Abstract
IMPORTANCE
Menopausal hormone therapy continues in clinical use but questions remain regarding its risks and benefits for chronic disease prevention.
OBJECTIVE
To provide a comprehensive, integrated overview of findings from the two Women’s Health Initiative (WHI) hormone therapy (HT) trials with extended post-intervention follow up.
DESIGN, SETTING, PARTICIPANTS, AND INTERVENTIONS
27,347 postmenopausal women, age 50–79 years, were enrolled at 40 US centers. Interventions were conjugated equine estrogens (CEE, 0.625 mg/day) with medroxyprogesterone acetate (MPA, 2.5 mg/day) for women with an intact uterus (N = 16,608) and CEE alone for women with hysterectomy (N= 10,739), or their placebos. Intervention continued for 5.6 and 7.2 years (median), respectively, with cumulative follow-up of 13 years through September 30, 2010.
MAIN OUTCOMES AND MEASURES
The primary efficacy and safety outcomes were coronary heart disease (CHD) and invasive breast cancer, respectively. A global index also included stroke, pulmonary embolism, colorectal cancer, endometrial cancer, hip fracture, and deaths. Secondary and quality-of-life outcomes were also assessed.
RESULTS
During the intervention phase for CEE+MPA, the hazard ratio (HR) for CHD was 1.18 (95% confidence interval [CI] 0.95–1.45) and overall risks outweighed benefits, with increases in invasive breast cancer, stroke, pulmonary embolism, and the global index. Other risks included increased dementia (in women >65 years), gallbladder disease, and urinary incontinence, while benefits included decreased hip fractures, diabetes, and vasomotor symptoms. Post-intervention, most risks and benefits dissipated, although some elevation in breast cancer risk persisted (cumulative hazard ratio [HR] =1.28; 95% confidence interval, 1.11–1.48). During intervention for CEE alone, risks and benefits were more balanced, with a HR for CHD of 0.94 (0.78–1.14), increased stroke and venous thrombosis, decreased hip fractures and diabetes, and over cumulative follow-up, decreased breast cancer (HR=0.79 [0.65–0.97]). Neither regimen affected all-cause mortality. With CEE, younger women (50–59 years) had more favorable results for all-cause mortality, myocardial infarction, and the global index (nominal P values for trend by age <0.05), but not for stroke and venous thrombosis. Absolute risks of adverse events (measured by the global index) per 10,000 women per year on CEE+MPA ranged from 12 excess cases for age 50–59 to 38 for age 70–79 and, for CEE, from 19 fewer cases for age 50–59 to 51 excess cases for age 70–79. Results for quality of life outcomes in both trials were mixed.
CONCLUSIONS AND RELEVANCE
Menopausal hormone therapy has a complex pattern of risks and benefits. While appropriate for symptom management in some women, its use for chronic disease prevention is not supported by the WHI randomized trials.
TRIAL REGISTRATION
clinical trials.gov Identifier: NCT00000611
INTRODUCTION
The Women’s Health Initiative (WHI) hormone therapy (HT) trials were designed to determine the benefits and risks of HT taken for chronic disease prevention by predominantly healthy postmenopausal women.1–3 Although originally prescribed primarily to treat vasomotor symptoms, menopausal HT had been increasingly viewed as a way to prevent many chronic diseases of aging, including coronary heart disease (CHD) and cognitive impairment4, 5 At least 40 percent of postmenopausal women in the United States were using HT shortly before the publication of the initial WHI findings.6 While observational studies had suggested net benefit for HT use,4, 5 no previous large-scale randomized prevention trial had addressed the balance of risks and benefits. In this context, the WHI HT trials were conceived and the most commonly used HT formulations in the U.S. at that time, conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) and CEE alone, were chosen as the interventions.1
Findings from the two HT trials have been published in numerous journals over the past decade2, 3, 7–15 (see full listing of previous reports in the online appendix), but no previous WHI publication has synthesized results for primary, secondary, and quality-of-life outcomes of the two trials over their intervention and post-intervention phases. In addition, for some endpoints, analyses have not been previously stratified by age or time since menopause. The goal of the present report is to provide a comprehensive, integrated overview of findings from the two WHI HT trials with extended post-intervention follow up (median, 13 years cumulative follow up) and stratification by age and other important variables.
METHODS
Study Design
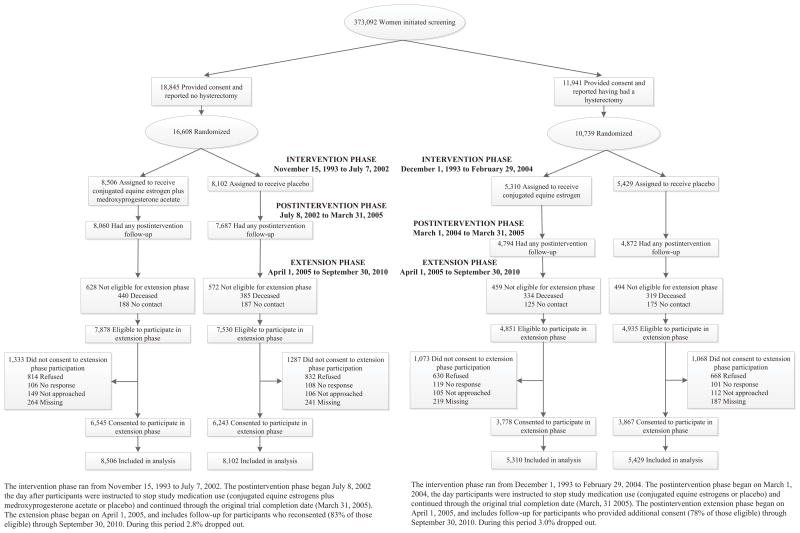
Details of the two WHI hormone therapy trial designs and outcome adjudication procedures have been published.1–3 Briefly, 27,347 postmenopausal women ages 50 to 79 were recruited from 1993 to 1998 at 40 U.S. clinical centers; 16,608 women with a uterus were randomized to daily oral CEE (0.625 mg) plus MPA (2.5 mg) (Prempro) or placebo and 10,739 women with hysterectomy were randomized to daily oral CEE (0.625 mg) alone (Premarin) or placebo. The primary efficacy and safety outcomes of the trial were CHD and invasive breast cancer, respectively. The sample sizes were based on power to detect effects on these outcomes.1 Race and ethnicity were self reported. Institutional review board approval was obtained at each clinical center and all participants provided written informed consent. Post-intervention follow-up through September 30, 2010 is based on 81.1% of surviving participants who provided written informed consent. Comparisons in the post-intervention phase need to be interpreted in the context of possible selection, due to effects in the preceding intervention phase and partial consent to further follow-up after 2005. .
Statistical Analysis
For each trial, intervention phase analyses included all randomized participants according to their randomization assignment until last intervention contact, using time-to-event methods based on the intention-to-treat principle. A global index of the monitored clinical events was calculated as time to first event for: coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, endometrial cancer (for estrogen-progestin only), hip fractures, and death from all other causes. Hazard ratios were estimated using Cox proportional hazards models stratified by age, prior disease (if appropriate), and randomization status in the WHI Dietary Modification trial. Models were constructed for each clinical endpoint: women contributed follow-up time until the end of the study phase, date of their first relevant clinical event, death, or withdrawal from the study, whichever came first. Comparisons in the post-intervention phase include randomized participants in active follow-up and at risk for an initial diagnosis of the relevant outcome. Cumulative results represent overall findings. Hazard ratios may exhibit time dependencies within or between phases, as previously reported.14, 15
All statistical tests are two-sided and nominal p values of 0.05 or less are regarded as significant. P-values do not adjust for multiple outcomes, sequential monitoring, or multiple subgroup comparisons; due to the large number of tests conducted, the p-values should be interpreted cautiously. Inference on subgroup analyses rely primarily on tests for interaction, which are also subject to multiple testing limitations when a large number of tests are conducted. Subgroup analyses, stratifying on age and time since menopause, are reported for most outcomes. Tests were based on a 1 degree-of-freedom test for trend where models included an interaction term between randomization arm and baseline group, which was coded ordinally. Adherence sensitivity analyses, conducted by censoring follow-up six months after non-adherence (taking <80% of study pills or starting non-protocol hormone therapy), included time-varying weights (inversely proportional to the estimated probability of continued adherence) in proportional-hazards models that adjusted for changes in the distribution of sample characteristics during follow-up. For secondary and quality-of-life outcomes, results are provided for the intervention phase and, where available, for the post-intervention and cumulative follow-up period. Additional analyses were conducted among women with no prior HT use before entry, as well as stratified by presence or absence of vasomotor symptoms at enrollment. All statistical analyses were conducted using SAS software version 9.3 (SAS Institute Inc., Cary, North Carolina) and R software version 2.15 (R Foundation for Statistical Computing, http://www.r-project.org/).
