Abstract
Purpose
To prospectively evaluate cosmetic outcomes in women treated with accelerated partial breast irradiation (APBI) using high-dose-rate (HDR) interstitial brachytherapy for early-stage breast cancer.
Methods and Materials
Between 2004 and 2008, 151 patients with early-stage breast cancer were enrolled in a phase II prospective clinical trial. Eligible patients had Tis-T2 tumors ≤3 cm, excised with negative margins, and with no nodal involvement. Patients received 3.4 Gy BID to a total dose of 34 Gy. Both patients and the treating radiation oncologist qualitatively rated cosmesis as excellent, good, fair, or poor over time and ascribed a cause for changes in cosmesis. Cosmetic outcome was evaluated quantitatively by the percentage breast retraction assessment (pBRA). Patients also reported their satisfaction with treatment over time.
Results
Median follow-up was 55 months. The rate of excellent/good cosmesis reported by patients and the treating radiation oncologist was as follows: 92% and 97% pretreatment, 91% and 97% at 3–4 months follow-up, 87% and 94% at 2 years, and 92% and 94% at 3 years. Breast infection and adjuvant chemotherapy were independent predictors of a fair/poor cosmetic outcome at 3 years. Compared to the pretreatment pBRA (7.35), there was no significant change in pBRA over time. V150 was the only significant predictor of pBRA. The vast majority of patients (86.6%) were completely satisfied with their treatment.
Conclusions
Patients and the treating physician reported a high rate of excellent/good cosmetic outcomes at all follow-up time points. Acute breast infection and chemotherapy were associated with worse cosmetic outcomes. Multicatheter interstitial brachytherapy does not significantly change breast size as measured by pBRA.
Keywords: Breast cancer, brachytherapy, partial breast, cosmesis
Introduction
Breast-conserving therapy (BCT) is a standard treatment for early-stage breast cancer. The use of whole-breast irradiation (WBI) after breast-conserving surgery has been shown to reduce the risk of ipsilateral breast tumor recurrence (IBTR) compared to breast conserving surgery alone (1). The majority of local recurrences after breast-conserving surgery are at or near the lumpectomy site (2). This observation has led to investigation of accelerated partial breast irradiation (APBI) as an alternative to WBI. APBI can be completed within several days as compared to several weeks for conventional WBI. Early studies have reported low ipsilateral breast tumor recurrence (IBTR) rates after APBI (3).
Cosmesis following BCT is an important outcome of treatment. Qualitative cosmetic outcomes after APBI have been reported in several large series (4–6). Unfortunately, qualitative cosmetic evaluations suffer from substantial interobserver variability, and patient and physician evaluations can differ considerably (7, 8). The breast retraction assessment (BRA) and percentage breast retraction assessment (pBRA) are objective measurements of the amount of breast retraction, determined by comparing nipple positions relative to the sternal notch between the treated and contralateral breast (8, 9). The pBRA has been shown to have a low intra- and interobserver variability. Lower values of pBRA have been shown to correlate with improved cosmetic outcomes, making pBRA a reliable, objective measure of cosmesis (8).
To our knowledge no prospective, objective, quantitative measurement of cosmetic outcome has been reported in patients who have received APBI. The current prospective study reports the qualitative and quantitative cosmetic outcomes of a large cohort of patients with early-stage breast cancer treated with APBI using high-dose-rate (HDR) multicatheter interstitial brachytherapy. We examined clinical and treatment-related factors that could predict poorer cosmesis.
METHODS AND MATERIALS
Between March 2004 and December 2008, 151 patients with early-stage breast cancer were enrolled in a prospective study evaluating APBI using HDR multicatheter interstitial brachytherapy at XXXX. Whole breast external beam radiation therapy was not given. Selection criteria included patients with unifocal American Joint Cancer Center Commission (AJCC, 6th edition) Tis-T2N0M0 breast cancers ≤3 cm treated with breast-conserving surgery. All but one of the 123 patients with invasive disease had a sentinel lymph node biopsy. Patients were ineligible if they had: 1) lobular carcinoma in-situ (LCIS), 2) multicentric carcinoma, 3) diffuse suspicious microcalcifications, 4) suspicious microcalcifications remaining on the post-lumpectomy mammogram, or 5) systemic lupus erythematosus, scleroderma, or dermatomyositis with a CPK level above normal or with an active skin rash. The XXXX Human Research Protection Office approved this study, and all patients provided written informed consent.
