Abstract
Selective serotonin reuptake inhibitor (SSRI) antidepressants are frequently used in the management of antenatal maternal mood disturbances. SSRIs readily cross the placenta and increase central serotonergic tone in the fetus. Given serotonin’s key neurodevelopmental role, such prenatal exposure raises concerns about its impact on child development. Preclinical studies report enduring molecular, physiological, and behavioral consequences of developmental SSRI exposure. In humans, sustained developmental outcomes remain largely unstudied, and distinguishing between the effects of prenatal SSRI exposure and the impact of maternal mental illness remains a key challenge.
INTRODUCTION
Exposure of the fetus to maternal depression during gestation occurs in ~15% of all pregnancies1 and may have lasting consequences for the cognitive and emotional development of the child.2 The management of mood disturbances during pregnancy frequently requires mothers and their clinicians to weigh the potential consequences of the mental illness against the consequences of psychopharmacotherapy. Because selective serotonin reuptake inhibitor (SSRI) antidepressants have not been reported to produce gross structural neuroteratogenic effects, they are often considered safe for antenatal use;3 this has led to an increase in their use for optimization of maternal mental health during pregnancy.1
SSRIs act primarily by blocking the serotonin transporter 5-HTT, thereby raising extracellular serotonin (5-HT) levels (Figure 1). Because SSRIs readily cross the placental and blood–brain barriers,4 SSRI treatment during pregnancy alters fetal central 5-HT signaling. 5-HT is a phylogenetically ancient neurotransmitter that is widely distributed throughout the entire brain. In the mature brain, 5-HT acts mainly as a modulatory neurotransmitter, regulating cognition, attention, emotion, learning, sleep, arousal, and stress responses. During neurodevelopment, 5-HT also acts as a trophic factor, regulating cell division, differentiation, migration, growth cone elongation, myelination, synaptogenesis, and dendritic pruning.5 Importantly, changing prenatal serotonergic tone affects these processes and alters in animal models.6
Figure 1.
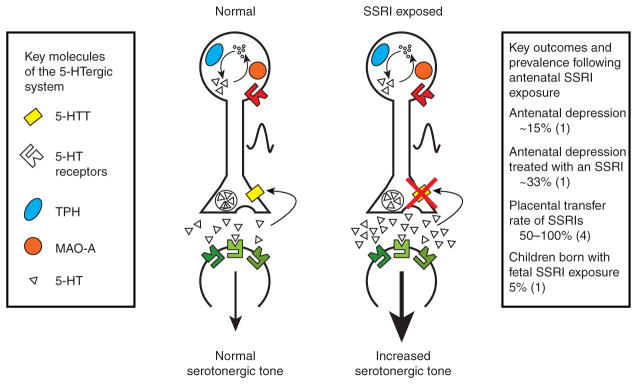
Molecular serotonin system components, effects of selective serotonin reuptake inhibitor (SSRI) exposure, and outcomes related to antenatal SSRI exposure. Pre- and postsynaptic components of the serotonin system with key molecules that regulate serotonin synthesis, release, reuptake, and degradation are shown under normal conditions (left) and under SSRI exposure (right). Blockade of the serotonin transporter leads to an increase in serotonergic tone. 5-HT, serotonin; 5-HTT, serotonin transporter; MAO-A, monoamine oxidase A; TPH, tryptophan hydroxylase.
Clinical studies investigating the effects of antenatal SSRI exposure on the child have been hampered by the difficulty of distinguishing between the consequences of drug treatment and the effects of antenatal maternal mood disturbances. To date, no gross SSRI-related neuroteratogenic outcomes have been identified, yet reports of subtle functional behavioral disturbances in early childhood associated with fetal SSRI exposure are beginning to emerge.
This article reviews the preclinical and clinical neurobehavioral consequences to the child of prenatal exposure to SSRIs and contrasts these outcomes with the consequences of undertreated antenatal maternal depression. The findings are presented in the context of a developmental model that reconciles conflicting associations between serotonin transporter inhibition, increased synaptic serotonin availability, and subsequent serotonergic deficits.
