Abstract
Modulation of serotonin transporter (5-HTT) function causes changes in affective behavior, both in humans and rodents. Stressful life events likewise affect emotional behavior. In humans, a low-expressing genetic 5-htt variant, the s allele of the 5-htt linked promoter region, has been associated with increased risk for depression only where there was a history of stressful life events. To investigate this gene by environment interaction in mice, we compared the effects of inescapable shocks on the behavior of wild-type (5-htt+/+), heterozygote (5-htt+/−), and serotonin transporter deficient (5-htt−/−) mice. Inescapable shocks induce behavioral changes including a shock escape deficit, in a subsequent test when escape is possible. Confirming a gene by environment interaction, we found that stress increases escape latencies in a gene-dose dependent manner (5-htt−/− > 5-htt+/− > 5-htt +/+), where as there were no differences among the genotypes in the unstressed condition. The vulnerability to increased escape latency could not be accounted for by enhanced fear learning, as 5-htt−/− mice did not show heightened fear conditioning. The interaction of 5-htt genotype and stress appeared to produce a selective behavioral vulnerability, because no interaction of 5-htt genotype and stress was observed in other measures of anxiety and depression-linked behavior, including the open field, novelty suppressed feeding, and forced swim tests. We replicated prior findings that the 5-htt−/− displays heightened anxiety and depression-like behavior at baseline (unstressed condition). In conclusion, our data offers the possibility for future investigation of the neural basis underlying 5-htt genotype by stress interaction demonstrated here.
Keywords: serotonin transporter, Slc6a4, shock escape, depression, anxiety, animal model
Introduction
The serotonin transporter (5-HTT) conveys serotonin from the extracellular to the intracellular space for reuse or degradation. By regulating extracellular serotonin concentration, the 5-HTT plays a key role in the activity of serotonin receptors. Modulation of 5-HTT function has long been implicated in psychiatric phenomena. For example, many antidepressants, such as serotonin selective reuptake inhibitors and tricyclic antidepressants, block 5-HTT function. In addition, a common polymorphism in the promoter region of the human 5-htt (the 5httlpr) influences 5-htt transcription and has been associated with elevated trait anxiety (Lesch et al., 1996) and increased vulnerability to affective disorders (Collier et al., 1996) in many but not all studies (Mendlewicz et al., 2004, Wray et al., 2009). Intriguingly, a variant of the 5httlpr may confer vulnerability to depression only in the presence of stressful life events (Caspi et al., 2003). Specifically, individuals carrying the low expressing “s” allele are more vulnerable to depression following stressful events than individuals carrying the higher expressing “l” allele. Replication efforts have yielded mixed results (e.g., yes: Ressler et al., 2009, no: Aslund et al., 2009), from which meta-analyses have concluded that there is no convincing evidence of the gene-environment interaction (Munafo et al., 2009, Risch et al., 2009). A significant strength of the Caspi study was that some information about stressful life events was collected at time points prior to the time point at which measures of depression were taken. The strength of this prospective approach is not present in many other studies. Using experimental animals to investigate a 5-htt genotype by environment interaction in anxiety and depression-like behavior offers the advantage that controlled levels of stressors can be introduced experimentally, with systematic and prospective observation of behavior. Further, if such an interaction can be identified, its neurobiological mechanism can also be studied with a broad range of experimental approaches.
To investigate the putative 5-htt genotype by stress interaction we employ mice with no (5-htt+/+), partial (5-htt+/−) and complete (5-htt−/−) loss of 5-HTT function. Naïve 5-htt−/− mice show a number of anxiety and depression-like phenotypes (Lira et al., 2003; Holmes et al., 2003). Notably, 5-htt+/− mice are behaviorally indistinguishable from 5-htt+/+ mice under standard laboratory rearing conditions, even though 5-htt+/− mice have a demonstrable reduction in 5-HTT function when compared to 5-htt+/+ mice (Mathews et al., 2004, Montanez et al., 2003).
An aversive series of inescapable shocks produces a constellation of behaviors consistent with increased anxiety and depression (Overmier & Seligman, 1967, Weiss, 1968). Naïve wild-type animals exposed to inescapable shocks show depression-like behavior such as increased immobility in the forced swim test and delayed escape from footshocks in a shuttlebox test where escape is possible (Maier & Watkins, 2005).
Our goal is to identify, in mice, a stress by serotonin transporter genotype interaction, which may serve as a model to understand such an interaction in humans. Particularly, we believe the comparison of stress's effects on 5-htt+/− mice, which have reduced but not zero levels of the transporter, and WT mice, which have a full complement of transporter, could be useful to understand the effect of stress on humans with less ('s' allele carriers) or more ('l' allele carriers) serotonin transporter expression. We hypothesize that stress will differentially affect mice depending on their genetic 5-htt complement. Here, we confirm this hypothesis, demonstrating that reduced number of functional 5-htt alleles confers a dose dependent vulnerability to the behavioral effects of inescapable footshocks.
Methods
Many of the methods here (Subjects, Open Field, Novelty Suppressed Feeding, Shock Escape, and statistics) have been previously used in the lab and their descriptions have been adapted from an earlier report (Ansorge et al., 2008).
Subjects
For the Interaction of 5HTT genotype and Inescapable Shock and 5HTT and Fear Conditioning experiments, mice heterozygous for the non-functional 5-htt mutation on a 129S6/SvEv background (founders from the Taconic Farms line), were crossed to produce wild-type, heterozygous, and knockout offspring. Pregnant females were housed 2 to a cage, and remained together after giving birth. To which of two mothers a pup belonged was not tracked. Pups of the same genotype from a given cage were distributed across experimental conditions as much as possible, but it was rare to have an equal number of pups of each genotype available per homecage to distribute evenly across each genotype-by-stress condition. Thus, the possibility that the results are partially skewed by litter effects exists, but is mitigated by the large number of litters used. For the Interaction of 5HTT genotype and Inescapable Shock experiment, 5-htt−/−, 5-htt+/−, and WT subjects came from 46 litter pairs. For the 5HTT and Fear Conditioning experiment, 5-htt−/− and WT subjects came from 22 litter pairs. Mice were weaned on P28, ear-tagged, and genotyped using PCR of genomic DNA isolated from tissue samples. Animals were separated by sex and group housed with all genotypes present in each cage. For the Inescapable Shock Session Intensity Assay, 129S6/SvEv male mice ordered from Taconic that arrived as adults to our facility were used. Mice were maintained on a 12 h light/dark cycle (light from 7:00 A.M. to 7:00 P.M.) and provided with food and water ad libitum (except as noted). Animal care and use was in accordance with the National Institutes of Health guide for the care and use of animals and approved by our institution's animal care committee.
Procedures
Inescapable shock stress
The procedure was conducted in a two chamber shuttle box (model ENV 010MD; Med Associates, St. Albans, VT) located within a sound-attenuated cubicle. The apparatus was equipped with a grid floor made of a stainless steel and connected to a shock generator. The scrambled shock generator (model ENV 414S, Med Associates) created varying electrical potential differences between bars preventing an animal from avoiding shock. For each shuttlebox two animals were administered the protocol at the same time; the central door was closed, with one animal in each side's chamber. After a 3 min habituation period, the shock deliveries began as described in the design section below. No-shock control animals were placed in pairs on each side of identical chambers, for the same duration, but no shocks were administered.