All patients were treated with HDR multicatheter interstitial brachytherapy using a high activity Iridium-192 source. The interstitial implants were placed using a free hand technique with the goal of encompassing the surgical cavity with a 2 cm margin of breast tissue in all directions. In general, an intraplane catheter spacing of 1.2 cm and an interplane spacing of 1.5 to 2.0 cm were used. All implants were multiplanar, and the use of more than two planes was common. Interstitial implants were placed intraoperatively with a reopened surgical cavity in thirteen patients, including the first eight patients in the trial. This was done as a separate operative procedure from the breast conserving surgery after the final pathology was known. Real-time ultrasound guidance with an unopened, surgical cavity was predominantly used after the first eight cases. Real-time ultrasound guided implantation was performed using a strict sterile technique and under a combination of narcotic and anxiolytic sedation with local anesthesia.
All patients underwent computed tomography (CT) simulation and three-dimensional treatment planning. The Plato Brachytherapy planning system (Nucletron BV, Veenendaall, The Netherlands) was used through November 2006, after which treatment planning was done using the BrachyVision treatment planning system (Varian Medical Systems Inc., Palo Alto, CA). The planning target volume (PTV) was created by adding a uniform 2 cm margin to the surgical cavity contour and subsequently limited to 5 mm away from the skin surface. The pectoral muscles, chest wall, and axilla were excluded from the PTV.
Patients received 3400 cGy delivered in 10 twice-daily fractions. Treatment was given over 5–7 days, with a minimum of 6 hours separation between fractions. Dosimetric goals were: ≥95% of the PTV receiving the prescribed dose, V150 ≤50 cm3, V200 ≤20 cm3, and dose homogeneity index, defined as 1-(V150/V100), ≥0.7, where Vx is the volume receiving x% of the prescription dose (10). If all dosimetric goals could not be achieved in a particular case, then coverage was optimized at the expense of exposure.
Chemotherapy and hormonal therapy were administered at the discretion of the consulting medical oncologist. Chemotherapy was started at least 4 weeks after completion of APBI. Hormonal therapy was allowed during brachytherapy, but in practice was rarely started prior to APBI.
Cosmetic Assessment
Cosmesis was evaluated pretreatment, 6–8 weeks post-treatment, 3–4 months post-treatment, 6–8 months post-treatment, then every 6 months for the next 4.5 years, and yearly thereafter. Late skin toxicity was graded according to the Common Terminology for Adverse Events version 3.0. Cosmesis was qualitatively evaluated by the treating radiation oncologist by comparing the treated breast with the untreated breast using the rating system described by Aaronson et al. (11). The global cosmetic result, appearance of the surgical scar, breast size, breast shape, skin color, location of the areola and nipple, and shape of the areola and nipple were scored on a 4-point scale: “0” excellent result (no difference), “1” good result (small difference), “2” fair result (moderate difference), “3” poor result (large difference). Patients evaluated the global cosmetic result qualitatively using the same 4-point scale at each time point. The BRA was calculated according to the method of Pezner et al., which yields the difference in distance from the sternal notch to nipple between the treated and untreated breasts (9). The percentage BRA (pBRA) is defined as (BRA/reference length) x 100, where reference length is the distance between the sternal notch and the untreated nipple (Fig. 1). Physical measurements to calculate BRA and pBRA were taken directly on each patient in a seated position with both arms alongside the body. Both BRA and pBRA were determined pretreatment and at each follow-up. The pBRA was used for analysis, because it reports retraction as a percentage rather than a distance, and is therefore less dependent on breast size. At each time point both the patients and treating physician reported the perceived cause of cosmesis changes as mostly due to radiation, mostly due to surgery, unknown, or no significant change. Patients also reported their level of satisfaction with treatment. The cosmetic questionnaire was presented to the patient by a trained radiation oncology nurse. The patient and physician questionnaires used in this study are presented in Appendices eI and eII.