PRECLINICAL FINDINGS
Consequences of stress during pregnancy
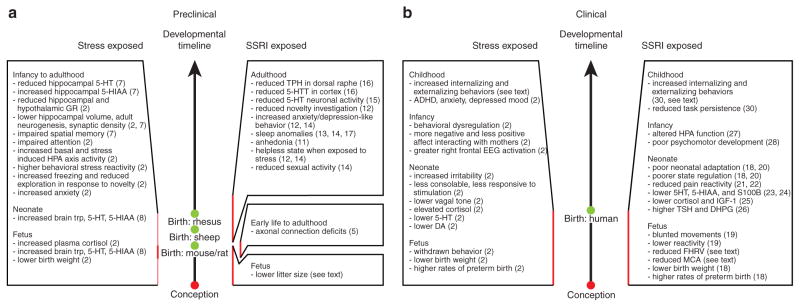
In attempting to understand the impact of prenatal SSRI exposure on fetal development, it is critical to distinguish between the impact of the medication itself and the maternal condition that required SSRI treatment (Figure 2a). Animal models allow for such a distinction to be made. Chronic antenatal stress, which in humans is often caused by maternal depression, increases the maternal and fetal plasma cortisol levels and has robust effects on fetal development.2 In rats, high, sustained gestational stress during the third week of pregnancy reduces hippocampal and hypothalamic glucocorticoid receptor levels, increases basal hypothalamic–pituitary–adrenal (HPA) axis activity, potentiates and prolongs HPA responses to stress in the offspring’s adulthood, and reduces hippocampal synaptic density.2,7 There are behavioral consequences as well. The fetal stress experience impairs spatial memory in young rats and reduces exploration in response to novelty in adulthood.2,7 Serotonin signaling is affected not only during prenatal stress but also long after, potentially causing changes in HPA activity and behavior. Specifically, gestational stress in rats increases the levels of tryptophan, 5-HT, and 5-hydroxyindoleacetic acid in the fetal brain until at least postnatal day P10;8 at P35, hippocampal 5-HT levels are reduced and 5-hydroxyindoleacetic acid levels are increased.7
Figure 2.
Comparison of preclinical and clinical findings associated with prenatal exposure to stress and selective serotonin reuptake inhibitor (SSRI) medications. (a) Preclinical and (b) clinical findings related to the consequences of exposure to stress or to SSRIs are summarized. Red vertical lines mark the time of exposure. In (a) the stress-exposed preclinical summary, light red represents the period of exposure in rhesus macaques and dark red represents the period of exposure in rats. 5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, serotonin; 5-HTT, serotonin transporter; ADHD, attention-deficit hyperactivity disorder; DA, dopamine; DHPG, dihydroxyphenylglycol; EEG, electroencephalography; FHRV, fetal heart rate variability; GR, glucocorticoid receptor; HPA, hypothalamic–pituitary–adrenal; IGF-1, insulin-like growth factor-1; MCA, middle cerebral artery; S100B, S100 calcium-binding protein B; TPH, tryptophan hydroxylase; trp, tryptophan; TSH, thyrotropin.
In nonhuman primates, prenatal stress results in reduced birth weights.2 The offspring of mothers who experienced stress during pregnancy display behavioral consequences at the juvenile, adolescent, and adult stages of life: impaired attention, increased anxiety and HPA-axis sensitivity, exacerbated responses to social isolation (e.g., increased adrenocorticotropic hormone levels, increased stereotypies), and blunted responses to subsequent reunion (e.g., reduced locomotor activity, exploration, and play).2 As in rodents, hippocampal anatomy is affected in nonhuman primates; gestational stress reduces hippocampal volume and adult hippocampal neurogenesis.2
Consequences of early 5-HTT blockade
Consequences of 5-HTT blockade on development have been investigated using pharmacological and genetic approaches in various species, including mice and rats, in which the first 10 postnatal days correspond to the third trimester of human fetal development.
Mice with genetically disrupted 5-HTT function (5-htt−/−) serve as an extreme model for lifelong SSRI exposure. In heterozygote breedings of this genotype, full blockade of 5-HTT function in fetal placental tissue has no effect on survival;9 however, prenatal fluoxetine reduces litter size (M.S.A., unpublished data).
