, and the cancers were larger and more advanced2; in addition, the frequency of abnormalities on mammograms and of breast biopsies was increased in the hormone-therapy group.3
After the release of our initial WHI report, in 2002,1 menopausal hormone use decreased considerably in the United States.4,5 Approximately a year later, a substantial drop in the incidence of breast cancer was observed.6,7 Although several countries subsequently reported similar decreases,8–10 others did not,11,12 and the cause of the decline remains controversial.13,14 The temporal relation between decreased use of menopausal hormones and the drop in the incidence of breast cancer suggested causality, although changes in the frequency of mammographic screening as well as other factors were recognized as possible contributors.6,7 When the WHI trial was stopped, more breast cancers were seen in the follow-up period (mean, 2.4 years) among women who had received estrogen plus progestin than in the placebo group,15 and it appeared that hormone therapy hindered the detection of breast cancer.2,3
METHODS
Details of the WHI study have been described elsewhere.16–18 Briefly, postmenopausal women between the ages of 50 and 79 years with an anticipated survival of at least 3 years were eligible. Additional criteria for eligibility were the absence of a history of invasive breast cancer or hysterectomy and a baseline mammogram and clinical breast examination with no suggestion of breast cancer. Women using menopausal hormone therapy were eligible after a 3-month washout period. Subjects were randomly assigned to receive a daily dose of conjugated equine estrogens (0.625 mg) plus medroxyprogesterone acetate (2.5 mg) (Prempro, Wyeth-Ayerst Pharmaceuticals) or an identical-appearing placebo. Randomization was performed by the WHI Clinical Coordinating Center. Study pills were distributed at clinical centers, with the use of a bar-code system to ensure that both staff and participants were unaware of the group assignments. The initial study population consisted of 16,608 women, with the first randomization on October 29, 1993; post-intervention follow-up data were available for 15,387 women, none of whom had received a diagnosis of breast cancer, and these women were therefore included in the postintervention analyses.
We also analyzed data collected for 41,449 women enrolled in another WHI investigation, an observational study with eligibility criteria similar to those of the clinical trial: no history of a hysterectomy or breast cancer and normal findings on a mammogram obtained within 2 years before study enrollment; 25,328 of the participants reported no use of menopausal hormone therapy and 16,121 reported use of estrogen plus progestin at baseline. The first enrollment occurred on September 19, 1994, and data were included through December 31, 2005. These women received no instruction from the WHI regarding menopausal hormone use but were sent a letter outlining the results of the estrogen-plus-progestin trial several weeks after the initial publication of the study findings.
All women in both studies gave written informed consent, and the protocols were approved by the institutional review board at each institution.
DATA COLLECTION
All participants in both studies provided information on demographic characteristics, risk factors for breast cancer, medical and family histories, and lifestyle using standardized self-reporting instruments. Information on past use of hormones was obtained by trained interviewers using structured questionnaires. Ongoing use of hormone therapy in the observational study was determined annually by means of questionnaires. Mammography was performed at WHI clinical centers and at community sites,19 and the reports were coded in accordance with radiologists’ recommendations. Mammographic findings were considered to be abnormal if a physician-directed intervention was recommended (either follow-up after a short interval or further evaluation because of a finding that was suspicious or highly suggestive of cancer).
FOLLOW-UP AND TERMINATION PROCEDURES
Clinical outcomes were reported in a self-administered questionnaire at 6-month intervals for the clinical trial and annually for the observational study. Breast cancers were confirmed by a review of pathology reports performed by local physician adjudicators, followed by adjudication at the Clinical Coordinating Center.3 In the clinical trial, mammograms and breast examinations were required annually, and the administration of study pills was continued only after adherence to the regimen during the previous year had been documented. In the observational study, mammography use was not defined by the protocol. Decisions regarding diagnostic procedures for breast cancer were made by community physicians. Recommendations to perform breast biopsies were based on clinical findings.
The intervention phase of the trial was terminated when an excessive net risk of combined hormone therapy was identified.1 All participants were instructed to stop taking the study pills (active or placebo) immediately, in a letter that was intended for receipt on the day before the trial results were published (July 8, 2002). This letter initiated the postintervention phase of the trial. The originally specified date of trial completion (March 31, 2005) was the end date for the current analyses. During the postintervention phase, participants were followed on the same schedule and were encouraged to continue having annual mammograms. Information on the frequency of mammography and on clinical outcomes was collected for the observational study during a similar time period.
STATISTICAL ANALYSIS
Baseline characteristics of participants in the clinical trial were compared with the use of the chi-square test, Fisher’s exact test, or Student’s t-test. Hazard ratios for the intervention and postintervention phases of the trial were estimated from Cox proportional-hazards analysis, stratified according to age and randomized assignment in the dietary-modification trial, a concurrent WHI clinical trial that participants could also enter.15 Additional analyses of the influence of menopausal hormones on the risk of invasive breast cancer depict linear, time-varying hazard ratios over the period of the intervention and postintervention phases. To determine whether temporal trends differed between the two phases, the equality of the slopes between the two linear estimates was tested. To confirm that the linear fits were reasonable, hazard ratios for the hormone-therapy and placebo groups were determined for sequential 6-month intervals and were visually compared with the linear hazard-ratio estimates. Sensitivity analyses for adherence were conducted after censoring of data for events that occurred 6 months after a woman became nonadherent (i.e., was using <80% of study pills). Time-varying weights, inversely proportional to the estimated probability of continued adherence, were used in the proportional-hazards models to maintain the distribution of the sample characteristics during follow-up.
In the observational study, the comparison of women who used estrogen plus progestin with nonusers, defined at enrollment, was based on calendar time. Since the study did not direct participants to stop using hormone therapy, we fitted a smooth, nonparametric hazard-ratio estimate for the influence of combined hormone therapy on the risk of breast cancer,20 with the results expressed as a test for trend with one degree of freedom. To confirm that the smoothed fit was reasonable, hazard ratios were calculated for sequential 6-month intervals and visually compared with the smoothed estimate. Both parametric and nonparametric regression models were adjusted for age, race or ethnic group, body-mass index, education, smoking status, use or nonuse of alcohol, health status, level of physical activity, presence or absence of a family history of breast cancer, estimated breast-cancer risk based on the Gail model,21 and bilateral oophorectomy and were stratified according to age.
We also performed sensitivity analyses for adherence in the observational study, censoring data for participants who changed their status with respect to estrogen-plus-progestin use from that reported at entry, before July 8, 2002, and comparing the incidence of breast cancer among the women who were regularly using hormones at entry with the incidence among those who were not. Linear, time-varying hazard ratios were calculated, with the use of the same regression-model adjustments as those listed above, for the intervention and postintervention periods, and the equality of slopes between the two linear estimates was tested. Time-varying weights, inversely proportional to the estimated probability of no change in status with regard to the use of estrogen plus progestin, were used to maintain the distribution of the sample characteristics during follow-up. SAS software, version 9.1.3 (SAS Institute), was used.