Fig 1.
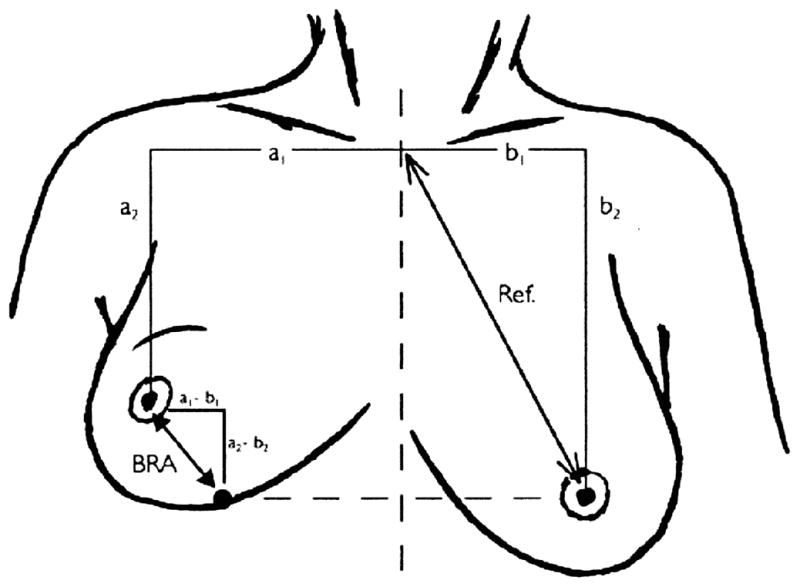
Illustration of the BRA measurements.
Illustration from Vreiling et al. (8) (copyright 1999 Elsevier Inc.).
Statistical Analysis
The pretreatment assessment was made after surgery and prior to brachytherapy. All time intervals were calculated from the date of completion of brachytherapy. For this analysis, cosmetic outcomes were analyzed up to three years after completion of therapy. Due to poor intraobserver and interobserver correlation reported with the four category qualitative cosmesis scale, the results were dichotomized into excellent/good and fair/poor for analysis (7). Agreement between patient and physician cosmetic evaluation was measured by the normalized kappa statistic (12). A kappa of less than 0.20 indicated poor agreement; 0.21–0.40 slight agreement; 0.41–0.60 fair agreement; 0.61–0.80 good agreement; 0.81–1.00 very good agreement. Logistic regression was used to examine clinical and treatment-related factors that could contribute to developing a fair/poor cosmetic outcome at the 3-year time point. The pBRA values were log-transformed to obtain a Gaussian distribution for statistical analysis. The effect of time on pBRA was assessed with a mixed repeated measures model using a heterogeneous autoregressive covariance structure. Student’s t-test was used to analyze the effect of symptomatic fat necrosis on pBRA. To describe the association between pBRA and qualitative cosmetic evaluations, Spearman correlation coefficients were calculated. The following interpretation of the correlation coefficient was used: 0.20–0.40 indicated slight correlation, 0.41–0.60 moderate correlation, 0.61–0.80 substantial correlation, and a coefficient above 0.80 indicated very strong correlation. A p value of <0.05 was considered statistically significant. All tests were two-sided. Statistical analyses were performed using StatView (version 5.0.1; SAS Institute Inc., Cary, NC) and SAS (version 9.2; SAS Institute Inc., Cary, NC).