Open field test
Mice were tested in Plexiglas activity chambers (model ENV-520; Med Associates) (43.2 wide by 43.2 deep by 30.5 cm high) equipped with infrared beams located 1.5 cm above the chamber floor and spaced 2.5 cm apart to detect horizontal activity. Vertical activity was detected by infrared beams 6 cm above the chamber floor and spaced 2.5 cm apart. Testing took place under bright ambient light conditions (800–900 lux). Mice were placed into the front left corner of the open field and activity was recorded for 30 min. To compare activity in the center versus periphery, the center of the open field was defined as the central 15 by 15 cm region.
Novelty-suppressed feeding
Animals were weighed and then food deprived for 24 h with free access to water. Then, each subject was placed in a brightly lit (800–900 lux) open box (51 wide by 35 deep by 15 cm high) containing clean wood chip bedding. A manila cardboard disk 125 mm in diameter was secured in the center of the field. One familiar food pellet weighing ~4 g was bound to the disk. Mice were removed from their home cage, placed in a holding cage for 30 min before the test, and then placed in one corner of the box. The latency to bite the pellet was recorded. After 10 s of sustained feeding (remaining in contact with the disk while biting or chewing) or after 12 minutes, the test ended. Between animals, fresh bedding was added and the disk was cleaned. Immediately after the NSF test, a subject was placed alone in its home cage with a single food pellet for 5 min. Food consumption was defined as the difference in weight of the pellet before and after the test. At the end of the test, the subject was weighed.
Forced Swim Test
Mice were placed in a 3L clear plastic bucket filled with 2.2L of water at 25 +/− 1 °C for 7 min. Afterward, animals were gently wiped with a paper towel and monitored until their coats were dry. Videotape of the test was scored for the amount of immobility (cessation of limb, tail, and trunk movements that propel the mouse). The last 4 minutes of scored data was used in the analysis.
Shock escape
Mice were tested in the same shuttlebox used in the inescapable shock procedure. The box consisted of two identical chambers (20 wide by 17 deep by 20 cm high), separated by an automated door that opened vertically. The shuttlebox was equipped with 8 infrared beams (4 on each side) for detecting position and activity of the animal. One mouse was placed into the chamber with the door raised and was allowed to freely explore both compartments for 3 min. Then the door was closed.
At the beginning of each trial, the door was raised. 5 seconds later a foot shock (0.4 mA) was delivered. The subject's exit from the shocked side ended the trial. If the subject did not exit after 15 seconds, the shock was turned off and the trial ended. The door was lowered at the end of the trial. A session consisted of 30 trials separated by a 30 s intertrial interval. Escape latencies were computed as the time from shock onset to the end of trial. If the subject failed to make a transition the maximum 15 s was used for the escape latency score.
Fear conditioning
The fear conditioning protocol consisted of 3 days of procedures. The auditory stimulus was a 20 s 2.5 kHz 89 dB tone. The aversive stimulus was a 2s 0.7 mA footshock. The stimuli were presented in the same chambers used for inescapable shocks described above, with the door kept open at all times. On the first 2 days there were two conditioning categories: paired, in which the shock came on at the end of the tone; and unpaired, in which the shock and tone presentations were separated in time. Half of the animals of each genotype were assigned to each of the conditioning categories. On day 1, in both conditioning categories shock onsets occurred at the following times after the procedure was initiated: 79, 239, and 359s. For the paired condition, tone onsets occurred at 60, 220, and 340 s. For the unpaired condition, tone onsets occurred at 120, 160 and 440 s. On day 2, shock onsets occurred at 199, 299, and 419s. For the paired condition tone onsets occurred at 180 280 and 400 s. In the unpaired condition tone onsets occurred at 240 320 and 360 s. On day 3, three 20 s tone presentations were administered over 480 s, in the absence of any footshock. The tone onsets occurred at 100 200 and 280 s. In order to alter the contextual information available on day 3, the chamber's walls were covered with a novel material, a novel odor was wiped on the surroundings, the grid floor was replaced by a tray covered in familiar bedding, and the experimenters used different textured gloves in handling the animals. Freezing behavior was defined as the absence of all but breathing related movement. The percent of time freezing in the 40s prior to any stimuli presentation on day 1 was used as an index of the baseline levels of the freezing behavior. Context conditioning was defined as freezing in the 60s prior to the first stimulus presentation on day 2. To compare response to the tone in the paired and unpaired conditions, the percentage of freezing in the 60s preceding the first tone onset on day 3 was subtracted from the percentage of freezing in the 60s following that tone onset.
Design
Separate cohorts of animals were used for each of the 3 following experiments.
Inescapable Shock Session Intensity Assay
At 3 months of age wild-type mice were assigned to one of 5 conditions of shock session intensity. The conditions chosen were no shocks (None); 40 shocks of 3s average duration, 0.6 mA, and an average intershock interval of 15 s (Low); 70 shocks of 3s average duration, 0.6 mA, and an average intershock interval of 15 s (Med); 90 shocks of 6s average duration, an average intershock interval of 17 s, and with the first 45 shocks at 0.6 mA and the latter 45 at 0.7 mA (MedHi); and 140 shocks of 6s average duration, an average intershock interval of 17 s, and with 0.6 mA for the first 46 shocks, 0.7 mA for the next 46 and 0.8 mA for the final 48 shocks (High). In all conditions, 3 sessions were administered: 24, 72, and 96 hours prior to the shock escape test.
Interaction of 5HTT genotype and Inescapable Shock
At 3 months of age experimental animals were administered 1 footshock session. A session consisted of 70 shocks of 0.6 mA current with mean duration of 3 s and a mean inter-shock interval of 15 s. Control animals were exposed to the chambers for the same length of time without shocks. 2 h later, the open field test was administered. 24 h later a second footshock session and control exposure procedure were administered. 2 hours later the Novelty Suppressed Feeding test was administered. 48 h after the second session, a third shock session and control exposure session were administered. 2 h later the Forced Swim Test was administered. 24 h later, the Shock Escape Test was administered.
5HTT and Fear Conditioning
At 3 months of age the fear conditioning procedure was administered. Wild-type and 5-htt knockout mice were included. Half of each genotype was assigned to the tone-shock condition, and the other half to the shock-only condition.
Statistical analysis
Data were analyzed using analyses of variance (ANOVA). The criterion for significance was p = 0.05. The criterion for a trend was 0.05 < p < 0.1. Post hoc pairwise comparisons were conducted using the Fisher Least Significant Difference test. To make pairwise comparisons for two-way ANOVA's, the analysis was split by one variable, retaining the other as the independent variable, and then Fisher Least Significant Difference tests were used on each of the two split ANOVA's. For the Interaction of 5HTT genotype and Inescapable Shock experiment, data from male and female mice were analyzed for sex effects. As with previously published data (Ansorge et al., 2008, Ansorge et al., 2004, Lira et al., 2003), no significant main nor three-way-interaction effects of sex were detected; so male and female data were combined in the presented analyses.