RESULTS
Baseline characteristics
Baseline characteristics for the two randomization groups in each trial were well balanced on demographic and disease risk factors (Table 1). When comparing characteristics between trials, however, several differences are seen. Compared to estrogen-progestin trial participants, women in the estrogen-alone trial were more racially diverse, more distant from menopause onset, had less favorable cardiovascular risk profiles, and more commonly had oophorectomy and prior HT use.
Table 1.
Baseline Characteristics of Participants in the Women’s Health Initiative Trials of Postmenopausal Hormone Therapy
| Characteristic | CEE+MPA Trial | CEE Alone Trial | ||||||
|---|---|---|---|---|---|---|---|---|
| Active (n=8506) | Placebo (n=8102) | Active (n=5310) | Placebo (n=5429) | |||||
| N | % | N | % | N | % | N | % | |
| Age at screening, y, mean ( SD) | 63.2 | (7.1) | 63.3 | (7.1) | 63.6 | (7.3) | 63.6 | (7.3) |
| Age group at screening, y, % | ||||||||
| 50–59 | 2837 | 33.4 | 2683 | 33.1 | 1639 | 30.9 | 1674 | 30.8 |
| 60–69 | 3854 | 45.3 | 3655 | 45.1 | 2386 | 44.9 | 2465 | 45.4 |
| 70–79 | 1815 | 21.3 | 1764 | 21.8 | 1285 | 24.2 | 1290 | 23.8 |
| Race/ethnicity, % | ||||||||
| White | 7141 | 84.0 | 6805 | 84.0 | 4009 | 75.5 | 4075 | 75.1 |
| Black | 548 | 6.4 | 574 | 7.1 | 781 | 14.7 | 835 | 15.4 |
| Hispanic | 471 | 5.5 | 415 | 5.1 | 319 | 6.0 | 332 | 6.1 |
| American Indian | 25 | 0.3 | 30 | 0.4 | 41 | 0.8 | 34 | 0.6 |
| Asian/Pacific Islander | 194 | 2.3 | 169 | 2.1 | 86 | 1.6 | 78 | 1.4 |
| Unknown | 127 | 1.5 | 109 | 1.3 | 74 | 1.4 | 75 | 1.4 |
| Years since menopause, y, % | ||||||||
| < 10 | 2780 | 36.2 | 2711 | 36.1 | 827 | 18.4 | 817 | 17.6 |
| 10 – <20 | 3049 | 39.7 | 2992 | 39.9 | 1438 | 32.0 | 1500 | 32.4 |
| ≥20 | 1850 | 24.1 | 1805 | 24.0 | 2230 | 49.6 | 2319 | 50.0 |
| Hormone use, % | ||||||||
| Never | 6277 | 73.8 | 6022 | 74.4 | 2769 | 52.2 | 2769 | 51.0 |
| Past | 1671 | 19.7 | 1587 | 19.6 | 1871 | 35.2 | 1947 | 35.9 |
| Current‡ | 554 | 6.5 | 490 | 6.1 | 669 | 12.6 | 709 | 13.1 |
| Vasomotor symptoms. % | ||||||||
| None | 5162 | 61.3 | 4928 | 61.5 | 2962 | 56.4 | 3004 | 56.0 |
| Mild | 2190 | 26.0 | 2115 | 26.4 | 1377 | 26.2 | 1441 | 26.9 |
| Moderate or severe | 1072 | 12.7 | 974 | 12.1 | 913 | 17.4 | 917 | 17.1 |
| Body mass index, kg/m2, median (IQR) | 27.5 (24.2–31.7) | 27.5 (24.3–31.7) | 29.2 (25.7–33.7) | 29.2 (25.7–33.5) | ||||
| Body mass index, kg/m2, % | ||||||||
| <25 | 2579 | 30.4 | 2479 | 30.8 | 1110 | 21.0 | 1096 | 20.3 |
| 25–29 | 2992 | 35.3 | 2835 | 35.2 | 1798 | 34.0 | 1915 | 35.5 |
| ≥30 | 2899 | 34.2 | 2737 | 34.0 | 2375 | 45.0 | 2385 | 44.2 |
| Systolic BP, mm Hg, mean (SD) | 127.6 | (17.6) | 127.8 | (17.5) | 130.4 | (17.5) | 130.2 | (17.6) |
| Diastolic BP, mm Hg, mean (SD) | 75.6 | (9.1) | 75.8 | (9.1) | 76.5 | (9.2) | 76.5 | (9.4) |
| Smoking, % | ||||||||
| Never | 4178 | 49.6 | 3999 | 50.0 | 2723 | 51.9 | 2705 | 50.4 |
| Past | 3362 | 39.9 | 3157 | 39.5 | 1986 | 37.8 | 2090 | 38.9 |
| Current | 880 | 10.5 | 838 | 10.5 | 542 | 10.3 | 571 | 10.6 |
| Never pregnant/no term pregnancy, % | 860 | 11.2 | 833 | 11.5 | 491 | 10.4 | 463 | 9.5 |
| Age at first birth (among those ever pregnant), y, % | ||||||||
| <20 | 1124 | 16.4 | 1117 | 17.3 | 1193 | 28.1 | 1234 | 28.0 |
| 20–29 | 4996 | 73.0 | 4698 | 73.0 | 2846 | 67.0 | 2914 | 66.1 |
| ≥30 | 727 | 10.6 | 624 | 9.7 | 210 | 4.9 | 260 | 5.9 |
| Age at hysterectomy, y, % | ||||||||
| <40 | 2100 | 39.8 | 2148 | 39.8 | ||||
| 40–49 | 2280 | 43.2 | 2275 | 42.2 | ||||
| 50–54 | 501 | 9.5 | 566 | 10.5 | ||||
| ≥55 | 401 | 7.6 | 404 | 7.5 | ||||
| Bilateral oophorectomy, % | 29 | 0.3 | 24 | 0.3 | 1938 | 39.5 | 2111 | 42.0 |
| Medical treatment, % | ||||||||
| Treated for diabetes | 374 | 4.4 | 360 | 4.4 | 410 | 7.7 | 412 | 7.6 |
| Treated for hypertension or BP ≥140/90 | 3377 | 43.2 | 3283 | 42.7 | 2651 | 53.3 | 2647 | 52.6 |
| Elevated cholesterol levels requiring medication | 1018 | 12.0 | 1027 | 12.7 | 763 | 14.4 | 829 | 15.3 |
| Statin use at baseline | 580 | 6.8 | 535 | 6.6 | 397 | 7.5 | 430 | 7.9 |
| Aspirin use (≥80 mg/d) at baseline | 1652 | 19.4 | 1654 | 20.4 | 1050 | 19.8 | 1081 | 19.9 |
| Medical history, % | ||||||||
| Myocardial infarction | 139 | 1.6 | 157 | 1.9 | 165 | 3.1 | 173 | 3.2 |
| Angina | 318 | 3.8 | 331 | 4.1 | 402 | 7.6 | 388 | 7.2 |
| CABG/PCI | 95 | 1.1 | 120 | 1.5 | 120 | 2.3 | 114 | 2.1 |
| Stroke | 61 | 0.7 | 77 | 1.0 | 76 | 1.4 | 92 | 1.7 |
| Deep vein thrombosis or pulmonary embolism | 79 | 0.9 | 62 | 0.8 | 87 | 1.6 | 84 | 1.5 |
| Family history of breast cancer | 1286 | 16.0 | 1175 | 15.3 | 892 | 17.9 | 870 | 17.1 |
| Fracture at age ≥55 | 1030 | 16.6 | 1027 | 16.7 | 676 | 17.3 | 643 | 16.4 |
| > High school/GED, % | 6272 | 74.1 | 5899 | 73.3 | 3488 | 66.3 | 3678 | 68.3 |
| Family income ≥ $50,000, % | 2447 | 30.4 | 2401 | 31.4 | 1148 | 22.9 | 1167 | 22.9 |
Required a 3-month washout prior to randomization
CABG/PCI=coronary artery bypass graft/percutaneous coronary intervention
Intervention in the CEE+MPA trial ended on July 7, 2002 (after a median [interquartile range] of 5.6 [4.8, 6.5] years) because of increased breast cancer risk and an unfavorable risk-benefit ratio with CEE+MPA2. Intervention in the CEE trial ended on February 29, 2004 (after a median of 7.2 [6.4, 8.1] years) because of increased stroke incidence not offset by lower CHD risk in the hormone group.3 Some HRs differ slightly from those previously reported due to the more complete outcome ascertainment. After intervention ended, follow-up continued through September 30, 2010 among surviving participants who provided written consent. The cumulative results reported here include a median post-intervention follow-up of 8.2 [6.6, 8.2]) years and median cumulative follow-up of 13.2 (10.5, 14.2) years for the CEE+MPA trial, and a median post-intervention follow-up of 6.6 [3.8, 6.6]) years and median cumulative follow-up of 13.0 (9.1, 14.1) years for the CEE-alone trial (see CONSORT diagram, Figure 1).