RESULTS
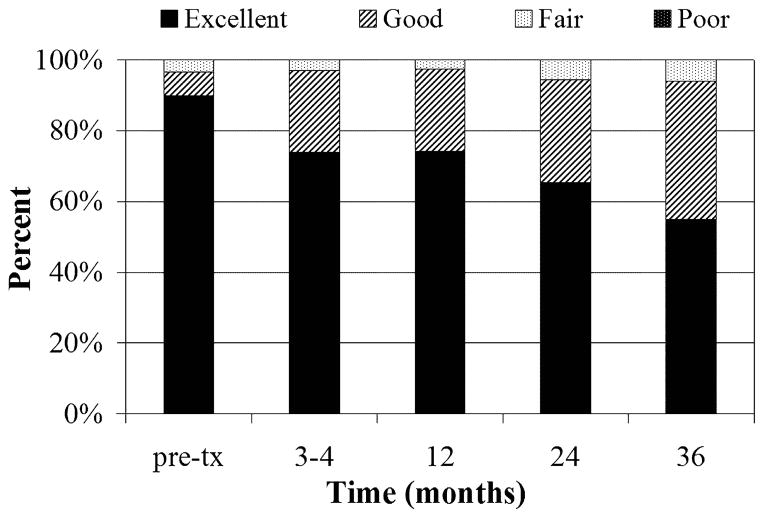
Patient, cancer, and treatment-related factors are summarized in Table 1. The median time from surgery to ISI placement was 5 weeks (range, 1–12 weeks). Dosimetric goals for V150 and/or V200 were not met for 28% of patients. The median follow-up was 55 months (range, 1–81 months). Eighty-two patients (54%) had cosmetic outcome data through 3 years of follow-up. Six patients experienced a breast infection after ISI and 19 developed symptomatic fat necrosis. The incidence of late skin toxicity was: grade 0 (76%), grade 1 (21%), grade 2 (1%), grade 3 (1%). There was no grade 4 or 5 late skin toxicity. Seven patients developed less than 1.0 cm2 regions of telangectasias. The global cosmetic results as graded by the patient and physician are presented in Figure 2.
Table 1.
Patient, tumor and treatment-related characteristics
| Characteristic | Findings |
|---|---|
| Age (y) | |
| Mean (range) | 60 (40–85) |
| 40–50 | 29 (19%) |
| 50–60 | 50 (33%) |
| >60 | 72 (48%) |
| Tumor Location | |
| UOQ | 54 (36%) |
| UIQ | 46 (30%) |
| LOQ | 30 (20%) |
| LIQ | 21 (14%) |
| Pathologic tumor stage | |
| Tis | 28 (19%) |
| T1mic | 1 (1%) |
| T1a | 19 (13%) |
| T1b | 52 (34%) |
| T1c | 38 (25%) |
| T2 | 13 (9%) |
| Sentinel lymph node biopsy (for patients with invasive cancer) | |
| Yes | 122 (99%) |
| No | 1 (1%) |
| Chemotherapy | |
| Yes | 30 (20%) |
| Anthracycline based chemotherapy | 26 (17%) |
| No | 121 (80%) |
| Hormonal therapy | |
| Yes | 116 (77%) |
| No | 35 (23%) |
| Method of catheter placement | |
| Open cavity | 13 (9%) |
| Closed cavity | 138 (91%) |
| Number of catheters | |
| Mean (range) | 21 (12–34) |
| V100 (cm3) | |
| Mean (range) | 246 (99.6–690) |
| V150 (cm3) | |
| Mean | 45.2 (19.1–129) |
| V200 (cm3) | |
| Mean | 16.1 (7–39.8) |
| DHI | |
| Mean | 0.808 (0.680–0.880) |
| IRAK | |
| Mean | 0.344 (0.199–0.659) |
| Toxicity | |
| Breast infection | 6 (4%) |
| Symptomatic fat necrosis | 19 (12.6%) |
Abbreviations: UOQ = upper outer quadrant; UIQ = upper inner quadrant; LOQ = lower outer quadrant; LIQ = lower inner quadrant; DHI = dose homogeneity index; IRAK = integrated reference air kerma
Fig 2.
Global cosmetic evaluation by (a) physician and (b) patient evaluation. Pretx=pretreatment
Dichotomizing the global cosmetic results into excellent/good or fair/poor, the percentage of patients reporting excellent/good cosmetic outcomes is as follows: 92% pretreatment, 91% 3–4 months after treatment, 87% at 2 years, and 92% at 3 years. The percentage of patients with excellent/good outcomes based on physician evaluation is as follows: 97% pretreatment, 97% 3–4 months after treatment, 94% at 2 years, and 94% at 3 years. The normalized kappa ranged from 0.72–0.84 for patient and physician agreement on qualitative cosmetic evaluation, indicating good to very good agreement.
