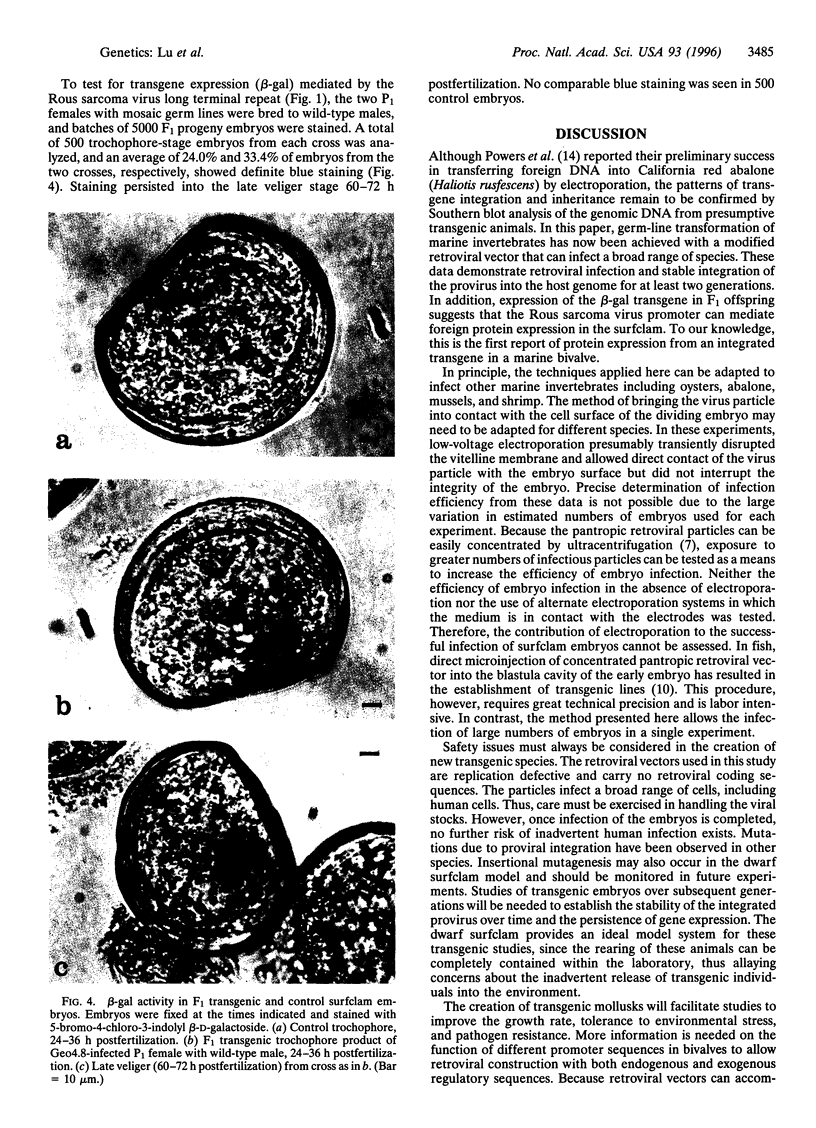
Abstract
A pantropic pseudotyped retroviral vector containing the envelope protein of vesicular stomatitis virus was used as a gene transfer vector in the dwarf surfclam, Mulinia lateralis. These pantropic retroviral vectors have an extremely broad host cell range and can infect many nonmammalian species. Newly fertilized dwarf surfclam eggs were electroporated at 700 V in the presence of 1 x 10(4) colony-forming units of pantropic pseudotyped retroviral particles. Infection was well tolerated and did not affect the survival rate of the embryos. Gametes collected from P1 presumptive transgenic animals were analyzed for the presence of provirus by PCR, and in different experiments 13-33% of the gamete pools were positive for the transgene. Dot blot hybridization of DNA samples from the F1 offspring of two different crosses between infected P1 and wild-type individuals revealed that 28% and 31% of F1 offspring were transgenic, respectively. Southern blot analysis of DNA isolated from PCR-positive F1 animals confirmed integration of a single copy of the provirus into the host genome. Thus, the germ lines of these two P1 transgenic animals were mosaic for the transgene. Expression of beta-galactosidase encoded by the provirus was detected in transgenic but not control surfclam embryos. Pantropic pseudotyped retroviral vectors provide a useful method for the stable introduction of foreign genetic information into surfclams and may facilitate the introduction of desirable genetic traits into commercially important shellfish and crustaceans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns J. C., Friedmann T., Driever W., Burrascano M., Yee J. K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. C., Matsubara T., Lozinski G., Yee J. K., Friedmann T., Washabaugh C. H., Tsonis P. A. Pantropic retroviral vector-mediated gene transfer, integration, and expression in cultured newt limb cells. Dev Biol. 1994 Sep;165(1):285–289. doi: 10.1006/dbio.1994.1253. [DOI] [PubMed] [Google Scholar]
- Inoue K., Yamashita S., Hata J., Kabeno S., Asada S., Nagahisa E., Fujita T. Electroporation as a new technique for producing transgenic fish. Cell Differ Dev. 1990 Feb;29(2):123–128. doi: 10.1016/0922-3371(90)90030-z. [DOI] [PubMed] [Google Scholar]
- Lin S., Gaiano N., Culp P., Burns J. C., Friedmann T., Yee J. K., Hopkins N. Integration and germ-line transmission of a pseudotyped retroviral vector in zebrafish. Science. 1994 Jul 29;265(5172):666–669. doi: 10.1126/science.8036514. [DOI] [PubMed] [Google Scholar]
- Lu J. K., Chen T. T., Chrisman C. L., Andrisani O. M., Dixon J. E. Integration, expression and germ-line transmission of foreign growth hormone genes in medaka (Oryzias latipes). Mol Mar Biol Biotechnol. 1992 Aug-Oct;1(4-5):366–375. [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers D. A., Kirby V. L., Cole T., Hereford L. Electroporation as an effective means of introducing DNA into abalone (Haliotis rufescens) embryos. Mol Mar Biol Biotechnol. 1995 Dec;4(4):369–375. [PubMed] [Google Scholar]
- Yee J. K., Miyanohara A., LaPorte P., Bouic K., Burns J. C., Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]