Figure 1.
CONSORT Diagram: Women’s Health Initiative trials of postmenopausal hormone therapy through extended follow-up. During the post-intervention and extension phases, fewer than 2% and 4% of women in the estrogen-progestin and estrogen-alone trials, respectively, reported use of hormone therapy.
Primary Endpoints in the Two Trials: Intervention and Post-Intervention Results
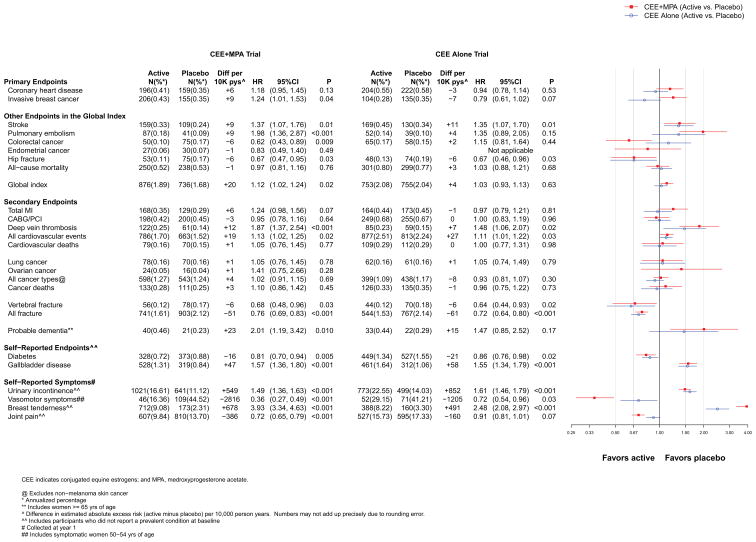
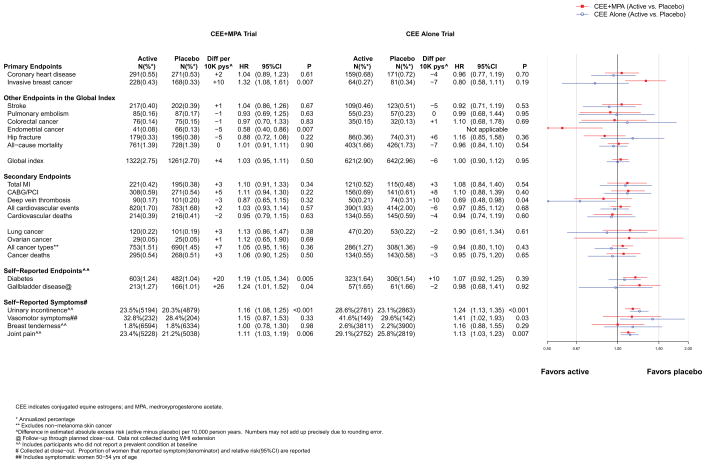
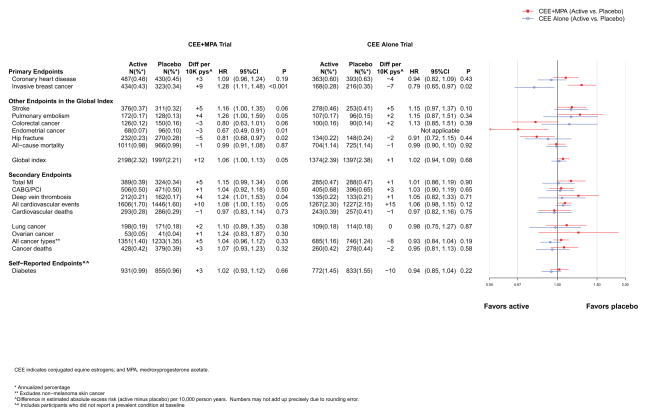
The intervention and post-intervention results for the primary efficacy (CHD) and safety (breast cancer) outcomes in the two trials are presented in Figures 2 and 3, respectively. Cumulative results for intervention plus post-intervention phases are shown in Figure 4. Figures include the number of incident cases, absolute risk differences (cases per 10,000 person-years [pys]) for each endpoint in the active minus placebo groups), hazard ratios (HRs), 95% confidence intervals (CIs), and a visual display of the HRs (95% CIs) for the two trials. The higher absolute risks in older than younger age groups are presented graphically in online eFigure 1. Results are summarized below.
Figure 2.
Number of events (annualized %), difference in absolute risks per 10,000 person–years, and hazard ratios (95%CI) for various health outcomes in the overall study population in the WHI Hormone Therapy Trials (intervention phase). The total cardiovascular disease outcome includes MI, CHD death, angina, heart failure, CABG/PCI, stroke, carotid artery disease, peripheral vascular disease, venous thromboembolism, and cardiovascular death.
Figure 3.
Number of events (annualized %), difference in absolute risks per 10,000 person–years, and hazard ratios (95%CI) for various health outcomes in the overall study population in the WHI Hormone Therapy Trials (postintervention phase). The total cardiovascular disease outcome is defined in the legend for Figure 2.
Figure 4.
Number of events (annualized %), difference in absolute risks per 10,000 person–years, and hazard ratios (95%CI) for various health outcomes in the overall study population in the WHI Hormone Therapy Trials (overall combined phases). The total cardiovascular disease outcome is defined in the legend for Figure 2.
Coronary heart disease (CHD)
CHD was defined as nonfatal myocardial infarction (MI) or coronary death. Results for total MI, a secondary endpoint, are reported separately below.
Intervention phase
Results for CHD differed between the two trials (Figure 2). Women assigned to CEE+MPA had a HR of 1.18 (95% CI, 0.95–1.45), as compared to placebo. The HR (95% CI) at year one was 1.80 (1.08–2.99), but was less elevated or neutral in subsequent years (p for trend by time = 0.03). Women assigned to CEE had a HR of 0.94 (95% CI, 0.78–1.14), as compared to placebo, and the HRs did not differ appreciably by year since randomization (p for trend by time = 0.21).
Post-intervention and cumulative follow-up phases
Post-intervention results in both trials were neutral (Figure 3). During cumulative 13-year follow-up, the HRs for CHD were 1.09 (0.96–1.24) for CEE+MPA and 0.94 (0.82–1.09) for CEE (Figure 4).