Results
Effect of inescapable shock session intensity on shock escape latency
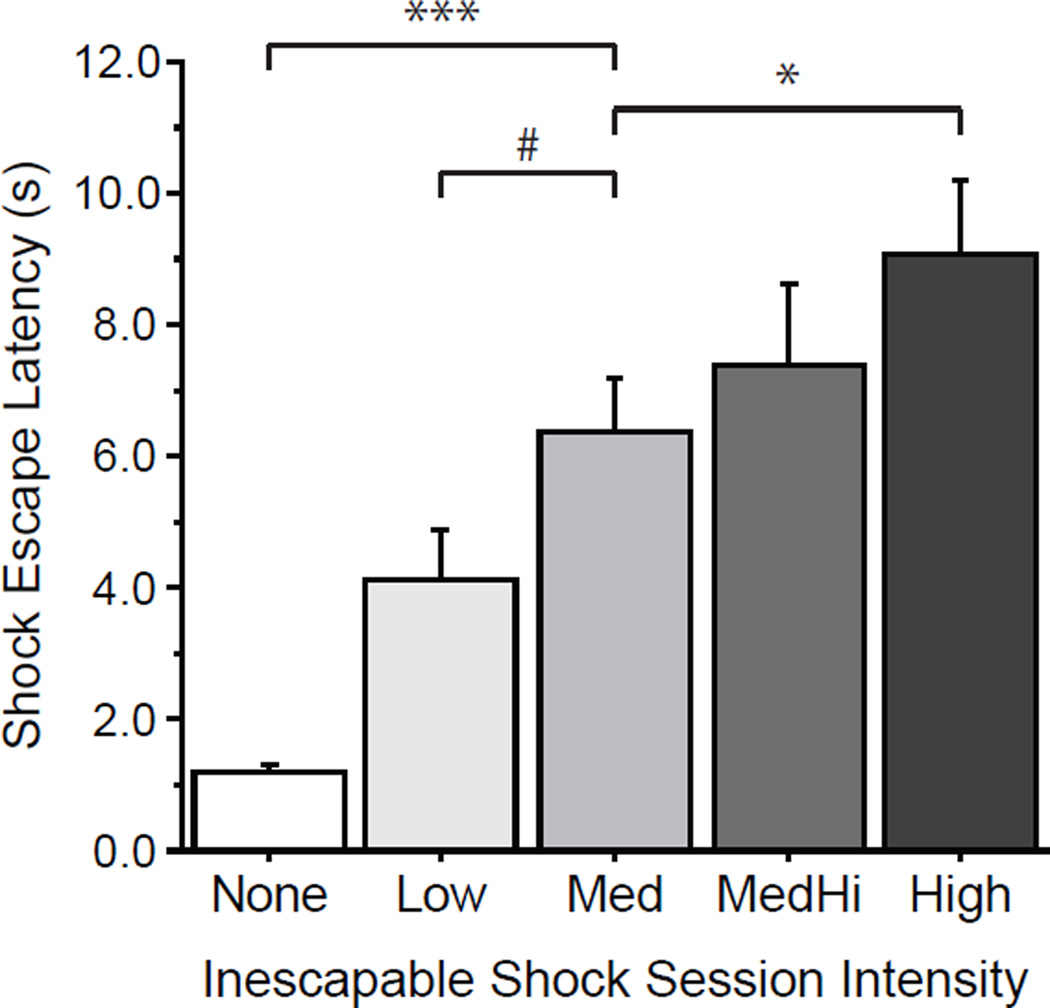
The higher the intensity of the preceding inescapable shock sessions, the slower animals were to escape from footshocks (F(4,74) = 11.7, p < .0001). Post hoc testing showed a number of conditions differed from each other: subjects in the no shock control condition escaped significantly faster than subjects in all inescapable shock conditions (vs. Low p = .022, compared to each of the other conditions p < .0001); the Low condition mean escape latency was quicker than that of the Medium condition at trend level (p = .074), and was significantly faster than the higher inescapable shock session intensity conditions (vs. Medium-High p = .011, High p = .002); and finally, the Medium condition’s mean escape latency was faster than that of the High condition (p = .038). The results from 79 wild-type mice were reported: 16 None, 16 Low, 16 Med, 16 Medium-High, and 15 High subjects. (See Figure 1.)
Figure 1. Inescapable Shock Session Intensity.
Mean (and standard error) latency in seconds to escape from a footshock by running through a door to the other side of a two-sided chamber is shown by group. Prior to the shock escape test, animals were exposed to inescapable shock sessions. Intensity of shock sessions was varied from no shocks (None) through a range of intensities, Low, Medium (Med), Medium High (MedHi), and High. Parameters of the intensity levels are found in the methods. The greater the intensity of inescapable shock session, the longer was the escape latency. The Medium intensity was selected for the 5-htt genotype testing. Symbols indicate groups that differed from the Medium condition: # p < .1, * p < .05, *** p < .001.
Effect of genotype and stress on shock escape
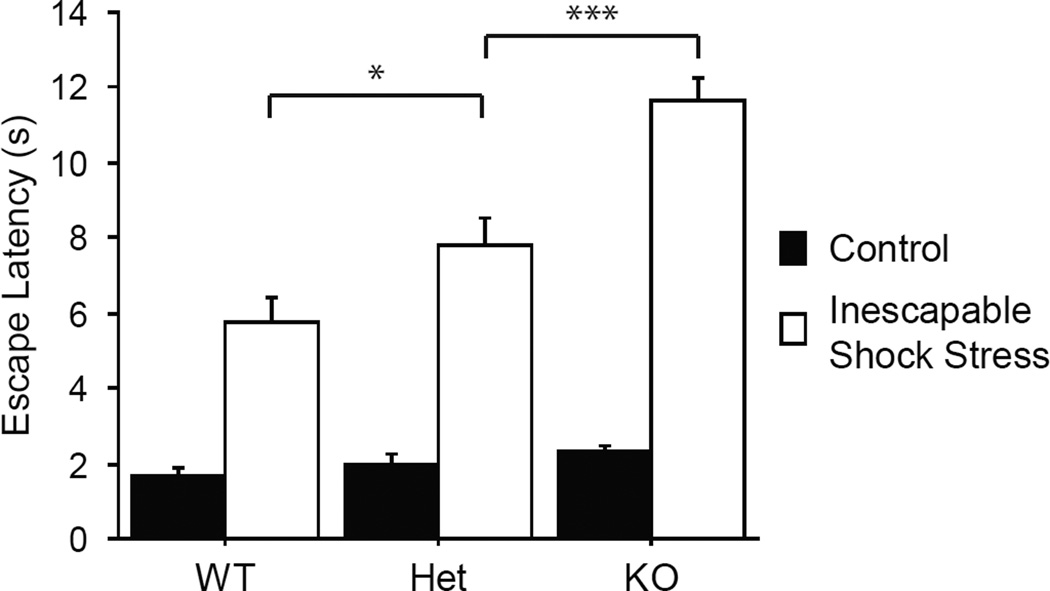
To investigate the consequences of stress as a function of 5-htt genotype, we administered inescapable shock sessions using the Medium intensity condition in 5-htt+/+, 5-htt+/−, and 5-htt−/− mice. The Medium intensity inescapable shock condition was selected to allow for the detection of possible positive or negative modifying effects of genotype. Confirming our previous results, we detected a main effect of stress F(1,146) = 216, p < .0001. Shock-exposed animals took longer to escape compared to controls. We also detected a main effect of genotype F(2,146) = 18.4, p < .0001. Importantly, we detected an interaction between stress and genotype F(2,146) = 12.4, p < .0001. Posthoc analysis revealed that stressed 5-htt+/− and 5-htt−/− mice took significantly longer to escape in the shuttle box shock escape test than did stressed WT animals. Further, stressed 5-htt−/− mice had longer escape latencies than 5-htt+/− mice (WT - 5-htt−/−, p < .0001; WT - 5-htt+/−, p = .044; 5-htt+/− - 5-htt−/−, p = .0002). There were however no such differences among genotypes in the unstressed control condition F(2,72) = 1.7, p = .19. The numbers of subjects in each condition were as follows: WT control n = 26, stressed n = 25; 5-htt+/− control n = 26, stressed n = 28; 5-htt−/− control n = 23, stressed n = 24. (See Figure 2.)