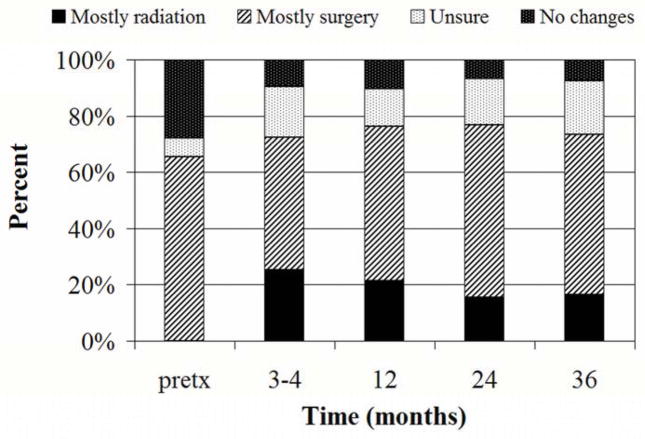
Patient impressions of the cause of cosmesis changes over time are shown in Figure 3. At 3 years, 16.5% of patients reported that cosmetic changes were mostly due to radiation, 57% reported that cosmetic changes were mostly due to surgery, 19% were unsure, and 7.5% of patients reported no cosmetic changes. Three years after treatment, 86.6% of patients were completely satisfied with the treatment and results. If they had to do it again, 98.8% of patients self reported that they would choose breast conserving surgery with APBI as received over mastectomy without radiation therapy.
Fig 3.
Causes of cosmetic changes reported by patients. Pretx=pretreatment.
Logistic regression was performed to determine whether there was an association between the clinical and treatment-related factors listed in Table 1 and a fair/poor global cosmetic outcome based on physician evaluation at 3 years. Univariate results indicated that breast infection was a significant predictor of a fair/poor cosmetic outcome (odds ratio (OR), 30.4; p=0.009), as was open cavity catheter placement (OR, 8.88; p=0.0125). Chemotherapy showed a trend toward significance (OR, 5.025; p=0.053). Multivariate analysis including breast infection, method of catheter placement and chemotherapy as variables indicated that breast infection (OR, 66; 95% confidence interval (CI) 4.1–999; p=0.003) and chemotherapy (OR, 9.9; 95% CI 1.5–67; p=0.019) were independent predictors of a fair/poor cosmetic outcome. Logistic regression assessing the association of clinical and treatment-related factors with a fair/poor cosmetic outcome based on patient evaluation at 3 years indicated that none of the factors included in Table 1 was significant.
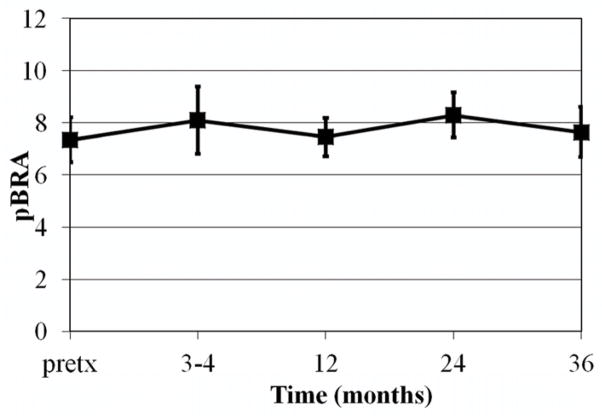
A graph of mean pBRA over time is shown in figure 4. The mean pretreatment pBRA was 7.35 (95% confidence interval (CI), 6.49–8.21). The mean pBRA 3 years after treatment was 7.64 (95% CI, 6.68–8.6). To assess the effect of time on pBRA, a mixed repeated measures model was utilized with pBRA measurements taken pretreatment, 3–4 months, 1 year, 2 years, and 3 years after treatment. There was no significant change in pBRA over the repeated measures taken from pretreatment up to 3 years after treatment. In addition, pBRA was not significantly different in the patients who developed symptomatic fat necrosis compared to those who did not. The relationship between the clinical and treatment-related factors listed in Table 1 and pBRA at 3 years was examined using linear regression. Results indicated that only V150 significantly correlated with pBRA (regression coefficient (β)=0.008; p=0.044).
Fig 4.
Mean pBRA over time. Error bars indicate 95% confidence interval. Pretx=pretreatment.