Stratified analyses (by age and time since menopause)
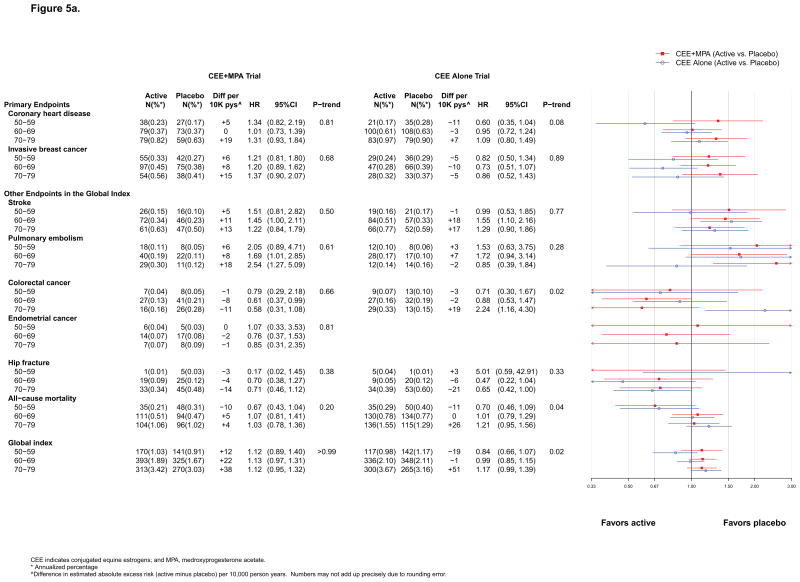
For CEE+MPA, HRs were similar by age (Figure 5a) but there was a non significant difference by time since menopause (p for trend =0.08), with significantly elevated risk among women more than 20 years past menopause onset (eFigure 2, online). For CEE, a trend for lower CHD risk in younger women was suggested (p for trend=0.08) (Figure 5a). Statistically significant differences by age or proximity to menopause for MI are described below.
Figure 5.
Figure 5a. Number of events (annualized %), difference in absolute risks per 10,000 person–years, and hazard ratios (95%CI) for various health outcomes in the WHI Hormone Therapy Trials (intervention phase) according to 10–year age groups at randomization.
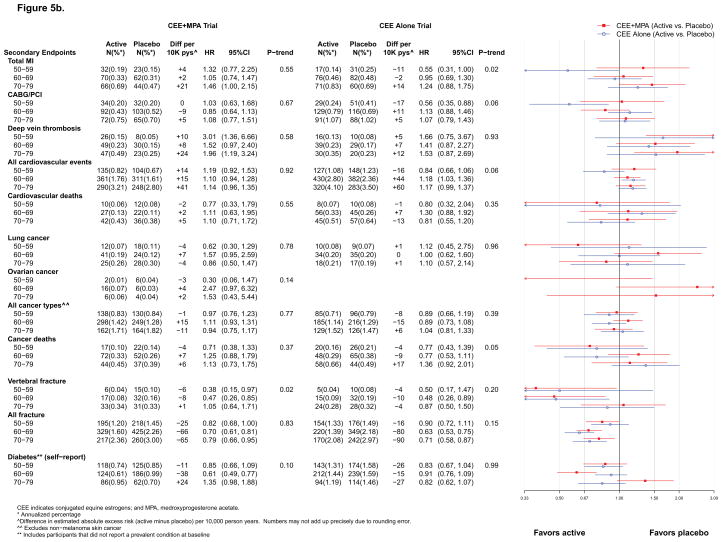
Figure 5b. Number of events (annualized %), difference in absolute risks per 10,000 person–years, and hazard ratios (95%CI) for secondary endpoints in the WHI Hormone Therapy Trials (intervention phase) according to 10–year age groups at randomization. The total cardiovascular disease outcome is defined in the legend for Figure 2.
Breast cancer
Intervention phase
Results for breast cancer differed between the two trials. The HR for breast cancer with CEE+MPA was 1.24 (1.01–1.53) (Figure 2). HRs progressively increased by time since randomization (p for time trend = 0.005), with cancers diagnosed at more advanced stages.16 In contrast, the HR for CEE was 0.79 (0.61–1.02) and HRs did not differ by time since randomization
Post-intervention and cumulative follow-up
The HR for breast cancer with CEE+MPA remained statistically significantly elevated during post-intervention and cumulative follow-up (HR for latter 1.28 [1.11–1.48]) (Figures 3 and 4), although more detailed time-dependent analyses identified risk attenuation with time since cessation of use.17 For CEE, the risk reduction became statistically significant during cumulative follow up (HR=0.79 [0.65–0.97]) (Figure 4).
Stratified analyses
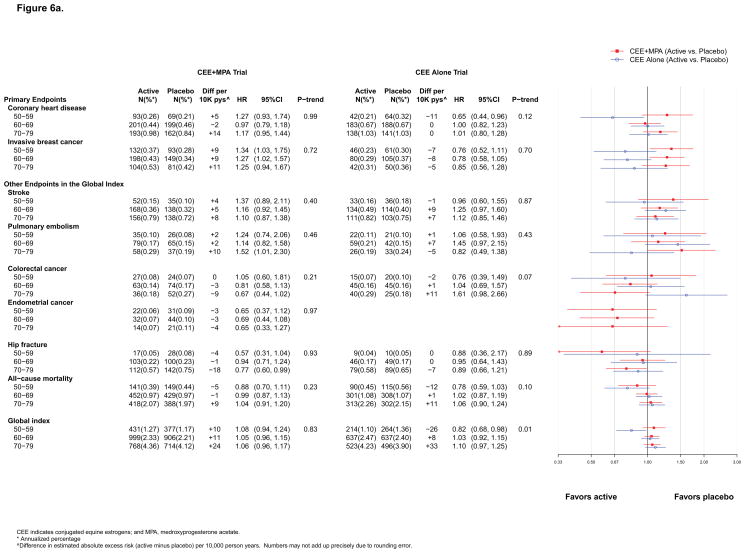
No appreciable differences by age or time since menopause emerged (Figure 5a, 6a and eFigure 2).
Figure 6.
Figure 6a. Number of events (annualized %), difference in absolute risks per 10,000 person–years, and hazard ratios (95%CI) for various health outcomes in the WHI Hormone Therapy Trials (overall combined phases) according to–10 year age groups at randomization.
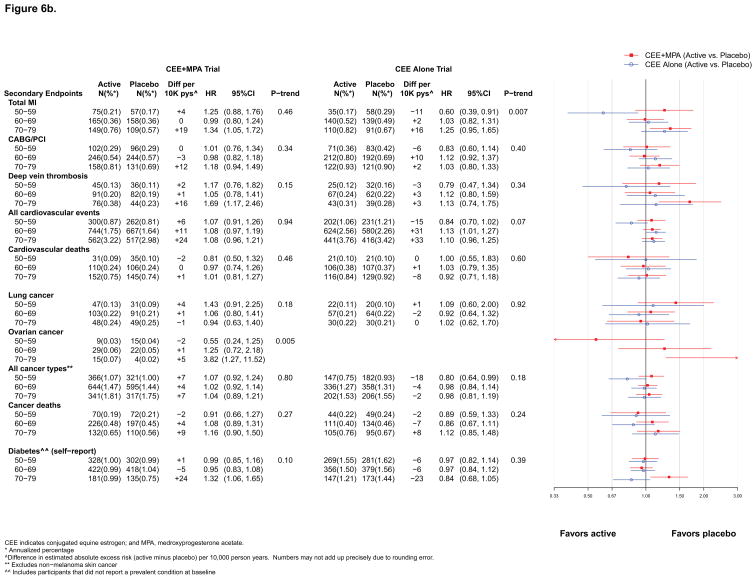
Figure 6b. Number of events (annualized %), difference in absolute risks per 10,000 person–years, and hazard ratios (95%CI) for secondary endpoints in the WHI Hormone Therapy Trials (overall combined phases) according to 10–year age groups at randomization. The total cardiovascular disease outcome is defined in the legend for Figure 2.