RESULTS
CLINICAL TRIAL
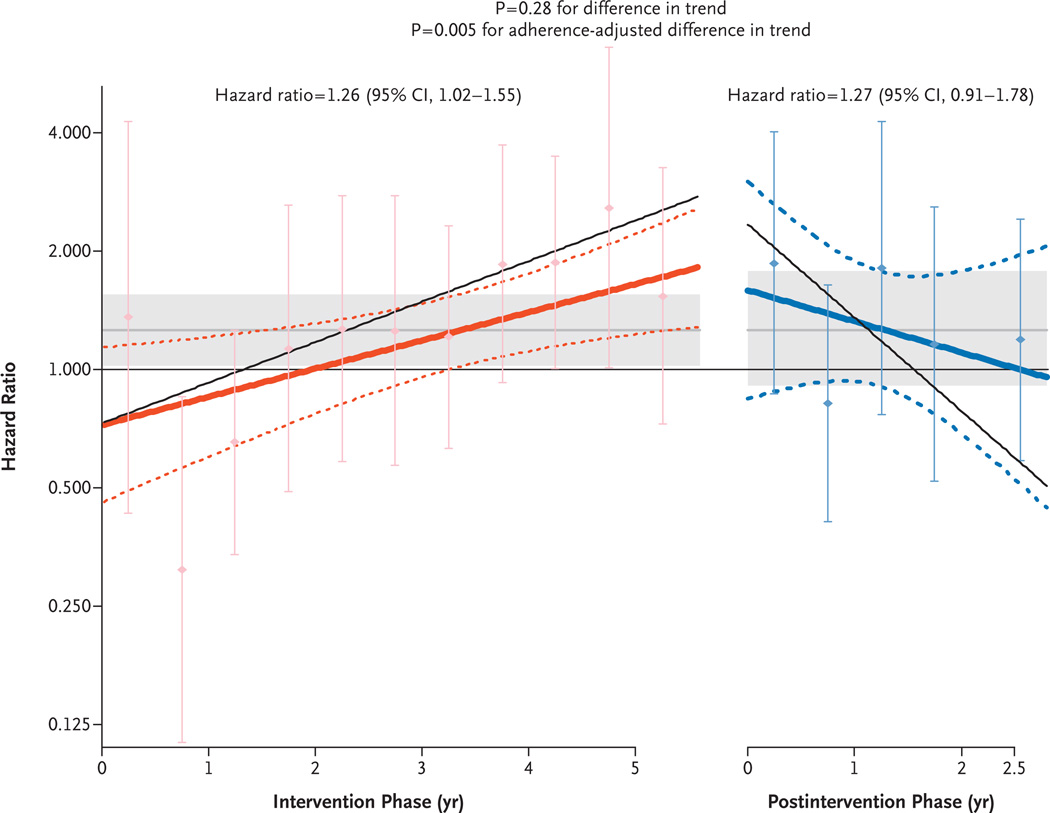
For the 15,387 participants in the clinical trial who had not previously received a diagnosis of invasive breast cancer and for whom any follow-up data during the postintervention phase were available, risk factors for breast cancer were balanced between the two randomized study groups (Table 1) (P>0.10 for all tests of association). As previously reported, the risk of invasive breast cancer was higher in the hormone-therapy group than in the placebo group during the intervention phase (199 cases vs. 150 cases; hazard ratio, 1.26; 95% confidence interval [CI], 1.02 to 1.55).2 The linear, time-varying hazard ratios used to calculate the influence of menopausal hormones on the risk of breast cancer were below 1.00 (but were not significantly different from 1.00) during the first 2 years of the trial, subsequently increased throughout the intervention phase, and decreased during postintervention (Fig. 1). In an intention-to-treat analysis, the difference in the hazard-ratio slopes for the effect of menopausal hormones on the risk of breast cancer during the intervention and postintervention phases was not significant (P = 0.28 for the difference in trend). In a sensitivity analysis that repeated these analyses with an adjustment for adherence, a significant difference in the hazard ratio slopes was detected (P = 0.005 for the difference in trend) (Fig. 1). The adherence-adjusted hazard ratio during the intervention was 1.62 (95% CI, 1.10 to 2.39) as compared with a hazard ratio after the intervention of 1.26 (95% CI, 0.73 to 2.20).
Table 1.
Characteristics of the Participants in the Postintervention Phase of the Clinical Trial.*
| Characteristic | Hormone-Therapy Group (N = 7854) |
Placebo Group (N = 7533) |
|---|---|---|
| no./total no. (%) | ||
| Age at screening | ||
| 50 to 59 yr | 2663 (33.9) | 2506 (33.3) |
| 60 to 69 yr | 3566 (45.4) | 3425 (45.5) |
| 70 to 79 yr | 1625 (20.7) | 1602 (21.3) |
| Race or ethnic group† | ||
| White | 6619 (84.3) | 6351 (84.3) |
| Black | 502 (6.4) | 524 (7.0) |
| Hispanic | 423 (5.4) | 379 (5.0) |
| American Indian | 23 (0.3) | 27 (0.4) |
| Asian or Pacific Islander | 172 (2.2) | 155 (2.1) |
| Unknown | 115 (1.5) | 97 (1.3) |
| Education | ||
| 0 to 8 yr | 174/7812 (2.2) | 160/7475 (2.1) |
| Some high school | 326/7812 (4.2) | 337/7475 (4.5) |
| High-school diploma or GED | 1501/7812 (19.2) | 1501/7475 (20.1) |
| Some schooling after high school | 3090/7812 (39.6) | 2810/7475 (37.6) |
| College degree or higher | 2721/7812 (34.8) | 2667/7475 (35.7) |
| Gail-model estimate of 5-yr breast-cancer risk | ||
| <1.25 | 2605 (33.2) | 2525 (33.5) |
| 1.25 to 1.75 | 2642 (33.6) | 2525 (33.5) |
| >1.75 | 2607 (33.2) | 2483 (33.0) |
| Age at menarche | ||
| ≤11 yr | 1582/7833 (20.2) | 1546/7501 (20.6) |
| 12 to 13 yr | 4252/7833 (54.3) | 4038/7501 (53.8) |
| ≥14 yr | 1999/7833 (25.5) | 1917/7501 (25.6) |
| Interval since menopause | ||
| <5 yr | 1239/7112 (17.4) | 1150/6986 (16.5) |
| 5 to <10 yr | 1378/7112 (19.4) | 1406/6986 (20.1) |
| 10 to <15 yr | 1509/7112 (21.2) | 1474/6986 (21.1) |
| ≥15 yr | 2986/7112 (42.0) | 2956/6986 (42.3) |
| No. of term pregnancies | ||
| Never had term pregnancy or was never pregnant | 795/7821 (10.2) | 759/7504 (10.1) |
| 1 | 632/7821 (8.1) | 611/7504 (8.1) |
| 2 | 1749/7821 (22.4) | 1586/7504 (21.1) |
| 3 | 1876/7821 (24.0) | 1830/7504 (24.4) |
| 4 | 1309/7821 (16.7) | 1316/7504 (17.5) |
| ≥5 | 1460/7821 (18.7) | 1402/7504 (18.7) |
| Age at birth of first child | ||
| Never had term pregnancy or was never pregnant | 795/7116 (11.2) | 759/6768 (11.2) |
| <20 yr | 1020/7116 (14.3) | 1033/6768 (15.3) |
| 20 to 29 yr | 4637/7116 (65.2) | 4395/6768 (64.9) |
| ≥30 yr | 664/7116 (9.3) | 581/6768 (8.6) |
| Period of breast-feeding | ||
| Never breast-fed | 3522/7767 (45.3) | 3400/7445 (45.7) |
| ≤12 mo | 2910/7767 (37.5) | 2760/7445 (37.1) |
| >12 mo | 1335/7767 (17.2) | 1285/7445 (17.3) |
| Use of oral contraceptives | ||
| No | 4396 (56.0) | 4291 (57.0) |
| Yes | 3458 (44.0) | 3242 (43.0) |
| Duration of use | ||
| <5 yr | 1837 (23.4) | 1682 (22.3) |
| 5 to <10 yr | 780 (9.9) | 765 (10.2) |
| ≥10 yr | 839 (10.7) | 792 (10.5) |
| Use of hormone therapy | ||
| Never | 5790/7850 (73.8) | 5594/7530 (74.3) |
| Previous | 1544/7850 (19.7) | 1467/7530 (19.5) |
| Current | 516/7850 (6.6) | 469/7530 (6.2) |
| Previous or current use of unopposed estrogen | ||