The correlation between pBRA and physician reported qualitative cosmetic results at 3 years is listed in Table e1. There was a slight correlation between pBRA and the physician rated global cosmetic score at 3 years (ρ=0.36). There was slight to moderate correlation between pBRA and the physician rated specific cosmetic items (ρ=0.37–0.47). The correlation between the patient rated global cosmetic score and pBRA was minimal (ρ 0.18).
DISCUSSION
We report the results of the first prospective study of cosmesis after APBI using both qualitative and quantitative evaluations. In previous reports, several methods of cosmetic evaluation have been used. The Harvard criteria (13) categorized the overall cosmetic outcome as excellent, good, fair or poor. Pezner et al. (7) reported low interobserver consensus with this 4-point scoring system, and found that substantial consensus was only achieved if the scoring system was reduced to two categories: excellent/good or fair/poor. Aaronson et al. (11) expanded the Harvard criteria to include appearance of the surgical scar, breast size, breast shape, skin color, location of the areola and nipple, and shape of the areola and nipple. Our study incorporated the Aaronson modification of the Harvard criteria for the physician cosmetic evaluation.
Through 3 years of follow-up, the percentage of patients with excellent/good cosmetic outcomes remained high for both physician and patient assessment (94% and 92%, respectively). Patients reported that changes in cosmesis were mostly due to surgery, and the vast majority were very satisfied with the overall treatment and results.
Other series evaluating cosmetic outcomes after APBI using interstitial brachytherapy have reported excellent/good cosmetic outcomes in 75–99% of patients, based on physician evaluation (4, 14, 15). Patient reported cosmetic evaluation has been limited. In the German-Austrian multicatheter brachytherapy trial, the rate of excellent/good cosmetic outcomes was similar for physician evaluation (90.1%) and patient evaluation (89.9%) (5).
Our results indicate that breast infection and adjuvant chemotherapy are independent predictors of a fair/poor cosmetic outcome at 3 years, based on physician evaluation. Of note, the vast majority (86.7%) of patients who received chemotherapy received Anthracycline-based chemotherapy. Due to the small number of patients with a fair/poor cosmetic result who also experienced a breast infection or underwent chemotherapy, the odds ratios of these significant predictors have a large confidence interval. There was no significant relationship between clinical and treatment-related variables and patient reported fair/poor cosmetic outcomes at 3 years. The lack of an association may be due to the limited number of patients (seven) who reported a fair/poor outcome.
Other investigators have found similar predictors of poor cosmesis. Chen et al. (4) and Wazer et al. (14) reported that chemotherapy was associated with worse cosmetic outcomes in their series of patients treated with APBI via ISI. Analysis of the American Society of Breast Surgeons MammoSite breast brachytherapy registry trial showed that breast infection was predictive of a worse cosmetic outcome at 3 years, but this association was no longer significant at 5 years (6). Symptomatic fat necrosis was not associated with fair/poor cosmesis in our study, in contrast Lovey et al. (16) reported a significant association in their series. Possible reasons for this difference include the subjectivity of qualitative cosmetic grading, a difference in length of follow-up, and differences in treatment dose and fractionation.
The pBRA is an objective measure of cosmetic outcome that quantitate the retraction of the treated breast compared to the untreated breast. In a group of healthy controls, Fabry et al. (17) reported a median pBRA of 6.1. Vrieling et al. (8) evaluated the pBRA in 731 patients treated in EORTC trial 22881/10822, in which patients with early-stage breast cancer received WBI and were randomized to receive a boost dose or no-boost dose. The mean pBRA was 8.3 in the boost group, significantly higher than the pBRA of 7.6 in the no-boost group. The mean pBRA in the present study is of similar magnitude to the results reported after WBI.
To our knowledge, this is the first report of the breast retraction assessment in patients treated with breast-conserving surgery and APBI. There was no significant change in pBRA from pretreatment through 3 years of follow-up. This indicates that HDR multicatheter interstitial brachytherapy did not cause significant changes in breast size or retraction. V150 was found to be significantly correlated with pBRA, such that each increase of V150 by 1 cm3 leads to an estimated 0.8% increase in pBRA. Symptomatic fat necrosis, a potential complication of treatment, did not cause significant breast retraction.