Other Endpoints in the Global Index: Intervention and Post-Intervention Results
Stroke
Intervention phase
Stroke risk was increased by 37% with CEE+MPA and by 35% with CEE (Figure 2), reflecting increased ischemic, but not hemorrhagic, stroke risk.10, 11
Post-intervention and cumulative follow-up
Post-intervention results were neutral in both trials (Figures 3 and 4). Cumulatively, the HRs for stroke were 15–16% higher in the hormone therapy groups in both trials (Figure 4).
Stratified analyses
No appreciable differences were seen in either trial (Figure 5a, 6a and eFigure 2).
Pulmonary embolism
Intervention phase
While a statistically significant doubling of pulmonary embolism (PE) risk was seen with CEE+MPA (Figure 2) the 35% higher PE risk with CEE was not statistically significant.
Post-intervention and cumulative follow-up
Post-stopping results were neutral in both trials (Figure 3). Cumulatively, the HRs were 1.26 (1.00–1.59) for CEE+MPA and 1.15 (0.87–1.51) for CEE (Figure 4).
Stratified analyses
No appreciable differences by age or time since menopause were seen in either trial (Figure 5a, 6a and eFigure 2).
Colorectal cancer
Intervention phase
Results for colorectal cancer differed between the two trials. For CEE+MPA, the HR was 0.62 (0.43–0.89) (Figure 2), but the cancers were diagnosed at a more advanced stage.18 CEE did not affect colorectal cancer incidence (HR =1.15 [0.81–1.64]).
Post-intervention and cumulative follow-up
Post-stopping and cumulative HRs were neutral in both trials (Figure 3 and 4).
Stratified analyses
For CEE, results were more adverse in older, compared to younger, women (p for trend = 0.02), but age differences were not apparent for CEE+MPA (Figure 5a).
Endometrial cancer
Intervention phase
Women in the CEE+MPA compared to placebo group had a HR of 0.83 (0.49–1.40) (Figure 2).
Post-intervention and cumulative follow-up
A reduced risk of endometrial cancer with CEE+MPA emerged post-intervention (HR=0.58 [0.40–0.86]) (Figure 3)) and for cumulative follow up (HR=0.67 [0.49–0.91]) (Figure 4).
Hip fracture
Intervention phase
Women in the CEE+MPA and CEE, compared to placebo, groups had statistically significant 33% reductions in hip fracture (Figure 2).
Post-intervention and cumulative follow-up
Post-intervention, the risk reductions were attenuated in both trials (Figure 3) but a significant fracture benefit persisted at 13 years for CEE+MPA (HR=0.81 [0.68–0.97]) (Figure 4).
Stratified analyses
Results in the CEE trial were more favorable for women with greater time since menopause (eFigure 2).
All-cause mortality
Intervention phase
Neither CEE+MPA nor CEE affected all-cause mortality (Figure 2).
Post-intervention and cumulative follow-up
All-cause mortality remained neutral post-intervention and during cumulative follow up in both trials (Figures 3 and 4) with cumulative follow-up HRs of 0.99 (0.91–1.08) for CEE+MPA and 0.99 (0.90–1.10) for CEE (Figure 4).
Stratified analyses
In both trials, patterns of more favorable results for all-cause mortality in younger than older women were apparent during the intervention phase, with HRs of 0.67 (0.43–1.04) and 0.70 (0.46–1.09) among women ages 50–59 in the CEE+MPA and CEE trials, respectively, but HRs ranged from 1.01 to 1.21 among women ages 60–79 (Figure 5a). The nominal p for trend by age was significant (p=0.04) only in the CEE trial. Trends with time since menopause were not significant (eFigure 2).
Global index
Intervention phase
Overall, the health risks of CEE+MPA significantly outweighed the benefits. For the global index of monitored events, which included the above outcomes, the HR was elevated at 1.12 (1.02–1.24) (Figure 2). In absolute terms, for every 10,000 women taking CEE+MPA per year, there were 6 more coronary events, 9 more strokes, 9 more pulmonary emboli, 9 more breast cancers, 6 fewer colorectal cancers, 1 fewer endometrial cancers, 6 fewer hip fractures, and 1 fewer death, yielding a net effect of 20 additional adverse events per 10,000 person-years (pys). The corresponding HR for the global index for CEE was 1.03, with a net of 4 adverse events.
Post-intervention and cumulative follow-up
As most risks became attenuated after stopping, the global index was neutral for both trials post-intervention and cumulatively (Figures 3 and 4).
Stratified analyses
The global index HR with CEE+MPA was not modified by age (p for trend >0.99) but for CEE was more favorable in younger women (p for trend = 0.02) (Figure 5a). In both trials, however, the absolute rates of adverse events were lower in younger than older women. For CEE+MPA compared to placebo, women aged 50–59, 60–69, and 70–79, had 12, 22, and 38 more adverse events per 10,000 pys, respectively. In contrast, for CEE compared to placebo, women aged 50–59 had 19 fewer adverse events, while women aged 70–79 had 51 more adverse events, per 10,000 pys (Figure 5a). Effect modification by age for CEE was more pronounced during cumulative follow-up (p for trend by age = 0.01; 26 fewer adverse events per 10,000 pys among women aged 50–59 and 33 more adverse events per 10,000 pys among women 70–79, for those assigned to CEE compared with placebo) (Figure 6a). Trends with time since menopause were not significant (eFigure 2).
Secondary Endpoints in the Two Trials: Intervention and Post-Intervention Results
The results for other clinical endpoints in the trial are summarized below, with more details available in the online Appendix.
Myocardial Infarction
Overall, results for MI were similar to those for CHD (Figure 2). However, differences by age or time since menopause emerged during the intervention phase of both trials. For CEE+MPA, statistically significant differences were apparent by time since menopause (HRs were 0.91, 1.16, and 1.99 with increasing decade past menopause, respectively; p for trend = 0.01) but not by age (Figure 5a). For CEE, the HRs increased with increasing decade of age (HRs 0.55, 0.95, and 1.24, respectively; p for trend by age = 0.02) (Figure 5b). Cumulatively, the differences by time since menopause for CEE+MPA persisted (p for trend =0.02) and the differences by age for CEE became more pronounced (p for trend =0.007) (Figure 6b).
Other secondary CVD outcomes
Results for CABG/PCI were neutral in both trials and findings for deep vein thrombosis generally paralleled those for pulmonary embolism described above (Figures 2–4). HRs were significantly elevated for total cardiovascular events during the intervention phase of both trials, but were neutral post-intervention. For cardiovascular death, results were neutral throughout (Figures 2–4).
Secondary cancer outcomes
The incidence of lung and ovarian cancer did not differ significantly between randomization groups in either trial (Figures 2–4). An adverse effect of CEE+MPA, but not CEE, on lung cancer mortality has been observed in WHI.19, 20 Neither intervention was associated with total cancer incidence (Figures 2–4); the cumulative HR was 1.04 (0.96–1.12) for CEE+MPA and 0.93 (0.84–1.04) for CEE (Figure 4). Younger women (aged 50–59) in the CEE compared to placebo group had a lower cumulative incidence of total cancer (HR=0.80 [0.64–0.99]) (Figure 6b). During intervention, total cancer mortality did not differ between randomization groups in either trial (Figure 2); during cumulative follow-up, the HRs were 1.07 (0.93–1.23) for CEE+MPA and 0.95 (0.81–1.13) for CEE. When examined by age, HRs for total cancer mortality in the CEE trial were more adverse for women above age 70 (HRs for increasing age groups were 0.77, 0.77, 1.36; p for trend = 0.05) (Figure 5b), but this trend was not significant in cumulative results. No effect modification by age or time since menopause was detected for cancer mortality in the CEE+MPA trial.
Clinical vertebral and total fractures
In both trials, results for clinical vertebral and total fractures paralleled those for hip fracture (Figures 2 and 5b).