5-HT−/− mice display behavioral, neurophysiological, neuroanatomical, and molecular alterations, some of which are developmental in origin, such as changes in neuronal cytoarchitecture, serotonergic physiology, neurobehavior, and sleep behavior. High central 5-HT levels during development (E15 to P7) in mice cause permanent axonal connection deficits in the somatosensory cortex and the lateral geniculate nucleus throughout life.5 In mice, increased 5-HT levels from P4 to P21 permanently reduce novelty investigation behavior, increase anxiety during conflict-associated tasks, and induce sleep anomalies, anhedonia, and a helpless state when exposed to stress.10–12 Interestingly, some of these behavioral effects have a peripubescent onset,10 suggesting that events in late brain maturation are required for neurobehavioral sequelae to manifest. In rats, P8 to P21 exposure to 5-HTT-blocking drugs also induces states of learned helplessness and sleep abnormalities and permanently reduces sexual activity.13 These behavioral changes correlate with reduced 5-HT neuronal firing activity and reduced tryptophan hydroxylase and 5-HTT immunoreactivity in the dorsal raphe and cortex, respectively.14,15 In sheep, intravenous infusion of fluoxetine during pregnancy decreases fetal low-voltage electrocortical activity and rapid eye movements and increases in utero quiet sleep.16
CLINICAL FINDINGS
Consequences of antenatal maternal mood
Exposure to maternal depression during pregnancy is among the earliest of adverse experiences and has long-term effects on the offspring (Figure 2b). Antenatal maternal stress disrupts fetal neurobehavioral development (i.e., alters reactivity in utero) and causes reduction in birth weight and an increase in the occurrence of premature births2. Exposure of the fetus to antenatal maternal depression has clinical consequences in the newborn infant: irritability, atypical frontal electroencephalography patterns, reduced vagal tone, elevated cortisol and norepinephrine levels, and lower dopamine and serotonin levels.2 The effects of antenatal maternal anxiety experienced in utero continue to influence cognitive, behavioral, and emotional outcomes throughout childhood.2 This finding holds true even after controlling for obstetric risk, psychosocial disadvantage, and post-natal maternal mood. Although the exact mechanism by which antenatal anxiety/stress influences fetal development remains unclear, the magnitude of the effect is clinically significant, with ~15% of the emotional behavioral problems in childhood being attributable to the contribution of antenatal stress/anxiety.2
Consequences of prenatal SSRI exposure
Soon after the introduction of SSRIs in 1988, their use to manage mood disorders during pregnancy led to reports of lower birth weight, higher rates of preterm births, and neurobehavioral disturbances in the infants.17 Subsequent studies have focused largely on neonatal outcomes, and only a limited number have investigated the effects of fetal SSRI exposure on cognition and behavior beyond the age of 4 years. Efforts to control for the effects of severity of maternal illness and related confounding factors (alcohol, other drug use, smoking) have been limited.
Fetal neurobehavior
Third-trimester exposure to SSRIs and maternal depression is associated with blunted fetal movement and lower reactivity to vibroacoustic stimuli.18 After controlling for maternal mood, prenatal exposure to an SSRI reduced fetal heart rate variability and middle cerebral artery flow resistance at 36 weeks gestation (K. Lim et al., personal communication). Whether these changes are transient and related to acute drug exposure or whether they are persistent and represent the early origins of altered neonatal or childhood neurobehavior remains to be determined.
Neonatal neurobehavior
A wide spectrum of neonatal neurobehavioral disturbances have been reported following gestational SSRI exposure, including tachypnea, temperature instability, poor feeding, irritability, weak or absent cry, increased motor activity, reduced heart rate variability, and altered behavioral state regulation.17,19 In response to an acute painful event, the duration of facial reaction and cardiac responses, particularly parasympathetic cardiac activity, is shorter and less intense in SSRI-exposed neonates.20 Altered response to pain persists at 2 months of age, even after controlling for drug levels and postnatal maternal mood.21
The severity of SSRI-induced neonatal behavioral disturbances is associated with reduced levels of 5-HT and 5-hydroxyindoleacetic acid in cord blood,22 reflecting altered central 5-HT activity. Neonates with fetal exposure to SSRIs also have lower cord blood levels of the astroglial-specific calcium-binding protein S100B, a biomarker of early brain maturation and central serotonergic function.23 Furthermore, fetal SSRI exposure is associated with increased norepinephrine metabolite (dihydroxyphenylglycol), and thyrotropin, and reduced cord blood levels of insulin-like growth factor-1.24,25 These findings might explain the impaired intrauterine growth in neonates exposed to SSRIs. Finally, prenatal SSRI exposure alters HPA stress response patterns and reduces basal cortisol levels in cord blood as well as in the early evening,26 even after controlling for pre- and postnatal maternal mood.