Figure 2. Shock Escape test of the serotonin genotype by stress environment experiment.
Mean (and standard error) latency in seconds to escape from a footshock by running through a door to the other side of a two-sided chamber is shown by group. Serotonin transporter (5-htt) knockout mice (KO) with no functional transporter, heterozygote animals (Het) with one functional and one disrupted allele, and wild-type subjects (WT) were either exposed previously to 3 sessions of inescapable footshocks (Inescapable Shock Stress) or just placed in chambers identical to those where inescapable shock sessions were conducted (Control). The prior inescapable shock stress increased escape latency. 5-htt deficient mice showed increased escape latencies. Stressed KO mice escaped more slowly than stressed WT animals. Most importantly for the Het mouse as a model of gene environment interaction, stressed Het mice were significantly slower to escape than stressed WT animals. Further, the Het animals showed an intermediate phenotype; mean escape latency among the Het mice was faster than that of the KO subjects. No differences among genotypes were observed in the no-shock control condition. * p < .05, *** p < .0001.
Effect of genotype on fear conditioning
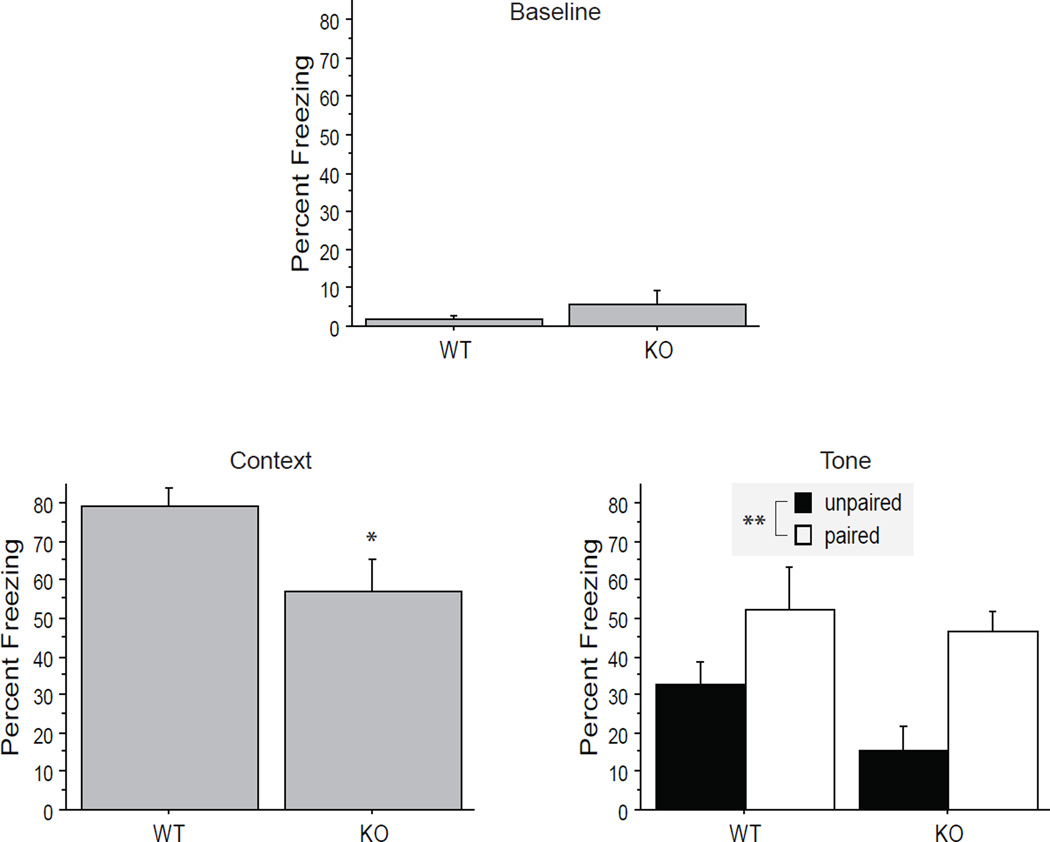
Whether the vulnerability of reduced serotonin transporter genotype to increased shock escape latency could be accounted for by enhanced fear conditioning was addressed next. In fact, no enhanced fear conditioning was observed. There was no significant difference between genotypes in the baseline condition, F(1,31) = 1.6, p = .21. 5-htt−/− mice froze less than WT animals to the context F(1,31) = 5.6, p = .025, but did not differ significantly in freezing to the tone F(1,29) = 2.4, p = .13. Animals exposed to paired tone-shock presentations on days 1 and 2 froze more to the tone (alone) on day 3 than animals exposed to unpaired tones and shocks on days 1 and 2, F(1,29) = 12.0, p = .0017. The numbers of subjects in each condition were as follows: WT unpaired n = 10, WT paired n = 8, 5-htt−/− unpaired n = 7, 5-htt−/− paired n = 8. (See Figure 3.)
Figure 3. Fear conditioning by serotonin transporter genotype.
Mean (and standard error) percent freezing data of wild-type (WT) and serotonin transporter knockout (KO) mice are shown for 3 conditions. Freezing is defined as the absence of all but breathing related movement. In the "Baseline" condition, percent freezing is shown for the period in the first day of conditioning, before any tones or shocks were presented. For the "Context" condition, freezing is shown for the period in the second day before any tones or shocks were presented. For the "Tone" condition, the difference in freezing percent is shown between the period following the first tone onset on the third day (on which the context was altered, and no shocks were presented) and the freezing percent observed in the period just prior to that tone onset. There was no significant difference between genotypes in the baseline condition. KO mice froze less than WT animals to the context, but did not differ significantly in freezing to the tone. Animals exposed to paired tone-shock presentations on days 1 and 2 froze more to the tone (alone) on day 3 than animals exposed to unpaired tones and shocks on days 1 and 2. * p < .05, ** p < .01.
Effect of genotype and stress on other behavior indices
The vulnerability of transporter deficient mice to uncontrollable shock stress was selective to the shock escape test. Other indices revealed no significant evidence that stress preferentially enhanced anxiety and depression-like behavior in transporter deficient mice. For each of the tests reviewed below, number of subjects, group means and standard errors are presented in Table 1.