| No | 7036/7854 (89.6) | 6740/7532 (89.5) |
| Yes | 818/7854 (10.4) | 793/7532 (10.5) |
| Duration of use | ||
| <5 yr | 612/7854 (7.8) | 609/7532 (8.1) |
| ≥5 yr | 206/7854 (2.6) | 183/7532 (2.4) |
| Previous or current use of estrogen plus progestin | ||
| No | 6437 (82.0) | 6216 (82.5) |
| Yes | 1417 (18.0) | 1317 (17.5) |
| Duration of use | ||
| <5 yr | 987 (12.6) | 942 (12.5) |
| ≥5 yr | 430 (5.5) | 375 (5.0) |
| Recency of hormone-therapy use | ||
| Current | 516/7850 (6.6) | 469/7530 (6.2) |
| Past, <5 yr | 678/7850 (8.6) | 634/7530 (8.4) |
| Past, ≥5 yr | 866/7850 (11.0) | 833/7530 (11.1) |
| No use | 5790/7850 (73.8) | 5594/7530 (74.3) |
| No. of first-degree relatives with breast cancer | ||
| 0 | 6436/7351 (87.6) | 6226/7045 (88.4) |
| 1 | 840/7351 (11.4) | 744/7045 (10.6) |
| ≥2 | 75/7351 (1.0) | 75/7045 (1.1) |
| No. of second-degree relatives with breast cancer | ||
| 0 | 6639/6929 (95.8) | 6408/6674 (96.0) |
| 1 | 280/6929 (4.0) | 263/6674 (3.9) |
| ≥2 | 10/6929 (0.1) | 3/6674 (0.0) |
| History of benign breast disease | ||
| No | 5881/7034 (83.6) | 5839/7022 (83.2) |
| Yes, 1 biopsy | 881/7034 (12.5) | 912/7022 (13.0) |
| Yes, ≥2 biopsies | 272/7034 (3.9) | 271/7022 (3.9) |
| Body-mass index‡ | ||
| <25 | 2384/7818 (30.5) | 2342/7486 (31.3) |
| 25 to <30 | 2757/7818 (35.3) | 2644/7486 (35.3) |
| ≥30 | 2677/7818 (34.2) | 2500/7486 (33.4) |
| Dietary energy | ||
| <1322 kcal/day | 2463/7599 (32.4) | 2435/7295 (33.4) |
| 1322 to 1841 kcal/day | 2591/7599 (34.1) | 2496/7295 (34.2) |
| >1841 kcal/day | 2545/7599 (33.5) | 2364/7295 (32.4) |
| Percent energy from fat | ||
| <29.64% | 2507/7599 (33.0) | 2427/7295 (33.3) |
| 29.64 to 37.19% | 2555/7599 (33.6) | 2503/7295 (34.3) |
| >37.19% | 2537/7599 (33.4) | 2365/7295 (32.4) |
| Physical activity | ||
| ≤3.5 METS/wk | 2444/7103 (34.4) | 2388/7068 (33.8) |
| 3.5 to 12.8 METS/wk | 2309/7103 (32.5) | 2312/7068 (32.7) |
| >12.8 METS/wk | 2350/7103 (33.1) | 2368/7068 (33.5) |
| Alcohol use | ||
| No | 3306/7824 (42.3) | 3187/7510 (42.4) |
| ≤1 drink/day | 3554/7824 (45.4) | 3347/7510 (44.6) |
| >1 drink/day | 964/7824 (12.3) | 976/7510 (13.0) |
| Smoking | ||
| Never smoked | 3920/7773 (50.4) | 3756/7437 (50.5) |
| Past smoker | 3080/7773 (39.6) | 2925/7437 (39.3) |
| Current smoker | 773/7773 (9.9) | 756/7437 (10.2) |
| Use of NSAIDs | 2628/7773 (33.5) | 2569/7437 (34.1) |
Because of rounding, percentages may not total 100. GED denotes general equivalency diploma, METS metabolic equivalents, and NSAID nonsteroidal antiinflammatory drug.
Race or ethnic group was self-reported.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Figure 1. Effects over Time of Estrogen plus Progestin on the Incidence of Breast Cancer in the WHI Clinical Trial.
Time-varying linear hazard ratios and 95% confidence intervals (solid and dashed lines, respectively) are shown for the effect of conjugated equine estrogens plus medroxyprogesterone acetate on the risk of breast cancer as compared with placebo during the intervention and postintervention phases of the study. The shaded areas indicate the overall mean and 95% confidence intervals for the hazard ratios in the intervention and postintervention phases. The I bars show hazard ratios and 95% confidence intervals according to an analysis based on events accumulated at 6-month intervals. The P value of 0.28 for a difference in trend is for the comparison of the hazard-ratio slopes in the two study phases in the primary, unadjusted analysis, and the P value of 0.005 is for a difference in trend from an analysis adjusted for adherence status, with censoring of events that occurred 6 months after a woman became nonadherent (defined as consuming <80% of study pills or starting hormone therapy). The thin solid lines show the adherence-adjusted, time-varying linear hazard ratios.
The annualized incidence of breast cancer was greater in the hormone-therapy group than in the placebo group during the later years of the intervention phase, but the number of breast-cancer diagnoses in the hormone-therapy group decreased by 28% from the last year of the intervention phase to the first year of the postintervention phase (from 48 cases [0.61%] to 34 cases [0.44%]) (Table 2). Mammography use was similar in the hormone-therapy and placebo groups throughout the trial, including the years immediately before and after the intervention ended (Table 2).
Table 2.