Infant and child neurobehaviors
Infants and toddlers who had been exposed to SSRIs in utero exhibited a poorer psychomotor development index (Bayley) between the ages of 6 and 40 months;27 however, at preschool ages they displayed typical intelligence, language, and behavioral patterns,28 when compared with children of nondepressed mothers. After controlling for pre- and postnatal maternal mood, both prenatal SSRI exposure and current maternal mood equally predicted increased internalizing behaviors in 3-year-olds (T.F. Oberlander., M. Papsdorf, U. Brain, S. Misri, C. Ross & R. Grunau, unpublished data). In a cohort of SSRI exposed 4-year-olds, externalizing behaviors and reduced persistence on a laboratory-based task were associated with increased cord blood drug levels and a history of neonatal withdrawal symptoms.29 These findings suggest that early neurobehavioral disturbances and pharmacological factors may predict an increased vulnerability to behavioral abnormalities in early childhood.
Other susceptibilities: genetic factors
Why some children are affected by prenatal SSRI exposure and others are not remains a pressing question. SSRI exposure and maternal mood likely interact with genetic factors to affect serotonergic tone during development and/or disease susceptibility later in life. A moderating role for genetic variables is beginning to emerge, but not all alleles carry the same risk. Among SSRI-exposed neonates, 5-min Apgar scores are lower and the risk for neuromotor symptoms is higher in carriers of short (s) promoter alleles of the 5-HTT gene, SLC6A4, which is associated with reduced SLC6A4 expression.30 In contrast, the risk for respiratory distress is higher in SSRI-exposed neonates with two copies of the long (l) allele.30 Infants with two high-activity MAO-A alleles have higher withdrawal-symptom scores than infants with at least one low-activity allele.24 Together, these findings may reflect “fetal serotonergic programming” arising from altered early levels of central 5-HT.
CONCLUSIONS
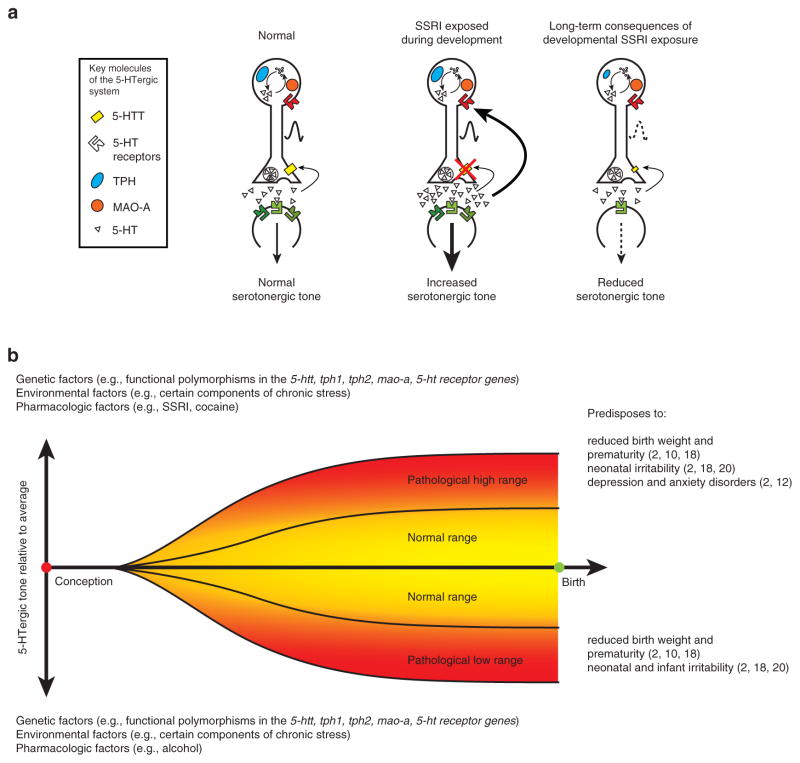
Studies of the neurobehavioral consequences of prenatal SSRI exposure have yielded two key findings. First, in animal models, early changes in serotonergic tone have molecular, neuroanatomical, and functional consequences, which are dependent on the timing (critical periods) and direction (increased or decreased) of change. Second, neurodevelopmental “main effects” following prenatal SSRI exposure in humans are few, and they are confounded by the difficulty in separating the impact of the antidepressants from the consequences of the maternal condition itself. Findings still support the hypothesis that, during brain development, auto-inhibitory feedback restrains the maturation of the 5-HT system. With feedback signaling being increased in the presence of high 5-HT tone (secondary to SSRI exposure), 5-HT system maturation is blunted (Figure 3a). If this hypothesis is true, it creates a clinical “catch-22” for the management of antenatal depression. Reducing antenatal maternal depressive symptoms using SSRIs elevates fetal 5-HT levels, ultimately restricting 5-HTergic system development. This would lead to decreased 5-HT signaling later in life, predisposing to psychopathology, such as depression, in the offspring. In this model, factors that influence fetal 5-HT levels, such as functional polymorphisms in serotonin system–related genes, environmental factors (e.g., prenatal stress), and pharmacologic factors (e.g., SSRIs, alcohol, cocaine), collectively set the net 5-HTergic tone5 (Figure 3b). Although this review focuses on the effects of increased developmental 5-HT tone, low 5-HT tone during development is also associated with neurobehavioral disturbances. Therefore, SSRI treatment during pregnancy could potentially have beneficial effects on fetal neurodevelopment by counteracting other factors that reduce fetal central 5-HTergic tone. Conversely, such exposure could also have detrimental effects, compounding the influence of other factors that increase fetal 5-HTergic tone. Emerging data reflecting an interaction between fetal SSRI exposure and genetic polymorphisms support this hypothesis. The central serotonergic auto-feedback model is far from being a unitary construct, and in order to further our understanding of factors that influence developmental 5-HT signaling, we need to investigate how functional alterations in specific raphe subregions and downstream effects on postsynaptic structures contribute to the complexity of behavioral outcomes.