Dementia
A subset of WHI participants aged ≥65 years at enrollment underwent cognitive testing in the WHI Memory Study (WHIMS).21, 22 HRs for probable dementia were 2.01 (1.19–3.42) during the intervention phase of the CEE+MPA trial and 1.47 (0.85–2.52) in the CEE trial (Figure 2). For women aged 50–55 years at randomization, cognitive assessments conducted an average of 7.2 years post-intervention showed neutral results.23
Self-Reported Endpoints, Self-Reported Symptoms, and Quality-of-Life Outcomes in the Two Trials
In both trials, women assigned hormones had significantly lower rates of treated diabetes than women assigned placebo (HR=0.81 [0.70–0.94] and 0.86 [0.76–0.98] for CEE+MPA and CEE, respectively) (Figure 2). Rates of gallbladder disease, however, were approximately 50% higher among women assigned hormones in both trials (Figure 2).). Self-reported urinary incontinence24 (at least once/week) was also higher in women assigned to CEE+MPA (HR=1.49 [1.36–1.63] or CEE (HR=1.61 [1.46–1.79] than to placebo (Figure 2) and were attenuated but still higher post-stopping in both trials (Figure 3). Post-intervention in both trials, the reductions in diabetes dissipated (Figures 3 and 4) while the HRs for gallbladder disease were attenuated but still elevated for CEE+MPA and became neutral for CEE. No significant differences by age group were observed for these outcomes.
Among younger women (ages 50–54) experiencing moderate or severe hot flashes and/or night sweats at enrollment (n=979), those in both the CEE+MPA and CEE groups had substantial reductions in symptoms (64% and 28%, respectively, compared to placebo at one year) (Figure 2). In the overall cohort, women assigned to CEE+MPA and CEE reported less sleep disturbances (assessed by a five-item validated scale25, 26), but more breast tenderness, than those receiving placebo (Figure 2 and eFigure 3). Women receiving CEE+MPA, but not CEE, were less likely to have joint pain than those receiving placebo. Regarding health-related quality of life (RAND-36 form) 9, 27 CEE+MPA, compared to placebo, was associated with a small but statistically significant benefit for physical functioning, role physical, bodily pain, and general health, and neutral results for the other subscales at one year (Appendix eFigure 3). CEE was associated with nominally significant adverse effects for social functioning and role emotional (eFigure 3). No significant differences in depressive symptom scores were observed. Post-intervention, symptoms of breast tenderness were similar between treatment arms in both trials but the direction of some of the other associations reversed (Figure 3), particularly joint pain. Additional discussion of these and other patient-reported outcomes are provided in the Appendix.
Additional Analyses Conducted in the Two Trials
Women without pre-randomization use of hormone therapy
Approximately one quarter of estrogen-progestin and one half of estrogen-alone trial participants had used HT pre-randomization. To simulate first initiation of HT in clinical practice, secondary analyses were conducted in women without pre-randomization HT use, stratified by age group (eFigure 4). The age-stratified findings remained similar to the primary analysis for CEE+MPA, but were slightly more favorable for younger women in the CEE trial. Among women aged 50–59 without prior HT use, the global index was significantly better for those assigned CEE compared to placebo (HR=0.71 [0.50–0.99]), with 40 fewer adverse events per 10,000 pys in the CEE group, compared to 34 excess events per 10,000 pys among women aged 70–79.
Analyses stratified by presence or absence of vasomotor symptoms at baseline
Women aged 70–79 with moderate-to-severe vasomotor symptoms at baseline assigned to CEE+MPA had a HR for CHD of 5.79 (1.29–25.97) while women in younger age groups (irrespective of vasomotor symptom status) did not have significantly elevated CHD risks (eFigure 5). Similarly, women aged 70–79 who had moderate-to-severe vasomotor symptoms and were assigned to CEE had a HR for CHD of 4.34 (1.43–13.14) compared to women assigned placebo, while women in younger age groups, with or without vasomotor symptoms, had no excess risk. Thus, CHD risk with both HT regimens was particularly high in the small group of women aged 70 and above with moderate-to-severe vasomotor symptoms (n=392; 4.8% and 8.7% of women in this age group in the CEE+MPA and CEE-alone trials, respectively), but the three-way interactions by age and vasomotor symptoms were nominally significant only for CEE (p=0.04). Such interactions were not observed for other disease outcomes.
Sensitivity analyses censoring for noncompliance with study pills
Secondary analyses among adherent women (censoring women within 6 months of reporting <80% compliance with study pills) were generally similar to intention-to-treat results but tended to accentuate the findings in each trial. For example, the intervention-phase adherence-adjusted HR for CHD was 1.32 (1.00–1.75) in the CEE+MPA trial and 0.85 (0.64–1.14) in the CEE trial, while the HR for breast cancer was 1.52 (1.15–2.00) in the CEE+MPA trial and 0.58 (0.39–0.84) in the CEE trial.
Other analyses
A detailed presentation of biomarker findings and analyses stratified by other baseline characteristics is beyond the scope of this manuscript. However, several additional analyses with potential relevance to clinical decision making about HT are summarized in the Appendix.
DISCUSSION
This report provides a comprehensive overview of findings from the intervention and extended post-intervention phases of the estrogen-progestin and estrogen-alone trials of the WHI, representing 13 years of cumulative follow up. Key findings include differences in the benefit-risk profile for CEE+MPA compared to CEE, the role of age and/or time since menopause in modifying HT effects on some outcomes, and the role of vasomotor symptoms in modifying CHD outcomes in older women using HT.
Overall, the risks of CEE+MPA during intervention outweighed the benefits. Most risks and benefits from CEE+MPA dissipated post-intervention; however, CVD events remained borderline elevated, a reduction in endometrial cancer emerged, and breast cancer HRs remained above unity. For CEE in women with prior hysterectomy, the benefits and risks during the intervention phase were more balanced, with increased risks of stroke and venous thrombosis, reduced risk of hip and total fractures, and a borderline reduction in breast cancer. Post-intervention with CEE, a significant decrease in breast cancer emerged and most other outcomes were neutral. Thus, breast cancer findings were divergent between the two trials and, for both cancer and CVD outcomes, results tended to be more adverse for CEE+MPA than for CEE.
HT effects on clinical outcomes were influenced in some cases by age or time since menopause. For CEE during the intervention phase, results were more favorable for younger than older women for all-cause mortality, MI, cancer deaths, and the global index. Both regimens, however, were associated with increased risk of stroke, venous thrombosis, gallbladder disease, and urinary incontinence, without clear differences by age. For CEE+MPA, breast cancer was an additional adverse effect and, although risk of MI varied by time since menopause, the overall risks outweighed benefits across all age groups. The potential influence of age or time since menopause on the relation between HT and vascular disease has received considerable attention.28–32 It has been postulated that estrogen may slow early stages of atherosclerosis and have favorable endothelial effects in recently menopausal women but have adverse and plaque-destabilizing effects on advanced atherosclerotic lesions.28, 32 Overall, the WHI findings suggest that HT has a harmful effect on CHD risk in older women, while the results in younger women remain inconclusive. Lower absolute risks of adverse events with HT in younger women, however, lead to lower attributable risks in these age groups. Whether menopausal hormone therapy has a particularly adverse effect on coronary risk in older women with vasomotor symptoms remains unclear.33–35 These symptoms have been associated with higher coronary risk in some reports,33, 35 and have been previously linked to adverse outcomes on HT among women with prevalent CHD.36 Due to the small sizes of these subgroups in the WHI and other studies, however, further research is needed.
CEE+MPA increased breast cancer incidence and the cancers were diagnosed at higher stage, likely reflecting diagnostic delay due to interference with mammographic detection 37. While a residual elevation in breast cancer risk was seen with CEE+MPA post-intervention, analyses demonstrated year-to year reductions in HRs after stopping. In contrast, the significant reduction in breast cancer seen with CEE38, 39 was unexpected and differs from results of many observational studies.40, 41 While differential mammography utilization in HT compared to non-HT users in observational studies may explain some of the differences, the opposite findings for CEE alone compared to CEE+MPA in the randomized trials points to a determinant influence of progestin on the breast epithelium.42 Full discussion of the complex processes mediating these differences43, 44 is beyond the scope of this report. Fewer colorectal cancers were diagnosed during CEE+MPA intervention but the cancers were diagnosed at higher stage, potentially reflecting differential detection (see Appendix).18 CEE+MPA reduced the risk of endometrial cancer but both HT regimens may increase ovarian cancer risk.45 CEE+MPA increased deaths from, but not incidence of, lung cancer, while CEE alone had no effect on these outcomes.20 Neither CEE+MPA nor CEE influenced total cancer incidence or total cancer mortality.