Table 1. Other outcome measures of the serotonin transporter genotype by stress environment (control or inescapable shocks) experiment.
For each outcome measure (named in the first column) there is an indication whether each effect was significant: genotype, stress, and interaction of stress and genotype.
| Outcome Measure |
Effect | Genotype | Treatment | n | Mean | SE | ||
|---|---|---|---|---|---|---|---|---|
| Genotype | Stress | G × E interaction |
||||||
| Ambulatory distance in OF | ** | * | ns | WT | Control | 24 | 974 | 85 |
| Inescapable Shock | 26 | 860 | 107 | |||||
| Het | Control | 27 | 980 | 75 | ||||
| Inescapable Shock | 27 | 718 | 92 | |||||
| KO | Control | 24 | 719 | 108 | ||||
| Inescapable Shock | 25 | 531 | 101 | |||||
| Percent distance in OF center | # | ** | ns | WT | Control | 25 | 1.33 | 0.52 |
| Inescapable Shock | 26 | 2.78 | 0.81 | |||||
| Het | Control | 26 | 0.69 | 0.19 | ||||
| Inescapable Shock | 25 | 2.43 | 0.83 | |||||
| KO | Control | 23 | 0.26 | 0.12 | ||||
| Inescapable Shock | 23 | 1.13 | 0.46 | |||||
| Bite latency in NSF (s) | *** | ns | * | WT | Control | 21 | 59.3 | 8.3 |
| Inescapable Shock | 22 | 66.0 | 9.6 | |||||
| Het | Control | 17 | 76.4 | 12 | ||||
| Inescapable Shock | 19 | 102 | 20 | |||||
| KO | Control | 21 | 194 | 28 | ||||
| Inescapable Shock | 20 | 129 | 20 | |||||
| 5 min pellet consumption (g) | ns | ns | ns | WT | Control | 20 | .135 | .015 |
| Inescapable Shock | 20 | .165 | .018 | |||||
| Het | Control | 18 | .128 | .023 | ||||
| Inescapable Shock | 19 | .111 | .025 | |||||
| KO | Control | 20 | .100 | .021 | ||||
| Inescapable Shock | 20 | .140 | .011 | |||||
| Percent weight lost in NSF | ns | ns | ns | WT | Control | 17 | 8.48 | .68 |
| Inescapable Shock | 17 | 8.58 | .62 | |||||
| Het | Control | 15 | 8.30 | .78 | ||||
| Inescapable Shock | 17 | 8.20 | .72 | |||||
| KO | Control | 18 | 7.50 | .96 | ||||
| Inescapable Shock | 17 | 7.32 | .76 | |||||
| Immobility min 3–7 in FST | *** | ns | ns | WT | Control | 27 | 148 | 13 |
| Inescapable Shock | 26 | 158 | 11 | |||||
| Het | Control | 26 | 139 | 11 | ||||
| Inescapable Shock | 27 | 157 | 10 | |||||
| KO | Control | 25 | 214 | 9 | ||||
| Inescapable Shock | 26 | 214 | 11 | |||||
p < .05,
p < .01,
p < .0001,
p < .1,
"ns" not significant.
In addition, for each outcome measure, group results including number of subjects, mean, and standard error of the group are indicated.
Abbreviations: KO – knockout with no functional serotonin transporter, Het – one functional and one disrupted 5HTT allele, WT – wild-type, OF – Open Field, NSF – Novelty Suppressed Feeding, FST – Forced Swim Test.
Open Field
Total locomotion
Inescapable shock stress reduced total locomotion in the 30 minute test. The main effect of stress was significant F(1,147) = 5.9, p = .017. Transporter deficient mice had reduced locomotion in the test. There was a main effect of 5-htt genotype F(2,147) = 5.0, p = .0077. Fisher PLSD post hoc tests showed that 5-htt−/− mice traveled significantly less than 5-htt+/− (p = .018) and WT (p = .0029) animals; 5-htt+/− and WT subjects did not differ significantly from each other (p = .49). There was no significant interaction of stress and transporter genotype F(2,147) = .31, p = .74.
Proportion in Center
Inescapable shock stress increased the proportion of total distance traveled in the center region of the open field. There was a main effect of stress F(1,140) = 8.7, p = .0038. There was a trend for transporter deficient mice to have a reduced proportion of their travel in the center F(2,140) = 2.9, p = .059. There was no evidence of an interaction between stress and transporter genotype for the proportion of locomotion in the center F(2,140) = .30, p = .74.
Novelty Suppressed Feeding
There was no main effect of stress on latency to bite a pellet lying in the center of an exposed field F(1,114) = .53, p = .47. We replicated our previous observation that 5-htt−/− mice show increased latency to bite; there was a main effect of 5-htt genotype F(2,114) = 16.6, p < .0001. 5-htt−/− mice had longer latencies than each of the other genotypes (5-htt+/− p = .0002; 5-htt+/+ p < .0001). WT and 5-htt+/− did not differ (p = .14). Inescapable shock interacted with 5-htt genotype in the novelty suppressed feeding test F(2,114) = 3.5, p = .034. The interaction likely arose from the different direction of change in bite latency among 5-htt genotypes. For WT and 5-htt+/− mice that were shocked, there was a non-significant increase in the latency to bite the pellet in the novelty suppressed feeding test (NSF). In 5-htt−/− animals, there was a trend for shock stress to reduce bite latency, p = .07. See Supplemental Figure S1.
The difference in bite latency between WT and 5-htt+/− subjects in the control condition was not significant, p = .54. The difference between WT and 5-htt+/− mice in the stressed group was also not significant, p = .13. In the unstressed control condition, 5-htt−/− mice were significantly different from both WT, and 5-htt+/− animals, each p < .0001; in the stressed condition, 5-htt−/− subjects were significantly different only from WT mice, p = .0095.
Forced Swim Test
There was no effect of inescapable shock on immobility duration in the forced swim test F(1,150) = 1.9, p = .17. There was no interaction of stress and 5-htt genotype F(2,150) = .17, p = .88. We did replicate our previous finding that 5-htt deficient mice are more immobile than WT animals. The main effect of genotype was significant F(2,150) = 28, p < .0001. This effect of genotype was carried by 5-htt−/− mice. Post hoc testing showed 5-htt−/− animals differed significantly from WT and 5-htt+/− subjects (p < .0001 for each in the control condition; and in the stress condition, p = .0002 for 5-htt+/− mice and p = .0003 for WT animals). 5-htt+/− and WT mice did not differ either in control or stress conditions (p = .56, p = .94, respectively).
Discussion
We demonstrated that, in wild-type mice, escape latency increases with the intensity of inescapable shock sessions. Further, among shocked animals, 5-htt−/− and 5-htt+/− mice had longer escape latencies than wild-type animals. Moreover, 5-htt+/− mice displayed an intermediate phenotype: longer escape latency than WT and shorter than 5-htt−/−. There were no differences among genotypes in the unstressed condition. The gene-environment interaction was selective to the shock escape test, as there were no similar interactions in the other assessments of anxiety and depression-like behavior.