Figure 3.
Long-term consequences of altered serotonergic tone. (a) Pre- and postsynaptic components of the serotonin system with key molecules that regulate serotonin synthesis, release, reuptake, and degradation are shown under normal conditions (left), SSRI exposure during development (middle), and in later life after developmental exposure to SSRIs. Although SSRI exposure during development leads to acute increases in serotonergic tone, it inhibits the development of the serotonin system and leads to reduced serotonin tone later in life. 5-HT, serotonin; 5-HTT, serotonin transporter; MAO-A, monoamine oxidase A; TPH, tryptophan hydroxylase. (b) Schematic representation of genetic, environmental, and pharmacological factors that collectively alter serotonergic tone during development. Examples of consequences of pathologically high or low serotonergic tone during development are listed. 5-ht, serotonin; 5-htt, serotonin transporter gene; mao-a, monoamine oxidase A gene; tph1, tryptophan hydroxylase 1 gene; tph2, tryptophan hydroxylase 2 gene.
Antenatal maternal mood disorders require urgent recognition and treatment. Yet, with questions about the sustained effects of prenatal SSRI exposure on the fetus remaining unanswered, the appropriateness of SSRIs for use during pregnancy remains unclear. Recognizing the dual risks associated with antenatal maternal mood disorders on the one hand and the management of such disorders with an SSRI on the other hand is critical for developing empirical, evidence-based approaches that identify the best fit between a pharmacological agent, nonpharmacological therapy, and maternal and neonatal factors so as to balance risks and benefits for mothers and their children.
Acknowledgments
We are grateful to Ursula Brain for her thoughtful editorial skill in the preparation of this manuscript. T.F.O. is supported by a HELP (Human Early Learning Partnership) Senior Career Award and holds the R. Howard Webster Professorship in Child Development (College for Interdisciplinary Studies, University of British Columbia). M.S.A. is supported by the Sackler Foundation, the National Alliance for Research on Schizophrenia and Depression, and the National Institute of Mental Health (NIMH). J.A.G. is supported by the Sackler Foundation and the NIMH.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63:898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- 2.Talge NM, Neal C, Glover V Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gentile S. SSRIs in pregnancy and lactation: emphasis on neurodevelopmental outcome. CNS Drugs. 2005;19:623–633. doi: 10.2165/00023210-200519070-00004. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, et al. Stereoselective disposition of fluoxetine and norfluoxetine during pregnancy and breast-feeding. Br J Clin Pharmacol. 2006;61:155–163. doi: 10.1111/j.1365-2125.2005.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 6.Ansorge MS, Hen R, Gingrich JA. Neurodevelopmental origins of depressive disorders. Curr Opin Pharmacol. 2007;7:8–17. doi: 10.1016/j.coph.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi A, Nagaoka M, Yamada K, Ichitani Y, Miake Y, Okado N. Maternal stress induces synaptic loss and developmental disabilities of offspring. Int J Dev Neurosci. 1998;16:209–216. doi: 10.1016/s0736-5748(98)00028-8. [DOI] [PubMed] [Google Scholar]
- 8.Peters DA. Maternal stress increases fetal brain and neonatal cerebral cortex 5-hydroxytryptamine synthesis in rats: a possible mechanism by which stress influences brain development. Pharmacol Biochem Behav. 1990;35:943–947. doi: 10.1016/0091-3057(90)90383-s. [DOI] [PubMed] [Google Scholar]