There was a slight to moderate correlation between physician cosmetic evaluation scores and the pBRA at 3 years. The strongest correlation was with position of the areola and nipple, which is unsurprising since the pBRA reflects the difference in nipple positions. There was minimal correlation between the patient reported cosmetic outcome and pBRA. The pBRA does not account for disturbing scars or skin changes. This limitation of the pBRA may explain the low correlation with global cosmetic assessment in our study.
Other potential limitations of this study include cosmetic evaluation by the treating radiation oncologist and length of follow-up. Several studies assessing cosmetic outcomes after APBI or WBI have found that cosmetic outcomes stabilize after 2–3 years (4, 18, 19). However data from the MammoSite brachytherapy registry trial indicate that cosmetic outcomes may continue to change up to 5 years after treatment (6). Longer follow-up may be necessary to further characterize the long-term cosmetic outcomes.
Conclusions
In this prospective study, patients with early-stage breast cancer treated with APBI using HDR multicatheter interstitial brachytherapy had a high rate of excellent/good cosmetic outcomes based on patient and physician cosmetic evaluations. Chemotherapy and breast infection were associated with worse cosmetic outcomes. There was no significant difference in pBRA up to 3 years after treatment. Furthermore, the magnitude of pBRA in our study was comparable to that seen in whole breast irradiation studies. HDR multicatheter interstitial brachytherapy does not significantly change breast size or retraction as measured by pBRA over time.
Supplementary Material
Table 2.
Agreement between patient and physician reported global cosmetic outcomes
| Percent agreement: dichotomized scoring | Normalized κ: dichotomized scoring | |
|---|---|---|
| Pretreatment | 92% | 0.83 |
| 3–4 months | 92% | 0.84 |
| 2 years | 90% | 0.78 |
| 3 years | 87% | 0.72 |
Abbreviation: κ = kappa statistic
SUMMARY.
In a prospective study, cosmetic outcomes of 151 women treated with accelerated partial breast irradiation using high-dose-rate interstitial brachytherapy for early-stage breast cancer were analyzed through 3 years of follow-up. Both patients and the treating physician reported a high rate of excellent/good cosmetic outcomes. Breast infection and chemotherapy were associated with worse cosmetic outcomes. Multicatheter interstitial brachytherapy did not significantly change cosmesis as measured by percentage breast retraction assessment (pBRA) over time.
Acknowledgments
We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Mo., for the use of the Clinical Trials Core, which provided regulatory services. The Siteman Cancer Center is supported in part by NCI Cancer Center Support Grant #P30 CA91842.
Footnotes
CONFLICT OF INTEREST NOTIFICATION: The authors declare that no actual or potential conflicts of interest exist.
USE OF COPYRIGHTED INFORMATION: Permission to use the copyrighted figure has been granted by Copyright Clearance Center.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 2.Fisher ER, Sass R, Fisher B, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (protocol 6). II. Relation of local breast recurrence to multicentricity. Cancer. 1986;57:1717–1724. doi: 10.1002/1097-0142(19860501)57:9<1717::aid-cncr2820570902>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) J Am Coll Surg. 2009;209:269–277. doi: 10.1016/j.jamcollsurg.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 4.Chen PY, Vicini FA, Benitez P, et al. Long-term cosmetic results and toxicity after accelerated partial-breast irradiation: a method of radiation delivery by interstitial brachytherapy for the treatment of early-stage breast carcinoma. Cancer. 2006;106:991–999. doi: 10.1002/cncr.21681. [DOI] [PubMed] [Google Scholar]