Year-to-Year Breast-Cancer Incidence and Means of Detection before and after Intervention Ended, According to Study Group.*
| Variable | Interval before Intervention Ended | Interval after Intervention Ended | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 to <3 yr | 1 to <2 yr | <1 yr | <1 yr | 1 to <2 yr | 2 yr to End of Trial | |||||||
|
Hormone Therapy |
Placebo |
Hormone Therapy |
Placebo |
Hormone Therapy |
Placebo |
Hormone Therapy |
Placebo |
Hormone Therapy |
Placebo |
Hormone Therapy |
Placebo | |
| Breast cancer | ||||||||||||
| No. at risk | 8245 | 7846 | 8113 | 7752 | 7990 | 7643 | 7854 | 7533 | 7647 | 7369 | 7466 | 7190 |
| Mean follow-up (mo) | 11.9 | 12.0 | 11.9 | 11.9 | 11.9 | 11.9 | 11.8 | 11.8 | 11.9 | 11.9 | 5.6 | 5.6 |
| No. of cases | 38 | 23 | 45 | 23 | 48 | 31 | 34 | 27 | 28 | 19 | 17 | 14 |
| Annualized incidence (%)† | 0.46 | 0.29 | 0.56 | 0.30 | 0.61 | 0.41 | 0.44 | 0.36 | 0.37 | 0.26 | 0.49 | 0.42 |
| Hazard ratio (95% CI)‡ | 1.59 (0.95–2.67) | 1.88 (1.14–3.11) | 1.48 (0.94–2.33) | 1.20 (0.72–1.99) | 1.43 (0.80–2.57) | 1.19 (0.59–2.42) | ||||||
| Means of detection — % | ||||||||||||
| Mammogram§ | 84 | 84 | 83 | 82 | 83 | 83 | 80 | 80 | 78 | 78 | 71 | 70 |
| Breast biopsy¶ | 2.7 | 1.5 | 2.8 | 1.5 | 3.0 | 1.6 | 3.3 | 1.8 | 2.2 | 1.4 | 1.5 | 1.1 |
Summary statistics for the intervention phase are based on all 16,608 participants in the trial, and those for the postintervention phase are based on the 15,387 participants for whom postintervention follow-up data were available.
Annualized incidence was computed according to time period, where the total number of events for patients at risk for invasive breast cancer during a particular time period was divided by the total follow-up time. Follow-up time is defined as the time elapsed between the start of each period and the first occurrence of invasive breast cancer or censoring (death, loss to follow-up, or the end of each time period).
Hazard ratios were calculated with the use of a Cox proportional-hazards model for the effect on the risk of breast cancer in the hormone-therapy group as compared with the placebo group, stratified according to age and randomized assignment in the diet-modification trial.
The data on mammograms are based on the trial protocol for scheduled annual mammograms.
The data on breast biopsies were self-reported.
Breast tumors diagnosed in the hormone-therapy group during the postintervention phase were significantly larger than those in the placebo group (mean [±SD] diameter, 1.5±1.2 cm vs. 1.1±0.8 cm; P = 0.03), but other tumor characteristics, including the stage, were similar. During the postintervention phase, mammograms with abnormalities were more common in the hormone-therapy group than in the placebo group (cumulative number, 780 [7.5%] vs. 589 [5.4%]; P<0.001), and breast biopsies were more frequent (cumulative number, 538 [2.3%] vs. 319 [1.4%]; P<0.001).
OBSERVATIONAL STUDY
Participants in the observational study who were taking estrogen plus progestin at study entry, as compared with those who were not, were more likely to be white, younger, more highly educated, and nonsmokers; they were also more likely to have a lower body-mass index, to consume more alcohol, to engage in more physical activity, and to have better self-reported health, no family history of breast cancer, and a lower Gail-model estimate of breast-cancer risk (P<0.001 for all comparisons) (data not shown). The mean duration of hormone therapy in the group of women taking hormones at entry was 6.9±5.4 years.
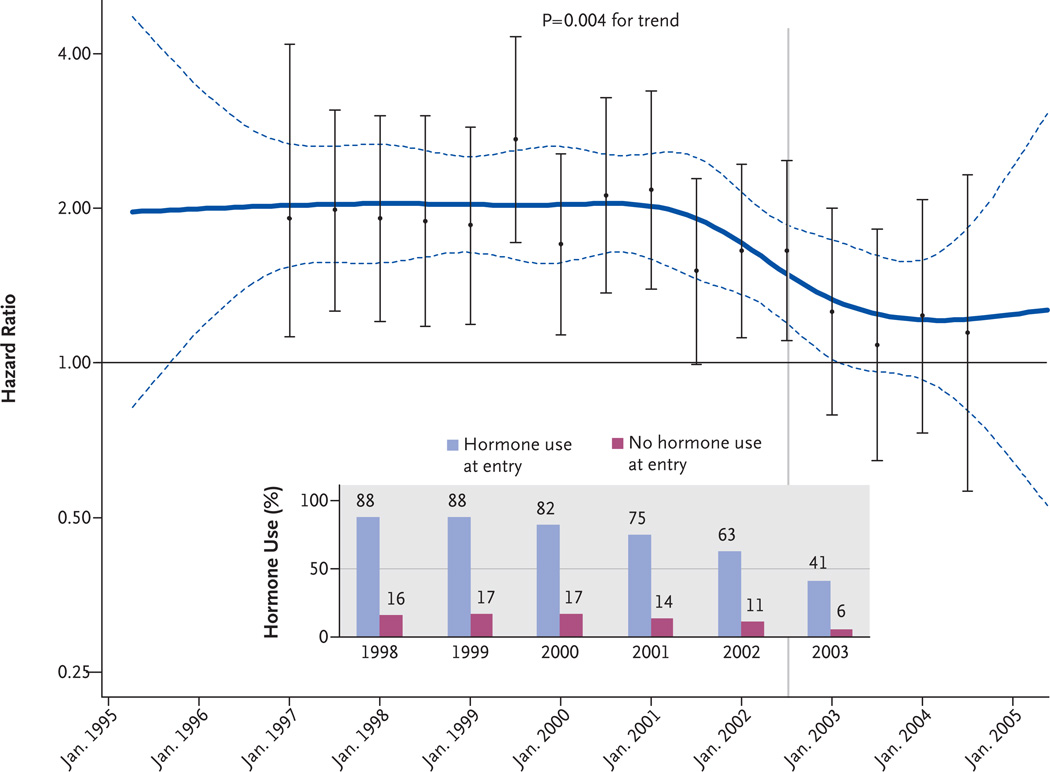
Figure 2 shows a smoothed, multivariable-adjusted, nonparametric estimate of the hazard ratios over time for women taking estrogen plus progestin, which summarizes the sequential, semiannual, multivariable-adjusted hazard ratios. Before 2002, the smoothed estimate of the hazard ratio was nearly 2.00. Between 2002 and the 2004, the hazard ratio decreased significantly (P = 0.004 for trend, for the difference in slope in the two intervals), ultimately reaching a value that did not differ significantly from 1.00.
Figure 2. Effects over Time of Estrogen plus Progestin on the Risk of Breast Cancer in the WHI Observational Study.
Smoothed time-varying, multivariable-adjusted hazard ratios and 95% confidence intervals (solid and dashed blue lines, respectively) for the comparison of participants who were taking estrogen plus progestin at study entry with those who were not are shown with the corresponding multivariable-adjusted hazard ratios and 95% confidence intervals from an analysis based on accumulated events at 6-month intervals (I bars). The variables used in this analysis are listed in the Methods section. The vertical line indicates the announcement of the results of the clinical trial in July 2002. The bar graph shows the year-to-year percentages of participants who were taking hormones and those who were not.