- 9.Lira A, et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- 10.Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 12.Popa D, Léna C, Alexandre C, Adrien J. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior. J Neurosci. 2008;28:3546–3554. doi: 10.1523/JNEUROSCI.4006-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel G, Neill D, Hagler M, Kors D. A new animal model of endogenous depression: a summary of present findings. Neurosci Biobehav Rev. 1990;14:85–91. doi: 10.1016/s0149-7634(05)80164-2. [DOI] [PubMed] [Google Scholar]
- 14.Kinney GG, Vogel GW, Feng P. Decreased dorsal raphe nucleus neuronal activity in adult chloral hydrate anesthetized rats following neonatal clomipramine treatment: implications for endogenous depression. Brain Res. 1997;756:68–75. doi: 10.1016/s0006-8993(97)00119-4. [DOI] [PubMed] [Google Scholar]
- 15.Maciag D, et al. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison JL, Chien C, Gruber N, Rurak D, Riggs W. Fetal behavioural state changes following maternal fluoxetine infusion in sheep. Brain Res Dev Brain Res. 2001;131:47–56. doi: 10.1016/s0165-3806(01)00255-3. [DOI] [PubMed] [Google Scholar]
- 17.Moses-Kolko EL, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. 2005;293:2372–2383. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- 18.Salisbury AL, Dipietro J. Prenatal serotonin reuptake inhibitor exposure: fetal and infant outcomes. Dev Psychobiol. 2006;48:624. [Google Scholar]
- 19.Zeskind PS, Stephens LE. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics. 2004;113:368–375. doi: 10.1542/peds.113.2.368. [DOI] [PubMed] [Google Scholar]
- 20.Oberlander TF, et al. Prolonged prenatal psychotropic medication exposure alters neonatal acute pain response. Pediatr Res. 2002;51:443–453. doi: 10.1203/00006450-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Oberlander TF, Grunau RE, Fitzgerald C, Papsdorf M, Rurak D, Riggs W. Pain reactivity in 2-month-old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics. 2005;115:411–425. doi: 10.1542/peds.2004-0420. [DOI] [PubMed] [Google Scholar]
- 22.Laine K, Heikkinen T, Ekblad U, Kero P. Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch Gen Psychiatry. 2003;60:720–726. doi: 10.1001/archpsyc.60.7.720. [DOI] [PubMed] [Google Scholar]
- 23.Pawluski JL, Galea LAM, Brain U, Papsdorf M, Oberlander TF. neonatal S100B protein levels after prenatal exposure to selective serotonin reuptake inhibitors. Pediatrics. 2009;124:e662–e670. doi: 10.1542/peds.2009-0442. [DOI] [PubMed] [Google Scholar]
- 24.Hilli J, et al. MAO-A and COMT genotypes as possible regulators of perinatal serotonergic symptoms after in utero exposure to SSRIs. Eur Neuropsychopharmacol. 2009;19:363–370. doi: 10.1016/j.euroneuro.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Davidson S, et al. Effect of exposure to selective serotonin reuptake inhibitors in utero on fetal growth: potential role for the IGF-I and HPA axes. Pediatr Res. 2009;65:236–241. doi: 10.1203/PDR.0b013e318193594a. [DOI] [PubMed] [Google Scholar]
- 26.Oberlander TF, et al. Hypothalamic-pituitary-adrenal (HPA) axis function in 3-month old infants with prenatal selective serotonin reuptake inhibitor (SSRI) antidepressant exposure. Early Hum Dev. 2008;84:689–697. doi: 10.1016/j.earlhumdev.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casper RC, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142:402–408. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- 28.Nulman I, et al. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med. 1997;336:258–262. doi: 10.1056/NEJM199701233360404. [DOI] [PubMed] [Google Scholar]
- 29.Oberlander TF, Reebye P, Misri S, Papsdorf M, Kim J, Grunau RE. Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med. 2007;161:22–29. doi: 10.1001/archpedi.161.1.22. [DOI] [PubMed] [Google Scholar]
- 30.Oberlander TF, Bonaguro RJ, Misri S, Papsdorf M, Ross CJ, Simpson EM. Infant serotonin transporter (SLC6A4) promoter genotype is associated with adverse neonatal outcomes after prenatal exposure to serotonin reuptake inhibitor medications. Mol Psychiatry. 2008;13:65–73. doi: 10.1038/sj.mp.4002007. [DOI] [PubMed] [Google Scholar]