Both CEE+MPA and CEE reduced diabetes risk during intervention, when improvements in measured glucose and insulin levels were also documented,46, 47 but the risk reductions dissipated post-intervention. Both regimens increased risks for venous thrombosis and gallbladder disease. Among participants aged ≥65 years, HT increased probable dementia risk, with results for CEE+MPA more adverse than for CEE. Women aged 50–54 with moderate-to-severe vasomotor symptoms at baseline experienced symptom reductions with hormone therapy, and women overall had fewer sleep disturbances and joint pain, although incidence of rheumatoid arthritis was not reduced.48 Overall, results for self-reported symptoms with both interventions were mixed and few additional quality-of-life benefits were observed.
Despite the large size and numerous strengths of the WHI randomized trial, some limitations warrant consideration. Only one dose, formulation and route of administration in each trial was assessed: thus, results are not necessarily generalizable to other hormone preparations. Also, event information collected post-stopping represents unblinded reporting and nearly 20% of surviving participants did not consent to extended follow-up. Multiple outcomes and subgroups, some with low power, were examined, potentially leading to both false positive and false negative results. Thus, the nominal p-values and confidence intervals presented here should be interpreted cautiously.
In summary, although HT remains a reasonable option for the management of moderate-to-severe menopausal symptoms among generally healthy women in early menopause, current WHI findings do not support the use of either estrogen-progestin or estrogen alone for chronic disease prevention. The risks of CEE+MPA outweigh the benefits irrespective of a woman’s age; whereas for CEE in women with prior hysterectomy, a more favorable risk:benefit ratio is seen in younger women. However, even for younger women, increased risks of stroke and venous thrombosis, as well as gallstones and urinary incontinence, remain a concern with both regimens. These concerns, in conjunction with the multiple testing limitations attending subgroup analyses, preclude a recommendation in support of CEE use for disease prevention even among younger women. Current findings also suggest caution when considering HT treatment in older age groups, even in the presence of persistent vasomotor symptoms, given the high risk of CHD and other outcomes associated with HT use in this setting.
Supplementary Material
Appendix eFigure 1. Absolute risks and risk differences (per 10,000 person-years) for various health outcomes in the WHI Hormone Therapy Trials, by 10-year age groups.
Appendix eFigure 2. Number of events (annualized %), difference in absolute risks per 10,000 person–years, and hazard ratios (95%CI) for various health outcomes in the WHI Hormone Therapy Trials (intervention phase) according to time since menopause (10–year groups) at randomization.
Appendix eFigure 3. Mean (95% CI) and effect sizes for other health–related quality of life variables in the WHI Hormone Therapy Trials by randomization arm at Year 1 (intervention phase).
Appendix eFigure 4. Number of events (annualized %), difference in absolute risks per 10,000 person–years, and hazard ratios (95%CI) for various health outcomes in the WHI Hormone Therapy Trials (intervention phase) among women with no prior HT Use according to 10–year age groups at randomization
Appendix eFigure 5. Number of Events (annualized %) and hazard ratios (95%CI) for CHD in the WHI Hormone Therapy Trials (intervention phase) according to vasomotor symptoms and age at randomization
Acknowledgments
Funding/Support: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 321115, 32118–32119, 32122, 42107–26, 42129–32, and 44221. Wyeth Ayerst donated the study drugs.
Role of the Sponsor: The Women’s Health Institute (WHI) Project Office at the National Heart, Lung, and Blood Institute (NHLBI), the Sponsor, had a role in the design and conduct of the study; interpretation of the data; review and approval of the manuscript; and decision to submit the manuscript for publication. Decisions concerning the above, as well as data collection, management, and analysis, resided with committees comprised of WHI investigators and included NHLBI representatives.
WHI Investigators and Study Participants
The authors thank the WHI investigators, staff, and the trial participants for their outstanding dedication and commitment.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Chlebowski reports receiving consulting fees or honoraria from Novartis, Amgen, and Astra-Zeneca, fees for participation in review activities from Pfizer, payment for lectures from Novartis, and payment for educational activities from Educational Concepts Group. Dr. Jackson reports receiving consulting fee from Merck for educational materials on clinical trials methods and a pending grant to her institution from Pfizer for health education activities using electronic health records. Dr. Gass reports serving as the Executive Director of the North American Menopause Society. Dr. Wassertheil-Smoller reports payment for DSMB activities related to the Olagen Collagen Matrix Study of glaucoma.
Short List of WHI Investigators
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller Clinical Coordinating Center: Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
For a list of all the investigators who have contributed to WHI science, see: https://cleo.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf
Author Contributions: Dr. Manson and Mr. Aragaki had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study and design concept: Manson, Stefanick, Rossouw, Prentice, Anderson, Howard, Thompson, Wactawski-Wende, Eaton, Johnson, Kooperberg, Lewis, Liu, Wallace
Acquisition of data: Manson, Chlebowski, Stefanick, Prentice, Anderson, Howard, LaCroix, Wactawski-Wende, Jackson, Limacher, Wassertheil-Smoller, Beresford, Gass, Hsia, Johnson, Kooperberg, Lewis, Liu, Ockene, O’Sullivan, Van Horn, Vitolins, Wallace
Analysis and interpretation of data: Manson, Chlebowski, Stefanick, Aragaki, Rossouw, Prentice, Anderson, Howard, LaCroix, Jackson, Limacher, Margolis, Wassertheil-Smoller, Beresford, Cauley, Hsia, Johnson, Kooperberg, Kuller, Liu, Martin, Simon, Van Horn, Vitolins
Drafting of manuscript: Manson, Aragaki, Rossouw
Critical revision of manuscript for important intellectual content: Manson, Chlebowski, Stefanick, Aragaki, Rossouw, Prentice, Anderson, Howard, Thomson, LaCroix, Wactawski-Wende, Jackson, Limacher, Margolis, Wassertheil-Smoller, Beresford, Cauley, Eaton, Gass, Hsia, Johnson, Kooperberg, Kuller, Lewis, Liu, Martin, Ockene, O’Sullivan, Simon, Van Horn, Vitolins, Wallace
Statistical analysis: Manson, Aragaki, Prentice, LaCroix, Kooperberg, Liu
Obtaining funding: Manson, Stefanick, Rossouw, Prentice, Anderson, Howard, LaCroix, Wactawski-Wende, Jackson, Limacher, Wassertheil-Smoller, Hsia, Johnson, Lewis, Ockene, Wallace
Administrative, technical, or material support: Manson, Chlebowski, Stefanick, Rossouw, Prentice, Thomson, Wactawski-Wende, Jackson, Limacher, Wassertheil-Smoller, Beresford, Cauley, Eaton, Hsia, Johnson, Lewis, O’Sullivan, Simon, Van Horn
Study supervision: Chlebowski, Rossouw, Prentice, Anderson, Wactawski-Wende, Beresford, Wallace
Other (specify): Howard, Thomson (Chairs, WHI P & P Committee)
References
- 1.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 2.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 4.Guidelines for counseling postmenopausal women about preventive hormone therapy. American College of Physicians. Ann Intern Med. 1992;117(12):1038–41. doi: 10.7326/0003-4819-117-12-1038. [DOI] [PubMed] [Google Scholar]
- 5.Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117(12):1016–37. doi: 10.7326/0003-4819-117-12-1016. [DOI] [PubMed] [Google Scholar]
- 6.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291(1):47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 7.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349(6):523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 8.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289(24):3243–53. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 9.Hays J, Ockene JK, Brunner RL, Kotchen JM, Manson JE, Patterson RE, et al. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348(19):1839–54. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- 10.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289(20):2673–84. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 11.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, et al. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006;113(20):2425–34. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 12.Jackson RD, Wactawski-Wende J, LaCroix AZ, Pettinger M, Yood RA, Watts NB, et al. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: results from the women’s health initiative randomized trial. J Bone Miner Res. 2006;21(6):817–28. doi: 10.1359/jbmr.060312. [DOI] [PubMed] [Google Scholar]