The incidence of breast cancer among the women taking hormones was relatively stable for the years 2000 through 2002 and was consistently greater than that among women not taking hormones (Table 3). Paralleling the year-toyear decrease in hormone use, which began in 2001 and accelerated in 2003 (Fig. 2), a lower annualized incidence of breast cancer began to emerge in the group of women taking hormones (Table 3), with a 43% reduction in the annualized incidence of breast cancer from 2002 to 2003 (122 cases [0.81%] vs. 68 cases [0.46%]). The annual frequency of mammography was lower in the group of women who were not taking hormones than in the group of women who were taking hormones, and this pattern was consistent over time (P<0.01).
Table 3.
Year-to-Year Breast-Cancer Incidence and Means of Detection in the Observational Study According to Whether the Participants Were Using Hormone Therapy.*
| Variable | 2000 | 2001 | 2002 | 2003 | 2004 | 2005† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taking Hormones at Entry |
Not Taking Hormones at Entry |
Taking Hormones at Entry |
Not Taking Hormones at Entry |
Taking Hormones at Entry |
Not Taking Hormones at Entry |
Taking Hormones at Entry |
Not Taking Hormones at Entry |
Taking Hormones at Entry |
Not Taking Hormones at Entry |
Taking Hormones at Entry |
Not Taking Hormones at Entry |
|
| Breast cancer | ||||||||||||
| No. at risk | 15,544 | 24,353 | 15,344 | 23,925 | 15,121 | 23,515 | 14,856 | 23,027 | 14,582 | 23,027 | 12,885 | 18,773 |
| Mean follow-up (mo) | 11.9 | 11.9 | 11.9 | 11.9 | 11.9 | 11.9 | 11.9 | 11.8 | 11.6 | 11.4 | 11.2 | 10.7 |
| No. of cases | 118 | 99 | 106 | 100 | 122 | 117 | 68 | 99 | 60 | 80 | 52 | 64 |
| Annualized incidence (%)‡ | 0.76 | 0.41 | 0.70 | 0.42 | 0.81 | 0.50 | 0.46 | 0.44 | 0.43 | 0.38 | 0.43 | 0.38 |
| Hazard ratio (95% CI)§ | 1.89 (1.40–2.54) | 1.79 (1.32–2.42) | 1.65 (1.25–2.19) | 1.18 (0.84–1.67) | 1.28 (0.87, 1.88) | 1.23 (0.82, 1.83) | ||||||
| Hazard ratio (95% CI) with adjustment for prior mammograms¶ | 1.91 (1.41–2.58) | 1.75 (1.29–2.38) | 1.62 (1.22–2.14) | 1.19 (0.84–1.69) | 1.25 (0.85, 1.84) | 1.23 (0.82, 1.84) | ||||||
| Means of detection — % | ||||||||||||
| Mammogram‖ | 86 | 79 | 85 | 77 | 85 | 76 | 83 | 75 | 83 | 75 | 75 | 67 |
| Breast biopsy** | 4.6 | 2.4 | 4.5 | 2.5 | 4.5 | 2.4 | 3.9 | 2.4 | 3.2 | 2.3 | 2.6 | 1.8 |
| Use of estrogen and progestin during follow-up with adjustment for age (%)†† | 82 | 16 | 75 | 14 | 63 | 11 | 41 | 6 | 17 | 2 | 12 | 2 |
Participants were categorized according to whether they were taking estrogen and progestin at the time of study entry; those who reported that they were not taking estrogen and progestin may or may not have done so before entering the study. All participants had an intact uterus, had no history of breast cancer, and had undergone mammography within 2 years before enrollment. At the time of enrollment, a total of 25,328 participants reported that they were using estrogen and progestin, and 16,121 reported that they were not using hormone therapy.
Data were included through December 31, 2005.
Annualized incidence was computed according to time period, where the total number of events for patients at risk for invasive breast cancer during a particular time period was divided by the total follow-up time. Follow-up time is defined as the time elapsed between the start of each period and the first occurrence of invasive breast cancer or censoring (death, loss to follow-up, or the end of each time period).
These hazard ratios were calculated with the use of a Cox proportional-hazards model for the effect on the risk of breast cancer among participants using hormone therapy as compared with those not using hormone therapy, stratified according to age and adjusted for age, race or ethnic group, body-mass index, education, smoking status, use or nonuse of alcohol, self-reported health, presence or absence of a family history of breast cancer, bilateral-oophorectomy status, level of physical activity, and Gail-model estimate of breast-cancer risk.
These hazard ratios were calculated with the use of a Cox proportional-hazards model stratified according to age and adjusted for age, race or ethnic group, body-mass index, education, smoking status, alcohol use, self-reported health, family history of breast cancer, bilateral oophorectomy status, level of physical activity, and Gail risk as well as for the number of annual mammograms (none, one, or two) reported by the participants during the previous 2 years.
The data on mammograms were self-reported.
The data on breast biopsies were self-reported.
Women in the WHI observational study initially consented to participation through March 31, 2005. To allow for further follow-up of this same cohort in an extension study, additional consent was required and was obtained from 71% of eligible participants. Data from the WHI extension study were not used in Figures 2 and 3.
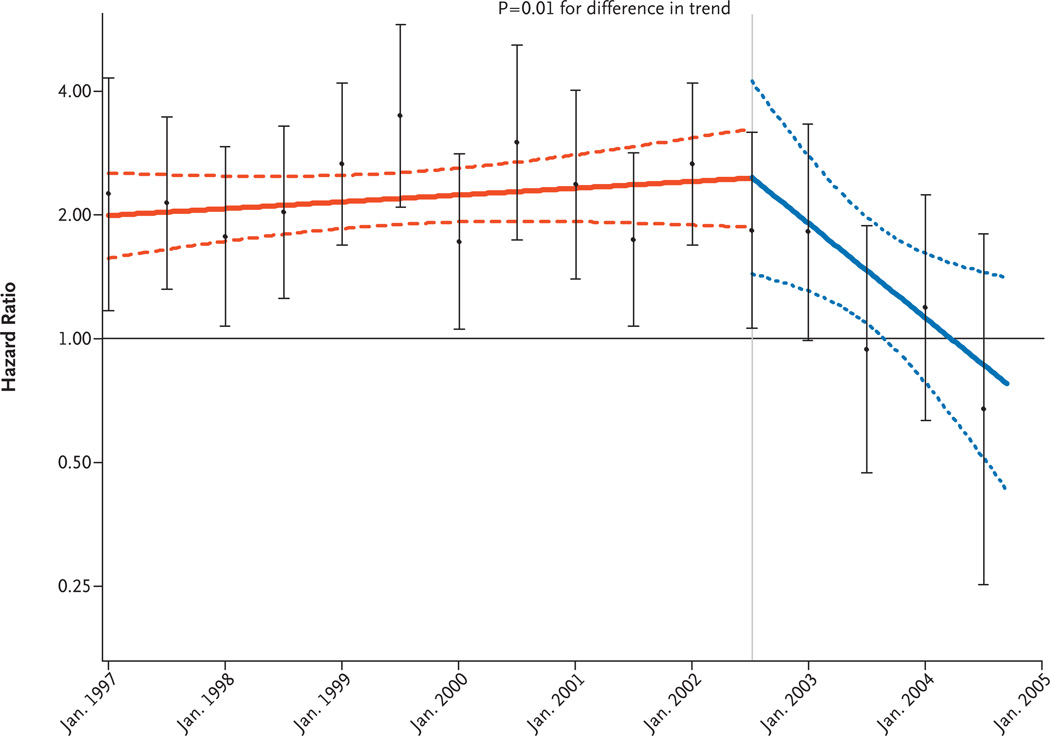
In a sensitivity analysis for adherence, which was adjusted for changes in hormone use after study entry and in which linear time-varying hazard-ratio models were used for the intervals before and after July 2002, corresponding to the announcement of trial results, we observed a decreasing trend in the hazard ratio in the hormone- therapy group (Fig. 3) (P = 0.01 for trend, for the difference in slope in the two intervals).