Previous findings regarding 5-htt genotype and inescapable shocks were replicated. 5-htt−/− mice showed reduced activity in the open field, increased latency to feed in a novel context, and increased immobility in a forced swim (Ansorge et al., 2004, Lira et al., 2003). We also observed the expected effect of inescapable shock sessions: shocked subjects took significantly longer to escape compared to unshocked control subjects. We assessed shock escape behavior using a current intensity (0.4 mA) less than that used in the inescapable shock sessions (0.6 mA). It is possible that the increased latency to escape in the shocked group reflects a familiarity with a more intense shock, and hence a relative low valuation of the aversiveness of the escapable shock compared to unshocked controls. There are many alternative explanations, including that the deficit could arise from reduced motivation arising from prior experience with uncontrollable stress. We can not distinguish among the alternatives with the present results. Inescapable shock stress decreased overall activity in the open field, as has been reported previously (Anderson et al., 1976, Plaznik et al., 1988, Van Den Berg et al., 1998, Van Dijken et al., 1992, Weyers et al., 1989). It is possible that stress-induced reduction in locomotor activity could contribute to increased shock escape latency. However, there was no significant interaction between stress and 5-htt genotype on locomotion in the open field, so it is unlikely that stressed-induced reduction in locomotion accounts for the increased escape deficit in the stressed 5-htt+/− mice compared to the stressed WT animals. Inescapable footshocks also increased center activity. Although many stress paradigms reduce center activity, increased center activity after repeated footshock has been reported (Lee & Huang, 1988, Lee et al., 1986). Thus, there is consistency both with previous 5-htt genotype results and inescapable shocks results.
We did not observe a main effect of stress in the forced swim test. Inescapable shock stress has been reported to increase immobility in the FST (Prince & Anisman, 1984). We explored whether there was in fact an effect of inescapable shock stress on forced swim behavior that could be revealed by separating shocked animals that showed a pronounced escape deficit from those animals that escaped relatively quickly. As others have done (Berton et al., 2007), we categorized stressed animals as resilient if they showed short escape latencies, and vulnerable if they showed long latencies (see supplemental material for methods and results and supplemental Figure S2). Vulnerable animals showed more immobility than resilient and non-stressed animals. No interaction between 5-htt genotype and resilient versus vulnerable status was present. The re-analysis of the forced swim data suggests that inescapable shock stress affects multiple depression-like measures, consistent with previous work.
The gene environment interaction experiment was designed to examine whether 5-htt genotype affected the response to inescapable shock stress on a number of tests of anxiety and depression-like behavior. We used a repeated testing design to gather as much data as possible from the significant investment in the 5-htt animals. We ran each of the behavioral tests within 24 hours of a shock session because inescapable shock session effects were shown to dissipate after 72 hours (Maier, 2001). There are several limitations of this design. First, only in the case of the shock escape and forced swim tests were the subjects exposed to all 3 inescapable shock sessions. Second, previously administered behavioral tests may have influenced the results of subsequent tests. We ordered the tests in what we believed was an increasing intensity of stress (open field, novelty suppressed feeding, forced swim, shock escape) to minimize the amount of exposure to stress unrelated to shock sessions before a given test. Regardless of these precautions, the inherent limitations of the repeated testing design were present. Since that a genotype by stress interaction has been demonstrated, future work can address these concerns: the inescapable shock sessions can be administered followed by a single behavioral test.
Enhanced effects of inescapable shocks in 5-htt deficient mice were observed in the shock escape test but not in the open field, novelty suppressed feeding, and forced swim tests. This is somewhat unexpected, given that the human 5httlpr polymorphism is associated with multiple anxiety and mood related phenomena. The absence of interactions in the open field and novelty suppressed feeding tests could be due to those tests not being preceded by as many inescapable shock sessions as was the case for the shock escape test. Mitigating against this explanation, the single shock session was effective: in the open field, stress reduced locomotion regardless of genotype. It could still be the case that the 5-htt deficient mice did not show an enhanced vulnerability to reduced locomotion because more intense, prolonged stress would be required to reveal the interaction. In any case, the effects of inescapable shock can vary by test and mouse strain (Shanks and Anisman, 1988). This raises the possibility that distinct brain circuits underlie behavior in different tests, and that the circuits are differentially affected by the interaction of 5-htt genotype and stress.
We next asked whether serotonin transporter genotype's role in the shock escape test might arise from augmented fear learning. It could have been that the 5-htt deficient mice showed increased escape latency in the shock escape test because they conditioned more to the shocks, and hence were freezing more instead of escaping as rapidly. In the fear conditioning experiment, 5-htt−/− mice did not differ from WT animals in freezing behavior to the chamber before conditioning. 5-htt−/− mice froze less to the context than WT subjects. There were no significant differences in conditioning to the tone cue. 5-htt deficient mice of a different background strain (C57bl6) did not differ in acquisition and expression of fear conditioning when compared to WT controls (Wellman et al., 2007). Hence, it is unlikely that enhanced fear conditioning can account for the vulnerability of transporter deficient mice to increased shock escape latency. Furthermore, it is unlikely that differences in shock sensitivity among 5-htt genotypes account for shock escape finding. In previously published results from our lab, 5-htt−/− mice showed no difference from wild-type animals in shock sensitivity (Lira et al., 2003). Changes in pain sensitivity and shock escape deficits have been previously shown to be dissociable consequences of inescapable shocks; notably, experimentally-induced reduction in pain sensitivity did not alter shock escape deficits induced by inescapable shock (Mah et al., 1980). Thus differences in fear conditioning and shock sensitivity likely do not account for the current results. Nonetheless, it would be informative, for its own sake, to investigate whether there is an interaction between 5-htt genotype and inescapable shock stress on shock sensitivity.
Given the state of the human literature, there is particular relevance for demonstrations that the interaction of 5-htt genotype and environment alters affective behavior in other animals. Among Macaque monkeys, there is also a polymorphism in the serotonin promoter region. The monkey allele homologous to the human low expressing allele confers increased risk taking (Suomi, 2006). When combined with poor maternal care, carriers of that allele show deficits in social interactions, increased banishment from the group and increased mortality.
Previous investigations of the interaction of environment with serotonin transporter genotype in mice reported mixed results. Wellman and colleagues (2007) found that 5-htt−/− mice show a repeated-testing induced increase in immobility in the forced swim test, whereas there was no difference in immobility between 5-htt−/− and WT mice in an initial forced swim test. This study did not include the 5-htt+/− mouse. The interaction, reported using the C57/bl6 mouse strain, is not present in the strain of mice used here, 129SvEv/S6, in which 5-htt−/− show increased immobility in the absence of environmental stressors. As noted, we observed no differences in stress-induced increases in immobility due to 5-htt genotype. Another study found no interaction between 10 days of footshocks in early postnatal life and 5-htt genotype (Carroll et al., 2007). Exposure to predator odor elicited more anxiety behavior in 5-htt−/− animals than either 5-htt+/− or WT mice, which did not differ from each other (Adamec et al., 2006). Importantly, 5-htt+/− and WT mice responded differently to variation in maternal care, on measures of anxiety and depression-like behavior (Carola et al., 2008). For example, in the open field test, 5-htt+/− mice with low maternal care spent less time than 5-htt+/− animals with high maternal care in the center region; and low maternal care 5-htt+/− mice spent more time in the field’s corners than both high maternal care 5-htt+/− subjects and the low maternal care WT animals. Another report demonstrated that 5-htt+/− mice were more avoidant during social interactions following a social stressor than were wild-type animals (Bartolomucci et al.). Thus, the current finding adds to a growing number of reports of 5-htt gene environment interaction in anxiety and depression-like behavior, but notably only with some stressors and revealed by different behaviors, depending on the stressor.