- 13.Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166(3):357–65. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 14.Heiss G, Wallace R, Anderson GL, Aragaki A, Beresford SA, Brzyski R, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299(9):1036–45. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 15.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–14. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chlebowski RT, Anderson G, Pettinger M, Lane D, Langer RD, Gilligan MA, et al. Estrogen plus progestin and breast cancer detection by means of mammography and breast biopsy. Arch Intern Med. 2008;168(4):370–7. doi: 10.1001/archinternmed.2007.123. quiz 345. [DOI] [PubMed] [Google Scholar]
- 17.Chlebowski RT, Kuller LH, Prentice RL, Stefanick ML, Manson JE, Gass M, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360(6):573–87. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough RJ, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350(10):991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 19.Chlebowski RT, Schwartz AG, Wakelee H, Anderson GL, Stefanick ML, Manson JE, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women’s Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet. 2009;374(9697):1243–51. doi: 10.1016/S0140-6736(09)61526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chlebowski RT, Anderson GL, Manson JE, Schwartz AG, Wakelee H, Gass M, et al. Lung cancer among postmenopausal women treated with estrogen alone in the women’s health initiative randomized trial. J Natl Cancer Inst. 2010;102(18):1413–21. doi: 10.1093/jnci/djq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 22.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 23.Espeland MA, Shumaker SA, Leng I, Manson JE, Brown CM, Leblanc ES, et al. Long-Term Effects on Cognitive Function of Postmenopausal Hormone Therapy Prescribed to Women Aged 50 to 55 Years. JAMA Intern Med. 2013:1429–1436. doi: 10.1001/jamainternmed.2013.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendrix SL, Cochrane BB, Nygaard IE, Handa VL, Barnabei VM, Iglesia C, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA. 2005;293(8):935–48. doi: 10.1001/jama.293.8.935. [DOI] [PubMed] [Google Scholar]
- 25.Levine DW, Kripke DF, Kaplan RM, Lewis MA, Naughton MJ, Bowen DJ, et al. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):137–48. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 26.Levine DW, Dailey ME, Rockhill B, Tipping D, Naughton MJ, Shumaker SA. Validation of the Women’s Health Initiative Insomnia Rating Scale in a multicenter controlled clinical trial. Psychosom Med. 2005;67(1):98–104. doi: 10.1097/01.psy.0000151743.58067.f0. [DOI] [PubMed] [Google Scholar]
- 27.Brunner RL, Gass M, Aragaki A, Hays J, Granek I, Woods N, et al. Effects of conjugated equine estrogen on health-related quality of life in postmenopausal women with hysterectomy: results from the Women’s Health Initiative Randomized Clinical Trial. Arch Intern Med. 2005;165(17):1976–86. doi: 10.1001/archinte.165.17.1976. [DOI] [PubMed] [Google Scholar]
- 28.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–7. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 29.Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17(2):242–55. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- 30.Aging menopause cardiovascular disease and HRT. International Menopause Society Consensus Statement. Climacteric. 2009;12(5):368–77. doi: 10.1080/13697130903195606. [DOI] [PubMed] [Google Scholar]
- 31.Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95(7 Suppl 1):s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res. 2002;53(3):605–19. doi: 10.1016/s0008-6363(01)00466-7. [DOI] [PubMed] [Google Scholar]
- 33.Gast GC, Pop VJ, Samsioe GN, Grobbee DE, Nilsson PM, Keyzer JJ, et al. Vasomotor menopausal symptoms are associated with increased risk of coronary heart disease. Menopause. 2011;18(2):146–51. doi: 10.1097/gme.0b013e3181f464fb. [DOI] [PubMed] [Google Scholar]
- 34.Lantto H, Haapalahti P, Tuomikoski P, Viitasalo M, Vaananen H, Sovijarvi AR, et al. Vasomotor hot flashes and heart rate variability: a placebo-controlled trial of postmenopausal hormone therapy. Menopause. 2012;19(1):82–8. doi: 10.1097/gme.0b013e318221bae8. [DOI] [PubMed] [Google Scholar]
- 35.Gast GC, Pop VJ, Samsioe GN, Grobbee DE, Nilsson PM, Keyzer JJ, et al. Hormone therapy and coronary heart disease risk by vasomotor menopausal symptoms. Maturitas. 2011;70(4):373–8. doi: 10.1016/j.maturitas.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Huang AJ, Sawaya GF, Vittinghoff E, Lin F, Grady D. Hot flushes, coronary heart disease, and hormone therapy in postmenopausal women. Menopause. 2009;16(4):639–43. doi: 10.1097/gme.0b013e31819c11e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chlebowski RT, Anderson GL. Changing concepts: Menopausal hormone therapy and breast cancer. J Natl Cancer Inst. 2012;104(7):517–27. doi: 10.1093/jnci/djs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stefanick ML, Anderson GL, Margolis KL, Hendrix SL, Rodabough RJ, Paskett ED, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006;295(14):1647–57. doi: 10.1001/jama.295.14.1647. [DOI] [PubMed] [Google Scholar]
- 39.Anderson GL, Chlebowski RT, Aragaki AK, Kuller LH, Manson JE, Gass M, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–86. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997;350(9084):1047–59. [PubMed] [Google Scholar]
- 41.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–27. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 42.Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34(1):130–62. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis-Wambi JS, Jordan VC. Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Cancer Res. 2009;11(3):206. doi: 10.1186/bcr2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jordan VC, Ford LG. Paradoxical clinical effect of estrogen on breast cancer risk: a “new” biology of estrogen-induced apoptosis. Cancer Prev Res (Phila) 2011;4(5):633–7. doi: 10.1158/1940-6207.CAPR-11-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SA, Pettinger M, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women’s Health Initiative randomized trial. JAMA. 2003;290(13):1739–48. doi: 10.1001/jama.290.13.1739. [DOI] [PubMed] [Google Scholar]
- 46.Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47(7):1175–87. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 47.Bonds DE, Lasser N, Qi L, Brzyski R, Caan B, Heiss G, et al. The effect of conjugated equine oestrogen on diabetes incidence: the Women’s Health Initiative randomised trial. Diabetologia. 2006;49(3):459–68. doi: 10.1007/s00125-005-0096-0. [DOI] [PubMed] [Google Scholar]
- 48.Walitt B, Pettinger M, Weinstein A, Katz J, Torner J, Wasko MC, et al. Effects of postmenopausal hormone therapy on rheumatoid arthritis: the women’s health initiative randomized controlled trials. Arthritis Rheum. 2008;59(3):302–10. doi: 10.1002/art.23325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix eFigure 1. Absolute risks and risk differences (per 10,000 person-years) for various health outcomes in the WHI Hormone Therapy Trials, by 10-year age groups.
Appendix eFigure 2. Number of events (annualized %), difference in absolute risks per 10,000 person–years, and hazard ratios (95%CI) for various health outcomes in the WHI Hormone Therapy Trials (intervention phase) according to time since menopause (10–year groups) at randomization.
Appendix eFigure 3. Mean (95% CI) and effect sizes for other health–related quality of life variables in the WHI Hormone Therapy Trials by randomization arm at Year 1 (intervention phase).
Appendix eFigure 4. Number of events (annualized %), difference in absolute risks per 10,000 person–years, and hazard ratios (95%CI) for various health outcomes in the WHI Hormone Therapy Trials (intervention phase) among women with no prior HT Use according to 10–year age groups at randomization
Appendix eFigure 5. Number of Events (annualized %) and hazard ratios (95%CI) for CHD in the WHI Hormone Therapy Trials (intervention phase) according to vasomotor symptoms and age at randomization