Figure 3. Adherence-Adjusted Effects over Time of Estrogen plus Progestin on the Risk of Breast Cancer in the WHI Observational Study.
Time-varying linear hazard ratios and 95% confidence intervals for the risk of breast cancer (solid and dashed lines, respectively) are shown for a multivariable-adjusted proportional-hazards model comparing women who were using hormones at study entry with those who were not, with censoring of follow-up data for women whose status with regard to hormone use changed after enrollment and before July 2002. The I bars show hazard ratios and 95% confidence intervals according to the use or nonuse of hormones in an analysis based on events accumulated at 6-month intervals. Inverse probability weights according to time were used to adjust for changing characteristics of the study sample over time. The vertical line indicates the announcement of the results of the clinical trial in July 2002.
DISCUSSION
Analysis of two WHI cohorts — the cohort in the WHI randomized trial comparing menopausal hormone use with placebo and the cohort in the WHI observational study — yielded a composite picture of the influence of estrogen plus progestin on the incidence of breast cancer and breast-cancer detection during the period when women were taking menopausal hormones as well as after they stopped taking them. In the clinical trial, the number of breast-cancer diagnoses in the hormone-therapy group was initially lower than that in the placebo group, perhaps reflecting the interference of hormonal effects on breast tissue with the interpretation of mammographic findings.3 The incidence increased as the duration of exposure increased and subsequently decreased in the postintervention period. In the observational study, the incidence of breast cancer was initially about twice as high among women who used hormones as among those who did not use them, a finding that probably reflected the longer duration of hormone exposure in this study than in the clinical trial.22,23 Nonetheless, the incidence decreased in parallel with the year-to-year reduction in the use of estrogen plus progestin. The rapid decrease in breast cancers during the postintervention period suggests that withdrawal of estrogen-plus-progestin therapy leads to a regression of preclinical cancers.7
Most participants stopped taking the study pills when instructed to do so on July 8, 2002, and 1 year later, only 4% of participants reported using hormone therapy that was unrelated to the study.24 In the observational study, hormone use in the group of women who reported its use at study entry began to decrease in 2001, perhaps reflecting concerns about safety in view of the results of the Heart and Estrogen/Progestin Replacement Study (HERS).25 The decline in the use of combined hormones subsequently accelerated, with a 50% decrease in 2003 as compared with 2000. The magnitude of this decrease reflects secular population trends,4 but the decrease began somewhat earlier in the observational study than in the general population, perhaps because the study participants were more aware of the HERS results.
A population-based change in the incidence of breast cancer might be attributable to factors other than discontinuation of hormone therapy. Such factors include the initial prevalence of hormone-therapy use, hormone prescribing practices (areas with relatively low use are likely to prescribe hormones mainly for women at low risk for breast cancer), the change in hormone-therapy use over the period of observation, the validity of the assessment of hormone-therapy use, the frequency of mammography, change in frequency of mammography use over the period of observation, and the validity of the estimate of mammography use.26
The decrease in breast-cancer incidence rates in the hormone-therapy groups in both the WHI clinical trial and the observational study after July 2002 cannot, however, be explained by differences in use of mammography. In the postintervention phase of the clinical trial, annual rates of mammography remained nearly identical in the hormone-therapy and placebo groups, whereas the incidence of breast cancer in the hormone-therapy group declined substantially. In the observational study, the difference in frequency of mammography use of 2% between 2002 and 2003 for women using hormones is insufficient to account for the 43% reduction in the incidence of breast cancer. In addition, the difference in frequency of mammography use between women who did and those who did not use hormones was constant over the entire interval, whereas the hazard ratio for breast cancer in the hormone-therapy group changed markedly. These findings suggest that the carryover effect of combined hormone therapy on the risk of breast cancer is time-limited and is not explained by changes in frequency of mammography use.
After the initial report of a decrease in the incidence of breast cancer beginning in 2003,6,7 subsequent reports have largely supported a relation between the use of menopausal hormone-replacement therapy and breast-cancer rates,8–10,27 but discordant results11,12 and controversy regarding causality13,14 remain. Prior analyses did not control for risk factors, frequency of mammography, or dose, schedule, and type of menopausal hormone replacement. Kerlikowske and colleagues reported a similar change in the incidence of breast cancer in a population followed after screening mammography,28 and a California report correlated county-by-county changes in hormone therapy with changes in the incidence of breast cancer.27 In the two WHI populations, detailed information regarding risk factors for breast cancer was collected at study entry, menopausal hormone-therapy use at entry was carefully assessed by means of a questionnaire administered by trained interviewers, data on frequency of mammography and findings were collected prospectively, and breast cancer was verified by central adjudication. In addition, the precise stopping date for menopausal hormone use is known in the clinical trial, and information on continuing hormone use was obtained annually in the observational study. The influence of these factors on the incidence of breast cancer was controlled by randomization in the clinical trial and by multivariate regression models in the observational study. Additional strengths of the current analysis include the randomized design of the clinical trial and the complementary nature of the independent findings in the well-characterized observational-study cohort.
The recent update of the Surveillance, Epidemiology, and End Results (SEER) Program shows that the reduction in the incidence of breast cancer among postmenopausal women in the United States that was initially observed in 2002 has been sustained through 2005 and that the reduction has occurred among women 50 to 69 years of age but not among older or younger women.29 Given breast-tumor doubling times of about 150 days,30–32 the absence of so-called catch-up incidence argues against the idea that differences in mammography practices can explain the decline in the incidence of breast cancer.
There is controversy regarding mammography use in the United States. A comparison of use between 2000 and 2005 suggested a decrease in annual mammography of about 5%,33 perhaps mainly among women between 40 and 59 years of age.34 However, a recent analysis of Medicare claims showed a 5% increase in mammography use over the same period for participants in Medicare fee-for-service plans.35
In summary, the increased risk of breast cancer associated with estrogen-plus-progestin therapy declined markedly soon after discontinuation of the therapy and was unrelated to a change in the use of mammography. This finding supports the hypothesis that the recent reduction in the incidence of breast cancer among women in certain age groups in the United States is predominantly related to a decrease in the use of combined estrogen plus progestin.
Acknowledgments
Supported by grants from the National Heart, Lung, and Blood Institute (N01WH22110, N01WH24152, N01WH32100-2, N01WH32105-6, N01WH32108-9, N01WH32111-13, N01WH32115, N01WH32118-32119, N01WH32122, N01WH42107-26, N01WH42129-32, and N01WH44221).