Genetically reduced complement of serotonin transporter could confer vulnerability to increased shock escape latency through at least two, possibly independent, mechanisms. First, reduced serotonin transporter levels could alter brain development. The adult brain of the 5-htt deficient mouse could be differently “wired” than that of the wild-type. In support of this developmental hypothesis, we have previously shown that disruption of the serotonin transporter only in early postnatal life increases adult anxiety and depression-like behavior (Ansorge et al., 2004). Alternatively, differences in the expression of the transporter could directly produce differences in the clearance of serotonin from the extracellular space during the critical events during inescapable shock sessions and shock escape testing. Increased shock escape latency is associated with increased serotonergic activity in the dorsal raphe nucleus (Grahn et al., 1999b, Maswood et al., 1998). Experimental enhancement of serotonergic activity there induces increased latency using a inescapable shock protocol that otherwise does not induced escape deficits (Grahn et al., 1999a, Maier et al., 1995a). Lesion (Maier et al., 1993) or disruption of serotonergic activity in the raphe (Maier et al., 1995b, Maier et al., 1994) blocks the induction and the expression of shock escape deficits. Hence the enhanced extracellular serotonin concentration of the 5-htt deficient mice could directly contribute to increased shock escape latency.
The interaction of genes and environment is critical to normal development and mature function. In the search for causes of mental illness, the importance of such interactions between gene and environment has been recognized. Here we show that a vulnerability to depression-like behavior can arise from the interaction between serotonin transporter genotype and adult stress. Finding a vulnerability to increased shock escape latency in the 5-htt+/− mouse offers the opportunity for investigation of its neural basis.
Supplementary Material
Supplemental Figure S1. Novelty suppressed feeding test. Mean latency (and standard error) to bite a food pellet is displayed by group. All subjects were food deprived for 24 hours, then placed in the corner of a box with familiar bedding. In the center was a disk, with a familiar food pellet secured to the disk. Time in seconds from placement in the box to a bite on the pellet was recorded. 5-htt−/− mice had longer latencies that 5-htt+/− and WT animals, which did not differ significantly. Stress changed latencies differently by genotype: stressed WT and 5-htt+/− latencies were higher than corresponding unstressed groups (not significantly), stressed 5-htt−/− animals had lower latencies than unstressed counterparts (not significantly). The different directions of effect of stress on 5-htt−/− mice compared to 5-htt+/− and WT subjects gave rise to a significant gene-stress interaction. There was no overall main effect of stress. Statistics for these analysis of variance effects are found in the main text. Shown in the figure are individual groups that differed significantly: WT mice differed from 5-htt−/− subjects in both the stressed and unstressed condition; 5-htt+/− mice differed from 5-htt−/− animals in the unstressed condition. ** p < .01, ** p < .001
Supplemental Figure S2. Forced swim immobility by shock escape latency category. Among animals exposed to inescapable shock sessions, subjects were categorized as "vulnerable" if the mean shock escape latency on trials 11 to 30 was greater than 7 seconds. Stressed animals with quicker escape latencies were called "resilient". The distribution of escape latencies by stress condition is shown in Panel A. "Vulnerable" animals showed more immobility than did "resilient" and unstressed subjects. (Panel B) Posthoc analysis of results showed that for the 5-htt+/− genotype, the vulnerable group differed from the resilient group. * < .05
Acknowledgements
Thanks to Richard Swedarsky, Pooja Shah, Juliet Wojciechowski and Sarah Eberle for their efforts with behavioral experiments, and to Tamara Harr for her editing of the manuscript. This work was supported by the NIMH grant R01-MH076026-01.
Footnotes
Disclosure/Conflict of Interest
No authors have financial interests or holdings that could be perceived as constituting a potential conflict of interest.
Citations
- Adamec R, Burton P, Blundell J, Murphy DL, Holmes A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behavioural brain research. 2006;170:126–140. doi: 10.1016/j.bbr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Anderson DC, Crowell C, Koehn D, Lupo JV. Different intensities of unsignalled inescapable shock treatments as determinants of non-shock-motivated open field behavior: a resolution of disparate results. Physiol Behav. 1976;17(3):391–394. doi: 10.1016/0031-9384(76)90096-2. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Aslund C, Leppert J, Comasco E, Nordquist N, Oreland L, Nilsson KW. Impact of the interaction between the 5HTTLPR polymorphism and maltreatment on adolescent depression. A population-based study. Behav Genet. 2009;39:524–531. doi: 10.1007/s10519-009-9285-9. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Carola V, Pascucci T, Puglisi-Allegra S, Cabib S, Lesch KP, Parmigiani S, Palanza P, Gross C. Increased vulnerability to psychosocial stress in heterozygous serotonin transporter knockout mice. Disease models & mechanisms. doi: 10.1242/dmm.004614. [DOI] [PubMed] [Google Scholar]
- Berton O, Covington HE, 3rd, Ebner K, Tsankova NM, Carle TL, Ulery P, Bhonsle A, Barrot M, Krishnan V, Singewald GM, Singewald N, Birnbaum S, Neve RL, Nestler EJ. Induction of deltaFosB in the periaqueductal gray by stress promotes active coping responses. Neuron. 2007;55:289–300. doi: 10.1016/j.neuron.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Carola V, Frazzetto G, Pascucci T, Audero E, Puglisi-Allegra S, Cabib S, Lesch KP, Gross C. Identifying molecular substrates in a mouse model of the serotonin transporter x environment risk factor for anxiety and depression. Biol Psychiatry. 2008;63:840–846. doi: 10.1016/j.biopsych.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Boyce-Rustay JM, Millstein R, Yang R, Wiedholz LM, Murphy DL, Holmes A. Effects of Mild Early Life Stress on Abnormal Emotion-related Behaviors in 5-HTT Knockout Mice. Behav Genet. 2007;37:214–222. doi: 10.1007/s10519-006-9129-9. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-HTT Gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Collier DA, Stober G, Li T, Heils A, Catalano M, Di Bella D, Arranz MJ, Murray RM, Vallada HP, Bengel D, Muller CR, Roberts GW, Smeraldi E, Kirov G, Sham P, Lesch KP. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol Psychiatry. 1996;1:453–460. [PubMed] [Google Scholar]
- Grahn RE, Maswood S, McQueen MB, Watkins LR, Maier SF. Opioid-dependent effects of inescapable shock on escape behavior and conditioned fear responding are mediated by the dorsal raphe nucleus. Behavioural brain research. 1999a;99:153–167. doi: 10.1016/s0166-4328(98)00101-6. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999b;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002;27:914–923. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- Lee EH, Huang SL. Role of lateral habenula in the regulation of exploratory behavior and its relationship to stress in rats. Behavioural brain research. 1988;30:265–271. doi: 10.1016/0166-4328(88)90169-6. [DOI] [PubMed] [Google Scholar]