- 5.Strnad V, Hildebrandt G, Potter R, et al. Accelerated partial breast irradiation: 5-year results of the German-Austrian multicenter phase II trial using interstitial multicatheter brachytherapy alone after breast-conserving surgery. Int J Radiat Oncol Biol Phys. 2011;80:17–24. doi: 10.1016/j.ijrobp.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Vicini F, Beitsch P, Quiet C, et al. Five-Year Analysis of Treatment Efficacy and Cosmesis by the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial in Patients Treated With Accelerated Partial Breast Irradiation. Int J Radiat Oncol Biol Phys. 2011;79:808–817. doi: 10.1016/j.ijrobp.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 7.Pezner RD, Lipsett JA, Vora NL, et al. Limited usefulness of observer-based cosmesis scales employed to evaluate patients treated conservatively for breast cancer. Int J Radiat Oncol Biol Phys. 1985;11:1117–1119. doi: 10.1016/0360-3016(85)90058-6. [DOI] [PubMed] [Google Scholar]
- 8.Vrieling C, Collette L, Bartelink E, et al. Validation of the methods of cosmetic assessment after breast-conserving therapy in the EORTC “boost versus no boost” trial. EORTC Radiotherapy and Breast Cancer Cooperative Groups. European Organization for Research and Treatment of Cancer. Int J Radiat Oncol Biol Phys. 1999;45:667–676. doi: 10.1016/s0360-3016(99)00215-1. [DOI] [PubMed] [Google Scholar]
- 9.Pezner RD, Patterson MP, Hill LR, et al. Breast retraction assessment: an objective evaluation of cosmetic results of patients treated conservatively for breast cancer. Int J Radiat Oncol Biol Phys. 1985;11:575–578. doi: 10.1016/0360-3016(85)90190-7. [DOI] [PubMed] [Google Scholar]
- 10.Arthur DWWD, Koo D, et al. The Importance of Dose Volume Histogram Evaluation in Partial Breast Brachytherapy: A Study of Dosimetric Parameters [abstract] Int J Radiat Oncol Biol Phys. 2003;52(Suppl):S361–362. [Google Scholar]
- 11.Aaronson NK, Bartelink H, van Dongen JA, et al. Evaluation of breast conserving therapy: clinical, methodological and psychosocial perspectives. Eur J Surg Oncol. 1988;14:133–140. [PubMed] [Google Scholar]
- 12.Lantz CA, Nebenzahl E. Behavior and interpretation of the kappa statistic: resolution of the two paradoxes. J Clin Epidemiol. 1996;49:431–434. doi: 10.1016/0895-4356(95)00571-4. [DOI] [PubMed] [Google Scholar]
- 13.Harris JR, Levene MB, Svensson G, et al. Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int J Radiat Oncol Biol Phys. 1979;5:257–261. doi: 10.1016/0360-3016(79)90729-6. [DOI] [PubMed] [Google Scholar]
- 14.Wazer DE, Kaufman S, Cuttino L, et al. Accelerated partial breast irradiation: an analysis of variables associated with late toxicity and long-term cosmetic outcome after high-dose-rate interstitial brachytherapy. Int J Radiat Oncol Biol Phys. 2006;64:489–495. doi: 10.1016/j.ijrobp.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 15.King TA, Bolton JS, Kuske RR, et al. Long-term results of wide-field brachytherapy as the sole method of radiation therapy after segmental mastectomy for T(is,1,2) breast cancer. Am J Surg. 2000;180:299–304. doi: 10.1016/s0002-9610(00)00454-2. [DOI] [PubMed] [Google Scholar]
- 16.Lovey K, Fodor J, Major T, et al. Fat necrosis after partial-breast irradiation with brachytherapy or electron irradiation versus standard whole-breast radiotherapy--4-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007;69:724–731. doi: 10.1016/j.ijrobp.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 17.Fabry HF, Zonderhuis BM, Meijer S, et al. Cosmetic outcome of breast conserving therapy after sentinel node biopsy versus axillary lymph node dissection. Breast Cancer Res Treat. 2005;92:157–162. doi: 10.1007/s10549-005-0321-z. [DOI] [PubMed] [Google Scholar]
- 18.Olivotto IA, Weir LM, Kim-Sing C, et al. Late cosmetic results of short fractionation for breast conservation. Radiother Oncol. 1996;41:7–13. doi: 10.1016/s0167-8140(96)91824-1. [DOI] [PubMed] [Google Scholar]
- 19.Rose MA, Olivotto I, Cady B, et al. Conservative surgery and radiation therapy for early breast cancer. Long-term cosmetic results. Arch Surg. 1989;124:153–157. doi: 10.1001/archsurg.1989.01410020023002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.