Dr. Chlebowski reports receiving consulting fees from Astra- Zeneca, Novartis, Pfizer, Eli Lilly, and Wyeth Pharmaceuticals, lecture fees from AstraZeneca, Novartis, and Abraxis, and grant support from Amgen, Eli Lilly, and Organon. Dr. Gass reports receiving consulting fees from Upsher-Smith Laboratories, Wyeth Pharmaceuticals, and Procter & Gamble and funding for multisite clinical trials from Procter & Gamble and Wyeth Pharmaceuticals. Dr. Rohan reports receiving consulting fees from legal firms regarding hormone-therapy issues. Dr. Hendrix reports receiving consulting fees from Meditrina Pharmaceuticals, lecture fees from Merck, and grant support from Boehringer Ingelheim and Organon.
APPENDIX
The following investigators participated in the Women’s Health Initiative studies: Program Office: National Heart, Lung, and Blood Institute, Bethesda, MD — E. Nabel, J. Rossouw, S. Ludlam, J. McGowan, N. Geller, L. Ford. Clinical Coordinating Center: Fred Hutchinson Cancer Research Center, Seattle — R. Prentice, G.L. Anderson, A. LaCroix, R. Patterson, A. McTiernan, B. Cochrane, J. Hunt, L. Tinker, C. Kooperberg, M. McIntosh, C.Y. Wang, C. Chen, D. Bowen, A. Kristal, J. Stanford, N. Urban, N. Weiss, E. White; Medical Research Laboratories, Highland Heights, KY — E. Stein, P. Laskarzewski; San Francisco Coordinating Center, San Francisco — S.R. Cummings, M. Nevitt, L. Palermo; University of Minnesota, Minneapolis — L. Harnack; Fisher BioServices, Rockville, MD — F. Cammarata, S. Lindenfelser; University of Washington, Seattle — B. Psaty, S. Heckbert. Clinical Centers: Albert Einstein College of Medicine, Bronx, NY — S. Wassertheil-Smoller, W. Frishman, J. Wylie-Rosett, D. Barad, R. Freeman; Baylor College of Medicine, Houston — A. Rajkovic, J. Hays, R. Young, H. Sangi-Haghpeykar; Brigham and Women’s Hospital, Harvard Medical School, Boston — J.E. Manson, K.M. Rexrode, B. Walsh, J.M. Gaziano, M. Bueche; Brown University, Providence, RI — C.B. Eaton, M. Cyr, G. Sloane; Emory University, Atlanta — L. Phillips, V. Butler, V. Porter; Fred Hutchinson Cancer Research Center, Seattle — S.A.A. Beresford, V.M. Taylor, N.F. Woods, M. Henderson, R. Andersen; George Washington University, Washington, DC — L. Martin, J. Hsia, N. Gaba, R. Katz; Los Angeles Biomedical Research Institute at Harbor–UCLA Research and Education Institute, Torrance, CA — R. Chlebowski, R. Detrano, A. Nelson, M. Geller; Kaiser Permanente Center for Health Research, Portland, OR — Y. Michael, E. Whitlock, V. Stevens, N. Karanja; Kaiser Permanente Division of Research, Oakland, CA — B. Caan, S. Sidney, G.B.J. Hirata; Medical College of Wisconsin, Milwaukee — J. Morley Kotchen, V. Barnabei, T.A. Kotchen, M.A.C. Gilligan, J. Neuner; MedStar Research Institute–Howard University, Washington, DC — B.V. Howard, L. Adams-Campbell, L. Lessin, C. Iglesia, L.K. Mickel; Northwestern University, Chicago–Evanston — L. Van Horn, P. Greenland, J. Khandekar, K. Liu, C. Rosenberg; Rush University Medical Center, Chicago — H. Black, L. Powell, E. Mason, M. Gulati; Stanford Prevention Research Center, Stanford, CA — M.L. Stefanick, M.A. Hlatky, B. Chen, R.S. Stafford, S. Mackey; State University of New York at Stony Brook, Stony Brook — D. Lane, I. Granek, W. Lawson, C. Messina, G. San Roman; Ohio State University, Columbus — R. Jackson, R. Harris, E. Paskett, W.J. Mysiw, M. Blumenfeld; University of Alabama at Birming- ham, Birmingham — C.E. Lewis, A. Oberman, J.M. Shikany, M. Safford; University of Arizona, Tucson–Phoenix — C.A. Thomson, T. Bassford, C. Ritenbaugh, Z. Chen, M. Ko; University at Buffalo, Buffalo, NY — J. Wactawski-Wende, M. Trevisan, E. Smit, S. Graham, J. Chang; University of California at Davis, Sacramento — J. Robbins, S. Yasmeen; University of California at Irvine, Irvine — F.A. Hubbell, G. Frank, N. Wong, N. Greep, B. Monk; University of California at Los Angeles, Los Angeles — L. Nathan, D. Heber, R. Elashoff, S. Liu; University of California at San Diego, La Jolla–Chula Vista — R.D. Langer, M.H. Criqui, G.T. Talavera, C.F. Garland, M.A. Allison; University of Cincinnati, Cincinnati — M. Gass, N. Watts; University of Florida, Gainesville–Jacksonville — M. Limacher, M. Perri, A. Kaunitz, R.S. Williams, Y. Brinson; University of Hawaii, Honolulu — J.D. Curb, H. Petrovitch, B. Rodriguez, K. Masaki, P. Blanchette; University of Iowa, Iowa City–Davenport — R. Wallace, J. Torner, S. Johnson, L. Snetselaar, J. Robinson; University of Massachusetts, Fallon Clinic, Worcester — J. Ockene, M. Rosal, I. Ockene, R. Yood, P. Aronson; University of Medicine and Dentistry of New Jersey, Newark — N. Lasser, B. Singh, V. Lasser, J. Kostis, P. McGovern; University of Miami, Miami — M.J. O’Sullivan, L. Parker, J. Potter, D. Fernandez, P. Caralis; University of Minnesota, Minneapolis — K.L. Margolis, R.H. Grimm, M.F. Perron, C. Bjerk, S. Kempainen; University of Nevada, Reno — R. Brunner, W. Graettinger, V. Oujevolk, M. Bloch; University of North Carolina, Chapel Hill — G. Heiss, P. Haines, D. Ontjes, C. Sueta, E. Wells; University of Pittsburgh, Pittsburgh — L. Kuller, J. Cauley, N.C. Milas; University of Tennessee Health Science Center, Memphis — K.C. Johnson, S. Satterfield, R. Li, S. Connelly, F. Tylavsky; University of Texas Health Science Center, San Antonio — R. Brzyski, R. Schenken; University of Wisconsin, Madison — G.E. Sarto, D. Laube, P. McBride, J. Mares, B. Loevinger; Wake Forest University School of Medicine, Winston-Salem, NC — M. Vitolins, G. Burke, R. Crouse, S. Washburn; Wayne State University School of Medicine–Hutzel Hospital, Detroit — M. Simon. Women’s Health Initiative Memory Study: Wake Forest University School of Medicine, Winston-Salem, NC — S. Shumaker, S. Rapp, C. Legault, M. Espeland, L. Coker. Former Principal Investigators and Project Officers: Baylor College of Medicine, Houston — J. Hays, J. Foreyt; Brown University, Providence, RI — A.R. Assaf; Emory University, Atlanta — D. Hall; George Washington University, Washington, DC — V. Miller; Kaiser Permanente Center for Health Research, Portland, OR — B. Valanis; Kaiser Permanente Division of Research, Oakland, CA — R. Hiatt; National Cancer Institute, Bethesda, MD — C. Clifford (deceased); National Heart, Lung, and Blood Institute, Bethesda, MD — L. Pottern; University of California at Irvine, Irvine — F. Meyskens, Jr.; University of California at Los Angeles, Los Angeles — H. Judd (deceased); University of Cincinnati, Cincinnati — J. Liu, N. Watts; University of Miami, Miami — M. Baum; University of Minnesota, Minneapolis — R. Grimm; University of Nevada, Reno — S. Daugherty; University of North Carolina, Chapel Hill — D. Sheps, B. Hulka; University of Tennessee Health Science Center, Memphis — W. Applegate; University of Wisconsin, Madison — C. Allen (deceased); Wake Forest University School of Medicine, Winston-Salem, NC — D. Bonds.