- Lee EH, Tsai MJ, Chai CY. Stress selectively influences center region activity of mice in an open field. Physiology & behavior. 1986;37:659–662. doi: 10.1016/0031-9384(86)90301-x. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF, Hofer MA, Hen R, Gingrich JA. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Mah C, Suissa A, Anisman H. Dissociation of antinociception and escape deficits induced by stress in mice. J Comp Physiol Psychol. 1980;94(6):1160–1171. doi: 10.1037/h0077742. [DOI] [PubMed] [Google Scholar]
- Maier SF. Exposure to the stressor environment prevents the temporal dissipation of behavioral depression/learned helplessness. Biol Psychiatry. 2001;49:763–773. doi: 10.1016/s0006-3223(00)01095-7. [DOI] [PubMed] [Google Scholar]
- Maier SF, Busch CR, Maswood S, Grahn RE, Watkins LR. The dorsal raphe nucleus is a site of action mediating the behavioral effects of the benzodiazepine receptor inverse agonist DMCM. Behav Neurosci. 1995a;109:759–766. doi: 10.1037//0735-7044.109.4.759. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Kalman BA, Sutton LC, Wiertelak EP, Watkins LR. The role of the amygdala and dorsal raphe nucleus in mediating the behavioral consequences of inescapable shock. Behav Neurosci. 1993;107:377–388. doi: 10.1037//0735-7044.107.2.377. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav Neurosci. 1995b;109:404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- Maier SF, Kalman BA, Grahn RE. Chlordiazepoxide microinjected into the region of the dorsal raphe nucleus eliminates the interference with escape responding produced by inescapable shock whether administered before inescapable shock or escape testing. Behav Neurosci. 1994;108:121–130. doi: 10.1037//0735-7044.108.1.121. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience and biobehavioral reviews. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Mendlewicz J, Massat I, Souery D, Del-Favero J, Oruc L, Nothen MM, Blackwood D, Muir W, Battersby S, Lerer B, Segman RH, Kaneva R, Serretti A, Lilli R, Lorenzi C, Jakovljevic M, Ivezic S, Rietschel M, Milanova V, Van Broeckhoven C. Serotonin transporter 5HTTLPR polymorphism and affective disorders: no evidence of association in a large European multicenter study. Eur J Hum Genet. 2004;12:377–382. doi: 10.1038/sj.ejhg.5201149. [DOI] [PubMed] [Google Scholar]
- Montanez S, Owens WA, Gould GG, Murphy DL, Daws LC. Exaggerated effect of fluvoxamine in heterozygote serotonin transporter knockout mice. J Neurochem. 2003;86:210–219. doi: 10.1046/j.1471-4159.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Overmier JB, Seligman MEP. Effects of inescapable shock upon subsequent escape and avoidance learning. Journal of Comparative Physiology and Psychology. 1967;63:28–33. doi: 10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- Plaznik A, Tamborska E, Hauptmann M, Bidzinski A, Kostowski W. Brain neurotransmitter systems mediating behavioral deficits produced by inescapable shock treatment in rats. Brain Res. 1988;447:122–132. doi: 10.1016/0006-8993(88)90972-9. [DOI] [PubMed] [Google Scholar]
- Prince CR, Anisman H. Acute and chronic stress effects on performance in a forced-swim task. Behav Neural Biol. 1984;42:99–119. doi: 10.1016/s0163-1047(84)90942-7. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Bradley B, Mercer KB, Deveau TC, Smith AK, Gillespie CF, Nemeroff CB, Cubells JF, Binder EB. Polymorphisms in CRHR1 and the serotonin transporter loci: Gene × Gene × Environment interactions on depressive symptoms. Am J Med Genet B Neuropsychiatr Genet. 2009 doi: 10.1002/ajmg.b.31052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. Jama. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Anisman H. Stressor-provoked behavioral changes in six strains of mice. Behav Neurosci. 1988;102:894–905. doi: 10.1037//0735-7044.102.6.894. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Risk, resilience, and gene × environment interactions in rhesus monkeys. Annals of the New York Academy of Sciences. 2006;1094:52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- Van den Berg CL, Lamberts RR, Wolterink G, Wiegant VM, Van Ree JM. Emotional and footshock stimuli induce differential long-lasting behavioural effects in rats; involvement of opioids. Brain Res. 1998;799:6–15. doi: 10.1016/s0006-8993(98)00397-7. [DOI] [PubMed] [Google Scholar]
- van Dijken HH, Mos J, van der Heyden JA, Tilders FJ. Characterization of stress-induced long-term behavioural changes in rats: evidence in favor of anxiety. Physiology & behavior. 1992;52:945–951. doi: 10.1016/0031-9384(92)90375-c. [DOI] [PubMed] [Google Scholar]
- Weiss JM. Effects of coping responses on stress. J Comp Physiol Psychol. 1968;65:251–260. doi: 10.1037/h0025562. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired Stress-Coping and Fear Extinction and Abnormal Corticolimbic Morphology in Serotonin Transporter Knock-Out Mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyers P, Bower DB, Vogel WH. Relationships of plasma catecholamines to open-field behavior after inescapable shock. Neuropsychobiology. 1989;22(2):108–116. doi: 10.1159/000118602. [DOI] [PubMed] [Google Scholar]
- Wray NR, James MR, Gordon SD, Dumenil T, Ryan L, Coventry WL, Statham DJ, Pergadia ML, Madden PA, Heath AC, Montgomery GW, Martin NG. Accurate, Large-Scale Genotyping of 5HTTLPR and Flanking Single Nucleotide Polymorphisms in an Association Study of Depression, Anxiety, and Personality Measures. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Novelty suppressed feeding test. Mean latency (and standard error) to bite a food pellet is displayed by group. All subjects were food deprived for 24 hours, then placed in the corner of a box with familiar bedding. In the center was a disk, with a familiar food pellet secured to the disk. Time in seconds from placement in the box to a bite on the pellet was recorded. 5-htt−/− mice had longer latencies that 5-htt+/− and WT animals, which did not differ significantly. Stress changed latencies differently by genotype: stressed WT and 5-htt+/− latencies were higher than corresponding unstressed groups (not significantly), stressed 5-htt−/− animals had lower latencies than unstressed counterparts (not significantly). The different directions of effect of stress on 5-htt−/− mice compared to 5-htt+/− and WT subjects gave rise to a significant gene-stress interaction. There was no overall main effect of stress. Statistics for these analysis of variance effects are found in the main text. Shown in the figure are individual groups that differed significantly: WT mice differed from 5-htt−/− subjects in both the stressed and unstressed condition; 5-htt+/− mice differed from 5-htt−/− animals in the unstressed condition. ** p < .01, ** p < .001
Supplemental Figure S2. Forced swim immobility by shock escape latency category. Among animals exposed to inescapable shock sessions, subjects were categorized as "vulnerable" if the mean shock escape latency on trials 11 to 30 was greater than 7 seconds. Stressed animals with quicker escape latencies were called "resilient". The distribution of escape latencies by stress condition is shown in Panel A. "Vulnerable" animals showed more immobility than did "resilient" and unstressed subjects. (Panel B) Posthoc analysis of results showed that for the 5-htt+/− genotype, the vulnerable group differed from the resilient group. * < .05