Footnotes
No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 3.Chlebowski RT, Anderson GL, Pettinger M, et al. Estrogen plus progestin and breast cancer detection by means of mammography and breast biopsy. Arch Intern Med. 2008;168:370–377. doi: 10.1001/archinternmed.2007.123. [DOI] [PubMed] [Google Scholar]
- 4.Hersh AL, Stefanick ML, Stafford RS. National use of menopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 5.Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy with the publication of clinical trial results. Ann Intern Med. 2004;140:184–188. doi: 10.7326/0003-4819-140-3-200402030-00009. [DOI] [PubMed] [Google Scholar]
- 6.Clarke CA, Glaser SL, Uratsu CS, Selby JV, Kushi LH, Herrinton LJ. Recent declines in hormone therapy utilization and breast cancer incidence: clinical and population-based evidence. J Clin Oncol. 2006;24(33):e49–e50. doi: 10.1200/JCO.2006.08.6504. [DOI] [PubMed] [Google Scholar]
- 7.Ravdin PM, Cronin K, Howlander N, et al. A sharp decrease in breast cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 8.Katalinic A, Rawal R. Decline in breast cancer incidence after decrease in utilisation of hormone replacement therapy. Breast Cancer Res Treat. 2008;107:427–430. doi: 10.1007/s10549-007-9566-z. [DOI] [PubMed] [Google Scholar]
- 9.Canfell K, Banks E, Moa AM, Beral V. Decrease in breast cancer incidence following a rapid fall in use of hormone replacement therapy in Australia. Med J Aust. 2008;188:641–644. doi: 10.5694/j.1326-5377.2008.tb01821.x. [DOI] [PubMed] [Google Scholar]
- 10.Allemand H, Seradour B, Weill A, Ricordeau P. Decline in breast cancer incidence in 2005 and 2006 in France: a paradoxical trend. Bull Cancer. 2008;95:11–15. doi: 10.1684/bdc.2008.0556. (In French.) [DOI] [PubMed] [Google Scholar]
- 11.Zahl PH, Maehlen J. A decline in breast cancer incidence. N Engl J Med. 2007;357:510–511. [PubMed] [Google Scholar]
- 12.Vaidya JS. Declines in invasive breast cancer and use of menopausal hormone therapy in a screening mammography population. J Natl Cancer Inst. 2008;100:598–599. doi: 10.1093/jnci/djn080. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast Cancer Res. 2007;9:R28. doi: 10.1186/bcr1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacMahon B, Cole P. Is the breast cancer incidence declining? Epidemiology. 2008;19:268–269. doi: 10.1097/EDE.0b013e31816334f5. [DOI] [PubMed] [Google Scholar]
- 15.Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299:1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 16.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 17.Hays J, Hunt JR, Hubbel FA, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(Suppl):S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 18.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 19.Yasmeen S, Romano PS, Pettinger M, et al. Frequency and predictive value of a mammographic recommendation for short-interval follow-up. J Natl Cancer Inst. 2003;95:429–436. doi: 10.1093/jnci/95.6.429. [DOI] [PubMed] [Google Scholar]
- 20.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 21.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 22.Prentice RL, Chlebowski RT, Stefanick ML, et al. Estrogen plus progestin therapy and breast cancer in recently postmenopausal women. Am J Epidemiol. 2008;167:1207–1216. doi: 10.1093/aje/kwn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [Erratum, Lancet 2003;362:1160.] [DOI] [PubMed] [Google Scholar]
- 24.Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294:183–193. doi: 10.1001/jama.294.2.183. [DOI] [PubMed] [Google Scholar]
- 25.Hulley S, Grady B, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 26.Ravdin PM, Cronin KA, Chlebowski RT. A decline in breast-cancer incidence. N Engl J Med. 2007;357:513. [Google Scholar]
- 27.Robbins AS, Clarke CA. Regional changes in hormone therapy use and breast cancer incidence in California from 2001 to 2004. J Clin Oncol. 2007;25:3437–3439. doi: 10.1200/JCO.2007.11.4132. [DOI] [PubMed] [Google Scholar]
- 28.Kerlikowske K, Miglioretti DL, Buist DS, Walker R, Carney PA. Declines in invasive breast cancer and use of menopausal hormone therapy in a screening mammography population. J Natl Cancer Inst. 2007;99:1335–1339. doi: 10.1093/jnci/djm111. [Erratum, J Natl Cancer Inst 2007;99:1493.] [DOI] [PubMed] [Google Scholar]
- 29.Surveillance, Epidemiology and End Results Program home page. [Accessed January 12, 2009]; at http://seer.cancer.gov/.)
- 30.Kuroishi T, Tominaga S, Morimoto T, et al. Tumor growth rate and prognosis of breast cancer mainly detected by mass screening. Jpn J Cancer Res. 1990;81:454–462. doi: 10.1111/j.1349-7006.1990.tb02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tilanus-Linthorst MM, Kriege M, Boetes C, et al. Hereditary breast cancer growth rates and its impact on screening policy. Eur J Cancer. 2005;41:1610–1617. doi: 10.1016/j.ejca.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 32.Peer PG, van Djick JA, Hendriks JH, Holland R, Verbeek AL. Age-dependent growth of primary breast cancer. Cancer. 1993;71:3547–3551. doi: 10.1002/1097-0142(19930601)71:11<3547::aid-cncr2820711114>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 33.Hyattsville, MD: National Center for Health Statistics; 2006. Health, United States 2006, with chart-book on trends in health of Americans. Table 84. (DHHS publication no. 2006-1232.) [PubMed] [Google Scholar]
- 34.Ryerson AB, Miller JW, Eheman CR, Leadbetter S, White MC. Recent trends in U.S. mammography use from 2000–2006: a population-based analysis. Prev Med. 2008;47:477–482. doi: 10.1016/j.ypmed.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Rao VM, Levin DC, Parker L, Frangos AJ. Recent trends in mammography utilization in the Medicare population: is there a cause for concern? J Am Coll Radiol. 2008;5:652–656. doi: 10.1016/j.jacr.2008.01.023. [DOI] [PubMed] [Google Scholar]